Abstract
Background:
Freshwater snails serve as intermediate hosts for a variety of trematodes that cause illness in the human and animal populations. Several species of freshwater snails in Thailand have been found to have larval trematode infections. We aimed to investigate a freshwater snail in Phitsanulok Province and report on its current status of larval trematode infection.
Methods:
Freshwater snails were collected from six localities (rice field and irrigation canal) by handpicking and using a count per unit of time sampling approach. The snails were identified by their external shell morphology. The shedding method was applied to observe the cercariae, which were photographed under a light microscope to determine their morphological types.
Results:
A total of 211 snails were classified into seven genera. The most abundant snail species was Lymnaea sp., representing 31.3% of the sample, followed by Physella sp., Bithynia sp., Pomacea canaliculata, Filopaludina martensi, Indoplanorbis exustus, and Melanoides tuberculata, in that order. From the sample, 21 snails (9.95%), including Bithynia sp., Lymnaea sp., I. exustus, and M. tuberculata, were infected with cercarial trematodes, which could be categorized into four types, namely amphistome, parapleurolophocercous, echinostome, and xiphidiocercaria. Amphistome emerged from Bithynia sp., and I. exustus was the most common cercaria to be recovered, representing 80.9% of all infected snails.
Conclusion:
This study presents the current prevalence of cercariae in infected snails within the studied area. It is important to manage intermediate host snails in order to restrict trematode life cycle completion.
Keywords: Cercaria, Freshwater snail, Intermediate host, Trematode, Thailand
Introduction
Trematode infections in humans and animals are a major helminthic disease and are major agents of morbidity and mortality worldwide, particularly in underdeveloped countries (1). Digenetic trematodes have a complicated life cycle, which required snails to be the first or the second intermediate host (2). When snails act as the first intermediate host, the larval stages of trematodes follow the sequence of sporocysts, rediae, and cercariae. The cercarial trematodes are released from the infected snail host to find a new host for their transmissions (3, 4).
Several species of freshwater snails become naturally infected by larval trematodes (5–9), especially in the north and northeastern part of Thailand where their prevalence is high (10–12). For example, Melanoides tuberculata was found to be infected with parapleurolophocercous, pleurolophocercous, and megalurous cercariae. Moreover, Bithynia siamensis siamensis was found to be infected with monostome, gymnocephalus, and virgulate cercariae. Moreover, snails in the Planorbidae family act as intermediate hosts of animal schistosomes in the family Schistosomatidae, which is the cause of cercarial dermatitis in humans (13, 14). Trematode infections have been reported among intermediate hosts in Thailand (15, 16).
However, only a few studies on snail diversity and cercarial infection have been reported in Phitsanulok Province, which is in the lower northern part of Thailand. This province is an important agricultural area with a large number of rice fields, which serve as a habitat for a variety of intermediate host snails. It is situated on the geographical line uniting the central and northern regions of Thailand. Phitsanulok consists of 9 districts where the mountain region which is one third covers the east and north part of province. We randomly selected 3 districts to collect the snail samples; namely Mueang Phitsanulok District, Bang Rakam, and Wang Thong District. These areas are the plain region with most rice paddy fields and irrigation canals.
Hence, the objectives of this study were to examine a freshwater snail from Phitsanulok province, Thailand, and report on its current larval trematode infection status. The results of this investigation will provide a foundation for preventing or controlling intermediate host snails, which may aid in limiting trematode infection in the study area.
Materials and Methods
Snail collection
The research was carried out in 4–28 February 2019. For the study, six sites in Thailand’s Phitsanulok province were surveyed for freshwater snails. A total of three rice fields in Mueang Phitsanulok District (RM1, RM2, and RM3), two rice fields in Bang Rakam District (RB5 and RB6), and one irrigation canal in Wang Thong District (CW4) shows in Fig. 1 and Table 1.
