Abstract
The coronavirus disease 2019 (COVID-19) has rapidly spread worldwide, leading to increased concerns about long-term patients’ neuropsychiatric consequences. This study aims to describe the presence of depressive and anxiety symptoms in severe COVID-19 survivors and to identify associated baseline, in-hospital and post-discharge factors. This study is part of the MAPA longitudinal project conducted with severe COVID-19 patients admitted in Intensive Care Medicine Department (ICMD) of a University Hospital (CHUSJ) in Porto, Portugal. Patients with ICMD length of stay ≤ 24 h, terminal illness, major auditory loss or inability to communicate at follow-up assessment were excluded. All participants were assessed by telephone post-discharge (median = 101 days), with a comprehensive protocol assessing depressive and anxiety symptoms, cognition, Intensive Care Unit (ICU) memories recall and health-related quality of life. Out of a sample of 56 survivors (median age = 65; 68% males), 29% and 23% had depressive and anxiety symptoms, respectively. Depressive and anxiety symptoms were significantly more prevalent among younger survivors and were associated with cognitive complaints, emotional and delusions ICU memories and fear of having COVID-19 sequelae, sleep problems and pain after discharge (all p < 0.05). An important proportion of these survivors suffers from depression and anxiety symptoms post-discharge, namely younger ones and those who reported more cognitive complaints, ICU memories, fear of having COVID-19 sequelae, sleep problems and pain. These findings highlight the importance of psychological consequences assessment and planning of appropriate and multidisciplinary follow-up care after hospitalization due to COVID-19.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11126-022-09998-z.
Keywords: COVID-19, Critical care, Depression, Anxiety, Survivors
Introduction
The coronavirus disease 2019 (COVID-19) has rapidly spread worldwide, leading to increased concerns about long-term patients’ health and neuropsychiatric consequences. In this context, the presence of several physical and mental health problems up to three months post-COVID-19, including fatigue, pain, anxiety and depression mainly among female patients and in those who have been admitted to intensive care units (ICU) have been reported [1].
It is known that 50–70% of severe non-COVID-19 patients develop at least one of the Post-Intensive Care Syndrome (PICS) symptoms, which is characterized by the presence of impairments in physical, cognitive and mental health function during or after critical illness. PICS can persist from months to years after hospitalization [2, 3]. Similar to other causes of intensive care admission, COVID-19 survivors who required ICU hospitalization are at high risk of the developing PICS [4].
Severe COVID-19 patients may be particularly prone to PICS. Not only because they are often sedated and ventilated for long periods of time, but also because they are frequently isolated, with restricted family visits [5, 6]. In addition, the fear of infecting their loved ones, death of close family members, survivor’s guilt, stigma and the impact of negative news on social media may increase the risk of PICS development among these patients.
The frequency of this syndrome is dependent upon patient characteristics and assessment methods. However, early reports about PICS after COVID-19 showed that its prevalence varies widely one to six months after discharge, with 28–87% of the cases presented physical impairments, 20–57% cognitive impairment, and 6–60% mental health problems [4]. Regarding the mental health domain, anxiety, depression and post-traumatic stress symptoms have been commonly reported, with a recent multicenter cohort study [7] showing that approximately 25% of severe COVID-19 survivors experienced substantial psychological distress, which remained similar six months after hospital discharge.
Despite data on COVID-19 have increased exponentially, the knowledge about long-term psychological consequences in severe COVID-19 survivors remains meager.
Bearing this in mind, this study aims to describe the presence of depressive and anxiety symptoms after discharge home in severe COVID-19 adults’ survivors. It also aims to identify which baseline patients’ characteristics, in-hospital and post-discharge factors, may be associated with these psychological symptoms.
Methods
Sample selection and procedures
The present study is part of a multidisciplinary single-center longitudinal research project (MAPA - Mental Health in Critically ill patients with COVID-19: Implementation of an Active Monitoring Program and Post-discharge Support), which is currently being carried out in the Intensive Care Medicine Department (ICMD) of a University Hospital (Centro Hospitalar Universitário São João - CHUSJ) in Porto, Portugal. All adult patients (≥ 18 years old) admitted with COVID-19 in ICMD between March and May 2020 (during COVID-19 first wave) were eligible for participation. Patients with ICMD length of stay (LoS) ≤ 24 h, terminal illness, major auditory loss or inability to communicate at the time of follow-up assessment were excluded.
After discharge home, all participants were assessed by telephone at the ICMD scheduled follow-up appointment . A multidisciplinary team, including an intensive care medicine physician and residents, an intensive care nurse, and a clinical psychologist, conducted patients assessment and data collection. A comprehensive protocol was administered, including: (1) the ICU Memory Tool (ICUMT) [8, 9] – recall of factual, emotional and delusional memories during ICU stay; (2) the Six-item Cognitive Impairment Test (6CIT) [10, 11] – cognitive impairment; (3) the Patient Health Questionnaire (PHQ-9) [12, 13] – depressive symptoms; (4) the General Anxiety Disorder Scale (GAD-7) [14, 15] – anxiety symptoms (5) the EuroQol 5-Dimension 5-Level questionnaire (EQ-5D-5L) – health-related quality of life (HRQoL), and the EQ-Visual Analogue Scale (EQ-VAS) – global health status patient record [16, 17].
Baseline demographic (age, gender, marital status, education, employment status, living situation) and clinical data [Charlson Comorbidity Index (CCI)] [18], medication, history of previous mental health care and psychotropic medication] were obtained from hospital electronic records and semi-structured clinical interviews. In-hospital data were also retrospectively collected, namely acute illness severity [Acute Physiology and Chronic Health Evaluation (APACHE-II) and Simplified Acute Physiology Score (SAPS-II)], deep sedation, type of respiratory support required [Invasive Mechanical Ventilation (IMV) and Extracorporeal Membrane Oxygenation (ECMO)] and major ICU complications (delirium, nosocomial infections, difficulty weaning from IMV). ICMD and hospital LoS, as well as destination after hospital discharge were also registered.
