Abstract
Social stress can contribute to the development of substance use disorders (SUDs) and increase the likelihood of relapse. Oxytocin (OT) is a potential pharmacotherapy that may buffer the effects of social stress on arousal and reward neurocircuitry. However, more research is needed to understand how OT moderates the brain’s response to social stress in SUDs. The present study examined the effect of intransasal OT (24 IU) versus placebo (PBO) on corticolimbic functional connectivity associated with acute social stress in individuals with cocaine use disorder (CUD; n = 67) and healthy controls (HC; n = 52). Psychophysiological interaction modeling used the left and right amygdala as seed regions with the left and right orbitofrontal and anterior cingulate cortex as a priori regions of interest. Moderators of the OT response included childhood trauma history and biological sex, which were examined in independent analyses. The main finding was that OT normalized corticolimbic connectivity (left amygdala-orbitofrontal and left amygdala-anterior cingulate) as a function of childhood trauma such that connectivity was different between trauma-present and trauma-absent groups on PBO, but not between trauma groups on OT. Effects of OT on corticolimbic connectivity were not different as a function of diagnosis (CUD vs HC) or sex. However, OT reduced subjective anxiety during social stress for CUD participants who reported childhood trauma compared to PBO and normalized craving response as a function of sex in CUD. The present findings add to some prior findings of normalizing effects of OT on corticolimbic circuitry in individuals with trauma histories and provide some initial support that OT can normalize subjective anxiety and craving in CUD.
Keywords: Montreal imaging stress task, Amygdala, Orbitofrontal cortex, Childhood trauma, Craving, Anxiety
Highlights
-
•
Social stress-related corticolimbic connectivity was affected by childhood trauma under placebo.
-
•
Under oxytocin, corticolimbic connectivity differences due to childhood trauma were absent.
-
•
Oxytocin reduced subjective anxiety in cocaine users with childhood trauma.
-
•
Oxytocin reduced subjective craving in male cocaine users.
1. Introduction
Social stress is increasingly recognized as a key factor in the development of substance use disorders (SUDs; [[1], [2], [3]]). Preclinical studies have found that chronic social stress increases the reinforcing properties of drugs of abuse. For example, socially isolated rodents readily self-administer cocaine as compared to rodents housed in groups [4] and social defeat exacerbates drug-seeking in male rodents [2]. Clinical studies have found that social stress increases drug craving in individuals with cocaine use disorder (CUD; [5,6]). Epidemiological data demonstrate a strong link between childhood maltreatment and the risk for the development of SUDs; indeed, childhood maltreatment accounts for up to 67% of the population attributable risk for SUDs [7].
Childhood maltreatment and trauma may stem from chronic social stress or isolated traumatic events of an interpersonal nature. Consequently, a history of childhood trauma and maltreatment can maladaptively alter the brain’s stress circuitry thereby increasing susceptibility to SUDs and other psychiatric disorders [8]. Sahani et al. [3] note that stress and addiction-related neurocircuitry significantly overlap and that a history of substance abuse combined with maltreatment history led to specific alterations in the structure and function of regions that support executive function as well as the hippocampal complex. In addition to increasing susceptibility for developing addictive disorders, alterations in stress and reward circuitry can increase vulnerability to relapse under conditions of social stress [9]. For these reasons, understanding the response to social stress in SUD should consider childhood trauma and maltreatment as an important moderator.
One aspect of brain function that is altered by social stress, social deprivation and chronic drug exposure is the release and regulation of oxytocin [10]. Consequently, the neuropeptide oxytocin (OT) has gained attention recently as a potential therapeutic to treat SUDs due, in part, to its actions on the neurocircuitry of reward and addiction [11,12]. Preclinical studies have yielded compelling findings that demonstrate the role of OT in reducing cocaine self-administration in rodents and in inhibiting cue-induced reinstatement [2]. However, emerging clinical findings are mixed. One study of OT in co-morbid opioid use disorder and CUD showed that OT can reduce cocaine craving and use [13]. Similarly, Joseph et al. (2020) reported that an acute intranasal dose of OT reduced amygdala reactivity to cocaine cues in men with CUD and a history of childhood trauma, which was also correlated with reduced cocaine craving. Conversely, OT exacerbated amygdala reactivity in women with CUD and a history of childhood trauma in the same sample [14]. Lee et al. (2014) also reported that OT induced a desire to use cocaine and increased cue-induced excitability in cocaine users, but in a predominantly male sample [15]. Still other studies have reported no effects of OT in reducing cocaine craving in CUD [16]. Taken together, clinical findings to date report mixed results of OT in reducing cocaine craving and use behaviors. Discrepant findings may be due to moderation of effects by sex or history of childhood trauma.
In addition to its effects on reward neurocircuitry, the benefit of OT for substance using populations may lie in its anxiolytic properties, which are well documented both in animals [e.g., Ref. [12]) and humans [e.g., Ref. [17]). Mechanistic preclinical studies have shown that OT may promote the release of serotonin transmission by acting on OT receptors in the amygdala, hippocampus, or dorsal raphe nucleus or inhibit hypothalamic-pituitary-adrenal (HPA) axis release of corticotrophin releasing factor [10]. Clinical studies using a social stress task have shown that acute doses of OT reduce stress reactivity in CUD. Specifically, Flanagan et al. (2016) showed that OT attenuated the association between childhood trauma and cortisol reactivity [18] and Sherman et al. (2020) reported that female cocaine users who received OT had a blunted cortisol response compared to men and compared to placebo (PBO; [16]).
Initial findings on the therapeutic value of OT for CUD indicate that OT may attenuate craving and stress responses at physiological, neural or subjective levels. However, these effects are often moderated by childhood trauma history, sex, or both. Moreover, prior studies investigating the effect of OT on brain response in substance-using populations have focused on cue reactivity [14,19,20] or the resting state [21], but to our knowledge, no prior studies used a social stress task in an fMRI setting. Given that the stress pathway is implicated in addictive behaviors and that OT can attenuate stress responses in humans, the present study used a social stress task adapted for the MRI environment, the Montreal Imaging Stress Task (MIST, [22]), in healthy controls (HC) and individuals with cocaine use disorder (CUD) who were not seeking treatment. Intranasal OT or placebo (PBO) was administered in a randomized, double-blind parallel design. The MIST task induces social stress by providing feedback on arithmetic problems in a social comparison context. Additional negative feedback from the experimenter in real time adds ecological validity to the task. Corticolimbic fMRI response during the MIST is associated with individual variation in physiological and subjective stress reactivity, with areas like the amygdala and various prefrontal cortex areas implicated [23,24].
The primary hypothesis was that OT would normalize corticolimbic connectivity in individuals with CUD, as indicated by an interaction between diagnostic group (CUD, HC) and treatment (OT, PBO). We hypothesized that CUD and HC groups on PBO would show significant differences in corticolimbic connectivity magnitude or direction, but CUD and HC groups on OT would not. In addition, childhood trauma history was expected to moderate these effects such that individuals with CUD who reported a history of childhood trauma (CUD/TRAUMA+) were expected to show the greatest difference in connectivity compared to other groups (CUD/TRAUMA-, HC/TRAUMA+, HC/TRAUMA-) on PBO. Sex was also explored as a moderator of the effect of OT on corticolimbic connectivity, given the culmination of preclinical and clinical findings of sex differences in drug-seeking and other addiction-related behaviors and outcomes [25].