Fig. 1:
Map of the Phitsanulok province showing areas from where freshwater snails were collected
Table 1:
Species and the number of freshwater snails collected from six localities in the Phitsanulok province
| Site of collection (code) | Number of snails | Total | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Bithynia sp. | M. tuberculata | P. canaliculata | F. matensi | Lymnaea sp. | Physella sp. | I. exustus | ||
| Rice field, Mueang Phitsanulok district (RM1) | 20 | - | 6 | 14 | 1 | - | 1 | 42 |
| Rice field, Mueang Phitsanulok district (RM2) | - | - | 1 | 1 | 25 | - | - | 27 |
| Rice field, Mueang Phitsanulok district (RM3) | - | 1 | 4 | 1 | 40 | - | - | 46 |
| Irrigation canal, Wang Thong District (CW4) | - | - | 1 | 3 | - | - | 1 | 5 |
| Rice field, Bang Rakam District (RB5) | - | - | - | - | - | 30 | - | 30 |
| Rice field, Bang Rakam District (RB6) | 15 | - | 19 | - | - | 26 | 1 | 61 |
| Total | 35 | 1 | 31 | 19 | 66 | 56 | 3 | 211 |
Collection and identification of freshwater snails
Snail specimens were collected by handpicking or using a wire-mesh scoop with a count per unit of time sampling method (17). Two researchers collected snail samples for 30 minutes at each site. All snails collected were cleaned, labelled, and stored in plastic bags. The snails were returned to the laboratory to be identified for genus or species based on external shell morphology using taxonomic keys (18). Following that, the snails were examined for trematode larvae (cercariae).
Examination for larval trematode infections
Using cercarial shedding procedures, the snails were investigated for trematode infections. Each snail was washed and placed in a plastic cup with 20 ml of dechlorinated tap water. Cercarial shedding was carried out for 24 hours at room temperature under natural light (19–21). Under the stereomicroscope, trematode cercariae were studied and their swimming activity was noted. The cercariae were collected using a plastic pasture pipette from unstained and mounted on slides with a heat fix. A camera was used to record images of immobile cercariae beneath a compound microscope. According to Schell, Yamaguti, and Ito, morphological types of cercariae were classified based on the observed characteristics (22–24).
Results
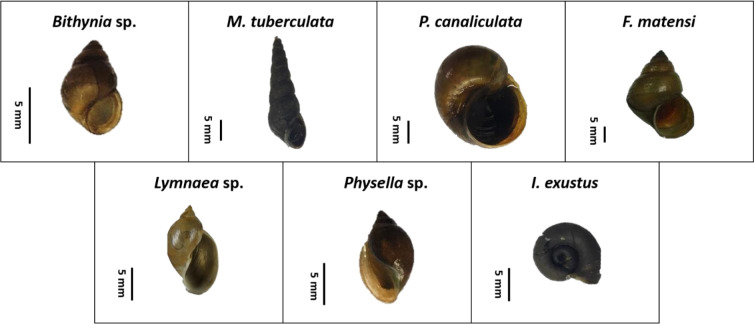
In the current investigation, a freshwater snail was collected in February 2019 from six distinct locations in Phitsanulok Province of Thailand. The collected snails were divided into seven families, seven genera, and seven species (Table 1 and 2). Lymnaea sp. was the most prevalent species, accounting for 31.3% of the sample, followed by Physella sp., Bithynia sp., P. canaliculata, F. matensi, I. exustus, and M. tuberculata (Fig. 2). Pomacea canaliculata was discovered in five locations, but M. tuberculata was discovered once in a rice field in Mueang Phitsanulok District (RM3). Snail diversity is abundant in rice fields, particularly in RM1, where five different kinds of snails were discovered.
Table 2:
Number of infected snails with larval trematodes
| Snails collected in the present study | No. of snail collected | No. of infected snail | Number of infected snail from eachsite | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Genus | Species | RM1 | RM2 | RM3 | CW4 | RB5 | RB6 | |||||||
| Bithyniidae | Bithynia | Bithynia sp. | 35 | 17 | 1 | - | - | - | - | 16 | |||||
| Physidae | Physella | Physella sp. | 56 | 2 | - | - | - | - | 2 | - | |||||
| Planorbidae | Indoplanorbis | I. exustus | 3 | 1 | - | - | - | - | - | 1 | |||||
| Thiaridae | Melanoides | M. tuberculata | 1 | 1 | - | - | 1 | - | - | - | |||||
| Viviparidae | Filopaludina | F. matensi | 19 | 0 | - | - | - | - | - | - | |||||
| Ampullariidae | Pomacea | P. canaliculata | 31 | 0 | - | - | - | - | - | - | |||||
| Lymnaeidae | Lymnaea | Lymnaea sp. | 66 | 0 | - | - | - | - | - | - | |||||
| Total | 211 | 21 | 1 | - | 1 | - | 2 | 17 | |||||||
Fig. 2:
The freshwater snails collected in this study
Cercariae infection was found in four species of snails, including Bithynia sp., Lymnaea sp., I. exustus, and M. tuberculata (21 out 211 snails or 9.95%) (Table 2). The observed cercariae were then classified into four categories (Fig. 3 and Table 3): amphistome, echinostome, parapleurolophocercous, and xiphidiocercaria. After echinostome cercaria, amphistome that emerged from Bithynia sp. and I. exustus was the most prevalent cercaria, accounting for 80.9% of all infected snails (9.5%). In each cercarial type, xiphidiocercaria and parapleurolophocercous cercaria constituted 4.7% of all infected snails. Bithynia sp. were reported to be infected with amphistome cercaria and xiphidiocercaria in two separate locations (RM1 and RB5, respectively).