Through the clinical interview, cognitive complaints self-reported by participants after ICMD and/or hospital discharge, such as memory, attention/concentration, orientation difficulties and slowed thinking, among others, were recorded.
Additional questions were included in the clinical interview aiming to assess participants’ concerns, regarding the fear of: (1) being discriminated against for having COVID-19; (2) infecting a relative and/or friend; (3) having COVID-19 disease again; and (4) having sequelae related to COVID-19. It was also asked if the participant had or have any family members with COVID-19 disease or if any family members had died from COVID-19.
At the follow-up assessment, the symptoms reported by the participants were also collected, based on a pre-defined checklist with the following symptoms: dyspnea, dysphagia, cough, tiredness/fatigue, muscular weakness, paresthesia, sleep difficulties and nightmares.
Ethical considerations
This project was conducted in accordance with the ethical principles of the Declaration of Helsinki, and was approved by the Ethics Committee for Health of the CHUSJ/Faculty of Medicine, University of Porto (FMUP) (authorization number 218/2020). Given the current contingencies and procedures adopted due to COVID-19 pandemic, informed consent was obtained orally, duly witnessed, and recorded in the participant’s medical record with their knowledge and permission.
Statistical analysis
Descriptive statistics were presented as raw frequencies and percentages for categorical variables and as median and range for continuous variables, in cases in which normality could not be assumed. Participants were divided into groups: with and without depressive (based on PHQ-9 scoring), as well as with and without anxiety symptoms (according to GAD-7 total). Cut-off points of ≥ 5 on PHQ-9 and GAD-7 were considered to indicate the presence of depressive and anxiety symptoms, respectively. Differences between groups regarding the socio-demographic and clinical variables collected were analyzed with the Mann-Whitney test for continuous variables, the Chi-square test for categorical variables and the Fisher´s exact test for dichotomous variables, at a significance level of 0.05. The statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 27.
Results
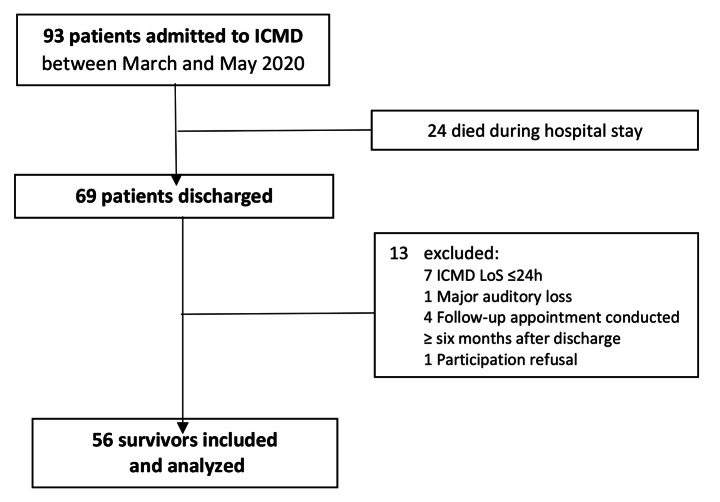
The flow diagram of the study and sampling process is presented in Fig. 1. Out of 93 patients admitted in ICMD between March and May 2020, 24 died during hospital stay and 13 were excluded. Thus, 56 survivors with a median age of 65 (range: 24–81) years old were included. The majority were male (68%), married (61%), with more than four years of education (54%), retired (54%) and lived with a partner and/or children (71%). The median (min-max) score of CCI was 3 (0–7) and 4 (0–16) for the total daily medication. About 43% of patients had history of previous mental health care and 36% were taking psychotropic medication before being hit by COVID-19.
Fig. 1.
Study flow-chart. Abbreviations: ICMD, Intensive Care Medicine Department; LoS, length of stay
Regarding in-hospital data, the median APACHE-II and SAPS-II scores were 16 (5–36) and 36 (7–77), respectively. Concerning major complications during ICMD stay, 70% had nosocomial infections, 50% delirium and 50% difficult weaning from IMV. Deep sedation was used in 75% of the patients (median = 20 days; range: 5–89). About 75% needed IMV and 21% were supported with ECMO. The median ICMD and hospital LoS were 22 (2-104) and 52 (9-394) days, respectively. Around 59% of these patients were discharged to home, 25% to other hospital and 16% to Integrated Continued Care National Network.
Follow-up assessment occurred with a median of 101 days (46-146) after discharge home. A total of 23 patients was professionally active before hospitalization. From these, 11 (47.8%) returned to work and the remainder were either on sick leave (39.1%), became unemployed (8.7%) or have retired (4.3%).
Based on ICU Memory Tool, 80% of patients recalled ate least one type of memories, particularly factual memories (71%). About emotional memories, the most recalled were feeling confused (45%) and anxious/frightened (43%). Moreover, nightmares (41%), dreams (38%) and guessing that people were trying to hurt them (25%) were the most frequently reported delusional memories.
Half reported having cognitive difficulties, including 40% memory impairment, 21% disorientation and 20% confusion. Based on 6CIT assessment, 18% of survivors had cognitive impairment, adjusted for education level.
