Neuroblastoma
Neuroblastoma (NB) is the most common extracranial tumor among children with an average age of 17 months. NB is a tumor of the autonomic nervous system originating from embryonic neural crest cells [1] in which the pathogenesis of this malignancy is characterized by a block of differentiation [2, 3]. Many factors converge in this heterogeneous disease, including age, disease stage, and genetic and molecular features that, in turn, influence whether NB will spontaneously regress or metastasize and become resistant to therapy [4, 5]. Among the genetic alterations described in NB, MYCN amplification is the most common genetic dysfunction and is also associated with poor outcome. Moreover, mutations affecting both the α-thalassemia/mental retardation syndrome X-linked (ATRX) gene [6] or anaplastic lymphoma receptor tyrosine kinase (ALK) [7] are also common in NB.
Current therapeutic strategies for NB are selected according to patient stratification in four prognostic groups: low, intermediate, high risk, and tumor stage 4 [8]. Patients with low-risk disease (stage 1) and 4S0 are subjected to surgery alone. Patients with intermediate-risk (stages 2) first receive chemotherapy followed by resectioning of the tumor mass. Overall, in both prognostic categories, the survival is greater than 90%. In contrast, patients with high-risk disease (stages 3–4) are treated with dose-intensive chemotherapy plus radiotherapy. In addition, these patients undergo differentiation therapy with isotretinoin and immunotherapy with antidisialoganglioside GD2 monoclonal antibodies [9]. However, even with this aggressive treatment, children have the lowest overall survival (40–50%) and may also face a reduced quality of life. Therefore, it is urgent to develop novel therapeutics for less toxic and effective therapies. At the molecular level, several genes have been implicated in the pathogenesis of the disease, including the family of p53 [10–15], and in particular p73 [16–18], redox regulators [19–21], or apoptotic regulators [22]. However, MYCN amplification and activation of its downstream signaling is the most robust clinical biomarker of the poor clinical outcome and is present in about 40% of high-risk cases [23]. Consequently, targeting MYCN downstream cellular processes, including metabolism, may be exploited as a potent strategy to overcome the difficulties of directly targeting MYCN [24, 25].
Metabolic alteration in neuroblastoma
Reprogramming of cellular metabolism is a hallmark of cancer. Indeed, cancer cells have deregulated glucose, lipids, and glutamine metabolism to sustain cell proliferation, control redox homeostasis, and overcome conditions of low nutrient and oxygen availability [26–30]. Positron emission tomography of cancer patients shows that NB tumors have high glucose uptake [31] and a high rate of lactic acid production, indicating the switch from OXPHOS to glycolysis [32]. In addition, a possible link between common genetic alterations in NB and metabolism has been explored. Deletion of the short arm of chromosome 1 (1p36) occurs in approximately 20–40% of primary NB [33]. Germline mutations of the succinate dehydrogenase enzymes complex (SDH), which is involved in the tricarboxylic acid cycle, are primarily predisposed to paraganglioma and phaeochromocytoma [34]. The gene encoding for the subunit B (SDHB) maps to the 1p36 region, and reduced mitochondrial activity has been described in undifferentiated neuroblastoma [35]. However, mutation analysis in 46 primary NB did not identify any germline or somatic SDHB mutations [36]. Gain of chromosome 17 is an additional genetic alteration in NB that correlates with high-stage disease and poor prognosis [37]. The antiapoptotic protein BIRC5/survivin, which maps to 17q25, is highly expressed in human NB and is associated with chemotherapy resistance and poor prognosis [38]. The underlying molecular mechanism associated with drug resistance is caused, in part, by a switch from oxidative phosphorylation to aerobic glycolysis [39].
Thus, MYCN amplification is another characteristic genetic alteration found in NB patients. MYCN protects NB cells from oxidative stress by increasing glutathione biosynthesis, and in vivo administration of glutathione biosynthesis inhibitors significantly potentiated the anticancer activity of cytotoxic chemotherapy against established tumors [40]. In addition, MYCN positively regulates the expression of solute carrier family 1 member 5 (ASCT2) to maintain sufficient levels of glutamine essential for the TCA cycle anaplerosis. Interestingly, glutamine transporter abundance correlates to poor prognosis in neuroblastoma patients [41]. In MYCN-amplified NB cells, MYCN cooperates with MondoA in regulating levels of proteins involved in lipid biosynthesis, and a subset of these proteins correlates with poor patient outcome [42]. Moreover, MYCN mediates structural changes in the mitochondrial network by increasing fusion, resulting in resistance to cell death induced by cisplatin [43].
