Abstract
Purpose
Acute ischemic stroke (AIS) is a devastating disease and remains the leading cause of death and disability. This retrospective study aims to investigate associations between systemic immune-inflammation index (SII) and all-cause mortality in patients with AIS. Patients and Methods. We used the data from Medical Information Mart for Intensive Care IV. A total of 1,181 patients with acute ischemic stroke (AIS) were included. Systemic immune-inflammation index (SII) was calculated as platelet count (/L) × neutrophil count (/L)/lymphocyte count (/L). The main outcomes were 30-day all-cause mortality. The association between SII with mortality was evaluated using the Cox proportional hazards regression model.
Results
After adjusting for potential covariates, the highest quartiles of SII versus the lowest quartiles of SII, the HR was 2.74 (CI 1.79–4.19, P < 0.001). Log-transformed SII was significantly associated with 30-day all-cause mortality (HR 2.44; CI 1.72–3.46, P < 0.001). Furthermore, we found that there is a nearly linear relationship (P=0.265) between logarithmic transformed SII with all-cause mortality.
Conclusion
Elevated SII of patients with acute ischemic stroke increased the risk of 30-day all-cause mortality. SII may serve as a useful marker to elucidate the role of thrombocytosis, inflammation, and immunity interaction in the development of AIS.
1. Introduction
Acute ischemic stroke (AIS) is a devastating disease; it leads to high morbidity and mortality. In mainland China, the in-hospital death rate was 1.9% for stroke patients [1]. The Ministry of Health China Stroke Prevention Project Committee (CSPPC) has established a stroke center network, stroke map, and stroke “Green Channel” to create three 1 h gold rescue circles, which led to a significant improvement in stroke [1, 2]. But this disease still causes us to suffer huge pain and losses.
The mechanism of AIS is complex and needs more investigation. Scientific evidence has revealed that immunity and inflammation play crucial roles in AIS, which are the main causes of the most cardiovascular event [3, 4]. Growing evidence from prospective cohort studies has shown that differential leukocyte counts, including counts of lymphocytes [5], neutrophils [6, 7], and other granulocyte [5], are associated with the risk of cardiovascular disease. A study has previously found that platelet counts were significantly associated with stroke events [8]. Emerging data have also shown that some inflammation and immune indices based on the complete blood count, such as platelet-lymphocyte ratio (PLR) and neutrophil-lymphocyte ratio (NLR), can serve as predictors of cardiovascular events in patients at risk of primary cardiovascular disease [9, 10]. However, there is a novel inflammation and immune marker, defined as a systemic immune-inflammation index (SII), which is calculated using platelet, neutrophil, and lymphocyte counts (P∗N/L). This marker was shown to reflect the severity of systemic inflammation in patients with carcinoma [11] and has high prognostic values in different types of cancer [12, 13]. SII was recently reported to be positively associated with mortality in patients with heart failure [14]. Some studies have shown that it is related to the incidence, severity, and prognosis of AIS [15, 16], but it is unknown whether it is related to the mortality of patients who stay in the intensive care unit. To explore the correlation between SII and AIS all-cause mortality, our study analyzed the data from Medical Information Mart for Intensive Care IV database.
2. Materials and Methods
2.1. Database
This study was a restrictive observation study from the Medical Information Mart for Intensive Care IV database (MIMIC-IV version 1.0) from 2008 to 2019. This database, an update to MIMIC-IV, is deidentified according to the Health Insurance Portability and Accountability Act Safe Harbor provision and has approval from the Massachusetts Institute of Technology and Institutional Review Board of Beth Israel Deaconess Medical Center (BIDMC). MIMIC-IV contains clinical information from patients in the Intensive Care Unit (ICU) at BIDMC. The author finished the Collaborative Institutional Training Initiative (CITI) program course named “Data or Specimens Only Research” and achieved access to the database.
2.2. Data Extraction
The Structured Query Language (SQL) with PostgreSQL (version 9.6) was introduced to extract the data from the MIMIC-IV database. Adult patients who were diagnosed with AIS based on the ninth and tenth revision of the International Classification of Diseases (ICD-9/10) code during their admissions were included in this study (ICD-9 codes: 34660, 34661, 34662, 34663, 43301, 43311, 43321, 43331, 43381, 43391, 43401, 43411, and 43491; ICD-10 code: I63). The following information was collected: general information, vital signs, scoring systems (the sequential organ failure assessment (SOFA) score, acute physiology score III (APS III) score, and simplified acute physiology score II (SAPS II)), Glasgow coma scale (GCS), comorbidities, laboratory data, and treatments. All of the laboratory results extracted were from the first test results after the patient entered the ICU. The primary outcome was 30-day all-cause mortality, and the secondary outcome was 90-day all-cause mortality.
2.3. Evaluation of Systemic Immune-Inflammation Index
The systemic immune-inflammation index was calculated from absolute peripheral platelet counts (P, ∗ 109/L), neutrophil count (N, ∗109/L), and lymphocyte counts (L, ∗ 109/L) using the following formula: SII = P ×N/L [16]. The SII value was also transformed to a logarithmic scale to minimize the skewness of the underlying distribution.
2.4. Inclusion Criteria
Patients from 2008 to 2019 were identified in the MIMIC-IV database. The inclusion criteria were as follows: adult patients (age, 18–89 years) with acute ischemic stroke. Exclusion criteria were as follows: nonfirst admission to ICU; age <18; a length of ICU stay less than 24 hours; missing platelet, neutrophil, or lymphocyte counts data at ICU admission; and accepted mechanical thrombectomy or intravenous thrombolysis. Figure 1 shows the detailed flowchart.
Figure 1.
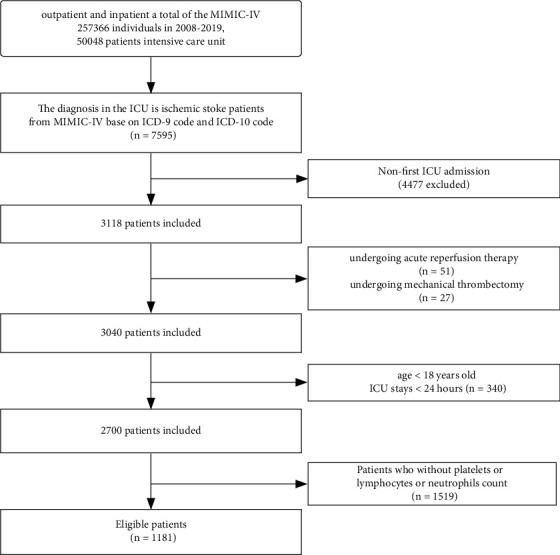
Flowchart of subject screening.
