Abstract
Mitomycin C (MC) is an antitumor antibiotic derived biosynthetically from 3-amino-5-hydroxybenzoic acid (AHBA), d-glucosamine, and carbamoyl phosphate. A gene (mitA) involved in synthesis of AHBA has been identified and found to be linked to the MC resistance locus, mrd, in Streptomyces lavendulae. Nucleotide sequence analysis showed that mitA encodes a 388-amino-acid protein that has 71% identity (80% similarity) with the rifamycin AHBA synthase from Amycolatopsis mediterranei, as well as with two additional AHBA synthases from related ansamycin antibiotic-producing microorganisms. Gene disruption and site-directed mutagenesis of the S. lavendulae chromosomal copy of mitA completely blocked the production of MC. The function of mitA was confirmed by complementation of an S. lavendulae strain containing a K191A mutation in MitA with AHBA. A second gene (mitB) encoding a 272-amino-acid protein (related to a group of glycosyltransferases) was identified immediately downstream of mitA that upon disruption resulted in abrogation of MC synthesis. This work has localized a cluster of key genes that mediate assembly of the unique mitosane class of natural products.
Streptomyces spp. are filamentous gram-positive soil bacteria with a nucleotide base composition greater than 70 mol% G+C (53). They produce a wide array of biologically active compounds, including over two-thirds of the commercially important natural-product metabolites (1, 10). Genetic information accumulated over the past 15 years has demonstrated that genes encoding enzymes for natural product assembly are clustered on the Streptomyces genome (38). In addition, one or more pathway-specific transcriptional regulatory genes and at least one resistance gene are typically found within the antibiotic biosynthetic gene cluster (14). Among the strategies for cloning antibiotic biosynthetic genes, heterologous hybridization with gene probes based on highly conserved biosynthetic-enzyme amino acid sequences has been very effective (25, 49, 56).
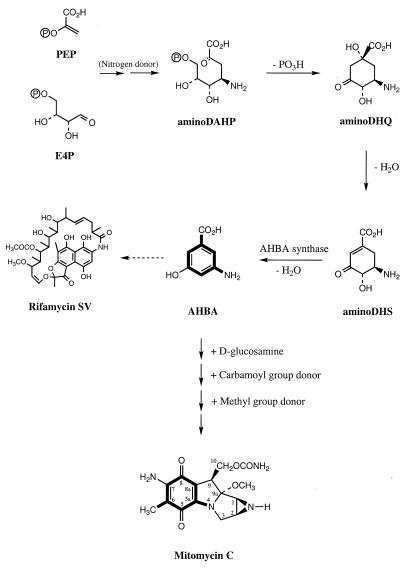
Streptomyces lavendulae produces the clinically important antitumor antibiotic mitomycin C (MC) (22). MC has become one of the most effective drugs against non-small-cell lung carcinoma, as well as other soft tumors (24). The molecule has an unusual structure, comprised of aziridine, pyrrolizidine, pyrrolo-(1,2a)-indole, and amino-methylbenzoquinone rings to give the mitosane nucleus (58). A significant amount of information on the biosynthesis of MC has accumulated since 1970. The mitosane core was shown to be derived from the junction of an amino-methylbenzoquinone (mC7N unit) and hexosamine (C6N unit) (27) (Fig. 1). The C6N unit consists of carbons 1, 2, 3, 9, 9a, and 10, with the aziridine nitrogen derived intact from d-glucosamine (29).
FIG. 1.
Proposed biosynthetic pathway leading to mitomycins.
The mC7N unit in MC and the ansamycins is derived from 3-amino-5-hydroxybenzoic acid (AHBA) (8, 33). AHBA was first shown to be incorporated into the ansamycin antibiotic actamycin (32). Subsequently, it was confirmed as an efficient precursor for rifamycin (21), geldanamycin (46), ansamitocin (23), ansatrienin (59), streptovaricin (54), and naphthomycin A (37). Anderson et al. demonstrated that [carboxy-13C]AHBA could be efficiently and specifically incorporated into the C-6 methyl group of porfiromycin, which contains the same mitosane core as MC (3). 14C-labeled precursor feeding studies with d-glucose, pyruvate, and d-erythrose indicated that de novo biosynthesis of AHBA resulted directly from the shikimate pathway. However, no incorporation into the mC7N unit of either MC (27) or the ansamycin antibiotics (15) was found from labeling studies with shikimic acid, the shikimate precursor 3-dehydroquinic acid, or the shikimate-derived amino acids. These results led to the hypothesis of a modified shikimate pathway, in which a 3-deoxy-d-arabino-heptulosonic acid-7-phosphate (DAHP) synthase-like enzyme catalyzes the conversion to 3,4-dideoxy-4-amino-d-arabino-heptulosonic acid-7-phosphate (aminoDAHP) to give the ammoniated shikimate pathway (34). Kim et al. provided strong support for this new variant of the shikimate pathway by showing that aminoDAHP, 5-deoxy-5-amino-3-dehydroquinic acid (aminoDHQ), and 5-deoxy-5-amino-3-dehydroshikimic acid (aminoDHS) could be efficiently converted into AHBA by a cell extract of Amycolatopsis mediterranei (the rifamycin producer), in contrast to the normal shikimate pathway intermediate DAHP, which was not converted (34, 35). Recently, the AHBA synthase gene (rifK) from A. mediterranei has been cloned, sequenced, and functionally characterized (36).
Since AHBA is a biosynthetic precursor for MC, we decided to use rifK as a probe to identify a corresponding gene from S. lavendulae that may be linked with one of the previously characterized MC resistance genes (4, 50). A 3.8-kb BamHI fragment from the S. lavendulae genome was identified, and its nucleotide sequence revealed three open reading frames (ORFs). One ORF (mitA) showed high similarity to previously identified AHBA synthase genes (36), while a second (mitB) showed sequence similarity to several procaryotic and eucaryotic glycosyltransferases. The involvement of both of these genes in MC biosynthesis was demonstrated by gene disruption, site-directed mutagenesis, and subsequent isolation of mutants blocked in antibiotic biosynthesis. MC production was restored when the mitA mutant strain was cultured in the presence of exogenous AHBA.
MATERIALS AND METHODS
Strains and culture conditions.
Escherichia coli DH5α was grown in either Luria broth or tryptic soy broth (TSB) (Difco) as liquid medium or agar plates. E. coli DH5αF′, the host for harvesting single-stranded DNA, was grown at 37°C on TBG (1.2% tryptone, 2.4% yeast extract, 0.4% glycerol, 17 mM KH2PO4, 55 mM K2HPO4, and 20 mM glucose). E. coli S17-1 (39), used for conjugation, was grown in TSB with 10 μg of streptomycin/ml. S. lavendulae was grown in TSB or on R5T plates (containing [grams per liter] sucrose, 121.2; K2SO4, 0.3; MgCl2 · 6H2O, 11.92; glucose, 11.8; yeast extract, 5.89; Casamino Acids, 0.12; agar, 25.9; and 2.35 ml of trace elements [26]; after the mixture was autoclaved, 0.5% KH2PO4 [11.8 ml], 5 M CaCl2 [4.71 ml], and 1 N NaOH [8.25 ml] were added). For MC production, S. lavendulae was grown in Nishikohri medium (containing [grams per liter] glucose, 15; soluble starch, 5; NaCl, 5; CaCO3, 3; and yeast extract, 5) for 72 h from a 1% (vol/vol) inoculum of frozen mycelia. Pulse feeding of AHBA to the disruption mutant, MV100, and the site-directed mutant, MV102, was done with feedings of 2.5 mg of a 20-mg/ml solution of the sodium salt of AHBA (pH 7.1) in three pulses at 24, 43, and 57 h of growth of a culture that was harvested at 76 h.
DNA preparation and amplification.