Recently, the metabolic network regulated by MYCN has been further extended. Indeed, several observations have highlighted the involvement of MYCN in regulating lipid metabolism in NB cancer. Inhibition of MYCN and the downstream signaling pathway by several means in NB cells results in intracellular lipid droplet accumulation as a consequence of mitochondrial dysfunction [44]. Furthermore, lipid accumulation was shown to be caused mainly by inhibition of β-oxidation, suggesting that aggressive NB tumors use fatty acid as an energy source. This observation was further supported by analyzing the metabolic features of NB tumors with MYCN amplified. MYCN amplification enhances oxidative phosphorylation in NB cells [45], and more importantly, gene expression profile and proteomic analysis in patients show that high levels of MYCN are associated with elevated expression of key enzymes involved in glycolysis, Krebs cycle, and electron transport chain proteins. Patients with high expression of these genes show poor overall survival.
Selective targeting lipid metabolism as therapeutic approach in neuroblastoma
Cancer cells are particularly dependent on lipid metabolism for energy production because they are key components of cellular membranes, storing precursors of biologically active lipid mediators [51–53]. Therefore, novel therapeutic strategies have been explored to inhibit lipid metabolism for improving clinical outcomes of cancer treatment [54]. In agreement with this, several clinical trials have assessed the possibility of inhibiting cholesterol biosynthesis by statins as a novel antitumor strategy [55]. However, this therapeutic approach has generated conflicting results [56, 57].
Inhibition of fatty acid oxidation
Several tumors use fatty acids as a source of mitochondrial energy production [58]. In NB, MYCN amplification correlates with high expression of key genes involved in the regulation of fatty acid oxidation (FAO), including hydroxyacyl-CoA dehydrogenase (HADH), indicating that MYCN-amplified NB tumors are more dependent on OXPHOS compared to non-MYCN-amplified tumors [45]. Interestingly, high expression of some of these enzymes correlates with poor prognosis in NB patients, suggesting that fatty acids are a major substrate for OXPHOS-based energy metabolism in NB. Carnitine palmitoyl-transferase 1a (CPT1a) is the β-oxidation rate-limiting enzyme, and high expression of the gene correlates with poor prognosis in NB patients. Etomoxir is a small-molecule irreversible inhibitor of CPT1a used widely in preclinical studies. Recently, it has been shown that etomoxir treatment was able to reduce in vivo tumor growth of MYCN-amplified NB cells. Although, in phase II clinical trials, etomoxir has shown hepatic toxicity and has been suspended, these experiments are proof of concept that inhibition of FAO could be used for the development of novel therapeutic strategies. In addition, the novel reversible CPT1a inhibitor, teglicar, which was recently developed, does not show as severe toxicity as etomoxir [59]. Further studies are needed to assess the possibility of using teglicar for cancer treatment.
Inhibition of de novo fatty acid synthesis
One common feature shared by almost all tumors is the reactivation of fatty acid synthesis (FAS) to support cancer cell proliferation, as cancer cells need lipids both as membrane components and as signaling molecules involved in cell homeostasis, cell death, and metastasis [54]. FAS, which takes place in cytoplasm, is regulated primarily by acetyl-CoA carboxylase (ACACA) and fatty acid synthase (FASN). Small-molecule inhibitors of these two enzymes, including TOFA and Soraphen A, target ACACA, and Cerulenin, Orlistat, and UB006 that target FASN are available and used largely in preclinical experiments that have shown promising antitumor activity [60]. Using these five inhibitors in different experimental approaches, including PDX-derived cell cultures and xenograft model of NB, it has been shown that inhibition of FAS resulted in decreased cell proliferation, reduced MYCN protein levels, and induction of neural differentiation [61]. Of note, these antitumor effects were independent from MYCN status. Among the inhibitors tested, only Orlistat has been approved by the FDA, although not for cancer treatment. Therefore, these observations strongly support the idea of developing more specific FAS inhibitors that can be used in the clinic.