Overall, 29% and 23% of the patients had depressive and anxiety symptoms, respectively. The co-existence of these symptoms occurred in 23% of the sample. The two most frequent depressive symptoms were feeling tired or having little energy (57%), and feeling down, depressed, or hopeless (50%). Moreover, 13% of patients had suicidal thoughts. Regarding anxiety, not being able to stop or control worrying (50%), and feeling nervous, anxious or on edge (48%) were the most reported symptoms.
In this sample, 77% stated they felt fear of having COVID-19 again, 63% of having sequelae related to this disease, 43% of infecting others and 20% of being discriminated against for having COVID-19. About 81% had a family member with COVID-19 and 6% had a relative who died from this infection.
In EQ-5D-5L evaluation, the majority of the patients described problems in the domains of pain/discomfort (70%), usual activities (66%) and mobility (63%). Concerning the self-rated health status with EQ-VAS, a median of 75 was found, ranging between 40 and 100 (the latter of which corresponds to the best health status).
Regarding the symptoms reported by the participants based on the pre-defined checklist used, muscular weakness (43%), tiredness/fatigue (41%), sleep difficulties (29%), nightmares (23%) and dyspnea (21%) were the most frequent.
Comparing patients with and without depressive symptoms regarding all variables assessed, the first group was significantly younger (median age = 63 vs. 68; p = 0.044), reported more cognitive complaints after ICMD discharge (80% vs. 37.8%; p = 0.006), more anxiety symptoms (median score = 8 vs. 1; p < 0.001) and pain/discomfort (93.8% vs. 60%; p = 0.022) at follow-up assessment. Also, patients with depressive symptoms were significantly more likely to report emotional and delusional memories related to ICMD stay, particularly “feeling down” (56% vs. 25%; p = 0.026), “feeling that people were trying to hurt him/her” (50% vs. 15%, p = 0.014) and “nightmares” (63% vs. 33%; p = 0.039). In this comparative analysis, it was also found that patients with depressive symptoms more often reported fear of having sequelae related to COVID-19 (87.5% vs. 52.5%; p = 0.015) (Table 1). Also, these patients more frequently described tiredness/fatigue (69% vs. 30%; p = 0.008), paresthesia (31% vs. 3%; p = 0.006) and sleep problems (56% vs. 18%; p = 0.007).
Table 1.
Comparison between group patients regarding all variables assessed
| With depressive symptoms (n = 16) |
Without depressive symptoms (n = 40) |
p-value | With anxiety symptoms (n = 13) |
Without anxiety symptoms (n = 43) |
p-value | |
|---|---|---|---|---|---|---|
| BASELINE | ||||||
| Age (years), median (min.-max.) | 63 (33–73) | 68 (30–80) | 0.044 (1) | 58 (33–73) | 67 (24–81) | 0.014 (1) |
| Male, n (%) | 9 (56.3) | 29 (72.5) | 0.239 (2) | 5 (38.5) | 13 (30.2) | 0.736 (3) |
| Married, n (%)* | 8 (50) | 26 (65) | 0.299 (2) | 7 (53.8) | 15 (34.9) | 0.220 (2) |
| Education (> 4 years), n (%)** | 7 (43.8) | 23 (57.5) | 0.351 (2) | 6 (46.2) | 24 (55.8) | 0.541 (2) |
| Active worker, n (%)*** | 7 (43.8) | 16(40) | 0.797 (2) | 7 (53.8) | 26 (60.5) | 0.671 (2) |
| Living alone, n (%)**** | 2 (12.5) | 5 (12.5) | 1.000 (3) | 12 (92.3) | 37 (86) | 1.000 (3) |
| Comorbidity (CCI), median (min.-max.) | 2 (0–5) | 3 (0–7) | 0.257 (1) | 2 (0–3) | 3 (0–7) | 0.071 (1) |
| Daily medication, median (min.-max.) | 4 (1–7) | 4.5 (0–11) | 0.949 (1) | 4 (0–11) | 4 (1–7) | 0.898 (1) |
| History of previous mental health care, n (%) | 8 (50) | 16 (40) | 0.495 (1) | 6 (46.2) | 18 (41.9) | 0.784 (2) |
| Previous psychotropic medication, n (%) | 8 (50) | 12 (30) | 0.158 (2) | 7 (53.8) | 13 (30.2) | 0.186 (3) |
| IN-HOSPITAL | ||||||
| APACHE-II, median (min.-max.) | 15.5 (6–36) | 16 (6–34) | 0.557 (1) | 14 (6–27) | 16 (5–36) | 0.204 (1) |
| SAPS-II, median (min.-max.) | 41.5 (18–68) | 39 (16–77) | 0.726 (1) | 34 (18–68) | 38.5 (7–77) | 0.648 (1) |
| Deep sedation, n (%) | 12 (75) | 30 (75) | 1.000 (3) | 9 (69.2) | 33 (76.7) | 0.717 (3) |
| Deep sedation (days), median (min.-max.) | 15 (6–62) | 20 (5–89) | 0.540 (1) | 15 (6–62) | 20 (5–89) | 0.509 (1) |
| IMV, n (%) | 12 (75) | 30 (75) | 1.000 (3) | 9 (69.2) | 33 (76.7) | 0.717 (3) |
| IMV (days), median (min.-max.) | 17 (7–70) | 24.5 (8–99) | 0.276 (1) | 17 (9–70) | 25 (7–99) | 0.307 (1) |
