Abstract
Despite an aggressive standard of care involving radiation therapy, temozolomide-based chemotherapy, and surgical resection, glioblastoma multiforme (GBM) continues to exhibit very high recurrence and mortality rates partly due to the highly plastic and heterogenous nature of the tumor. In recent years, activation of the immune system has emerged as a promising strategy in cancer therapies. However, despite recent successes in other fields, immunotherapeutic approaches continue to encounter challenges in GBM. In this review, we first discuss immunotherapies targeting the most well-studied immune checkpoint proteins, CTLA-4 and PD-1, followed by discussions on therapies targeting immune-stimulatory molecules and secreted metabolic enzymes. Finally, we address the major challenges with immunotherapy in GBM and the potential for combination and neoadjuvant immunotherapies to tip the scales in the fight against glioblastoma
Keywords: Checkpoint proteins, Immunotherpay, Glioblastoma
Introduction
With the introduction of immunomodulators targeted to T cell checkpoint proteins, immunotherapy has been revitalized as a cancer treatment in the past couple of decades. The focus first shifted to immunotherapy as an effective treatment with the introduction of inhibitors of negative immune regulators, primarily CTLA-4 and PD-1. The long-term impact of these immunomodulators on the progression of cancer became imminent with the awarding of the 2018 Nobel Prize to James P. Allison and Tasuku Honjo who both independently discovered strategies for inhibiting negative immune regulators that could be used in the treatment of cancer.1,2 By creating ICIs (immune checkpoint inhibitors) that were monoclonal antibodies against CTLA-4 and PD-1, the “brakes” to T cell activation could be removed allowing for cancer cells to be attacked and thereby showed promising results for future curative treatments. Proteins such as TIM3 and LAG3 were also found to have cancer immunotherapy potential through regulation of T cell signaling, along with a myriad of other negative regulatory checkpoint proteins.3 With the establishment of the therapeutic potential of ICIs, the field started to branch into other regulators of T cell function in relation to cancer progression. Rather than inhibiting the negative immunomodulators of T cells, there was a shift to also focus on the co-stimulatory signaling on T cells and whether agonists of these signal cascades could cause similar or enhanced effects of the ICIs. Focus on costimulatory molecules such as OX40 and ICOS expanded the potential of T cell regulatory immunotherapy through not only ICIs but also through agonists of positive T cell regulatory signal cascades.4 The final significant breakthrough of the field was the discovery of how secreted metabolic enzymes, such as IDO and arginase, could impact the regulation of TILs (tumor infiltrating lymphocytes) and how these could change the immune response to the host cancer progression.
One major topic of discussion in the immunotherapy field is the fact that although these therapies have had clinical success, that triumph does not apply to all subsets of cancer. One type of cancer that has been notoriously difficult to treat is glioblastoma (GBM), the most aggressive brain cancer in adult patients. Patients diagnosed with GBM undergo surgical resection and chemo/radiotherapy, but almost uniformly recur.5 Also, because GBM is heterogeneous, the recurrent tumor is resistant to chemotherapy which eventually leads to most patients succumbing to cancer progression. Since there is no current effective treatment to halt recurrence or treat the primary tumor, there was a lot of hope early on that immunotherapy may be the path to treating GBM. However, with recent clinical trials for CTLA-4 and PD-1 inhibitors resulting in little to no therapeutic effect as presented in Table 1, questions remain regarding whether immunotherapy has a future in GBM. If so, we need to understand the path forward of understanding the mechanistic reasoning of such failure in order to create effective immunotherapy against GBM. In this review, we cover the checkpoint proteins, and their impact on the three major subsections of T cell immune regulation – negative immune costimulatory signaling, and secreted metabolic enzymes within GBM as we aim to identify the future role of immunotherapy for GBM.
Table 1.
Ongoing and completed clinical trials of immune checkpoint inhibitor combined with other treatment modalities.
| Trial ID | Intervention | Immune Checkpoint-related Target | Antagonist or agonist | Phase | Outcome |
|---|---|---|---|---|---|
| NCT02667587 | TMZ + Nivolumab + RT | PD1 | Antagonist | 3 | Ongoing |
| NCT02617589 | Nivolumab + TMZ + RT | PD1 | Antagonist | 3 | No sig. difference in median OS Ongoing |
| NCT02017717 | Nivolumab + Bevacizumab + Ipilimumab | CTLA4, PD1 | Antagonist | 3 | Ongoing |
| NCT03347097 | Transgenic TILs | PD1 | Antagonist | 1 | Ongoing |
| NCT04888611 | Camrelizumab + GSC-DCV | PD1 | Antagonist | 2 | Ongoing |
| NCT04583020 | Camrelizumab + radiation + Temozolomide | PD1 | Antagonist | 2 | Ongoing |
| NCT04225039 | INCMGA00012 + INCAGN01876 + SRS | PD1 | Antagonist | 2 | Ongoing |
| NCT04977375 | Pembrolizumab + SRS + Resection | PD1 | Antagonist | 1b/2 | Ongoing |
| NCT03961971 | SRS + MBG453 | PD1 | Antagonist | 1 | Ongoing |
| NCT04656535 | AB122 + AB154 | PD1 | Antagonist | 1 | Ongoing |
| NCT03726515 | CART-EGFRvIII T cells + Pembrolizumab | PD1 | Antagonist | 1 | Completed but not yet published |
| NCT04201873 | Pembrolizumab + ALT-DC vaccine | PD1 | Antagonist | 1 | Ongoing |
| NCT02852655 | MK-3475 | PD1 | Antagonist | 1 | Ongoing |
| NCT04013672 | Pembrolizumab + SurVaxM | PD1 | Antagonist | 2 | Ongoing |
| NCT03743662 | Nivolumab + Bevacizumab + RT | PD1 | Antagonist | 2 | Ongoing |
| NCT02658981 | BMS-986016, Urelumab + Nivolumab | PD1 | Antagonist | 1 | Ongoing |
| NCT04606316 | Nivolumab + Ipilimumab + Surgery | CTLA4, PD1 | Antagonist | 1 | Ongoing |
| NCT03233152 | Nivolumab + Ipilimumab | CLTA4, PD1 | Antagonist | 1 | Ongoing |
| NCT02529072 | Nivolumab until resection (group 1) vs nivolumab + DC vaccine until resection (group 2) | PD1 | Antagonist | 1 | Approx. 7 month improvement in median OS for group 2 |
| NCT02287428 | RT + Personalized NeoAntigen Vax + Pembrolizumab + Temozolomide | PD1 | Antagonist | 1 | Ongoing |
| NCT02335918 | Varlilumab + nivolumab | PD1 | Antagonist | 2 | Ongoing |
| NCT03493932 | Nivolumab + BMS-986016 | PD1 | Antagonist | 1 | Ongoing |
| NCT03899857 | Pembrolizumab | PD1 | Antagonist | 2 | Ongoing |
| NCT02968940 | Avelumab + Hypofractionated RT | PD1 | Antagonist | 2 | Ongoing |
| NCT03491683 | INO-5401 + INO-9012 + Cemiplimab + Temozolomide + RT | PD1 | Antagonist | 2 | Ongoing |
| NCT02798406 | DNX-2401 + Pembrolizumab | PD1 | Antagonist | 2 | Ongoing |
| NCT03718767 | Nivolumab | PD1 | Antagonist | 2 | Ongoing |
| NCT03576612 | AdV-tk + Valacyclovir + RT + TMZ + Nivolumab | PD1 | Antagonist | 1 | Ongoing |
| NCT04429542 | BCA101 + Pembrolizumab | PD1 | Antagonist | 1 | Ongoing |
| NCT03797326 | Pembrolizumab + Lenvatinib | PD1 | Antagonist | 2 | Ongoing |
| NCT04704154 | Regoafenib + Nivolumab | PD1 | Antagonist | 2 | Ongoing |
| NCT02794883 | Durvalumab + Tremelimumab | CTLA4, PD1 | Antagonist | 2 | Ongoing |
| NCT04145115 | Ipilimumab + Nivolumab | CTLA4, PD1 | Antagonist | 2 | Ongoing |
| NCT04003649 | IL13Ralpha2-CRT T cells + Ipilimumab + Nivolumab | CTLA4, PD1 | Antagonist | 1 | Ongoing |
| NCT02311920 | Ipilimumab + Nivolumab + TMZ | CTLA4, PD1 | Antagonist | 1 | Ongoing |
| NCT04323046 | Ipilimumab + Nivolumab | CTLA4, PD1 | Antagonist | 1 | Ongoing |
| NCT04047706 | BMS-986205 + Nivolumab + RT + TMZ | IDO1, PD1 | Antagonist | 1 | Ongoing |
| NCT02052648 | Indoximod + TMZ + Bevacizumab + Stereotactic RT | IDO | Antagonist | 1 | 3.7 mo. increase in median OS |
| NCT04049669 | Indoximod + TMZ + Cyclophosphamide + Etoposide + Lomustine + RT | IDO | Antagonist | 2 | Ongoing |
ICI
The primary method of cancer immunotherapy currently used is via immune checkpoint inhibition. With the first use of checkpoint inhibitors against CTLA-4 and PD-1 in melanoma, immunotherapy expanded the possibilities of effective therapies in cancer.1,2 Although the use of these checkpoint inhibitors has had success in a variety of other cancers, this effectiveness shows minimal success in the context of GBM. Here, we summarize the most well-studied checkpoint inhibitors – against CTLA-4 and PD-1 – and how they are used in GBM therapy as well as the potential for these antagonists to lead to a successful GBM treatment.
