Abstract
Histone deacetylases (HDACs) play important roles in regulating gene expression of tumorigenesis and tumor maintenance, and they have been considered as key targets in cancer therapy. As an novel category of antitumor agents, the histone deacetylase inhibitors (HDACis) can induce cell cycle arrest, apoptosis, and differentiation in cancer cells, and then combat cancer. Despite the treatment of certain cancers have been approved, the therapeutic efficacy of HDACis with solid tumors is always unsatisfactory and the drug resistance may occur. To enhance the therapeutic efficacy and reverse acquired drug resistance, numerous combination therapies involving HDACis in synergy with other antitumor therapies have been studied. In this review, we have made a brief classification of HDACs. Moreover, the antitumor mechanism of the HDACis for targeting key cellular processes of cancers (cell cycle, apoptosis, angiogenesis, DNA repair, and immune response) has been summarized. In addition, we have also outlined the major developments of other antitumor therapies in combination with HDACis, including chemotherapy, radiotherapy, phototherapy, targeted therapy, and immunotherapy. Finally, we have discussed the current state and challenges of HDACis-drugs combinations in future clinical trials, with the aim of optimizing the antitumor effect of such combinations.
Keywords: histone deacetylations, cancer, histone deacetylases inhibitors, combination therapies
Graphical Abstract
1. Introduction
Epigenetic abnormalities, such as histone deacetylases (HDACs), play important roles in modulating cellular processes and regulating gene expression [1, 2]. Abnormal activity in histone acetylation are often associated with tumor progression and HDACs are recognized as key targets to the onset and maintenance of various cancers [3, 4]. HDACs are a family of human enzymes that can tight bind of DNA to the histone proteins and upregulate the deacetylation of protein substrates, thereby favoring compact chromatin and preventing gene transcription [5, 6]. Based on homology, HDACs enzymes are commonly divided into four different categories: class I (HDACs 1-3, and 8), which are mostly located in the nucleus; class II (class IIa: HDACs 4, 5, 7, and 9, class IIb: HDACs 6 and 10), which are mostly located in both the cytoplasm and nucleus; class III (sirtuins 1- 7), which are located in the cytoplasm, mitochondria, and nucleus; and class IV (HDAC11) [7, 8]. Classes I, II, and IV HDACs require zinc molecule as an essential cofactor, while class III HDACs are NAD+ dependent protein deacetylases [9, 10]. Particularly, the gene expression levels of HDACs in cancer cells are always higher than that in normal cells [11, 12]. Recently, several studies on HDACs have received intensive attention in both scientific researches and clinical trials [13, 14].
The histone deacetylase inhibitors (HDACis) can inhibit the growth of cancer cells by inducing cell cycle arrest, apoptosis, angiogenesis, DNA repair, and enhancement of immunogenicity [15, 16]. Therefore, a series of HDACis have been studied in clinical trials for developing novel antitumor strategies [17]. To date, five HDACis have been approved by the USA food and drug administration (FDA) for the treatment of various cancers: including vorinostat (SAHA), panobinostat (LBH-589), belinostat (PXD-101), valproic acid (VPA), and romidepsin (FK-228) [18, 19]. Despite a single agent of HDACis have been applied and conducted for treating tumors, the most of them are ineffective in the clinic [20]. When in combination with other antitumor therapies, HDACis might be effective for sensitizing tumor cells to a certain therapy, overcoming drug resistance and realizing synergistic effect [21, 22]. Therefore, to reverse the drug resistance and enhance the therapeutic potential of HDACis in solid tumors, combining HDACis with other antitumor therapies (HDACis-drugs) have been investigated in the clinic [23, 24]. Therefore, this review will firstly make the description and classification of HDACs, outline the antitumor mechanisms of HDACis, and then highlight various combinations of HDACs with other therapies in cancer treatment, such as chemotherapy, radiotherapy, phototherapy, targeted therapy and immunotherapy. We also summarize the current clinical state and challenges of HDACis-drugs combinations, aiming at rational design of HDACis for enhanced efficacy in future clinical trials.
2. Role of HDACis in cancer therapy
HDACs are a class of enzymes which play a major role in many physiological processes, such as gene expressions transcription, cell differentiation, proliferation, metastasis, and survival [25, 26]. In recent years, HDACs have been found to be overexpressed in several human cancer cell lines, including breast, liver, gastric, and lung cancers [27, 28]. By regulating the activity or silencing gene expression of numerous proteins in cancer progression, HDACs are regarded as highly attractive drug targets for cancer therapy [29, 30].
2.1. HDACs
HDACs are ancient protease found in various organisms of mammals, which play important roles in gene expression regulation and chromosome structural modification [31]. The acetylation and deacetylation processes of histone are in regulation with both HDACs and histone acetyltransferase (HATs) and sustain in dynamic equilibrium in the cell nucleus [32]. On one hand, acetylation of histone is conductive to generating a looser nucleosome structure which interferes with the repair and recombination process of DNA, and the gene transcription is promoted [33]. On the other hand, deacetylation of histones is beneficial for the ionic interactions between DNA with negative charge and histone octamers with positive charge, thereby a compact nucleosome structure is produced [34, 35]. By preventing the specifically access of various transcription factors to the DNA binding site, the gene transcription is inhibited [36]. This epigenetic information can regulate gene expression and enhance the activity of transcription factors in various physiological processes, which has become the most attractive target related to disorders and diseases [37, 38].
As shown in Table 1, HDACs have been identified in 18 isoforms and generally classified into four categories relying on subcellular localization, substrate recognition, expression patterns, and physiological implications: including class I (HDACs 1-3, and 8), class II (class IIa: HDACs 4, 5, 7, and 9, class IIb: HDACs 6 and 10), class III (sirtuins 1- 7), and class IV (HDAC11) [39, 40]. Among all human HDACs, eleven kinds of the HDACs (HDACs 1-11) are zinc-dependent (Zn2+) metalloenzymes, which water can be served as a nucleophile to hydrolyze the amide bond. Class I HDACs possess an amino acid sequence of 400-500 residues [41]. Class I HDACs molecules are expressed in most types of cells and can affect both non-histone and histone substrates [42]. Class I HDACs, including HDACs 1-3 and 8, are usually localized in the cell nucleus (HDAC8 shuttles between the cell nucleus and cytoplasm), showing sequence homology with the gene of yeast reduced potassium dependency-3 (Rpd3) [43]. Class II HDACs are further classified into two subgroups: class IIa (HDAC 4, 5, 7 and 9) with a large C-terminus, and class IIb (HDAC 6 and 10) with two deacetylase domains [44]. Class II HDACs, including class IIa and class IIb, share sequence homology with the yeast histone deacetylases 1 (Hda1) gene, are mostly localized in the cytoplasm [45, 46]. Composed of an amino acid sequence of 1000 residues, class II HDACs exhibit certain tissue and cell specificity when compared to class I HDACs. Except for HDAC6, other class II HDACs (HDACs 4, 5, 7 and 9) could also shuttle between the cell nucleus and cytoplasm with the conduction of specific cellular signals [47]. Class IV HDACs molecules have only one member (HDAC11) and are mainly located in the cell nucleus. Composed of 347 amino acids, the structures of class IV HDACs are similar to class I HDACs [48]. Class III HDACs molecules, which always called “sirtuins”, composed of sirtuins 1-7 (sirt 1-7). Compared with other HDACs of Zn2+ dependent, Class III HDACs employ nicotine-adenine dinucleotide (NAD+) as a cofactor for their enzyme activity, displaying biochemical and structural specificity [49]. Generally, Class III HDACs are efficient in regulating cell metabolism, including DNA repair, cell apoptosis, and inflammatory response [50].
Table 1.
Classification of HDACs.
| Class | HDACs | Localization | Dependent | Amino Acid | Physiological Implications |
|---|---|---|---|---|---|
| I | HADC 1 | Nucleus | Zn2+ | 483 | Cell survival, proliferation |
| HADC 2 | Nucleus | 488 | Insulin resistance | ||
| HADC 3 | Nucleus/Cytoplasm | 428 | Cell survival, proliferation | ||
| HADC 8 | Nucleus | 377 | Cell proliferation | ||
|
| |||||
| IIa | HADC 4 | Nucleus/Cytoplasm | Zn2+ | 1084 | Skeletogenesis |
| HADC 5 | Nucleus/Cytoplasm | 1122 | Cardiovascular growth | ||
| HADC 7 | Nucleus/Cytoplasm | 952 | Endothelial growth | ||
| HADC 9 | Nucleus/Cytoplasm | 1011 | Thymocyte differentiation | ||
|
| |||||
| IIb | HADC 6 | Nucleus/Cytoplasm | Zn2+ | 1215 | Cell motility |
| HADC 10 | Nucleus/Cytoplasm | 669 | Cell survival | ||
|
| |||||
| III | Sirtuin 1 | Nucleus/Cytoplasm | NAD+ | 747 | Aging, redox regulation, apoptosis, metabolism |
| Sirtuin 2 | Nucleus | 389 | |||
| Sirtuin 3 | Mitochondria | 399 | |||
| Sirtuin 4 | Mitochondria | 314 | |||
| Sirtuin 5 | Mitochondria | 310 | |||
| Sirtuin 6 | Nucleus | 355 | |||
| Sirtuin 7 | Nucleus | 400 | |||
|
| |||||
| IV | HADC 11 | Nucleus | Zn2+ | 347 | Immunomodulators |
Epigenetic dysregulation can promote the occurrence and progression of various diseases [51]. Class I HDACs could regulate cell proliferation effectively. Among these isozymes, HDAC 1, a key regulator of tumor suppressor p53, is a promising target for the treatment of lung, breast, and gastric cancer [52]; by binding to nuclear receptors, HDAC 2 could not only simulate insulin release in the diabetes therapy, but also promote cell progression and prevent apoptosis by inhibiting the p53 [53]; deacetylation of HDAC 3 is related to cellular proliferation, DNA damage and cell apoptosis, which is overexpressed in various cancers, such as colon cancer, breast cancer, and cervical cancer [54]; HDAC 8 acts on non-histone proteins correlated with cancers, such as p53, inversion fusion protein, estrogen-related receptor alpha, and cortactin, and only overexpressed in neuroblastoma [55]. Class II HDACs could be served as versatile regulators. Among these isozymes, by modulating the activity of RunX2, HDAC 4 can regulate bone hypertrophy during skeletogenesis [56]; HDAC 5 is an inhibitor of angiogenesis, which determines the development of the cardiovascular system [57]; by silencing the formation of endothelial progenitor cells, HDAC 7 plays an important role in maintaining vascular integrity [58]; HDAC 9 can moderate the development of T-cells at various stages of thymocyte differentiation, which is a target for immunotherapy [59]; HDAC 6 can specially bind to α-tubulin proteins and block its fibrillar action, thereby regulating the actin dependent motility [60]; HDAC 10 downregulates the cell survival by increasing the efflux of drugs and inhibiting DNA damage, serving as the target genes in neuroblastoma acute promyelocytic leukemia [61]. Class III HDACs (sirtuins) are eligible for various biological functions, including aging. Among these isozymes, redox homeostasis, metabolism regulation, and DNA repair. Under both physiological and pathological environment, sirtuins could regulate different metabolic and redox pathways, which are pivotal steps in several disorders [62]. By blocking the expression of gene encoding interleukin 10 (IL-10), Class IV HDACs (HDAC 11) is an immunoregulator, which may be a target for immunotherapy [63]. Because that many HDACs relate to various cancers, the inhibitors of HDACs can be considered as effective tools in cancer treatment.
2.2. HDACis
As HDACs-targeting inhibitors, several HDACis have been widely recognized for enhancing the acetylation of cellular proteins [64]. Due to altered HDACs expression, several types of cancers are associated with disrupted acetylation of histone/nonhistone proteins, resulting in inhibition of angiogenesis, cell cycle arrest, cell apoptosis, and modulate immune response [65, 66]. Attractively, HDACis can regulate the expression of several tumor suppressor genes or oncogenes, and they are necessary for regulating important genes related to survival and growth of cancer cells but not in normal cells [67]. Therefore, HDACis may produce additional therapeutic efficacy especially in cancer cells, bringing more breakthroughs in the field of tumor treatment. With the development of epigenetics research, HDACis have been identified as a promising treatment modality for cancer therapy with high efficacy and low toxicity.
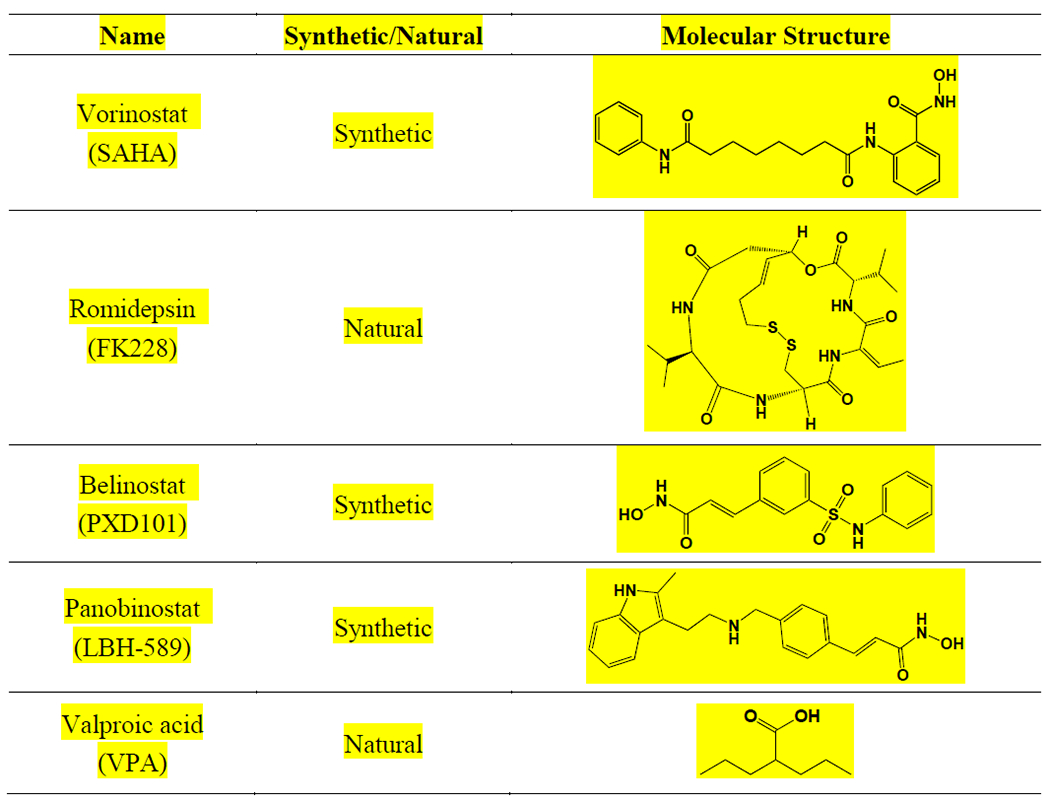
According to chemical structures, currently discovered natural or chemically synthesized HDACis can be classified into four main categories (Fig. 1): hydroxamic acids, such as SAHA and trichostatin A (TSA); fatty acids, such as VPA and butyric acid; benzamides, such as entinostat (MS-275) and chidamide (CS055); cyclic peptides, such as natural products FK228 and apicidin [68, 69]. Hydroxamic acids is the largest one family among them, and its members including SAHA, LBH589, and PDX101, have been approved by the FDA [70]. TSA, the first natural hydroxamate to inhibit HDACs, exhibits a similar structure to SAHA. Short chain fatty acids, including VPA and butyric acid, target mainly class I HDACs and class IIa HDACs [71]. Benzamides and cyclic peptides families to a great extent target class I HDACs [72]. Recently, several HDACis have been approved by FDA as anticancer agents, including SAHA, FK228, PXD101, LBH-589 and VPA (Table 2) [73]. Besides, some other HDACis have been applied in clinical trials, such as CS055, TSA, MS-275, and curcumin.