Differentiation therapy (elovanoids)
The highest-risk group of patients is often treated with isotretinoin (13-cis-retinoic acid) to induce terminal differentiation of NB cells, thus reducing the risk of relapse [62]. However, the benefits of using retinoids are uncertain [8, 63]. Therefore, it would be important to develop novel agents able to induce differentiation for treatments of NB. In this respect, elovanoids (ELVs) are a novel class of endogenous lipid mediators that protect against excitotoxicity and cell damage and modulate neuronal homeostasis [47, 50]. Recently, it has also been shown that ELV-N34:6 may have pharmacological activity against glioblastoma multiforme (GBM). Indeed, in the orthotopic model of GBM treatment with LAU-0901 (a platelet-activating factor receptor antagonist), ELV-N34:6, and Avastin (angiogenesis inhibitors) individually and with all three compounds in combination resulted in a reduction of tumor size [64, 65]. Therefore, since ELV-N34:6 has shown in vivo pharmacological activity, it is possible to hypothesize that ELV-N34:6 may be exploited for NB treatment in alternative or in combination with isotretinoin for inducing neural differentiation and improving patient outcome.
Very-long-chain polyunsaturated fatty acids (VLC-PUFA) play an important role in the maintenance of the homeostasis of several tissues, including neural tissue [46]. Among the family of PUFA products [47], ELVs represent a class of recently characterized lipid mediators that sustain cellular integrity against hemostasis disturbances [48, 49]. The enzyme ELOVL4 (elongation of VLC fatty acids–4), which is responsible for the elongation of > C28 FAs, mediates the biosynthesis of the precursors of elovanoids (ELV-N32 and ELV-N34). The expression of ELOVL4, which is repressed by MYCN, increases during neuronal differentiation of NB cells, and its presence is necessary for the progression of the differentiation. In addition, ELOVL4 expression is required to increase the lipid droplet number and VLC-PUFAs in differentiated cells [50]. Interestingly, more differentiated tumors (low- and intermediate-risk) show higher expression of ELOVL4 when compared with those less differentiated tumors (high-risk), and high levels of ELOVL4 identify subsets of NB patients with a better prognosis. Therefore, these observations suggest that dysregulation of ELOVL4 expression may participate in the lipid alterations that occur in NB. Overall, there is compelling experimental evidence that cellular metabolism, including lipid metabolism, is deregulated in NB cells and may therefore provide a novel, potential therapeutic target (see Fig. 1). Here we discuss some possible clinical applications of targeting lipid metabolism in NB that may be explored for therapeutic purposes.
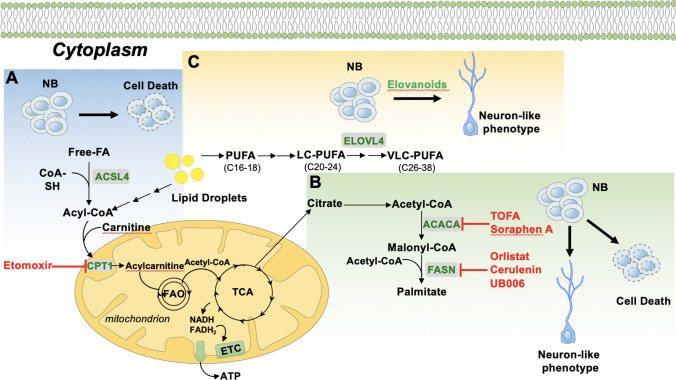
Fig. 1.