2.5. Statistical Analysis
The R software (version 3.42) and Free Statistics software (version 1.3) were used. P values less than 0.05 (two-sided) were considered statistically significant. In our statistical analysis, we followed the methods of Bin Hu et al. [17]. 1,181 participants were divided into four groups according to SII quartiles calculated at baseline. Normally distributed continuous variables are presented as mean ± standard deviation, nonnormally distributed continuous variables as medians with their interquartile ranges, and categorical variables as total number and percentage. We used the chi-square test for categorical variables and the Kruskal–Wallis test for continuous variables to compare groups. To examine the link between SII and the risk of all-cause mortality in patients with AIS, we introduced three different models by the univariate and multivariate Cox proportional hazards regression model, including the unadjusted model, the minimally adjusted model, and the fully adjusted model. SII was converted into LogSII by log-transformed because of nonnormally distribution. Cumulative hazards across SII quartiles are shown using Kaplan–Meier curves. The log-rank test was used to compare the curves. Accounting for the nonlinear correlation between SII and all-cause mortality of AIS patients, we also used a generalized additive model and the smooth curve fitting (penalized spline method) to address nonlinearity. Subgroup analyzes were conducted using a stratified Cox proportional hazard regression model. To test the robustness of our results, we performed a sensitivity analysis. We divided SII into four groups as categorical variables and calculated P for the trend to verify the result of SII as the continuous variable. Missing values for all variables are less than 5%, and missing values are imputed by the median or mean. All data were analyzed using the R software (version 3.42) and Free Statistics software (version 1.3). P values less than 0.05 (two-sided) were considered statistically significant.
3. Results
The detailed process of patient selection is shown in Figure 1. A total of 3118 patients satisfied the diagnostic criteria of AIS, and of these, 1181 patients fulfilled the inclusion criteria for the study. Table 1 provides the baseline characteristics of the enrolled patients. SII was divided into quartiles based on the distribution of baseline SII in patients (Q1: <667.5, Q2 : 667.6–1243.2, Q3: 1243.3–2242.0, and Q4: >2242). The baseline characteristics divided by SII are given in Table 1. According to Table 1, we found that there are significant differences in age, heart rate, respiratory rate, hyperlipidemia, COPD, paraplegia, neutrophils, lymphocytes, platelets, WBC, RBC, hemoglobin, RDW, anion gap, calcium, chloride, BUN, glucose, ALT, AST, INR, PT, PTT, NOAC, mechanical ventilation, PEGJ, APS III, SAPS II, OASIS, ICU.LODS, SOFA, GCSMIN, LOS.ICU, and LOS.hospital.
Table 1.
Baseline characteristics of participants by quartiles of the systemic immune-inflammation index (N = 1181).
| Variables | Total | Quartiles of the systemic immune-inflammation index (109/L) | P value | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| <667.5 | 667.5–1243.2 | 1243.2–2242 | >2242 | |||
| Participants (N) | 1181 | 295 | 295 | 295 | 296 | |
|
| ||||||
| Characteristics | ||||||
| Age, years | 69.1 ± 15.6 | 71.2 ± 15.1 | 69.3 ± 16.5 | 67.7 ± 15.0 | 68.2 ± 15.8 | 0.035 |
| Female, n (%) | 581 (49.2) | 144 (48.8) | 146 (49.5) | 151 (51.2) | 140 (47.3) | 0.82 |
| Heart rate | 83.7 ± 18.7 | 78.8 ± 16.5 | 81.7 ± 17.7 | 84.4 ± 19.3 | 89.7 ± 19.2 | <0.001 |