Fig. 1.
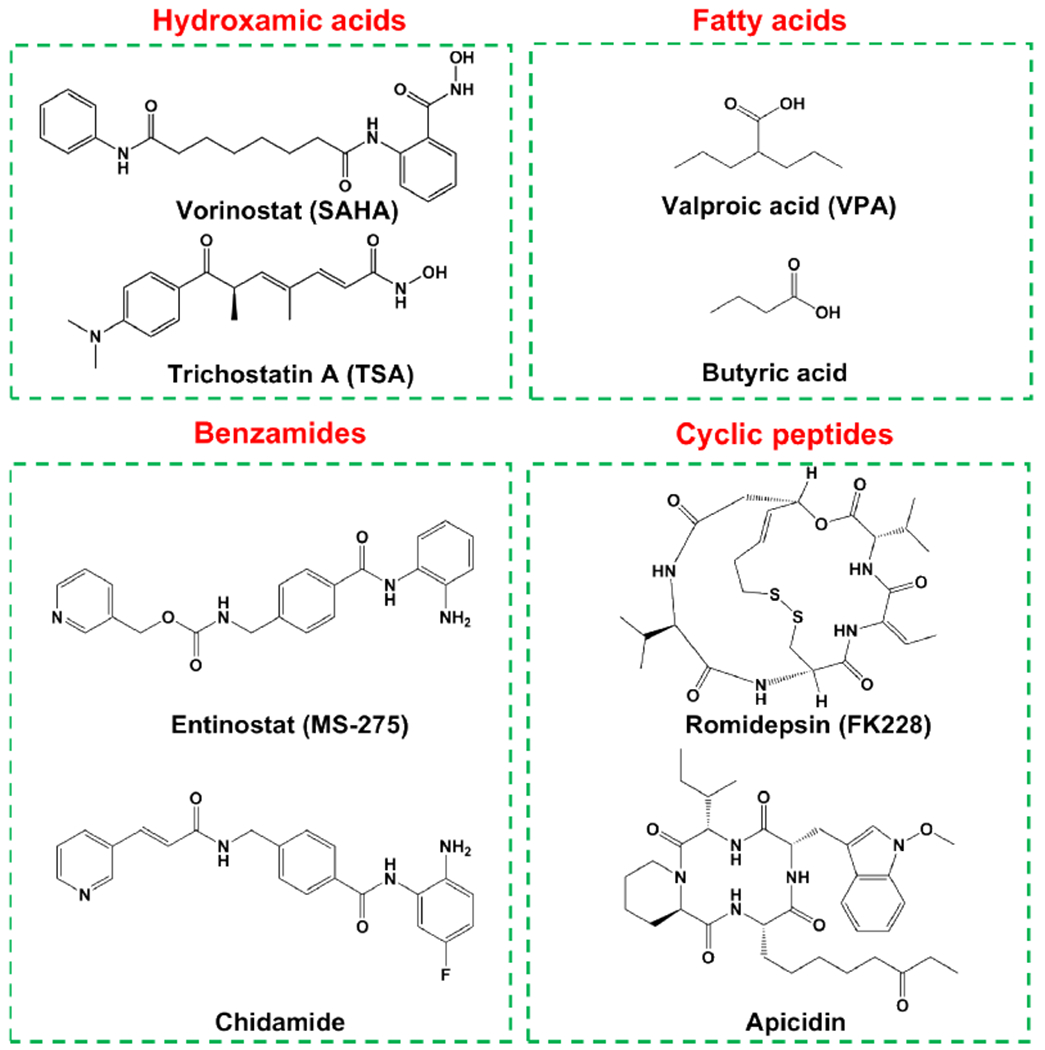
Classification of HDACis and the structures of representative compounds.
Table 2.
FDA approved anticancer HDACis.
|
2.3. Effect of HDACis in cancer therapy
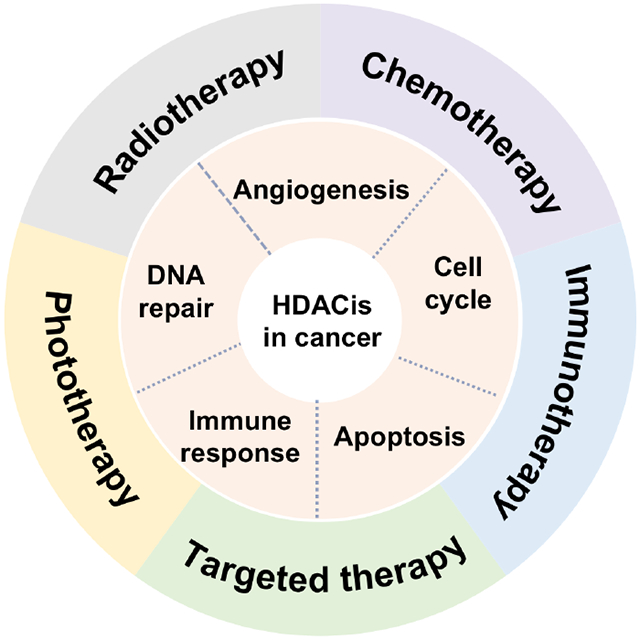
Due to the large number of oncogenic genes regulated by HDACs, HDACis have been extensively served as alternatives to anticancer agents, which exert a good antitumor efficacy in vitro and in vivo [74]. In many cases, inhibition of abnormal acetylation on histones can emerge as a therapeutic tool with antitumor activity, inducing the cell cycle arrest, sensitivity to cell apoptosis, inhibition of angiogenesis, DNA damage repair, and modulating immune response [75]. Herein, the major cellular/physiological pathways affected by HDACs have been presented below (Fig. 2).
Fig. 2.
The major cellular processes affected by HDACis in cancer cells.
2.3.1. Cell cycle
By upregulating the expression of cyclin-dependent kinase (CDK) inhibitor, such as the protein p21, HDACis can induce cell cycle arrest (in G0/G1, G1/S or G2/M phase) [76, 77]. Induction of p21 protein disrupts the production of dimers from cyclins and CDK, thereby inhibiting cell differentiation. Besides, another CDK inhibitor, the protein p53, could regulate the expression of p21 protein via binding to its promotor [78]. The deacetylation of p53 protein inhibited by HDACis can further improve its interaction with p21 protein. Dysregulated growth of cells has been known as an important factor in cancers, therefore this epigenetic modification may produce essential influences in oncogenesis [79].
2.3.2. Apoptosis
HDACis play an important role in modulating apoptosis pathways of both the intrinsic and extrinsic, resulting in the elimination of damaged cells [80]. On one hand, HDACis can increase the expression of certain death receptor (DR4, DR5) in cancer cells as the extrinsic pathway [81]. The caspase 8 and 10 could be activated by the interaction of DR4 and DR5 with tumor necrosis factor (TNF) receptor and their ligands, thus inducing cell apoptosis [82]. On the other hand, HDACis can also regulate intracellular apoptotic pathways by regulating apoptosis inhibitor, or modulating the interaction of death domain with the death-inducing signaling complex [83]. HDACis have been proved to upregulate the expression of the anti-apoptotic BCL-2 proteins or BH3-only proteins [84]. In addition, HDACis could induce the intrinsic apoptotic pathway by promoting cytochrome c release from the membrane of mitochondrial, resulting in the activation of caspase-9 [85].
2.3.3. Angiogenesis
With a mainly anti-angiogenic action, HDACis have been shown to modulate angiogenesis via various mechanisms [86]. HDACis can not only downregulate the expression of hypoxia-inducible factor-1α (HIF-1α), but also upregulate the expression of vascular endothelial growth factor (VEGF) in several types of cancers, resulting in the degradation of HIF-1α [87]. Besides, HDACis can also increase the level of anti-angiogenic proteins, such as activin A and thrombospondin-1, therefore influence angiogenesis of endothelial cells. VPA could upregulate the expression of both anti-angiogenic genes by decreasing the level of proangiogenic factors [88].
2.3.4. DNA repair
Due to DNA damage repair (DDR) machinery, cancer cells exhibit inefficient sensitivity to chemotherapy and radiotherapies [89]. When combining with HDACis, an increase DNA damage could be induced, demonstrating that the inhibition of HDACs blocks the capacity to repair double strand break of DNA. Some HDACis, such as TSA and SAHA, can downregulate the expression of recombination protein A and breast cancer 1, thus inhibiting the mechanisms of homologous/non-homologous recombination end joining DNA damage repair [90, 91]. Besides, HDACis can upregulate the generation of reactive oxygen species (ROS), which induce DNA damages and cell death in apoptosis-resistant cells [92].
2.3.5. Immune response
HDACis can regulate the expression of immune response molecules, such as costimulatory molecules, thereby upregulating antigen presentation, which in turn activating T cells [93]. Generally, the inhibitors of class I HDACs have been proved to target adaptive immunity through advancing the functionality of CD8+ cells and natural killer [94]. Moreover, class II HDACis can target Tregs. In addition, the inhibitor of HDAC6 could improve immune checkpoint blockade (ICB) in melanoma [95].
3. HDACis as anticancer agents for combination therapy
Low plasma concentration of HDACis have shown no significant clinical efficacy in solid tumors [96]. To enhance the therapeutic efficacy, the development of drug combinations has become a promising approach. Targeting two or multiple different pathways involved in cancers often results in synergistic effects and low drug resistance [97]. For example, Vyxeos (a FDA-approved liposomal nanomedicine), which is constructed by cytarabine and daunorubicin in fixed ratio, could effectively prolong the survival time of cancer patients [98]. When compared to individual drugs, only smaller drugs doses are needed to realize excellent therapeutic effects in cancer therapy, thereby decreasing side and toxic effects [99]. Given that, designing combination therapy integrating with HDACis and other therapeutic modalities may further enhance the therapeutic effect of cancer therapy.
Generally, several studies have found that HDACs would result in tumor resistance to chemotherapeutic drugs, and inhibitors targeting HDACs could increase their sensitivity to chemotherapy [100]. Moreover, HDACis can enhance antitumor immune response via several endogenous mechanisms [101]. In recent years, combination therapy of HDACis with other therapeutic drugs have demonstrated to realize enhanced biological effects in preclinical trials [102, 103]. Various combinations of HDACis with other anticancer therapies can enhance the antitumor efficacy of several major treatment of cancers, such as chemotherapy, radiotherapy, phototherapy, targeted therapy and immunotherapy [104, 105]. We will summarize the synergistic therapeutic mechanisms and corresponding examples of HDACis-drugs combinations in the following sections.
3.1. Chemotherapy
HDACis have been proved cumulative or synergistic antitumor efficacy in combination with other chemotherapeutic agents, and the synergistic effect are mostly been realized by damaging DNA or interfering with DDR [106]. Generally, the antitumor effect of chemotherapy is always limited by drug resistance to cytotoxic agents of cancer cells [107]. The combined use of HDACis at low doses might reverse resistance to chemotherapeutic drugs via removing some certain resistance phenotype [108]. When combined with topoisomerase II inhibitors, HDACis could improve the activity of topoisomerase II inhibitor to cleave DNA by promoting decondensation of chromatin, finally resulting in synergistic therapeutic effect [109]. Similar results have been found with other chemotherapeutic drugs, such as oxaliplatin, gemcitabine, 5-fluorouracil, and doxorubicin (Dox). ACY1215, a HDAC6 inhibitor, could block cell proliferation and cause apoptosis [110]. Therefore, the antitumor effect of oxaliplatin and gemcitabine could be enhanced by combining with ACY1215 [111]. These studies have supported the use of HDACi-drug combinations in cancer chemotherapy.
The current therapy of sarcomas are always with poor prognosis and have shown limited therapeutic efficacy [112]. Available therapeutic agents for sarcoma patients with metastatic tumors are chemotherapy based on anthracyclines regimens, such as Dox and epirubicin [113]. However, the antitumor effect is still insufficient, and new strategies need to be developed to improve the management of sarcomas patients. Martile and co-workers have found that ITF2357, an HDACi, potently inhibited the survival of cancer cells via a p53-independent manner by using sarcoma cells with different subtypes [114]. BH3-only proteins were effectors of mitochondrial apoptosis, and showed capability to activate Bax and Bak via direct or indirect mechanisms. Attested by a both BCL-2 and caspases-dependent mechanism and upregulating the expression of pro-apoptotic BH3-only proteins, ITF2357 activated mitochondrial pathway of apoptosis and finally cell death. Notably, co-treatment of ITF2357 with Dox strongly inhibited cell death of sarcoma cells in vitro. Compared to single treatments, the combination treatment effectively reduced xenograft tumor growth in vivo, demonstrating that combination of ITF2357 with Dox was promising to increase sensitization in preclinical and primary patient-derived sarcoma cells. Based on these results, this work highlights the potential therapeutic values of ITF2357 in combination therapies with anthracyclines regimens, aiming at further treating bone or soft tissue sarcomas.
Recently, many kinds of antitumor Pt(IV) prodrugs have been designed and synthesized, such as oxaliplatin, cisplatin and carboplatin [115]. Sabbatini and co-workers designed a cyclohexane-1R,2R-diamine-based Pt(IV) derivative containing 2-(2-propynyl)octanoic acid (POA), and (OC-6-44)-acetatodichlorido(cyclohexane-1R,2R-diamine)(rac-2-(2-propynyl)octanoato)platinum(IV), 1, were prepared [116]. Besides, its isomers, (OC-6-44)-acetatodichlorido(cyclohexane-1R,2R-diamine)(2R-(2-propynyl)octanoato)-platinum(IV), named 1R, and (OC-6-44)-acetatodichlorido-(cyclohexane-1R,2R-diamine)(2S-(2-propynyl)octanoato)-platinum(IV), named 1S, were synthesized as comparation. Among them, POA could serve as an inhibitor of HDACs. The synthesis process, characterization, and antitumor activity of these complexes were investigated in 3 human cancer cell lines (HCT 116, SW480, and HT-29) and 1 mouse colon cancer cell lines (CT26). Compared to 1R and 1S, the complex 1 exhibited higher accumulation, retention, drug release and inhibition in tumors as well as less nephro- and hepato- toxicities in in vivo experiments when administered intravenously. POA reversed the immune escape genes involved and induced ICD by activating cytotoxic CD8+ T lymphocytes, therefore the following Pt(IV) treatment provided Prodrug 1 an effective tumor mass invasion. The remarkable therapeutic effect of epigenetic drugs based Pt(IV) prodrugs paves the way for the therapy of colorectal cancer.
TSA can downregulate the expression of tumor suppressor genes and decrease tumor cell proliferation [117]. Cai and co-workers investigated the therapeutic effect of TSA in combination with gemcitabine against pancreatic ductal adenocarnoma (PDAC) cells (Fig. 3) [118]. The responsiveness and antitumor effect of PDAC cells to SAHA, TSA, and gemcitabine was evaluated. TSA or SAHA downregulated HDACs 1, 7 and 8, increased the levels of E-cadherin mRNA and protein, and inhibited the pluripotency transcription factors (Sox-2, Oct-4, Nanog), finally caused cell death. By improving the accumulation of acetylated histones (histone 3 lysine 4 or 9 dimethylation levels) in PDAC cells, TSA silenced the expression of HDACs 1, 7 and 8, inhibited of cancer stemness, decreased cancer metastasis, and enhanced sensitivity to gemcitabine. Compared to SAHA, TSA regulated the oncogenic activity of PDAC cancer cells mediated by epigenetically, and potentiated therapeutic activity of gemcitabine. This finding makes a case for further research of HDACis alone or in combination with chemotherapeutic drugs in personalized medicine applications.
Fig. 3.