Fig. 3:
The cercariae emerged from freshwater snails. (A) amphistome cercaria from Bithynia sp., (B) parapleurolophocercous cercaria, (C) amphistome cercaria from I. exustus, (D) echinostome cercaria, and (E) xiphidiocercaria
Table 3:
Cercariae types in the infected snails
| Cercariae types | No. of infected snail | Infected snail species |
|---|---|---|
| Amphistome cercaria | 16 | Bithynia sp. |
| Xiphidiocercaria | 1 | Bithynia sp. |
| Echinostome cercaria | 2 | Physella sp. |
| Amphistome cercaria | 1 | I. exustus |
| Parapleurolophocercous cercaria | 1 | M. tuberculata |
Discussion
Our research determines the current prevalence of trematode infection in a freshwater snail in Phitsanulok province of Thailand. A total of 9.95% (21 out of 211) infected snails, with a species distribution of Bithynia sp., Lymnaea sp., I. exustus, and M. tuberculata, were discovered. The prevalence rate (9.95%) of freshwater snails with trematode infection in the current study is comparable to that determined by a survey conducted in Chiang Mai (9.6% or 19 out 352 samples) (25). The rate is higher than several previous studies, for example, 1.38% (17 out of 1,227 samples) in Chiang Rai province (8), 6.20% in five provinces of Northern Thailand (24), and 1.69% (35 out of 2,076 samples) in Ubon Ratchathani Province (26). In Chiang Mai Province, however, the infection rate of snails with trematodes was 17.27% (11). The prevalence of cercariae infections in freshwater snails is well-reported globally, such as 16% in Sri Lanka (27), 4.3% in Nepal (28), 27.9% in Iran (29), and 6.6% in Zimbabwe (30). When compared to earlier studies, the infection rate reported in the current study is rather high. This may involve different factors, including differences in geographical localities of water reservoirs, season, snail species in each water area, and cercarial type (31–32).
In general, snail populations in different geographical areas fluctuate depending on the amount of rainfall, since their habitats are usually swept away by heavy rains. The surviving snails take a few months to resettle and reproduce in order to increase their populations (31–32). The snail specimens collected for this study were collected in the month of February, which is a few months away from the rainy season in Thailand. Seven species of snails were found in the studied area, namely Lymnaea sp., Physella sp., Bithynia sp., P. canaliculata, F. martensi, I. exustus and M. tuberculata. The most abundant species was Lymnaea sp., representing 31.3% of the entire sample, in contrast to other provinces where Bithynia sp. was found to be the most abundant (8, 11). Snail populations were higher in rice fields, with five snail species being found in one locality. Similar to a previous study, snail diversity was higher in rice fields than in other habitats (8). The distribution pattern of freshwater snails was rather erratic as a result of differences in water habitat, anthropocene alteration of water environments, and agricultural activities such as transplanting or harvesting and using chemicals, some of which may contain molluscicide (33).
Cercarial emergence was seen in four different species: Bithynia sp., Lymnaea sp., I. exustus, and M. tuberculata. In this investigation, the remaining three species, P. canaliculate, F. martensi, and Physella sp., were not infected by any type of cercariae. Amphistome cercariae were found in the greatest abundance in Bithynia sp. and I. exustus. In Nepal, they were discovered in I. exustus, Gabbia orcula, and Gyraulus euphraticus (28). This cercaria is responsible for snail-borne illnesses, amphistomiasis in domestic animals, and on rare occasions, amphistomiasis in humans (34–35). Xiphidiocercaria was observed in Bithynia sp., correlating with previous reports that this form of cercariae was found in three snail species, namely, B. siamensis, M. tuberculata, and T. granifera (11). Furthermore, B. siamensis goniomphalos could act as an intermediate host for seven different species of cercariae (25). This shows that Bithynia sp. snails are trematode intermediate hosts. Moreover, Lymnaea caillaudi, Cleopatra bulinoides, Lonistes carnatus, Thiara tuberculata, and Thiara granifera were hosted by xiphidiocercaria (36–37).