| MAP, mmHg | 76.3 ± 19.3 | 75.8 ± 18.6 | 75.2 ± 18.8 | 78.2 ± 21.0 | 76.2 ± 18.6 | 0.282 |
| Respiratory rate | 19.2 ± 5.6 | 18.3 ± 5.0 | 18.5 ± 4.8 | 19.7 ± 6.1 | 20.4 ± 6.0 | <0.001 |
| Temperature (°C) | 36.8 ± 0.8 | 36.7 ± 0.7 | 36.8 ± 0.7 | 36.8 ± 0.7 | 36.8 ± 0.9 | 0.117 |
| SPO2% | 97.2 ± 3.7 | 97.8 ± 2.7 | 97.3 ± 3.8 | 96.9 ± 3.8 | 96.8 ± 4.2 | 0.003 |
|
| ||||||
| Comorbidities, n (%) | ||||||
| Hypertension, n (%) | 602 (51.0) | 152 (51.5) | 158 (53.6) | 145 (49.2) | 147 (49.7) | 0.701 |
| Hyperlipidemia, n (%) | 613 (51.9) | 170 (57.6) | 165 (55.9) | 160 (54.2) | 118 (39.9) | <0.001 |
| Atrial fibrillation, n (%) | 450 (38.1) | 98 (33.2) | 115 (39) | 119 (40.3) | 118 (39.9) | 0.252 |
| Myocardial infarct, n (%) | 200 (16.9) | 43 (14.6) | 50 (16.9) | 52 (17.6) | 55 (18.6) | 0.607 |
| CHF, n (%) | 300 (25.4) | 68 (23.1) | 69 (23.4) | 86 (29.2) | 77 (26) | 0.291 |
| PVD, n (%) | 134 (11.3) | 36 (12.2) | 33 (11.2) | 31 (10.5) | 34 (11.5) | 0.933 |
| Dementia, n (%) | 59 (5.0) | 18 (6.1) | 20 (6.8) | 8 (2.7) | 13 (4.4) | 0.102 |
| CPD, n (%) | 233 (19.7) | 48 (16.3) | 45 (15.3) | 72 (24.4) | 68 (23) | 0.007 |
| Rheumatoid disease, n (%) | 35 (3.0) | 8 (2.7) | 10 (3.4) | 5 (1.7) | 12 (4.1) | 0.373 |
| Peptic ulcer disease, n (%) | 18 (1.5) | 6 (2) | 2 (0.7) | 4 (1.4) | 6 (2) | 0.475 |
| Diabetes mellitus, n (%) | 396 (33.5) | 109 (36.9) | 100 (33.9) | 94 (31.9) | 93 (31.4) | 0.751 |
| Paraplegia, n (%) | 592 (50.1) | 141 (47.8) | 163 (55.3) | 159 (53.9) | 129 (43.6) | 0.015 |
| Malignancy, n (%) | 85 (7.2) | 19 (6.4) | 20 (6.8) | 21 (7.1) | 25 (8.4) | 0.796 |
| Severe liver disease, n (%) | 17 (1.4) | 7 (2.4) | 3 (1) | 4 (1.4) | 3 (1) | 0.437 |
| Renal disease, n (%) | 228 (19.3) | 61 (20.7) | 56 (19) | 55 (18.6) | 56 (18.9) | 0.922 |
| Metastatic solid tumor, n (%) | 39 (3.3) | 4 (1.4) | 9 (3.1) | 15 (5.1) | 11 (3.7) | 0.084 |
| Charlson comorbidity | 7.1 ± 2.8 | 7.2 ± 2.7 | 7.1 ± 2.7 | 7.2 ± 3.0 | 7.0 ± 2.8 | 0.787 |
|
| ||||||
| Laboratory | ||||||
| Neutrophils, 109/L | 8.7 ± 4.4 | 5.4 ± 2.8 | 7.2 ± 2.9 | 9.7 ± 3.6 | 12.7 ± 4.3 | <0.001 |
| Lymphocytes, 109/L | 1.6 ± 0.9 | 2.3 ± 1.1 | 1.7 ± 0.7 | 1.3 ± 0.7 | 1.0 ± 0.5 | <0.001 |
| Platelets, 109/L | 226.0 ± 89.2 | 180.9 ± 77.7 | 215.4 ± 71.4 | 223.0 ± 83.2 | 284.5 ± 90.7 | <0.001 |
| WBC, 109/L | 10.0 (7.7, 13.6) | 7.7 (6.2, 10.1) | 8.9 (7.5, 11.1) | 11.1 (8.8, 13.8) | 13.8 (10.5, 17.6) | <0.001 |
| RBC, mean ± SD | 4.1 ± 0.8 | 4.0 ± 0.8 | 4.1 ± 0.8 | 4.2 ± 0.8 | 4.2 ± 0.8 | 0.008 |
| Hemoglobin, g/L | 12.3 ± 2.4 | 11.9 ± 2.4 | 12.3 ± 2.4 | 12.5 ± 2.3 | 12.3 ± 2.4 | 0.032 |
| RDW (%) | 14.3 ± 1.9 | 14.1 ± 1.9 | 14.3 ± 1.9 | 14.3 ± 1.8 | 14.6 ± 2.1 | 0.007 |
| Anion gap, mmol/L | 15.6 ± 4.3 | 14.6 ± 4.4 | 15.1 ± 3.7 | 16.1 ± 4.1 | 16.6 ± 4.7 | <0.001 |
| Bicarbonate, mmol/L | 22.9 ± 3.9 | 23.1 ± 3.7 | 23.3 ± 3.5 | 22.5 ± 3.9 | 22.6 ± 4.5 | 0.05 |
| Calcium, mg/dL | 8.7 ± 0.7 | 8.8 ± 0.8 | 8.8 ± 0.6 | 8.6 ± 0.8 | 8.6 ± 0.8 | <0.001 |
| Chloride, mmol/L | 103.1 ± 5.5 | 104.3 ± 5.2 | 102.9 ± 5.4 | 102.8 ± 5.2 | 102.5 ± 6.0 | <0.001 |
| Sodium, mmol/L | 138.9 ± 4.5 | 139.5 ± 4.1 | 139.0 ± 4.5 | 138.7 ± 4.1 | 138.6 ± 5.4 | 0.098 |
| Potassium, mmol/L | 4.3 ± 0.8 | 4.3 ± 0.7 | 4.3 ± 0.8 | 4.3 ± 1.0 | 4.3 ± 0.8 | 0.933 |
| Creatinine, mEq/L | 1.0 (0.8, 1.3) | 1.0 (0.8, 1.3) | 0.9 (0.8, 1.2) | 1.0 (0.8, 1.3) | 1.0 (0.8, 1.4) | 0.487 |
| BUN, mg/dL | 18.0 (14.0, 26.0) | 18.0 (14.0, 26.0) | 18.0 (12.0, 25.0) | 18.0 (14.0, 26.0) | 20.0 (14.5, 30.0) | 0.043 |
| Glucose, mg/dL | 126.0 (104.0, 166.0) | 114.0 (96.5, 143.0) | 117.0 (101.0, 154.0) | 127.0 (106.0, 165.5) | 148.0 (119.0, 193.2) | <0.001 |
| ALT, U/L | 20.0 (14.0, 31.0) | 18.0 (13.0, 26.8) | 19.0 (13.0, 28.0) | 21.0 (14.0, 33.0) | 22.0 (16.0, 39.0) | 0.001 |