The discovery and role of CTLA-4 in cancer immunotherapy
Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), was first discovered as a member of the B7/CD28 immunoglobulin superfamily in 1987.6 In 1991, it was then identified as a novel receptor for CD80 and CD86.7 However, for several years the role of CTLA-4 in the immune system remained controversial. One theory, supported by data showing that anti-CTLA-4 antibodies enhanced CD28-stimulation, argued that CTLA-4 promoted T cell proliferation.8 Another concept argued that CTLA-4 inhibits T cell proliferation by cross-linking with the T cell receptor (TCR) and CD28,9 Ultimately, the controversy was resolved by Tak Mak and Arlene Sharpe when they proved the inhibitory function of CTLA-4 through the use of deficient mouse strains which rapidly developed severe lymphoproliferative diseases, suggesting the presence of CTLA-4 does have an anti-proliferative effect on T cells.10,11
CTLA-4 is expressed by many different T cell compartments including T regulatory cells (Tregs) and activated CD4+ T cells. Exhausted T cells are also often characterized by CTLA-4 overexpression. CTLA-4 primarily located in intracellular vesicles awaiting its induction by TCR stimulation and exhibits a high degree of homology to the T cell stimulatory protein CD28.7,12 However, CTLA-4 binding to CD80 and CD86 is significantly more favorable than CD28 binding.13 Thus, it is currently theorized that CTLA-4 exerts its anti-proliferative effects on T cells by outcompeting CD28. While the exact molecular basis for CTLA-4 signaling still under investigation, it has been proposed that CTLA-4 may also remove CD80 and CD86 from the cell surfaces of antigen-presenting cells via trans-endocytosis, further reducing the frequency of stimulatory CD28-CD80/86 interactions.14
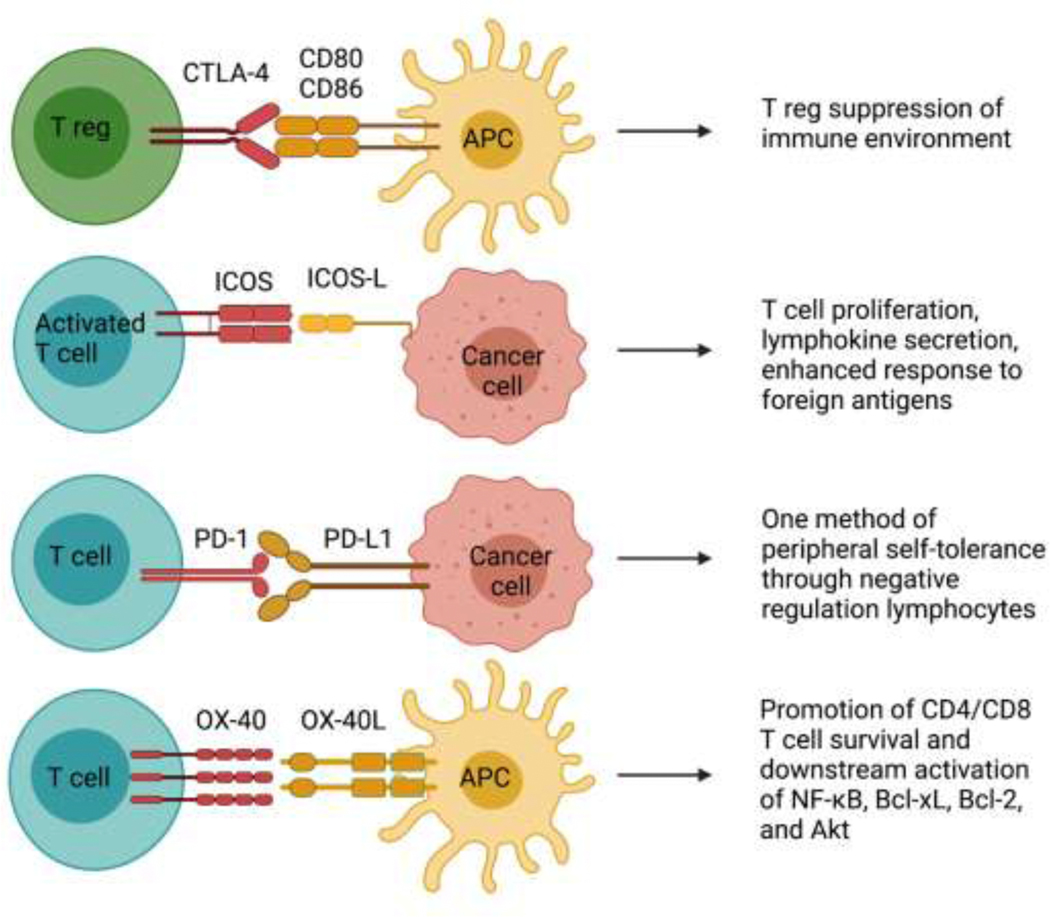
It has been demonstrated that CTLA-4 is also constitutively expressed on T regulatory cells (Tregs), activated effector T cells and a variety of nonlymphoid cells such as placental fibroblasts, muscle cells, and monocytes.15,16 CTLA-4 primarily functions within the Treg compartment seems to be integral for Treg regulation of suppression (Figure 1).17–19 Although the expression of a checkpoint inhibitor protein such as CTLA-4 on effector T cells may appear contradictory, it has been shown that CTLA-4 plays an inhibitory role on effector T cells and on Tregs, perhaps as an evolved defense against lymphoproliferative disorders.21 In healthy immune systems, CTLA-4 thus plays an important role in preventing autoimmunity and maintaining immune response within physiologically sustainable levels.
Figure 1. Immune checkpoint inhibition of CTLA-4, ICOS, PD1 and OX-40 in GBM.
Multiple signals, both inhibitory and stimulatory, modulate the activation of T cells by GBM cells or antigen-presenting cells.
In cancer, CTLA-4 expression is hijacked by tumor cells to create an immunosuppressive microenvironment to facilitate their uncontrol growth. CTLA-4 is constitutively expressed in tumor cell lines in breast, colon, renal, lung, ovarian, uterine, and bladder carcinomas, as well as melanomas, osteosarcomas, and neuroblastomas.16 The widespread expression of CTLA-4 in cancer makes it a very attractive target for immunotherapies.
In recent years, there have been a few breakthroughs with anti-CTLA-4 immunotherapies. Ipilimumab, a monoclonal antibody, is the first FDA-approved CTLA-4 blocker for the treatment of metastatic melanoma.20–22 Tremelimumab, another anti-CTLA-4 monoclonal antibody, is currently in clinical trials for the treatment of many cancers including advanced hepatocellular carcinoma, head and neck squamous cell carcinoma, small-cell lung cancer, and mesothelioma.23–26 Unfortunately, in both head and neck squamous cell carcinoma (HNSCC) and mesothelioma, despite some evidence of upregulated immune responses median overall safety of patients was not improved compared to placebos.27,28
In the glioblastoma space, preclinical experiments with CTLA-4 inhibiting antibodies show promise in murine models.27 Additionally, combination therapies – such as those targeting IDO, CTLA-4, and PD-L1 simultaneously – led to a decrease in the number of Treg cells in mouse glioma models; however, the overall survival benefit remained minimal.28 This may be in part because large molecules such as antibodies are generally incapable of crossing the blood brain barrier (BBB) on their own.29 It has been shown in GBM that there is a significant difference in therapy efficacy when injecting monotherapies directly into the tumor core as opposed to peripheral antibody treatment.30 Various approaches have been explored to overcome the BBB issue. A BBB permeable nanoparticles with poly(β-L-malic acid) scaffolds covalently bound to aCTLA-4 or a-PD-1 molecules have been developed to improve CTLA-4 inhibitor delivery in glioblastoma models. The 2019 study by Galstyan et al. demonstrates the efficacy of these nano immunoconjugates (NICs) in penetrating the blood brain barrier, stimulating T cell and macrophage responses, the downregulation of FoxP3+ cells in tumors, and, perhaps most promisingly, a 40% increase in the median survival of mice bearing GBM GL261 tumors.31 Another exciting innovation by those working to conquer CNS pathologies is the use of focused ultrasound to transiently disrupt the BBB by locally detaching tight junctions on the capillary wall without neuronal damage.32 Despite these advances, however, the BBB remains one of the paramount challenges to improving the delivery of both conventional and immunotherapeutic drugs to brain pathologies such as GBM.