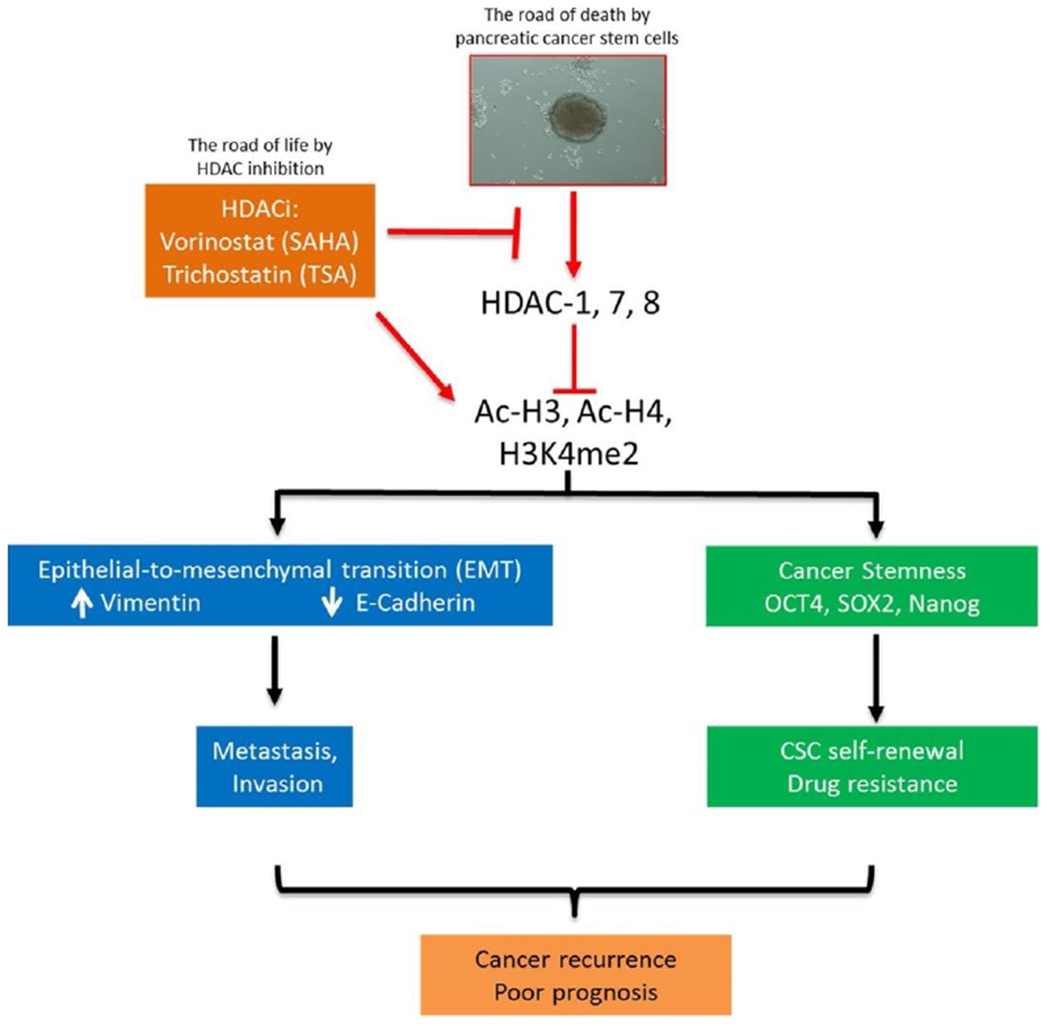
A schematic summary showing SAHA or TSA inhibition of cancer invasion, and increased sensitivity to chemotherapy. Adapted with permission from Ref. [118].
Artemisinin and its derivatives (ARTs) exhibit heme-dependent anticancer activity, and many clinical trials of artesunate (ARS) against solid tumors have been carried out [119]. However, the antitumor effect of ARS is always ineffective. It is worth noting that HDACis can promote the production of heme in erythroid cells, however, their regulatory effect on heme in non-erythroid tumor cells is still unknown. Chen and co-workers attempted to develop a combination of ARS and HDACi for antitumor therapy and investigate its therapeutic effect [120]. HDACi (SAHA or LBH589) has been found to increase the production of heme by upregulating the expression of the increase 5-aminolevulinate synthase (ALAS1) for heme synthesis, thereby enhancing the anti-tumor activity of ARS. Notably, due to the negative feedback regulation effect of heme on ALAS1, HDACi alone could only temporarily increase the expression of ALAS1. However, when used in combination with HDACi, ARS could maintain consuming the newly generated free heme, thereby weakening its negative feedback regulation effect on ALAS1 and increasing the expression of ALAS1 regulated by HDACi. Therefore, the antitumor efficacy of ARS was synergistically sensitized via continuous production of heme. These results not only preliminarily confirmed the feasibility of ARS-HDACi combination in the cancer treatment, but also provided the possibility of combining ARS with other heme synthesis promoters to combat solid tumors.
3.2. Radiotherapy
Clinical success of radiotherapy (RT) is always limited by two factors, insufficient DNA damage during cancer treatment and rapid DNA repair after treatment, respectively [121]. Therefore, strategies focus on both enhancing DNA damage and subsequent inhibiting DNA repair are crucial in improving the therapeutic outcome of RT. By disrupting the cell cycle and upregulating the generation of ROS, HDACis have been demonstrated to potentiate therapeutic activity of RT in preclinical studies [122].
Both generation of DNA damage and subsequent inhibition of DNA repair are key factors influencing the therapeutic effect of RT [123]. Jiang and co-workers have designed and developed a DNA-dual-targeting nanomedicine (NM) aimed at simultaneously promoting the formation of DNA lesion and preventing the subsequent repair (Fig. 4) [124]. Specifically, the cisplatin prodrug (PtIV) loaded in the NM formed platinated DNA in cell nuclei, making cancer cells DNA more sensitive to the ionizing radiation produced by RT. Due to the reduced expression of DNA repair-associated proteins, the release of vorinostat from NM prolonged the formation of double-strand breaks, generated ROS, and caused cell apoptosis. Besides, this nanomedicine exhibited magnetic resonance imaging and fluorescence techniques, enabling accurate trafficking and real-time imaging-guided precision RT of the NM. Finally, the in vitro and in vivo studies revealed that this nanomedicine realized an obviously improved radiotherapeutic outcome. This DNA-dual-targeting and imaging-guided design provided a promising strategy for precision RT of cancers.
Fig. 4.
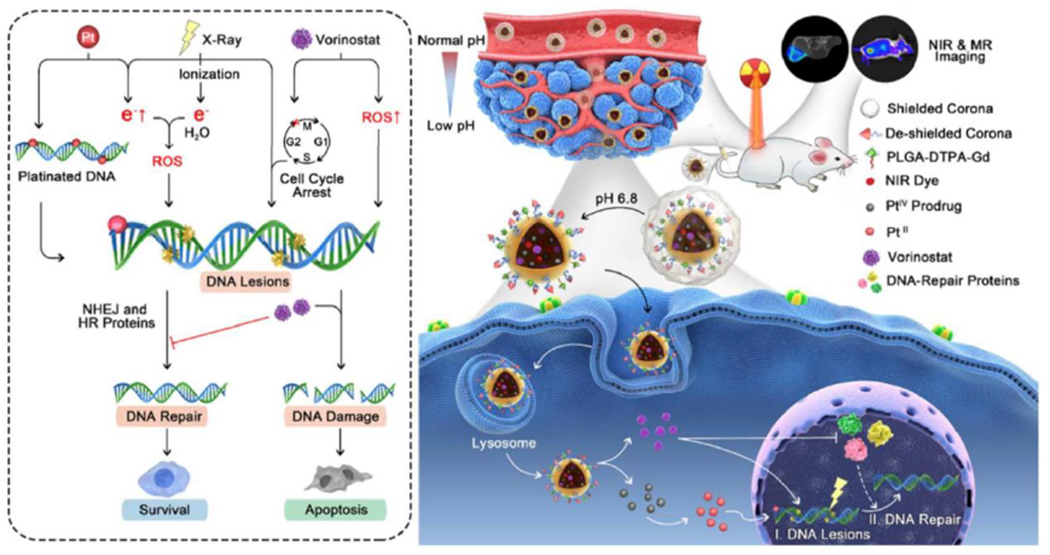
Schematic of an imaging-guided nanomedicine for targeted cancer chemoradiotherapy via increased DNA lesions formation and inhibited DNA damage response. Adapted with permission from Ref. [124].
Melanocortin 1 receptor (MC1R) is a major target for metastatic melanoma imaging and therapy [125]. Li and co-workers have used BRAF inhibitors (BRAFi) and HDACis to pharmacologically upregulate the expression of MC1R and enhance the delivery of radiolabeled peptide, [212Pb]DOTA-MC1L, in metastatic melanoma cancers (Fig. 5) [126]. Two HDACis, SAHA and 4-phenylbutyrate, were used as class I and II HDACs inhibitors. SAHA has been FDA-approved for clinical treatment of cutaneous T-cell lymphoma. 4-phenylbutyrate, a fatty acidic HDACi has also been FDA-approved for treatment cancers, urea cycle disorders, motor neuron diseases, hemoglobinopathies, and cystic fibrosis. SAHA and 4-phenylbutyrate have been proved to improve the sensitivity and activity to ionizing radiation in cancer cells via regulating gene expression of pre-radiation and post-radiation. HDACis treatments could not only enhance the sensitivity of tumors to 212Pb α-particle mediated radiotherapy, but also increase the radiation dose in the tumor microenvironment by upregulating gene expression of MC1R in tumors. Dabrafenib and vemurafenib, two BRAFis, were studied in this work for inhibiting mitogen activated protein kinase (MAPK) pathway. Moreover, BRAFi and HDACi obvious enhanced the expression of MC1R (up to 4-fold in protein levels and 10-fold in mRNA) via microphthalmia associated transcription factor (MITF)-dependent pathways, thereby resulting in increased ligand binding on the surface of cancer cells. The combination of BRAFi/HDACi based MC1R-targeted radionuclide therapy exhibited improved overall survival and superior tumor inhibition in both BRAFV600E A2058 and BRAFWT MEWO melanoma. These findings demonstrate that the combination of HDACi and BRAFi could realize effect therapeutic efficacy of MC1R-targeted RT via upregulated MC1R.
Fig. 5.
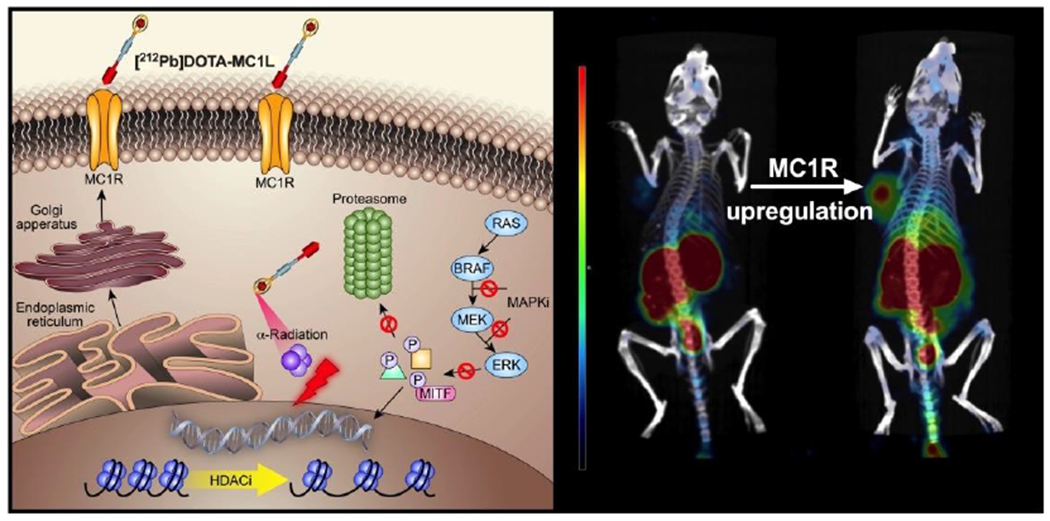
The synergistic mechanism and in vivo imaging of BRAFi/HDACi based MC1R-targeted RT (2 hour post-injection SPECT/CT imaging of A2058 melanoma in mice treated with SAHA and 4-phenylbutyrate). Adapted with permission from Ref. [126].
3.3. Phototherapy
Recently, the potential synergy of phototherapy and other antitumor therapies has also attracted research attention [127]. In particular, sodium butyrate (SB), an HDACi, has been proved to augment sensitivity to phototherapy/RT in cancer cells by decreasing the gene expression of double-strand breaks repair [128]. SAHA could also enhance radio-cytotoxicity via inducing the formation of an open chromatin, thereby upregulating the number of binding sites for free radicals and photosensitizers (PSs) to cause DNA damage [129]. In cutaneous T cell lymphomas, the combinations of photochemotherapy and HDACi could significantly enhance apoptosis, promote DNA double-strand breaks, and decrease cell viability [130].
Photodynamic therapy (PDT) is a non-invasive treatment methodology for cancers [131]. However, the poor bioavailability, low physiological solubility, and acquired resistance, limit the effectiveness of PDT [132]. Liu and co-workers have designed a smart therapeutic nanomedicine based on Chlorin 6 (Ce6) as novel PSs and tumor-responsive nanogel as drug carriers (Fig. 6) [133]. Compared to free Ce6, the as-prepared nanogel-Ce6 nanoplatform exhibited highly effective ROS yield for PDT treatment of cancer in the responsive tumor environment. Moreover, SAHA was loaded for enhanced synergistic therapy of prostate cancer. SAHA could reverse PDT resistance by inhibiting HIF-1α and VEGF pathways. Notably, the combination of SAHA and nanogel-Ce6 exhibited enhanced antitumor efficacy against the tumor cells, which exhibited a strong pro-apoptotic effect both in vitro and in vivo. This study offers a synergistic strategy to address the main limitations to the application of PDT and paves the way for imaging-guided combination therapy.
Fig. 6.
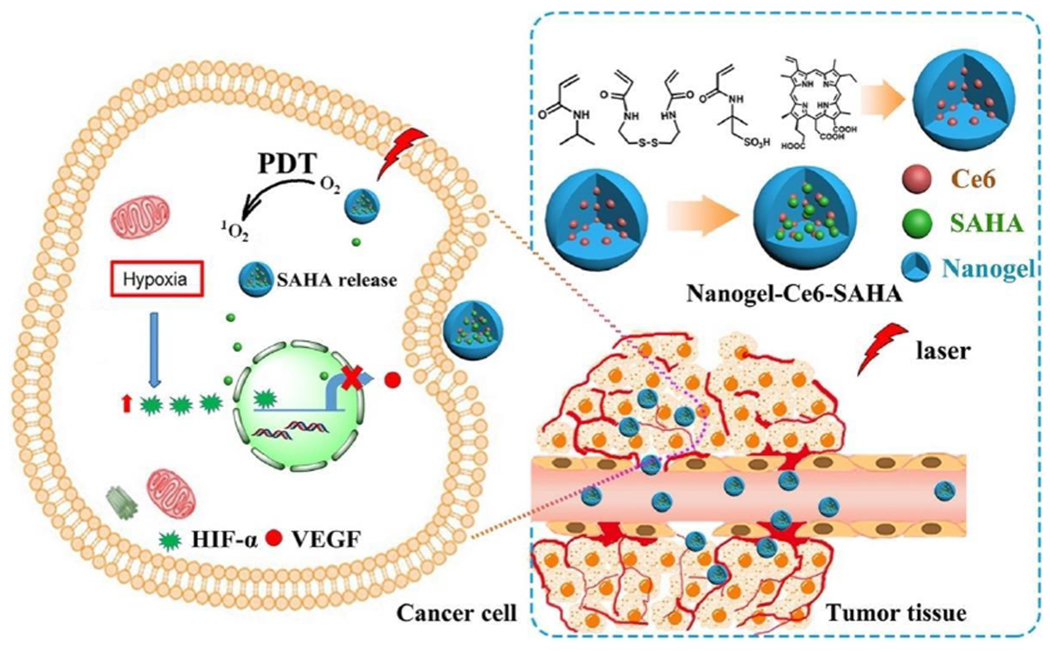
The synthesis route of nanogel-Ce6 nanoplatform and the combination of Ce6 and SAHA for synergistic therapy. Adapted with permission from Ref. [133].