| AST, U/L | 27.0 (19.0, 43.0) | 25.0 (19.0, 37.0) | 25.0 (19.0, 39.0) | 29.0 (19.0, 45.0) | 29.0 (20.0, 53.0) | 0.002 |
| INR | 1.1 (1.0, 1.3) | 1.1 (1.0, 1.3) | 1.1 (1.0, 1.3) | 1.2 (1.1, 1.3) | 1.1 (1.1, 1.3) | 0.02 |
| PT seconds | 12.5 (11.4, 14.4) | 12.4 (11.3, 14.5) | 12.2 (11.3, 13.9) | 12.8 (11.6, 14.6) | 12.7 (11.7, 14.4) | 0.002 |
| PTT seconds | 28.8 (25.7, 32.1) | 29.8 (26.2, 33.0) | 28.4 (25.7, 32.0) | 28.7 (25.3, 33.0) | 28.3 (25.7, 31.8) | 0.031 |
|
| ||||||
| Drugs use and treatment, n (%) | ||||||
| Warfarin, n (%) | 271 (22.9) | 60 (20.3) | 67 (22.7) | 75 (25.4) | 69 (23.3) | 0.534 |
| NOAC, n (%) | 152 (12.9) | 44 (14.9) | 45 (15.3) | 39 (13.2) | 24 (8.1) | 0.035 |
| Antiplatelet agents, n (%) | 897 (76.0) | 221 (74.9) | 224 (75.9) | 236 (80) | 216 (73) | 0.235 |
| MV, n (%) | 441 (37.3) | 95 (32.2) | 87 (29.5) | 111 (37.6) | 148 (50) | <0.001 |
| PEGJ, n (%) | 88 (7.5) | 7 (2.4) | 18 (6.1) | 22 (7.5) | 41 (13.9) | <0.001 |
|
| ||||||
| Scoring systems | ||||||
| APS III | 48.4 ± 24.4 | 43.1 ± 22.4 | 44.3 ± 22.0 | 47.3 ± 22.8 | 58.9 ± 27.0 | <0.001 |
| SAPS II | 34.8 ± 13.4 | 33.4 ± 13.0 | 33.3 ± 13.7 | 34.4 ± 12.7 | 38.0 ± 13.7 | <0.001 |
| OASIS | 33.8 ± 9.3 | 31.8 ± 8.9 | 32.7 ± 9.1 | 33.6 ± 8.8 | 37.2 ± 9.6 | <0.001 |
| LODS days | 4.0 (2.0, 7.0) | 4.0 (1.5, 6.0) | 4.0 (2.0, 6.0) | 4.0 (2.0, 7.0) | 5.0 (3.0, 8.0) | <0.001 |
| SOFA | 4.0 (2.0, 7.0) | 4.0 (2.0, 6.0) | 4.0 (2.0, 6.0) | 4.0 (2.0, 6.5) | 5.0 (3.0, 7.0) | <0.001 |
| GCS | 10.8 ± 3.8 | 11.5 ± 3.6 | 11.2 ± 3.6 | 10.8 ± 3.8 | 9.5 ± 4.0 | <0.001 |
| HASBLED | 1.4 (1.0, 1.8) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 0.332 |
| LOS.ICU | 3.7 (1.9, 7.4) | 3.1 (1.8, 6.1) | 3.2 (1.9, 6.8) | 3.8 (2.0, 8.3) | 4.4 (2.3, 9.1) | <0.001 |
| LOS.hospital | 8.7 (4.8, 15.8) | 7.3 (4.0, 13.2) | 8.3 (4.6, 14.8) | 9.0 (5.2, 17.4) | 11.2 (5.5, 18.8) | <0.001 |
|
| ||||||
| Death, n (%) | ||||||
| ICU mortality, n (%) | 134 (11.3) | 21 (7.1) | 20 (6.8) | 40 (13.6) | 53 (17.9) | <0.001 |
| 30-day mortality, n (%) | 208 (17.6) | 31 (10.5) | 35 (11.9) | 53 (18) | 89 (30.1) | <0.001 |
| 90-day mortality, n (%) | 226 (19.1) | 34 (11.5) | 38 (12.9) | 61 (20.7) | 93 (31.4) | <0.001 |
Data were expressed as mean ± SD/median (interquartile ranges (IQR)) for continuous variables and percentage for categorical variables. SD, standard deviation; IQR, interquartile range; MAP, mean arterial pressure; SPO2, saturation of percutaneous oxygen; CHF, congestive heart failure; PVD, peripheral vascular disease; CPD, chronic pulmonary disease; WBC, white blood cell; RBC, red blood cell; RDW, red blood cell distribution width; BUN, blood urea nitrogen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio; PT, prothrombin time; PTT, partial thromboplastin time; MV, mechanical ventilation; PEGJ, percutaneous gastrojejunostomy; NOAC, new oral anticoagulants; APS III, acute physiology score III; SAPS II, simplified acute physiology score; OASIS, Oxford acute severity of illness score; LODS, logistic organ dysfunction score; SOFA, sequential organ failure assessment; GCS, Glasgow coma scale; LOS.ICU, length of stay in the ICU; LOS.hospital, length of stay in the hospital.
Table 2 provides the results of the univariate Cox regression analysis. Through the results, the covariates included age, heart rate, respiratory rate, Charlson comorbidity, neutrophils, WBC, anion gap, bicarbonate, warfarin, NOAC, antiplatelet agents, PEGJ, mechanical ventilation, APS III, SAPS II, OASIS, SOFA, GCSMIN, HASBLED, ICU.LODS, LOS.ICU, and LOS.hospital were associated with 30-day all-cause mortality.
Table 2.
Univariate Cox regression analysis.
