Abstract
Living gymnosperms comprise four major groups: cycads, Ginkgo, conifers, and gnetophytes. Relationships among/within these lineages have not been fully resolved. Next generation sequencing has made available a large number of sequences, including both plastomes and single-copy nuclear genes, for reconstruction of solid phylogenetic trees. Recent advances in gymnosperm phylogenomic studies have updated our knowledge of gymnosperm systematics. Here, we review major advances of gymnosperm phylogeny over the past 10 years and propose an updated classification of extant gymnosperms. This new classification includes three classes (Cycadopsida, Ginkgoopsida, and Pinopsida), five subclasses (Cycadidae, Ginkgoidae, Cupressidae, Pinidae, and Gnetidae), eight orders (Cycadales, Ginkgoales, Araucariales, Cupressales, Pinales, Ephedrales, Gnetales, and Welwitschiales), 13 families, and 86 genera. We also described six new tribes including Acmopyleae Y. Yang, Austrocedreae Y. Yang, Chamaecyparideae Y. Yang, Microcachrydeae Y. Yang, Papuacedreae Y. Yang, and Prumnopityeae Y. Yang, and made 27 new combinations in the genus Sabina.
Keywords: Classification, Gymnosperms, Morphology, New tribe, Phylogenomics, Taxonomy
Highlights
• A new classification of extant gymnosperms is proposed that includes three classes (Cycadopsida, Ginkgoopsida, and Pinopsida), five subclasses (Cycadidae, Ginkgoidae, Cupressaceae, Pinidae, and Gnetidae), eight orders (Cycadales, Ginkgoales, Araucariales, Cupressales, Pinales, Ephedrales, Gnetales, and Welwitschiales), 13 families, and 86 genera.
• Six new tribes are described, including Acmopyleae, Austrocedreae, Chamaecyparideae, Microcachrydeae, Papuacedreae, and Prumnopityeae, and 27 new combinations are made in the genus Sabina.
1. Introduction
Gymnosperms are a group of early-diverging seed plants defined by having ovules or seeds completely or partly exposed (vs. ovules enclosed in carpels in angiosperms) (Yang et al., 2017). Gymnosperms constitute one of the four major groups of land plants (the other three being bryophytes, ferns and fern allies, and angiosperms) and possess some characters similar to ferns (e.g., circinnate young leaves in Cycas L., and presence of archegonia in female gametophytes and spermatozoids in cycads and Ginkgo L.), as well as similarities to angiosperms (e.g., possessing ovules/seeds and pollen tubes) (Christenhusz et al., 2011; Stevenson, 2013; Yang et al., 2017).
The origin of gymnosperms can be dated back to the mid-Devonian; Runcaria heinzelinii Stockmans is one of the oldest seed-like structures from Belgium with an age of ca. 385 myr (million years) (Gerrienne et al., 2004). This non-flowering seed plant group radiated and dominated land vegetation at the end of the Paleozoic (Rothwell and Scheckler, 1988). Almost all the living conifer families have a fossil record by the middle Jurassic (Taylor et al., 2009; Rothwell et al., 2012; Spencer et al., 2015; Farjon, 2018). The dominance of gymnosperms in the terrestrial vegetation has gradually declined since the origin and diversification of flowering plants in the Early Cretaceous. Today, gymnosperms retain their dominance in ca. 39% forests on Earth, being represented by four groups including 86 genera and over 1000 species (Christenhusz et al., 2011; Yang et al., 2017). The low species diversity of modern gymnosperms is largely explained by Cenozoic extinction of ancient lineages (Crisp and Cook, 2011); however many extant species originated from recent (Miocene) re-diversification (Davis and Schaefer, 2011; Nagalingum et al., 2011).
Relationships at the family level or above have been subject to debate. Is the family Ginkgoaceae related to cycads or to conifers? Are gnetophytes sister to Pinaceae or to conifers? Is the family Cephalotaxaceae nested within Taxaceae or not? (Wang and Ran, 2014; Yang et al., 2017; Ji et al., 2021). Recent phylogenomic data address these problems (Wu et al., 2013; Ran et al., 2018a; Stull et al., 2021). In addition, new progress has been made in a few families including Cycadaceae (Nagalingum et al., 2011; Salas-Leiva et al., 2013; Condamine et al., 2015), Ginkgoaceae (Zhao et al., 2019; Liu et al., 2021), Pinaceae (Ran et al., 2018b), Cupressaceae (Mao et al., 2012, 2019; Yang et al., 2012; Qu et al., 2017), Podocarpaceae (Knopf et al., 2012; Klaus and Matzke, 2020), and Taxaceae (Majeed et al., 2019; Ji et al., 2021; Xiong et al., 2021).
Taxonomy should be based on phylogeny. The most recent linear systematic arrangement of the gymnosperms is that of Christenhusz et al. (2011). This classification is widely adopted, although it contains a number of flaws. First, the classification is based on phylogenetic results using only a few molecular markers, so relationships among certain groups are not well-resolved (Chaw et al., 1997, 2000; Ran et al., 2010). Second, these authors ranked the four morphological groups into four subclasses, which do not reflect the latest phylogenomic advances concerning relationships of the four groups. For instance, whether Ginkgo is close to cycads or to conifers is unclear in their classification. Third, the subclass Pinidae is paraphyletic according to recent studies, with Gnetidae nested within Pinidae and they together form a monophyletic group sister to a clade including the remaining conifers (conifer II, Ran et al., 2018a; Stull et al., 2021). Recent phylogenomic results have reinforced this gnepine hypothesis (Ran et al., 2018a; Stull et al., 2021; Liu et al., 2022). Fourth, the sequence of the three families within the Gnetidae is not in accordance with their relationships. Christenhusz et al. (2011) treated the Welwitschiaceae at the beginning of the Gnetidae, followed by Gnetaceae and Ephedraceae successively. However, the Ephedraceae as the basal family within the Gnetidae is well-established (Lu et al., 2014; Ran et al., 2018a; Stull et al., 2021), so the sequence should be Ephedraceae, Gnetaceae, and Welwitschiaceae. Fifth, recent phylogenetic advances require that some generic changes are made, e.g., the Xanthocyparis complex and the Callitris complex (Terry et al., 2012; Zhu et al., 2018; Mao et al., 2019). Here we have summarized new advances in gymnosperm phylogeny and propose a new classification of extant gymnosperms.
2. Recent advances
2.1. Relationships of Ginkgo
Ginkgo represents an ancient lineage of seed plants containing a single relict species native to China (Lyu, 2019; Zhao et al., 2019). Recent phylogenomic studies have shown that a few natural populations of this relict species are sporadically scattered in eastern, southern and southwestern China (Gong et al., 2008; Zhao et al., 2019). Its phylogenetic relationships with other lineages have been controversial. Some authors have argued that Ginkgo is close to cycads (Wu et al., 2013; Li et al., 2017; Ran et al., 2018a; Stull et al., 2021), which is supported by a number of reproductive characters, e.g., motile spermatozoids, branched pollen tubes functioning as haustoria, boat-shaped pollen, fleshy seeds with the integument differentiating into three layers (outer fleshy sarcotesta, middle sclerotesta, and inner membranous endotesta), and slow growth of pollen tubes in the female gametophyte (Gifford and Foster, 1989). Other researchers have suggested that Ginkgo is close to conifers plus gnetophytes (Gugerli et al., 2001; Ran et al., 2010), because they all possess simple leaves, monopodial branching, pycnoxylic wood, female organs organized into a compound female cone (the spur shoot) with the long ovulate peduncle axillary to scale leaves that are helically arranged on the spur shoot (vs. large compound leaves, bifurcate branching or unbranched stems, manoxylic wood, female cones simple with megasporophylls directly and helically arranged) (Gifford and Foster, 1989; Douglas et al., 2007). Christenhusz et al. (2011) treated Ginkgo as a separate subclass (Ginkgoidae) parallel to the other three subclasses including Cycadidae, Pinidae, and Gnetidae. Recent phylogenomics based on thousands of single-copy nuclear genes have consistently suggested that ginkgophyte is sister to cycadophyte (Ran et al., 2018a; Stull et al., 2021; Liu et al., 2022). Considering its unusual morphology and phylogeny, we treat Ginkgo as a class here in this updated classification of gymnosperms.
2.2. Relationships of gnetophytes and conifers
Gnetophytes have a number of unusual characters, e.g., bisexual cones, vessels in secondary wood, ovules partially enclosed in one or two outer envelopes, dicot-like pinnately veined broad leaves in Gnetum L., modified to a membranous sheath in Ephedra L. and giant strap-shaped leaves in Welwitschia Hook. f., archegonia absent in Gnetum and Welwitschia but present in Ephedra (Gifford and Foster, 1989). This unique set of characters has led many people to believe that the gnetophytes are direct ancestors (pseudanthial hypothesis) or relatives of ancestors of the angiosperms (anthophyte and euanthial hypotheses) (Yang et al., 2004; Friis et al., 2011). However, molecular phylogenetic studies have indicated that gnetophytes constitute a monophyletic group that is either sister to the Pinaceae (gnepine hypothesis, Gugerli et al., 2001; Ran et al., 2018a; Stull et al., 2021; Liu et al., 2022), or to the Cupressophytes, including Sciadopityaceae, Cupressaceae and Taxaceae (gnecup hypothesis, Wu et al., 2013), or to the conifers (gnetifer hypothesis, Ran et al., 2010), or to other living gymnosperms (Wang and Ran, 2014), or to other living seed plants (Wang and Ran, 2014; Song et al., 2021; Niu et al., 2022). Nuclear genomes of Gnetaceae and Welwitschiaceae were published recently (Wan et al., 2018, 2021). Phylogenomic results based on thousands of single-copy nuclear genes reinforce the gnepine hypothesis and negate the monophyly of conifers (Ran et al., 2018a; Stull et al., 2021; Liu et al., 2022). Accordingly, the traditional concept of conifers should be revised in a new classification. Here we classify conifers and gnetophytes in the class Pinopsida, and further divide the class into three subclasses representing the three major lineages.