2. Methods
2.1. Participants
Participants took part in a larger study investigating the impact of OT on subjective and neural responses to stressors. The current analysis included data only from the MIST fMRI task. Non-treatment seeking CUD individuals and healthy controls responded to local media advertisements over a 54-month period. Written informed consent was obtained before study assessments were administered. All procedures were conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki, and the protocol received Institutional Review Board (IRB) approval. General exclusion criteria for all participants included (1) history of significant hematological, endocrine, cardiovascular, pulmonary, renal, gastrointestinal, or neurological disease including diabetes, (2) history of or current psychotic disorder or bipolar affective disorder, (3) current major depressive disorder or post-traumatic stress disorder, (4) psychotropic medications, including SSRI’s or other antidepressants, opiates or opiate antagonists, but excluding trazodone or non-benzodiazepine hypnotics for sleep, (5) pregnant, nursing or of childbearing potential and not practicing an effective means of birth control, (6) a BMI that would preclude them from fitting comfortably in the scanner, (7) Persons with ferrous metal implants or pacemaker, (8) claustrophobia, (9) significant psychiatric or medical problems that would impair participation or limit ability to complete the scanning session, (10) Subjects that require maintenance or acute treatment with any psychoactive medication including anti-seizure medications which could potentially interfere with fMRI data acquisition.
Two-hundred ninety individuals were consented. Of this number, 192 met eligibility requirements and were randomized to treatment, 136 began procedures, and 130 participants completed the MRI scanning portion. Of these, 7 participants were excluded due to excessive head motion, and 3 participants had missing or incomplete data, leaving 120 participants who had usable MRI data. One participant did not complete the CTQ, leaving a final sample of 119 subjects.
2.2. Assessment
Participants meeting pre-screening criteria were evaluated for study eligibility with the Mini-International Neuropsychiatric Interview (MINI) [26] to assess for potential psychiatric exclusions. Participants who met criteria for current and lifetime substance use disorders according to the substance use module of the Structured Clinical Interview for DSM-IV (SCID-IV) [27] were included in the CUD group; participants who did not meet criteria for substance use disorders were included in the HC group. Nicotine and cannabis use disorders were not exclusionary for either the CUD or HC group, but cannabis use disorder in the last year was exclusionary for HC. Due to the high comorbidity of alcohol and cocaine use disorders, individuals with alcohol use disorder were included in the CUD group if they did not require medically supervised detoxification. The reason for this was that participants were asked to abstain from alcohol and other substances for three days prior to the study, which could present a safety concern for individuals requiring medically supervised detoxification.
Substance use in the ninety days prior to the study for participants in the CUD group was assessed using the Time-Line Follow-Back [28]. The Childhood Trauma Questionnaire (CTQ; [29]) was used to assess the extent to which individuals experienced five domains of childhood abuse and neglect (sexual abuse, physical abuse, emotional abuse, emotional neglect, and physical neglect). Participants answered each of 25 questions using a 5-point Likert scale ranging from 1 (never true) to 5 (very often true); a total score cut-off of 37 or greater is used to indicate some childhood trauma. A medical history and physical examination were completed to assess for medical exclusions. Participants meeting inclusion criteria and no exclusion criteria were scheduled to complete the study procedures and instructed to not use cocaine or other drugs of abuse for a minimum of three days prior to the test sessions.
2.3. Study procedures
Participants completed one fMRI session that included the MIST, an implicit facial affect task, and a resting state scan, which were completed in a counterbalanced order across subjects. Only results from the MIST are reported here. On the testing day, upon arrival at the research clinic urine pregnancy tests were administered. Smokers were offered a nicotine patch. Self-reports, urine drug screens (Roche Diagnostics, Indianapolis, Indiana), and breathalyzer tests (AlcoSensor III, Intoximeters, Inc., St. Louis, Missouri) were used to assess abstinence. If the pregnancy and drug tests were negative (with the exception of THC), study procedures continued and baseline subjective ratings were obtained. Participants provided a blood draw to measure endogenous OT. A modified version of the Within Session Rating Scale was used to assess subjective ratings of craving, mood, anxiety, and stress [30]. This 1-10 visual analogue scale is anchored with the adjectival modifiers (“not at all,” “mildly,” “moderately,” and “extremely”).
Participants self-administered 24 IUs of OT nasal spray or matching placebo under observation by research staff 45 min prior to scanning. This dose was selected based on previous studies using similar doses of OT in studies of SUD, childhood trauma or anxiety disorders [15,19,20,[31], [32], [33]]. Doses of OT between 18 and 40 IU intranasally have been shown to be safe for short-term use in clinical populations [34]. Timing of administration was also based on previous studies showing central activity of OT approximately 40 min after intranasal administration (e.g., Ref. [15]).
Intranasal OT and matching PBO (saline spray) were compounded by the MUSC Investigational Drug Service (IDS). USP certified OT powder was acquired by the MUSC IDS through the Professional Compounding Centers of America (PCCA). IDS used the potency data provided in the PCCA provided certificate of analysis to prepare the dose required in the final intranasal spray product; the potency of all intranasal batches were further validated by external testing by Eagle Analytics Laboratory. The OT vehicle consists of glycerin and preserved water; the delivery device utilized was the Professional Compounding Centers of America - PCCA #35–1453 Metered Nasal Spray Pump 20 MM, which delivers 0.1 cc per pump. The total volume delivered was based on potency testing; participants administered one pump to each nares, alternating between pumps, and waiting a minimum of 10 s between each administration. The range of sprays administered was typically between 3 and 8 total sprays, with an oxytocin potency range of 27.2–49.1 IU/ml.
Subjective measures were repeated 5 min prior to scanning. During initial scanner tuning, localizing, and structural scanning, participants were shown relaxation images (i.e., 20 scenic pictures, each displayed for 30 s, and repeated if necessary).
fMRI data were acquired on a Siemens Trio or PRISMA 3.0 T scanner (Siemens Medical, Erlangen, Germany). Two scanners were used due to a system upgrade during the study. While the acquisition parameters were nearly identical for TRIO and PRISMA scanners, a 12-channel head coil was used on the TRIO and a 32-channel head coil was used on the PRISMA. Scanner model was used as a covariate in analyses. A high-resolution T1-weighted MPRAGE anatomical scan (TR = 1.9 ms, TE = 2.2 ms, flip angle = 9°, field of view = 256 mm, 1.0 mm) covering the entire brain and positioned using a sagittal scout image was acquired for co-registration and normalization of functional images. T2*-weighted gradient echo EPI images were acquired with the following parameters: TR = 2200 ms, TE = 35 ms, flip angle = 90°, 36 axial slices, FOV = 192 × 192 mm, thickness = 3 mm, in interleaved order. A gradient field map image was collected to match the spatial parameters of the EPI images. 162 brain volumes were collected on the TRIO scanner and 164 vol were collected on the PRISMA.