| Item | HR (95% CI) | P |
|---|---|---|
| Gender: male vs. female | 0.92 (0.7, 1.21) | 0.567 |
| Age, years | 1.02 (1.01, 1.03) | <0.001 |
| Heart rate | 1.01 (1.01, 1.02) | <0.001 |
| MAP, mmHg | 0.9968 (0.9897, 1.004) | 0.383 |
| Respiratory rate | 1.05 (1.02, 1.07) | <0.001 |
| Temperature (°C) | 0.97 (0.82, 1.14) | 0.679 |
| SPO2 | 0.99 (0.95, 1.02) | 0.357 |
| Hypertension | 1.13 (0.86, 1.49) | 0.371 |
| Hyperlipidemia | 0.76 (0.58, 1) | 0.051 |
| Atrial fibrillation | 1.13 (0.86, 1.49) | 0.379 |
| Myocardial infarct | 1.2 (0.86, 1.67) | 0.281 |
| Congestive heart failure | 0.95 (0.71, 1.29) | 0.755 |
| Charlson comorbidity | 1.07 (1.02, 1.12) | 0.006 |
| Neutrophils (109/L) | 1.08 (1.05, 1.11) | <0.001 |
| Lymphocytes (109/L) | 0.87 (0.74, 1.03) | 0.1 |
| Platelets (109/L) | 1.0003 (0.9989, 1.0018) | 0.638 |
| WBC (109/L) | 1.05 (1.03, 1.07) | <0.001 |
| Hemoglobin (g/L) | 0.9982 (0.9455, 1.0539) | 0.949 |
| RDW (109/L) | 1.02 (0.95, 1.08) | 0.624 |
| BUN (mg/dL) | 1.0043 (0.9988, 1.0099) | 0.124 |
| Anion gap (mmol/L) | 1.05 (1.03, 1.08) | <0.001 |
| Bicarbonate (mmol/L) | 0.95 (0.92, 0.98) | 0.002 |
| Chloride (mmol/L) | 0.9955 (0.9733, 1.0181) | 0.692 |
| Sodium (mmol/L) | 1.0051 (0.9781, 1.0327) | 0.716 |
| Potassium (mmol/L) | 0.93 (0.79, 1.09) | 0.346 |
| Glucose (mg/dL) | 1.0018 (1.0008, 1.0028) | <0.001 |
| INR | 0.95 (0.78, 1.17) | 0.659 |
| PT seconds | 0.9963 (0.9763, 1.0167) | 0.719 |
| PTT seconds | 1.0036 (0.9964, 1.0108) | 0.33 |
| Warfarin | 0.2 (0.12, 0.34) | <0.001 |
| NOAC | 0.1 (0.03, 0.3) | <0.001 |
| Antiplatelet agents | 0.46 (0.35, 0.61) | <0.001 |
| PEGJ | 0.1 (0.03, 0.31) | <0.001 |
| Mechanical ventilation | 2.49 (1.86, 3.33) | <0.001 |
| APS III | 1.02 (1.02, 1.02) | <0.001 |
| SAPS II | 1.04 (1.03, 1.05) | <0.001 |
| OASIS | 1.07 (1.05, 1.08) | <0.001 |
| LODS (days) | 1.16 (1.12, 1.2) | <0.001 |
| SOFA | 1.09 (1.06, 1.13) | <0.001 |
| GCSMIN | 0.89 (0.86, 0.92) | <0.001 |
| LOS.ICU | 0.94 (0.92, 0.97) | <0.001 |
| LOS.hospital | 0.81 (0.78, 0.84) | <0.001 |
HR, hazard ratio; CI, confidence interval; MAP, mean arterial pressure; SPO2, saturation of percutaneous oxygen; WBC, white blood cell; RDW, red blood cell distribution width; BUN, blood urea nitrogen; INR, international normalized ratio; PT, prothrombin time; PTT, partial thromboplastin time; NOAC, new oral anticoagulants; PEGJ, percutaneous gastrojejunostomy; APS III, acute physiology score III; SAPS II, simplified acute physiology score; OASIS, Oxford acute severity of illness score; LODS, logistic organ dysfunction score; SOFA, sequential organ failure assessment; GCS, Glasgow coma scale; LOS.ICU, length of stay in the ICU; LOS.hospital, length of stay in the hospital.
Table 3 provides the results of the association analysis between SII and all-cause mortality for 30-day and 90-day. Data are expressed as the hazard ratio (HR) and 95% confidence interval (CI). Log-transformed SII was described as a risk factor for AIS patients (30-day: nonadjusted model 2.35 (1.63–3.37), minimally adjusted model 2.51 (1.76–3.59), and fully adjusted model 2.43 (1.72–3.46); 90-day: nonadjusted model 2 (1.56–3.11), minimally adjusted model 2.42 (1.72–3.41), and fully adjusted model 1.92 (1.36–2.72)). Furthermore, compared to low SII (Q1 group), higher SII (Q4 group) was associated with an increased risk of 30-day all-cause mortality (nonadjusted model: 2.28 (1.51–3.43); minimally adjusted model: 2.56 (1.69–3.86); and fully adjusted model: 2.74 (1.79–4.19)). On 90-day mortality, Q4 group expressed such results (nonadjusted model: 2.14 (1.44–3.17); minimally adjusted model: 2.45 (1.65–3.64); and fully adjusted model: 2.63 (1.74–3.96)). A similar trend was found in the Q2 group and Q3 group.
Table 3.
Multivariate Cox regression analysis between quartiles of the systemic immune-inflammation index and 30-day mortality.
| Variable | Nonadjusted (HR (95% CI), P) | Adjusted I (HR (95% CI), P) | Adjusted II (HR (95% CI), P) |
|---|---|---|---|
| 30-day SII (quartile) | |||
| Q1 | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Q2 | 1.03 (0.63–1.67), 0.91 | 1.08 (0.66–1.75) 0.765 | 1.3 (0.79–2.12) 0.297 |
| Q3 | 1.42 (0.91–2.21), 0.123 | 1.61 (1.03–2.51) 0.037 | 1.86 (1.18–2.93) 0.007 |
| Q4 | 2.28 (1.51–3.43), <0.001 | 2.56 (1.69–3.86) <0.001 | 2.72 (1.78–4.17) <0.001 |
| Log SII | 2.35 (1.63–3.37), <0.001 | 2.51 (1.76–3.59) <0.001 | 2.43 (1.71–3.45) <0.001 |
|
| |||
| 90-day SII (quartile) | |||
| Q1 | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Q2 | 1 (0.63–1.58), 0.99 | 1.04 (0.65–1.65), 0.884 | 1.25 (0.78–2), 0.355 |
| Q3 | 1.45 (0.95–2.21), 0.081 | 1.68 (1.1–2.57), 0.016 | 1.87 (1.22–2.87), 0.004 |
| Q4 | 2.14 (1.44–3.17), <0.001 | 2.45 (1.65–3.64), <0.001 | 2.63 (1.74–3.96), <0.001 |
| Log SII | 2 (1.56–3.11), <0.001 | 2.42 (1.72–3.41), <0.001 | 1.92 (1.36–2.72), <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 |
Nonadjusted: no covariates were adjusted; adjusted I: we only adjusted for age and sex; adjusted II: we adjusted for age, gender, heart rate, respiratory rate, Charlson comorbidity, anion gap, bicarbonate, glucose, warfarin, NOAC, antiplatelet agents, PEGJ, mechanical ventilation, SOFA, and LOS.ICU. HR, hazard ratio; CI, confidence interval; Ref, reference.
The Kaplan–Meier curve for the SII quartile is shown in Figure 2. The figure indicates that survival rates of groups Q1 and Q2 were higher than groups Q3 and Q4, even though group Q4 described lowest survival probability at the time point of 30-day. Additionally, we plotted the survival rates at the time point of 30-day and 90-day (in Supplementary material 1).
Figure 2.