| ECMO, n (%) | 4 (25) | 8 (20) | 0.726 (3) | 4 (30.8) | 8 (18.6) | 0.443 (3) |
| Major complications, n (%): | ||||||
| Delirium | 7 (43.8) | 21 (52.5) | 0.554 (1) | 4 (30.8) | 24 (55.8) | 0.114 (2) |
| Nosocomial infections | 10 (62.5) | 29 (72.5) | 0.527 (3) | 8 (61.5) | 31 (72.1) | 0.504 (3) |
| Difficulty weaning from IMV | 7 (43.8) | 21 (52.5) | 0.554 (2) | 5 (38.5) | 23 (53.5) | 0.342 (2) |
| ICMD LoS, median (min.-max.) | 21.5 (9–66) | 35.5 (2-104) | 0.813 (1) | 20 (3–66) | 23 (2-104) | 0.727 (1) |
| Hospital LoS, median (min.-max.) | 50 (19–133) | 66.5 (21–255) | 0.765 (1) | 49 (19–133) | 52 (9-394) | 0.831 (1) |
| POST-DISCHARGE | ||||||
| Follow-up time (days), median (min.-max.) | 100 (74–139) | 95 (46–146) | 0.238 (1) | 95 (53–134) | 106 (46–146) | 0.356 (1) |
| Return to work, n (%) | 2 (28.6) | 9 (56.3) | 0.371 (3) | 2 (33.3) | 9 (52.9) | 0.640 (3) |
| ICU Memories, n (%) | 14 (87.5) | 31 (77.5) | 0.483 (3) | 11 (84.6) | 34 (79.1) | 1.000 (3) |
| Patient-reported cognitive complaints, n (%) | 12 (80) | 14 (37.8) | 0.006 (2) | 9 (75) | 17 (42.5) | 0.048 (2) |
| Cognitive impairment (6CIT), n (%) | 4 (25) | 6 (15) | 0.448 (3) | 4 (30.8) | 6 (14) | 0.218 (3) |
| Depressive symptoms (PHQ-9), median (min.-max.) | - | - | - | 10 (6–24) | 2 (0–12) | < 0.001 (2) |
| Anxiety symptoms (GAD-7), median (min.-max.) | 8 (2–21) | 1 (0–4) | < 0.001 (2) | - | - | - |
| HRQoL (EQ-5D-5L) (with problems), n (%): | ||||||
| Mobility | 13 (81.3) | 22 (55) | 0.067 (2) | 10 (76.9) | 25 (58.1) | 0.330 (3) |
| Self-care | 4 (25) | 12 (30) | 1.000 (3) | 3 (23.1) | 13 (30.2) | 0.737 (3) |
| Usual activities | 13 (81.3) | 24(60) | 0.129 (2) | 10 (76.9) | 27 (62.8) | 0.507 (3) |
| Pain/discomfort | 15 (93.8) | 24 (60) | 0.022 (3) | 12 (92.3) | 27 (62.8) | 0.082 (3) |
| Anxiety/depression | 16 (100) | 17 (42.5) | < 0.001 (2) | 13 (100) | 20 (46.5) | < 0.001 (2) |
| EQ-VAS, median (min.-max.) | 55 (40–80) | 75 (40–100) | 0.146 (1) | 70 (40–100) | 75 (40–100) | 0.256 (1) |
| After discharge, fear of: n (%) | ||||||
| Being discriminated against for having COVID-19 | 6 (37.5) | 5 (12.5) | 0.059 (3) | 5 (38.5) | 6 (14) | 0.104 (3) |
| Infecting a relative/friend | 8 (50) | 16 (40) | 0.495 (2) | 6 (46.2) | 18 (41.9) | 0.784 (2) |
| Having COVID-19 disease again | 14 (87.5) | 29 (72.5) | 0.308 (3) | 11 (84.6) | 32 (74.4) | 0.710 (3) |
| Having sequelae related to COVID-19 | 14 (87.5) | 21 (52.5) | 0.015 (2) | 12 (92.3) | 23 (53.5) | 0.020 (3) |
| Relative with COVID-19 disease, n (%) | 13 (86.7) | 30 (78.9) | 0.706 (3) | 10 (83.3) | 33 (80.5) | 1.000 (3) |
| Relative who died from COVID-19, n (%) | 0 (0) | 3 (7.9) | 0.550 (3) | 0 (0) | 3 (7.3) | 1.000 (3) |
Note: min.-max: minimum-maximum; CCI: Charlson Comorbidity Index; APACHE-II: Acute Physiology and Chronic Health Evaluation II; SAPS-II: The Simplified Acute Physiology score; IMV: Invasive Mechanical Ventilation; ECMO: Extracorporeal Membrane Oxygenation; ICMD: Intensive Care Medicine Department; LoS: Length of Stay; ICU: Intensive Care Unit; 6CIT: Six-item Cognitive Impairment test; PHQ-9: Patient Health Questionnaire; GAD-7: General Anxiety Disorder scale; EQ-5D-5L: EuroQol 5-Dimension 5-Level questionnaire; EQ-VAS: EQ-Visual Analogue scale; *Married vs. Other (single, divorced, widowed); **0–4 vs. > 4 Years of education; ***Active worker vs. Other professional situation (sick leave, unemployed, retired); ****Living alone vs. Other situation (living with partner/children, other family member); (1)Mann–Whitney test; (2)Chi-Square Independent test (3)Fisher’s exact test
Similarly, patients with anxiety symptoms were also significantly younger (median age = 58 vs. 67; p = 0.014) and reported more cognitive complaints after discharge (75% vs. 42.5%; p = 0.048). These patients had a higher depression score (median = 10 vs. 2; p < 0.001) and more often stated fear of having sequelae related to COVID-19 (92.3% vs. 53.5%; p = 0.020), comparing to those without anxiety symptoms (Table 1). They were also significantly more likely to recall emotional memories concerning “feeling down” (62% vs. 26%; p = 0.023), “being uncomfortable” (54% vs. 19%; p = 0.027) and “feeling panic” (31% vs. 7%, p = 0.043) during ICMD hospitalization, as well as to express more sleep problems at follow-up evaluation (54% vs. 21%; p = 0.035).