In order to enhance the anticancer efficacy of PDT, Aru and co-workers have developed and characterized a silicon phthalocyanine (SiPc) substituted with HDACi, 3-hydroxypyridin-2-thione (3-HPT), and then SiPc-HDACi was prepared [134]. The chemical properties and its anticancer efficacy on different breast cancer cell lines (MCF-7 cells and MDA-MB-231 cells) and a healthy human endothelial cell line (HUVEC cells) were investigated. Due to high ROS yield and HDACs downregulating properties, SiPc-HDACi effectively decreased the levels of HDAC6 and HDAC8 protein, induced cell cycle arrest and cell death. In addition, the decreasing protein levels of CD44 (a adhesion receptor that is overexpressed in surface of many cancers cells) and CCR7 (a protein involved in EMT process) proved that HDACis in combination with PDT may prevent metastases in vivo.
Recent research has demonstrated that the expression of HDACs in residual cancer cells would be increased after PDT, resulting in the potential metastasis of tumor [135]. To solve these questions, Zhang and co-workers have synthesized a series of novel cytotoxic derivatives based on chlorin as dual photosensitizers (PSs) and HDACis, and then investigated their biological activity [136]. Among them, compound 15e showed good inhibitory activities to HDAC 2 and HDAC 10 via increasing the expression of acetyl-H4. However, compound 15e showed weak inhibition activities to some other HDACs (including HDAC 1, HDAC 3, HDAC 6 and HDAC 8), displaying selective inhibition activities towards HDACs. Compared to talaporfin alone as a PS and SAHA alone as an HDACi, compound 15e showed higher phototoxicity and dark-toxicity. The in vitro studies displayed that compound 15e was much higher (almost 9 fold) cellular uptake than that of talaporfin, demonstrating that the compound 15e was more easily to accumulate in A549 cells. Moreover, compound 15e could significantly improve the production of intracellular ROS under light irradiation, which may due to the higher cellular uptake capability. The cellular uptake experiments displayed that compound 15e was liable to localized in multiple organelles of A549 cells, including lysosomes, mitochondria, golgi and endoplasmic reticulum (ER), which probably triggered various apoptosis pathways and cell destruction. Besides, compound 15e induced autophagy of cancer cell effectively as a dual PS and HDACi. All data exhibited that HDACis could be applied as synergistic agents for PDT.
3.4. Targeted therapy
Recently, dual-target or multitarget therapies have attracted a great deal of interest form medical researchers in cancers [137]. To maximize the efficacy of single-target drugs, the design of multitarget ligands has become an approach with clear advantages over the past decade [138]. It is well known that several cancers could produce obvious resistance against any individual inhibitor of protein or kinase, so that developing dual-target ligands is necessary to improve the effect and reduce the side effects of single-inhibitor based target therapy [139]. The combinational inhibition of different cancer-related target pathways can cause additive or synergistic effects and inhibit drug resistance in cancer cells [140]. HDACs have been demonstrated a target for clinical cancer treatment, therefore, designing dual inhibitors of HDACs and other cancer-related target, such as DNA methyltransferases (DNMTs), EGFRs, Janus kinases (JAKs), receptor tyrosine kinases (RTKs), becomes a new trend of cancer treatment [141].
The overexpression of DNMTs aberrant always induces epigenetic dysregulations in a variety of cancer types, making them promising as cancer targets [142]. However, the short-term stability, limited specificity of action, and low accumulation and retention of DNMTs greatly influenced their efficacy against solid tumors in clinical trials [143]. Wang and co-workers have designed a epigenetic modulatory drugs combination via a self-assembly method based on inhibitors of DNMTs and HDACs (Fig. 7) [144]. The nanofibers were developed through a simple ultrasonic mixture of a DNMT inhibitor (5-Aza), SAHA, and zinc ions. The drug conjugates could self-assemble into nanofibers with improved structure stability. The as-prepared nanofibers inhibited DNA methylation and histone deacetylation synergistically, subsequently promoted apoptosis of gastric cancer cells. The molecular mechanism of nanofibers was clarified by RNA-Seq technologies. Moreover, the nanofibers exhibited prolonged accumulation and retention in tumors and the reduced off-target effects, thereby showing superior therapeutic efficacy in vivo. Therefore, this combined inhibitors of DNMTs and HDACs may develop a promising strategy for tumor treatment.
Fig. 7.
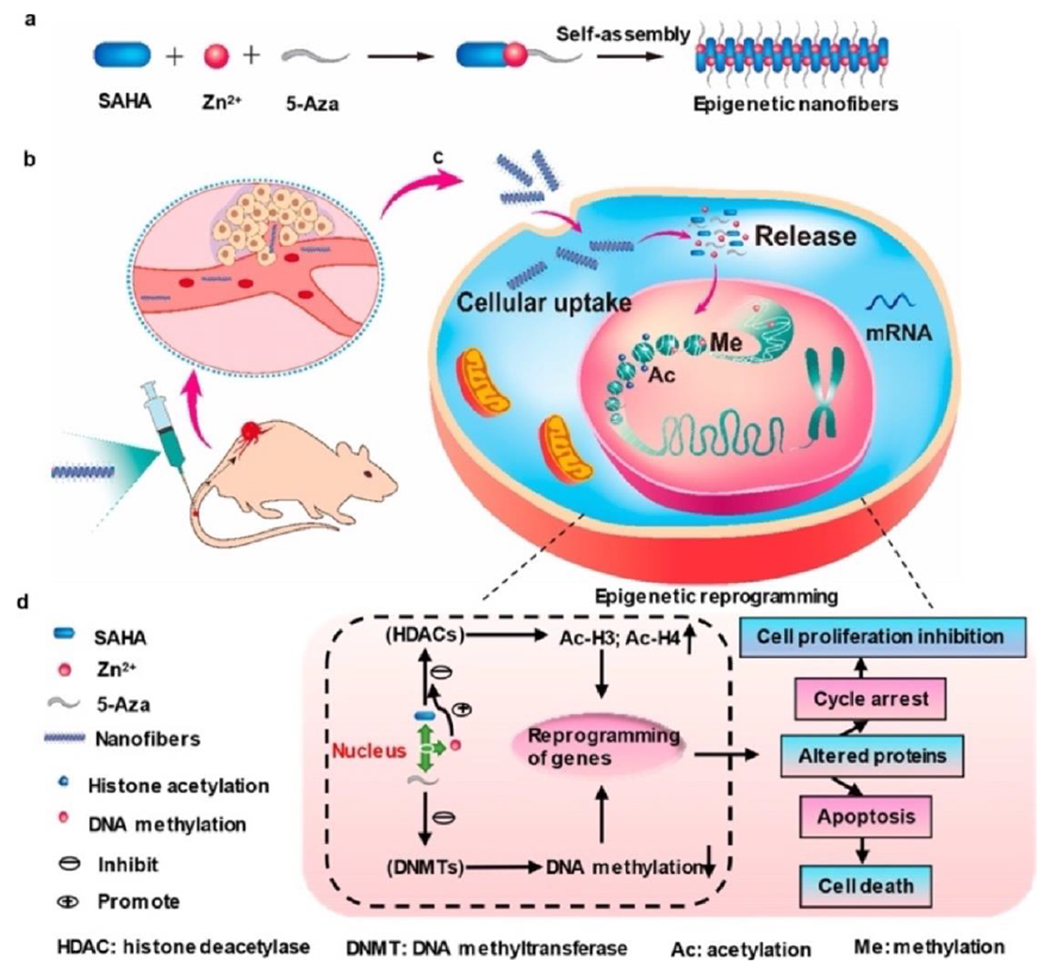
Development of nanofibers and the synergistic mechanism via epigenetic reprogramming. Adapted with permission from Ref. [144].
The overexpression of EGFR is tightly binding with the occurrence of head and neck cancer (HNC) and has been proved an important therapeutic target for this cancer [145]. However, due to the acquired resistance caused by increased epithelial to mesenchymal transition (EMT), the use of EGFR inhibitors exhibits limited therapeutic efficacy [146]. In recent years, SAHA has shown promising capability to revert EMT in different cancers [147]. Citro and co-workers studied the cooperative activity of SAHA and gefitinib (an inhibitor of EGFR tyrosine kinase) in HNC cells [148]. The in vitro and in vivo studies showed that both SAHA and gefitinib exhibited antitumor effect in HNC cells of both HPV-positive and HPV-negative, and the combination produced a synergistic therapeutic efficacy in inhibiting tumor growth. SAHA inhibited tumor-promoting TGFβ signals which initiated EMT and decreased the expression of mesenchymal transcription factor ΔNp63α. Downregulated of ΔNp63α suppressed the level of EGFR protein, and then inhibited cell proliferation and TGFβ-dependent mesenchymal transcription factors in HNC cells of both HPV-positive and HPV-negative. By clarifying a molecular mechanism clearly, this work provides a rationale for the combination of SAHA with gefitinib in HNC patients.
JAKs, a family of nonreceptor tyrosine kinases, play major roles in transducing cytokine-mediated signals and are associate with various hematological malignancies or autoimmune diseases [149]. Inhibition of JAKs and HDACs at the same time may improve the efficacy of the HDACis in the cancer treatment and inhibitor resistance of HDACis in some types of tumors [150]. Liang and co-workers have designed and synthesized a novel series of pyrimidin-2-amino-pyrazol hydroxamate derivatives as dual inhibitors of JAKs (ruxolitinib) and HDACs (SAHA), and evaluated their characteristics and antitumor effect [151]. Among them, compound 8m possessed balanced activities and maximal inhibitory efficacy against both JAK2 and HDAC 6. Compound 8m showed enhanced antiproliferative and proapoptotic activities over ruxolitinib and SAHA in several cancer cell lines. Notably, compound 8m exhibited synergistic and more potent antiproliferation effect in JAK2V617F mutated HEL cells. Pharmacokinetic studies displayed that compound 8m exhibited excellent bioavailability after intraperitoneal administration. In addition, high antitumor effect and less significant toxicity was found in 8m treated HEL xenograft model. These results confirmed the antitumor potential of JAK/HDAC dual inhibitors in malignancies, providing valuable leads for further optimization of antitumor strategies in the clinic.
3.5. Immunotherapy
Even though cancer immunotherapies have received extensive research attention for cancer treatment, the low response rate of patients and severe adverse effects related to immune remain problems [152]. The antitumor efficacy of immunotherapy is always decreased by the immunosuppressive tumor microenvironment (TME). How to comprehensively inhibit the TME and enhance antitumor immune response mediated by immune cells (T cells, natural killer (NK) cells, antigen- presenting cells, etc.) is still a challenge for cancer immunotherapy [153]. The HDAC6 inhibitor, ACY241, can activate the AKT/mTOR/p65 pathways in cancer cells, and then increase the number of antigen-specific CD8+ T cells to suppress cancer progression [154, 155]. Moreover, HDACis can upregulate the expression of NK cells receptor, thereby enhancing the recognition of cancers by NK cells [156]. Besides, HDACis can also enhance the sensitivity of cancer cells against ICB by activating immune surveillance mechanism [157, 158].
Despite tumor immune disease remains a big challenge in clinal trails, immunotherapy in combination with HDACis have realized success [159, 160]. Shen and co-workers have designed and prepared a series of inhibitors to HDAC6, and then investigated their structural and biological characterization [161]. Among them, SS-208, a novel HDAC6-selective inhibitor which composed of a hydrophobic linker and isoxazole-3-hydroxamate moiety was identified. SS-208 showed inefficient therapeutic effects in murine SM1 melanoma cells in vitro, while significantly upregulated the levels of Ac-α-Tubulin. Moreover, SS-208 efficiently decreased Y705 phosphorylation of STAT3 mediated by IL-6, and reduced the PD-L1 expression in melanoma cells. Furthermore, SS-208 significantly inhibited tumor growth in a murine SM1 syngeneic melanoma mouse in vivo, which was mediated by the enhanced infiltration of NK+ T and CD8+ cells and the increased ratio of M1/M2 macrophages in the TME. The combination of a selective HDAC6i, and PD-1 immune blockade has been proved significant enhanced in antitumor immune responses, resulting in the further inhibition of tumor growth compared to single immunotherapy.
Despite ICB exhibits remarkable clinical success against PD-1/PD-L1 pathway, this therapy has encountered limitations in most patients by the upregulation of certain tumor immunosuppressive factors (myeloid-derived suppressor cells, MDSCs) [162]. HDACis could reverse the resistance of ICB by reducing the immunosuppressive activity of MDSCs and enhanced PD-L1 expression in cancer cells [163]. In order to realize enhanced antitumor efficacy, Adeshakin and co-workers combined VPA and anti-PD-L1 antibody aiming at promoting the polarization into M-MDSCs from bone marrow-derived precursor cells [164]. Both the in vitro and in vivo results demonstrated that VPA promoted the reactivation of CD8+ T cells response and generated TNFα, which further converted M-MDSCs to a stimulatory phenotype from an immunosuppressive phenotype. The combined therapy of VPA with anti-PD-L1 antibody increased the accumulation of M-MDSCs and enhanced the proportions of CD4+ T cells and CD8+ T cells in spleen. Besides, VPA promoted the PD-L1 blockade therapy by upregulating IRF1/IRF8 transcriptional axis in cancer cells, resulting in reduced immunosuppressive function of MDSCs via decreasing the expression of IL-6, IL-10, and ARG1. In addition, the possible synergistic effect of VPA with antiPD-L1 antibody in suppressing tumor growth was also investigated. Compared to the mice group treated with anti-PD-L1 antibody, the combination-treated group showed a significant reduction in tumor weight on day 20, displaying a synergistic antitumor effect. These studies provide a potential rationale for the synergistic therapy of HDACis with anti-PD-L1 antibody in the preclinic.
By improving antitumor immune responses, indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors can control and eradicate tumor growth [165]. However, IDO1 inhibitors only showed moderate therapeutic activity when served as single antitumor agents. Indeed, IDO1 inhibitors are generally used combining with other antitumor agents in preclinical and clinical trials [166]. Fang and co-workers have designed and developed a dual inhibitors of IDO1 and HDAC1 (compound 10) for enhanced antitumor efficacy (Fig. 8) [167]. The highly active compound 10 exhibited balanced and excellent activity against IDO1 and HDAC1, which IC50 were 69.0 nM and 66.5 nM, respectively. The dual targeting mechanisms were investigated and validated in tumor cells. As an orally active antitumor agent, compound 10 significantly decreased the 1-kynurenine level in plasma with good pharmacokinetic profiles. Compound 10 also possessed synergistic antitumor efficacy and low toxicity in the murine LLC tumor model. This work clarified the mechanisms and relationships between immunotherapy and epigenetics of cancer, providing a valuable suggestion for the design of novel antitumor agents.
Fig. 8.