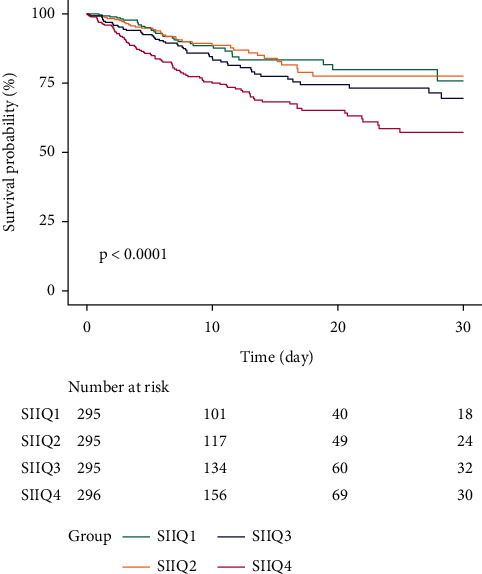
Kaplan–Meier survival curve for quartiles of SII.
We did not find an obvious nonlinear relationship between LogSII and 30-day mortality after adjusting for age, sex, heart rate, respiratory rate, Charlson comorbidity, anion gap, bicarbonate, glucose, warfarin, NOAC, antiplatelet agents, PEGJ, mechanical ventilation, SOFA, and LOS.ICU. Figure 3 shows that the association between LogSII and 30-day mortality of AIS patients was nearly linear (P=0.265).
Figure 3.
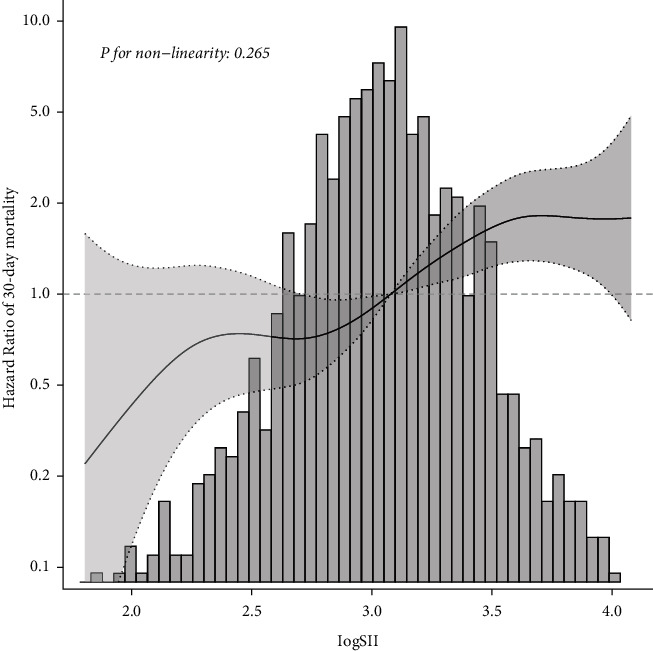
Smooth curve fitting for LogSII to HR of 30-day mortality.
Figure 4 shows the results of subgroup analyzes regarding the outcome of 30-day mortality. The interactions tests were not statistically significant for age, sex, hypertension, hyperlipidemia, atrial fibrillation, myocardial infarction, congestive heart failure, diabetes mellitus, warfarin, antiplatelet agents, or mechanical ventilation use (P for interaction = 0.146, 0.275, 0.065, 0.302, 0.896, 0.696, 0.696, 0.291, 0.482, 0.884, and 0.264).
Figure 4.
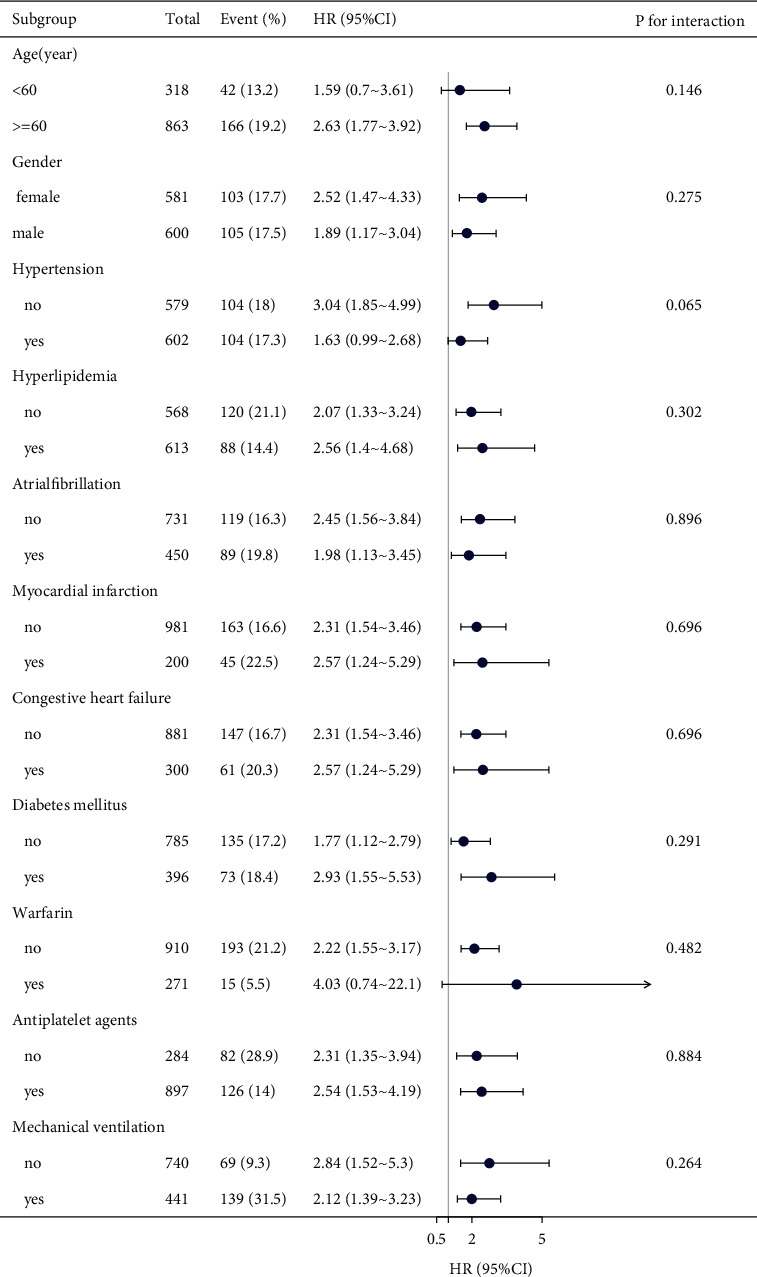
Subgroup analysis of the associations between SII and 30-day mortality.