Isolation and purification of DNA was performed by standard methods (47). S. lavendulae NRRL 2564 genomic DNA was isolated by using the modified Chater protocol (26). Plasmid DNA was isolated from E. coli by using the alkaline-sodium dodecyl sulfate method.
pDHS2002 was constructed as follows. The 1.1-kb thiostrepton resistance gene (tsr) fragment was removed from pDHS5000 by SmaI-BamHI digestion, blunt ended with the large fragment of DNA polymerase (Gibco BRL), and ligated to MscI restriction enzyme-digested pDHS7601 to yield pDHS2001. MscI digestion of pDHS7601 resulted in the removal of 155 nucleotides at the C terminus of the mitA gene, and ligation of the blunt-ended BamHI site of the tsr adjacent to the MscI site of pDHS7601 resulted in regeneration of the BamHI site in pDHS2001. The 4.9-kb EcoRI-HindIII fragment from pDHS2001 containing the tsr-disrupted mitA gene was removed and ligated into EcoRI-HindIII-digested pKC1139 to yield pDHS2002.
Primer-mediated site-directed mutagenesis was employed to construct pDHS2015 containing a K191A mutation in mitA. Primer 1 (5′-GGCAAGGCATGCGAGGGTCGC-3′) and primer 2 (5′-TTCCAGAACGGCGCCCTGATGACCGCCGGC-3′) were used to amplify the 691-bp fragment of the 5′ end of mitA. The 3′ end of mitA was amplified with primer 3 (5′-GCCGGCGGTCATCAGGGCGCCGTTCTGGAA-3′) and primer 4 (5′-TCAGAATTCGGATCCGAGGGCCGGAGT-3′) to generate a 1,151-bp band. A second round of PCR was performed, with the overlapping 691- and 1,151-bp units as the initial templates, with primer 1 and primer 4 to afford a 1.8-kb fragment. The final product, containing mutagenized mitA, was digested with EcoRI-SphI and ligated to the 2.1-kb HindIII-SphI fragment from pDSH2004 and the EcoRI-HindIII-digested pKC1139 to yield pDSH2015. The site-directed mutation of MitA K191A in pDHS2015 was confirmed by sequencing with forward primer (5′-ACCTACTGCCTCGATGCC-3′) and reverse primer (5′-CTGATCCTTCAAGCG-3′).
The mitB disruption vector pDHS7702 was constructed as follows. pDHS7601 was digested with BstBI, blunt ended, and ligated with the 1.4-kb neomycin resistance gene fragment from pFD666 (17) (ApaL1-HindIII digestion; blunt ended). The 5.2-kb EcoRI-HindIII fragment from the resulting construct, pDHS7701, was subcloned into pKC1139 to create pDHS7702.
DNA library construction and screening.
S. lavendulae NRRL 2564 genomic DNA was partially digested with Sau3AI, and a fraction containing 30- to 50-kb fragments was recovered by sucrose gradient centrifugation and ligated into the calf intestinal alkaline phosphatase-treated BglII site of the E. coli-Streptomyces shuttle vector pNJ1 (55) and then packaged with the Packagene Lambda DNA packaging system (Promega). The cosmid library was constructed by transfecting E. coli DH5α, and colonies that appeared on the Luria broth plates containing 100 μg of ampicillin/ml were transferred to a BioTrace NT nitrocellulose blotting membrane (Gelman Sciences). Colony hybridization was performed as specified by the manufacturer. A PCR-amplified 0.7-kb DNA fragment from plasmid pKN108 (Table 1) was used to screen the library. The primers used for PCR were 5′-GCGTCCGTGCTGCGCGCGCA-3′ and 5′-TGCGCGCGCAGCACGGACGC-3′. The cosmids from the positive colonies were confirmed by Southern blot hybridization, and a 1.7-kb AflIII-BamHI fragment from pDHS3001 containing the mitomycin resistance determinant (mrd) (50) was used as a probe to establish genetic linkage.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | F−recA φ80 dlacZΔM15 | Gibco BRL |
| DH5αF′ | F′ φ80 dlacZΔM15 | Gibco BRL |
| S17-1 | Contains RP4 integrated into the chromosome; Strr | 39 |
| A. mediterranei ATCC 27643 | Rifamycin producer | ATCC |
| S. lavendulae | ||
| NRRL 2564 | MC producer | ATCC |
| MV100b | mitA insertional disruption mutant of NRRL 2564 | This study |
| MV102b | mitA site-directed mutant (K191A) of NRRL 2564 | This study |
| MV103 | MV100 with plasmid pDHS2003 | This study |
| MM101b | mitB insertional disruption mutant of NRRL 2564 | This study |
| Plasmids | ||
| pNJ1 | Thr Apr bifunctional E. coli and Streptomyces shuttle vector | 55 |
| pUC119 | AprlacZα MCS; E. coli cloning vector | 61 |
| pKC1139 | AmrlacZα MCS oriT rep(Ts) | 12 |
| pDHS3001 | pIJ702 with 4.1-kb BclI DNA insert; contains mrd locus | 50 |
| pKN108 | Contains rifamycin AHBA synthase gene | 34, 35 |
| pFD666 | Neor bifunctional E. coli and Streptomyces shuttle cosmid | 17 |
| pSL301 | AprlacZα MCS; E. coli cloning vector | Invitrogen |
| pDHS7529 | pNJ1 with 37-kb inserted fragment from S. lavendulae; contains mrd and mitABC locus | This study |
| pDHS7601 | pUC119 with 3.8-kb BamHI subclone from pDHS7529; contains mitABC locus | This study |
| pDHS5000 | pUC119 with 1.1-kb SmaI-PstI fragment of tsr gene from pNJ1; blunt ended and subcloned in SmaI site | This study |
| pDHS2001 | mitA disruption construct; 1.1-kb SmaI-BamHI (tsr) fragment from pDHS5000 was blunt ended and inserted into the two MscI sites of pDHS7601 | This study |
| pDHS2002 | mitA disruption vector; 4.9-kb EcoRI-HindIII insert from pDHS2001 was subcloned into pKC1139 | This study |
| pDHS2003 | pKC1139 with 3.8-kb EcoRI-HindIII insert from pDHS7601 | This study |
| pDHS2004 | pSL301 vector with 3.8-kb PstI-EcoRI fragment from pDHS7601 inserted. Makes SphI site in mitABC unique. | This study |
| pDHS2015 | mitA site-directed disruption vector with lysine 191 replaced by alanine | This study |
| pDHS7701 | mitB disruption construct; 1.4-kb neo-resistant fragment ApaL1-HindIII was blunt ended and subcloned into the BstBI-digested, blunt ended pDHS7601 | This study |
| pDHS7702 | mitB disruption vector; 5.2-kb EcoRI-HindIII insert from pDHS7701 subcloned into pKC1139 | This study |
Amr, apramycin resistance; Apr, ampicillin resistance; MCS, multiple cloning site; Neor, neomycin resistance; rep(Ts), temperature-sensitive replicon for Streptomyces; Strr, streptomycin resistance; Thr, thiostrepton resistance; tsr, thiostrepton resistance gene.
See Materials and Methods.
DNA sequencing and analysis.
Deletion subclones from pDHS7601 were made with the exonuclease III Erase-a-Base system (Promega). Sequencing was accomplished with the PRISM dye terminator cycle-sequencing ready reaction kit (Applied Biosystems) and analyzed on an Applied Biosystems 377 DNA sequencer at the University of Minnesota Advanced Genetic Analysis Center. For generating single-stranded DNA, deletion subclones in pUC119 were transformed into E. coli DH5αF′, and M13K07 helper phage (Gibco BRL) was used. Nucleotide sequence data were analyzed with Wisconsin Genetics Computer Group software (version 9.0) (18) and GeneWorks software version 2.51 (Oxford Molecular Group).