Novel potential pharmacological approaches in neuroblastoma treatment. A MYCN-amplified neuroblastoma (NB) cells rely on fatty acid–dependent mitochondrial respiration. Inhibition of fatty acid oxidation (FAO) with inhibitor targeting carnitine palmitoyl-transferase 1a (CPT1) reduces tumor growth by inducing cell death. B Reactivation of fatty acid synthesis (FASN) in cancer cells is required for providing lipid building blocks for sustained cell proliferation. Inhibition of FASN by small molecules decreases cell proliferation and results in neural differentiation of NB cells. C Elongation of very-long-chain fatty acids–4 (ELOVL4) is required for the proper neural differentiation of NB cells (Rugolo et al. 2021). We hypothesize that NB cells treated with elovanoids (ELVs) may induce differentiation of NB cells. NB: neuroblastoma; ACSL4: acyl-CoA synthetase long-chain family member 4; TCA: tricarboxylic acid cycle; ETC: electron transport chain; ACACA: acetyl-CoA carboxylase alpha; FASN: fatty acid synthase; PUFA: polyunsaturated fatty acid; LC-PUFA: long-chain polyunsaturated fatty acid; VLC-PUFA: long-chain poly unsaturated fatty acid
Author contribution
MA, GM, and NGB conceived the study. All authors contributed to the manuscript.
Funding
This project was supported by the Eye Ear Nose Throat Foundation of New Orleans.
Data availability
N/A.
Code availability
N/A.
Declarations
Ethics approval
N/A
Consent to participate
N/A
Consent for publication
N/A
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Massimiliano Agostini, Email: nbazan@lsuhsc.edu, Email: m.agostini@med.uniroma2.it.
Gerry Melino, Email: melino@uniroma2.it.
Bola Habeb, Email: bhabeb@lsuhsc.edu.
Jorgelina M. Calandria, Email: jcalan@lsuhsc.edu
Nicolas G. Bazan, Email: nbazan@lsuhsc.edu, Email: m.agostini@med.uniroma2.it
References
- 1.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet (London, England) 2007;369(9579):2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 2.Thiele CJ. Biology of pediatric peripheral neuroectodermal tumors. Cancer metastasis reviews. 1991;10(4):311–319. doi: 10.1007/BF00554793. [DOI] [PubMed] [Google Scholar]
- 3.Pieraccioli M, Nicolai S, Pitolli C, Agostini M, Antonov A, Malewicz M, Melino G. ZNF281 inhibits neuronal differentiation and is a prognostic marker for neuroblastoma. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(28):7356–7361. doi: 10.1073/pnas.1801435115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amelio I, Bertolo R, Bove P, Candi E, Chiocchi M, Cipriani C, Melino G. Cancer predictive studies. Biology direct. 2020;15(1):18. doi: 10.1186/s13062-020-00274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolai S, Pieraccioli M, Peschiaroli A, Melino G, Raschellà G. Neuroblastoma: Oncogenic mechanisms and therapeutic exploitation of necroptosis. Cell death & disease. 2015;6:e2010. doi: 10.1038/cddis.2015.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molenaar JJ, Koster J, Zwijnenburg DA, van Sluis P, Valentijn LJ, van der Ploeg I, Versteeg R. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature. 2012;483(7391):589–593. doi: 10.1038/nature10910. [DOI] [PubMed] [Google Scholar]
- 7.Mossé YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, Maris JM. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455(7215):930–935. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohn SL, Pearson ADJ, London WB, Monclair T, Ambros PF, Brodeur GM, Task INRG, Force. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. Journal of clinical oncology: Official journal of the American Society of Clinical Oncology. 2009;27(2):289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ragupathi G, Livingston PO, Hood C, Gathuru J, Krown SE, Chapman PB, Hwu W-J. Consistent antibody response against ganglioside GD2 induced in patients with melanoma by a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21. Clinical cancer research: An official journal of the American Association for Cancer Research. 2003;9(14):5214–5220. [PubMed] [Google Scholar]