As shown in the present study, parapleurolophocercous cercariae were found in M. tuberculata. Earlier, parapleurolophocercous was observed in thiarid snails, including M. tuberculata, M. jugicostis, Thiara scabra, T. granifera, and Sermyla riquetii (8, 11, 38). The morphological characteristic of parapleurolophocercous cercaria was identified as belonging to the family Heterophyidae, which includes Haplorchis taichui, Haplorchis pumilio, Stellantchasmus falcatus, Centrocestus caninus, and Procerovum sp. (39–44). Echinostome cercariae were discovered in physellid snails, whereas several studies reported its emergence in viviparid, planorbid, lymnaeid, and thiarid snails (8, 45–47), which were reported to be the cercarial stage of the intestinal trematodes in the family Echinostomatidae (48).
The four larval trematodes found in the present study showed the ability to infect several snail species. Similarly, snails collected for this study were shown to be intermediate hosts that are susceptible to harboring a wide spectrum of cercarial types. As a result, freshwater snails can be considered an important determinant for monitoring the health of human and animal populations.
Conclusion
We report the prevalence of larval trematodes in freshwater snails in the Phitsanulok Province of Thailand. Four types of larval trematodes with health-related implications for humans and animals were found in freshwater snails in the studied area. These findings suggest that it is necessary to control the snail population in this area in order to check the prevalence of snail-borne diseases.
Acknowledgments
We would like to thank Ms. Paramaporn Muangpat and Ms. Ketsarin Tipphet for their help with the snail collection. This study was funded by Thailand Science Research and Innovation (TSRI) through Naresuan University (FRB640025 or R2564B004).
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Andrews RH, Sithithaworn P, Petney TN. Opisthorchis viverrini: an underestimated parasite in world health. Trends Parasitol. 2008;24(11):497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elsheikha HM, Elshazly AM. Host-dependent variations in the seasonal prevalence and intensity of heterophyid encysted metacercariae (Digenea: Heterophyidea) in brackish water fish in Egypt. Veterinary Parasitology. 2008;153:65–72. [DOI] [PubMed] [Google Scholar]
- 3.Skála V, Bulantová J, Walker AJ, et al. Insights into the development of Notocotylus attenuatus (Digenea: Notocotylidae) in Lymnaea stagnalis: from mother sporocyst to cercariae. Parasitol Int. 2014;63(1):94–9. [DOI] [PubMed] [Google Scholar]
- 4.Tesana S, Srisawangwong T, Sithithaworn P, et al. Angiostrongylus cantonensis: experimental study on the susceptibility of apple snails, Pomacea canaliculata compared to Pila polita. Exp Parasitol. 2008;118(4):531–5. [DOI] [PubMed] [Google Scholar]
- 5.Burch JB, Lohachit C. Snail of medical importance in Thailand. Walkerana (Ann Arbor Mich); 1983. p.395–398. [Google Scholar]
- 6.Woodruff DS, Upatham ES. Snail transmitted diseases of medical and veterinary importance in Thailand and the Mekong valley. J Med Appl Malacol. 1993;4:1–12. [Google Scholar]
- 7.Sri-Aroon P. Freshwater snails of medical importance in Thailand. Thailand: Mollusk Museum, Applied Malacology Center, Department of Social and Environmental Medicine, Mahidol University. 2011, p. 6–8. [Google Scholar]
- 8.Chantima K, Suk-Ueng K, Kampan M. Freshwater snail diversity in Mae Lao agricultural basin (Chiang Rai, Thailand) with a focus on larval trematode infections. Korean J Parasitol. 2018;56(3):247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sithithaworn P, Haswell-Elkins M. Epidemiology of Opisthorchis viverrini. Acta Trop. 2003;88(3):187–94. [DOI] [PubMed] [Google Scholar]
- 10.Boonchot K, Wongsawad C. A survey of helminths in cyprinoid fish from the Mae Ngad Somboonchon Reservoir, Chiang Mai Province, Thailand. Southeast Asian J Trop Med Public Health. 2005;36(1):103–7. [PubMed] [Google Scholar]