Conjugation from E. coli S17-1 to S. lavendulae.
To transfer plasmid from E. coli S17-1 to S. lavendulae, the procedure of Bierman et al. (12) was used with the following modification. A single colony of E. coli S17-1/pDHS2002 was used to inoculate 2 ml of TSB containing 100 μg of apramycin/ml and 10 μg of streptomycin/ml. Following overnight incubation at 37°C, a 1:100 inoculation was made into TSB with 100 μg of apramycin/ml and 10 μg of streptomycin/ml. This culture was grown for 3 h at 37°C, and the cells were washed twice with TSB and resuspended in 2 ml of TSB to provide the donor E. coli culture. The recipient S. lavendulae culture was generated by inoculating 9 ml of TSB with 1 ml of frozen wild-type culture. Following overnight (16-h) incubation at 29°C, the culture was homogenized by sonication, and 2 ml of this culture was used to inoculate 18 ml of TSB. Following overnight growth at 29°C and sonication treatment to homogenize the culture, a 1-ml inoculum was placed in 9 ml of TSB. This culture was grown for 3 h, and the mycelia were washed with TSB and resuspended in 2 ml of TSB to provide the stock recipient culture.
The donor and recipient cultures were mixed together in 9:1, 1:1, and 1:10 donor/recipient ratios, and 100 μl of the cell mixture was spread on AS1 plates (5). The plates were incubated overnight at 29°C and overlaid with 1 ml of water containing a suspension of 500 μg each of thiostrepton, apramycin, and nalidixic acid/ml. For the pKC1139 control, only apramycin and nalidixic acid were overlaid, while for pDHS7702, 500 μg of kanamycin/ml was used instead of thiostrepton. S. lavendulae exconjugants appeared in approximately 11 to 13 days at a frequency ranging from 10−7 to 10−5. pKC1139 has a temperature-sensitive Streptomyces replication origin, which is unable to replicate at temperatures above 34°C (41), while the S. lavendulae host grows well at 42°C. Thus, after the conjugants were propagated at 39°C for several generations, double-crossover mutants were readily generated. The presence of the plasmid was determined by transformation of E. coli DH5α with plasmid extracts from S. lavendulae transconjugants.
Double-crossover selection procedure.
A single colony of S. lavendulae/pDHS2002 grown on R5T plates (50 μg [each] of thiostrepton and apramycin/ml) was used to inoculate TSB broth containing 20 μg of thiostrepton/ml. After 72 h of incubation at 39°C, 10−4, 10−5, and 10−6 diluted aliquots were used to inoculate R5T plates containing 50 μg of thiostrepton/ml. Following 48 h of growth at 39°C, 84 colonies were picked randomly and each colony was patched out on separate R5T plates containing 50 μg of thiostrepton/ml and 50 μg of apramycin/ml. One of the 84 colonies displayed the double-crossover phenotype of thiostrepton resistance and apramycin sensitivity. The integration of the tsr-disrupted mitA gene and the loss of plasmid pDHS2002 were confirmed by Southern hybridization analysis.
MitA K191A site-directed mutants (MV102) were selected by propagating MV100/pDHS2015 on R5T plates for two generations at 37°C. The colonies were replicated to plates containing 50 μg of thiostrepton/ml and plates without antibiotics. Of the 108 colonies replicated in the first round, one had the correct (thiostrepton-sensitive) phenotype. To confirm the K191A mutation, the mitA gene was amplifed from the chromosome with primers 1 and 4. Mutation of the conserved lysine codon (AAG) to an alanine codon (GCC) was verified with the same sequencing primers employed to confirm the correct construction of pDHS2015. The alanine codon was observed in both the forward and reverse sequence data.
Mutants for mitB (MM101) were selected as follows: S. lavendulae/pDHS7702 was propagated on R5T plates for five generations at 39°C before single colonies were replicated on R5T plates as described above. Of the 300 colonies tested, 12 clones displayed the correct phenotype (kanamycin resistance and apramycin sensitivity). The genotypes of selected mitB mutants were confirmed by Southern blot hybridization of S. lavendulae genomic DNA.
Analysis of MC production.
All cultures intended for MC extraction were grown in Nishikohri medium (42) for a period of 72 h. In all cases, a wild-type S. lavendulae culture was grown concurrently with the mutant cultures to provide MC production reference points. A 72-h, 50-ml culture (250-ml flask) of the MitA K191A MV102 mutant strain was supplemented with 125 μl of a 20-mg/ml solution of the sodium salt of AHBA (pH. 7.05) at 24, 43, and 55 h. In each case, the culture broth was separated from mycelia by centrifugation and then extracted three times with equal volumes of ethyl acetate. The ethyl acetate extracts were pooled, and the solvent was removed by vacuum to provide the crude broth extract. The preliminary screen for MC production involved thin-layer chromatography (TLC) on silica gel plates (Whatman K6) eluted with 9:1 chloroform-methanol. Production of MC was monitored by high-performance liquid chromatography (HPLC) (C18 reverse-phase column) with a gradient of 80% 50 mM Tris buffer (pH 7.2)–20% methanol to 40% 50 mM Tris buffer (pH 7.2)–60% methanol with the UV detector set to 363 nm.
Bioassay detection of MC was performed by loading a 1-cm disk with fractions eluting at the mitomycin retention time from HPLC injections of wild-type, MV100, pKC1139 vector control crude extracts and MC standards. The disks were placed on antibiotic medium no. 2 agar plates (Difco) with Bacillus subtilis spores added directly to the medium. The plates were incubated overnight at 29°C and examined for zones of inhibition. To confirm the production of MC by MV102 in the presence of exogenous AHBA, the fraction eluting at the MC retention time was collected, dried down, desalted, and submitted for desorption ionization mass spectrometric analysis on a Bio-Ion 20R DS-MS instrument (Applied Biosystems). The MC (molecular weight, 334)-sodium (molecular weight, 23) adduct peak, [M+Na]+, of 357 was diagnostic for the presence of MC in the AHBA-supplemented culture.
Nucleotide sequence accession number.
The GenBank accession number for mitABC is AF115779.
RESULTS
The mrd and ahbas genes are linked in the S. lavendulae genome.
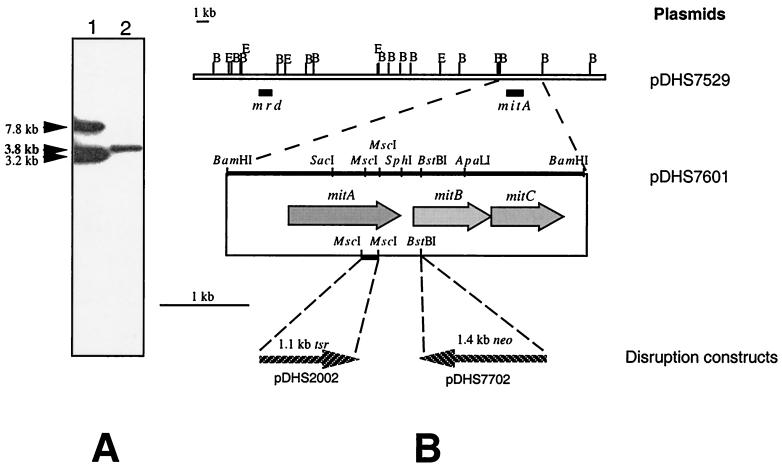
Southern blot analysis with the A. mediterranei AHBA synthase gene (rifK) probe (36) showed a single 3.8-kb band that hybridized with BamHI-digested S. lavendulae genomic DNA (Fig. 2). Subsequently, a S. lavendulae genomic DNA library was constructed with the E. coli-Streptomyces shuttle cosmid pNJ1 (55). Of the 5,000 colonies screened, 21 positive clones were identified, with 6 of them hybridizing with the mrd gene probe (none hybridized with the mcr gene probe [reference 4 and data not shown]). Restriction enzyme mapping and reciprocal hybridization of the cosmid clones established that the mrd and S. mediterranei AHBA synthase homologous genes were ∼20 kb apart in the S. lavendulae genome. The 3.8-kb BamHI fragment bearing a putative S. lavendulae AHBA synthase gene was subcloned, and its nucleotide sequence was determined.