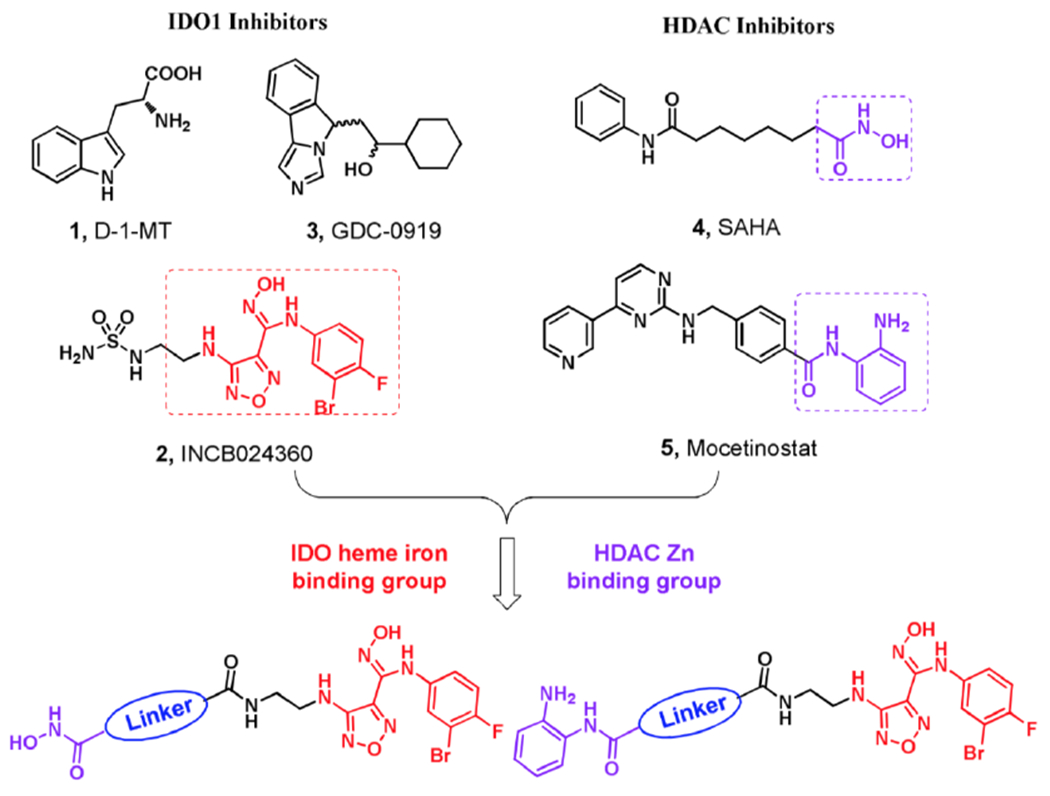
Design and chemical structure of dual IDO1 and HDAC inhibitors. Adapted with permission from Ref. [167].
4. Conclusions
As the first found epigenetic therapeutic strategy against cancer, HDACis have been attracted increasing research attentions in cancer research. However, recent studies of HDACis are always difficult to meet the desired antitumor efficacy. In the recent years, a great deal of mechanistic evidence proves that the combination therapy containing HDACis can synergize with other anticancer agents, thereby exciting great interests of scientists. The combination treatment can target to multiple signaling pathways related to cancers simultaneously, and then reverse drug resistance in various preclinical models. Therefore, the advantages of combination strategy or dual targeting agents based on HDACs may become a promising trend for cancer therapy.
Nevertheless, the efficacy of HADCis based combination therapy tested in clinical trials remains unsatisfactory. Since this strategy involves two or multiple antitumor therapies or targets, it is crucial to rational design the selected compounds. The challenges for these HDACis-drugs are maintaining the balancing of two pharmacophores with safety profiles, limited tolerability, and desired pharmacological properties. Taken the safety profiles, selectivity, pharmacological properties, and membrane permeability of antitumor agents into consideration, the rational development of HDACis-drugs may enhance the therapeutic index. Therefore, more explorations are needed to develop sequential and targeted delivery of drugs for combination therapy with lower doses, and it is hopeful that more and more HDACis-drugs combinations will be advanced to further clinical stage.
Histone deacetylations (HDACs) are novel anticancer drug targets, and a brief classification of HDACs is made.
The antitumor mechanism of histone deacetylases inhibitors (HDACis) have been summarized.
The major developments of other antitumor therapies in combination with HDACis are outlined, including chemotherapy, radiotherapy, phototherapy, targeted therapy, and immunotherapy.
The current clinical state and challenges of HDACis-drugs combinations are discussed, aiming at rational design of such combinations for enhanced efficacy in future clinical trials.
Acknowledgement
This work was supported by the National Natural Science Foundation of China [Grant No. 51903201]; Fundamental Research Funds for the Central Universities [Grant No. xzy012019077]; the Youth Natural Science Fund of Jiangsu Province [Grant No. BK20200977]; the Jiangsu Key Laboratory for Carbon Based Functional Materials & Devices, Soochow University [Grant No. KJS1907].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Hanif M, Arshad J, Astin JW, Rana Z, Zafar A, Movassaghi S, Leung E, Patel K, Söhnel T, Reynisson J, Sarojini V, Rosengren RJ, Jamieson SMF, Hartinger CG, A multitargeted approach: Organorhodium anticancer agent based on vorinostat as a potent histone deacetylase inhibitor, Angew. Chem. Int. Ed 59 (2020) 14609–14614. 10.1002/anie.202005758. [DOI] [PubMed] [Google Scholar]
- [2].Bjornson YM, Huang CY, Castaneda G, Yamomoto E, Histone deacetylase inhibitors enhance CRISPR-Cas9 cutting efficiency, FASEB. J 34 (2020) 1–10. 10.1096/fsb2.21134. [DOI] [Google Scholar]
- [3].Jia M, Wang T, Xu S, Liu J, Wen T, Meng J, Xu H, Arsenic sulfide nanoformulation induces megakaryocytic differentiation through histone deacetylase inhibition, Adv. Ther 3 (2020) 1900151. 10.1002/adtp.201900151. [DOI] [Google Scholar]
- [4].Lee JA, An J, Taniguchi J, Kashiwazaki G, Pandian GN, Parveen N, Kang TM, Sugiyama H, De D, Kim KK, Targeted epigenetic modulation using a DNA-based histone deacetylase inhibitor enhances cardiomyogenesis in mouse embryonic stem cells, J. Cell. Physiol 236 (2021) 3946–3962. 10.1002/jcp.30140. [DOI] [PubMed] [Google Scholar]
- [5].Sinatra L, Bandolik JJ, Roatsch M, Sönnichsen M, Schoeder CT, Hamacher A, Schöler A, Borkhardt A, Meiler J, Bhatia S, Kassack MU, Hansen FK, Hydroxamic acids immobilized on resins (HAIRs): Synthesis of dual-targeting HDAC inhibitors and HDAC degraders (PROTACs), Angew. Chem. Int. Ed 59 (2020) 22494–22499. 10.1002/anie.202006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang J, Peng J, Huang Y, Meng L, Li Q, Xiong F, Li X, Identification of histone deacetylase (HDAC)-associated proteins with DNA-programmed affinity labeling, Angew. Chem. Int. Ed 59 (2020) 17525–17532. 10.1002/anie.202001205. [DOI] [PubMed] [Google Scholar]
- [7].Ho TCS, Chan AHY, Ganesan A, Thirty years of HDAC inhibitors: 2020 insight and hindsight, J. Med. Chem 63 (2020) 12460–12484. 10.1021/acs.jmedchem.0c00830. [DOI] [PubMed] [Google Scholar]
- [8].Li Z, Tu J, Han G, Liu N, Sheng C, Novel carboline fungal histone deacetylase (HDAC) inhibitors for combinational treatment of azole-resistant candidiasis, J. Med. Chem 64 (2021) 1116–1126. 10.1021/acs.jmedchem.0c01763. [DOI] [PubMed] [Google Scholar]
- [9].Yang F, Zhao N, Hu Y, Jiang CS, Zhang H, The development process: From SAHA to hydroxamate HDAC inhibitors with branched CAP region and linear linker, Chem. Biodivers 17 (2020) e1900427. 10.1002/cbdv.201900427. [DOI] [PubMed] [Google Scholar]
- [10].McIntyre RL, Daniels EG, Molenaars M, Houtkooper RH, Janssens GE, From molecular promise to preclinical results: HDAC inhibitors in the race for healthy aging drugs, EMBO Mol. Med 11 (2019) e9854. 10.15252/emmm.201809854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shouksmith AE, Shah F, Grimard ML, Gawel JM, Raouf YS, Geletu M, Berger-Becvar A, de Araujo ED, Luchman HA, Heaton WL, Bakhshinyan D, Adile AA, Venugopal C, O’Hare T, Deininger MW, Singh SK, Konieczny SF, Weiss S, Fishel ML, Gunning PT, Identification and characterization of AES-135, a hydroxamic acid-based HDAC inhibitor that prolongs survival in an orthotopic mouse model of pancreatic cancer, J. Med. Chem 62 (2019) 2651–2665. 10.1021/acs.jmedchem.8b01957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Romanelli A, Stazi G, Fioravanti R, Zwergel C, Di Bello E, Pomella S, Perrone C, Battistelli C, Strippoli R, Tripodi M, del Bufalo D, Rota R, Trisciuoglio D, Mai A, Valente S, Design of first-in-class dual EZH2/HDAC inhibitor: Biochemical activity and biological evaluation in cancer cells, ACS Med. Chem. Lett 11 (2020) 977–983. 10.1021/acsmedchemlett.0c00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shankar E, Pandey M, Verma S, Abbas A, Candamo M, Kanwal R, Shukla S, MacLennan GT, Gupta S, Role of class I histone deacetylases in the regulation of maspin expression in prostate cancer, Mol. Carcinog 59 (2020) 955–966. 10.1002/mc.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kovacs-Kasa A, Kovacs L, Cherian-Shaw M, Patel Y, Meadows ML, Fulton DJ, Su Y, Verin AD, Inhibition of class II HDACs improves endothelial barrier function in endotoxin-induced acute lung injury, J. Cell. Physiol 236 (2021) 2893–2905. 10.1002/jcp.30053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rodrigues DA, Pinheiro P.d.S.M., Sagrillo FS, Bolognesi ML, Fraga CAM, Histone deacetylases as targets for the treatment of neurodegenerative disorders: Challenges and future opportunities, Med. Res. Rev 40 (2020) 2177–2211. 10.1002/med.21701. [DOI] [PubMed] [Google Scholar]
- [16].Ling Y, Feng J, Luo L, Guo J, Peng YF, Wang TT, Ge X, Xu QB, Wang XY, Dai H, Zhang YN, Design and synthesis of C3-substituted β-carboline-based histone deacetylase inhibitors with potent antitumor activities, ChemMedChem 12 (2017) 646–651. 10.1002/cmdc.201700133. [DOI] [PubMed] [Google Scholar]
- [17].McGuire JJ, Nerlakanti N, Lo CH, Tauro M, Utset-Ward TJ, Reed DR, Lynch CC, Histone deacetylase inhibition prevents the growth of primary and metastatic osteosarcoma, Int. J. Cancer 147 (2020) 2811–2823. 10.1002/ijc.33046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ediriweera MK, Cho SK, Targeting miRNAs by histone deacetylase inhibitors (HDACi): Rationalizing epigenetics-based therapies for breast cancer, Pharmacol. Therapeut 206 (2020) 107437. 10.1016/j.pharmthera.2019.107437. [DOI] [PubMed] [Google Scholar]
- [19].Ângelo de Souza L, Silva e Bastos M, de Melo Agripino J, Souza Onofre T, Apaza Calla LF, Heimburg T, Ghazy E, Bayer T, Ferraz da Silva VH, Dutra Ribeiro P, Licursi de Oliveira L, Costa Bressan G, de Almeida Lamêgo MR, Silva-Júnior A, de Souza Vasconcellos R, Suarez-Fontes AM, Almeida-Silva J, Vannier-Santos MA, Pierce R, Sippl W, Lopes Rangel Fietto J, Histone deacetylases inhibitors as new potential drugs against leishmania braziliensis, the main causative agent of new world tegumentary leishmaniasis, Biochem. Pharmacol 180 (2020) 114191. 10.1016/j.bcp.2020.114191. [DOI] [PubMed] [Google Scholar]
- [20].Islam S, Espitia CM, Persky DO, Carew JS, Nawrocki ST, Resistance to histone deacetylase inhibitors confers hypersensitivity to oncolytic reovirus therapy, Blood Adv. 4 (2020) 5297–5310. 10.1182/bloodadvances.2020002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Borgonetti V, Galeotti N, Combined inhibition of histone deacetylases and BET family proteins as epigenetic therapy for nerve injury-induced neuropathic pain, Pharmacol. Res 165 (2021) 105431. 10.1016/j.phrs.2021.105431. [DOI] [PubMed] [Google Scholar]
- [22].He Y, Chen D, Yi Y, Zeng S, Liu S, Li P, Xie H, Yu P, Jiang G, Liu H, Histone deacetylase inhibitor sensitizes ERCC1-high non-small-cell lung cancer cells to cisplatin via regulating miR-149, Mol. Ther-Oncolytics 17 (2020) 448–459. 10.1016/j.omto.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gao Y, Zhang H, Lirussi F, Garrido C, Ye X-Y, Xie T, Dual inhibitors of histone deacetylases and other cancer-related targets: A pharmacological perspective, Biochem. Pharmacol 182 (2020) 114224. 10.1016/j.bcp.2020.114224. [DOI] [PubMed] [Google Scholar]
- [24].Gronemeyer H, Promising strategies: Combination of epigenetic inhibitors for cancer therapy, Int. J. Cancer 147 (2020) 2656–2657. 10.1002/ijc.33142. [DOI] [PubMed] [Google Scholar]
- [25].Yruela I, Moreno-Yruela C, Olsen CA, Zn2+-Dependent histone deacetylases in plants: Structure and evolution, Trends Plant. Sci (2021). 10.1016/j.tplants.2020.12.011. [DOI] [PubMed] [Google Scholar]
- [26].Zhao TC, Wang Z, Zhao TY, The important role of histone deacetylases in modulating vascular physiology and arteriosclerosis, Atherosclerosis 303 (2020) 36–42. 10.1016/j.atherosclerosis.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang XH, Qin M, Wu HP, Khamis MY, Li YH, Ma LY, Liu HM, A review of progress in histonedeacetylase 6 inhibitors research: Structural specificity and functional diversity, J. Med. Chem 64 (2021) 1362–1391. 10.1021/acs.jmedchem.0c01782. [DOI] [PubMed] [Google Scholar]
- [28].Li X, Peterson YK, Inks ES, Himes RA, Li J, Zhang Y, Kong X, Chou CJ, Class I HDAC inhibitors display different antitumor mechanism in leukemia and prostatic cancer cells depending on their p53 status, J. Med. Chem 61 (2018) 2589–2603. 10.1021/acs.jmedchem.8b00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Romero D, HDAC inhibitors tested in phase III trial, Nat. Rev. Clin. Oncol 16 (2019) 465–465. 10.1038/s41571-019-0224-2. [DOI] [PubMed] [Google Scholar]
- [30].Ling Y, Wang XM, Wang CN, Xu CJ, Zhang W, Zhang YH, Zhang YN, Hybrids from farnesylthiosalicylic acid and hydroxamic acid as dual ras-related signaling and histone deacetylase (HDAC) inhibitors: Design, synthesis and biological evaluation, ChemMedChem 10 (2015) 971–976. 10.1002/cmdc.201500019. [DOI] [PubMed] [Google Scholar]
- [31].Khurana I, Maxwell S, Royce S, Mathiyalagan P, Karagiannis T, Mazarakis N, Vongsvivut J, K.N H, Okabe J, Al-Hasani K, Samuel C, El-Osta A, SAHA attenuates takotsubo-like myocardial injury by targeting an epigenetic Ac/Dc axis, Signal Transduct. Tar 6 (2021) 159. 10.1038/s41392-021-00546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee M, Nam HY, Kang HB, Lee WH, Lee GH, Sung GJ, Han MW, Cho KJ, Chang EJ, Choi KC, Kim SW, Kim SY, Epigenetic regulation of p62/SQSTMl overcomes the radioresistance of head and neck cancer cells via autophagy-dependent senescence induction, Cell Death Dis. 12 (2021) 250. 10.1038/s41419-021-03539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bao Y, Xu Q, Wang L, Wei Y, Hu B, Wang J, Liu D, Zhao L, Jing Y, Studying histone deacetylase inhibition and apoptosis induction of psammaplin a monomers with modified thiol group, ACS Med. Chem. Lett 12 (2021) 39–47. 10.1021/acsmedchemlett.0c00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rabal O, José-Enériz ES, Agirre X, Sánchez-Arias JA, de Miguel I, Ordoñez R, Garate L, Miranda E, Sáez E, Vilas-Zornoza A, Pineda-Lucena A, Estella A, Zhang F, Wu W, Xu M, Prosper F, Oyarzabal J, Design and synthesis of novel epigenetic inhibitors targeting histone deacetylases, DNA methyltransferase 1, and lysine methyltransferase G9a with in vivo efficacy in multiple myeloma, J. Med. Chem 64 (2021) 3392–3426. 10.1021/acs.jmedchem.0c02255. [DOI] [PubMed] [Google Scholar]