- 10.Billon N, Terrinoni A, Jolicoeur C, McCarthy A, Richardson WD, Melino G, Raff M. Roles for p53 and p73 during oligodendrocyte development. Development (Cambridge, England) 2004;131(6):1211–1220. doi: 10.1242/dev.01035. [DOI] [PubMed] [Google Scholar]
- 11.de Laurenzi V, Melino G. Evolution of functions within the p53/p63/p73 family. Annals of the New York Academy of Sciences. 2000;926:90–100. doi: 10.1111/j.1749-6632.2000.tb05602.x. [DOI] [PubMed] [Google Scholar]
- 12.Bellomaria, A., Barbato, G., Melino, G., Paci, M., & Melino, S. (2010). Recognition of p63 by the E3 ligase ITCH: Effect of an ectodermal dysplasia mutant. Cell cycle (Georgetown, Tex.), 9(18), 3730–9. [PubMed]
- 13.Candi E, Terrinoni A, Rufini A, Chikh A, Lena AM, Suzuki Y, Melino G. p63 is upstream of IKK alpha in epidermal development. Journal of cell science. 2006;119(Pt 22):4617–4622. doi: 10.1242/jcs.03265. [DOI] [PubMed] [Google Scholar]
- 14.Candi E, Cipollone R, di Val R, Cervo P, Gonfloni S, Melino G, Knight R. p63 in epithelial development. Cellular and molecular life sciences: CMLS. 2008;65(20):3126–3133. doi: 10.1007/s00018-008-8119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Candi E, Agostini M, Melino G, Bernassola F. How the TP53 family proteins TP63 and TP73 contribute to tumorigenesis: Regulators and effectors. Human mutation. 2014;35(6):702–714. doi: 10.1002/humu.22523. [DOI] [PubMed] [Google Scholar]
- 16.Agostini M, Tucci P, Killick R, Candi E, Sayan BS, di Val R, Cervo P, Melino G. Neuronal differentiation by TAp73 is mediated by microRNA-34a regulation of synaptic protein targets. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(52):21093–21098. doi: 10.1073/pnas.1112061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velletri, T., Romeo, F., Tucci, P., Peschiaroli, A., Annicchiarico-Petruzzelli, M., Niklison-Chirou, M. V., Agostini, M. (2013). GLS2 is transcriptionally regulated by p73 and contributes to neuronal differentiation. Cell cycle (Georgetown, Tex.), 12(22), 3564–73. 10.4161/cc.26771 [DOI] [PMC free article] [PubMed]
- 18.Tomasini R, Tsuchihara K, Tsuda C, Lau SK, Wilhelm M, Rufini A, Mak TW. TAp73 regulates the spindle assembly checkpoint by modulating BubR1 activity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(3):797–802. doi: 10.1073/pnas.0812096106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amelio I, Melino G. The p53 family and the hypoxia-inducible factors (HIFs): Determinants of cancer progression. Trends in biochemical sciences. 2015;40(8):425–434. doi: 10.1016/j.tibs.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Nepravishta R, Sabelli R, Iorio E, Micheli L, Paci M, Melino S. Oxidative species and S-glutathionyl conjugates in the apoptosis induction by allyl thiosulfate. The FEBS journal. 2012;279(1):154–167. doi: 10.1111/j.1742-4658.2011.08407.x. [DOI] [PubMed] [Google Scholar]
- 21.Mauretti A, Neri A, Kossover O, Seliktar D, di Nardo P, Melino S. Design of a novel composite H2 S-releasing hydrogel for cardiac tissue repair. Macromolecular bioscience. 2016;16(6):847–858. doi: 10.1002/mabi.201500430. [DOI] [PubMed] [Google Scholar]
- 22.Hattori T, Takahashi Y, Chen L, Tang Z, Wills CA, Liang X, Wang H-G. Targeting the ESCRT-III component CHMP2A for noncanonical caspase-8 activation on autophagosomal membranes. Cell death and differentiation. 2021;28(2):657–670. doi: 10.1038/s41418-020-00610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brodeur, G. M., Seeger, R. C., Schwab, M., Varmus, H. E., & Bishop, J. M. (1984). Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science (New York, N.Y.), 224(4653), 1121–4. 10.1126/science.6719137 [DOI] [PubMed]