The MIST presents a series of arithmetic tasks with difficulty concurrently tuned by the program to the performance of the subject [22]. The task was run on a Mac book Pro synchronized with the scanner. Participants practiced the MIST before entering the scanner to become familiar with task structure and response entry. Three MIST runs were acquired in the scanner. Each run lasted 6 min, with the first and third runs followed by negative experimenter feedback which was scripted to highlight the artificially induced poor performance of the participant to induce social anxiety. Runs were modeled in a block design and contained three repeating elements. First, an active control or practice condition lasting 50 s (and of equivalent difficulty as task/experimental condition albeit without feedback or time limits). The screen presented an arithmetic problem to be solved in a large somewhat centered white box. A color bar at the top of the screen was divided into red, yellow and green components to provide feedback on performance relative to a fictional opponent (red indicated performance that was much worse, yellow indicated worse and green indicated better performance). This color bar was presented but not used during practice. Below the arithmetic problem to the right a rotary wheel with digits from 0 to 9 was used to provide the answer to each problem (all problems resolved to a single digit). Participants used two buttons in a response box to navigate and enter a response. Below the arithmetic problem to left was a box that provided feedback for each problem (correct/incorrect/timeout; timeout was only applicable for the experimental condition). Second, an experimental condition lasted 50 s with impending time limit and negative onscreen feedback reflecting relatively poor subject performance regardless of task performance. The display was the same as in the practice condition except that the color bar updated on each trial to show the participant’s performance relative to the opponent. In addition, a horizontal bar that moved from left to right showed the remaining time for each problem. Participants responded with a CURDES fORP 932 interface and Pyka handpad (CURRENT DESIGNS INC., Philadelphia, PA, USA). If participants responded within the time limit, on-screen feedback was provided in the feedback box (correct/incorrect) and the color bar updated. Another arithmetic problem was presented immediately. If the participant did not respond in time, the feedback box showed “timeout”, the color bar updated and the next problem appeared. The time limit and problem difficulty dynamically changed depending on a participant’s performance to adjust to greater or lesser difficulty accordingly. Consequently, some participants completed more problems than others but all task blocks lasted 50 s. However, the difficulty gradient for the mental arithmetic and the control condition speed that is built into the algorithm were both set to medium for all participants. Third, a rest condition of 20 s was presented which showed the color bar at the top, the rotary response display and blank boxes for the arithmetic problem and feedback. No responses were required during the rest block. After each MIST run and experimenter verbal feedback, participants were asked to rate their craving, stress and anxiety levels using a hand pad; ratings ranged from zero (“none”) to four (“severe”), with responses recoded from 1 to 5 for analysis. The rating period lasted 6 s. Following scanning, participants were debriefed and compensated.
2.4. Design
This study used a randomized, double-blind parallel design. The randomization of treatment assignment, study drug (OT) and placebo (PBO), was double-blind with 1:1 allocation. Randomization was implemented with a stratified random block design with half of the CUD and HC participants randomized to receive OT and half randomized to receive PBO.
2.5. Data analysis
The primary hypothesis of this study was that corticolimbic connectivity during acute social stress would be different between CUD and HC groups in the PBO condition, but not in the OT condition. The study was powered to detect a between-group difference (CD-PBO vs. HC-PBO) in the PPI beta estimate of OFC-amygdala connectivity (d = .9) and required n = 40 CD and 40 HC; 20 each randomized to OT and PBO in each diagnosis group, to achieve 80% power and a type 1 error rate of 5%. Further, to assure power for secondary study aims, the final sample size was determined to be able to detect a smaller effect size for subjective craving and anxiety (d = 0.75; d = 0.5) responses assessed across 3 MIST sessions (n = 40 per diagnosis/treatment group). The amygdala was chosen as a seed region with two orbitofrontal and one anterior cingulate regions of interest for analysis given their importance in stress and cue reactivity and moderation by childhood trauma in prior neuroimaging studies [14,21,32,[35], [36], [37], [38], [39]].
2.5.1. fMRI preprocessing
Data were analyzed using FMRI Expert Analysis Tool (FEAT) Version 6.00, part of FMRIB's Software Library (FSL; www.fmrib.ox.ac.uk/fsl). Preprocessing included motion correction using MCFLIRT [40], fieldmap-based EPI unwarping using PRELUDE + FUGUE [41,42]; non-brain removal using BET [43]; spatial smoothing using a Gaussian kernel of FWHM 7.0 mm; grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor; high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 120s). Registration to the MNI template using the high resolution structural scan was carried out using FLIRT [44].
2.5.2. Psychophysiological interaction (PPI) analysis
A PPI analysis [45] was conducted to examine amygdala functional connectivity changes due to social stress (MIST task versus practice conditions). Following initial preprocessing for each MIST run separately, left and right amygdala seeds for the PPI analysis were defined based on the left and right amygdala as defined in the Harvard-Oxford Subcortical Structural Atlas then thresholded at 25% probability and binarized. Time series were extracted in each amygdala seed region after resampling the seed regions into each subject’s native space.
Left and right amygdala time series served as the physiological regressors (with no convolution, temporal derivatives or temporal filtering applied). Each experimental condition (MIST task, practice, which were psychological regressors) was an explanatory variable with double-gamma HRF convolution applied and temporal derivatives added. Two interactions (PPI EV’s) were used for the a priori regions of interest (ROI) analysis described below: Left-amygdala seed x Stress Task > Practice contrast and right-amygdala seed x Stress Task > Practice contrast. The six rotations and displacements of head movement and head motion outliers (isolated using fsl_motion_outliers) were added as confound variables. Each MIST run was analyzed separately at the subject-level; however, only the two MIST runs that followed negative feedback from the experimenter were used in analyses in order to maximize effects of an acute social stressor. To maximize the sample size, participants with one or two usable MIST runs contributed data to this analysis.
ROIs were defined in the left orbitofrontal, right orbitofrontal, and anterior cingulate cortex using the Harvard-Oxford Cortical Structural Atlas regions thresholded at 60% probability and binarized. Featquery was used to extract parameter estimates (PEs) in each ROI for each PPI EV, each subject, and each usable MIST run. PEs reflected connectivity between the seed and ROI modulated by the Stress Task > Practice Task contrast, and connectivity could be positive or negative. For those participants with 2 usable MIST runs, PE’s were averaged over both usable runs. For those participants with only one usable run, PE values from the single usable run were submitted to the generalized linear models (GLMMs) described below. Hence, each participant with at least one usable MIST run contributed a PE value for each seed/ROI combination (left amygdala-ROFC; left amygdala-LOFC, left amygdala-ACC, right amygdala-ROFC, right amygdala-LOFC, right amygdala-ACC).
2.5.3. Analysis of amygdala connectivity
Generalized linear mixed effects regression models (GLMM) were developed for each seed/ROI combination for a total of 6 GLMMs (IBM SPSS Statistics v. 25, Chicago, IL). Diagnosis (CUD, HC), Treatment (OT, PBO), and Trauma Group (trauma absent, trauma present) served as predictors of functional connectivity (PE values) with sex, age, smoking status, education, scanner, absolute head motion included as model covariates. The primary analysis focused on childhood trauma as a moderator of treatment effects and/or diagnostic group. Wald χ2 values were reported for effects and interactions significant at p < = .05 and effect sizes are reported using Hedge’s g for unequal sample sizes. Because only a handful of studies have reported effects of OT on neural substrates in SUD, effect sizes are also reported for the effect of OT versus PBO in the context of significant interactions with treatment in order to inform the design of future studies on OT in SUD. Further, biological sex was examined as a possible moderator of treatment and diagnosis effects on study outcomes. False discovery rate (FDR; [46]) at 5% was used to control for multiple testing.
