Abstract
Background/Objective
Patients with an insulinoma, a type of pancreatic neuroendocrine tumor, typically present with fasting hypoglycemia but can rarely present exclusively with postprandial hypoglycemia.
Case Report
A 69-year-old man presented with episodes of postprandial blurry vision, sweating, and confusion for the last 2 years that were becoming more frequent over the last several weeks. Home blood glucose measurements revealed postprandial hypoglycemia (glucose level, 45-70 mg/dL), and symptoms were consistent with the Whipple triad. Continuous glucose monitoring revealed only postprandial hypoglycemia within 2 hours following meals. An outpatient fast was conducted with detectable insulin (6 μIU/mL) and C-peptide (2.0 ng/mL) levels with an elevated proinsulin (20.8 pmol/L) level when the serum blood glucose level dropped to 47 mg/dL (21 hours after the initiation of the fast). A computed tomography scan of the abdomen and pelvis showed a 1.6-cm hyperenhancing lesion in the distal body of the pancreas. He underwent endoscopic ultrasonography with fine-needle aspiration. Pathology revealed a low-grade, well-differentiated, neuroendocrine tumor with lymphovascular invasion and regional lymph node metastases, confirming the diagnosis of a pancreatic neuroendocrine tumor.
Discussion
Exclusive postprandial hypoglycemia is estimated to occur in 6% of the insulinomas. Patients with postprandial hypoglycemia may be initially managed as those with reactive hypoglycemia; however, this case highlights the importance of evaluating for an insulinoma in a patient who has failed treatment for reactive hypoglycemia. This case also demonstrates the importance of including proinsulin levels in that evaluation.
Conclusion
Pancreatic neuroendocrine tumor should be considered in postprandial hypoglycemia, even in the absence of fasting hypoglycemia. Measuring proinsulin levels is essential in the diagnostic workup of insulinoma causing hypoglycemia.
Key words: insulinoma, neuroendocrine tumor, postprandial hypoglycemia
Abbreviation: NET, neuroendocrine tumor
Highlights
-
•
Although insulin-secreting neuroendocrine tumors (NET) classically present with fasting hypoglycemia, they can occasionally present with postprandial hypoglycemia.
-
•
If insulin levels are low or normal, a proinsulin-producing NET should be suspected.
-
•
It is important to obtain both insulin and proinsulin levels when evaluating for an insulinoma.
Clinical Relevance
Our case is a unique presentation of pancreatic neuroendocrine tumors for two reasons: (1) hypoglycemia occurred postprandially, and (2) this was a proinsulin-producing tumor. For this reason, insulin and proinsulin levels should be obtained when insulinoma is being considered as a cause of postprandial hypoglycemia.
Introduction
Patients with an insulinoma, a type of pancreatic neuroendocrine tumor (NET), typically present with fasting hypoglycemia.1, 2, 3, 4, 5, 6, 7, 8, 9 Hypoglycemia is caused by inappropriate secretion of insulin and/or its precursors such as proinsulin. We present the case of a patient with metastatic proinsulin-secreting tumor presenting with exclusive postprandial hypoglycemia.
Case Report
A 69-year-old man with a medical history of hypertension and obstructive sleep apnea presented to his primary care physician with episodes of postprandial blurry vision and confusion that were occurring for the last 2 years. Although the symptoms began 2 years prior, they were becoming more frequent over the last several weeks and would occur 2 to 3 hours after eating. He was asymptomatic during the night. His primary care physician recommended that he begin to measure his blood glucose level when the symptoms would occur, using a home glucose monitor. Blood glucose values during these symptomatic episodes at home ranged from 30 to 55 mg/dL. His symptoms would improve with having juice or fruit, with blood sugar levels correcting to 60 to 80 mg/dL. He was then referred to the endocrinology department for further evaluation. At his first endocrinology consultation (performed as a telehealth appointment), he had a body mass index of 25.7 kg/m2 (obtained from prior visits) and normal skin color without pallor. He was alert and oriented, with coherent and linear thought process. He was breathing normally and did not appear to be in any distress. He was prescribed a continuous glucose monitor and counseled on dietary changes with a diagnosis of reactive hypoglycemia. Laboratory investigations performed at approximately 8:00 am showed normal adrenocorticotropic hormone and cortisol levels as well as normal renal and thyroid function. With a fasting blood glucose level of 97 mg/dL (reference range, 70-99 mg/dL), his insulin level returned at 7.8 μIU/mL (reference range, 2-21 μIU/mL), C-peptide level was 1.67 ng/mL (reference range, 1.1-4.4 ng/mL), and acetone levels were undetectable (reference undetectable) (Table 1). His proinsulin level was 39.1 pmol/L (reference value, ≤18.8 pmol/L).