- 24.Dang C, v, Reddy, E. P., Shokat, K. M., & Soucek, L. Drugging the “undruggable” cancer targets. Nature reviews. Cancer. 2017;17(8):502–508. doi: 10.1038/nrc.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gouw AM, Margulis K, Liu NS, Raman SJ, Mancuso A, Toal GG, Felsher DW. The MYC oncogene cooperates with sterol-regulated element-binding protein to regulate lipogenesis essential for neoplastic growth. Cell metabolism. 2019;30(3):556–572.e5. doi: 10.1016/j.cmet.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Science advances. 2016;2(5):e1600200. doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindner AU, Salvucci M, McDonough E, Cho S, Stachtea X, O’Connell EP, Prehn JHM. An atlas of inter- and intra-tumor heterogeneity of apoptosis competency in colorectal cancer tissue at single-cell resolution. Cell death and differentiation. 2022;29(4):806–817. doi: 10.1038/s41418-021-00895-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosa N, Ivanova H, Wagner LE, Kale J, la Rovere R, Welkenhuyzen K, Bultynck G. Bcl-xL acts as an inhibitor of IP3R channels, thereby antagonizing Ca2+-driven apoptosis. Cell death and differentiation. 2022;29(4):788–805. doi: 10.1038/s41418-021-00894-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humpton TJ, Hall H, Kiourtis C, Nixon C, Clark W, Hedley A, Vousden KH. p53-mediated redox control promotes liver regeneration and maintains liver function in response to CCl4. Cell death and differentiation. 2022;29(3):514–526. doi: 10.1038/s41418-021-00871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiorillo M, Scatena C, Naccarato AG, Sotgia F, Lisanti MP. Bedaquiline, an FDA-approved drug, inhibits mitochondrial ATP production and metastasis in vivo, by targeting the gamma subunit (ATP5F1C) of the ATP synthase. Cell death and differentiation. 2021;28(9):2797–2817. doi: 10.1038/s41418-021-00788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shulkin BL, Mitchell DS, Ungar DR, Prakash D, Dole MG, Castle VP, Hutchinson RJ. Neoplasms in a pediatric population: 2-[F-18]-fluoro-2-deoxy-D-glucose PET studies. Radiology. 1995;194(2):495–500. doi: 10.1148/radiology.194.2.7824731. [DOI] [PubMed] [Google Scholar]
- 32.Levy AG, Zage PE, Akers LJ, Ghisoli ML, Chen Z, Fang W, Zweidler-McKay PA. The combination of the novel glycolysis inhibitor 3-BrOP and rapamycin is effective against neuroblastoma. Investigational new drugs. 2012;30(1):191–199. doi: 10.1007/s10637-010-9551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White PS, Thompson PM, Seifried BA, Sulman EP, Jensen SJ, Guo C, Brodeur GM. Detailed molecular analysis of 1p36 in neuroblastoma. Medical and pediatric oncology. 2001;36(1):37–41. doi: 10.1002/1096-911X(20010101)36:1<37::AID-MPO1010>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 34.King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: Linking mitochondrial dysfunction and cancer. Oncogene. 2006;25(34):4675–4682. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- 35.Feichtinger RG, Zimmermann F, Mayr JA, Neureiter D, Hauser-Kronberger C, Schilling FH, Kofler B. Low aerobic mitochondrial energy metabolism in poorly- or undifferentiated neuroblastoma. BMC Cancer. 2010;10:149. doi: 10.1186/1471-2407-10-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Astuti D, Morris M, Krona C, Abel F, Gentle D, Martinsson T, Maher ER. Investigation of the role of SDHB inactivation in sporadic phaeochromocytoma and neuroblastoma. British journal of cancer. 2004;91(10):1835–1841. doi: 10.1038/sj.bjc.6602202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bown N, Cotterill S, Lastowska M, O’Neill S, Pearson AD, Plantaz D, van Roy N. Gain of chromosome arm 17q and adverse outcome in patients with neuroblastoma. The New England journal of medicine. 1999;340(25):1954–1961. doi: 10.1056/NEJM199906243402504. [DOI] [PubMed] [Google Scholar]