- 11.Chontananarth T, Wongsawad C. Epidemiology of cercarial stage of trematodes in freshwater snails from Chiang Mai province, Thailand. Asian Pac J Trop Biomed. 2013;3(3):237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chai JY, Sohn WM, Na BK, et al. Echinostoma revolutum: metacercariae in Filopaludina snails from Nam Dinh Province, Vietnam, and adults from experimental hamsters. Korean J Parasitol. 2011;49(4):449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolářová L, Horák P, Skírnisson K, et al. Cercarial dermatitis, a neglected allergic disease. Clin Rev Allergy Immunol. 2013;45(1):63–74. [DOI] [PubMed] [Google Scholar]
- 14.Nak-ai W. (2020). The prevalence of helminthes infection among host and intermediate hosts at Mae-soi water gate project, Lampoon province year 2016–17. jdpc7kk. 2020;27:22–37. [Google Scholar]
- 15.Wiroonpan P, Chontananarth T, Purivirojkul W. Cercarial trematodes in freshwater snails from Bangkok, Thailand: prevalence, morphological and molecular studies and human parasite perspective. Parasitology. 2021;148(3):366–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunchom N, Pilap W, Suksavate, et al. Trematode infection in freshwater snails from Maha Sarakham province, Thailand. Southeast Asian J Trop Med Public Health. 2020;50(4).518–527. [Google Scholar]
- 17.Oliver L, Schneiderman M. A method for estimating the density of aquatic snail populations. Exp Parasitol. 1956;5(2):109–17. [DOI] [PubMed] [Google Scholar]
- 18.Brandt RAM. The non-marine aquatic Mollusca of Thailand. Arch Mollusken; 1974. P. 1–423 . [Google Scholar]
- 19.Kaewkes S, Kaewkes W, Boonmars T, et al. Effect of light intensity on Opisthorchis viverrini cercarial shedding levels from Bithynia snails-a preliminary study. Parasitol Int. 2012;61(1):46–8. [DOI] [PubMed] [Google Scholar]
- 20.Sripa J, Kiatsopit N, Piratae S. Prevalence of trematode larvae in intermediate hosts: snails and fish in Ko Ae sub-district of Khueang Nai, Ubon Ratchathani province, Thailand. Southeast Asian J Trop Med Public Health. 2016;47(3):399–409. [PubMed] [Google Scholar]
- 21.Schell SC. Parasitology Laboratory Manual. Wiley. New York, USA; 1963. [Google Scholar]
- 22.Yamaguti S. A Synoptical Review of Life Histories of Digenetic Trematodes of Vertebrates. Tokyo, Japan; 1975. p.1–590. [Google Scholar]
- 23.Ito J. Studies on Cercariae in Japan. Shizuoka University. Shizuoka, Japan; 1980. [Google Scholar]
- 24.Mard-arhin N, Prawang T, Wongsawad C. Helminths of freshwater animals from five provinces in northern Thailand. Southeast Asian J Trop Med Public Health. 2001;32(2):206–9. [PubMed] [Google Scholar]
- 25.Ngern-klun R, Sukontason KL, Tesana S, et al. Field investigation of Bithynia funiculata, intermediate host of Opisthorchis viverrini in northern Thailand. Southeast Asian J Trop Med Public Health. 2006;37(4):662–72. [PubMed] [Google Scholar]
- 26.Haruay S, Piratae S. Situation and cercarial infection of freshwater mollusk from Sirindhorn Reservoir, Ubon Ratchathani Province, Thailand. Iran J Parasitol. 2019;14(3):421–429 [PMC free article] [PubMed] [Google Scholar]
- 27.Jayawardena UA, Rajakaruna R, Amerasinghe P. Cercariae of trematodes in freshwater snails in three climatic zones in Sri Lanka. Cey J Sci (Bio. Sci.). 2011; 39(2):95–108. [Google Scholar]
- 28.Devkota R, Budha PB, Gupta R. Trematode cercariae infections in freshwater snails of Chitwan district, central Nepal. Himalayan J H Sci. 2011;7(9):9–14. [Google Scholar]
- 29.Kalat-Meimari M, Shamseddin J, Salahi-Moghaddam A. Ecological and Parasitological Study on Cerithidea cingulata (Gastropoda) in Hormoz Strait Littoral, South of Iran. Iran J Parasitol. 2018;13(2):285–292. [PMC free article] [PubMed] [Google Scholar]
- 30.Chingwena G, Mukaratirwa S, Kristensen TK, et al. Larval trematode infections in freshwater snails from the Highveld and Lowveld areas of Zimbabwe. J Helminthol. 2002;76(4):283–93. [DOI] [PubMed] [Google Scholar]