Discussion
In this cohort study of patients hospitalized with severe COVID-19, approximately 29% and 23% experienced depressive and anxiety symptoms, respectively, when assessed after discharge. Similar rates were reported in previous studies conducted with general ICU patients [19–21]. Regarding prior research with COVID-19 survivors [4, 7, 22–29], the results showed rates between 10% and 40.5% for depressive and 10.4%-29.6% for anxiety symptoms, being globally in line with the data presented here. Nevertheless, some caution is needed in these comparisons due to methodological heterogeneity, including the lower number of ICU patients representated in most of these studies and the use of different assessment tools and follow-up periods. Despite this, studies with mixed samples concluded that ICU survivors had a higher risk of presenting psychological symptoms, comparing to non-ICU patients [24, 27].
Another important finding is that younger patients with severe COVID-19 were more likely to develop symptoms of depression and anxiety after discharge, which is in conformity with previous investigations [22, 28, 30, 31]. In Cai et al. [22] study, older COVID-19 survivors reported fewer emotional and anxiety symptoms during early convalescence, comparing to younger ones. According to these authors, this may be understood from the cognitive theory perspective, which states that previous experiences make older people less likely to incorporate traumatic events as a central part of their identity. In addition, psychological maturation benefits older people in the development of coping mechanisms with greater resilience to psychological distress compared to younger people [32]. It should also be underlined that younger patients are at a life stage with more social and financial responsibilities and events such as ICU admission, often associated with physical impairments after discharge, leading to persistent disabilities in activities of daily living, will undoubtedly make more difficult the return to work and cause financial and family impact.
Moreover, as expected, survivors who had more anxiety and depressive symptoms were also the ones who more frequently expressed fear of having sequelae related to COVID-19 and reported more symptoms after discharge, mostly tiredness/fatigue, paresthesia and sleep problems, which is in alignment with other studies with COVID-19 survivors [1, 4, 24, 25, 33].
Pain was the most frequently reported HRQoL domain, particularly in patients who scored for depressive symptoms. Literature has shown that both sleep disturbance and pain are very common during and following critical illness [34–37], with recent investigations confirming the same pattern for COVID-19 patients [1]. Moreover, it is well known that both conditions often coexist with mental health problems, such as depression and anxiety, sharing common causes and having a bidirectional relationship. In the case of sleep disturbances, the strongest path is the disruption of sleep as a causal factor for the development of other psychiatric conditions [38]. Bearing this in mind, COVID-19 survivors are likely to benefit from an early treatment of sleep disturbances, since literature showed that its successful treatment contributes to the reduction of mental health problems [38]. Considering that there are some promising results achieved with cognitive-behavioral therapy in the management of pain and depressive symptoms [39], COVID-19 patients may also benefit from this approach, as well as from an early pain screening and adequate management.
Regarding the concerns of COVID-19 survivors, it should also be noted that almost 80% of participants were afraid of having this disease again and almost half were afraid of infecting family members. Similar to some previous studies [34–33, 40], these worries were mostly reported by survivors who also presented depressive or anxiety symptoms, although a statistically significant difference was not achieved in the present study. Nevertheless, these findings are relevant, highlighting that these survivors are likely to have psychosocial difficulties after discharge, since these concerns regarding their own health and their beloved ones might lead to reduced social interactions, increased self-isolation and loneliness, which may have a detrimental impact on mental health [22, 40, 41]. In addition, the media spreading of uncontrolled news about COVID-19, with an increased risk of fake news, as well as the uncertainty about the long-term sequelae of COVID-19, could also contribute or exacerbate these concerns [41]. This was particularly relevant among the first wave COVID-19 patients, during which scientific grounded information was still scarce.
Regarding ICU memories, emotional and delusional ones were associated with depressive and/or anxiety symptoms. These findings are in line with previous research with general ICU survivors [42–46], which points out that delusional memories are reported as one of the most traumatizing aspects of intensive care experience [47], being related with post-discharge psychological symptomatology. In COVID-19 survivors, studies are still scarce. Nevertheless, Mongodi et al. [48] found that feeling of loneliness due to restrictions of communication with families/health staff, as well as nightmares, feeling of derealization and belief of being in a fake hospital were the most frequently ICU memories recalled by COVID-19 survivors.
It should also be emphasized that half of sample reported at least one cognitive complaint after ICMD and/or hospital discharge, and 18% showed objective cognitive impairment at follow-up assessment. Although the underlying mechanisms are yet unknown, it is thought that these symptoms might be the result of central nervous system injury due to COVID-19 [49]. Furthermore, a higher proportion of patients with depression and anxiety symptoms have also presented more cognitive complains and cognitive impairment, which is in accordance with a previous similar work [50], despite the statistical significance has only been achieved for complaints.
Notwithstanding of some earlier evidence pointing to the association of female gender, previous psychiatric history, as well as ICU-specific factors (such as delirium, sedation, mechanical ventilation, LoS) with a higher prevalence of psychological symptoms in COVID-19 patients [1, 27, 28, 31, 33, 51], no significant statistically differences were observed in this study, which may be partially explained by the small sample size.
One strength of this study is the contribution for the accumulating data on the long-term mental health consequences of severe COVID-19 and their associated factors. Secondly, a wide-range of psychosocial and clinical factors were collected, which enabled a detailed characterization of this sample. Thirdly, as far as the authors are aware, this is the first study exploring the association between ICU memories recall and psychological symptomatology in COVID-19 survivors. Finally, the present findings highlight the need to be aware of long-term psychological consequences and its early detection, as well as to plan appropriate and multidisciplinary follow-up care after COVID-19.