Table 1.
Patient’s Initial Laboratory Values After His First Endocrinology Consultation
| Test | 8:30 am | Reference range |
|---|---|---|
| Glucose | 97 mg/dL | 70-99 mg/dL |
| Blood urea nitrogen | 19 mg/dL | 6-20 mg/dL |
| Creatinine | 0.78 mg/dL | 0.76-1.27 mg/dL |
| Sodium | 138 mEq/L | 134-144 mEq/L |
| Potassium | 4.6 mEq/L | 3.5-5.2 mEq/L |
| Chloride | 104 mEq/L | 96-106 mEq/L |
| Carbon dioxide | 26 mEq/L | 20-29 mEq/L |
| Calcium | 9.4 mg/L | 8.7-10.2 mg/dL |
| Acetone blood | None detected | None detected |
| Adrenocorticotropic hormone | 20 pg/mL | 6-50 pg/mL |
| Total cortisol | 13.4 μg/dL | 7-9 am: 4.0-22.0 μg/dL |
| Insulin | 7.8 μIU/mL | 2-21 μIU/ML |
| Proinsulin | 39.1 pmol/L | ≤18.8 pmol/L |
| C-peptide | 1.67 ng/mL | 1.1-4.4 ng/mL |
| Thyroid-stimulating hormone | 1.38 mIU/L | 0.350-5.500 mIU/mL |
| Free thyroxine | 1.0 ng/dL | 0.8-1.8 ng/dL |
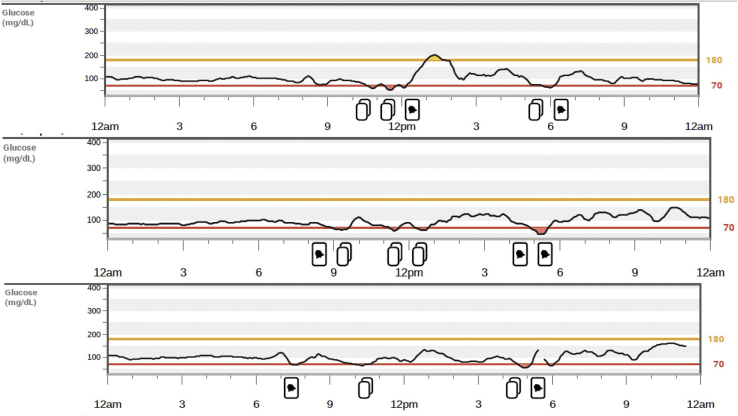
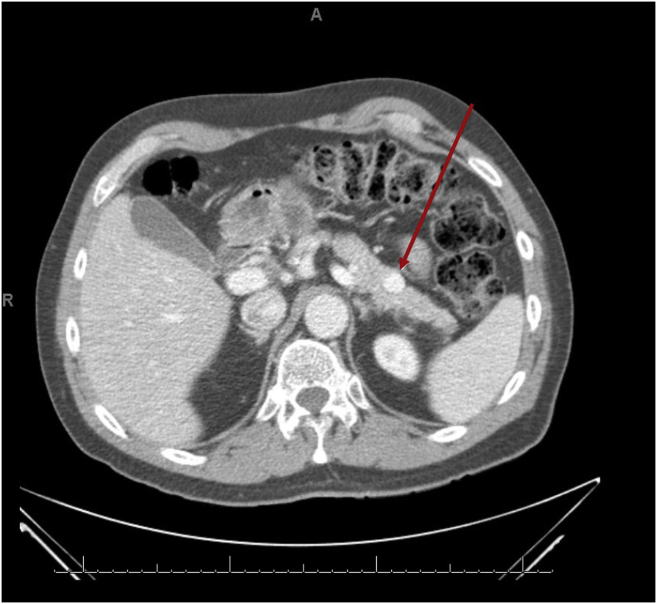
Continuous glucose monitoring over 14 days revealed only postprandial hypoglycemia within 1 to 2 hours after meals (Fig. 1). An oral glucose tolerance test was performed after the ingestion of 75 g of glucose, and it showed the lowest glucose level at 3 hours of 45 mg/dL and an insulin level of 17 μIU/mL (reference range, 2-21 μIU/mL). An outpatient fast was then conducted in the clinic, and when the serum blood glucose level dropped to 47 mg/dL (21 hours after the initiation of the fast), all appropriate laboratory investigations were performed, which revealed an insulin level of 6 μIU/mL, C-peptide level of 2.0 ng/mL, and proinsulin level of 20.8 pmol/L, supporting the presence of inappropriate insulin and proinsulin secretion (Table 2). A computed tomography scan of the abdomen and pelvis showed a 1.6-cm hyperenhancing lesion in the distal body of the pancreas (Fig. 2). He underwent endoscopic ultrasonography with fine-needle aspiration, which confirmed the presence of a pancreatic NET. Distal pancreatectomy and splenectomy were performed to resect the tumor, which led to resolution of his symptoms. Surgical histopathology showed the tumor positive for synaptophysin and chromogranin with a Ki-67 index of 2.3%, with lymphovascular invasion and 5 of 22 regional lymph nodes positive. He was diagnosed with a proinsulin-secreting metastatic NET. He has had no recurrent symptoms following surgery. After the surgery, continuous glucose monitoring showed an average blood glucose level of 112 mg/dL, with 99% of values in range without any measured hypoglycemia. At 6 months and 1 year following his diagnosis, repeat computed tomography scan and positron emission tomography scan did not show any residual disease. Prolactin and calcium levels were also evaluated to screen for other multiple endocrine neoplasia type 1–related tumors with the values of 8.9 ng/mL (reference range, 4-26 ng/mL) and 9.7 mg/dL (reference range, 8.5-10.5 mg/dL), respectively.
Fig. 1.
Excerpts from the G6 Continuous Glucose Monitoring System (Dexcom) reading demonstrating postprandial hypoglycemia.
Table 2.
Outpatient Fasting Challenge Laboratory Values
| Test | Result | Reference range |
|---|---|---|
| Glucose | 47 mg/dL | 70-99 mg/dL |
| Insulin | 6 μIU/mL | 2-21 μIU//mL |
| Proinsulin | 20.8 pmol/L | ≤8.0 pmol/L |