- 38.Islam A, Kageyama H, Takada N, Kawamoto T, Takayasu H, Isogai E, Nakagawara A. High expression of survivin, mapped to 17q25, is significantly associated with poor prognostic factors and promotes cell survival in human neuroblastoma. Oncogene. 2000;19(5):617–623. doi: 10.1038/sj.onc.1203358. [DOI] [PubMed] [Google Scholar]
- 39.Hagenbuchner J, Kuznetsov A, v, Obexer, P., & Ausserlechner, M. J. BIRC5/survivin enhances aerobic glycolysis and drug resistance by altered regulation of the mitochondrial fusion/fission machinery. Oncogene. 2013;32(40):4748–4757. doi: 10.1038/onc.2012.500. [DOI] [PubMed] [Google Scholar]
- 40.Carter DR, Sutton SK, Pajic M, Murray J, Sekyere EO, Fletcher J, Marshall GM. Glutathione biosynthesis is upregulated at the initiation of MYCN-driven neuroblastoma tumorigenesis. Molecular oncology. 2016;10(6):866–878. doi: 10.1016/j.molonc.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren P, Yue M, Xiao D, Xiu R, Gan L, Liu H, Qing G. ATF4 and N-Myc coordinate glutamine metabolism in MYCN-amplified neuroblastoma cells through ASCT2 activation. The Journal of pathology. 2015;235(1):90–100. doi: 10.1002/path.4429. [DOI] [PubMed] [Google Scholar]
- 42.Carroll PA, Diolaiti D, McFerrin L, Gu H, Djukovic D, Du J, Eisenman RN. Deregulated Myc requires MondoA/Mlx for metabolic reprogramming and tumorigenesis. Cancer Cell. 2015;27(2):271–285. doi: 10.1016/j.ccell.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casinelli G, LaRosa J, Sharma M, Cherok E, Banerjee S, Branca M, Graves JA. N-Myc overexpression increases cisplatin resistance in neuroblastoma via deregulation of mitochondrial dynamics. Cell death discovery. 2016;2:16082. doi: 10.1038/cddiscovery.2016.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zirath H, Frenzel A, Oliynyk G, Segerström L, Westermark UK, Larsson K, Henriksson MA. MYC inhibition induces metabolic changes leading to accumulation of lipid droplets in tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(25):10258–10263. doi: 10.1073/pnas.1222404110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliynyk, G., Ruiz-Pérez, M. V., Sainero-Alcolado, L., Dzieran, J., Zirath, H., Gallart-Ayala, H., … Arsenian-Henriksson, M. (2019). MYCN-enhanced oxidative and glycolytic metabolism reveals vulnerabilities for targeting neuroblastoma. iScience, 21, 188–204. 10.1016/j.isci.2019.10.020 [DOI] [PMC free article] [PubMed]
- 46.Kihara A. Very long-chain fatty acids: Elongation, physiology and related disorders. Journal of biochemistry. 2012;152(5):387–395. doi: 10.1093/jb/mvs105. [DOI] [PubMed] [Google Scholar]
- 47.Bhattacharjee S, Jun B, Belayev L, Heap J, Kautzmann M-A, Obenaus A, Bazan NG. Elovanoids are a novel class of homeostatic lipid mediators that protect neural cell integrity upon injury. Science advances. 2017;3(9):e1700735. doi: 10.1126/sciadv.1700735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jun B, Mukherjee PK, Asatryan A, Kautzmann M-A, Heap J, Gordon WC, Bazan NG. Elovanoids are novel cell-specific lipid mediators necessary for neuroprotective signaling for photoreceptor cell integrity. Scientific reports. 2017;7(1):5279. doi: 10.1038/s41598-017-05433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agbaga M-P, Brush RS, Mandal MNA, Henry K, Elliott MH, Anderson RE. Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(35):12843–12848. doi: 10.1073/pnas.0802607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rugolo F, Bazan NG, Calandria J, Jun B, Raschellà G, Melino G, Agostini M. The expression of ELOVL4, repressed by MYCN, defines neuroblastoma patients with good outcome. Oncogene. 2021;40(38):5741–5751. doi: 10.1038/s41388-021-01959-3. [DOI] [PubMed] [Google Scholar]