PD-1 (Programmed cell death protein 1)
The PD-1 gene was first characterized by Tasuku Honjo and colleagues during their attempt to find a gene associated with programmed cell death in immature T lymphocytes.2 However, what they found was that the PD-1/PD-L1 pathway, despite its name, actually mediated peripheral self-tolerance through the negative regulation of lymphocytes (Figure 1).35–38 This coincidental discovery would lead to the later revolutionary finding that this pathway allowed for cancer cells to evade immune recognition through the negative regulation of T cells.36
The current understanding of the PD-1/PD-L1 pathway is that the finding supports its involvement in suppressing only a limited number of immune responses that PD-1 knockout mice develop mild autoimmune diseases only a year after birth.33 Although it is thought that PD-1 functions to promote peripheral self-tolerance, the exact mechanism of this process remains unknown. A likely scenario seems to be the accumulation of PD-L1 on aged cells that have acquired slight somatic mutations over time to signal that the cell should still be recognized as self.37 By binding to PD-1 on T cells and signaling a blockade of their activation, the host can evade activation of the immune system in response to slightly altered aged cells, a hypothesis supported by the observation that autoimmunity only occurs in older PD-1 knockout mice.33 However, CD80 – a marker of activation of antigen presenting cells (APCs) – has been shown to inactivate this pathway, suggesting that the PD-1/PD-L1 system may not be designed to function for peripheral self-tolerance in apparent non-self-recognition, but rather as one designed to limit autoimmunity.38
After the discovery and characterization of PD-1 and the associated PD-L1, there was great interest in how a PD-1 blockade could be enhanced as a curative treatment of cancer. Although PD-1 inhibition has shown success in some cancer subtypes, interestingly the clinical trials of nivolumab (anti-PD-1 inhibitor) in GBM showed no difference between the group treated with TMZ and radiation alone (Table 1).39 This lack of a result was less than expected considering there was some success in increasing the survivability of murine GBM models with some form of PD-1 blockade and highlighted the importance of the preclinical animal model.40–42 Following the failed monotherapy clinical trial, there has been a race to discover why monotherapy with anti-PD-1 is so ineffective in GBM. After profiling GBM patients treated with PD-1 inhibitors nivolumab or pembrolizumab, immunotherapy in GBM showed clear associations with changes that reflected the clonal evolution of the tumor.43 This suggested that even though GBM could initially be sensitive to anti-PD-1 therapy, the ability of GBM to adapt renders therapy essentially ineffective. The current suggestion for evaluating the effectiveness of checkpoint blockade, but mostly within PD-1/PD-1L specific blockade models, is by measuring the tumor mutational burden (TMB). Some suggest that high TMB in the context of anti-PD-1/PD-L1 monotherapy is a marker of the effective response to the immunotherapy.44,45 However, others have indicated that high TMB is not necessarily better.46,47
There was an investigation into the effectiveness of a PD-1 blockade in conjunction with other therapies to prevent tumor immune evasion, which shows promising murine model survival results.48–55 The timing of the ICI monotherapy in GBM has also been a new concept proposed to explain why anti-PD-1 therapy may have failed. Patients treated with neoadjuvant+adjuvant (before surgical resection and following) nivolumab or pembrolizumab showed increased survival, greater T cell expansion, and less cell cycling in the tumor compared to adjuvant therapy alone.56,57 This suggests that although previous clinical trials hint at the inefficacy of PD-1 immunotherapies in GBM, the combination of this therapy with other agonists or antagonists as well as the timing of the therapy could very well be the difference between ineffective and effective GBM immunotherapy.
Co-stimulatory
Although antagonists against immune inhibitory checkpoint pathways have primarily been the focus of most immunotherapy investigation for GBM, the use of agonists for co-stimulators molecules have been shown to elevate the immune response to GBM in the context of immune checkpoint inhibitors and independently. The use of co-stimulatory agonists has been more recently described as an effective therapy in combination with other immune checkpoint agonists or antagonists to achieve increased survival in GBM models, especially where the GBM models may be resistant to the previously described checkpoint inhibitor therapies. In our review, we focus on checkpoint co-stimulatory molecules ICOS and OX40, and the role agonists against these proteins have on GBM progression.
The discovery and role of OX40 in cancer immunotherapy
The tumor necrosis factor receptor superfamily member OX40 was first discovered in 1987 as a potential augmenter of T cell proliferation on rat lymphocytes that co-express the CD4 antigen.58 In 1994, its cognate ligand, OX40L, was then identified on EBV-transformed B-cell lines that were activated by PMA/ionomycin.59
OX40 is primarily expressed on activated CD4+ T cells, but has also been reported on memory and regulatory CD4+ T cells. OX40-deficient mice have reduced memory antibody responses.60 A 2007 study also found that, although OX40 can be expressed by both T effector cells and Foxp3+ Tregs, the stimulation of OX40 on Foxp3+ Tregs strongly represses their ability to suppress T effector cell proliferation. This indicating that OX40 is a potent regulator of immune responses.61 Constitutive expression of OX40 in murine models has been linked to the induction of inflammatory diseases such as interstitial pneumonia and inflammatory bowel disease.62 Furthermore, OX40L has been shown to increase the activity of T follicular helper cells and encourage the formation of systemic lupus erythematosus in humans.63 OX40/ OX40L has also been found to be higher in the colon and jejunum of patients with inflammatory bowel disease.64
OX40 is induced on activated CD4 and CD8 T cells following antigen stimulation and peaks from 1 – 5 days after initial stimulation. Signaling by TCR is generally sufficient to invoke OX40; however, CD28-B7 interactions have been shown to augment OX40 induction.65,66 Mechanistically, it is believed that OX40 does not actually enhance or promote initial activation or proliferation directly. Instead, it provides downstream signaling through members of the TNF receptor associated factor (TRAF) family that maintain late-stage proliferation and prolong the survival of effector T cells. More specifically, OX40 has been shown to activate NF-κB and promote downstream expression of Bcl-xL, Bcl-2, Akt, and contribute to in CD4+ and CD8+ T cells survival (Figure 1).67,68,69,70
OX40L is expressed on glioblastoma cell lines and connected to stronger antitumor adaptive immunity. Additionally, subcutaneous vaccinations with OX86, an anti-OX40 monoclonal antibody, in mouse glioma models to force the activation of OX40/OX40L signaling resulted in substantial survival benefits.71 In non-small cell lung cancer, high OX40 expression measured by immunohistochemistry was correlated with better overall survival independent of CD8, PD-L1, and ICOS expression.72 OX40 expression has also been found to be positively correlated with overall survival in gastric cancer and hepatocellular carcinomas.73,74
Given the importance of OX40 in antitumor immune responses, considerable work is being undertaken to develop OX40-agonistic immunotherapies. In a 2020 study, combination therapies involving PD-1 inhibitors and an OX40 agonist resulted in a very strong survival benefit in murine models of pancreatic cancer.75 However, in 2021 Phase I/IIa human trials involving a different OX40 agonist, BMS-986178, combination therapies with the PD-1 antagonist nivolumab and the CTLA-4 antagonist ipilimumab, did not result in enhanced efficacy beyond what was expected for nivolumab and ipilimumab alone.75,76 Another 2021 study, utilizing neoadjuvant anti-OX40 agonistic therapy, showed an increase in the number of TILs.76
While the need for significant breakthroughs in immunotherapies against glioma remains, a few studies provide a reason for hope. A 2018 study combining vaccination (GVAX, whole tumor cell vaccination) with irradiated granulocyte macrophage colony stimulating factor (GM-CSF) with an agonist OX40 antibody improved survival in murine models of glioma.77 The same group was able to further improve long-term survival in murine glioma models with triple combination immunotherapy combining GVAX (a whole tumor cell vaccination), an anti-OX40 agonist, and an anti-PD-1 antagonist.78 The combination of anti-OX40 with anti-PD-1 significant increased survival compared to monotherapy of anti-PD-1 alone.