| C-peptide | 2.0 ng/mL | 1.1-4.4 ng/mL |
| Insulin autoantibody | <0.4 U/mL | <0.5 U/mL |
| Acetohexamide | Not detected | Not detected |
| Chlorpropamide | Not detected | Not detected |
| Glimepiride | Not detected | Not detected |
| Glipizide | Not detected | Not detected |
| Glyburide, serum | Not detected | Not detected |
| Nateglinide | Not detected | Not detected |
| Repaglinide | Not detected | Not detected |
| Tolazamide | Not detected | Not detected |
| Tolbutamide | Not detected | Not detected |
Fig. 2.
A computed tomography scan of the abdomen and pelvis with and without contrast showing a 1.6-cm hyperenhancing tumor in the distal body of the pancreas.
Discussion
Insulinomas are NETs that present with hypoglycemia due to inappropriate secretion of insulin and/or its precursors. Patients usually present with the Whipple triad, defined as symptoms of hypoglycemia, plasma glucose concentration of <55 mg/dL, and resolution of those symptoms with increased blood glucose levels.10 In patients with insulinoma, these symptoms classically occur in a fasting state; however, as exemplified by this case report, these symptoms may be present postprandially (ie, within 4 hours of a meal). Symptoms of hypoglycemia include autonomic and neuroglycopenic symptoms. Autonomic symptoms include palpitations, diaphoresis, tremors, and irritability; these generally occur when the plasma glucose levels are <60 mg/dL.2 Neuroglycopenic symptoms include dizziness, paresthesia, changes in vision, concentration difficulties, seizures, and loss of consciousness; these are generally seen with the plasma glucose levels of <50 mg/dL.2
In cases in which hypoglycemia occurs outside the medical setting, hypoglycemia is “induced” by either a 72-hour fast or a mixed-meal test under the supervision of health care providers, followed by drawing of venous blood for plasma glucose, insulin, C-peptide, proinsulin, and hydroxybutyrate concentrations and screening for oral hypoglycemic agents and insulin antibodies.3 Therefore, a 72-hour fast has long been considered the “gold standard” diagnostic test, with endogenous hyperinsulinism confirmed by the insulin levels of >3.0 μIU/mL (18 pmol/L), C-peptide levels of >0.6 ng/mL (0.2 nmol/L), and proinsulin levels of >5.0 pmol/L.1 If insulin levels are low or normal, a proinsulin-producing NET should be suspected.4,11, 12, 13 Once endogenous hyperinsulinism is confirmed, localization of inappropriate insulin production, usually from a NET, is then pursued. This is usually done with imaging, followed by acquisition of a tissue sample.5,14,15 When tissue sample is achieved, staining for chromogranin and synaptophysin confirm that tissue is composed of neuroendocrine cells, with more specific staining confirming pancreatic origin. Additionally, staining for Ki-67 and calculation of Ki-67 index allows for staging of the tumor, with a higher proliferation index having poorer prognosis.16,17 Management of insulinoma depends on the location of the tumor, the size of the tumor, and the presence of metastases. Surgical resection of the tumor is the first-line treatment for localized tumors.6 In metastatic or unresectable cases, a multimodal approach is considered with radiofrequency ablation, chemoembolization, and/or liver resection.6 Hypoglycemia related to the insulinoma can be managed with diazoxide and/or octreotide until definitive treatment is completed or if remission cannot be achieved.3