2.5.4. Analysis of subjective ratings
Subjective ratings of anxiety, stress (both CUD and HC) and craving (CUD group only) were analyzed with GLMMs. Subjective ratings following experimenter feedback were expressed as a difference score relative to the baseline ratings collected prior to the MIST. For stress and anxiety rating difference scores, Diagnosis (CUD, HC), Trauma Group (TRAUMA-, TRAUMA+) and Treatment Group (OT, PBO) served as predictors; for craving rating difference scores, predictors were Trauma Group and Treatment. For all analyses, sex, age, smoking status, education, scanner, absolute head motion were entered as covariates. GLMMs using sex as a moderator rather than trauma group were also conducted with trauma group, age, smoking status, education, scanner, absolute head motion added as covariates.
3. Results
3.1. Demographics and clinical characteristics
Demographic and clinical characteristics for the randomized sample stratified by diagnosis and treatment are presented in Table 1. The CUD group was older (M = 49.3, SD = 10.8) than HC (M = 43.2, SD = 10.0, p = .002), had fewer college graduates (16%) than HC (35%, p = .021) and a higher percentage of African Americans (81%) than HC (17%, p < .00001). The treatment groups were not different on any of the demographic or clinical measures. The cocaine use and history variables were not different across the two treatment groups among CUD participants.
Table 1.
Demographics and clinical characteristics.
| Cocaine Use Disorder (CUD) |
Healthy Controls (HC) |
||||
|---|---|---|---|---|---|
| Full Sample |
(n = 67) |
(n = 52) |
|||
| Measure | (n = 119) | PBO (n = 31) | OT (n = 36) | PBO (n = 25) | OT (n = 27) |
| Demographics | |||||
| Age in years (SD)a | 46.6 (10.8) | 48.1 (11.3) | 49.7 (10.5) | 40.9 (10.6) | 45.3 (9.1) |
| Female % (n) | 42 (50) | 45 (14) | 31 (11) | 36 (9) | 59 (16) |
| Educationb - College | |||||
| graduate % (nc) | 25 (29) | 17 (5) | 17 (6) | 36 (9) | 35 (9) |
| CigaretteSmoker % (nd) | 81 (95) | 77 (24) | 86 (31) | 76 (19) | 81 (21) |
| African American% (n)e | 53 (63) | 77 (24) | 83 (30) | 20 (5) | 15 (4) |
| Caucasian % (n) | 42 (50) | 23 (7) | 17 (6) | 68 (17) | 74 (20) |
| Other Race % (n) | 5 (6) | 0 (0) | 0 (0) | 12 (3) | 11 (3) |
| CTQ Total Score (SD) | 41.4 (17.3) | 43 (17.8) | 41 (15.8) | 38.8 (17.8) | 42.4 (19.1) |
| Cocaine Use Characteristics | |||||
| Age at 1st Use | 22.9 (6.6) | 22.7 (8.0) | 23.2 (5.2) | ||
| Total Years of Use | 22.6 (11.2) | 21.8 (11.9) | 23.2 (10.8) | ||
| Age at Dependence | |||||
| Onset | 32.9 (10.5) | 31.1 (10.9) | 34.6 (10.1) | ||
| Using Days | 42.2 (27.8) | 37.9 (29.3) | 45.8 (26.4) | ||
| CUD % Severe (n) | 74 (49) | 65 (20) | 83 (29) | ||
| Subjective Measures (change from baseline) | |||||
| Craving | -.15 (2.1) | -.15 (2.5) | -.14 (1.7) | ||
| Stress | 2.6 (2.9) | 3.0 (3.1) | 2.4 (3.1) | 2.4 (2.4) | 2.4 (2.9) |
| Anxiety | 2.1 (2.8) | 2.9 (3.0) | 1.4 (2.8) | 2.3 (2.3) | 2.1 (2.9) |
CUDs were older than HCs, t(117) = 3.17, p = .002.
CUDs had fewer college graduates than HCs, χ2(1, 117) = 5.35, p = .021.
Education was missing for 1 CUD and 1 HC participant.
Smoking status was missing for 1 HC participant.
CUDs were more likely to be African American, χ2(1, 119) = 47.1, p < .00001.
3.2. Psychophysiological interaction analysis ROI results
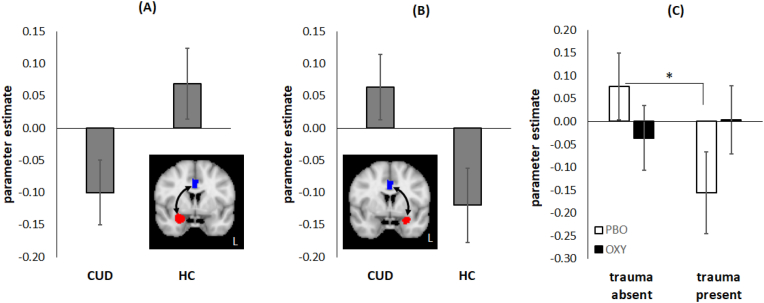
As shown in Fig. 1A–B, connectivity of the right amygdala and ACC was moderated by diagnosis, χ21 = 4.73, p = .03, with CUD showing negative connectivity and HC showing positive connectivity (g = 0.46). This pattern was reversed for left amygdala-ACC connectivity, with CUD showing positive and HC showing negative connectivity, χ21 = 4.68, p = .031, g = 0.46. These effects, however, were not significant after FDR correction.
Fig. 1.
Amygdala-Anterior Cingulate Cortex (ACC) connectivity results. The y-axis shows the parameter estimate for the psychophysiological interaction (PPI) of Task v. Practice contrast x Right/Left amygdala seed time series. The Task v. Practice contrast is the average connectivity over the two runs of the Montreal Imaging Stress Test (MIST) that followed experimenter feedback. (A) Main effect of diagnosis for the right amygdala seed (shown in red on inset) and ACC region of interest (ROI, shown in blue on inset). The cocaine use disorder (CUD) group shows negative connectivity whereas the healthy control (HC) group shows positive connectivity. (B) Main effect of diagnosis for the left amygdala seed (shown in red on inset) and ACC ROI (shown in blue on inset). The cocaine use disorder (CUD) group shows positive connectivity whereas the HC group shows negative connectivity. (C) Trauma Group × Treatment interaction for left amygdala seed and ACC ROI (see inset for panel B). Trauma-absent and trauma-present groups show significantly different connectivity on placebo (PBO) treatment, but not on oxytocin (OT) treatment. Error bars reflect standard error of the mean. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Left amygdala-ACC connectivity was also moderated by a Treatment Group x Trauma Group interaction, χ21 = 3.86, p = .05 (Fig. 1C). Connectivity was significantly different between TRAUMA-/PBO and TRAUMA+/PBO (p = .022, g = 0.60), but not significantly different for TRAUMA-/OT and TRAUMA+/OT (p = .13). Treatment effect size (Hedge’s g) was 0.33 for TRAUMA- and 0.41 for TRAUMA+, but the effect of treatment in each group was not significant (p’s > 0.13). This interaction, however, was not significant after FDR correction.