ICOS (inducible T-cell costimulator)
One of the costimulatory proteins recently found to belong to the family of regulatory receptors on T cells is ICOS. This protein was found to be de novo induced on the surface of activated T cells. It functioned similarly to CD28 in that it enhanced T cell response to foreign antigens through the promotion of T cell proliferation, secretion of lymphokines, cell-to-cell interactions, and antibody secretion by B cells (Figure 1).79,80 Further investigation showed that this CD28 homolog was also critical for class switching through the CD40/CD40L pathway, as well necessary for IL-4 production and the prevention of certain inflammatory autoimmune development (Figure 1).80,81,82
Due to the apparent role of ICOS in the promotion of T cell activation and regulation of effector responses, it became a target of possible immunotherapy in the developing field of cancer treatment.83 Another use of ICOS has also been noted as a marker of immune activation when other immunotherapies are given.84 The connection of this function to effective cancer immunotherapy is that agonists for the ICOS/ICOSL pathway have been implicated in potentiating the effects of immune checkpoint blockade and shown promising results in recent clinical trials.85 Although the information regarding ICOS stimulation specifically in GBM is slim, its success in complementing other immune regulator agonists and antagonists shows an interesting future possibility for ICOS use in GBM combo therapy. One group saw that their glioma mouse model treated with Delta24-RGD (a tumor-selective adenovirus that increases PD-1 and ICOS expression on CD8+ T cells by mimicking viral infection) and anti-PD-1 antibody saw a significant increase in survival compared to Delta24-RGD treatment alone. Although they did not have endpoint survival analysis on mice treated with the anti-ICOS agonist, they saw there was no significant difference between the IFNγ production of splenocytes from glioma-bearing mice, the Delta24-RGD treatment and the Delta24-RGD+anti-ICOS treatment.86 This however does not rule out the possibility that the treatment of anti-ICOS could have heightened the effects of the other treatments in combo therapy. As the field develops into a more combination therapy-directed approach against GBM, it will be interesting to see how specifically anti-ICOS agonists fit into this scheme.
Secreted metabolic enzymes
A more recent development in cancer immunotherapy is the discovery and use of secreted metabolic enzymes that alter the function of surrounding lymphocytes. The function of these secreted enzymes involves the depletion of amino acids that are necessary for lymphocytes to differentiate and develop effector functions that aid in their control of the tumor. The focus of following section is primarily on IDO and arginase and the recent role these enzymes have been found to play in the alteration of the immune tumor microenvironment (TME).
The discovery and role of metabolic enzymes IDO and arginase in cancer immunotherapy
The discovery of the role these enzymes played in the TME came soon after the role of the amino acids they catabolize became well charecterized. IDO and arginase promote tumor growth by degrading amino acid tryptophan and promoting the arginine-ornithine-polyamine axis respectively.10,87 Tryptophan is known to be important for the differentiation and proliferation since T lymphocytes arrest when deprived of this amino acid.88,89 IDO is involved in the tryptophan catabolism as the enzyme of the rate-limiting conversion of tryptophan to kynurenine. The metabolite kynurenine and its associated degradation products have been shown to prevent tumor clearance, inhibit T cell-mediated antitumor immunity, and promote Treg accumulation.88,90,91 On the other hand, arginase, likely through polyamine production, leads to an inhibition of cytokine production, T cell proliferation, and promotion of an immunosuppressive environment that facilitates tumor growth in a variety of cancers including glioblastoma10,92,93
Given the importance of certain amino acids in T cell proliferation and effector function, there was the push to see if inhibition of enzymes that facilitate these pathways would reverse the immunosuppressive environment in cancer. IDO, which is constitutively expressed by most cancers, was first isolated as a drug target when its inhibition by 1-methyl-L-tryptophan (1MT) led to the rejection of IDO-expressing P815 tumor cells injected into P1A-immunized mice that were previously able to grow.94 This was directly related to the T cell environment since the growth of injected IDO-expressing tumor cells was equal between 1MT-treated and non-treated T cell-depleted mice.94 IDO is expressed in a large majority of GBM tumor samples, and a greater than 50% proportion of the tumor cells in these samples are also IDO-positive.94,95 Therefore, it is understandable that the inhibition of IDO in GBM mouse models has been associated with decreased Treg populations, decreased tryptophan consumption, and increased T cell mediated survival.28,96
Arginase is also expressed in human GBM, with the majority of expression being found within neutrophils.97. Arginase leads to downstream polyamine production which has been shown to drive myeloid cell survival by buffering intracellular pH to promote the immunosuppressive environment of glioblastoma.10 It is then understandable that inhibition of arginase by CB-1158 led to greater infiltration of CD8+ T cells and NK cells, inflammatory cytokines, and expression of interferon-inducible genes within a GBM model.98
The ability of these inhibitors to work clinically in GBM is still up for debate, as there are currently no clinical trials investigating IDO or arginase inhibition at this date. Overall, the pre-clinical evidence is promising, however, IDO and arginase inhibitor use in GBM models is limited in its characterization and most of the information regarding its inhibition is in the context of regulating other immune checkpoint proteins. There is also the observation that GBM does not have a limitation of amino acids, but rather an increase with tumor progression, which is counterintuitive to the possible role of IDO in GBM therapy.99
Future of checkpoint inhibitors in GBM
The overall understanding of immunotherapy in cancer treatment has been fast developing in recent years. Although the inhibitors and agonists that we have discussed have shown promising results in a variety of cancers to date, the ability of these same drugs to work in the context of GBM has been met with varying success. The treatment of GBM is notoriously difficult, with its heterogeneity leading to the development of resistance against most therapies. Although immunotherapy is a developing approach with wide success in other cancer subtypes, the immune environment of GBM is that of a “cold” tumor–containing few TILs. In this environment, the immunotherapy approach of altering T cell function and proliferation faces an additional challenge. With the highly anticipated GBM clinical trials of CTLA-4 and PD-1 inhibitors being met with failure (Table 1), it is clear that if immunotherapy is to have a future in GBM, there needs to be a shift from the current therapeutic approach. The primary questions that need to be resolved for the development of effective therapy are the following: Is immune monotherapy a realistic approach? Does the timing of treatment matter? What is the optimal preclinical animal model to test the immunotherapies against GBM? Will immunotherapy be applicable for all GBM patients?
Immune combination therapies in GBM models have shown promising pre-clinical results as explained previously. It is also important to keep in mind that other immune targets not mentioned in this review may be necessary for the sensitization of glioblastoma to therapy such as ICIs. For example, Miska et al. showed the ability of anti-GITR to promote immunity against malignant glioma and Amoozgar et al. demonstrated the potential of this antibody to sensitize GBM to other immunotherapy treatments.30,100 Because there are already few TILs in the immunosuppressive environment of GBM, it is understandable that the combination of multiple approaches may be necessary to achieve a clinically significant effect in the cold tumor environment. This may explain why ICIs alone may not be enough to potentiate effective results, and why it may also be necessary to have positive stimulation of TILs and a microenvironment that is optimal for TIL effector function. Recent pre-clinical studies utilizing combination immunotherapies have all shown success in promoting the function of the TILs as well as increasing the lifespan of the associated mouse models.48,49,86,101,102 Although there was a recent clinical trial (Table 1) that aimed to test the triple combination of INCB001158 (arginase inhibitor), epacadostat (IDO inhibitor), and anti-PD-1, the trial had to be halted prematurely due to the side effects of combining epacadostat and anti-PD-1 therapy.
A more recent understanding of GBM immunotherapy is that the timing of said therapy may be integral to the effectiveness of the treatment. The current standard treatment for adult glioblastoma is radiotherapy and temozolomide (TMZ) chemotherapy with surgical resection followed by maintenance TMZ chemotherapy.103 However, TMZ has immunosuppressive effects on the TME. This means that immunotherapy may be unable to promote TIL effector functions if the already minimal amount of T cells in the environment have already become exhausted and/or developed tolerance against tumor antigens. Recently, it has been described that while consistent antigen stimulation is significant for reaching exhausted states, the cells retain a chronic scar signature such that they maintain an exhausted state with cessation of chronic antigen stimulation.99,104 This means T cells that have reached this exhausted state may be beyond reinvigoration via immunotherapies within cancer, providing the basis of why a therapy that targets the cancer earlier may be more successful. Neoadjuvant immunotherapy – providing drug treatment before chemoradiotherapy and surgery resection – serves to address this complication associated with the immunosuppressive environment of GBM. Cloughesy et al. and De Groot et al. have shown that neoadjuvant+adjuvant therapy with anti-PD-1 inhibitor pembrolizumab results in T cell and IFNγ gene upregulation and decreased cell cycling within the recurrent GBM tumor thereby promoting the antitumor response.56,105 Duhen et al. also demonstrated the potential of neoadjuvant therapy using an anti-OX40 agonist (MEDI6469) in HNSCC, which increased the proportion of TILs that were activated, proliferating, and demonstrated antitumor activity.76 While the majority of patients in these studies had progressive disease, the timing of the immunotherapy potentiated an effect on the TME, which is indicative of the future potential of immune regulatory therapy in GBM if timed to be given when its most effective.