2.3. Araucariaceae and Podocarpaceae
The sister relationship of the conifer families Podocarpaceae and Araucariaceae has been firmly established. However, traditional classification based on morphology classified them into different orders (Pilger and Melchior, 1954; Delectis Florae Reipublicae Popularis Sinicae Agendae Academiae Sinicae Edita, 1978) as the two families possess quite different morphology of female cones: Podocarpaceae possess reduced and usually fleshy cones, while Araucariaceae have typical woody cones. However, recent phylogenetic studies have consistently suggested that Podocarpaceae and Araucariaceae form a clade sister to another clade including Sciadopityaceae, Cupressaceae, and Taxaceae (Li et al., 2017; Ran et al., 2018a; Stull et al., 2021; Liu et al., 2022). Hence, we classify the two families in the order Araucariales. In addition, we classified the family Podocarpaceae according to a recent phylogenomic study that resulted in robust intergeneric relationships within the family (Chen et al., 2022).
2.4. Cupressaceae, Sciadopityaceae, and Taxaceae
Traditionally, Sciadopitys Siebold & Zucc. was included in Taxodiaceae, which was kept separate from Cupressaceae s.s. (Delectis Florae Reipublicae Popularis Sinicae Agendae Academiae Sinicae Edita, 1978). Most phylogenetic studies have given rise to the clade including Sciadopityaceae, Cupressaceae s.l., and Taxaceae s.l. (Sciadopityaceae are sister to a clade including Cupressaceae s.l. and Taxaceae s.l.) (Ran et al., 2018a; Stull et al., 2021), though occasionally Sciadopityaceae are considered as the sister of the Podocarpaceae-Araucariaceae clade (Lu et al., 2014). Taxodiaceae, excluding Sciadopitys, are paraphyletic, and members of Taxodiaceae plus Cupressaceae s.s. constitute a monophyletic group (Gadek et al., 2000; Li and Yang, 2002; Lu et al., 2014; Ran et al., 2018a). These molecular phylogenetic results support a separation of Sciadopityaceae from Cupressaceae s.l. and incorporation of Taxodiaceae into Cupressaceae s.l. This phylogenetic result is tenable because it shows a tendency to reduction of female cones, i.e., Sciadopitys and the basal lineages of Cupressaceae usually having typical female cones, while late-diverged groups possess reduced female cones. In Taxaceae s.s., the female cones are so reduced and specialized that they have lost the seed scale complex typically present in other conifer families.
Phylogenetic relationships of Cephalotaxus Siebold & Zucc., the sole genus in Cephalotaxaceae, have been controversial. Lu et al. (2014) and Majeed et al. (2019) indicated that Cephalotaxus is nested within Taxaceae s.s., thus supporting an incorporation of Cephalotaxaceae into Taxaceae, i.e., Taxaceae s.l. However, a few recent studies determined that Cephalotaxus alone constitutes a clade sister to Taxaceae s.s. including Austrotaxus Compton, Taxus L., Pseudotaxus W.C. Cheng, Amentotaxus Pilg., and Torreya Arn. (Ran et al., 2018a; Ji et al., 2021; Stull et al., 2021; Liu et al., 2022), thus supporting a separation of Cephalotaxaceae from Taxaceae s.s. In an extreme case, the family Cephalotaxaceae was found to be sister to a clade including Taxaceae and Cupressaceae (Ran et al., 2010), which shows the necessity to separate Cephalotaxaceae from Taxaceae.
Morphologically, the two families are very different from one another in their female reproductive organs. In Cephalotaxaceae, the female organs are organized into a cone which consists of a number of morphological units, each of which consists of a vegetative bract subtending two axillary ovules. The ovule possesses a fleshy aril developed from the receptacle, the funiculus is more or less elongated into a short pedicel in the process of ripening. The family Taxaceae is a conifer without typical female cones-the female cone is highly reduced and specialized and consists of only a single seed having a fleshy aril; no seed scale complex is found in the family, whereas other conifer families possess female cones comprising bract scale and seed scale complexes. Some researchers have proposed that the aril of Taxaceae s.s. is a modified seed scale complex (see Taylor et al., 2009). However, a recent teratological and ontogenetic study suggests that the aril of Pseudotaxus is actually derived from a pair of leaves, not from a modified branch or integument (Dörken et al., 2018). This new observation is interesting and falsifies a long-standing hypothesis regarding the origin of the aril in Taxaceae s.s. Based on that study, it is clear that there is no seed scale complex in the family Taxaceae s.s. Despite the structural difference of reproductive organs, however, for taxonomic purposes, a separation of Cephalotaxaceae from Taxaceae seems reasonable, because most recent phylogenetic results support a sister relationship between Cephalotaxaceae and Taxaceae s.s., and the female cones are quite different in the two families.
2.5. Relationships of the Callitropsis−Cupressus−Hesperocyparis–Xanthocyparis complex
Farjon and his collaborators described a new genus collected from Vietnam, i.e., Xanthocyparis Farjon & T.H. Nguyên (Farjon et al., 2002). The nomenclature of this genus and subsequent molecular systematic studies resulted in debates and taxonomic chaos within Cupressus L. and related genera (Farjon et al., 2002; Little, 2006; de Laubenfels, 2009; Zhu et al., 2018). Farjon et al. (2002) included Callitropsis Oerst. in the genus Xanthocyparis, i.e., Xanthocyparis nootkatensis (D. Don) Farjon & Harder. This inclusion made the name Xanthocyparis superfluous and illegitimate in nomenclature. For further use of Xanthocyparis, Mill and Farjon (2006) proposed to conserve Xanthocyparis against Callitropsis, and the nomenclature committee accepted their proposal (Brummitt, 2007). Little (2006) constructed a phylogeny of Xanthocyparis, Callitropsis, and Cupressus s.s., and found that Cupressus is diphyletic; he thus treated Xanthocyparis, Callitropsis and the New World Cupressus as a single genus. He adopted Callitropsis s.l. as the correct generic name and made a number of new combinations. But phylogenetic relationships among these generic clades were not resolved in that study. Christenhusz et al. (2011) took a conservative option and incorporated Xanthocyparis, Callitropsis, and Hesperocyparis Bartel & R.A. Price into Cupressus, which is paraphyletic with respect to Juniperus (Mao et al., 2019). Terry et al. (2012), Zhu et al. (2018), and Mao et al. (2019) resolved the phylogenetic relationships of these genera and recognized Xanthocyparis s.s., Callitropsis s.s., Hesperocyparis (New World Cupressus) and Cupressus s.s. (Old World Cupressus). Callitropsis s.s. and Xanthocyparis s.s. do not form a clade in these recent phylogenies; thus, Farjon's incorporation of Callitropsis s.s. into Xanthocyparis s.l. is untenable.
2.6. Arceuthos, Juniperus and Sabina
In Flora Reipublicae Popularis Sinicae, Sabina Mill. was treated as a separate genus from Juniperus L. (Wang et al., 1978), but in Flora of China, Sabina was incorporated into the latter. Sabina is easily distinguished from Juniperus s.s. based on morphology, i.e., presence of acicular leaves in Juniperus s.s. (vs. existence of both acicular and scale leaves in Sabina), acicular leaves having joints at the base in Juniperus s.s. (vs. no joints at the base in Sabina), terminal buds prominent in Juniperus s.s. (vs. inconspicuous in Sabina), seed scales ternately arranged in Juniperus s.s. (vs. decussately or ternately arranged in Sabina), and ovules between seed scales in Juniperus s.s. (vs. ovules inserted on the ventral side of seed scales in Sabina). Adams (2008) reconstructed a phylogeny using nrITS and plastome trnC-trnD sequences which supported a subdivision of the genus into three sections: sect. Caryocedrus Endl., sect. Juniperus, and sect. Sabina (Mill.) Spach. Mao et al. (2010) obtained a well-resolved phylogeny of Juniperus s.l. and confirmed these three sections. Adams (2008) tabulated the morphological differences between the three sections including the leaf shape, female cone size, female cone texture, and female cone color. Reproductive differences between the three sections were corroborated in Jagel and Dörken (2015). Considering the agreement between the phylogenetic results and the morphological differences, it seems reasonable to divide the genus Juniperus into three genera, Juniperus s.s. (sect. Juniperus), Sabina (sect. Sabina), and Arceuthos Antoine & Kotschy (sect. Caryocedrus). Arceuthos possesses winter terminal buds, leaves with a basal abscission zone, large female cones (18–25 mm) with 3 whorls of ternately arranged seed scales and seeds fused together in a hard bony-textured nut. Juniperus s.s. is similar to Arceuthos in the presence of winter terminal buds and basal abscission zone of leaves, having valvate seed scales and bearing one seed on each fertile scale, but differs from the latter in the female cone being smaller (6–15 mm vs. 18–25 mm in Arceuthos) and having three free seeds (vs. fused seeds in Arceuthos). Sabina is markedly distinguished from Arceuthos and Juniperus s.s. by having leaves decurrent down stem, female cones with peltate seed scales and free, unfused seeds, but lacking winter terminal buds and basal abscission zone (Adams, 2008, 2014).