- [35].Noonepalle S, Shen S, Ptaček J, Tavares MT, Zhang G, Stránský J, Pavliček J, Ferreira GM, Hadley M, Pelaez G, Bařinka C, P Kozikowski A, Villagra A, Rational design of suprastat: A novel selective histone deacetylase 6 inhibitor with the ability to potentiate immunotherapy in melanoma models, J. Med. Chem 63 (2020) 10246–10262. 10.1021/acs.jmedchem.0c00567. [DOI] [PubMed] [Google Scholar]
- [36].Danilenko N, Lercher L, Kirkpatrick J, Gabel F, Codutti L, Carlomagno T, Histone chaperone exploits intrinsic disorder to switch acetylation specificity, Nat. Commun 10 (2019) 3435. 10.1038/s41467-019-11410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang Y, Chen Q, Jiao F, Shi C, Pei M, Wang L, Gong Z, Histone deacetylase 2 regulates ULK1 mediated pyroptosis during acute liver failure by the K68 acetylation site, Cell Death Dis. 12 (2021) 55. 10.1038/s41419-020-03317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zeng Y, Hua YQ, Wang W, Zhang H, Xu XL, Modulation of SIRT1-mediated signaling cascades in the liver contributes to the amelioration of nonalcoholic steatohepatitis in high fat fed middle-aged LDL receptor knockout mice by dihydromyricetin, Biochem. Pharmacol 175 (2020) 113927. 10.1016/j.bcp.2020.113927. [DOI] [PubMed] [Google Scholar]
- [39].Rodrigues DA, Pinheiro PSM, Fraga CAM, Multitarget inhibition of histone deacetylase (HDAC) and phosphatidylinositol-3-kinase (PI3K): Current and future prospects, ChemMedChem 16 (2021) 448–457. 10.1002/cmdc.202000643. [DOI] [PubMed] [Google Scholar]
- [40].Rajan A, Shi H, Xue B, Class I and II histone deacetylase inhibitors differentially regulate thermogenic gene expression in brown adipocytes, Sci. Rep 8 (2018) 13072. 10.1038/s41598-018-31560-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].McCaw TR, Goel N, Brooke DJ, Katre AA, Londoño AI, Smith HJ, Randall TD, Arend RC, Class I histone deacetylase inhibition promotes CD8 T cell activation in ovarian cancer, Cancer Med. 10 (2021) 709–717. 10.1002/cam4.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chelladurai P, Dabral S, Basineni SR, Chen CN, Schmoranzer M, Bender N, Feld C, Nötzold RR, Dobreva G, Wilhelm J, Jungblut B, Zhao L, Bauer UM, Seeger W, Pullamsetti SS, Isoform-specific characterization of class I histone deacetylases and their therapeutic modulation in pulmonary hypertension, Sci. Rep 10 (2020) 12864. 10.1038/s41598-020-69737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wei W, Liu X, Chen J, Gao S, Lu L, Zhang H, Ding G, Wang Z, Chen Z, Shi T, Li J, Yu J, Wong J, Class I histone deacetylases are major histone decrotonylases: Evidence for critical and broad function of histone crotonylation in transcription, Cell Res. 27 (2017) 898–915. 10.1038/cr.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhu Y, Huang M, Bushong E, Phan S, Uytiepo M, Beutter E, Boemer D, Tsui K, Ellisman M, Maximov A, Class IIa HDACs regulate learning and memory through dynamic experience-dependent repression of transcription, Nat. Commun 10 (2019) 3469. 10.1038/s41467-019-11409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Laws MT, Bonomi RE, Kamal S, Gelovani DJ, Llaniguez J, Potukutchi S, Lu X, Mangner T, Gelovani JG, Molecular imaging HDACs class IIa expression-activity and pharmacologic inhibition in intracerebral glioma models in rats using PET/CT/(MRI) with [18F]TFAHA, Sci. Rep 9 (2019) 3595. 10.1038/s41598-019-40054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hogg SJ, Beavis PA, Dawson MA, Johnstone RW, Targeting the epigenetic regulation of antitumour immunity, Nat. Rev. Drug Discov 19 (2020) 776–800. 10.1038/s41573-020-0077-5. [DOI] [PubMed] [Google Scholar]
- [47].Trzeciakiewicz H, Ajit D, Tseng J-H, Chen Y, Ajit A, Tabassum Z, Lobrovich R, Peterson C, Riddick NV, Itano MS, Tripathy A, Moy SS, Lee VMY, Trojanowski JQ, Irwin DJ, Cohen TJ, An HDAC6-dependent surveillance mechanism suppresses tau-mediated neurodegeneration and cognitive decline, Nat. Commun 11 (2020) 5522. 10.1038/s41467-020-19317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Heim CE, Bosch ME, Yamada KJ, Aldrich AL, Chaudhari SS, Klinkebiel D, Gries CM, Alqarzaee AA, Li Y, Thomas VC, Seto E, Karpf AR, Kielian T, Lactate production by staphylococcus aureus biofilm inhibits HDAC11 to reprogramme the host immune response during persistent infection, Nat. Microbiol 5 (2020) 1271–1284. 10.1038/s41564-020-0756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Vaquero A, Stemglanz R, Reinberg D, NAD+-dependent deacetylation of H4 lysine 16 by class III HDACs, Oncogene 26 (2007) 5505–5520. 10.1038/sj.onc.1210617. [DOI] [PubMed] [Google Scholar]
- [50].Chadha S, Wang L, Hancock WW, Beier UH, Sirtuin-1 in immunotherapy: A janus-headed target, J. Leukocyte Biol 106 (2019) 337–343. 10.1002/JLB.2RU1118-422R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tian S, Zhang H, Zhang P, Kalmbach M, Lee JH, Ordog T, Hampel PJ, Call TG, Witzig TE, Kay NE, Klee EW, Slager SL, Yan H, Ding W, Epigenetic alteration contributes to the transcriptional reprogramming in T-cell prolymphocytic leukemia, Sci. Rep 11 (2021) 8318. 10.1038/s41598-021-87890-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pacheco M, Nielsen TO, Histone deacetylase 1 and 2 in mesenchymal tumors, Mod. Pathol 25 (2012) 222–230. 10.1038/modpathol.2011.157. [DOI] [PubMed] [Google Scholar]
- [53].Krämer OH, HDAC2: a critical factor in health and disease, Trends Pharmacol. Sci 30 (2009) 647–655. 10.1016/j.tips.2009.09.007. [DOI] [PubMed] [Google Scholar]
- [54].Adhikari N, Amin S, Trivedi P, Jha T, Ghoshb B, HDAC3 is a potential validated target for cancer: An overview on the benzamide-based selective HDAC3 inhibitors through comparative SAR/QSAR/QAAR approaches, Eur. J. Med. Chem 157 (2018) 1127–1142. 10.1016/j.ejmech.2018.08.081. [DOI] [PubMed] [Google Scholar]
- [55].Haider S, Joseph CG, Neidle S, Fierke CA, Fuchter MJ, On the function of the internal cavity of histone deacetylase protein 8: R37 is a crucial residue for catalysis, Bioorg. Med. Chem. Lett 21 (2011) 2129–2132. 10.1016/j.bmcl.2011.01.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton JM, Richardson JA, Karsenty G, Olson EN, Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis, Cell 119 (2004) 555–566. 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- [57].Urbich C, Rossig L, Kaluza D, Potente M, Boeckel JN, Knau A, Diehl F, Geng JG, Hofmann WK, Zeiher AM, Dimmeler S, HDAC5 is a repressor of angiogenesis and determines the angiogenic gene expression pattern of endothelial cells, Blood 113 (2009) 5669–5679. 10.1182/blood-2009-01-196485. [DOI] [PubMed] [Google Scholar]
- [58].Yu DD, Chen WH, Ren JH, Zhang T, Yang KY, Wu G, Liu HL, VEGF-PKD1-HDAC7 signaling promotes endothelial progenitor cell migration and tube formation, Microvasc. Res 91 (2014) 66–72. 10.1016/j.mvr.2013.10.006. [DOI] [PubMed] [Google Scholar]
- [59].Kotian S, Liyanarachchi S, Zelent A, Parvin JD, Histone deacetylases 9 and 10 are required for homologous recombination, J. Biol. Chem 286 (2011) 7722–7726. 10.1074/jbc.c110.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Barneda-Zahonero B, Parra M, Histone deacetylases and cancer, Mol. Oncol 6 (2012) 579–589. 10.1016/S0040-4020(99)00215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Peng L, Seto E, Deacetylation of nonhistone proteins by HDACs and the implications in cancer, Handb. Exp. Pharmacol 206 (2011) 39–56. 10.1007/978-3-642-21631-2_3. [DOI] [PubMed] [Google Scholar]
- [62].Ding Y, Gong WW, Zhang SP, Shen JR, Liu X, Wang YQ, Chen Y, Meng GL, Protective role of sirtuin3 against oxidative stress and NLRP3 inflammasome in cholesterol accumulation and foam cell formation of macrophages with ox-LDL-stimulation, Biochem. Pharmacol 192 (2021) 114665. 10.1016/j.bcp.2021.114665. [DOI] [PubMed] [Google Scholar]
- [63].Liu SS, Wu F, Jin YM, Chang WQ, Xu TM, HDAC11: a rising star in epigenetics, Biomed. Pharmacother 131 (2020) 110607. 10.1016/j.biopha.2020.110607. [DOI] [PubMed] [Google Scholar]
- [64].Ramaiah MJ, Tangutur AD, Manyam RR, Epigenetic modulation and understanding of HDAC inhibitors in cancer therapy, Life Sci. 277 (2021) 119504. 10.1016/j.lfs.2021.119504. [DOI] [PubMed] [Google Scholar]
- [65].Voss SR, Ponomareva LV, Dwaraka VB, Pardue KE, Baddar NWAH, Rodgers AK, Woodcock MR, Qiu Q, Crowner A, Blichmann D, Khatri S, Thorson JS, HDAC regulates transcription at the outset of axolotl tail regeneration, Sci. Rep 9 (2019) 6751. 10.1038/s41598-019-43230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhai XG, Qiao HW, Guan W, Li ZQ, Cheng YY, Jia X, Zhou YJ, Curcumin regulates peroxisome proliferator-activated receptor-γ coactivator-1α expression by AMPK pathway in hepatic stellate cells in vitro, Eur. J. Pharmacol 746 (2015) 56–62. 10.1016/j.ejphar.2014.10.055. [DOI] [PubMed] [Google Scholar]
- [67].Yang F, Sun S, Wang C, Haas M, Yeo S, Guan JL, Targeted therapy for mTORC1-driven tumours through HDAC inhibition by exploiting innate vulnerability of mTORC1 hyper-activation, Brit. J. Cancer 122 (2020) 1791–1802. 10.1038/s41416-020-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhang L, Han Y, Jiang Q, Wang C, Chen X, Li X, Xu F, Jiang Y, Wang Q, Xu W, Trend of histone deacetylase inhibitors in cancer therapy: Isoform selectivity or multitargeted strategy, Med. Res. Rev 35 (2015) 63–84. 10.1002/med.21320. [DOI] [PubMed] [Google Scholar]
- [69].Zagni C, Floresta G, Monciino G, Rescifina A, The search for potent, small-molecule HDACIs in cancer treatment: a decade after vorinostat, Med. Res. Rev 37 (2017) 1373–1428. 10.1002/med.21437. [DOI] [PubMed] [Google Scholar]
- [70].Li J, Zhu Y, Xie M, Zhang Q, Du W, Design, synthesis, and biological evaluation of target water-soluble hydroxamic acid-based HDACi derivatives as prodrugs, Chem. Biol. Drug Des 94 (2019) 1760–1767. 10.1111/cbdd.13577. [DOI] [PubMed] [Google Scholar]
- [71].Ho RH, Chan JCY, Fan H, Kioh DYQ, Lee BW, Chan ECY, In silico and in vitro interactions between short chain fatty acids and human histone deacetylases, Biochemistry 56 (2017) 4871–4878. 10.1021/acs.biochem.7b00508. [DOI] [PubMed] [Google Scholar]
- [72].Jones PA, Issa JPJ, Baylin S, Targeting the cancer epigenome for therapy, Nat. Rev. Genet 17 (2016) 630–641. 10.1038/nrg.2016.93. [DOI] [PubMed] [Google Scholar]
- [73].Saijo K, Imamura J, Narita K, Oda A, Shimodaira H, Katoh T, Ishioka C, Biochemical, biological and structural properties of romidepsin (FK228) and its analogs as novel HDAC/PI3K dual inhibitors, Cancer Sci. 106 (2015) 208–215. 10.1111/cas.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bayat S, Shekari Khaniani M, Choupani J, Alivand MR, Mansoori Derakhshan S, HDACis (class I), cancer stem cell, and phytochemicals: Cancer therapy and prevention implications, Biomed. Pharmacother 97 (2018) 1445–1453. 10.1016/j.biopha.2017.11.065. [DOI] [PubMed] [Google Scholar]
- [75].Ediriweera MK, Tennekoon KH, Samarakoon SR, Emerging role of histone deacetylase inhibitors as anti-breast-cancer agents, Drug Discov. Today 24 (2019) 685–702. 10.1016/j.drudis.2019.02.003. [DOI] [PubMed] [Google Scholar]
- [76].Liao Y, Niu X, Chen B, Edwards H, Xu L, Xie C, Lin H, Polin L, Taub JW, Ge Y, Qin Z, Synthesis and antileukemic activities of piperlongumine and HDAC inhibitor hybrids against acute myeloid leukemia cells, J. Med. Chem 59 (2016) 7974–7990. 10.1021/acs.jmedchem.6b00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ren Y, Sun QS, Yuan ZG, Jiang YY, Combined inhibition of HDAC and DNMT1 induces p85α/MEK-mediated cell cycle arrest by dual target inhibitor 208 in U937 cells, Chinese Chem. Lett 30 (2019) 1233–1236. 10.1016/j.cclet.2019.03.029. [DOI] [Google Scholar]
- [78].Li X, Jiang Y, Peterson YK, Xu T, Himes RA, Luo X, Yin G, Inks ES, Dolloff N, Halene S, Chan SSL, Chou CJ, Design of hydrazide-bearing HDACIs based on panobinostat and their p53 and FLT3-ITD dependency in antileukemia activity, J. Med. Chem 63 (2020) 5501–5525. 10.1021/acs.jmedchem.0c00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Morales Torres C, Wu MY, Hobor S, Wainwright EN, Martin MJ, Patel H, Grey W, Grönroos E, Howell S, Carvalho J, Snijders AP, Bustin M, Bonnet D, Smith PD, Swanton C, Howell M, Scaffidi P, Selective inhibition of cancer cell selfrenewal through a quisinostat-histone H1.0 axis, Nat. Commun 11 (2020) 1792. 10.1038/s41467-020-15615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mithraprabhu S, Khong T, Spencer A, Overcoming inherent resistance to histone deacetylase inhibitors in multiple myeloma cells by targeting pathways integral to the actin cytoskeleton, Cell Death Dis. 5 (2014) e1134–e1134. 10.1038/cddis.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Zhao L, Okhovat J-P, Hong EK, Kim YH, Wood GS, Preclinical studies support combined inhibition of BET family proteins and histone deacetylases as epigenetic therapy for cutaneous T-Cell lymphoma, Neoplasia 21 (2019) 82–92. 10.1016/j.neo.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Inoue S, MacFarlane M, Harper N, Wheat LMC, Dyer MJS, Cohen GM, Histone deacetylase inhibitors potentiate TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in lymphoid malignancies, Cell Death Differ. 11 (2004) S193–S206. 10.1038/sj.cdd.4401535. [DOI] [PubMed] [Google Scholar]