- 31.Upatham ES, Sornmani S, Kitikoon V, et al. Identification key for the fresh-and brackish-water snails of Thailand. Malacol Rev. 1983;16:107–13. [Google Scholar]
- 32.Nithiuthai S, Wiwanitkit V, Suwansaksri J, et al. A survey of trematode cercariae in Bithynia goniomphalos in northeast Thailand. Southeast Asian J Trop Med Public Health. 2002;33:106–9. [PubMed] [Google Scholar]
- 33.Dung BT, Madsen H, The DT. Distribution of freshwater snails in family-based VAC ponds and associated water bodies with special reference to intermediate hosts of fish-borne zoonotic trematodes in Nam Dinh Province, Vietnam. Acta Trop. 2010;116:15–23. [DOI] [PubMed] [Google Scholar]
- 34.Malek EA, Cheng TC. Medical and economic malacology. Academic press, New York; 1974. [Google Scholar]
- 35.Phiri AM, Phiri IK, Monrad J. Prevalence of amphistomiasis and its association with Fasciola gigantica infections in Zambian cattle from communal grazing areas. J Helminthol. 2006;80(1):65–8. [DOI] [PubMed] [Google Scholar]
- 36.Brinesh R, Janardanan KP. Three new species of xiphidiocercariae from the thairid snail Thaira tuberculata in Palakkad, India. J Parasit Dis. 2011; 35(1):42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bdir S, Adwan G. Three new species of cercariae from Melanopsis praemorsa (L. 1758, Buccinum) snails in Al-Bathan fresh water body, Palestine. Asian Pacific Journal of Tropical Biomedicine. 2012; S1064–S1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chontananarth T, Wongsawad C. The pleurophocercous cercariae infection in snail Family Thiaridae Grey, 1847 Northern, Thailand. Asian Pacific Journal of Tropical Disease. 2017;7:205–210. [Google Scholar]
- 39.Wivitchuta D, Glaubrecht M, Krailas D. Natural Trematode Infections of Freshwater Snail Melanoides jugicostis Hanley & Theobald, 1876 (Family Thiaridae), the First Intermediate Host of Animal and Human Parasites in Thailand. Silpakorn U Science & Tech J. 2017;11:9–16. [Google Scholar]
- 40.Chontananarth T, Wongsawad C. Haplorchis taichui infection of the freshwater snails and molecular identification. Trends Res Sci Tec. 2010;2:7–12. [Google Scholar]
- 41.Chuboon S, Wongsawad C. Molecular identification of larval trematode in intermediate hosts from Chiang Mai, Thailand. Southeast Asian J Trop Med Public Health. 2009; 40:1216–20. [PubMed] [Google Scholar]
- 42.Yousif F, Lbrahim A, Bardicy SE, et al. Morphology of new eleven cercariae procured from Melanoides tuberculata snails in Egypt. Australian Journal of Basic and Applied Sciences. 2010;4(4):1482–1494. [Google Scholar]
- 43.Dechruksa W, Krailas D, Ukong S, et al. Trematode infections of the freshwater snail family Thiaridae in the Khek River, Thailand. Southeast Asian J Trop Med Public Health. 2007;38(6):1016–28. [PubMed] [Google Scholar]
- 44.Krailas D, Namchote S, Rattanathai P. Human intestinal flukes Haplorchris taichui and Haplorchris pumilio in their intermediate hosts, freshwater snails of the families Thiaridae and Pachychilidae, in Southern Thailand. Zoosystematics and Evolution. 2011;87:349–360 [Google Scholar]
- 45.Krailas D, Namchote S, Koonchornboon T, et al. Trematodes obtained from the thiarid freshwater snail Melanoides tuberculata (Müller, 1774) as vector of human infections in Thailand. Zoosystematics and Evolution. 2014;90:57–86 [Google Scholar]
- 46.Fernández MV, Hamann MI, Ostrowski-de Núñez M. Echinostome cercariae from Biomphalaria straminea (Mollusca: Planorbidae) in a rice field from northeastern Argentina. Rev Mex Bio divers. 2014;85:1024–31. [Google Scholar]
- 47.Anucherngchai S, Tejangkura T, Chontananarth T. Epidemiological situation and molecular identification of cercarial stage in freshwater snails in Chao-Phraya Basin, Central Thailand. Asian Pacific J Trop Biomed. 2016;6:539–545. [Google Scholar]
- 48.Esteban JG, Muñoz-Antoli C. Echinostomes: systematics and life cycles. In: Toledo RM, Fried B. (eds.) The Biology of Echinostomes: From the Molecule to the Community. New York, USA. Springer; 2009. p.1–34. [Google Scholar]