2.7. Callitris, Actinostrobus and Neocallitropsis
Recent phylogenetic studies have suggested that Actinostrobus Miq. and Neocallitropsis Florin are nested within Callitris Vent. (Piggin and Bruhl, 2010; Larter et al., 2017). This result inevitably leads to an inclusion of the oligo-specific Actinostrobus and the monotypic Neocallitropsis into Callitris. Here we have treated Actinostrobus and Neocallitropsis as synonyms of Callitris.
3. An updated classification
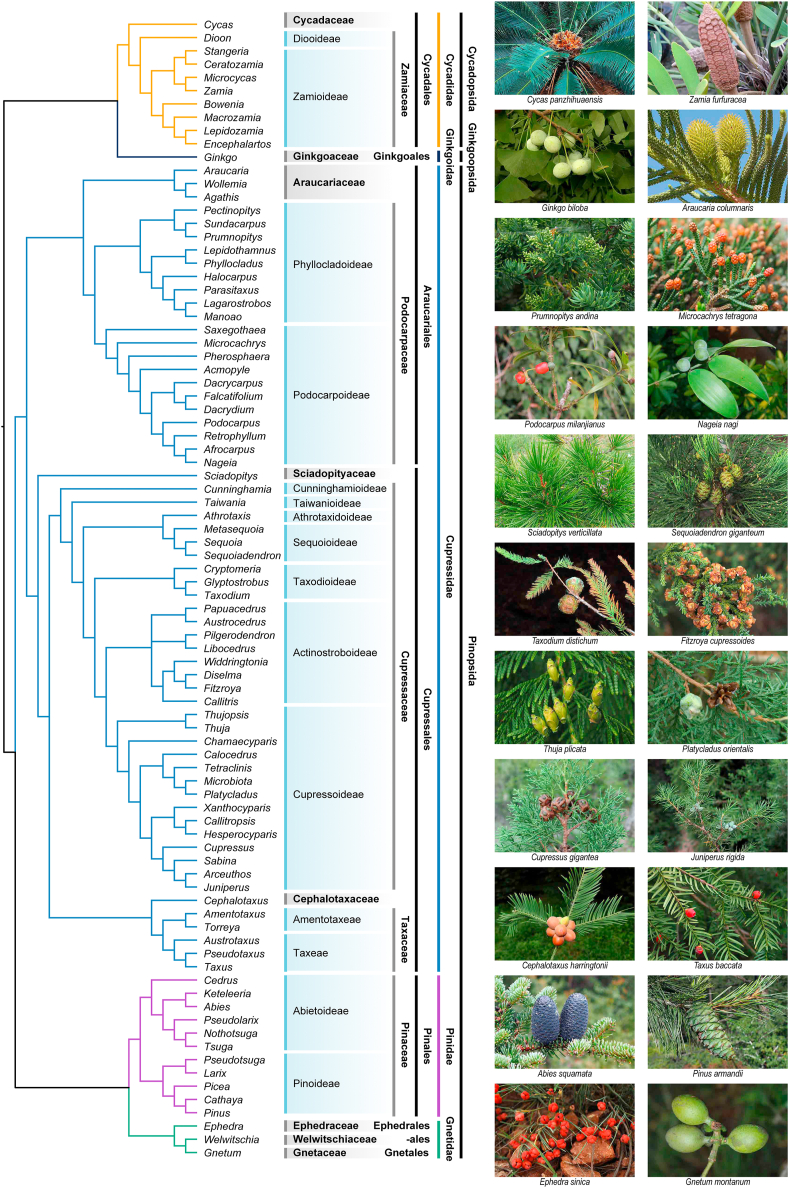
This new classification of extant gymnosperms (Acrogymnospermae/Pinophytina) contains three classes, five subclasses, eight orders, 13 families, and 86 genera (Table 1; Fig. 1). Our classification differs from Christenhusz et al. (2011) in a number of aspects. First, we divide the extant gymnosperms into three classes, i.e., Cycadopsida including cycads, Ginkgoopsida encompassing Ginkgo, and Pinopsida containing conifers and gnetophytes. There is no class category in the classification of Christenhusz et al. (2011). Second, we divide the living gymnosperms into five subclasses, Cycadidae, Ginkgoidae, and Gnetidae are the same as those in Christenhusz et al. (2011), but we classify conifers into two subclasses: Pinidae including Pinaceae, and Cupressidae containing the remaining conifer families (Araucariaceae, Podocarpaceae, Sciadopityaceae, Cupressaceae, Cephalotaxaceae, and Taxaceae). The linear sequence of the five subclasses is basically according to their phylogenetic relationships and morphological specialization, i.e., Cycadidae, Ginkgoidae, Cupressidae, Pinidae, and Gnetidae. Third, we treat Cephalotaxaceae as a separate family from Taxaceae; thus, there are 13 families in our new classification. Fourth, we recognize Arceuthos, Callitropsis, Hesperocyparis, Pectinopitys C.N. Page, Sabina, and Xanthocyparis, and accept Actinostrobus and Neocallitropsis as synonyms of Callitris, and Fokienia as synonymous with Chamaecyparis (Rushforth, 2007; Page, 2019; Wang et al., 2022). Fifth, we describe six new tribes (Acmopyleae, Austrocedreae, Chamaecyparideae, Microcachrydeae, Papuacedreae, and Prumnopityeae).
Table 1.
Diversity of gymnosperm families.
| Family | Genus | Species |
|---|---|---|
| Araucariaceae | 3 | 40 |
| Cephalotaxaceae | 1 | 10 |
| Cupressaceae | 31 | 169 |
| Cycadaceae | 1 | 126 |
| Ephedraceae | 1 | 70 |
| Ginkgoaceae | 1 | 1 |
| Gnetaceae | 1 | 46 |
| Pinaceae | 11 | 272 |
| Podocarpaceae | 20 | 181 |
| Sciadopityaceae | 1 | 1 |
| Taxaceae | 5 | 29 |
| Welwitschiaceae | 1 | 1 |
| Zamiaceae | 9 | 255 |
| Total | 86 | 1201 |
Fig. 1.
Cladogram displaying phylogenetic relationships among extant gymnosperms. Clade color indicates subclasses. Relationships are basically according to recent gymnosperm phylogenies (Lu et al., 2014; Ran et al., 2018a; Stull et al., 2021), Cupressaceae (Mao et al., 2010, 2012, 2019), Cycadales (Condamine et al., 2015), Pinaceae (Ran et al., 2018b), Podocarpaceae (Chen et al., 2022).
3.1. Taxonomic treatment
A synoptic classification is provided here; and the new classification with synonyms is in Appendix 1. In addition, we also provide a key and diagnoses of families in Appendix 2, and a global species list of extant gymnosperms in Appendix 3.
Pinophytina Cronquist, Takht. & Zimmerm. ex Reveal (松亚门)
Cl. 1. Cycadopsida Brongn. (苏铁纲)
Subcl. 1. Cycadidae Pax (苏铁亚纲)
Ord. 1. Cycadales Pers. ex Bercht. & J. Presl (苏铁目)
Fam. 1. Cycadaceae Pers. (苏铁科)
Cycas L.