4. Discussion
In this retrospective study, we found that SII was positively correlated with all-cause mortality in AIS patients using the unadjusted and adjusted Cox regression models. In particular, groups with a higher SII expressed higher mortality. We also found that the relationship between LogSII and 30-day mortality of patients with AIS was nearly linear. These results indicate that SII may be a new predictor of mortality of AIS.
As a new marker of inflammation, SII was proved to be used for the prediction of tumor prognosis prediction [12]. In recent years, some correlation with AIS is due to the mechanisms of immunity, inflammation, and atherosclerosis [18, 19]. SII integrates neutrophils, platelets, and lymphocytes, which cover the three aspects of inflammation, immunity, and thrombosis. Neutrophils, which are systemic inflammation markers, infiltrate the ischemic brain within 30 minutes to a few hours [20]; it releases several cytokines and proinflammatory mediators such as inducible nitric oxide synthase and matrix metalloproteinases to contribute to inflammation in the lesion [21]. Neutrophils also participate in the early destruction of the blood-brain barrier [22]. As one hallmark of brain ischemic injury, clinical studies have shown that the infiltration of neutrophils after ischemic stroke was correlated with the severity of the injury [23, 24]. At the time of ischemic stroke, platelets arrive at the injured vasculature in the brain first. Except for thrombosis, platelets can directly interact with circulatory leukocytes by changing the surface expression of P-selection or CD40 to form platelet-leukocyte aggregates activating the innate immune response to ischemia. Platelets also mediate dendritic cells antigen presentation to T cells. Platelets form heterotypic aggregates with neutrophils as a function of TLR7, TLR2, and TLR4 activation in the circulation [25]. The role of lymphocytes in the pathogenesis of AIS remains controversial. Lymphocytes are elevated in the ischemic brain later than neutrophils (3–6 days after AIS) [26]. Lymphocytes are a prognostic marker for cardiovascular disease. Several studies reported that lymphocytes play an important role in the healing and repair effects of inflammation [27]. Lymphocytes including B and T cells, especially CD4+, CD8+ T cells, and γδT cells, can produce proinflammatory cytokines such as interferon-γ and IL-17; however, Treg cell (CD4+CD25+Foxp3+Treg cell) is beneficial for inflammation by releasing anti-inflammatory cytokines such as IL-10 that are neuroprotective through the IL-10/JAK/STAT, PI3K and MAPK pathway [24, 28].
In our study, the high-level SII group was found to have higher neutrophils, platelets, and lower lymphocytes, which is consistent with our previous hypothesis. In addition, we performed a sensitivity analysis to determine the stability of our results and found no interaction among covariate subgroups, which may require larger sample sizes to verify.
Our study has some strengths. A key finding was that our study was the first study to reveal the association of SII with 30-day mortality in patients with AIS. Second, this study enrolled 1,181 patients, which is a large sample size for the clinical study of AIS. Third, we explored both the linear and nonlinear relationships. Fourth, we analyzed the exposure variable (SII) as not only a continuous variable but also a categorical variable and calculated the hazard ratio using Cox regression models. Such a method can minimize the incidence of contingency in statistical analysis and enhance the reliability of the final results.
There are some limitations to our study. First, SII was calculated using only the first test results after the patient entered the ICU. The optimal time point needs to be explored. Second, our study lacked some information at the time of participants' blood sample collection, including indicators of the acute inflammatory state (i.e., CRP, IL-1, IL-6, and TNF-α) [29–31], some other drugs use such as the use of steroids and nonsteroidal anti-inflammatory drugs, previous use of antibiotics and therapy durations, and information about other diseases that could cause inflammation, such as pneumonia, all of which may influence baseline leukocyte counts. Finally, as a single-center retrospective study, selection bias was inevitable; therefore, prospective studies are needed to confirm this finding.
5. Conclusions
SII is positively correlated with 30-day mortality in patients with AIS.
Data Availability
The datasets used to support this study are publicly available at https://mimic.physionet.org/.
Ethical Approval
This study was approved by the Ethics Committee of Changde City and was performed in accordance with the Declaration of Helsinki (No. 2022-004-01).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
In supplementary material 1, 30-day and 90-day death rate in the SII quartile were graphed and found that death outcome was mostly concentrated at the time point of 30-day.