FIG. 2.
Southern hybridization and restriction enzyme map of the mrd and rifK hybridizing regions from S. lavendulae. (A) Southern hybridization with the rifK gene probe (36). Lane 1, A. mediterranei ATCC 27643 genomic DNA digested with BamHI; lane 2, S. lavendulae NRRL 2564 genomic DNA digested with BamHI revealed a 3.8-kb hybridization band. (B) Physical map showing the mitA, mitB, and mitC genes. The locations of mrd and rifK hybridizing genes in cosmid pDHS7529 are indicated by solid bars. E, EcoRI; B, BamHI. The sequenced 3.8-kb BamHI fragment containing mitA, mitB, and mitC is enlarged (wide arrows). The thin arrows below show sites of resistance gene integration for disruption experiments.
Three ORFs identified within the 3.8-kb BamHI fragment.
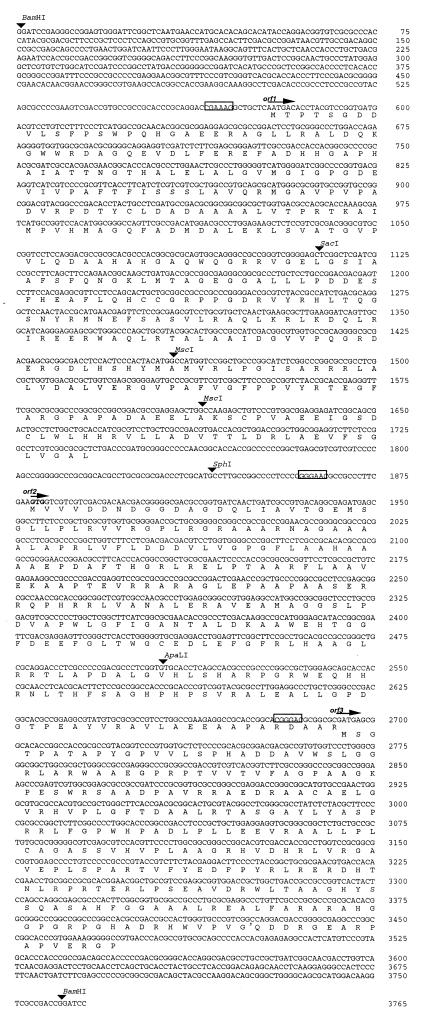
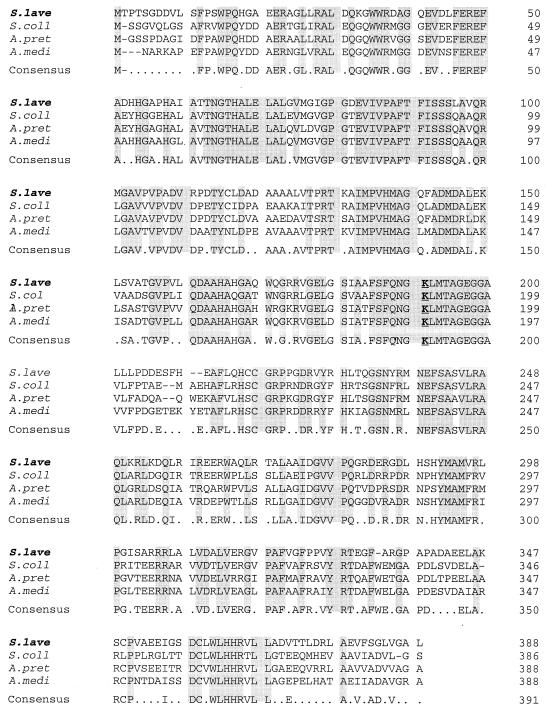
Three ORFs (mitA, mitB, and mitC) were identified within the sequenced 3.8-kb BamHI fragment (Fig. 2 and 3). mitA comprises 1,164 nucleotides and starts from an ATG (position 579 of the sequenced fragment) that is preceded by a potential ribosome binding site (RBS), GAAAGG. The deduced product of the mitA gene encodes a hydrophilic protein of 388 amino acids with a predicted mass of 41,949 Da and a calculated pI of 5.62. A BLAST (2) search showed that the predicted MitA protein has high sequence similarity (∼71% identity and 80% similarity) to AHBA synthases, from the rifamycin producer, A. mediterranei (36), and other ansamycin-producing actinomycetes, including Actinosynnema pretiosum (ansamitocin) and Streptomyces collinus (naphthomycin A and ansatrienin) (Fig. 4). A conserved pyridoxal phosphate (PLP) coenzyme binding motif (GX3DX7AX8EDX14GX13KX4–5geGGX19G) including the conserved lysine residue (boldface and underlined) can also be found in these four proteins (45).
FIG. 3.
Nucleotide sequence of the 3.8-kb DNA fragment containing mitABC. The deduced gene products are indicated in the one-letter code under the DNA sequence. Possible RBSs are boxed. The presumed translational start site and direction of transcription for each ORF are indicated by a labeled arrow. Restriction enzyme sites are marked by arrowheads.
FIG. 4.
Alignment of MitA with three other AHBA synthases. The deduced amino acid sequence comparison from AHBA synthase genes derived from S. lavendulae (mitA), S. collinus (Z54208), A. pretiosum (I39657), and A. mediterranei (I39657) is shown with the conserved lysine in the PLP-binding motif in boldface and underlined. Residues conserved in all sequences are shaded.
The mitB gene is predicted to start at a GTG (position 1879) that is preceded by a presumed RBS (GGAACG). This gene encodes a 272-amino-acid protein with a deduced mass of 28,648 Da and a deduced pI of 6.06. Database sequence homology searches revealed that the protein product of mitB shows local sequence similarity to a group of O-glycosyltransferases involved in polysaccharide biosynthesis. One segment of 70 amino acid residues at the N terminus of MitB has 43% similarity (36% identity) to the two glycosyltransferases SpsL and SpsQ from Sphingomonas sp. strain S88 and ExoO from Rhizobium meliloti, involved in polysaccharide (S88) and succinoglycan biosynthesis, respectively (7, 60). Another 60 amino acid residues located at the C terminus displayed 30% identity with UDP-GalNAc–polypeptide N-acetylgalactosaminyltransferase from Mus musculus and Homo sapiens (9).
The third ORF, mitC, starts from the ATG at position 2694, which is coupled to the stop codon, TGA, of mitB, and encodes a putative protein of 260 amino acids with a predicted molecular mass of 27,817 Da and a pI of 10.45. Database searches with the deduced protein product showed significant similarity over the first 90 amino acids (38% identity and 40% similarity) to the lmbE gene product (unknown function) from Mycobacterium leprae (U15183).
Insertional disruption of the mitA and mitB genes in S. lavendulae.
To test the dependence of functional mitA and mitB genes for MC biosynthesis, gene disruption constructs were generated for subsequent isolation of the corresponding S. lavendulae isogenic mutant strains.