Fam. 2. Zamiaceae Horan. (泽米铁科)
Subfam. 1. Diooideae Pilg. (双子铁亚科)
Trib. 1. Dioeae J. Schust. (双子铁族)
Dioon Lindl., nom. cons. (双子铁属)
Subfam. 2. Zamioideae Potonié (泽米铁亚科)
Trib. 2. Bowenieae J. Schust. (多羽铁族)
Bowenia Hook. f. (多羽铁属)
Trib. 3. Zamieae Miq. (泽米铁族)
Ceratozamia Brongn. (角状铁属)
Microcycas (Miq.) A. DC. (小苏铁属)
Stangeria T. Moore (蕨铁属)
Zamia L., nom. cons. (泽米铁属)
Trib. 4. Encephalarteae Miq. (非洲铁族)
Encephalartos Lehm. (非洲铁属)
Lepidozamia Regel (鳞木铁属)
Macrozamia Miq. (澳洲铁属)
Cl. 2. Ginkgoopsida Engl. (银杏纲)
Subcl. 2. Ginkgoidae Engl. (银杏亚纲)
Ord. 2. Ginkgoales Gorozh. (银杏目)
Fam. 3. Ginkgoaceae Engl., nom. cons. (银杏科)
Ginkgo L. (银杏属)
Cl. 3. Pinopsida Burnett (松纲)
Subcl. 3. Cupressidae Doweld (柏亚纲)
Ord. 3. Araucariales Gorozh. (南洋杉目)
Fam. 4. Araucariaceae Henkel et W. Hochst., nom. cons. (南洋杉科)
Agathis Salisb., nom. cons. (贝壳杉属)
Araucaria Juss. (南洋杉属)
Wollemia W.G. Jones et al. (凤尾杉属)
Fam. 5. Podocarpaceae Endl., nom. cons. (罗汉松科)
Subfam. 1. Phyllocladoideae W. Hochst. (叶枝杉亚科)
Trib. 1. Phyllocladeae Dumort. (叶枝杉族)
Halocarpus Quinn (白袍杉属)
Lagarostrobos Quinn (泣松属)
Lepidothamnus Phil. (沼银松属)
Manoao Molloy (白银松属)
Parasitaxus de Laub. (寄生松属)
Phyllocladus Rich. ex Mirb., nom. cons. (叶枝杉属)
Trib. 2. Prumnopityeae Y. Yang, trib. nov. (核果杉族)
Pectinopitys C.N. Page (梳叶杉属)
Prumnopitys Phil. (核果杉属)
Sundacarpus (J. Buchholz et N.E. Gray) C.N. Page (巽他杉属)
Subfam. 2. Podocarpoideae Beilschm. (罗汉松亚科)
Trib. 3. Saxegothaeeae Gordon (卓杉族)
Saxegothaea Lindl., nom. cons. (卓杉属)
Trib. 4. Microcachrydeae Y. Yang, trib. nov. (寒寿松族)
Microcachrys Hook. f. (寒寿松属)
Trib. 5. Pherosphaereae Pilg. (小泣松族)
Pherosphaera W. Archer bis (小泣松属)
Trib. 6. Acmopyleae Y. Yang, trib. nov. (绒袍杉族)
Acmopyle Pilg. (绒袍杉属)
Trib. 7. Dacrydieae Gordon (陆均松族)
Dacrycarpus (Endl.) de Laub. (鸡毛松属)
Dacrydium Sol. ex G. Forst. (陆均松属)
Falcatifolium de Laub. (镰叶杉属)
Trib. 8. Podocarpeae Dumort. (罗汉松族)
Afrocarpus (J. Buchholz & N.E. Gray) C.N. Page (非洲杉属)
Nageia Gaertn. (竹柏属)
Podocarpus L’Hér. ex Pers., nom. cons. (罗汉松属)
Retrophyllum C.N. Page (扭叶杉属)
Ord. 4. Cupressales Link (柏目)
Fam. 6. Sciadopityaceae Luerss. (金松科)
Sciadopitys Siebold & Zucc. (金松属)
Fam. 7. Cupressaceae Gray, nom. cons. (柏科)
Subfam. 1. Cunninghamioideae Silba (杉木亚科)
Cunninghamia R. Br., nom. cons. (杉木属)
Subfam. 2. Taiwanioideae L.Chu Li (台湾杉亚科)
Taiwania Hayata (台湾杉属)
Subfam. 3. Athrotaxidoideae L.Chu Li (密叶杉亚科)
Athrotaxis D. Don (密叶杉属)
Subfam. 4. Sequoioideae Quinn (红杉亚科)
Metasequoia Hu & W.C. Cheng, nom. cons. (水杉属)
Sequoia Endl., nom. cons. (红杉属)
Sequoiadendron J. Buchholz (巨杉属)
Subfam. 5. Taxodioideae Endl. ex K. Koch (落羽杉亚科)
Cryptomeria D. Don (柳杉属)
Glyptostrobus Endl. (水松属)
Taxodium Rich. (落羽杉属)
Subfam. 6. Actinostroboideae Koehne (星鳞柏亚科)
Trib. 1. Papuacedreae Y. Yang, trib. nov. (巴布亚柏族)
Papuacedrus H.L. Li (巴布亚柏属)
Trib. 2. Austrocedreae Y. Yang, trib. nov. (智利翠柏族)
Austrocedrus Florin & Boutelje (智利翠柏属)
Trib. 3. Libocedreae H.L. Li (甜柏族)
Libocedrus Endl. (甜柏属)
Pilgerodendron Florin (火地柏属)
Trib. 4. Diselmeae Henkel & W. Hochst. (寒寿柏族)
Diselma Hook. f. (寒寿柏属)
Fitzroya Hook. f. ex Lindl., nom. cons. (智利乔柏属)
Widdringtonia Endl. (南非柏属)
Trib. 5. Actinostrobeae Henkel et W. Hochst. (星鳞柏族)
Callitris Vent. (澳柏属)
Subfam. 7. Cupressoideae Sweet (柏木亚科)
Trib. 6. Thujopsideae Henkel et W. Hochst. (罗汉柏族)
Thuja L. (崖柏属)
Thujopsis Siebold et Zucc. ex Endl., nom. cons. (罗汉柏属)
Trib. 7. Chamaecyparideae Y. Yang, trib. nov. (扁柏族)
Chamaecyparis Spach (扁柏属)
Trib. 8. Tetraclineae H.L. Li (香漆柏族)
Calocedrus Kurz (翠柏属)
Microbiota Kom. (胡柏属)
Platycladus Spach (侧柏属)
Tetraclinis Mast. (香漆柏属)
Trib. 9. Cupresseae Dumort. (柏木族)
Arceuthos Antoine et Kotschy (合子刺柏属)
Callitropsis Oerst. (北美金柏属)
Cupressus L. (柏木属)
Hesperocyparis Bartel & R.A. Price (美洲柏木属)
Juniperus L. (刺柏属)
Sabina Mill. (圆柏属)
Xanthocyparis Farjon et T.H. Nguyên, nom. cons. (金柏属)
Fam. 8. Cephalotaxaceae Neger (三尖杉科)
Cephalotaxus Siebold et Zucc. ex Endl. (三尖杉属)
Fam. 9. Taxaceae Gray, nom. cons. (红豆杉科)
Trib. 1. Amentotaxeae W.C. Cheng et C.D. Chu (穗花杉族)
Amentotaxus Pilg. (穗花杉属)
Torreya Arn., nom. cons. (榧属)
Trib. 2. Taxeae Rich. ex Duby (红豆杉族)
Austrotaxus Compton (南紫杉属)
Pseudotaxus W.C. Cheng (白豆杉属)
Taxus L. (红豆杉属)
Subcl. 4. Pinidae Cronquist, Takht. et W. Zimm. (松亚纲)
Ord. 5. Pinales Gorozh. (松目)
Fam. 10. Pinaceae Spreng. ex F. Rudolphi, nom. cons. (松科)
Subfam. 1. Abietoideae Sweet (冷杉亚科)
Trib. 1. Cedreae Tiegh. (雪松族)
Cedrus Trew, nom. cons. (雪松属)
Trib. 2. Abieteae Dumort. (冷杉族)
Abies Mill. (冷杉属)
Keteleeria Carrière (油杉属)
Trib. 3. Pseudolariceae L. Chu Li (金钱松族)
Nothotsuga Hu ex C.N. Page (长苞铁杉属)
Pseudolarix Gordon, nom. cons. (金钱松属)
Tsuga (Endl.) Carrière (铁杉属)
Subfam. 2. Pinoideae W. Hochst. (松亚科)
Trib. 4. Lariceae Rouy (落叶松族)
Larix Mill. (落叶松属)
Pseudotsuga Carrière (黄杉属)
Trib. 5. Pineae Bluff et Fingerh. (松族)
Cathaya Chun et Kuang, nom. cons. (银杉属)
Picea A. Dietr. (云杉属)
Pinus L. (松属)
Subcl. 5. Gnetidae Pax (买麻藤亚纲)
Ord. 6. Ephedrales Dumort. (麻黄目)
Fam. 11. Ephedraceae Dumort., nom. cons. (麻黄科)
Ephedra Tourn. ex L. (麻黄属)
Ord. 7. Welwitschiales Skottsb. ex Reveal (百岁兰目)
Fam. 12. Welwitschiaceae Caruel, nom. cons. (百岁兰科)
Welwitschia Hook. f., nom. cons. (百岁兰属)
Ord. 8. Gnetales Mart. (买麻藤目)
Fam. 13. Gnetaceae Blume, nom. cons. (买麻藤科)
Gnetum L. (买麻藤属)
3.2. Description of new taxa
3.2.1. Acmopyleae Y. Yang, trib. nov. (绒袍杉族 新拟)
Type: Acmopyle Pilg.
Diagnosis. Dioecious small trees, evergreen. Leaves spirally arranged; dimorphic: small and scale-like on leading and reproductive shoots, bigger and foliar on lateral vegetative shoots. Male cones consisting of spirally arranged, triangular microsporophylls; pollen bisaccate. Female cones solitary, forming an irregular fleshy and verrucose receptacle. Seeds solitary, nearly erect at maturity, partially covered with a bluish, fleshy epimatium.
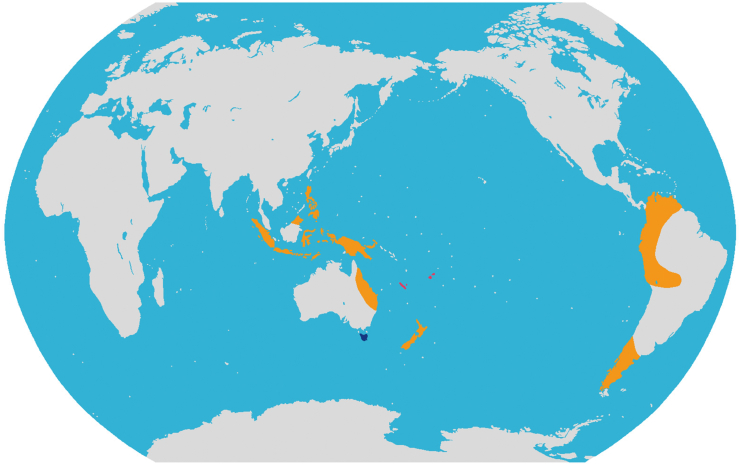
Diversity and distribution. The monotypic tribe belongs to Podocarpaceae and has two species that are disjunctly distributed in New Caledonia and Fiji (Fig. 2).