References
- 1.Tu W. J., Chao B. H., Ma L., et al. Case-fatality, disability and recurrence rates after first-ever stroke: a study from bigdata observatory platform for stroke of China. Brain Research Bulletin . 2021;175:130–135. doi: 10.1016/j.brainresbull.2021.07.020. [DOI] [PubMed] [Google Scholar]
- 2.Chao B. H., Yan F., Hua Y., et al. Stroke prevention and control system in China: CSPPC-Stroke Program. International Journal of Stroke . 2021;16(3):265–272. doi: 10.1177/1747493020913557. [DOI] [PubMed] [Google Scholar]
- 3.Moriya J. Critical roles of inflammation in atherosclerosis. Journal of Cardiology . 2019;73(1):22–27. doi: 10.1016/j.jjcc.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Awan Z., Genest J. Inflammation modulation and cardiovascular disease prevention. European Journal of Preventive Cardiology . 2015;22(6):719–733. doi: 10.1177/2047487314529350. [DOI] [PubMed] [Google Scholar]
- 5.Shah A. D., Denaxas S., Nicholas O., Hingorani A. D., Hemingway H. Low eosinophil and low lymphocyte counts and the incidence of 12 cardiovascular diseases: a CALIBER cohort study. Open Heart . 2016;3(2) doi: 10.1136/openhrt-2016-000477.e000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zia E., Melander O., Björkbacka H., Hedblad B., Engstrom G. Total and differential leucocyte counts in relation to incidence of stroke subtypes and mortality: a prospective cohort study. Journal of Internal Medicine . 2012;272(3):298–304. doi: 10.1111/j.1365-2796.2012.02526.x. [DOI] [PubMed] [Google Scholar]
- 7.Shah A. D., Denaxas S., Nicholas O., Hingorani A. D., Hemingway H. Neutrophil counts and initial presentation of 12 cardiovascular diseases: a caliber cohort study. Journal of the American College of Cardiology . 2017;69(9):1160–1169. doi: 10.1016/j.jacc.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He S., Lei W., Li J., et al. Relation of platelet parameters with incident cardiovascular disease (the dongfeng-tongji cohort study) The American Journal of Cardiology . 2019;123(2):239–248. doi: 10.1016/j.amjcard.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Trakarnwijitr I., Li B., Adams H., Layland J., Garlick J., Wilson A. Age modulates the relationship between platelet-to-lymphocyte ratio and coronary artery disease. International Journal of Cardiology . 2017;248:349–354. doi: 10.1016/j.ijcard.2017.06.127. [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Zhang G., Jiang X., Zhu H., Lu Z., Xu L. Neutrophil to lymphocyte ratio in relation to risk of all-cause mortality and cardiovascular events among patients undergoing angiography or cardiac revascularization: a meta-analysis of observational studies. Atherosclerosis . 2014;234(1):206–213. doi: 10.1016/j.atherosclerosis.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Wang B. L., Tian L., Gao X. H., et al. Dynamic change of the systemic immune inflammation index predicts the prognosis of patients with hepatocellular carcinoma after curative resection. Clinical Chemistry and Laboratory Medicine . 2016;54(12):1963–1969. doi: 10.1515/cclm-2015-1191. [DOI] [PubMed] [Google Scholar]
- 12.Hu B., Yang X. R., Xu Y., et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clinical Cancer Research . 2014;20(23):6212–6222. doi: 10.1158/1078-0432.ccr-14-0442. [DOI] [PubMed] [Google Scholar]
- 13.Yang R., Chang Q., Meng X., Gao N., Wang W. Prognostic value of Systemic immune-inflammation index in cancer: a meta-analysis. Journal of Cancer . 2018;9(18):3295–3302. doi: 10.7150/jca.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan M., Ren F., Gao D. The value of SII in predicting the mortality of patients with heart failure. Disease Markers . 2022;2022:1–10. doi: 10.1155/2022/3455372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou D., Wang C., Luo Y., et al. Systemic immune-inflammation index (SII) but not platelet-albumin-bilirubin (PALBI) grade is associated with severity of acute ischemic stroke (AIS) International Journal of Neuroscience . 2021;131(12):1203–1208. doi: 10.1080/00207454.2020.1784166. [DOI] [PubMed] [Google Scholar]
- 16.Xu M., Chen R., Liu L., et al. Systemic immune-inflammation index and incident cardiovascular diseases among middle-aged and elderly Chinese adults: the Dongfeng-Tongji cohort study. Atherosclerosis . 2021;323:20–29. doi: 10.1016/j.atherosclerosis.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Hu B., Cao J., Hu Y., Qin Z., Wang J. The association between serum anion gap and all-cause mortality in disseminated intravascular coagulation patients: a retrospective analysis. International Journal of General Medicine . 2021;14:4535–4544. doi: 10.2147/ijgm.s318334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ammirati E., Moroni F., Magnoni M., Camici P. G. The role of T and B cells in human atherosclerosis and atherothrombosis. Clinical and Experimental Immunology . 2015;179(2):173–187. doi: 10.1111/cei.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anrather J., Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics . 2016;13(4):661–670. doi: 10.1007/s13311-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin R., Yang G., Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. Journal of Leukocyte Biology . 2010;87(5):779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iadecola C., Anrather J. The immunology of stroke: from mechanisms to translation. Nature Medicine . 2011;17(7):796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark R. K., Lee E. V., White R. F., Jonak Z., Feuerstein G., Barone F. Reperfusion following focal stroke hastens inflammation and resolution of ischemic injured tissue. Brain Research Bulletin . 1994;35(4):387–392. doi: 10.1016/0361-9230(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 23.Zhou W., Liesz A., Bauer H., et al. Postischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models. Brain Pathology . 2013;23(1):34–44. doi: 10.1111/j.1750-3639.2012.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akopov S. E., Simonian N. A., Grigorian G. S. Dynamics of polymorphonuclear leukocyte accumulation in acute cerebral infarction and their correlation with brain tissue damage. Stroke . 1996;27(10):1739–1743. doi: 10.1161/01.str.27.10.1739. [DOI] [PubMed] [Google Scholar]
- 25.Blair P., Rex S., Vitseva O., et al. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circulation Research . 2009;104(3):346–354. doi: 10.1161/circresaha.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G. Z., Zhong D., Yang L. M., et al. Expression of interleukin-17 in ischemic brain tissue. Scandinavian Journal of Immunology . 2005;62(5):481–486. doi: 10.1111/j.1365-3083.2005.01683.x. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz M., Moalem G. Beneficial immune activity after CNS injury: prospects for vaccination. Journal of Neuroimmunology . 2001;113(2):185–192. doi: 10.1016/s0165-5728(00)00447-1. [DOI] [PubMed] [Google Scholar]
- 28.Ooboshi H., Ibayashi S., Shichita T., et al. Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation . 2005;111(7):913–919. doi: 10.1161/01.cir.0000155622.68580.dc. [DOI] [PubMed] [Google Scholar]
- 29.Duan R., Wang N., Shang Y., et al. TNF-Α (G-308A) polymorphism, circulating levels of TNF-α and IGF-1: risk factors for ischemic stroke-an updated meta-analysis. Frontiers in Aging Neuroscience . 2022;14 doi: 10.3389/fnagi.2022.831910.831910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Angelantonio E., Pepys M. B., Thompson S. G., Collins R., Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet (London, England) . 2010;375(9709):132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwan J., Horsfield G., Bryant T., et al. IL-6 is a predictive biomarker for stroke associated infection and future mortality in the elderly after an ischemic stroke. Experimental Gerontology . 2013;48(9):960–965. doi: 10.1016/j.exger.2013.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In supplementary material 1, 30-day and 90-day death rate in the SII quartile were graphed and found that death outcome was mostly concentrated at the time point of 30-day.
Data Availability Statement
The datasets used to support this study are publicly available at https://mimic.physionet.org/.