Although insulin-secreting NETs classically present with fasting hypoglycemia, there is growing evidence that postprandial hypoglycemia may be the only presenting symptom of an insulinoma. According to the retrospective data from Mayo Clinic, which reviewed data of 237 patients with insulinoma, fasting hypoglycemia occurred 73% of the time, exclusive postprandial hypoglycemia occurred 6% of the time (total of 13 patients), and both occurred 21% of the time.1 There was a male predominance in those with exclusive postprandial hypoglycemia. The diagnosis was made using 1 of the following 3 ways in this patient group: a mixed-meal test, laboratory testing during an episode of spontaneous hypoglycemia, or a 72-hour fast.1 Several case reports discuss exclusive postprandial hypoglycemia as a presentation for a pancreatic NET.7, 8, 9 Shreenivas et al7 reported the case of a 47-year-old woman with postprandial hypoglycemia with isolated insulinoma diagnosed by a mixed-meal test, with insulin being the predominant hormone during hypoglycemia. Madathil et al8 reported the case of a 60-year-old man with type 2 diabetes mellitus with elevated insulin levels at the time of postprandial hypoglycemia. Qian et al9 discussed postprandial hyperinsulinemic hypoglycemia in a 63-year-old man who underwent fundoplication; he was diagnosed with a mixed-meal test. To our knowledge, ours is the only case that describes exclusive postprandial hypoglycemia with a tumor that predominantly secretes proinsulin.
Wiesli et al18 described the cytoplasmic distribution of proinsulin in tumor cells obtained from a patient with postprandial hypoglycemia. They found that proinsulin is located in secretory granules that are released by calcium signaling as a response to glucose or glucagon, which may explain a theoretical mechanism for postprandial hypoglycemia in proinsulin-predominant insulinomas.18 However, this does not clinically seem to be the case for other proinsulin-secreting tumors that presented with fasting hypoglycemia.4,11,13 In a case series by Murtha et al reviewing 16 cases of proinsulinomas, all cases presented with fasting hypoglycemia, with the exception of 2 cases in which the presentation involved both fasting and postprandial hypoglycemia.4 Hence, our case was unique as there was only postprandial hypoglycemia. Similar to the patient in our case, the patient in the case reported by Gutelius et al13 was also initially managed as reactive hypoglycemia but hypoglycemia persisted.
Our case highlights the importance of considering insulinoma when evaluating postprandial hypoglycemia in the absence of fasting or overnight hypoglycemia in a patient with normal weight, without a history of bariatric surgery, and who has persistent postprandial hypoglycemia despite diet changes. A thorough investigation with a fasting test should be done in patients presenting with exclusive postprandial hypoglycemia with a clinical suspicion of a NET. In these patients, failure to measure proinsulin levels may lead to a missed or delayed diagnosis.
Disclosure
The authors have no multiplicity of interest to disclose.