Fig. 2.
Distribution of the three new tribes of Podocarpaceae. Pink: Acmopyleae Y. Yang, trib. nov.; Blue: Microcachrydeae Y. Yang, trib. nov.; Orange: Prumnopityeae Y. Yang, trib. nov.
3.2.2. Austrocedreae Y. Yang, trib. nov. (智利翠柏族 新拟)
Type. Austrocedrus Florin & Boutelje
Diagnosis. Dioecious trees, evergreen. Leaves scale-like; lateral leaves thick, curving inwards at the apex; facial leaves blunt, having an indistinct gland on the adaxial surface and white stomatal bands on the abaxial surface; facial leaves slightly smaller than lateral ones. Female cones solitary, consisting of two pairs of seed scales, the lower pair smaller and reflexed; seed scales having a subapical bract apex. Seeds unequally 2-winged.
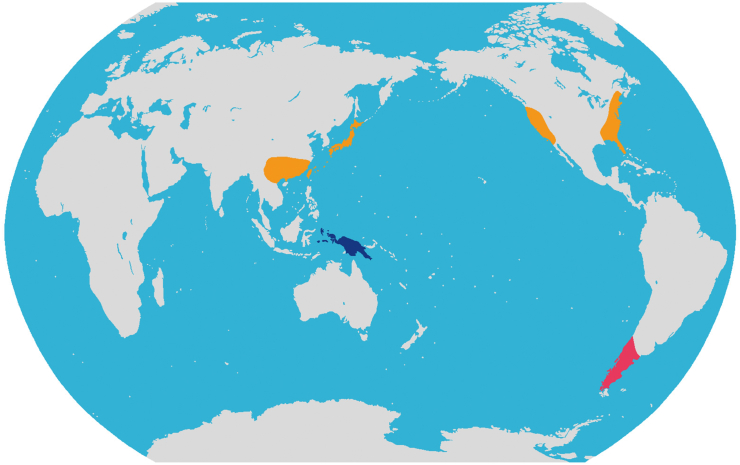
Diversity and distribution. The monotypic tribe belongs to Cupressaceae and is distributed in southern South America including S Argentina (Chubut, Neuquen, Rio Negro) and S Chile (Valparaiso, O´Higgins, Maule, Bio Bio, Araucania, Los Lagos, Reg. Metropolitana) (Fig. 3).
Fig. 3.
Distribution map of the three new tribes of Cupressaceae. Pink: Austrocedreae Y. Yang, trib. nov.; Orange Chamaecyparideae Y. Yang, trib. nov.; Blue: Papuacedreae Y. Yang, trib. nov.
3.2.3. Chamaecyparideae Y. Yang, trib. nov. (扁柏族 新拟)
Type. Chamaecyparis Spach
Diagnosis. Monoecious trees or rarely shrubs. Branchlets usually dorsiventrally flattened in fan-shaped or pinnately flattened sprays. Leaves opposite in four series, juvenile leaves subulate, mature leaves scale-like, green above, possessing white or greenish white stomatal bands below. Male cones ovoid or oblong, consisting of 2–3 pairs of microsporophylls. Female cones woody, globose to ovoid-globose, consisting of 4–8 pairs of decussate, peltate, woody seed scales, terminal pair fused. Seeds normally 2–4 per seed scale, possessing two lateral membranous wings.
Diversity and distribution. The monotypic tribe belongs to Cupressaceae and contains only one genus and is disjunctly distributed in E Asia (Japan and southern China) and N America (Fig. 3).
3.2.4. Microcachrydeae Y. Yang, trib. nov. (寒寿松族 新拟)
Type. Microcachrys Hook.f.
Diagnosis. Monoecious prostrate shrubs, evergreen. Branches spreading. Twigs 4-angled in cross section. Leaves triangular, small, usually lanceolate and decurrent on whip shoots, triangular and keeled on lateral twigs. Male cones terminal. Seed cones terminal, ovoid to globose, fleshy and bright red when ripe. Only one inverted seed per fertile seed scale, partially covered by an asymmetrical cup-like epimatium at the base.
Diversity and distribution. This monotypic tribe belongs to Podocarpaceae and includes only Microcachrys (one species), which is distributed in W Tasmania, Australia (Fig. 2).
3.2.5. Papuacedreae Y. Yang, trib. nov. (巴布亚柏族 新拟)
Type. Papuacedrus H.L. Li.
Diagnosis. Tall trees, rarely shrubs. Branches and twigs usually flattened, glabrous, with flattened leaves. Leaves on lateral twigs scale-like, decussate or in whorls of four; facial leaves smaller than lateral leaves; rhombic, lanceolate to oblong; two broad stomatal bands on abaxial surface. Male cones cylindrical, consisting of 8–30 peltate microsporophylls. Female cones terminal, consisting of two decussate pairs of seed scales, upper pair larger; seed scales having a small and recurved bract apex in the middle part. Seeds 2–4, angular ovoid or oblique; wings 2 on opposite sides, membranous.
Diversity and distribution. This monotypic tribe belongs to Cupressaceae and contains only Papuacedrus (one species), which is restricted to New Guinea and Maluku (the Moluccas) (Fig. 3).
3.2.6. Prumnopityeae Y. Yang, trib. nov. (核果杉族 新拟)
Type. Prumnopitys Phil.
Diagnosis. Dioecious trees, evergreen. Leaves flattened, linear, 1-veined, spirally arranged, appearing distichous, decurrent. Pollen cones aggregated into spikes. Seed cones consisting of one to a few spirally arranged bracts; only one single erect ovule axillary to the small distal bracts; lacking any fleshy receptacle at maturity. Seeds ovoid, with a drupe-like, thick, fleshy, colored, and globose or ovoid-elliptic epimatium.
Diversity and distribution. This tribe belongs to Podocarpaceae and includes eight species in three genera, i.e., Pectinopitys, Prumnopitys, Sundacarpus. These genera are distributed in SE Asia, East Australia, New Caledonia and New Zealand, and from Chile to Venezuela and Costa Rica (Fig. 2). Pectinopitys has six species that are distributed in South America (2), Costa Rica (1), New Zealand (1), New Caledonia (1), and Australia (1). Prumnopitys has three species that are distributed in South America (2), Fiji (1), and New Zealand (1). Sundacarpus is monotypic and distributed in Australia (NE-Queensland); New Guinea (Irian Jaya, Papua New Guinea); Bismarck Arch. (New Britain, New Ireland); Moluccas (Buru, Halmaheira, Morotai); Lesser Sunda Isl. (Timor, Flores, West Sumbawa, Lombok); Java; C-Sulawesi; SW-Sulawesi; Borneo; Sumatra; Philippines.
3.3. New combinations
We treat Sabina as a separate genus, and transfer 27 names from Juniperus to Sabina.
3.3.1. Sabina angosturana (R.P. Adams) Y. Yang et K.S. Mao, comb. nov. (安古斯图拉圆柏)
Basionym: Juniperus angosturana R.P. Adams, Biochem. Syst. Ecol. 22(7): 704 (1994).
Synonym: Juniperus monosperma var. gracilis Martínez, Anales Inst. Biol. Univ. Nac. Autón. México, Bot. 17: 111 (1946).
Distribution: Mexico (Coahuila, Hidalgo, Nuevo Leon, San Luis Potosí, Tamaulipas).
3.3.2. Sabina arizonica (R.P. Adams) Y. Yang et K.S. Mao, comb. nov. (亚利桑那圆柏)
Basionym: Juniperus coahuilensis var. arizonica R. P. Adams, Biochem. Syst. Ecol. 22(7): 708 (1994).
Synonym: Juniperus arizonica (R.P. Adams) R.P. Adams, Phytologia 88(3): 306 (2006).
Distribution: Mexico (Sonora); United States (Arizona, New Mexico).
3.3.3. Sabina ashei (J. Buchholz) Y. Yang et K.S. Mao, comb. nov. (阿什圆柏)
Basionym: Juniperus ashei J. Buchholz, Bot. Gaz. 9: 329, Figs. 1 and 2 (1930).
Distribution: Mexico (Coahuila); United States (Arkansas, Missouri, Oklahoma, Texas).
3.3.4. Sabina blancoi (Martínez) Y. Yang et K.S. Mao, comb. nov. (布兰科圆柏)
Basionym: Juniperus blancoi Martínez, Anales Inst. Biol. Univ. Nac. México 17: 73, Figs. 59−63 (1946).
Distribution: Mexico (Chihuahua, Durango, Mexico, Sonora).
3.3.5. Sabina coahuilensis (Martínez) Y. Yang et K.S. Mao, comb. nov. (科阿韦拉圆柏)
Basionym: Juniperus erythrocarpa var. coahuilensis Martínez, Anales Inst. Biol. Univ. Nac. México 17: 114, Figs. 95–97 (1946).
Synonym: Juniperus coahuilensis (Martínez) Gaussen ex R. P. Adams, Phytologia 74: 413 (1993).
Distribution: Mexico (Chihuahua, Coahuila, Durango, Nayarit, Tamaulipas, Zacatecas); United States (Texas).