An interesting concept specific to GBM is that the necessity for combination and neoadjuvant therapies seems to be rooted in the fact that there is so little T cell infiltration in GBM.106,107 Trying to develop immunotherapy can be difficult if the targets of that immunotherapy are scarce to begin with. The impact of the size of the TIL population is most apparent by the stark contrast of widespread success of pre-clinical monotherapy treatments in murine models but failure in clinical testing. Murine models of GBM are generally more hot tumor environments in comparison to the cold tumor environment in GBM patients. This is the reasoning why there may be so much preclinical success since these studies rely on murine models of GBM, but that same therapy may be unsuccessful in human clinical trials.
This is a critical consideration since the majority of GBM murine models that are used to evaluate the ICIs failed to recapitulate the complexity and heterogeneity of the patient’s GBM microenvironment.108,109 For example, GL261, a widely used chemically-induced murine model of GBM, contained a higher frequency of activated microglia and total T cells, a lower frequency of Tregs than GBM microenvironment in patients. Since the infiltrative tumor lymphocytes (TIL) population is minimal in the “cold” immunosuppressive environment in patient’s GBM, CTLA-4 and other immunotherapies failed to repeat the success of the preclinical models. Such difference in the immune-microenvironment could contribute to the observed efficacy of immunotherapy that targets the T-cell population in these models compared to the GBM patient population.110 Thus, the mouse model to evaluate immune-based therapies in the preclinical setting thus must be carefully considered. Until the patient GBM environment is able to be shifted from cold to hot, these immunotherapies are severely limited in their clinical capacity.111
Another consideration specific to the TME is the consumption of glucose by tumor cells. While tumor cells were classically known to go through Warburg metabolism–recently it has been found that glutamine metabolism is the limiting factor for cancer in the TME rather than glucose, a molecule required for TIL fuctiion.112,113 Whether this is the case in GBM is to be seen, but if glutamine-specific uptake is necessary for the TME it may be an interesting target of therapy that could enhance the effect of other more direct therapies like ICIs.
Overall, the potential for T cell regulatory immunotherapy in GBM is great. With the appropriate timing and combination of therapy, there may be a way to transform the cold immune environment of GBM into a hot tumor environment, with TILs that can effectively mount an antitumor attack. Through these methods and targeting all corners of T cell regulation–immune checkpoint blockade, costimulatory signaling, and amino acid depletion–the appropriately timed combination immunotherapy may be able to overcome the immunosuppression within GBM to effectively treat this disease.
Acknowledgements
This work was supported by the National Institute of Neurological Disorders and Stroke grant 1R01NS096376, 1R01NS112856 (to A.U.A.), and P50CA221747 SPORE for Translational Approaches to Brain Cancer.
Funding Source
All sources of funding should also be acknowledged and you should declare any involvement of study sponsors in the study design; collection, analysis and interpretation of data; the writing of the manuscript; the decision to submit the manuscript for publication. If the study sponsors had no such involvement, this should be stated.
Footnotes
Conflict of Interest
A conflicting interest exists when professional judgement concerning a primary interest (such as patient’s welfare or the validity of research) may be influenced by a secondary interest (such as financial gain or personal rivalry). It may arise for the authors when they have financial interest that may influence their interpretation of their results or those of others. Examples of potential conflicts of interest include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
competing interests
Authors declare no competing interest.
Declarations
Seminars in Cancer Biology requires that all authors sign a declaration of conflicting interests. If you have nothing to declare in any of these categories then this should be stated.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996. Mar 22;271(5256):1734–6. doi: 10.1126/science.271.5256.1734.PMID: 8596936. [DOI] [PubMed] [Google Scholar]
- 2.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992. Nov;11(11):3887–95. PMID: 1396582; PMCID: PMC556898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monney L. et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415, 536–541 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Sanmamed MF, Pastor F, Rodriguez A, Perez-Gracia JL, Rodriguez-Ruiz ME, Jure-Kunkel M, Melero I. Agonists of Co-stimulation in Cancer Immunotherapy Directed Against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol. 2015. Aug;42(4):640–55. doi: 10.1053/j.seminoncol.2015.05.014. Epub 2015 Jun 11. PMID: 26320067. [DOI] [PubMed] [Google Scholar]
- 5.Loeffler JS, Alexander E 3rd, Hochberg FH, Wen PY, Morris JH, Schoene WC, Siddon RL, Morse RH, Black PM. Clinical patterns of failure following stereotactic interstitial irradiation for malignant gliomas. Int J Radiat Oncol Biol Phys. 1990. Dec;19(6):1455–62. doi: 10.1016/03603016(90)90358-q. PMID: 2262370. [DOI] [PubMed] [Google Scholar]
- 6.Brunet JF, et al. (1987). “A new member of the immunoglobulin superfamily--CTLA-4.” Nature 328(6127): 267–270. [DOI] [PubMed] [Google Scholar]
- 7.Linsley PS, et al. (1991). “CTLA-4 is a second receptor for the B cell activation antigen B7.” J Exp Med 174(3): 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linsley PS, et al. (1992). “Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes.” J Exp Med 176(6): 1595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krummel MF and Allison JP (1995). “CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation.” J Exp Med 182(2): 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miska J, Rashidi A, Lee-Chang C, Gao P, Lopez-Rosas A, Zhang P, Burga R, Castro B, Xiao T, Han Y, Hou D, Sampat S, Cordero A, Stoolman JS, Horbinski CM, Burns M, Reshetnyak YK, Chandel NS, Lesniak MS. Polyamines drive myeloid cell survival by buffering intracellular pH to promote immunosuppression in glioblastoma. Sci Adv. 2021. Feb 17;7(8):eabc8929. doi: 10.1126/sciadv.abc8929. PMID: 33597238; PMCID: PMC7888943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995. Nov;3(5):541–7. doi: 10.1016/10747613(95)90125-6. PMID: 7584144. [DOI] [PubMed] [Google Scholar]
- 12.Harper K, et al. (1991). “CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location.” J Immunol 147(3): 1037–1044. [PubMed] [Google Scholar]
- 13.Collins AV, et al. (2002). “The interaction properties of costimulatory molecules revisited.” Immunity 17(2): 201–210. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi OS, et al. (2011). “Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4.” Science 332(6029): 600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ha D, et al. (2019). “Differential control of human Treg and effector T cells in tumor immunity by Fc-engineered anti-CTLA-4 antibody.” Proc Natl Acad Sci U S A 116(2): 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contardi E, et al. (2005). “CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction.” Int J Cancer 117(4): 538–550. [DOI] [PubMed] [Google Scholar]
- 17.Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000. Jul 17;192(2):295–302. doi: 10.1084/jem.192.2.295. PMID: 10899916; PMCID: PMC2193261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000. Jul 17;192(2):303–10. doi: 10.1084/jem.192.2.303. PMID: 10899917; PMCID: PMC2193248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004. Nov;34(11):29963005. doi: 10.1002/eji.200425143. PMID: 15468055. [DOI] [PubMed] [Google Scholar]
- 20.Hodi FS, et al. (2010). “Improved survival with ipilimumab in patients with metastatic melanoma.” N Engl J Med 363(8): 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Day SJ, et al. (2010). “Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study.” Ann Oncol 21(8): 1712–1717. [DOI] [PubMed] [Google Scholar]