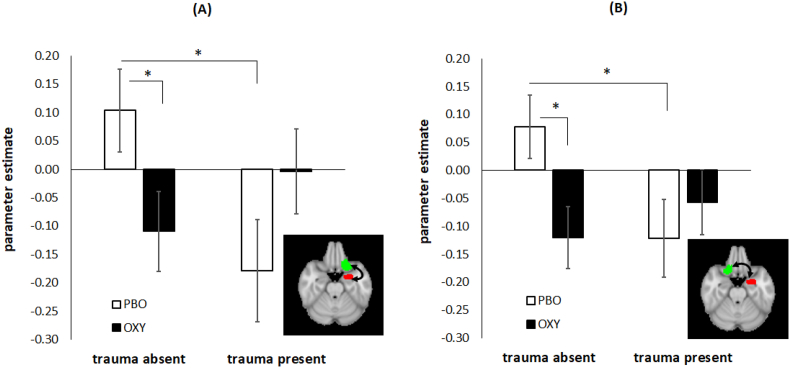
The same Trauma Group x Treatment Group interaction was observed for left amygdala-left OFC connectivity, χ21 = 7.3, p = .007 (Fig. 2A), with connectivity significantly different between TRAUMA-/PBO and TRAUMA+/PBO (p = .007, g = 0.47), but not significantly different for TRAUMA-/OT and TRAUMA+/OT (p = .13). Connectivity was significantly different on OT versus PBO for TRAUMA- (p = .016, g = 0.61) but not for TRAUMA+ (p = .13, g = 0.42). This interaction was significant after FDR correction (p = .021).
Fig. 2.
Amygdala-Orbitofrontal Cortex (OFC) connectivity results. The y-axis shows the parameter estimate for the psychophysiological interaction (PPI) of Task versus Practice contrast x Left amygdala seed time series. The Task v. Practice contrast is the average connectivity over the two runs of the Montreal Imaging Stress Test (MIST) that followed experimenter feedback. (A) Trauma Group × Treatment interaction for left amygdala seed (shown in red on inset) and left OFC region of interest (ROI, shown in green on inset). Trauma-absent and trauma-present groups show significantly different connectivity on placebo (PBO) treatment, but not on oxytocin (OT) treatment. (B) Trauma Group × Treatment interaction for left amygdala seed (shown in red on inset) and right OFC ROI (shown in green on inset). Trauma-absent and trauma-present groups show significantly different connectivity on PBO treatment, but not on OT treatment. Error bars reflect standard error of the mean. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Finally, left amygdala-right OFC connectivity was also moderated by the same Trauma Group x Treatment Group interaction, χ21 = 7.3, p = .007 (Fig. 2B). Connectivity was significantly different for TRAUMA-/PBO and TRAUMA+/PBO (p = .008, g = 0.70), but not significantly different for TRAUMA-/OT and TRAUMA+/OT (p = .253). Connectivity was significantly different on OT versus PBO for TRAUMA- (p = .003, g = 0.75) but not for TRAUMA+ (p = .28, g = 0.29). This interaction was significant after FDR correction (p = .021).
The analysis that included sex as a moderator yielded no significant main effects or interactions.
3.3. Subjective ratings
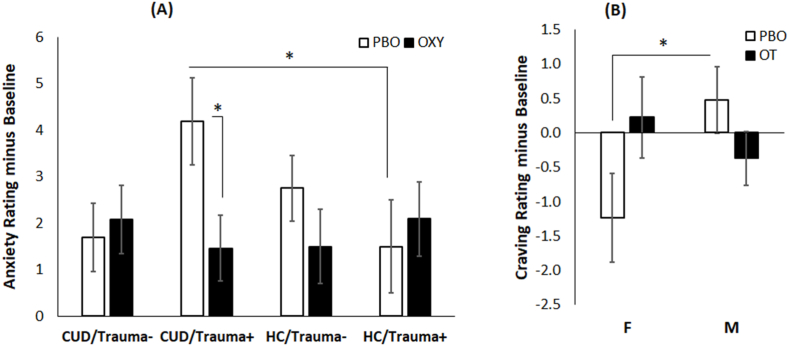
A significant Diagnosis x Trauma Group x Treatment Group interaction emerged for anxiety ratings, χ21 = 3.92, p = .048. As shown in Fig. 3A, the CUD/TRAUMA+/PBO group reported a greater increase in anxiety from baseline on the MIST runs that followed experimenter negative feedback compared to HC/TRAUMA+/PBO (p = .037) and compared to CUD/TRAUMA+/OT (p = .011, g = 0.86). Stress ratings did not differ significantly by diagnosis, trauma group or treatment group and craving ratings in CUD showed no effect of trauma or treatment group.
Fig. 3.
Results for subjective ratings. (A) The significant Diagnosis x Trauma group × Treatment interaction for anxiety ratings. The y-axis is the average anxiety rating following experimenter feedback after the first and second MIST runs minus the baseline anxiety rating collected prior to the MIST. The cocaine use disorder (CUD) with trauma present (Trauma+) group on PBO showed significantly higher anxiety ratings than the CUD/Trauma + group on OT and compared to the healthy control (HC) trauma present group on PBO. (B) The significant Sex × Treatment interaction for cocaine craving ratings in the CUD group. The y-axis is the average craving rating following experimenter feedback after the first and second MIST runs minus the baseline craving rating collected prior to the MIST. Females and males show significantly different cocaine craving ratings on PBO but not on OT. Error bars reflect standard error of the mean.
The analysis that included sex as a moderator yielded a main effect of sex for anxiety ratings, χ21 = 4.03, p = .045, g = 0.39, but no other main effects or interactions. Females reported higher anxiety (M = 2.8, SE = 0.41) than males (M = 1.7, SE = 0.34). Similarly, the main effect of sex was significant for stress ratings, χ21 = 5.8, p = .016, g = 0.47, with females reporting higher stress (M = 3.4, SE = 0.43) than males (M = 2.1, SE = 0.36). For craving ratings, there was a significant Treatment × Sex interaction, χ21 = 4.3, p = .038, as shown in Fig. 3B. CUD females who received PBO showed a reduction in craving (relative to baseline) during the MIST compared to CUD males who received PBO (p = .039, g = 0.87) but there were no sex differences for groups that received OT. The treatment effect size was g = 0.75 for CUD females and g = 0.44 for CUD males.
4. Discussion
The present study examined the effect of OT or PBO on functional connectivity of stress neurocircuitry using a social stress task during fMRI scanning, the Montreal Imaging Stress Test (MIST), in individuals with CUD and healthy controls. The primary hypothesis was that OT would normalize corticolimbic connectivity during the MIST in individuals with CUD, particularly in those who reported a history of childhood trauma and maltreatment. OT normalized connectivity with respect to childhood trauma status but not with respect to diagnosis (CUD v. HC). Left amygdala connectivity was significantly different for TRAUMA- and TRAUMA + who were administered PBO, but connectivity did not differ for TRAUMA- and TRAUMA + on OT. Although connectivity was not moderated by diagnosis, subjective ratings of anxiety during the MIST were higher for the CUD/TRAUMA+/PBO group compared to the HC/TRAUMA+/PBO group and compared to the CUD/TRAUMA+/OT. In addition, although sex was not a significant moderator of treatment effects on amygdala connectivity, stress and anxiety ratings were higher in females, and CUD males and females showed significantly different subjective craving response on PBO but not on OT. These findings are discussed more below in the context of other related literature findings and conceptual frameworks.