3.3.6. Sabina comitana (Martínez) Y. Yang et K.S. Mao, comb. nov. (恰帕斯圆柏)
Basionym: Juniperus comitana Martínez, Anales Inst. Biol. Univ. Nac. México 15: 12, Figs. 5−8 (1944).
Distribution: Guatemala; Mexico (Chiapas).
3.3.7. Sabina compacta (Martínez) Y. Yang et K.S. Mao, comb. et stat. nov. (墨西哥圆柏)
Basionym: Juniperus monticola f. compacta Martínez, Anales Inst. Biol. Univ. Nac. México 17: 85, Figs. 71−73 (1946).
Synonyms: Juniperus monticola subsp. compacta (Martínez) Silba, J. Int. Conifer Preserv. Soc. 13(1): 12 (2006); Juniperus compacta (Martínez) R. P. Adams, Phytologia 89(3): 368 (2007), nom. inval.
Distribution: Guatemala; Mexico (Guerrero, Hidalgo, Jalisco, Mexico City, Mexico, Michoacán, Nuevo Leon, Puebla, Veracruz).
3.3.8. Sabina coxii (A.B. Jacks.) Y. Yang et K.S. Mao, comb. nov. (小果垂枝圆柏)
Basionym: Juniperus coxii A.B. Jacks., New Fl. & Silva v. 33 (1932).
Synonym: Juniperus recurva var. coxii (A.B. Jacks.) Melville, Kew Bull. 13: 533 (1959).
Distribution: Bhutan; China (Xizang, Yunnan); India (Sikkim); Myanmar.
3.3.9. Sabina deppeana (Steud.) Y. Yang et K.S. Mao, comb. nov. (鳄柏)
Basionym: Juniperus deppeana Steud., Nomencl. Bot. [Steudel] ed. 2, 835 (1841).
Distribution: Mexico (Chihuahua, Coahuila, Durango, Hidalgo, Oaxaca, Puebla, Sonora, Veracruz, Zacatecas); United States (Arizona, New Mexico, Texas).
3.3.10. Sabina durangensis (Martínez) Y. Yang et K.S. Mao, comb. nov. (杜兰戈圆柏)
Basionym: Juniperus durangensis Martínez, Anales Inst. Biol. Univ. Nac. México 17: 94, Figs. 80−84 (1946).
Distribution: Mexico (Aguascalientes, Chihuahua, Durango, Jalisco, Sonora, Zacatecas).
3.3.11. Sabina erectopatens (W.C. Cheng et L.K. Fu) Y. Yang et K.S. Mao, comb. nov. (松潘圆柏)
Basionym: Sabina vulgaris var. erectopatens W.C. Cheng et L.K. Fu, Acta Phytotax. Sin. 13(4): 86 (1975).
Synonyms: Juniperus sabina var. erectopatens (W.C. Cheng et L.K. Fu) Y.F. Yu et L.K. Fu, Novon 7(4): 444 (1998); Juniperus erectopatens (W.C. Cheng et L.K. Fu) R.P. Adams, Biochem. Syst. Ecol. 27(7): 723 (1999).
Distribution: China (Sichuan).
3.3.12. Sabina gracilior (Pilg.) Y. Yang et K.S. Mao, comb. nov. (海地圆柏)
Basionym: Juniperus gracilior Pilg., Symb. Antill. (Urban). 7(4): 481 (1913).
Distribution: Dominican Republic; Haiti.
3.3.13. Sabina grandis (R.P. Adams) Y. Yang et K.S. Mao, comb. nov. (加州大圆柏)
Basionym: Juniperus grandis R.P. Adams, Phytologia 88(3): 306 (2006).
Synonyms: Juniperus occidentalis subsp. australis Vasek, Brittonia 18: 352 (1966); Juniperus occidentalis var. australis (Vasek) A.H. Holmgren et N.H. Holmgren, Intermount. Fl. [Cronquist et al.] 239 (1972).
Distribution: United States (California, Nevada).
3.3.14. Sabina jaliscana (Martínez) Y. Yang et K.S. Mao, comb. nov. (哈利斯科圆柏)
Basionym: Juniperus jaliscana Martínez, Anales Inst. Biol. Univ. Nac. México 17: 69, Figs. 55−58 (1946).
Distribution: Mexico (Durango, Jalisco).
3.3.15. Sabina jarkendensis (Kom.) Y. Yang et K.S. Mao, comb. nov. (昆仑圆柏)
Basionym: Juniperus jarkendensis Kom., Bot. Mater. Gerb. Glavn. Bot. Sada R.S.F.S.R. 4: 181 (1923).
Synonyms: Sabina vulgaris var. jarkendensis (Kom.) C.Y. Yang, Fl. Reipubl. Popularis Sin. 7: 360 (1978); Juniperus sabina var. jarkendensis (Kom.) Silba, Phytologia 68(1): 33 (1990); Juniperus semiglobosa var. jarkendensis (Kom.) R.P. Adams, Phytologia 94(3): 354 (2012).
Distribution: China (Xinjiang, Xizang).
3.3.16. Sabina maritima (R.P. Adams) Y. Yang et K.S. Mao, comb. nov. (温哥华圆柏)
Basionym: Juniperus maritima R.P. Adams, Phytologia 89(3): 278 (2007).
Distribution: Canada (Alberta, British Columbia); Mexico (Chihuahua, Coahuila); United States (Arizona, Nebraska, Nevada, New Mexico, North Dakota, South Dakota, Utah).
3.3.17. Sabina martinezii (Pérez de la Rosa) Y. Yang et K.S. Mao, comb. nov. (马丁内斯圆柏)
Basionym: Juniperus martinezii Pérez de la Rosa, Phytologia 57: 81 (1985).
Synonyms: Juniperus flaccida var. martinezii (Pérez de la Rosa) Silba, Phytologia 58: 367 (1985); Juniperus flaccida subsp. martinezii (Pérez de la Rosa) Silba, J. Int. Conifer Preserv. Soc. 13(1): 9 (2006).
Distribution: Mexico (Jalisco: Cuatralba Mountains).
3.3.18. Sabina monticola (Martínez) Y. Yang et K.S. Mao, comb. nov. (中美山圆柏)
Basionym: Juniperus monticola Martínez, Anales Inst. Biol. Univ. Nac. México 17: 79 (1946).
Distribution: Guatemala; Mexico (Guerrero, Hidalgo, Jalisco, Mexico City, Mexico, Michoacán, Nuevo Leon, Puebla, Veracruz, Peña Sierra Nevada, Tamaulipas, San Luis Potosí).
3.3.19. Sabina morrisonicola (Hayata) Y. Yang et K.S. Mao, comb. nov. (玉山圆柏)
Basionym: Juniperus morrisonicola Hayata, J. Linn. Soc., Bot. 38: 298 (1908).
Synonym: Juniperus squamata var. morrisonicola (Hayata) H.L. Li et H. Keng, Taiwania 5: 81 (1954).
Distribution: China (Taiwan).
3.3.20. Sabina mucronata (R.P. Adams) Y. Yang et K.S. Mao, comb. nov. (短尖圆柏)
Basionym: Juniperus mucronata R.P. Adams, Biochem. Syst. Ecol. 28(2): 158 (2000).
Synonym: Juniperus blancoi var. mucronata (R.P. Adams) Farjon, World Checkl. & Bibliogr. Conifers ed. 2, 60 (2001).
Distribution: Mexico (Sonora, Chihuahua).
3.3.21. Sabina pinchotii (Sudw.) Y. Yang et K.S. Mao, comb. nov. (平肖圆柏)
Basionym: Juniperus pinchotii Sudw., Forest. Irrig. 11: 204, Figs. 1−4 (1905).
Synonym: Juniperus monosperma var. pinchotii (Sudw.) Melle, Phytologia 4: 29 (1952).
Distribution: United States (Oklahoma, Arizona, New Mexico, Texas); Mexico (Chihuahua, Coahuila, Durango, Nuevo Leon, Sonora, Tamaulipa Sacatecas, Zacatecas).
3.3.22. Sabina poblana (Martínez) Y. Yang et K.S. Mao, comb. nov. (普埃布罗圆柏)
Basionym: Juniperus flaccida var. poblana Martínez, Anales Inst. Biol. Univ. Nac. México 17: 31 (1946).
Synonym: Juniperus poblana (Martínez) R.P. Adams, Phytologia 88(3): 239 (2006); Juniperus flaccida subsp. poblana (Martínez) Silba, J. Int. Conifer Preserv. Soc. 13(1): 10 (2006).
Distribution: Mexico (Coahuila, Guerrero, Jalisco, Michoacán, Nuevo Leon, Oaxaca, Puebla, Zacatecas).
3.3.23. Sabina saltillensis (M.T. Hall) Y. Yang et K.S. Mao, comb. nov. (萨尔迪罗圆柏)
Basionym: Juniperus saltillensis M. T. Hall, Fieldiana, Bot. 34: 45, Figs. 1−7 (1971).
Synonym: Juniperus ashei var. saltillensis (H. M. Hall) Silba, Phytologia Mem. VII: 32 (1984).
Distribution: Mexico (Chihuahua, Coahuila, Nuevo Leon, Zacatecas).
3.3.24. Sabina saxicola (Britton et P. Wilson) Y. Yang et K.S. Mao, comb. nov. (岩生圆柏)
Basionym: Juniperus saxicola Britton et P. Wilson, Bull. Torrey Bot. Club 50: 35 (1923).