- 22.Wolchok JD, et al. (2013). “Nivolumab plus ipilimumab in advanced melanoma.” N Engl J Med 369(2): 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffy AG, et al. (2017). “Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma.” J Hepatol 66(3): 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferris RL, et al. (2020). “Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study.” Ann Oncol 31(7): 942–950. [DOI] [PubMed] [Google Scholar]
- 25.Goldman JW, et al. (2021). “Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial.” Lancet Oncol 22(1): 51–65. [DOI] [PubMed] [Google Scholar]
- 26.Calabrò L, et al. (2018). “Tremelimumab combined with durvalumab in patients with mesothelioma (NIBIT-MESO-1): an open-label, non-randomised, phase 2 study.” Lancet Respir Med 6(6): 451–460. [DOI] [PubMed] [Google Scholar]
- 27.Fecci PE, et al. (2007). “Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function.” Clin Cancer Res 13(7): 2158–2167. [DOI] [PubMed] [Google Scholar]
- 28.Wainwright DA, et al. (2014). “Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors.” Clin Cancer Res 20(20): 5290–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poduslo JF, et al. (1994). “Macromolecular permeability across the blood-nerve and blood-brain barriers.” Proc Natl Acad Sci U S A 91(12): 5705–5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miska J, Rashidi A, Chang AL et al. Anti-GITR therapy promotes immunity against malignant glioma in a murine model.Cancer Immunol Immunother 65, 1555–1567 (2016). 10.1007/s00262-016-1912-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galstyan A, et al. (2019). “Blood-brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy.” Nat Commun 10(1): 3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croft M, So T, Duan W, Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229(1):173–191. doi: 10.1111/j.1600-065X.2009.00766.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999. Aug;11(2):141–51. doi: 10.1016/s1074-7613(00)80089-8. PMID: 10485649. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001. Jan 12;291(5502):319–22. doi: 10.1126/science.291.5502.319. PMID: . [DOI] [PubMed] [Google Scholar]
- 35.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001. Nov 20;98(24):13866–71. doi: 10.1073/pnas.231486598. Epub 2001 Nov 6. PMID: 11698646; PMCID: PMC61133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002. Sep 17;99(19):12293–7. doi: 10.1073/pnas.192461099. Epub 2002 Sep 6. PMID: 12218188; PMCID: PMC129438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishida Y. PD-1: Its Discovery, Involvement in Cancer Immunotherapy, and Beyond. Cells. 2020;9(6):1376. Published 2020 Jun 1. doi: 10.3390/cells9061376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugiura D, Maruhashi T, Okazaki IM, Shimizu K, Maeda TK, Takemoto T, Okazaki T. Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science. 2019. May 10;364(6440):558–566. doi: 10.1126/science.aav7062. Epub 2019 Apr 18. PMID: 31000591. [DOI] [PubMed] [Google Scholar]
- 39.PD-1 Blockade in GBM: Uncovering Response Clues. Cancer Discov. 2019. Jun;9(6):687–688. doi: 10.1158/2159-8290.CD-ND2019-001. Epub 2019 Apr 12. PMID: 30979702. [DOI] [PubMed] [Google Scholar]
- 40.Passaro C, Alayo Q, De Laura I, McNulty J, Grauwet K, Ito H, Bhaskaran V, Mineo M, Lawler SE, Shah K, Speranza MC, Goins W, McLaughlin E, Fernandez S, Reardon DA, Freeman GJ, Chiocca EA, Nakashima H. Arming an Oncolytic Herpes Simplex Virus Type 1 with a Single-chain Fragment Variable Antibody against PD-1 for Experimental Glioblastoma Therapy. Clin Cancer Res. 2019. Jan 1;25(1):290–299. doi: 10.1158/1078-0432.CCR-18-2311. Epub 2018 Oct 2. Erratum in: Clin Cancer Res. 2020 Feb 1;26(3):758. PMID: 30279232; PMCID: PMC6800097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu X, Castano AP, Darr H, Henderson DB, Bouffard AA, Larson RC, Scarfò I, Bailey SR, Gerhard GM, Frigault MJ, Leick MB, Schmidts A, Sagert JG, Curry WT, Carter BS, Maus MV. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J Immunother Cancer. 2019. Nov 14;7(1):304. doi: 10.1186/s40425-019-0806-7. PMID: 31727131; PMCID: PMC6857271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakazawa T, Natsume A, Nishimura F, Morimoto T, Matsuda R, Nakamura M, Yamada S, Nakagawa I, Motoyama Y, Park YS, Tsujimura T, Wakabayashi T, Nakase H. Effect of CRISPR/Cas9-Mediated PD-1-Disrupted Primary Human Third-Generation CAR-T Cells Targeting EGFRvIII on In Vitro Human Glioblastoma Cell Growth. Cells. 2020. Apr 16;9(4):998. doi: 10.3390/cells9040998. PMID: 32316275; PMCID: PMC7227242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao J, Chen AX, Gartrell RD, Silverman AM, Aparicio L, Chu T, Bordbar D, Shan D, Samanamud J, Mahajan A, Filip I, Orenbuch R, Goetz M, Yamaguchi JT, Cloney M, Horbinski C, Lukas RV, Raizer J, Rae AI, Yuan J, Canoll P, Bruce JN, Saenger YM, Sims P, Iwamoto FM, Sonabend AM, Rabadan R. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med. 2019. Mar;25(3):462–469. doi: 10.1038/s41591-019-0349-y. Epub 2019 Feb 11. Erratum in: Nat Med. 2019 Apr 17;: PMID: 30742119; PMCID: PMC6810613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, Stephens PJ, Daniels GA, Kurzrock R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther. 2017. Nov;16(11):2598–2608. doi: 10.1158/15357163.MCT-17-0386. Epub 2017 Aug 23. PMID: 28835386; PMCID: PMC5670009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, Peters S. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019. Jan 1;30(1):44–56. doi: 10.1093/annonc/mdy495. PMID: 30395155; PMCID: PMC6336005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, Peters S. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019. Jan 1;30(1):44–56. doi: 10.1093/annonc/mdy495. PMID: 30395155; PMCID: PMC6336005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snyder A, Wolchok JD. Successful Treatment of a Patient with Glioblastoma and a Germline POLE Mutation: Where Next? Cancer Discov. 2016. Nov;6(11):1210–1211. doi: 10.1158/2159-8290.CD16-1056. PMID: 27807100; PMCID: PMC5109825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flores-Toro JA, Luo D, Gopinath A, Sarkisian MR, Campbell JJ, Charo IF, Singh R, Schall TJ, Datta M, Jain RK, Mitchell DA, Harrison JK. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc Natl Acad Sci U S A. 2020. Jan 14;117(2):1129–1138. doi: 10.1073/pnas.1910856117. Epub 2019 Dec 26. PMID: 31879345; PMCID: PMC6969504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li M, Kirtane AR, Kiyokawa J, Nagashima H, Lopes A, Tirmizi ZA, Lee CK, Traverso G, Cahill DP, Wakimoto H. Local Targeting of NAD+Salvage Pathway Alters the Immune Tumor Microenvironment and Enhances Checkpoint Immunotherapy in Glioblastoma. Cancer Res. 2020. Nov 15;80(22):5024–5034. doi: 10.1158/0008-5472.CAN-20-1094. Epub 2020 Sep 30. Erratum in: Cancer Res. 2021 Apr 1;81(7):1922. PMID: 32998997; PMCID: PMC7669613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antonios JP, Soto H, Everson RG, Moughon D, Orpilla JR, Shin NP, Sedighim S, Treger J, Odesa S, Tucker A, Yong WH, Li G, Cloughesy TF, Liau LM, Prins RM. Immunosuppressive tumorinfiltrating myeloid cells mediate adaptive immune resistance via a PD-1/PD-L1 mechanism in glioblastoma. Neuro Oncol. 2017. Jun 1;19(6):796–807. doi: 10.1093/neuonc/now287. PMID: 28115578; PMCID: PMC5464463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karachi A, Yang C, Dastmalchi F, Sayour EJ, Huang J, Azari H, Long Y, Flores C, Mitchell DA, Rahman M. Modulation of temozolomide dose differentially affects T-cell response to immune checkpoint inhibition. Neuro Oncol. 2019. Jun 10;21(6):730–741. doi: 10.1093/neuonc/noz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kinoh H, Quader S, Shibasaki H, Liu X, Maity A, Yamasoba T, Cabral H, Kataoka K. Translational Nanomedicine Boosts Anti-PD1 Therapy to Eradicate Orthotopic PTEN-Negative Glioblastoma. ACS Nano. 2020. Aug 25;14(8):10127–10140. doi: 10.1021/acsnano.0c03386. Epub 2020 Aug 6. PMID: 32806051. [DOI] [PubMed] [Google Scholar]