4.1. Moderation of stress reactivity by oxytocin and childhood trauma
The primary finding of the present study was that the effect of OT on corticolimbic connectivity during an acute social stress task was moderated by childhood trauma history rather than by CUD diagnosis or sex. Specifically, OT equalized left amygdala connectivity between trauma groups. This finding replicates other literature findings that report normalizing effects of OT on amygdala/prefrontal connectivity in anxiety disorders [31,47]. In addition, Flanagan et al. (2016) reported that higher childhood trauma was associated with higher cortisol in CUD participants who received intranasal PBO, but OT attenuated this association [18]. OT also reduced subjective anxiety ratings in the CUD group that reported childhood trauma in the present study. Other studies report that OT reduced withdrawal-related anxiety in alcohol use disorder [48] and reduced alcohol craving in anxiously attached individuals [49]. Collectively, these findings together with the present results indicate that OT can alleviate anxiety-related neurophysiological and functional brain response in clinical samples.
In the present study, the direction of corticolimbic connectivity was different for the two trauma groups on PBO. The TRAUMA-group showed positive left amygdala connectivity but the TRAUMA + group showed negative connectivity, which replicates prior findings of reduced amygdala-pregenual ACC resting state connectivity in individuals with early life stress [32]. Other studies report deactivation of the ventromedial PFC during the MIST in people with a history of childhood maltreatment [37] and deactivation of the hippocampus, amygdala, ventromedial PFC and ACC in individuals who show increased cortisol in response to the MIST [23]. The present study adds to these findings by showing altered functional connectivity in people with childhood trauma histories in some of the same corticolimbic circuitry. Importantly, the present study showed that these alterations are not significant when individuals received intranasal OT.
The finding of negative amygdala-prefrontal connectivity in the TRAUMA + group on PBO may seem at odds with other findings in the literature that amygdala-prefrontal connectivity is often stronger in individuals who report greater state anxiety [50] or in anxiety disorders [51]. However, given that self-reported anxiety during the MIST was not higher in the TRAUMA + participants in the present study (no main effect of trauma group, nor a significant Trauma × Treatment interaction for anxiety ratings), the TRAUMA + group may not be comparable to the groups that report greater state or trait anxiety in other studies that observe stronger or positive amygdala-prefrontal connectivity. In addition, other studies report that reduced amygdala-prefrontal connectivity in response to cocaine cues is associated with higher anxiety [35], and that amygdala-prefrontal connectivity is reduced in the resting state in generalized anxiety disorder [52]. Consequently, positivity or negativity of amygdala-prefrontal connectivity is malleable and appears to depend on the task at hand.
It should be noted that the effect size for OT treatment varied by region and trauma group in the present study. The OT effect size for left amygdala-ACC connectivity was numerically greater for the trauma present (g = .41) than for the trauma absent (g = 0.33) group, whereas the OT effect size for left amygdala-OFC connectivity was greater for the trauma absent (g’s = .61, .75) than for the trauma present group (g’s = 0.29, 0.42). Fan et al. (2014) reported stronger modulation of amygdala-ACC resting state connectivity by OT in individuals with lower early life stress compared to those reporting higher early life stress [32]. Importantly, this moderation of amygdala-ACC connectivity by OT was only in the context of a social stress task. The present study also showed a greater effect of OT on amygdala-prefrontal connectivity, but in this case, it was left amygdala to OFC connectivity whereas Fan et al. reported this effect for right amygdala to ACC connectivity. While Fan et al. did not find that OT moderated functional connectivity in individuals with early life stress, the present study found a larger effect size for OT on left amygdala-ACC connectivity in the trauma present group (but this effect did not survive FDR correction). Differences between the studies may be driven by differences in sex composition of the samples. Fan et al. included only males whereas the present study included both sexes. The greater representation of women in the present study may have skewed the findings toward significant effects of OT on left rather than right amygdala connectivity. Indeed, prior findings have suggested that the left amygdala is more strongly implicated in emotional processing in females and the right amygdala is more strongly recruited for males [33,53].
4.2. Moderation of stress reactivity by oxytocin and CUD diagnosis
The MIST task is designed to induce an acute social stress response which is more likely to engage stress neurocircuitry rather than reward pathways. This may explain stronger moderation of stress reactivity by childhood trauma history than by CUD diagnosis in the present study. The present focus on orbitofrontal and ACC regions of interest was justified by prior studies of social stress that used the MIST (e.g., Ref. [23]); however, these ROIs may not have targeted the circuitry most affected by OT in CUD. Habets et al. (2021) reported that brain areas differentially affected by OT in fMRI studies show a high correspondence with areas with high oxytocin gene expression, and that subcortical regions show greater correspondence than cortical areas across all fMRI task types that were examined [54]. In support of this, resting state fMRI and task-based functional connectivity findings report moderation of connectivity by childhood trauma or by OT in substance users in subcortical regions like the nucleus acumbens and striatum [20,21,35]. Kaag et al. (2018) also reported that amygdala-prefrontal coupling was not different between CUD and healthy controls, similar to the present finding, but this coupling was moderated by state anxiety. Amygdala-striatal coupling, however, was different between CUD and controls and that difference was further moderated by childhood trauma [35]. Had the present study used regions-of-interest from subcortical regions, particularly those that are part of the mesolimbic reward pathway, differences between CUD and HC may have emerged.
Although there was no differential effect of OT for CUD versus HC, there was a significant main effect of diagnostic group on connectivity of the left or right amygdala with the ACC, where right amygdala-ACC connectivity was negative in CUD and positive in HC, with the pattern reversed for left amygdala-ACC connectivity. Crunelle et al. (2015) report reduced amygdala-ACC connectivity in CUD compared to HC in an emotional face matching task, which they interpreted as evidence for poorer regulation of amygdala reactivity by the prefrontal cortex [36]. While that explanation is reasonable and could potentially explain the present finding for right amygdala ACC connectivity, it would not explain the opposite finding for stronger left amygdala-ACC connectivity in the present study. Differences between Crunelle et al. and the present study include different tasks (emotional face matching v. MIST) and samples – Crunelle et al. only included males but the present study included both sexes. As noted above, functional lateralization of the amygdala for emotional processing differs by sex. While sex differences and interactions did not emerge for amygdala connectivity in the present study, the inclusion of both men and women may have contributed to some of the opposite lateralization effects.
4.3. Moderation of stress reactivity by oxytocin and sex
In light of some other findings on sex differences in CUD stress reactivity [14,21,25], it was somewhat surprising that there were no sex differences or moderation of amygdala connectivity by sex in the present study. For example, Joseph et al. (2020) showed that CUD males with childhood trauma had greater amygdala reactivity in response to cocaine cues on PBO and OT reduced this reactivity whereas the opposite was true for CUD females with childhood trauma. The present study was not able to examine the Trauma × Sex interaction that was reported by Joseph et al. due to small cell sizes. In addition, Joseph et al. used a cross-over design for OT/PBO administration, but the present study used a parallel design. These differences across studies may explain why sex did not moderate corticolimbic connectivity for the MIST task. Another potential explanation for lack of sex differences at the neural level is that ovarian hormones (high v. low estradiol) can affect patterns of activation on the MIST [55]. Women in the present study may have had greater variability in functional connectivity during the MIST due to different hormone levels, which may have masked detecting sex differences.