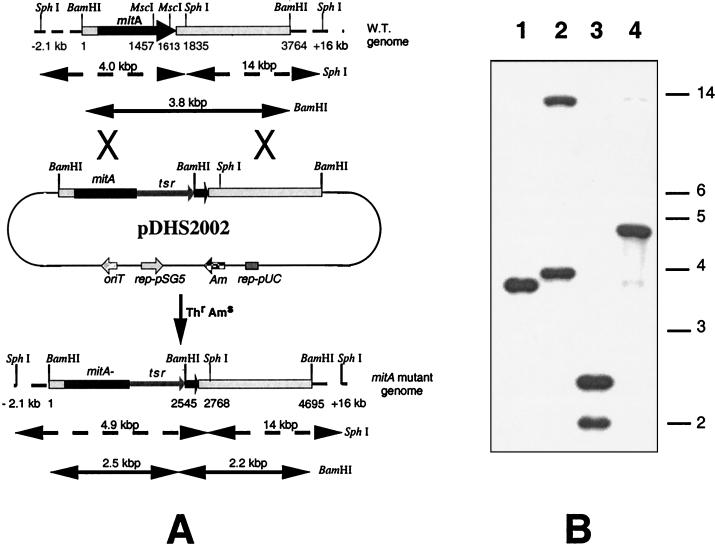
The mitA disruption construct was made by replacing a 155-bp fragment between the two MscI sites (located at the C terminus of the mitA gene in pDHS7601) with the 1.1-kb SmaI-BamHI fragment containing a thiostrepton resistance gene from pDHS5000 (Fig. 5A). This replacement regenerated a BamHI site at the junction, and the resulting construct was then subcloned into the E. coli-Streptomyces conjugative shuttle plasmid pKC1139, followed by conjugation into S. lavendulae. A double-crossover mutant strain (MV100) was selected based on the expected phenotype (thiostrepton resistant and apramycin sensitive) and further confirmed by Southern blot hybridization. Genomic DNAs from wild-type S. lavendulae and MV100 were digested with BamHI and SphI and hybridized with the 4.9-kb EcoRI-HindIII tsr-disrupted mitA fragment from pDHS2001. As expected, the 4.0-kb SphI-hybridized band in the wild-type strain was shifted to 4.9 kb in MV100, whereas the 3.8-kb BamHI hybridization band in the wild type was converted to two bands (2.2 and 2.5 kb) in the mutant (Fig. 5B).
FIG. 5.
Southern blot analysis of the mitA mutant strain MV100. (A) Construction of mitA disruption mutant and restriction map of the wild type and mitA disruption mutant showing expected band sizes in the Southern blot. The maps are not drawn to scale. (B) S. lavendulae genomic DNAs from the wild type (lanes 1 and 2) and the double-crossover mutant MV100 (lanes 3 and 4) were digested with BamHI (lanes 1 and 3) and SphI (lanes 2 and 4), respectively. The 4.9-kb EcoRI-HindIII fragment from pDHS2001 containing tsr-disrupted mitA was used as the probe.
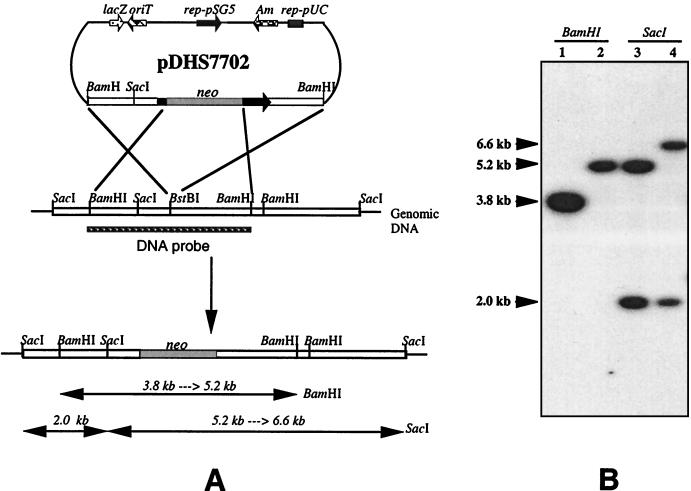
The mitB gene was disrupted by inserting a neomycin resistance gene (aphII) into the BstBI site (located at the 5′ end of mitB) (Fig. 6A). Transconjugants were selected on kanamycin-apramycin plates, and a double-crossover mutant strain (MM101) with a kanamycin-resistant, apramycin-sensitive phenotype was identified and subsequently confirmed by Southern blot hybridization. As expected, the 3.8-kb BamHI hybridization band in wild-type S. lavendulae was shifted to 5.2 kb in MM101, whereas a 5.2-kb SacI hybridization band was shifted to 6.6 kb (Fig. 6B).
FIG. 6.
(A) Construction of mitB disruption mutant and restriction map of the wild type and mitB disruption mutant showing expected band sizes in the Southern blot. (B) Southern blot analysis of mitB mutant MM101. S. lavendulae genomic DNAs from the wild type (lanes 1 and 3) and mitB mutant MM101 (lanes 2 and 4) were digested with BamHI (lanes 1 and 2) and SacI (lanes 3 and 4). The DNA probe was the 3.8-kb BamHI fragment insert from pDHS7601. (B) Southern blot hybridization of S. lavendulae genomic DNA of the double-crossover disruption mutant showing the expected hybridization bands.
mitA- and mitB-disrupted strains (MV100 and MM101) are blocked in MC biosynthesis.
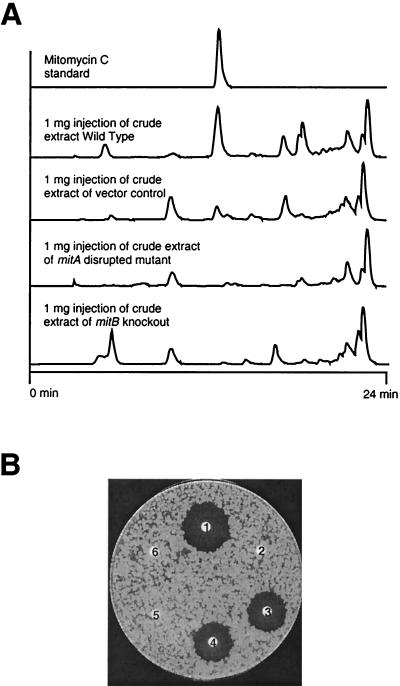
The growth characteristics and morphologies of MV100 and MM101 in liquid medium and on agar plates were identical to those of wild-type S. lavendulae. HPLC was used to quantify production of MC in MV100 and MM101 (Fig. 7A), and culture extracts were used in a biological assay to test for the presence of the drug (Fig. 7B). Injection of 1 mg of wild-type S. lavendulae culture extract gave a peak in the HPLC that eluted with the same retention time as the MC standard. Upon injection of 1 mg of culture extract from the mitA- or mitB-disrupted strains (MV100 and MM101), no MC peak was observed. To corroborate the lack of production of MC, the HPLC eluant obtained from the MV100 culture extracts was collected over the retention time range determined for MC. This eluant completely lacked biological activity against B. subtilis (the MC target strain), while the fraction collected from the same retention time region of wild-type S. lavendulae and the vector control strain culture extracts showed substantial levels of biological activity (Fig. 7B).
FIG. 7.
Chemical analysis and biological activities of extracts from S. lavendulae wild-type and mutant strains. (A) HPLC analysis of authentic mitomycin C standard, mitomycin C production in the wild-type S. lavendulae, wild type with vector control, and mitA and mitB disruption mutants of S. lavendulae. For each analysis, 1 mg of crude extract was injected; 1 μg of MC was injected as a standard. (B) B. subtilis bioassay of MC production in mitA disruption mutant strain of S. lavendulae. Filter discs: 1, 100-μg injection of wild type (collected from 12.5 to 13.5 min); 2, 100-μg injection of mitA disruption mutant (collected from 12.5 to 13.5 min); 3, 100-μg injection of the wild type containing the vector (collected from 12.5 to 13.5 min); 4, 1 μg of MC collected from HPLC from 12.5 to 13.5 min; 5, Tris buffer negative control; 6) methanol solvent negative control.