- 53.Yamaguchi I, Nakajima K, Shono K, Mizobuchi Y, Fujihara T, Shikata E, Yamaguchi T, Kitazato K, Sampetrean O, Saya H, Takagi Y. Downregulation of PD-L1 via FKBP5 by celecoxib augments antitumor effects of PD-1 blockade in a malignant glioma model. Neurooncol Adv. 2019. Dec 26;2(1):vdz058. doi: 10.1093/noajnl/vdz058. PMID: 32642723; PMCID: PMC7212915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang F, He Z, Duan H, Zhang D, Li J, Yang H, Dorsey JF, Zou W, Ali Nabavizadeh S, Bagley SJ, Abdullah K, Brem S, Zhang L, Xu X, Byrne KT, Vonderheide RH, Gong Y, Fan Y. Synergistic immunotherapy of glioblastoma by dual targeting of IL-6 and CD40. Nat Commun. 2021. Jun 8;12(1):3424. doi: 10.1038/s41467-021-23832-3. PMID: 34103524; PMCID: PMC8187342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim JE, Patel MA, Mangraviti A, Kim ES, Theodros D, Velarde E, Liu A, Sankey EW, Tam A, Xu H, Mathios D, Jackson CM, Harris-Bookman S, Garzon-Muvdi T, Sheu M, Martin AM, Tyler BM, Tran PT, Ye X, Olivi A, Taube JM, Burger PC, Drake CG, Brem H, Pardoll DM, Lim M. Combination Therapy with Anti-PD-1, Anti-TIM-3, and Focal Radiation Results in Regression of Murine Gliomas. Clin Cancer Res. 2017. Jan 1;23(1):124–136. doi: 10.1158/1078-0432.CCR-151535. Epub 2016 Jun 29. PMID: 27358487; PMCID: PMC5735836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, Wang AC, Ellingson BM, Rytlewski JA, Sanders CM, Kawaguchi ES, Du L, Li G, Yong WH, Gaffey SC, Cohen AL, Mellinghoff IK, Lee EQ, Reardon DA, O’Brien BJ, Butowski NA, Nghiemphu PL, Clarke JL, Arrillaga-Romany IC, Colman H, Kaley TJ, de Groot JF, Liau LM, Wen PY, Prins RM. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019. Mar;25(3):477–486. doi: 10.1038/s41591-018-0337-7. Epub 2019 Feb 11. PMID: 30742122; PMCID: PMC6408961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, López-Janeiro A, Porciuncula A, Idoate MA, Inogés S, de Andrea C, López-Diaz de Cerio A, Tejada S, Berraondo P, Villarroel-Espindola F, Choi J, Gúrpide A, Giraldez M, Goicoechea I, Gallego Perez-Larraya J, Sanmamed MF, PerezGracia JL, Melero I. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019. Mar;25(3):470–476. doi: 10.1038/s41591-018-0339-5. Epub 2019 Feb 11. PMID: 30742120. [DOI] [PubMed] [Google Scholar]
- 58.Paterson DJ, et al. (1987). “Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts.” Mol Immunol 24(12): 1281–1290. [DOI] [PubMed] [Google Scholar]
- 59.Godfrey WR, et al. (1994). “Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor.” J Exp Med 180(2): 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim MY, et al. (2003). “CD4(+)CD3(−) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells.” Immunity 18(5): 643–654. [DOI] [PubMed] [Google Scholar]
- 61.Vu MD, et al. (2007). “OX40 costimulation turns off Foxp3+ Tregs.” Blood 110(7): 2501–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacquemin C, et al. (2015). “OX40 Ligand Contributes to Human Lupus Pathogenesis by Promoting T Follicular Helper Response.” Immunity 42(6): 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacquemin C, et al. (2015). “OX40 Ligand Contributes to Human Lupus Pathogenesis by Promoting T Follicular Helper Response.” Immunity 42(6): 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murata K, et al. (2002). “Constitutive OX40/OX40 ligand interaction induces autoimmune-like diseases.” J Immunol 169(8): 4628–4636 [DOI] [PubMed] [Google Scholar]
- 65.Ohshima Y, et al. (1998). “OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors.” Blood 92(9): 3338–3345. [PubMed] [Google Scholar]
- 66.Lane P. (2000). “Role of OX40 signals in coordinating CD4 T cell selection, migration, and cytokine differentiation in T helper (Th)1 and Th2 cells.” J Exp Med 191(2): 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rogers PR, et al. (2001). “OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells.” Immunity 15(3): 445–455. [DOI] [PubMed] [Google Scholar]
- 68.Song J, et al. (2004). “The costimulation-regulated duration of PKB activation controls T cell longevity.” Nat Immunol 5(2): 150–158. [DOI] [PubMed] [Google Scholar]
- 69.Song J, et al. (2005). “Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion.” Immunity 22(5): 621–631. [DOI] [PubMed] [Google Scholar]
- 70.Kawamata S, et al. (1998). “Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-kappaB activation.” J Biol Chem 273(10): 5808–5814. [DOI] [PubMed] [Google Scholar]
- 71.Shibahara I, et al. (2015). “OX40 ligand expressed in glioblastoma modulates adaptive immunity depending on the microenvironment: a clue for successful immunotherapy.” Mol Cancer 14: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Massarelli E, et al. (2019). “High OX-40 expression in the tumor immune infiltrate is a favorable prognostic factor of overall survival in non-small cell lung cancer.” Journal for ImmunoTherapy of Cancer 7(1): 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ohmura H, et al. (2020). “OX40 and LAG3 are associated with better prognosis in advanced gastric cancer patients treated with anti-programmed death-1 antibody.” British Journal of Cancer 122(10): 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xie K, et al. (2018). “OX40 expression in hepatocellular carcinoma is associated with a distinct immune microenvironment, specific mutation signature, and poor prognosis.” Oncoimmunology 7(4): e1404214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma Y, et al. (2020). “Combination of PD-1 Inhibitor and OX40 Agonist Induces Tumor Rejection and Immune Memory in Mouse Models of Pancreatic Cancer.” Gastroenterology 159(1): 306–319.e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duhen R, et al. (2021). “Neoadjuvant anti-OX40 (MEDI6469) therapy in patients with head and neck squamous cell carcinoma activates and expands antigen-specific tumor-infiltrating T cells.” Nature Communications 12(1): 1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jahan N, et al. (2018). “Agonist OX40 immunotherapy improves survival in glioma-bearing mice and is complementary with vaccination with irradiated GM-CSF-expressing tumor cells.” Neuro Oncol 20(1): 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jahan N, et al. (2019). “Triple combination immunotherapy with GVAX, anti-PD-1 monoclonal antibody, and agonist anti-OX40 monoclonal antibody is highly effective against murine intracranial glioma.” Oncoimmunology 8(5): e1577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999. Jan 21;397(6716):263–6. doi: 10.1038/16717. PMID: 9930702. [DOI] [PubMed] [Google Scholar]
- 80.Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G, Odermatt B, Ho A, Itie A, Horan T, Whoriskey JS, Pawson T, Penninger JM, Ohashi PS, Mak TW. ICOS is essential for effective T-helper-cell responses. Nature. 2001. Jan 4;409(6816):105–9. doi: 10.1038/35051113. PMID: 11343123. [DOI] [PubMed] [Google Scholar]
- 81.McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001. Jan 4;409(6816):102–5. doi: 10.1038/35051107. PMID: 11343122. [DOI] [PubMed] [Google Scholar]
- 82.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS costimulatory receptor is essential for T-cell activation and function. Nature. 2001. Jan 4;409(6816):97–101. doi: 10.1038/35051100. PMID: 11343121. [DOI] [PubMed] [Google Scholar]
- 83.Gonzalo JA, Tian J, Delaney T, Corcoran J, Rottman JB, Lora J, Al-garawi A, Kroczek R, Gutierrez-Ramos JC, Coyle AJ. ICOS is critical for T helper cell-mediated lung mucosal inflammatory responses. Nat Immunol. 2001. Jul;2(7):597–604. doi: 10.1038/89739. PMID: 11429543. [DOI] [PubMed] [Google Scholar]
- 84.Xiao Z, Mayer AT, Nobashi TW, Gambhir SS. ICOS Is an Indicator of T-cell-Mediated Response to Cancer Immunotherapy. Cancer Res. 2020. Jul 15;80(14):3023–3032. doi: 10.1158/00085472.CAN-19-3265. Epub 2020 Mar 10. PMID: 32156777. [DOI] [PubMed] [Google Scholar]
- 85.Abrahao A, et al. (2019). “First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound.” Nature Communications 10(1): 4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Belcaid Z, Berrevoets C, Choi J, van Beelen E, Stavrakaki E, Pierson T, Kloezeman J, Routkevitch D, van der Kaaij M, van der Ploeg A, Mathios D, Sleijfer S, Dirven C, Lim M, Debets R, Lamfers MLM. Low-dose oncolytic adenovirus therapy overcomes tumor-induced immune suppression and sensitizes intracranial gliomas to anti-PD-1 therapy. Neurooncol Adv. 2020. Feb 3;2(1):vdaa011. doi: 10.1093/noajnl/vdaa011. PMID: 32642679; PMCID: PMC7212906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Opitz C, Litzenburger U, Sahm F. et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478, 197–203 (2011). 10.1038/nature10491 [DOI] [PubMed] [Google Scholar]