Although sex differences were not observed at the neural level, females reported higher stress and anxiety than males, which has also been reported elsewhere for social stress tasks [16]. Another sex difference in the present study was that OT treatment had different effects on craving in CUD males and females. CUD/OT males showed reduced cocaine craving compared to CUD/PBO, whereas CUD/OT females showed higher craving compared to CUD/PBO females during the MIST task. A similar interaction was found for stress ratings where males with cannabis dependence showed reduced subjective stress on OT (vs. PBO) but females with cannabis dependence showed higher stress on OT in response to an acute social stressor; however, there were no sex differences in subjective craving [56]. Although Joseph et al. (2020) also reported no sex differences in cocaine craving, CUD men and women with childhood trauma showed opposite effects of OT on cocaine cue amygdala reactivity, similar to the present findings for cocaine craving. In addition, Joseph et al. reported a significant correlation between OT-related reduction in craving and OT-related reduction in amygdala response to cocaine cues only in CUD/TRAUMA + males and not in CUD/TRAUMA + females. A preclinical study similarly demonstrated that OT reduced food intake in both male and female rats but this reduction was greater in males [57]. In addition, that same study showed that estrogen disrupted the reduction in food intake in female rats. While the picture is still quite complicated, all of these studies demonstrate that OT can affect subjective craving, food rewardand stress responses differently in males and females and that reductions in craving or stress due to OT are more pronounced in males.
4.4. Limitations
The present study had some limitations that should be considered in interpreting the findings. The sample size was relatively small and did not enable testing a model in which diagnosis, trauma history and sex interactions were included in a single model as moderators of corticolimbic connectivity during acute social stress. Consequently, some of the variance carried by the trauma group variable could have been shared with sex or diagnosis. Future studies examining these moderators should use a larger sample size to ensure sufficient power to detect these effects and interactions. The present CUD sample was a non-treatment seeking sample which may have had less severe CUD than a treatment-seeking sample. This may have inadvertently hindered detection of treatment effect differences between CUD and HC. Also, the present study did not address some important potential sex-linked moderators of stress reactivity and subjective craving and stress, such as menstrual cycle phase or ovarian hormone levels. The present study also used a parallel design for OT and PBO administration rather than a cross-over design which has the built-in advantage of controlling for subject-specific variables when considering effects of OT treatment. The present analyses were restricted to a priori regions-of-interest in accord with the original aims of the funded study. However, this approach may have missed some important neural targets that were affected by OT or moderated by diagnosis, trauma history or sex. Future analyses should include voxel-wise analyses to examine a full spectrum of brain regions. Finally, not all individuals show a positive physiological stress response (e.g., increased cortisol; [23]) on the MIST, but the present analyses did not differentiate responders from non-responders. Future studies that investigate the effects of social stress using the MIST in CUD should be powered sufficiently to examine responders versus non-responders.
5. Conclusions
The present study demonstrated that OT has the potential to attenuate neural and subjective stress responses in an acute social stress paradigm. The effects of OT on corticolimbic connectivity were in the small to medium range (g = 0.294-0.747) and were moderated by childhood trauma history. Altlhough these stress-buffering effects were not specific to CUD diagnosis or sex., individuals with CUD and a history of childhood trauma reported reduced anxiety during acute social stress, an effect size that was quite strong (g = .867) indicating some benefit that may be translated into clinical use in the future. In fact, preclinical studies demonstrate that the central amygdala may be a major target mediating the anxiolytic effects of OT [38] and that infusion of OT directly into the central amygdala can attenuate stress-related alcohol reinstatement in rats [39]. The present study demonstrated that OT can modify amygdala connectivity in humans, which has potential clinical implications for OT as a therapeutic for social stress management in CUD.
Funding
This work was supported by the National Institutes of Health (R01 DA037968, K24 DA038240, U54 DA016511, UL1 TR001450) which was not involved in the design, data collection, analysis or interpretation of study results and was not involved in manuscript preparation and submission.
Author contributions
N. Baker, K. Brady, K. Hartwell, J. Joseph, M. Moran Santa Maria were involved in the conception and design of the study. J. Flanagan, K. Hartwell, J. Joseph, A. McRae-Clark, M. Moran Santa Maria were involved in the acquisition of data. N. Baker, N. Bustos, K. Crum, J. Joseph, A. McRae-Clark, were involved in data analysis and interpretation of data. N. Baker, K. Crum, J. Flanagan, J. Joseph, A. McRae-Clark were involved in drafting the article and revising the article for important intellectual content.
Declarations of competing interest
None.
Data statement
Raw or summarized data from this project are available upon request.
References
- 1.Volkow N.D., et al. Addiction circuitry in the human brain. Annu. Rev. Pharmacol. Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrer-Pérez C., et al. Oxytocin signaling as a target to block social defeat-induced increases in drug abuse reward. Int. J. Mol. Sci. 2021;22(5) doi: 10.3390/ijms22052372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahani V., Hurd Y.L., Bachi K. In: Miczek K.A., Sinha R., editors. Vol. 54. Springer; Cham: 2021. Neural Underpinnings of Social Stress in Substance Use Disorders. (Neuroscience of Social Stress. Current Topics in Behavioral Neurosciences). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schenk S., et al. Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neurosci. Lett. 1987;81(1–2):227–231. doi: 10.1016/0304-3940(87)91003-2. [DOI] [PubMed] [Google Scholar]
- 5.Sinha R., Catapano D., O'Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999;142(4):343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- 6.Waldrop A.E., et al. Community-dwelling cocaine-dependent men and women respond differently to social stressors versus cocaine cues. Psychoneuroendocrinology. 2010;35(6):798–806. doi: 10.1016/j.psyneuen.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dube S.R., et al. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111(3):564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- 8.Peverill M., et al. Atypical prefrontal-amygdala circuitry following childhood exposure to abuse: links with adolescent psychopathology. Child. Maltreat. 2019;24(4):411–423. doi: 10.1177/1077559519852676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Back S.E., et al. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106(1):21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Che X., et al. Oxytocin signaling in the treatment of drug addiction: therapeutic opportunities and challenges. Pharmacol. Ther. 2021;223 doi: 10.1016/j.pharmthera.2021.107820. [DOI] [PubMed] [Google Scholar]
- 11.McGregor I.S., Bowen M.T. Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm. Behav. 2012;61(3):331–339. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Leong K.C., et al. Oxytocin and rodent models of addiction. Int. Rev. Neurobiol. 2018;140:201–247. doi: 10.1016/bs.irn.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stauffer C.S., et al. A two-week pilot study of intranasal oxytocin for cocaine-dependent individuals receiving methadone maintenance treatment for opioid use disorder. Addiction Res. Theor. 2016;24(6):490–498. doi: 10.3109/16066359.2016.1173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph J.E., et al. Neural correlates of oxytocin and cue reactivity in cocaine-dependent men and women with and without childhood trauma. Psychopharmacology (Berl) 2019:1–13. doi: 10.1007/s00213-019-05360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee M.R., et al. Complexity of oxytocins effects in a chronic cocaine dependent population. Eur. Neuropsychopharmacol. 2014;24(9):1483–1491. doi: 10.1016/j.euroneuro.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherman B.J., et al. The effect of oxytocin, gender, and ovarian hormones on stress reactivity in individuals with cocaine use disorder. Psychopharmacology (Berl) 2020;237(7):2031–2042. doi: 10.1007/s00213-020-05516-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon S., Kim Y.K. The role of the oxytocin system in anxiety disorders. Adv. Exp. Med. Biol. 2020;1191:103–120. doi: 10.1007/978-981-32-9705-0_7. [DOI] [PubMed] [Google Scholar]