Potential limitations of this study are also recognized, including a single-center design and a relatively small sample size that may limit the generalizability of these results. Furthermore, some factors associated with psychological morbidity were not explored, such as COVID-19 symptoms in acute phase, acute stress in ICU, or use of physical restraints. Moreover, the lack of a control group and of a pre-COVID-19 neuropsychological assessment in this sample do not allow to conclude if the symptoms found pre-existed, were exacerbated or acutely developed due to the stress of having COVID-19. Despite this, as no statistically significant differences were found between groups of patients with and without depression or anxiety symptoms regarding history of previous mental health care and of psychotropic medication, it is thought that these post-discharge psychological symptoms may possibly stem from the experience of being admitted in ICU with severe COVID-19.
In conclusion, an important proportion of severe COVID-19 survivors suffers from depressive and anxiety symptoms after discharge. These psychological symptoms were more prevalent among younger survivors and were associated with cognitive complaints, traumatic memories related to ICU stay and fear of having sequelae related to COVID-19, as well as with the presence of sleep problems and pain after discharge. Similar findings have also been reported in other ICU populations, suggesting comparable psychological symptoms.
The current findings should be interpreted as exploratory and need to be validated in future studies with larger multicenter samples and including other potential risk factors. As mentioned, this study is part of an ongoing larger research project, which will further allow the clarification of these neuropsychological sequelae of COVID-19 survivors and the assessment of how this symptomatology may change over time.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank to all patients for their collaboration.
Authors Contribution
Sónia Martins: Conceptualization; Methodology; Formal analysis; Investigation; Data Curation; Writing - Original Draft; Writing - Review & Editing. Ana Rita Ferreira: Conceptualization; Methodology; Formal analysis; Writing - Original Draft; Writing - Review & Editing. Joana Fernandes: Investigation; Writing - Review & Editing. Tatiana Vieira: Investigation; Writing - Review & Editing. Liliana Fontes: Investigation; Writing - Review & Editing. Isabel Coimbra: Investigation; Writing - Review & Editing. José Artur Paiva: Conceptualization; Methodology; Writing - Review & Editing; Supervision. Lia Fernandes: Conceptualization; Methodology; Writing - Review & Editing; Supervision; Project administration.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Statements and Declarations
Competing Interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shanbehzadeh S, Tavahomi M, Zanjari N, Ebrahimi-Takamjani I, Amiri-Arimi S. Physical and mental health complications post-COVID-19: Scoping review. J Psychosom Res. 2021;147:110525. doi: 10.1016/j.jpsychores.2021.110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, Zawistowski C, Bemis-Dougherty A, Berney SC, Bienvenu OJ, Brady SL, Brodsky MB, Denehy L, Elliott D, Flatley C, Harabin AL, Jones C, Louis D, Meltzer W, Muldoon SR, Palmer JB, Perme C, Robinson M, Schmidt DM, Scruth E, Spill GR, Storey CP, Render M, Votto J, Harvey MA. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502–509. 10.1097/CCM.0b013e318232da75. [DOI] [PubMed]
- 3.Mikkelsen M, Netzer G, Iwashyna T. Post-intensive care syndrome (PICS). UpToDate. Wolters Kluwer; 2021. https://www.uptodate.com/contents/post-intensive-care-syndrome-pics.
- 4.Nakanishi N, Liu K, Kawakami D, Kawai Y, Morisawa T, Nishida T, Sumita H, Unoki T, Hifumi T, Iida Y, Katsukawa H, Nakamura K, Ohshimo S, Hatakeyama J, Inoue S, Nishida O. Post-Intensive Care Syndrome and Its New Challenges in Coronavirus Disease 2019 (COVID-19) Pandemic: A Review of Recent Advances and Perspectives. J Clin Med. 2021;10(17):3870. doi: 10.3390/jcm10173870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J, Kremer S, Merdji H, Schenck M, Severac F, Clere-Jehl R, Studer A, Radosavljevic M, Kummerlen C, Monnier A, Boulay C, Fafi-Kremer S, Castelain V, Ohana M, Anheim M, Schneider F, Meziani F. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care. 2020;24(1):491. doi: 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson JE, Mart MF, Cunningham C, Shehabi Y, Girard TD, MacLullich A, Slooter A, Ely EW, Delirium Nat Rev Dis Primers. 2020;6(1):90. doi: 10.1038/s41572-020-00223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlake JH, Wesselius S, van Genderen ME, van Bommel J, Boxma-de Klerk B, Wils EJ. Psychological distress and health-related quality of life in patients after hospitalization during the COVID-19 pandemic: A single-center, observational study. PLoS ONE. 2021;16(8):e0255774. doi: 10.1371/journal.pone.0255774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones C, Humphris G, Griffiths RD. Preliminary validation of the ICUM tool: a tool for assessing memory of the intensive care experience. Clin Intensive Care. 2000;11(5):251–5. doi: 10.3109/tcic.11.5.251.255. [DOI] [Google Scholar]
- 9.Granja C. Outcomes in intensive care: patients’ physical and neuropsychological sequelae and their health-related quality of life. Doctoral Thesis. Faculdade de Medicina da Universidade do Porto; 2005.