- 88.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999. May 3;189(9):1363–72. doi: 10.1084/jem.189.9.1363. PMID: 10224276; PMCID: PMC2193062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kudo Y, Boyd CA, Sargent IL, Redman CW. Tryptophan degradation by human placental indoleamine 2,3-dioxygenase regulates lymphocyte proliferation. J Physiol. 2001. Aug 15;535(Pt 1):207–15. doi: 10.1111/j.1469-7793.2001.00207.x. PMID: 11507170; PMCID: PMC2288791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol. 2013. May;14(5):500–8. doi: 10.1038/ni.2556. Epub 2013 Mar 24. Erratum in: Nat Immunol. 2014 Jan;15(1):109. PMID: 23525088; PMCID: PMC3672957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brody JR, Costantino CL, Berger AC, Sato T, Lisanti MP, Yeo CJ, Emmons RV, Witkiewicz AK. Expression of indoleamine 2,3-dioxygenase in metastatic malignant melanoma recruits regulatory T cells to avoid immune detection and affects survival. Cell Cycle. 2009. Jun 15;8(12):1930–4. doi: 10.4161/cc.8.12.8745. Epub 2009 Jun 15. PMID: 19448397. [DOI] [PubMed] [Google Scholar]
- 92.Mussai F, Egan S, Hunter S, Webber H, Fisher J, Wheat R, McConville C, Sbirkov Y, Wheeler K, Bendle G, Petrie K, Anderson J, Chesler L, De Santo C. Neuroblastoma Arginase Activity Creates an Immunosuppressive Microenvironment That Impairs Autologous and Engineered Immunity. Cancer Res. 2015. Aug 1;75(15):3043–53. doi: 10.1158/0008-5472.CAN-14-3443. Epub 2015 Jun 8. PMID: 26054597; PMCID: PMC4527662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mussai F, De Santo C, Abu-Dayyeh I, Booth S, Quek L, McEwen-Smith RM, Qureshi A, Dazzi F, Vyas P, Cerundolo V. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood. 2013. Aug 1;122(5):749–58. doi: 10.1182/blood-2013-01-480129. Epub 2013 Jun 3. PMID: 23733335; PMCID: PMC3731930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uyttenhove C, Pilotte L, Théate I. et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 9, 1269–1274 (2003). 10.1038/nm934 [DOI] [PubMed] [Google Scholar]
- 95.Mitsuka K, Kawataki T, Satoh E, Asahara T, Horikoshi T, Kinouchi H. Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery. 2013. Jun;72(6):1031–8; discussion 1038–9. doi: 10.1227/NEU.0b013e31828cf945. PMID: 23426156. [DOI] [PubMed] [Google Scholar]
- 96.Miyazaki T, Moritake K, Yamada K, Hara N, Osago H, Shibata T, Akiyama Y, Tsuchiya M. Indoleamine 2,3-dioxygenase as a new target for malignant glioma therapy. Laboratory investigation. J Neurosurg. 2009. Aug;111(2):230–7. doi: 10.3171/2008.10.JNS081141. PMID: 19199463. [DOI] [PubMed] [Google Scholar]
- 97.Zhang I, Alizadeh D, Liang J, Zhang L, Gao H, Song Y, Ren H, Ouyang M, Wu X, D’Apuzzo M, Badie B. Characterization of Arginase Expression in Glioma-Associated Microglia and Macrophages. PLoS One. 2016. Dec 9;11(12):e0165118. doi: 10.1371/journal.pone.0165118. PMID: 27936099; PMCID: PMC5147798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Steggerda SM, Bennett MK, Chen J, Emberley E, Huang T, Janes JR, Li W, MacKinnon AL, Makkouk A, Marguier G, Murray PJ, Neou S, Pan A, Parlati F, Rodriguez MLM, Van de Velde LA, Wang T, Works M, Zhang J, Zhang W, Gross MI. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J Immunother Cancer. 2017. Dec 19;5(1):101. doi: 10.1186/s40425-017-0308-4. PMID: 29254508; PMCID: PMC5735564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hensel N, Gu Z, Sagar et al. Memory-like HCV-specific CD8+ T cells retain a molecular scar after cure of chronic HCV infection. Nat Immunol 22, 229–239 (2021). 10.1038/s41590-020-00817-w [DOI] [PubMed] [Google Scholar]
- 100.Amoozgar Z, Kloepper J, Ren J. et al. Targeting Treg cells with GITR activation alleviates resistance to immunotherapy in murine glioblastomas. Nat Commun 12, 2582 (2021). 10.1038/s41467-021-22885-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu A, Maxwell R, Xia Y, Cardarelli P, Oyasu M, Belcaid Z, Kim E, Hung A, Luksik AS, Garzon-Muvdi T, Jackson CM, Mathios D, Theodros D, Cogswell J, Brem H, Pardoll DM, Lim M. Combination anti-CXCR4 and anti-PD-1 immunotherapy provides survival benefit in glioblastoma through immune cell modulation of tumor microenvironment. J Neurooncol. 2019. Jun;143(2):241249. doi: 10.1007/s11060-019-03172-5. Epub 2019 Apr 25. PMID: 31025274. [DOI] [PubMed] [Google Scholar]
- 102.Chan HY, Choi J, Jackson C, Lim M. Combination immunotherapy strategies for glioblastoma. J Neurooncol. 2021. Feb;151(3):375–391. doi: 10.1007/s11060-020-03481-0. Epub 2021 Feb 21. PMID: 33611705. [DOI] [PubMed] [Google Scholar]
- 103.Lim M, Xia Y, Bettegowda C. et al. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol 15, 422–442 (2018). 10.1038/s41571-018-0003-5 [DOI] [PubMed] [Google Scholar]
- 104.Tonnerre P, Wolski D, Subudhi S. et al. Differentiation of exhausted CD8+ T cells after termination of chronic antigen stimulation stops short of achieving functional T cell memory. Nat Immunol 22, 1030–1041 (2021). 10.1038/s41590-021-00982-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Groo J, Penas-Prado M, Alfaro-Munoz K, Hunter K, Pei BL, O’Brien B, Weathers SP, Loghin M, Kamiya Matsouka C, Yung WKA, Mandel J, Wu J, Yuan Y, Zhou S, Fuller GN, Huse J, Rao G, Weinberg JS, Prabhu SS, McCutcheon IE, Lang FF, Ferguson SD, Sawaya R, Colen R, Yadav SS, Blando J, Vence L, Allison J, Sharma P, Heimberger AB. Window-of-opportunity clinical trial of pembrolizumab in patients with recurrent glioblastoma reveals predominance of immune-suppressive macrophages. Neuro Oncol. 2020. Apr 15;22(4):539–549. doi: 10.1093/neuonc/noz185. PMID: 31755915; PMCID: PMC7158647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brooks WH, Roszman TL, Mahaley MS, Woosley RE. Immunobiology of primary intracranial tumours. II. Analysis of lymphocyte subpopulations in patients with primary brain tumours. Clin Exp Immunol. 1977. Jul;29(1):61–6. PMID: 330067; PMCID: PMC1541043. [PMC free article] [PubMed] [Google Scholar]
- 107.Han S, Ma E, Wang X, Yu C, Dong T, Zhan W, Wei X, Liang G, Feng S. Rescuing defective tumor-infiltrating T-cell proliferation in glioblastoma patients. Oncol Lett. 2016. Oct;12(4):2924–2929. doi: 10.3892/ol.2016.4944. Epub 2016 Aug 3. PMID: 27703529; PMCID: PMC5038909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khalsa JK, Cheng N, Keegan J, Chaudry A, Driver J, Bi WL, Lederer J, Shah K. Immune phenotyping of diverse syngeneic murine brain tumors identifies immunologically distinct types. Nat Commun. 2020. Aug 6;11(1):3912. doi: 10.1038/s41467-020-17704-5. PMID: 32764562; PMCID: PMC7411074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wouters R, Bevers S, Riva M, De Smet F, Coosemans A. Immunocompetent Mouse Models in the Search for Effective Immunotherapy in Glioblastoma. Cancers (Basel). 2020. Dec 23;13(1):19. doi: 10.3390/cancers13010019. PMID: 33374542; PMCID: PMC7793150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Genoud V, Marinari E, Nikolaev SI, Castle JC, Bukur V, Dietrich PY, Okada H, Walker PR. Responsiveness to anti-PD-1 and anti-CTLA-4 immune checkpoint blockade in SB28 and GL261 mouse glioma models. Oncoimmunology. 2018. Sep 5;7(12):e1501137. doi: 10.1080/2162402X.2018.1501137. PMID: 30524896; PMCID: PMC6279422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013. Aug 28;5(200):200ra116. doi: 10.1126/scitranslmed.3006504. PMID: 23986400; PMCID: PMC4136707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vander Heiden MG, DeBerardinis RJ. Understanding the Intersections between Metabolism and Cancer Biology. Cell. 2017. Feb 9;168(4):657–669. doi: 10.1016/j.cell.2016.12.039. PMID: 28187287; PMCID: PMC5329766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reinfeld BI, Madden MZ, Wolf MM et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 593, 282–288 (2021). 10.1038/s41586-021-03442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]