It is important to note that the presence of the vector pKC1139 in S. lavendulae reduced the percentage of MC in the total crude extract while simultaneously increasing the total amount of material extractable by ethyl acetate. The combination of these two effects reduces the absolute amount of MC by approximately 25% in the vector control culture crude extract compared to that in the wild-type crude extract.
Exogenous AHBA can restore MC production in the MC-deficient MitA K191A mutant.
Although complementation of MV100 (the mitA insertional disruptant) was attempted by providing exogenous AHBA in the culture medium, MC production was not restored as measured by HPLC or biological assay. A polar effect on genes downstream of tsr-disrupted mitA in MV100 appeared likely, since supplying mitA in trans on a medium-copy-number plasmid (MV103) also failed to restore MC production. Therefore, site-directed mutagenesis was employed to generate a MitA K191A mutant, resulting in strain MV102. Kim et al. had demonstrated that the AHBA synthase from A. mediterranei is PLP dependent and catalyzes the aromatization of aminoDHS (36). Thus, the nitrogen of conserved lysine 191 is supposed to form a Schiff base with the PLP cofactor. Replacement of lysine 191 with alanine prevents binding of the cofactor and eliminates enzymatic activity. Replacement of the AGG encoding lysine 191 in wild-type S. lavendulae with a GCC codon in MV102 was confirmed by nucleotide sequence analysis. As expected, MV102 did not produce MC; however, when the culture medium was supplemented with exogenous AHBA, MC production was restored, as determined by MS ([M+Na]+ = 357), HPLC, and TLC analysis (Table 2).
TABLE 2.
Complementation results with or without AHBA
| S. lavendulae strain | MC productiona
|
|
|---|---|---|
| With AHBA | Without AHBA | |
| Wild type | + | + |
| MV100 | − | − |
| MV103 | − | − |
| MV102 | + | − |
+, production; −, no production.
DISCUSSION
An effective strategy for the identification of natural product biosynthetic gene clusters in actinomycetes has included cloning of antibiotic resistance genes followed by investigation of adjacent DNA for the presence of structural and regulatory genes (13, 20, 40, 57). Although linkage of antibiotic resistance and biosynthetic genes appears to be a general feature in procaryotes, a growing number of examples involve the existence of multiple-resistance loci that may be linked or unlinked to the biosynthetic gene cluster (16, 49, 51). The identification and characterization of two genetically unlinked resistance loci (4, 50) for MC created a dilemma for mounting an effective search for the MC biosynthetic gene cluster. However, the use of the AHBA synthase gene from A. mediterranei provided an effective probe for identifying cosmid clones bearing a linked MC resistance gene. Thus, the isolation of several cosmid clones from an S. lavendulae genomic DNA library that hybridized to both the A. mediterranei AHBA synthase gene and the S. lavendulae mrd gene indicated that the MC biosynthetic gene cluster resided on DNA adjacent to mrd. DNA sequence analysis of the 3.8-kb BamHI fragment revealed three ORFs whose deduced protein sequences corresponded to an AHBA synthase, a glycosyltransferase, and an lmbE-like product.
As determined by precursor feeding experiments, the mitosane core is formed through the condensation of AHBA and d-glucosamine (27). AHBA is thought to be derived from the ammoniated shikimate pathway from PEP and E4P, in which the last step from aminoDHS to AHBA is catalyzed by AHBA synthase (Fig. 1) (35, 36). Meanwhile, the reaction of attaching an activated sugar residue to a core compound is usually catalyzed by a group of enzymes called glycosyltransferases as specified by macrolide, glycopeptide antibiotic, and polysaccharide biosynthesis (31, 43, 52, 60). In principle, the condensation of AHBA with d-glucosamine can be initiated in two ways (Fig. 1). One would involve the formation of the C-8a–C-9 bond by an electrophilic aromatic alkylation or acylation. A second possibility would be formation of a Schiff base between the nitrogen of AHBA and the d-glucosamine C-1 aldehyde, followed by ring closure at C-8a–C-9. In either case, a C- or N-glycosyltransferase instead of an O-glycosyltransferase is expected. Although previously described glycosyltransferases display a high degree of sequence divergence (60), the mechanistic similarity with O-glycosyl transfer may suggest that mitB encodes a N-glycosyltransferase that initiates the formation of the mitosane system by linking glucosamine to AHBA. The mitA and mitB genes and their corresponding products are likely candidates to mediate formation of AHBA and the mitosane ring system, respectively. However, the possible function of the lmbE-like protein remains unclear, since its current role within the lincomycin biosynthetic pathway of Streptomyces lincolnensis is not known (44).
The involvement of AHBA synthase (encoded by mitA) and the putative glycosyltransferase (encoded by mitB) in MC biosynthesis was established by gene disruption to create mutants with MC biosynthesis blocked. This required development of a method to introduce DNA into S. lavendulae NRRL 2564, since the strain remains refractory to traditional Streptomyces protoplast- and electroporation-mediated transformation procedures. The modified protocol of Bierman et al. (12) was used to effect efficient conjugative transfer into S. lavendulae by using the E. coli-Streptomyces shuttle plasmid pKC1139. This result is significant because it has enabled the development of an effective system for analyzing in detail the genes involved in MC biosynthesis.
The function of mitA was probed by providing strains MV100 and MV102 with exogenous AHBA in the culture medium. Despite repeated attempts to complement MV100, MC production was not restored as measured by HPLC or biological assay. It is believed that insertion of the tsr gene into mitA resulted in disruption of biosynthetic genes immediately downstream, since supplying mitA in trans on a medium-copy-number plasmid also failed to restore MC production to MV100. This putative polar effect was eliminated by generating the MitA K191A mutant strain MV102. Providing exogenous AHBA to this mutant strain of S. lavendulae restored production of MC as shown by TLC, HPLC, and mass spectrometry. When MV102 was grown in the absence of AHBA there was no detectable production of MC. The ability of AHBA to complement the mutant MitA protein further supports the function of MitA as an AHBA synthase, as indicated by the database protein sequence alignment and previous studies of rifK (36).
Although our studies have focused on the identification of genes involved in the early steps of MC biosynthesis, further characterization of the genes and functions that mediate individual steps in the pathway will provide information required to understand more fully the details of gene regulation, molecular assembly, and cellular resistance for this important molecule.
ACKNOWLEDGMENTS
Yingqing Mao and Mustafa Varoglu contributed equally to this work.
We sincerely thank Heinz G. Floss, University of Washington, for providing the rifamycin AHBA synthase gene (rifK) probe.
This work was supported in part by the Biological Process Technology Institute Seed Grant Program, University of Minnesota (to D.H.S.). M.V. was the recipient of a Postdoctoral Fellowship from the National Cancer Institute (training grant CA09138).