Synonyms: Juniperus barbadensis subsp. saxicola (Britton et P. Wilson) Borhidi, Acta Bot. Hung. 37: 90 (1992); Juniperus barbadensis var. saxicola (Britton et P. Wilson) Silba, J. Int. Conifer Preserv. Soc. 7(1): 25 (2000).
Distribution: Cuba.
3.3.25. Sabina standleyi (Steyerm.) Y. Yang et K.S. Mao, comb. nov. (斯坦利圆柏)
Basionym: Juniperus standleyi Steyerm., Publ. Field Mus. Nat. Hist., Bot. Ser. 23: 3 (1943).
Distribution: Guatemala; Mexico (Chiapas).
3.3.26. Sabina tsukusiensis (Masam.) Y. Yang et K.S. Mao, comb. nov. (清水圆柏)
Basionym: Juniperus tsukusiensis Masam., Bot. Mag. (Tokyo) 44: 50 (1930).
Synonyms: Juniperus chinensis var. tsukushiensis (Masam.) Masam., J. Soc. Trop. Agric. 2: 152 (1930); Juniperus chinensis subsp. tsukusiensis (Masam.) Silba, J. Int. Conifer Preserv. Soc. 13(1): 6 (2006).
Distribution: Japan (Kyushu); China (Taiwan).
3.3.27. Sabina zanonii (R.P. Adams) Y. Yang et K.S. Mao, comb. nov. (扎罗尼圆柏)
Basionym: Juniperus zanonii R.P. Adams, Phytologia 92(1): 112, Figs. 1–5 (2010).
Distribution: Mexico (Nuevo Leon).
Author contributions
YY conceived the idea and prepared the manuscript; BL prepared the cladograms; DKF and KR polished the English; YY, DKF, BL, KSM, LMG, SZZ, TW, KR, and ZXZ discussed, revised, and finalized the manuscript.
Declaration of competing interest
We declare that we have no conflict of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31970205, 31870206) and the Metasequoia funding of the Nanjing Forestry University, China. We thank Y.H.Ji and two other anonymous reviewers for their valuable suggestions.
Footnotes
Peer review under responsibility of Editorial Office of Plant Diversity.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2022.05.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Adams R.P. 2nd edition. Trafford Publishing Co.; Vancouver: 2008. Junipers of the World: the Genus Juniperus. [Google Scholar]
- Adams R.P. 4nd edition. Trafford Publishing Co.; Bloomington: 2014. Junipers of the World: the Genus Juniperus. [Google Scholar]
- Brummitt R.K. Report of the nomenclature committee for vascular plants: 59. Taxon. 2007;56:1289–1296. [Google Scholar]
- Chaw S.M., Parkinson C.L., Cheng Y., et al. Seed plant phylogeny inferred from all three plant genomes: monophyly of extant gymnosperms and origin of Gnetales from conifers. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4086–4091. doi: 10.1073/pnas.97.8.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaw S.M., Zharkikh A., Sung H.M., et al. Molecular phylogeny of extant gymnosperms and seed plant evolution: analysis of nuclear 18S rRNA sequences. Mol. Biol. Evol. 1997;14:56–68. doi: 10.1093/oxfordjournals.molbev.a025702. [DOI] [PubMed] [Google Scholar]
- Chen L., Jin W.T., Liu X.Q., et al. New insights into the phylogeny and evolution of Podocarpaceae inferred from transcriptomic data. Mol. Phylogenet. Evol. 2022;166:107431. doi: 10.1016/j.ympev.2021.107341. [DOI] [PubMed] [Google Scholar]
- Christenhusz M.J.M., Reveal J.L., Farjon A., et al. A new classification and linear sequence of extant gymnosperms. Phytotaxa. 2011;19:55–70. [Google Scholar]
- Condamine F.L., Nagalingum N.S., Marshall C.R., et al. Origin and diversification of living cycads: a cautionary tale on the impact of the branching process prior in Bayesian molecular dating. BMC Evol. Biol. 2015;15:65. doi: 10.1186/s12862-015-0347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp M.D., Cook L.G. Cenozoic extinctions account for the low diversity of extant gymnosperms compared with angiosperms. New Phytol. 2011;192:997–1009. doi: 10.1111/j.1469-8137.2011.03862.x. [DOI] [PubMed] [Google Scholar]
- Davis C.C., Schaefer H. Plant evolution: pulses of extinction and speciation in gymnosperm diversity. Curr. Biol. 2011;21:R995–R998. doi: 10.1016/j.cub.2011.11.020. [DOI] [PubMed] [Google Scholar]
- de Laubenfels D.J. Nomenclatural actions for the new world cypresses (Cupressaceae) Novon. 2009;19:300–306. [Google Scholar]
- Delectis Florae Reipublicae Popularis Sinicae Agendae Academiae Sinicae Edita . Science Press; Beijing: 1978. Flora Reipublicae Popularis Sinicae (Tom. 7). Gymnospermae; pp. 1–542. [Google Scholar]
- Dörken V.M., Nimsch H., Rudall P.J. Origin of the Taxaceae aril: evolutionary implications of seed-cone teratologies in Pseudotaxus chienii. Ann. Bot. 2018;123:133–143. doi: 10.1093/aob/mcy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas A.W., Stevenson D.W., Little D.P. Ovule development in Ginkgo biloba L., with emphasis on the collar and nucellus. Int. J. Plant Sci. 2007;168:1207–1236. [Google Scholar]
- Farjon A. Conifers of the world. Kew Bull. 2018;73:8. [Google Scholar]
- Farjon A., Hiep N.T., Harder D.K., et al. A new genus and species in Cupressaceae (Coniferales) from northern Vietnam, Xanthocyparis vietnamensis. Novon. 2002;12:179–189. [Google Scholar]
- Friis E.M., Crane P.R., Pedersen K.R. Cambridge University Press; Cambridge: 2011. Early Flowers and Angiosperm Evolution. [Google Scholar]
- Gadek P.A., Alpers D.L., Heslewood M.M., et al. Relationships within Cupressaceae sensu lato: a combined morphological and molecular approach. Am. J. Bot. 2000;87:1044–1057. [PubMed] [Google Scholar]
- Gerrienne P., Meyer-Berthaud B., Fairon-Demaret M., et al. Runcaria, a Middle Devonian seed plant precursor. Science. 2004;306:856–858. doi: 10.1126/science.1102491. [DOI] [PubMed] [Google Scholar]
- Gifford E.M., Foster A.S. W.H.Freeman and Company; New York: 1989. Morphology and Evolution of Vascular Plants. [Google Scholar]
- Gong W., Chen C., Dobes C., et al. Phylogeography of a living fossil: pleistocene glaciations forced Ginkgo biloba L. (Ginkgoaceae) into two refuge areas in China with limited subsequent postglacial expansion. Mol. Phylogenet. Evol. 2008;48:1094–1105. doi: 10.1016/j.ympev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Gugerli F., Sperisen C., Buchler U., et al. The evolutionary split of Pinaceae from other conifers: evidence from an intron loss and a multigene phylogeny. Mol. Phylogenet. Evol. 2001;21:167–175. doi: 10.1006/mpev.2001.1004. [DOI] [PubMed] [Google Scholar]
- Jagel A., Dörken V. Morphology and morphogenesis of the seed cones of the Cupressaceae-part Ii: Cupressoideae. Bull. Cupressus Conserv. Proj. 2015;4:51–78. [Google Scholar]
- Ji Y.H., Liu C.K., Landis J.B., et al. Plastome phylogenomics of Cephalotaxus (Cephalotaxaceae) and allied genera. Ann. Bot. 2021;127:697–708. doi: 10.1093/aob/mcaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus K.V., Matzke N.J. Statistical comparison of trait-dependent biogeographical models indicates that Podocarpaceae dispersal is influenced by both seed cone traits and geographical distance. Syst. Biol. 2020;69:61–75. doi: 10.1093/sysbio/syz034. [DOI] [PubMed] [Google Scholar]
- Knopf P., Schulz C., Little D.P., et al. Relationships within Podocarpaceae based on DNA sequence, anatomical, morphological, and biogeographical data. Cladistics. 2012;28:271–299. doi: 10.1111/j.1096-0031.2011.00381.x. [DOI] [PubMed] [Google Scholar]
- Larter M., Pfautsch S., Domec J.C., et al. Aridity drove the evolution of extreme embolism resistance and the radiation of conifer genus Callitris. New Phytol. 2017;215:97–112. doi: 10.1111/nph.14545. [DOI] [PubMed] [Google Scholar]
- Li C.X., Yang Q. Divergence time estimates for major lineages of Cupressaceae (s.l.) Acta Phytotax. Sin. 2002;40:323–333. [Google Scholar]
- Li Z., de la Torre A.R., Sterck L., et al. Single-copy genes as molecular markers for phylogenomic studies in seed plants. Genome Biol. Evol. 2017;9:1130–1147. doi: 10.1093/gbe/evx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little D.P. Evolution and circumscription of the true cypresses (Cupressaceae: Cupressus) Syst. Bot. 2006;31:461–480. [Google Scholar]