- [83].Lernoux M, Schnekenburger M, Dicato M, Diederich M, Epigenetic mechanisms underlying the therapeutic effects of HDAC inhibitors in chronic myeloid leukemia, Biochem. Pharmacol 173 (2020) 113698. 10.1016/j.bcp.2019.113698. [DOI] [PubMed] [Google Scholar]
- [84].Herbaux C, Kornauth C, Poulain S, Chong SJF, Collins MC, Valentin R, Hackett L, Tournilhac O, Lemonnier F, Dupuis J, Daniel A, Tomowiak C, Laribi K, Renaud L, Roos-Weil D, Rossi C, Van Den Neste E, Leyronnas C, Merabet F, Malfuson JV, Tiab M, Ysebaert L, Ng S, Morschhauser F, Staber PB, Davids MS, BH3 profiling identifies ruxolitinib as a promising partner for venetoclax to treat T-cell prolymphocytic leukemia, Blood (2021). 10.1182/blood.2020007303. [DOI] [PubMed] [Google Scholar]
- [85].Fujii K, Suzuki N, Jimura N, Idogawa M, Kondo T, Iwatsuki K, Kanekura T, HSP72 functionally inhibits the anti-neoplastic effects of HDAC inhibitors, J. Dermatol. Sci 90 (2018) 82–89. 10.1016/j.jdermsci.2018.01.002. [DOI] [PubMed] [Google Scholar]
- [86].Ribatti D, Tamma R, Epigenetic control of tumor angiogenesis, Microcirculation 27 (2020) e12602. 10.1111/micc.12602. [DOI] [PubMed] [Google Scholar]
- [87].Hou F, Li D, Yu H, Kong Q, The mechanism and potential targets of class II HDACs in angiogenesis, J. Cell. Biochem 119 (2018) 2999–3006. 10.1002/jcb.26476. [DOI] [PubMed] [Google Scholar]
- [88].Kuendgen A, Gattermann N, Valproic acid for the treatment of myeloid malignancies, Cancer 110 (2007) 943–954. 10.1002/cncr.22891. [DOI] [PubMed] [Google Scholar]
- [89].Goswami U, Kandimalla R, Kalita S, Chattopadhyay A, Ghosh SS, Polyethylene glycol-encapsulated histone deacetylase inhibitor drug-composite nanoparticles for combination therapy with artesunate, ACS Omega 3 (2018) 11504–11516. 10.1021/acsomega.8b02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].McGivern TJP, Slator C, Kellett A, Marmion CJ, Innovative DNA-targeted metallo-prodrug strategy combining histone deacetylase inhibition with oxidative stress, Mol. Pharmaceutics 15 (2018) 5058–5071. 10.1021/acs.molpharmaceut.8b00652. [DOI] [PubMed] [Google Scholar]
- [91].Du JT, Guo J, Kang DW, Li ZH, Wang G, Wu JB, Zhang Z, Fang H, Hou XB, Huang ZJ, Li GB, Lu XY, Liu XY, Ouyang L, Rao L, Zhan P, Zhang XJ, Zhang YH, New techniques and strategies in drug discovery, Chinese Chem. Lett 31 (2020) 1695–1708. 10.1016/j.cclet.2020.03.028. [DOI] [Google Scholar]
- [92].Liao PH, Hsu HH, Chen TS, Chen MC, Day CH, Tu CC, Lin YM, Tsai FJ, Kuo WW, Huang CY, Phosphorylation of cofilin-1 by ERK confers HDAC inhibitor resistance in hepatocellular carcinoma cells via decreased ROS-mediated mitochondria injury, Oncogene 36 (2017) 1978–1990. 10.1038/onc.2016.357. [DOI] [PubMed] [Google Scholar]
- [93].Li A, Chen P, Leng Y, Kang J, Histone deacetylase 6 regulates the immunosuppressive properties of cancer-associated fibroblasts in breast cancer through the STAT3-COX2-dependent pathway, Oncogene 37 (2018) 5952–5966. 10.1038/s41388-018-0379-9. [DOI] [PubMed] [Google Scholar]
- [94].Jones PA, Ohtani H, Chakravarthy A, De Carvalho DD, Epigenetic therapy in immune-oncology, Nat. Rev. Cancer 19 (2019) 151–161. 10.1038/s41568-019-0109-9. [DOI] [PubMed] [Google Scholar]
- [95].Knox T, Sahakian E, Banik D, Hadley M, Palmer E, Noonepalle S, Kim J, Powers J, Gracia-Hernandez M, Oliveira V, Cheng F, Chen J, Barinka C, Pinilla-Ibarz J, Lee NH, Kozikowski A, Villagra A, Selective HDAC6 inhibitors improve anti-PD-1 immune checkpoint blockade therapy by decreasing the anti-inflammatory phenotype of macrophages and down-regulation of immunosuppressive proteins in tumor cells, Sci. Rep 9 (2019) 6136. 10.1038/s41598-019-42237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bolden JE, Peart MJ, Johnstone RW, Anticancer activities of histone deacetylase inhibitors, Nat. Rev. Drug Discov 5 (2006) 769–784. 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- [97].Su GX, Miao DD, Yu YY, Zhou M, Jiao PF, Cao XL, Yan B, Zhu HY, Mesoporous silica-coated gold nanostars with drug payload for combined chemo-photothermal cancer therapy, J. Drug Targeting 27 (2019) 201–210. 10.1080/1061186X.2018.1499746. [DOI] [PubMed] [Google Scholar]
- [98].Tzogani K, Penttilä K, Lapveteläinen T, Hemmings R, Koenig J, Freire J, Márcia S, Cole S, Coppola P, Flores B, Barbachano Y, Roige SD, Pignatti F, EMA review of daunorubicin and cytarabine encapsulated in liposomes (Vyxeos, CPX-351) for the treatment of adults with newly diagnosed, therapy-related acute myeloid leukemia or acute myeloid leukemia with myelodysplasia-related changes, Oncologist 25 (2020) e1414–e1420. 10.1634/theoncologist.2019-0785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Thomas O, Weber W, Overcoming physiological barriers to nanoparticle delivery-are we there yet? Front. Bioeng. Biotech 7 (2019) 415. 10.3389/fbioe.2019.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Morel D, Jeffery D, Aspeslagh S, Almouzni G, Postel-Vinay S, Combining epigenetic drugs with other therapies for solid tumours-past lessons and future promise, Nat. Rev. Clin. Oncol 17 (2020) 91–107. 10.1038/s41571-019-0267-4. [DOI] [PubMed] [Google Scholar]
- [101].Kularatne RN, Washington KE, Bulumulla C, Calubaquib EL, Biewer MC, Oupicky D, Stefan MC, Histone deacetylase inhibitor (HDACi) conjugated polycaprolactone for combination cancer therapy, Biomacromolecules 19 (2018) 1082–1089. 10.1021/acs.biomac.8b00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Li X, Su X, Liu R, Pan Y, Fang J, Cao L, Feng C, Shang Q, Chen Y, Shao C, Shi Y, HDAC inhibition potentiates anti-tumor activity of macrophages and enhances anti-PD-L1-mediated tumor suppression, Oncogene 40 (2021) 1836–1850. 10.1038/s41388-020-01636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Gagliano T, Brancolini C, Targeting histone deacetylases for combination therapies in neuroendocrine tumors, Cancer Gene Ther. (2020). 10.1038/s41417-020-00260-x. [DOI] [PubMed] [Google Scholar]
- [104].Blagitko-Dorfs N, Schlosser P, Greve G, Pfeifer D, Meier R, Baude A, Brocks D, Plass C, Lübbert M, Combination treatment of acute myeloid leukemia cells with DNMT and HDAC inhibitors: Predominant synergistic gene downregulation associated with gene body demethylation, Leukemia 33 (2019) 945–956. 10.1038/s41375-018-0293-8. [DOI] [PubMed] [Google Scholar]
- [105].Peng H, Chen B, Huang W, Tang Y, Jiang Y, Zhang W, Huang Y, Reprogramming tumor-associated macrophages to reverse EGFRT790M resistance by dual-targeting codelivery of gefitinib/vorinostat, Nano Lett. 17 (2017) 7684–7690. 10.1021/acs.nanolett.7b03756. [DOI] [PubMed] [Google Scholar]
- [106].Yan B, Chen Q, Shimada K, Tang M, Li H, Gurumurthy A, Khoury JD, Xu B, Huang S, Qiu Y, Histone deacetylase inhibitor targets CD123/CD47-positive cells and reverse chemoresistance phenotype in acute myeloid leukemia, Leukemia 33 (2019) 931–944. 10.1038/s41375-018-0279-6. [DOI] [PubMed] [Google Scholar]
- [107].Miao DD, Yu YY, Chen Y, Liu Y, Su GX, Facile construction of i-motif DNA-conjugated gold nanostars as near-infrared and pH dual-responsive targeted drug delivery systems for combined cancer therapy, Mol. Pharmaceutics 17 (2020) 1127–1138. 10.1021/acs.molpharmaceut.9b01159. [DOI] [PubMed] [Google Scholar]
- [108].Huang M, Zhang J, Yan C, Li X, Zhang J, Ling R, Small molecule HDAC inhibitors: Promising agents for breast cancer treatment, Bioorg. Chem 91 (2019) 103184. 10.1016/j.bioorg.2019.103184. [DOI] [PubMed] [Google Scholar]
- [109].Skok Ž, Zidar N, Kikelj D, Ilaš J, Dual inhibitors of human DNA topoisomerase II and other cancer-related targets, J. Med. Chem 63 (2020) 884–904. 10.1021/acs.jmedchem.9b00726. [DOI] [PubMed] [Google Scholar]
- [110].Hesham HM, Lasheen DS, Abouzid KAM, Chimeric HDAC inhibitors: Comprehensive review on the HDAC-based strategies developed to combat cancer, Med. Res. Rev 38 (2018) 2058–2109. 10.1002/med.21505. [DOI] [PubMed] [Google Scholar]
- [111].Ruan Y, Wang L, Lu Y, HDAC6 inhibitor, ACY1215 suppress the proliferation and induce apoptosis of gallbladder cancer cells and increased the chemotherapy effect of gemcitabine and oxaliplatin, Drug Develop. Res (2021). 10.1002/ddr.21780. [DOI] [PubMed] [Google Scholar]
- [112].Wang FF, Chen L, Zhang R, Chen ZP, Zhu L, RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer, J. Controlled Release 196 (2014) 222–233. 10.1016/j.jconrel.2014.10.012. [DOI] [PubMed] [Google Scholar]
- [113].Van Tine BA, Agulnik M, Olson RD, Walsh GM, Klausner A, Frank NE, Talley TT, Milhem MM, A phase II clinical study of 13-deoxy, 5-iminodoxorubicin (GPX-150) with metastatic and unresectable soft tissue sarcoma, Cancer Med. 8 (2019) 2994–3003. 10.1002/cam4.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Di Martile M, Desideri M, Tupone MG, Buglioni S, Antoniani B, Mastroiorio C, Falcioni R, Ferraresi V, Baldini N, Biagini R, Milella M, Trisciuoglio D, Del Bufalo D, Histone deacetylase inhibitor ITF2357 leads to apoptosis and enhances doxorubicin cytotoxicity in preclinical models of human sarcoma, Oncogenesis 7 (2018) 20. 10.1038/s41389-018-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Gibson D, Platinum(IV) anticancer agents; are we enroute to the holy grail or to a dead end?, J. Inorg. Biochem 217 (2021) 111353. 10.1016/j.jinorgbio.2020.111353. [DOI] [PubMed] [Google Scholar]
- [116].Sabbatini M, Zanellato I, Ravera M, Gabano E, Perin E, Rangone B, Osella D, Pt(IV) bifunctional prodrug containing 2-(2-propynyl)octanoato axial ligand: Induction of immunogenic cell death on colon cancer, J. Med. Chem 62 (2019) 3395–3406. 10.1021/acs.jmedchem.8b01860. [DOI] [PubMed] [Google Scholar]
- [117].Marek L, Hamacher A, Hansen FK, Kuna K, Gohlke H, Kassack MU, Kurz T, Histone deacetylase (HDAC) inhibitors with a novel connecting unit linker region reveal a selectivity profile for HDAC4 and HDAC5 with improved activity against chemoresistant cancer cells, J. Med. Chem 56 (2013) 427–436. 10.1021/jm301254q. [DOI] [PubMed] [Google Scholar]
- [118].Cai MH, Xu XG, Yan SL, Sun Z, Ying Y, Wang BK, Tu YX, Depletion of HDAC1, 7 and 8 by histone deacetylase inhibition confers elimination of pancreatic cancer stem cells in combination with gemcitabine, Sci. Rep 8 (2018) 1621. 10.1038/s41598-018-20004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Fröhlich T, Kiss A, Wölfling J, Mernyák E, Kulmány ÁE, Minorics R, Zupkó I, Leidenberger M, Friedrich O, Kappes B, Hahn F, Marschall M, Schneider G, Tsogoeva SB, Synthesis of artemisinin-estrogen hybrids highly active against HCMV, P. falciparum, and cervical and breast cancer, ACS Med. Chem. Lett 9 (2018) 1128–1133. 10.1021/acsmedchemlett.8b00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Chen CP, Chen K, Feng Z, Wen X, Sun H, Synergistic antitumor activity of artesunate and HDAC inhibitors through elevating heme synthesis via synergistic upregulation of ALAS1 expression, Acta Pharm. Sin. B 9 (2019) 937–951. 10.1016/j.apsb.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Jiang W, Li Q, Xiao L, Dou J, Liu Y, Yu W, Ma Y, Li X, You YZ, Tong Z, Liu H, Liang H, Lu L, Xu X, Yao Y, Zhang G, Wang Y, Wang J, Hierarchical multiplexing nanodroplets for imaging-guided cancer radiotherapy via DNA damage enhancement and concomitant DNA repair prevention, ACS Nano 12 (2018) 5684–5698. 10.1021/acsnano.8b01508. [DOI] [PubMed] [Google Scholar]
- [122].Zhu Y, Das K, Wu J, Lee MH, Tan P, RNH1 regulation of reactive oxygen species contributes to histone deacetylase inhibitor resistance in gastric cancer cells, Oncogene 33 (2014) 1527–1537. 10.1038/onc.2013.104. [DOI] [PubMed] [Google Scholar]
- [123].Schaue D, McBride WH, Opportunities and challenges of radiotherapy for treating cancer, Nat. Rev. Clin. Oncol 12 (2015) 527–540. 10.1038/nrclinonc.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Jiang W, Li Q, Zhu Z, Wang Q, Dou J, Zhao Y, Lv W, Zhong F, Yao Y, Zhang G, Liu H, Wang Y, Wang J, Cancer chemoradiotherapy duo: Nano-enabled targeting of DNA lesion formation and DNA damage response, ACS Appl. Mater. Interfaces 10 (2018) 35734–35744. 10.1021/acsami.8bl0901. [DOI] [PubMed] [Google Scholar]