REFERENCES
- 1.Alderson G, Ritchie D A, Cappellano C, Cool R H, Ivanova N M, Huddleston A S, Flaxman C S, Kristufek V, Lounes A. Physiology and genetics of antibiotic production and resistance. Res Microbiol. 1993;144:665–672. doi: 10.1016/0923-2508(93)90072-a. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Anderson M G, Kibby J J, Rickards R W, Rothschild J M. Biosynthesis of the mitomycin antibiotics from 3-amino-5-hydroxybenzoic acid. J Chem Soc Chem Commun. 1980;1980:1277–1278. [Google Scholar]
- 4.August P R, Flickinger M C, Sherman D H. Cloning and analysis of a locus (mcr) involved in mitomycin C resistance in Streptomyces lavendulae. J Bacteriol. 1994;176:4448–4454. doi: 10.1128/jb.176.14.4448-4454.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baltz R H. Genetic recombination in Streptomyces fradiae by protoplast fusion and cell regeneration. Dev Ind Microbiol. 1980;21:43–54. doi: 10.1099/00221287-107-1-93. [DOI] [PubMed] [Google Scholar]
- 6.Baltz R H, Hosted T J. Molecular genetic methods for improving secondary-metabolite production in actinomycetes. Trends Biotechnol. 1996;14:245–250. doi: 10.1016/0167-7799(96)10034-2. [DOI] [PubMed] [Google Scholar]
- 7.Becker A, Kleickmann A, Keller M, Arnold W, Puhler A. Identification and analysis of the Rhizobium meliloti exoAMONP genes involved in exopolysaccharide biosynthesis and mapping of promoters located on the exoHKLAMONP fragment. Mol Gen Genet. 1993;241:367–379. doi: 10.1007/BF00284690. [DOI] [PubMed] [Google Scholar]
- 8.Becker A M, Herlt A J, Hilton G L, Kibby J J, Rickards R W. 3-Amino-5-hydroxybenzoic acid in antibiotic biosynthesis. VI. Directed biosynthesis studies with ansamycin antibiotics. J Antibiot. 1983;36:1323–1328. doi: 10.7164/antibiotics.36.1323. [DOI] [PubMed] [Google Scholar]
- 9.Bennett E P, Hassan H, Clausen H. cDNA cloning and expression of a novel human UDP-N-acetyl-alpha-d-galactosamine. Polypeptide N-acetylgalactosaminyltransferase, GalNAc-t3. J Biol Chem. 1996;271:17006–17012. doi: 10.1074/jbc.271.29.17006. [DOI] [PubMed] [Google Scholar]
- 10.Berdy J. Are actinomycetes exhausted as a source of secondary metabolites? In: Debabov V, Dudnik Y, Danlienko V, editors. Proceedings of the 9th International Symposium on the Biology of Actinomycetes. Moscow, Russia: All-Russia Scientific Research Institute for Genetics and Selection of Industrial Microorganisms; 1995. pp. 13–14. [Google Scholar]
- 11.Bezanson G S, Vining L C. Studies on the biosynthesis of mitomycin C by Streptomyces verticillatus. Can J Biochem. 1971;49:911–918. doi: 10.1139/o71-131. [DOI] [PubMed] [Google Scholar]
- 12.Bierman M, Logan R, O’Brien K, Seno E T, Rao R N, Schoner B E. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene. 1992;116:43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- 13.Butler M J, Friend E J, Hunter I S, Kaczmarek F S, Sugden D A, Warren M. Molecular cloning of resistance genes and architecture of a linked gene cluster involved in biosynthesis of oxytetracycline by Streptomyces rimosus. Mol Gen Genet. 1989;215:231–238. doi: 10.1007/BF00339722. [DOI] [PubMed] [Google Scholar]
- 14.Chater K F. Genetic regulation of secondary metabolic pathways in Streptomyces. Ciba Found Symp. 1992;171:144–156. doi: 10.1002/9780470514344.ch9. [DOI] [PubMed] [Google Scholar]
- 15.Chiao J S, Xia T H, Mei B G, Jin Z K, Gu W L. Rifamycin SV and related ansamycins. In: Vining L C, Stuttard C, editors. Genetics and biochemistry of antibiotic production. Newton, Mass: Butterworth-Heinemann; 1995. pp. 477–498. [DOI] [PubMed] [Google Scholar]
- 16.Cundliffe E, Merson-Davies L A, Keleman G H. Aspects of tylosin production and resistance in Streptomyces fradiae. In: Baltz R H, Heyeman G D, Skatrud P L, editors. Industrial microorganisms: basic and applied molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 235–243. [Google Scholar]
- 17.Denis F, Brzezinski R. A versatile shuttle cosmid vector for use in Escherichia coli and actinomycetes. Gene. 1992;111:115–118. doi: 10.1016/0378-1119(92)90611-r. [DOI] [PubMed] [Google Scholar]
- 18.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewick P M. The biosynthesis of shikimate metabolites. Nat Prod Rep. 1995;12:579–607. doi: 10.1039/np9951200579. [DOI] [PubMed] [Google Scholar]
- 20.Donadio S, Staver M J, McAlpine J B, Swanson S J, Katz L. Modular organization of genes required for complex polyketide biosynthesis. Science. 1991;252:675–679. doi: 10.1126/science.2024119. [DOI] [PubMed] [Google Scholar]
- 21.Ghisalba O, Nuesch J. A genetic approach to the biosynthesis of the rifamycin-chromophore in Nocardia mediterranei. IV. Identification of 3-amino-5-hydroxybenzoic acid as a direct precursor of the seven-carbon amino starter-unit. J Antibiot. 1981;34:64–71. doi: 10.7164/antibiotics.34.64. [DOI] [PubMed] [Google Scholar]
- 22.Hata T, Sano Y, Sugawara R, Matsumae A, Kanamori K, Shima T, Hoshi T. Mitomycin, a new antibiotic from Streptomyces. J Antibiot Ser A. 1956;9:141–146. [PubMed] [Google Scholar]
- 23.Hatano K, Akiyama S, Asai M, Rickards R W. Biosynthetic origin of aminobenzenoid nucleus (C7N-unit) of ansamitocin, a group of novel maytansinoid antibiotics. J Antibiot. 1982;35:1415–1417. doi: 10.7164/antibiotics.35.1415. [DOI] [PubMed] [Google Scholar]
- 24.Henderson I C. Recent advances in the usage of mitomycin. Proceedings of a symposium. Hawaii, March 21–24, 1991. Oncology. 1993;1:1–83. doi: 10.1159/000227240. [DOI] [PubMed] [Google Scholar]
- 25.Hopwood D A. Genetic contributions to understanding polyketide synthases. Chem Rev. 1997;97:2465–2497. doi: 10.1021/cr960034i. [DOI] [PubMed] [Google Scholar]
- 26.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H S. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, United Kingdom: John Innes Institute; 1985. [Google Scholar]
- 27.Hornemann U. Biosynthesis of the mitomycins. In: Corcoran J W, editor. Biosynthesis. London, United Kingdom: The Chemical Society; 1981. pp. 295–312. [Google Scholar]
- 28.Hornemann U, Eggert J H. Utilization of the intact carbamoyl group of l-(NH2CO-13C,15N) citrulline in mitomycin biosynthesis by Streptomyces verticillatus. J Antibiot. 1975;28:841–843. doi: 10.7164/antibiotics.28.841. [DOI] [PubMed] [Google Scholar]
- 29.Hornemann Y, Kehrer J P, Nunez C S, Ranieri R L. d-Glucosamine and l-citrulline, precursors in mitomycin biosynthesis by Streptomyces verticillatus. J Am Chem Soc. 1974;96:320–322. doi: 10.1021/ja00808a087. [DOI] [PubMed] [Google Scholar]
- 30.Iyer V N, Szybalski W. Mitomycin or porfiromycin: chemical mechanism of activation and cross-linking of DNA. Science. 1964;145:55–56. doi: 10.1126/science.145.3627.55. [DOI] [PubMed] [Google Scholar]