- 10.Brooke P, Bullock R. Validation of a 6 item cognitive impairment test with a view to primary care usage. Int J Geriatr Psychiatry. 1999;14(11):936–40. doi: 10.1002/(SICI)1099-1166(199911)14:11<936::AID-GPS39>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Apóstolo J, Paiva D, Silva R, Santos E, Schultz TJ. Adaptation and validation into Portuguese language of the six-item cognitive impairment test (6CIT) Aging Ment Health. 2018;22(9):1184–9. doi: 10.1080/13607863.2017.1348473. [DOI] [PubMed] [Google Scholar]
- 12.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monteiro S, Torres A, Pereira A, Albuquerque E, Morgadinho R. Preliminary validation study of a Portuguese version of the patient health questionnaire (PHQ-9) Eur Psychiatry. 2013;28(S1):2077. [Google Scholar]
- 14.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 15.Sousa TV, Viveiros V, Chai MV, Vicente FL, Jesus G, Carnot MJ, Gordo AC, Ferreira PL. Reliability and validity of the Portuguese version of the Generalized Anxiety Disorder (GAD-7) scale. Health Qual Life Outcomes. 2015;13:50. doi: 10.1186/s12955-015-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EuroQol Group A new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira PL, Antunes P, Ferreira LN, Pereira LN, Ramos-Goñi JM. A hybrid modelling approach for eliciting health state preferences: the Portuguese EQ-5D-5L value set. Qual Life Res. 2019;28(12):3163–75. doi: 10.1007/s11136-019-02226-5. [DOI] [PubMed] [Google Scholar]
- 18.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 19.Nikayin S, Rabiee A, Hashem MD, Huang M, Bienvenu OJ, Turnbull AE, Needham DM. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2016;43:23–9. doi: 10.1016/j.genhosppsych.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabiee A, Nikayin S, Hashem MD, Huang M, Dinglas VD, Bienvenu OJ, Turnbull AE, Needham DM. Depressive Symptoms After Critical Illness: A Systematic Review and Meta-Analysis. Crit Care Med. 2016;44(9):1744–53. doi: 10.1097/CCM.0000000000001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marra A, Pandharipande PP, Girard TD, Patel MB, Hughes CG, Jackson JC, Thompson JL, Chandrasekhar R, Ely EW, Brummel NE. Co-Occurrence of Post-Intensive Care Syndrome Problems among 406 Survivors of Critical Illness. Cri Care Med. 2018;46(9):1393–401. doi: 10.1097/CCM.0000000000003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai X, Hu X, Ekumi IO, Wang J, An Y, Li Z, Yuan B. Psychological Distress and Its Correlates Among COVID-19 Survivors During Early Convalescence Across Age Groups. Am J Geriatr Psychiatry. 2020;28(10):1030–9. doi: 10.1016/j.jagp.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ismael F, Bizario JCS, Battagin T, Zaramella B, Leal FE, Torales J, Ventriglio A, Marziali ME, Martins SS, Castaldelli-Maia JM. Post-infection depressive, anxiety and post-traumatic stress symptoms: A prospective cohort study in patients with mild COVID-19. Prog. Neuro-psychopharmacolo. Biol Psychiatry. 2021;111:110341. doi: 10.1016/j.pnpbp.2021.110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu D, Baumeister RF, Veilleux JC, Chen C, Liu W, Yue Y, Zhang S. Risk factors associated with mental illness in hospital discharged patients infected with COVID-19 in Wuhan, China. Psychiatry Res. 2020;292:113297. doi: 10.1016/j.psychres.2020.113297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernández-de-Las-Peñas C, Gómez-Mayordomo V, de-la-Llave-Rincón AI, Palacios-Ceña M, Rodríguez-Jiménez J, Florencio LL, Velasco-Arribas M, Fuensalida-Novo S, Cigarán-Méndez M, Ambite-Quesada S, Guijarro C, Cuadrado ML, Arias-Navalón JA, Ortega-Santiago R, Elvira-Martínez CM, Molina-Trigueros LJ, Torres-Macho J, Sebastián-Viana T, Canto-Diez MG, Hernández-Barrera V. Palacios-Ceña D. Anxiety, depression and poor sleep quality as long-term post-COVID sequelae in previously hospitalized patients: A multicenter study. J Infect. 2021;83(4):496–522. doi: 10.1016/j.jinf.2021.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandes J, Fontes L, Coimbra I, Paiva JA. Health-Related Quality of Life in Survivors of Severe COVID-19 of a University Hospital in Northern Portugal. Acta Med Port. 2021;34(9):601–7. doi: 10.20344/amp.16277. [DOI] [PubMed] [Google Scholar]
- 27.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang J, Zhang D, Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–32. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, Melloni EMT, Furlan R, Ciceri F, Rovere-Querini P, COVID-19 BioB Outpatient Clinic Study group. Benedetti F. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martillo MA, Dangayach NS, Tabacof L, Spielman LA, Dams-O’Connor K, Chan CC, Kohli-Seth R, Cortes M, Escalon MX. 2021. Postintensive Care Syndrome in Survivors of Critical Illness Related to Coronavirus Disease 2019: Cohort Study from a New York City Critical Care Recovery Clinic. [DOI] [PubMed]
- 30.Wang C, Pan R, Wan X, Tan Y, Xu L, McIntyre RS, Choo FN, Tran B, Ho R, Sharma VK, Ho C. A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav Immun. 2020;87:40–8. doi: 10.1016/j.bbi.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.COVID-19 Mental Disorders Collaborators Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398(10312):1700–12. doi: 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knight BG, Gatz M, Heller K, Bengtson VL. Age and emotional response to the Northridge earthquake: a longitudinal analysis. Psychol Aging. 2000;15(4):627–34. doi: 10.1037//0882-7974.15.4.627. [DOI] [PubMed] [Google Scholar]