- [125].Zhou Y, Mowlazadeh Haghighi S, Liu Z, Wang L, Hruby VJ, Cai M, Development of ligand-drug conjugates targeting melanoma through the overexpressed melanocortin 1 receptor, ACS Pharmacol. Transl. Sci 3 (2020) 921–930. 10.1021/acsptsci.0c00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Li M, Liu D, Lee D, Kapoor S, Gibson-Corley KN, Quinn TP, Sagastume EA, Mott SL, Walsh SA, Acevedo MR, Johnson FL, Schultz MK, Enhancing the efficacy of melanocortin 1 receptor-targeted radiotherapy by pharmacologically upregulating the receptor in metastatic melanoma, Mol. Pharmaceutics 16 (2019) 3904–3915. 10.1021/acs.molpharmaceut.9b00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Yu YY, Zhou M, Zhang W, Huang L, Miao DD, Zhu HY, Su GX, Rattle-type gold nanorods/porous-SiO2 nanocomposites as near-infrared light-activated drug delivery systems for cancer combined chemo-photothermal therapy, Mol. Pharmaceutics 16 (2019) 1929–1938. 10.1021/acs.molpharmaceut.8b01298. [DOI] [PubMed] [Google Scholar]
- [128].Wang F, Wu H, Fan M, Yu R, Zhang Y, Liu J, Zhou X, Cai Y, Huang S, Hu Z, Jin X, Sodium butyrate inhibits migration and induces AMPK-mTOR pathway-dependent autophagy and ROS-mediated apoptosis via the miR-139-5p/Bmi-1 axis in human bladder cancer cells, FASEB. J 34 (2020) 4266–4282. 10.1096/fj.201902626R. [DOI] [PubMed] [Google Scholar]
- [129].Felisbino MB, Alves da Costa T, Gatti MSV, Mello MLS, Differential response of human hepatocyte chromatin to HDAC inhibitors as a function of microenvironmental glucose level, J. Cell. Physiol 231 (2016) 2257–2265. 10.1002/jcp.25343. [DOI] [PubMed] [Google Scholar]
- [130].Sung JJ, Ververis K, Karagiannis TC, Histone deacetylase inhibitors potentiate photochemotherapy in cutaneous T-cell lymphoma MyLa cells, J. Photoch. Photobio. B 131 (2014) 104–112. 10.1016/j.jphotobiol.2014.01.009. [DOI] [PubMed] [Google Scholar]
- [131].Li W, Yang J, Luo L, Jiang M, Qin B, Yin H, Zhu C, Yuan X, Zhang J, Luo Z, Du Y, Li Q, Lou Y, Qiu Y, You J, Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death, Nat. Commun 10 (2019) 3349. 10.1038/s41467-019-11269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Turubanova VD, Mishchenko TA, Balalaeva IV, Efimova I, Peskova NN, Klapshina LG, Lermontova SA, Bachert C, Krysko O, Vedunova MV, Krysko DV, Novel porphyrazine-based photodynamic anti-cancer therapy induces immunogenic cell death, Sci. Rep 11 (2021) 7205. 10.1038/s41598-021-86354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Liu N, Liu H, Chen H, Wang G, Teng H, Chang Y, Polyphotosensitizer nanogels for GSH-responsive histone deacetylase inhibitors delivery and enhanced cancer photodynamic therapy, Colloids Surf. B 188 (2020) 110753. 10.1016/j.colsurfb.2019.110753. [DOI] [PubMed] [Google Scholar]
- [134].Aru B, Günay A, Şenkuytu E, Yanıkkaya Demirel G, Gürek AG, Atilla D, A translational study of a silicon phthalocyanine substituted with a histone deacetylase inhibitor for photodynamic therapy, ACS Omega 5 (2020) 25854–25867. 10.1021/acsomega.0c03180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Wang X, Wang X, Jin S, Muhammad N, Guo Z, Stimuli-responsive therapeutic metallodrugs, Chem. Rev 119 (2019) 1138–1192. 10.1021/acs.chemrev.8b00209. [DOI] [PubMed] [Google Scholar]
- [136].Zhang XJ, Liu MH, Luo YS, Han GY, Ma ZQ, Huang F, Wang Y, Miao ZY, Zhang WN, Sheng CQ, Yao JZ, Novel dual-mode antitumor chlorin-based derivatives as potent photosensitizers and histone deacetylase inhibitors for photodynamic therapy and chemotherapy, Eur. J. Med. Chem 217 (2021) 113363. 10.1016/j.ejmech.2021.113363. [DOI] [PubMed] [Google Scholar]
- [137].Zhu HY, Zhang SY, Ling Y, Meng GL, Yang Y, Zhang W, pH-responsive hybrid quantum dots for targeting hypoxic tumor siRNA delivery, J. Controlled Release 220 (2015) 529–544. 10.1016/j.jconrel.2015.ll.017. [DOI] [PubMed] [Google Scholar]
- [138].Liang T, Zhou Y, Elhassan RM, Hou X, Yang X, Fang H, HDAC-bax multiple ligands enhance bax-dependent apoptosis in hela cells, J. Med. Chem 63 (2020) 12083–12099. 10.1021/acs.jmedchem.0c01454. [DOI] [PubMed] [Google Scholar]
- [139].Zhao LW, Gu CY, Gan Y, Shao LL, Chen HW, Zhu HY, Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis, J. Controlled Release 318 (2020) 1–15. 10.1016/jjconrel.2019.12.005. [DOI] [PubMed] [Google Scholar]
- [140].Huang Y, Dong G, Li H, Liu N, Zhang W, Sheng C, Discovery of janus kinase 2 (JAK2) and histone deacetylase (HDAC) dual inhibitors as a novel strategy for the combinational treatment of leukemia and invasive fungal Infections, J. Med. Chem 61 (2018) 6056–6074. 10.1021/acs.jmedchem.8b00393. [DOI] [PubMed] [Google Scholar]
- [141].Liu T, Wan Y, Xiao Y, Xia C, Duan G, Dual-target inhibitors based on HDACs: Novel antitumor agents for cancer therapy, J. Med. Chem 63 (2020) 8977–9002. 10.1021/acs.jmedchem.0c00491. [DOI] [PubMed] [Google Scholar]
- [142].Lyko F, The DNA methyltransferase family: A versatile toolkit for epigenetic regulation, Nat. Rev. Genet 19 (2018) 81–92. 10.1038/nrg.2017.80. [DOI] [PubMed] [Google Scholar]
- [143].San José-Enériz E, Agirre X, Rabal O, Vilas-Zornoza A, Sanchez-Arias JA, Miranda E, Ugarte A, Roa S, Paiva B, Estella-Hermoso de Mendoza A, Alvarez RM, Casares N, Segura V, Martín-Subero JI, Ogi FX, Soule P, Santiveri CM, Campos-Olivas R, Castellano G, de Barrena MGF, Rodriguez-Madoz JR, García-Barchino MJ, Lasarte JJ, Avila MA, Martinez-Climent JA, Oyarzabal J, Prosper F, Discovery of first-in-class reversible dual small molecule inhibitors against G9a and DNMTs in hematological malignancies, Nat. Commun 8 (2017) 15424. 10.1038/ncomms15424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Wang L, Zhang C, Hong Y, Li X, Li T, Gao A, Pan S, Liu B, Jin H, Cui D, Integrating epigenetic modulators in nanofibers for synergistic gastric cancer therapy via epigenetic reprogramming, Nano Lett. 21 (2021) 298–307. 10.1021/acs.nanolett.0c03665. [DOI] [PubMed] [Google Scholar]
- [145].Yang W, Cai Q, Lui VWY, Everley PA, Kim J, Bhola N, Quesnelle KM, Zetter BR, Steen H, Freeman MR, Grandis JR, Quantitative proteomics analysis reveals molecular networks regulated by epidermal growth factor receptor level in head and neck cancer, J. Proteome Res 9 (2010) 3073–3082. 10.1021/pr901211j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Colecchia D, Nicolato E, Ravagli C, Faraoni P, Strambi A, Rossi M, Doumett S, Mosconi E, Locatelli E, Comes Franchini M, Balzi M, Baldi G, Marzola P, Chiariello M, EGFR-targeted magnetic nanovectors recognize, in vivo, head and neck squamous cells carcinoma-derived tumors, ACS Med. Chem. Lett 8 (2017) 1230–1235. 10.1021/acsmedchemlett.7b00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Librizzi M, Caradonna F, Cruciata I, Dębski J, Sansook S, Dadlez M, Spencer J, Luparello C, Molecular signatures associated with treatment of triple-negative MDA-MB231 breast cancer cells with histone deacetylase inhibitors JAHA and SAHA, Chem. Res. Toxicol 30 (2017) 2187–2196. 10.1021/acs.chemrestox.7b00269. [DOI] [PubMed] [Google Scholar]
- [148].Citro S, Bellini A, Miccolo C, Ghiani L, Carey TE, Chiocca S, Synergistic antitumour activity of HDAC inhibitor SAHA and EGFR inhibitor gefitinib in head and neck cancer: a key role for ΔNp63α, Br. J. Cancer 120 (2019) 658–667. 10.1038/s41416-019-0394-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Zhu Y, Liu Z, Peng YP, Qiu YH, Interleukin-10 inhibits neuroinflammation-mediated apoptosis of ventral mesencephalic neurons via JAK-STAT3 pathway, Int. Immunopharmacol 50 (2017) 353–360. 10.1016/j.intimp.2017.07.017. [DOI] [PubMed] [Google Scholar]
- [150].Yao L, Mustafa N, Tan EC, Poulsen A, Singh P, Duong-Thi MD, Lee JXT, Ramanujulu PM, Chng WJ, Yen JJY, Ohlson S, Dymock BW, Design and synthesis of ligand efficient dual inhibitors of janus kinase (JAK) and histone deacetylase (HDAC) based on ruxolitinib and vorinostat, J. Med. Chem 60 (2017) 8336–8357. 10.1021/acs.jmedchem.7b00678. [DOI] [PubMed] [Google Scholar]
- [151].Liang X, Zang J, Li X, Tang S, Huang M, Geng M, Chou CJ, Li C, Cao Y, Xu W, Liu H, Zhang Y, Discovery of novel janus kinase (JAK) and histone deacetylase (HDAC) dual inhibitors for the treatment of hematological malignancies, J. Med. Chem 62 (2019) 3898–3923. 10.1021/acs.jmedchem.8b01597. [DOI] [PubMed] [Google Scholar]
- [152].Zhang B, Wu Q, Zhou YL, Guo XY, Ge J, Fu JJ, Immune-related adverse events from combination immunotherapy in cancer patients: A comprehensive meta-analysis of randomized controlled trials, Int. Immunopharmacol 63 (2018) 292–298. 10.1016/j.intimp.2018.08.014. [DOI] [PubMed] [Google Scholar]
- [153].Lai Y, Tang F, Huang Y, He C, Chen C, Zhao J, Wu W, He Z, The tumour microenvironment and metabolism in renal cell carcinoma targeted or immune therapy, J. Cell. Physiol 236 (2021) 1616–1627. 10.1002/jcp.29969. [DOI] [PubMed] [Google Scholar]
- [154].Ray A, Das DS, Song Y, Hideshima T, Tai YT, Chauhan D, Anderson KC, Combination of a novel HDAC6 inhibitor ACY-241 and anti-PD-L1 antibody enhances anti-tumor immunity and cytotoxicity in multiple myeloma, Leukemia 32 (2018) 843–846. 10.1038/leu.2017.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Bae J, Hideshima T, Tai YT, Song Y, Richardson P, Raje N, Munshi NC, Anderson KC, Histone deacetylase (HDAC) inhibitor ACY241 enhances anti-tumor activities of antigen-specific central memory cytotoxic T lymphocytes against multiple myeloma and solid tumors, Leukemia 32 (2018) 1932–1947. 10.1038/s41375-018-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Ni L, Wang L, Yao C, Ni Z, Liu F, Gong C, Zhu X, Yan X, Watowich SS, Lee DA, Zhu S, The histone deacetylase inhibitor valproic acid inhibits NKG2D expression in natural killer cells through suppression of STAT3 and HDAC3, Sci. Rep 7 (2017) 45266. 10.1038/srep45266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Díaz-Carballo D, Saka S, Acikelli AH, Homp E, Erwes J, Demmig R, Klein J, Schröer K, Malak S, D’Souza F, Noa-Bolaño A, Menze S, Pano E, Andrioff S, Teipel M, Dammann P, Klein D, Nasreen A, Tannapfel A, Grandi N, Tramontano E, Ochsenfarth C, Strumberg D, Enhanced antitumoral activity of TLR7 agonists via activation of human endogenous retroviruses by HDAC inhibitors, Commun. Biol 4 (2021) 276. 10.1038/s42003-021-01800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Ellmeier W, Seiser C, Histone deacetylase function in CD4+ T cells, Nat. Rev. Immunol 18 (2018) 617–634. 10.1038/s41577-018-0037-z. [DOI] [PubMed] [Google Scholar]
- [159].Pan X, Zheng L, Epigenetics in modulating immune functions of stromal and immune cells in the tumor microenvironment, Cell Mol. Immunol 17 (2020) 940–953. 10.1038/s41423-020-0505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Qin Y, Vasilatos SN, Chen L, Wu H, Cao ZS, Fu YM, Huang M, Vlad AM, Lu BF, Oesterreich S, Davidson NE, Huang Y, Inhibition of histone lysine-specific demethylase 1 elicits breast tumor immunity and enhances antitumor efficacy of immune checkpoint blockade, Oncogene 38 (2019) 390–405. 10.1038/s41388-018-0451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Shen S, Hadley M, Ustinova K, Pavlicek J, Knox T, Noonepalle S, Tavares MT, Zimprich CA, Zhang G, Robers MB, Bařinka C, Kozikowski AP, Villagra A, Discovery of a new isoxazole-3-hydroxamate-based histone deacetylase 6 inhibitor SS-208 with antitumor activity in syngeneic melanoma mouse models, J. Med. Chem 62 (2019) 8557–8577. 10.1021/acs.jmedchem.9b00946. [DOI] [PubMed] [Google Scholar]
- [162].DeNardo DG, Ruffell B, Macrophages as regulators of tumour immunity and immunotherapy, Nat. Rev. Immunol 19 (2019) 369–382. 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Franco F, Jaccard A, Romero P, Yu YR, Ho PC, Metabolic and epigenetic regulation of T-cell exhaustion, Nat. Metab 2 (2020) 1001–1012. 10.1038/s42255-020-00280-9. [DOI] [PubMed] [Google Scholar]
- [164].Adeshakin AO, Yan D, Zhang M, Wang L, Adeshakin FO, Liu W, Wan X, Blockade of myeloid-derived suppressor cell function by valproic acid enhanced anti-PD-L1 tumor immunotherapy, Biochem. Biophys. Res. Commun 522 (2020) 604–611. 10.1016/j.bbrc.2019.ll.155. [DOI] [PubMed] [Google Scholar]
- [165].Liu D, Chen B, Mo Y, Wang Z, Qi T, Zhang Q, Wang Y, Redox-activated porphyrin-based liposome remote-loaded with indoleamine 2,3-dioxygenase (IDO) inhibitor for synergistic photoimmunotherapy through induction of immunogenic cell death and blockage of IDO pathway, Nano Lett. 19 (2019) 6964–6976. 10.1021/acs.nanolett.9b02306. [DOI] [PubMed] [Google Scholar]
- [166].Lu J, Liu X, Liao Y-P, Wang X, Ahmed A, Jiang W, Ji Y, Meng H, Nel AE, Breast cancer chemo-immunotherapy through liposomal delivery of an immunogenic cell death stimulus plus interference in the IDO-1 pathway, ACS Nano 12 (2018) 11041–11061. 10.1021/acsnano.8b05189. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [167].Fang K, Dong G, Li Y, He S, Wu Y, Wu S, Wang W, Sheng C, Discovery of novel indoleamine 2,3-dioxygenase 1 (IDO1) and histone deacetylase (HDAC) dual inhibitors, ACS Med. Chem. Lett 9 (2018) 312–317. 10.1021/acsmedchemlett.7b00487. [DOI] [PMC free article] [PubMed] [Google Scholar]