- 31.Kahler C M, Carlson R W, Rahman M M, Martin L E, Stephens D S. Two glycosyltransferase genes, lgtF and rfaK, constitute the lipooligosaccharide ice (inner core extension) biosynthesis operon of Neisseria meningitidis. J Bacteriol. 1996;178:6677–6684. doi: 10.1128/jb.178.23.6677-6684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kibby J J, McDonald I A, Rickards R W. 3-Amino-5-hydroxybenzoic acid as a key intermediate in ansamycin and maytansinoid biosynthesis. J Chem Soc Chem Commun. 1980;1980:768–769. [Google Scholar]
- 33.Kibby J J, Rickards R W. The identification of 3-amino-5-hydroxybenzoic acid as a new natural aromatic amino acid. J Antibiot. 1981;34:605–607. doi: 10.7164/antibiotics.34.605. [DOI] [PubMed] [Google Scholar]
- 34.Kim C G, Kirschning A, Bergon P, Ahn Y, Wang J J, Shibuya M, Floss H G. Formation of 3-amino-5-hydroxybenzoic acid, the precursor of mC7N units in ansamycin antibiotics, by a new variant of the shikimate pathway. J Am Chem Soc. 1992;114:4941–4943. [Google Scholar]
- 35.Kim C G, Kirschning A, Bergon P, Zhou P, Su E, Sauerbrei B, Ning S, Ahn Y, Breuer M, Leistner E, Floss H G. Biosynthesis of 3-amino-5-hydroxybenzoic acid, the precursor of mC7N units in ansamycin antibiotics. J Am Chem Soc. 1996;118:7486–7491. [Google Scholar]
- 36.Kim C G, Yu T W, Frygle C B, Handa S, Floss H G. 3-Amino-5-hydroxybenzoic acid (AHBA) synthase, the terminal enzyme in the formation of the precursor of mC7N units in rifamycin and related antibiotics. J Biol Chem. 1998;273:6030–6040. doi: 10.1074/jbc.273.11.6030. [DOI] [PubMed] [Google Scholar]
- 37.Lee J P, Tsao S W, Chang C J, He X G, Floss H G. Biosynthesis of naphthomycin A in Streptomyces collinus. Can J Chem. 1994;72:182–187. [Google Scholar]
- 38.Martin J F. Clusters of genes for the biosynthesis of antibiotics: regulatory genes and overproduction of pharmaceuticals. J Ind Microbiol. 1992;9:73–90. doi: 10.1007/BF01569737. [DOI] [PubMed] [Google Scholar]
- 39.Mazodier P, Petter R, Thompson C. Intergeneric conjugation between Escherichia coli and Streptomyces species. J Bacteriol. 1989;171:3583–3585. doi: 10.1128/jb.171.6.3583-3585.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Motamedi H, Hutchinson C R. Cloning and heterologous expression of a gene cluster for the biosynthesis of tetracenomycin C, the anthracycline antitumor antibiotic of Streptomyces glaucescens. Proc Natl Acad Sci USA. 1987;84:4445–4449. doi: 10.1073/pnas.84.13.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muth G, Nussbaumer B, Wohlleben W, Publer A. A vector system with temperature-sensitive replication for gene disruption and mutational cloning in streptomycetes. Mol Gen Genet. 1989;219:341–348. [Google Scholar]
- 42.Nishikohri K, Fukui S. Biosynthesis of mitomycin in Streptomyces caespitosus. Relationship of intracellular vitamin B12 level to mitomycin synthesis and enzymatic methylation of a demethylated derivative of mitomycin. Eur J Appl Microbiol. 1975;2:129–145. [Google Scholar]
- 43.Otten S L, Liu X, Ferguson J, Hutchinson C R. Cloning and characterization of the Streptomyces peucetius dnrQS genes encoding a daunosamine biosynthesis enzyme and a glycosyl transferase involved in daunorubicin biosynthesis. J Bacteriol. 1995;177:6688–6692. doi: 10.1128/jb.177.22.6688-6692.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peschke U, Schmidt H, Zhang H Z, Piepersberg W. Molecular characterization of the lincomycin-production gene cluster of Streptomyces lincolnensis 78-11. Mol Microbiol. 1995;16:1137–1156. doi: 10.1111/j.1365-2958.1995.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 45.Piepersberg W. Pathway engineering in secondary metabolite-producing actinomycetes. Crit Rev Biotechnol. 1994;14:251–285. doi: 10.3109/07388554409079835. [DOI] [PubMed] [Google Scholar]
- 46.Potgieter M. Biosynthetic studies on geldanamycin and pactamycin. Ph.D. thesis. University of Illinois, Urbana; 1983. [Google Scholar]
- 47.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 48.Sartorelli A C, Hodnick W F, Belcourt M F, Tomasz M, Haffty B, Fischer J J, Rockwell S. Mitomycin C: a prototype bioreductive agent. Oncol Res. 1994;6:501–508. [PubMed] [Google Scholar]
- 49.Seno E T, Baltz R H. Structural organization and regulation of antibiotic biosynthesis and resistance genes in actinomycetes. In: Shapiro S, editor. Regulation of secondary metabolism in actinomycetes. Boca Raton, Fla: CRC Press; 1989. [Google Scholar]
- 50.Sheldon P J, Johnson D A, August P J, Liu H-W, Sherman D H. Characterization of a mitomycin-binding drug resistance mechanism from the producing organism, Streptomyces lavendulae. J Bacteriol. 1997;179:1796–1804. doi: 10.1128/jb.179.5.1796-1804.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith T M, Jiang Y F, Shipley P, Floss H G. The thiostrepton-resistance-encoding gene in Streptomyces laurentii is located within a cluster of ribosomal protein operons. Gene. 1995;164:137–142. doi: 10.1016/0378-1119(95)00442-9. [DOI] [PubMed] [Google Scholar]
- 52.Solenberg P J, Matsushima P, Stack D R, Wilkie S C, Thompson R C, Baltz R H. Production of hybrid glycopeptide antibiotics in vitro and in Streptomyces toyocaensis. Chem Biol. 1997;4:195–202. doi: 10.1016/s1074-5521(97)90288-x. [DOI] [PubMed] [Google Scholar]
- 53.Stackebrandt E, Woese C R. Towards a phylogeny of the actinomycetes and related organisms. Curr Microbiol. 1981;5:197–202. [Google Scholar]
- 54.Staley A L, Rinehart K L. Biosynthesis of the streptovaricins: 3-amino-5-hydroxybenzoic acid as a precursor to the meta-C7N unit. J Antibiot. 1991;44:218–224. doi: 10.7164/antibiotics.44.218. [DOI] [PubMed] [Google Scholar]
- 55.Tuan J S, Weber J M, Staver M J, Leung J O, Donadio S, Katz L. Cloning of the genes involved in erythromycin biosynthesis from Saccharopolyspora erythraea using a novel actinomycete-Escherichia coli cosmid. Gene. 1990;90:21–29. doi: 10.1016/0378-1119(90)90435-t. [DOI] [PubMed] [Google Scholar]
- 56.Turgay K, Marahiel M A. A general approach for identifying and cloning peptide synthetase genes. Peptide Res. 1994;7:238–241. [PubMed] [Google Scholar]
- 57.Vara J, Malpartida F, Hopwood D A, Jimenez A. Cloning and expression of a puromycin N-acetyl transferase gene from Streptomyces alboniger in Streptomyces lividans and Escherichia coli. Gene. 1985;33:197–206. doi: 10.1016/0378-1119(85)90094-0. [DOI] [PubMed] [Google Scholar]
- 58.Webb J S, Cosalich D B, Mowat T H, Patrick J B, Broschard R W, Meyor W E, Williams R P, Wolf C F, Fulmor W, Pidacks C, Lancaster J E. The structure of mitomycins A, B, and C and porfiromycin. Part I. J Am Chem Soc. 1962;84:3185–3188. [Google Scholar]
- 59.Wu T S, Duncan J, Tsao S W, Chang C J, Keller P J, Floss H G. Biosynthesis of the ansamycin antibiotic ansatrienin (mycotrienin) by Streptomyces collinus. J Nat Prod. 1987;50:108–118. doi: 10.1021/np50049a015. [DOI] [PubMed] [Google Scholar]
- 60.Yamazaki M, Thorne L, Mikolajczak M, Armentrout R W, Pollock T J. Linkage of genes essential for synthesis of a polysaccharide capsule in Sphingomonas strain S88. J Bacteriol. 1996;178:2676–2687. doi: 10.1128/jb.178.9.2676-2687.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]