- 18.Flanagan J.C., et al. Effects of adverse childhood experiences on the association between intranasal oxytocin and social stress reactivity among individuals with cocaine dependence. Psychiatr. Res. 2015;229(1–2):94–100. doi: 10.1016/j.psychres.2015.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansson A.C., et al. Oxytocin reduces alcohol cue-reactivity in alcohol-dependent rats and humans. Neuropsychopharmacology. 2018;43(6):1235–1246. doi: 10.1038/npp.2017.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bach P., et al. Oxytocin modulates alcohol-cue induced functional connectivity in the nucleus accumbens of social drinkers. Psychoneuroendocrinology. 2019;109 doi: 10.1016/j.psyneuen.2019.104385. [DOI] [PubMed] [Google Scholar]
- 21.Joseph J.E., et al. Oxytocin-induced changes in intrinsic network connectivity in cocaine use disorder: modulation by gender, childhood trauma, and years of use. Front. Psychiatr. 2019;10:502. doi: 10.3389/fpsyt.2019.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dedovic K., et al. The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J. Psychiatr. Neurosci. 2005;30(5):319–325. [PMC free article] [PubMed] [Google Scholar]
- 23.Pruessner J.C., et al. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol. Psychiatr. 2008;63(2):234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 24.Orem T.R., et al. Amygdala and prefrontal cortex activity varies with individual differences in the emotional response to psychosocial stress. Behav. Neurosci. 2019;133(2):203–211. doi: 10.1037/bne0000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin E.L., et al. Consideration of sex as a biological variable in the translation of pharmacotherapy for stress-associated drug seeking. Neurobiol. Stress. 2021;15 doi: 10.1016/j.ynstr.2021.100364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehan D.V., et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatr. 1998;59(Suppl 20):22–33. ;quiz 34-57. [PubMed] [Google Scholar]
- 27.First M.B., et al. patient edition. 2002. Structured Clinical Interview for DSM-IV-TR axis I Disorders, Research Version. SCID-I/P. [Google Scholar]
- 28.Sobell L.C., Sobell M.B. In: Timeline Follow-back: A Technique for Assessing Self-Reported Alcohol Consumption in Measuring Alcohol Consumption: Psychosocial and Biomedical Methods. Litten R.Z., Allen J.P., editors. Humana Press; Totawa, NJ: 1992. pp. 41–72. [Google Scholar]
- 29.Bernstein D.P., Fink L. The Psychological Corporation; San Antonio, TX: 1998. Childhood Trauma Questionnaire: A Retrospective Self-Report Manual. [Google Scholar]
- 30.Childress A.R., McLellan A.T., O'Brien C.P. Conditioned responses in a methadone population: a comparison of laboratory, clinic, and natural settings. J. Subst. Abuse Treat. 1986;3(3):173–179. doi: 10.1016/0740-5472(86)90018-8. [DOI] [PubMed] [Google Scholar]
- 31.Dodhia S., et al. Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology. 2014;39(9):2061–2069. doi: 10.1038/npp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan Y., et al. Early life stress modulates amygdala-prefrontal functional connectivity: implications for oxytocin effects. Hum. Brain Mapp. 2014;35(10):5328–5339. doi: 10.1002/hbm.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sippel L.M., et al. Effects of intranasal oxytocin on threat- and reward-related functional connectivity in men and women with and without childhood abuse-related PTSD. Psychiatry Res. Neuroimaging. 2021;317 doi: 10.1016/j.pscychresns.2021.111368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacDonald E., et al. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36(8):1114–1126. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Kaag A.M., et al. Enhanced amygdala-striatal functional connectivity during the processing of cocaine cues in male cocaine users with a history of childhood trauma. Front. Psychiatr. 2018;9:70. doi: 10.3389/fpsyt.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crunelle C.L., et al. Dysfunctional amygdala activation and connectivity with the prefrontal cortex in current cocaine users. Hum. Brain Mapp. 2015;36(10):4222–4230. doi: 10.1002/hbm.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong X., et al. Childhood maltreatment experience influences neural response to psychosocial stress in adults: an fMRI study. Front. Psychol. 2019;10:2961. doi: 10.3389/fpsyg.2019.02961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huber D., Veinante P., Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308(5719):245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 39.Ballas H.S., et al. Oxytocin attenuates the stress-induced reinstatement of alcohol-seeking in male rats: role of the central amygdala. Biomedicines. 2021;9(12) doi: 10.3390/biomedicines9121919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenkinson M., et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 41.Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn. Reson. Med. 2003;49(1):193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- 42.Jenkinson M. Improving the registration of B0-disorted Epi images using calculated cost function weights: we 202. Neuroimage. 2004;22:e1544–e1545. [Google Scholar]
- 43.Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenkinson M., Smith S.M. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 45.Friston K.J., et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 46.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B. 1995;57(1):289–300. [Google Scholar]
- 47.Koch S.B., et al. Intranasal oxytocin normalizes amygdala functional connectivity in posttraumatic stress disorder. Neuropsychopharmacology. 2016;41(8):2041–2051. doi: 10.1038/npp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pedersen C.A. Oxytocin, tolerance, and the dark side of addiction. Int. Rev. Neurobiol. 2017;136:239–274. doi: 10.1016/bs.irn.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell J.M., et al. Intranasal oxytocin selectively modulates social perception, craving, and approach behavior in subjects with alcohol use disorder. J. Addiction Med. 2016;10(3):182–189. doi: 10.1097/ADM.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 50.Gold A.L., Morey R.A., McCarthy G. Amygdala-prefrontal cortex functional connectivity during threat-induced anxiety and goal distraction. Biol. Psychiatr. 2015;77(4):394–403. doi: 10.1016/j.biopsych.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gold A.L., et al. Amygdala-cortical connectivity: associations with anxiety, development, and threat. Depress. Anxiety. 2016;33(10):917–926. doi: 10.1002/da.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makovac E., et al. Alterations in amygdala-prefrontal functional connectivity account for excessive worry and autonomic dysregulation in generalized anxiety disorder. Biol. Psychiatr. 2016;80(10):786–795. doi: 10.1016/j.biopsych.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 53.Cahill L., et al. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an FMRI investigation. Learn. Mem. 2004;11(3):261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Habets P.C., McLain C., Meijer O.C. Brain areas affected by intranasal oxytocin show higher oxytocin receptor expression. Eur. J. Neurosci. 2021;54(7):6374–6381. doi: 10.1111/ejn.15447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Albert K., Pruessner J., Newhouse P. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology. 2015;59:14–24. doi: 10.1016/j.psyneuen.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reed S.C., et al. Sex differences in stress reactivity after intranasal oxytocin in recreational cannabis users. Pharmacol. Biochem. Behav. 2019;176:72–82. doi: 10.1016/j.pbb.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu C.M., et al. Sex differences and estrous influences on oxytocin control of food intake. Neuroscience. 2020;447:63–73. doi: 10.1016/j.neuroscience.2019.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw or summarized data from this project are available upon request.