References
- 1.Placzkowski K.A., Vella A., Thompson G.B., et al. Secular trends in the presentation and management of functioning insulinoma at the Mayo Clinic, 1987-2007. J Clin Endocrinol Metab. 2009;94(4):1069–1073. doi: 10.1210/jc.2008-2031. [DOI] [PubMed] [Google Scholar]
- 2.Mitrakou A., Ryan C., Veneman T., et al. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol. 1991;260(1 Pt 1):E67–E74. doi: 10.1152/ajpendo.1991.260.1.E67. [DOI] [PubMed] [Google Scholar]
- 3.Cryer P.E., Axelrod L., Grossman A.B., et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94(3):709–728. doi: 10.1210/jc.2008-1410. [DOI] [PubMed] [Google Scholar]
- 4.Murtha T.D., Lupsa B.C., Majumdar S., Jain D., Salem R.R. A systematic review of proinsulin-secreting pancreatic neuroendocrine tumors. J Gastrointest Surg. 2017;21(8):1335–1341. doi: 10.1007/s11605-017-3428-8. [DOI] [PubMed] [Google Scholar]
- 5.Virgolini I., Traub-Weidinger T., Decristoforo C. Nuclear medicine in the detection and management of pancreatic islet-cell tumours. Best Pract Res Clin Endocrinol Metab. 2005;19(2):213–227. doi: 10.1016/j.beem.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Okabayashi T., Shima Y., Sumiyoshi T., et al. Diagnosis and management of insulinoma. World J Gastroenterol. 2013;19(6):829–837. doi: 10.3748/wjg.v19.i6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shreenivas A.V., Leung V. A rare case of insulinoma presenting with postprandial hypoglycemia. Am J Case Rep. 2014;15:488–491. doi: 10.12659/AJCR.891336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madathil A., Weaver J. Insulinoma presenting as postprandial hypoglycaemia. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.07.2011.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian S.Y., Hare M.J.L., Pham A., Topliss D.J. Insulinoma presenting with post-prandial hypoglycaemia following fundoplication. Endocrinol Diabetes Metab Case Rep. 2018;2018(1):17–0131. doi: 10.1530/EDM-17-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Service F.J. Diagnostic approach to adults with hypoglycemic disorders. Endocrinol Metab Clin North Am. 1999;28(3):519–532.vi. doi: 10.1016/s0889-8529(05)70086-4. [DOI] [PubMed] [Google Scholar]
- 11.Alsever R.N., Roberts J.P., Gerber J.G., Mako M.E., Rubenstein A.H. Insulinoma with low circulating insulin levels: the diagnostic value of proinsulin measurements. Ann Intern Med. 1975;82(3):347–350. doi: 10.7326/0003-4819-82-3-347. [DOI] [PubMed] [Google Scholar]
- 12.Hiura A., Kim E.C., Ikahara T., et al. Insulinoma with hyperproinsulinemia during hypoglycemia and loss of expression of vacuolar-type H(+)-ATPase (V-ATPase) in the tumor tissue. Int J Pancreatol. 1999;25(1):11–16. doi: 10.1385/IJGC:25:1:11. [DOI] [PubMed] [Google Scholar]
- 13.Gutelius B.J., Korytkowski M.T., Carty S.E., Hamad G.G. Diagnosis and minimally invasive resection of an insulinoma: report of an unusual case and review of the literature. Am Surg. 2007;73(5):520–524. [PubMed] [Google Scholar]
- 14.Noone T.C., Hosey J., Firat Z., Semelka R.C. Imaging and localization of islet-cell tumours of the pancreas on CT and MRI. Best Pract Res Clin Endocrinol Metab. 2005;19(2):195–211. doi: 10.1016/j.beem.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Kittah N.E., Vella A. Management of endocrine disease: pathogenesis and management of hypoglycemia. Eur J Endocrinol. 2017;177(1):R37–R47. doi: 10.1530/EJE-16-1062. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton N.A., Liu T.C., Cavatiao A., et al. Ki-67 predicts disease recurrence and poor prognosis in pancreatic neuroendocrine neoplasms. Surgery. 2012;152(1):107–113. doi: 10.1016/j.surg.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf P., Winhofer Y., Smajis S., et al. Clinical presentation in insulinoma predicts histopathological tumour characteristics. Clin Endocrinol (Oxf) 2015;83(1):67–71. doi: 10.1111/cen.12777. [DOI] [PubMed] [Google Scholar]
- 18.Wiesli P., Schmid C., Perren A., Pfammatter T., Spinas G.A., Keller U. Hypoglycemia in response to glucose and glucagon in insulinoma patients with a negative prolonged fast: functional and morphological properties. J Endocrinol Invest. 2004;27(9):832–838. doi: 10.1007/BF03346277. [DOI] [PubMed] [Google Scholar]