- 51.Chen X, Kang R, Kroemer G, Tang D. Organelle-specific regulation of ferroptosis. Cell death and differentiation. 2021;28(10):2843–2856. doi: 10.1038/s41418-021-00859-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang L, Hou Y, Du Y-E, Li Q, Zhou F, Li Y, Liu M. Mirtronic miR-4646-5p promotes gastric cancer metastasis by regulating ABHD16A and metabolite lysophosphatidylserines. Cell death and differentiation. 2021;28(9):2708–2727. doi: 10.1038/s41418-021-00779-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui W, Liu D, Gu W, Chu B. Peroxisome-driven ether-linked phospholipids biosynthesis is essential for ferroptosis. Cell death and differentiation. 2021;28(8):2536–2551. doi: 10.1038/s41418-021-00769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snaebjornsson MT, Janaki-Raman S, Schulze A. Greasing the wheels of the cancer machine: The role of lipid metabolism in cancer. Cell metabolism. 2020;31(1):62–76. doi: 10.1016/j.cmet.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 55.Göbel, A., Rauner, M., Hofbauer, L. C., & Rachner, T. D. (2020). Cholesterol and beyond - The role of the mevalonate pathway in cancer biology. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer, 1873(2), 188351. 10.1016/j.bbcan.2020.188351 [DOI] [PubMed]
- 56.Dickerman BA, García-Albéniz X, Logan RW, Denaxas S, Hernán MA. Avoidable flaws in observational analyses: An application to statins and cancer. Nature medicine. 2019;25(10):1601–1606. doi: 10.1038/s41591-019-0597-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGregor GH, Campbell AD, Fey SK, Tumanov S, Sumpton D, Blanco GR, Kamphorst JJ. Targeting the metabolic response to statin-mediated oxidative stress produces a synergistic antitumor response. Cancer research. 2020;80(2):175–188. doi: 10.1158/0008-5472.CAN-19-0644. [DOI] [PubMed] [Google Scholar]
- 58.Kant S, Kesarwani P, Prabhu A, Graham SF, Buelow KL, Nakano I, Chinnaiyan P. Enhanced fatty acid oxidation provides glioblastoma cells metabolic plasticity to accommodate to its dynamic nutrient microenvironment. Cell Death & Disease. 2020;11(4):253. doi: 10.1038/s41419-020-2449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conti R, Mannucci E, Pessotto P, Tassoni E, Carminati P, Giannessi F, Arduini A. Selective reversible inhibition of liver carnitine palmitoyl-transferase 1 by teglicar reduces gluconeogenesis and improves glucose homeostasis. Diabetes. 2011;60(2):644–651. doi: 10.2337/db10-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Makowski K, Mir JF, Mera P, Ariza X, Asins G, Hegardt FG, Serra D. (-)-UB006: A new fatty acid synthase inhibitor and cytotoxic agent without anorexic side effects. European journal of medicinal chemistry. 2017;131:207–221. doi: 10.1016/j.ejmech.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 61.Ruiz-Pérez, M. V., Sainero-Alcolado, L., Oliynyk, G., Matuschek, I., Balboni, N., Ubhayasekera, S. J. K. A., Arsenian-Henriksson, M. (2021). Inhibition of fatty acid synthesis induces differentiation and reduces tumor burden in childhood neuroblastoma. iScience, 24(2), 102128. 10.1016/j.isci.2021.102128 [DOI] [PMC free article] [PubMed]
- 62.Matthay, K. K., Villablanca, J. G., Seeger, R. C., Stram, D. O., Harris, R. E., Ramsay, N. K., Reynolds, C. P. (1999). Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. The New England journal of medicine, 341(16), 1165–73. 10.1056/NEJM199910143411601 [DOI] [PubMed]
- 63.Peinemann, F., van Dalen, E. C., Enk, H., & Berthold, F. (2017). Retinoic acid postconsolidation therapy for high-risk neuroblastoma patients treated with autologous haematopoietic stem cell transplantation. The Cochrane database of systematic reviews, 8, CD010685. 10.1002/14651858.CD010685.pub3 [DOI] [PMC free article] [PubMed]
- 64.Cruz Flores VA, Menghani H, Mukherjee PK, Marrero L, Obenaus A, Dang Q, Bazan NG. Combined therapy with avastin, a PAF receptor antagonist and a lipid mediator inhibited glioblastoma tumor growth. Frontiers in pharmacology. 2021;12:746470. doi: 10.3389/fphar.2021.746470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A.
N/A.