- 33.Wu C, Hu X, Song J, Yang D, Xu J, Cheng K, Chen D, Zhong M, Jiang J, Xiong W, Lang K, Tao Y, Lin X, Shi G, Lu L, Pan L, Xu L, Zhou X, Song Y, Wei M, Zheng J, Du C. Mental health status and related influencing factors of COVID-19 survivors in Wuhan, China. Clin Tansl Med. 2020;10(2):e52. doi: 10.1002/ctm2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Battle CE, Lovett S, Hutchings H. Chronic pain in survivors of critical illness: a retrospective analysis of incidence and risk factors. Crit Care. 2013;17(3):R101. doi: 10.1186/cc12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyranou M, Puntillo K. The transition from acute to chronic pain: might intensive care unit patients be at risk? Ann Intensive Care. 2012;2(1):36. doi: 10.1186/2110-5820-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altman MT, Knauert MP, Pisani MA. Sleep Disturbance after Hospitalization and Critical Illness: A Systematic Review. Ann Am Thorac Soc. 2017;14(9):1457–68. doi: 10.1513/AnnalsATS.201702-148SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mäkinen OJ, Bäcklund ME, Liisanantti J, Peltomaa M, Karlsson S, Kalliomäki ML. Persistent pain in intensive care survivors: a systematic review. Br J Anaesth. 2020;125(2):149–58. doi: 10.1016/j.bja.2020.04.084. [DOI] [PubMed] [Google Scholar]
- 38.Freeman D, Sheaves B, Waite F, Harvey AG, Harrison PJ. Sleep disturbance and psychiatric disorders. Lancet Psychiatry. 2020;7(7):628–37. doi: 10.1016/S2215-0366(20)30136-X. [DOI] [PubMed] [Google Scholar]
- 39.IsHak WW, Wen RY, Naghdechi L, Vanle B, Dang J, Knosp M, Dascal J, Marcia L, Gohar Y, Eskander L, Yadegar J, Hanna S, Sadek A, Aguilar-Hernandez L, Danovitch I, Louy C. Pain and Depression: A Systematic Review. Harv Rev Psychiatry. 2018;26(6):352–63. doi: 10.1097/HRP.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 40.Park HY, Jung J, Park HY, Lee SH, Kim ES, Kim HB, Song KH. Psychological Consequences of Survivors of COVID-19 Pneumonia 1 Month after Discharge. J Korean Med Sci. 2020;35(47):e409. doi: 10.3346/jkms.2020.35.e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiorillo A, Gorwood P. The consequences of the COVID-19 pandemic on mental health and implications for clinical practice. Eur Psychiatry. 2020;63(1):e32. doi: 10.1192/j.eurpsy.2020.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones C, Griffiths RD, Humphris G, Skirrow PM. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med. 2001;29(3):573–80. doi: 10.1097/00003246-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 43.Jones C, Skirrow P, Griffiths RD, Humphris GH, Ingleby S, Eddleston J, Waldmann C, Gager M. Rehabilitation after critical illness: a randomized, controlled trial. Crit Care Med. 2003;31(10):2456–61. doi: 10.1097/01.CCM.0000089938.56725.33. [DOI] [PubMed] [Google Scholar]
- 44.Samuelson KA, Lundberg D, Fridlund B. Stressful memories and psychological distress in adult mechanically ventilated intensive care patients - a 2-month follow-up study. Acta Anaesthesiol Scand. 2007;51(6):671–8. doi: 10.1111/j.1399-6576.2007.01292.x. [DOI] [PubMed] [Google Scholar]
- 45.Weinert CR, Sprenkle M. Post-ICU consequences of patient wakefulness and sedative exposure during mechanical ventilation. Intensive Care Med. 2008;34(1):82–90. doi: 10.1007/s00134-007-0829-2. [DOI] [PubMed] [Google Scholar]
- 46.Kiekkas P, Theodorakopoulou G, Spyratos F, Baltopoulos GI. Psychological distress and delusional memories after critical care: a literature review. Int Nurs Rev. 2010;57(3):288–96. doi: 10.1111/j.1466-7657.2010.00809.x. [DOI] [PubMed] [Google Scholar]
- 47.Wade DM, Brewin CR, Howell DC, White E, Mythen MG, Weinman JA. Intrusive memories of hallucinations and delusions in traumatized intensive care patients: An interview study. Br J Health Psychol. 2015;20(3):613–31. doi: 10.1111/bjhp.12109. [DOI] [PubMed] [Google Scholar]
- 48.Mongodi S, Salve G, Tavazzi G, Politi P, Mojoli F. COVID-19 Post-ICU team, COVID-19 Pavia Crisis Unit. High prevalence of acute stress disorder and persisting symptoms in ICU survivors after COVID-19. Intensive Care Med. 2021;47(5):616–8. doi: 10.1007/s00134-021-06349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Writing Committee for the COMEBAC Study Group. Morin L, Savale L, Pham T, Colle R, Figueiredo S, Harrois A, Gasnier M, Lecoq AL, Meyrignac O, Noel N, Baudry E, Bellin MF, Beurnier A, Choucha W, Corruble E, Dortet L, Hardy-Leger I, Radiguer F, Sportouch S, Verny C, Wyplosz B, Zaidan M, Becquemont L, Montani D, Monnet X. Four-Month Clinical Status of a Cohort of Patients after Hospitalization for COVID-19. JAMA. 2021;325(15):1525–34. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Méndez R, Balanzá-Martínez V, Luperdi SC, Estrada I, Latorre A, González-Jiménez P, Feced L, Bouzas L, Yépez K, Ferrando A, Hervás D, Zaldívar E, Reyes S, Berk M, Menéndez R. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J Intern Med. 2021;290(3):621–31. doi: 10.1111/joim.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prével R, Coelho J, Orieux A, Philip P, Gruson D, Bioulac S. Psychological evaluation and support in COVID-19 critically ill patients: a feasibility study. Crit Care. 2021;25(1):218. doi: 10.1186/s13054-021-03642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.