- Liu H.L., Wang X.B., Wang G.B., et al. The nearly complete genome of Ginkgo biloba illuminates gymnosperm evolution. Nat. Plants. 2021;7:748–756. doi: 10.1038/s41477-021-00933-x. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang S., Li L., et al. The Cycas genome and the early evolution of seed plants. Nat. Plants. 2022;8:389–401. doi: 10.1038/s41477-022-01129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu J. Ginkgo history told by genomes. Nat. Commun. 2019;10:4201. doi: 10.1038/s41477-019-0529-2. [DOI] [PubMed] [Google Scholar]
- Lu Y., Ran J.H., Guo D.M., et al. Phylogeny and divergence times of gymnosperms inferred from single-copy nuclear genes. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0107679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed A., Singh A., Choudhary S., et al. RNAseq-based phylogenetic reconstruction of Taxaceae and Cephalotaxaceae. Cladistics. 2019;35:461–468. doi: 10.1111/cla.12362. [DOI] [PubMed] [Google Scholar]
- Mao K.S., Hao G., Liu J.Q., et al. Diversification and biogeography of Juniperus (Cupressaceae): variable diversification rates and multiple intercontinental dispersals. New Phytol. 2010;188:254–272. doi: 10.1111/j.1469-8137.2010.03351.x. [DOI] [PubMed] [Google Scholar]
- Mao K.S., Milne R.I., Zhang L.B., et al. Distribution of living Cupressaceae reflects the breakup of Pangea. Proc. Natl. Acad. Sci. U.S.A. 2012;109:7793–7798. doi: 10.1073/pnas.1114319109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao K.S., Ruhsam M., Ma Y.Z., et al. A transcriptome-based resolution for a key taxonomic controversy in Cupressaceae. Ann. Bot. 2019;123:153–167. doi: 10.1093/aob/mcy152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill R.R., Farjon A. (1710) Proposal to conserve the name Xanthocyparis against Callitropsis Oerst. (Cupressaceae) Taxon. 2006;55:229–231. [Google Scholar]
- Nagalingum N.S., Marshall C.R., Quental T.B., et al. Recent synchronous radiation of a living fossil. Science. 2011;334:796–799. doi: 10.1126/science.1209926. [DOI] [PubMed] [Google Scholar]
- Niu S.H., Li J., Bo W.H., et al. The Chinese pine genome and methylome unveil key features of conifer evolution. Cell. 2022;185:204–217. doi: 10.1016/j.cell.2021.12.006. [DOI] [PubMed] [Google Scholar]
- Page C.N. New and maintained genera in the taxonomic alliance of Prumnopitys s.l. (Podocarpaceae), and circumscription of a new genus: Pectinopitys. New Zeal. J. Bot. 2019;57:137–153. [Google Scholar]
- Piggin J., Bruhl J.J. Phylogeny reconstruction of Callitris Vent. (Cupressaceae) and its allies leads to inclusion of Actinostrobus within Callitris. Austral. Syst. Bot. 2010;23:69–93. [Google Scholar]
- Pilger R., Melchior H. In: A. Engler's Syllabus der Pflanzenfamilien. Band 1. Allgemeiner Teil Bakterien bis Gymnospermen. Melchior H., Werdermann E., editors. Gebruder Borntraeger; Berlin-Nikolassee: 1954. XVI: abteilung: gymnospermae. Nacktsamer. (Archispermae) pp. 312–344. (Allgemeiner Teil Bakterien bis Gymnospermen). [Google Scholar]
- Qu X.J., Wu C.S., Chaw S.M., et al. Insights into the existence of isomeric plastomes in Cupressoideae (Cupressaceae) Genome Biol. Evol. 2017;9:1110–1119. doi: 10.1093/gbe/evx071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran J.H., Gao H., Wang X.Q. Fast evolution of the retroprocessed mitochondrial rps3 gene in conifer II and further evidence for the phylogeny of gymnosperms. Mol. Phylogenet. Evol. 2010;54:136–149. doi: 10.1016/j.ympev.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Ran J.H., Shen T.T., Wang M.M., et al. Phylogenomics resolves the deep phylogeny of seed plants and indicates partial convergent or homoplastic evolution between Gnetales and angiosperms. Proc. Roy. Soc. B Biol. Sci. 2018;285:20181012. doi: 10.1098/rspb.2018.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran J.H., Shen T.T., Wu H., et al. Phylogeny and evolutionary history of Pinaceae updated by transcriptomic analysis. Mol. Phylogenet. Evol. 2018;129:106–116. doi: 10.1016/j.ympev.2018.08.011. [DOI] [PubMed] [Google Scholar]
- Rothwell G.W., Mapes G., Stockey R.A., et al. The seed cone Eathiestrobus gen. nov.: fossil evidence for a Jurassic origin of Pinaceae. Am. J. Bot. 2012;99:708–720. doi: 10.3732/ajb.1100595. [DOI] [PubMed] [Google Scholar]
- Rothwell G.W., Scheckler S.E. In: Origin and Evolution of Gymnosperms. Beck C.B., editor. Columbia University Press; New York: 1988. Chapter 2: biology of ancestral gymnosperms; pp. 85–134. [Google Scholar]
- Rushforth K. Notes on the Cupressaceae in Vietnam. Tap Chí Sinh Hoc. J. Biol. Hanoi, Vietnam. 2007;29:32–39. [Google Scholar]
- Salas-Leiva D.E., Meerow A.W., Calonje M., et al. Phylogeny of the cycads based on multiple single-copy nuclear genes: congruence of concatenated parsimony, likelihood and species tree inference methods. Ann. Bot. 2013;112:1263–1278. doi: 10.1093/aob/mct192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Fu F.F., Yang L.L., et al. Taxus yunnanensis genome offers insights into gymnosperm phylogeny and taxol production. Commun. Biol. 2021;4:1203. doi: 10.1038/s42003-021-02697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer A.T., Mapes G., Bateman R.M., et al. Middle Jurassic evidence for the origin of Cupressaceae: a paleobotanical context for the roles of regulatory genetics and development in the evolution of conifer seed cones. Am. J. Bot. 2015;102:942–961. doi: 10.3732/ajb.1500121. [DOI] [PubMed] [Google Scholar]
- Stevenson D.W. Chapter 5: gymnosperms. Ann. Plant Rev. 2013;45:141–162. [Google Scholar]
- Stull G.W., Qu X.J., Parins-Fukuchi C., et al. Gene duplications and phylogenomic conflict underlie major pulses of phenotypic evolution in gymnosperms. Nat. Plants. 2021;7:1015–1025. doi: 10.1038/s41477-021-00964-4. [DOI] [PubMed] [Google Scholar]
- Taylor T., Taylor E.L., Krings M. second ed. Academic Press; Boston: 2009. Paleobotany: the Biology and Evolution of Fossil Plants. [Google Scholar]
- Terry R.G., Bartel J.A., Adams R.P. Phylogenetic relationships among the New World cypresses (Hesperocyparis; Cupressaceae): evidence from noncoding chloroplast DNA sequences. Plant Syst. Evol. 2012;298:1987–2000. [Google Scholar]
- Wan T., Liu Z.M., Leitch I.J., et al. The Welwitschia genome reveals a unique biology underpinning extreme longevity in deserts. Nat. Commun. 2021;12:4247. doi: 10.1038/s41467-021-24528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan T., Liu Z.M., Li L.F., et al. A genome for gnetophytes and early evolution of seed plants. Nat. Plants. 2018;4:82–89. doi: 10.1038/s41477-017-0097-2. [DOI] [PubMed] [Google Scholar]
- Wang W.T., Cheng W.C., Fu L.K., et al. Delectis Florae Reipublicae Popularis Sinicae Agendae Academiae Sinicae Edita (Ed) Flora Reipublicae Popularis Sinicae (Tom. 7) Gymnospermae. Science Press; Beijing: 1978. Juniperoideae Pilger; pp. 347–398. [Google Scholar]
- Wang X.Q., Ran J.H. Evolution and biogeography of gymnosperms. Mol. Phylogenet. Evol. 2014;75:24–40. doi: 10.1016/j.ympev.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Wang Y., Ruhsam M., Milne R., et al. Incomplete lineage sorting and local extinction shaped the complex evolutionary history of the Paleogene relict conifer genus, Chamaecyparis (Cupressaceae) Mol. Phylogenet. Evol. 2022;172:107485. doi: 10.1016/j.ympev.2022.107485. [DOI] [PubMed] [Google Scholar]
- Wu C.S., Chaw S.M., Huang Y.Y. Chloroplast phylogenomics indicates that Ginkgo biloba is sister to cycads. Genome Biol. Evol. 2013;5:243–254. doi: 10.1093/gbe/evt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X.Y., Gou J.B., Liao Q.G., et al. The Taxus genome provides insights into paclitaxel biosynthesis. Nat. Plants. 2021;7:1026–1036. doi: 10.1038/s41477-021-00963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Fu D.Z., Wang Q. Origin of flowers: hypotheses and evidence. Acta Bot. Boreal.-Occident. Sin. 2004;24:2366–2380. [Google Scholar]
- Yang Y., Wang Z.H., Xu X.T. Shanghai Scientific and Technical Publishers; Shanghai: 2017. Taxonomy and Distribution of Global Gymnosperms. [Google Scholar]
- Yang Z.Y., Ran J.H., Wang X.Q. Three genome-based phylogeny of Cupressaceae s.l.: further evidence for the evolution of gymnosperms and Southern Hemisphere biogeography. Mol. Phylogenet. Evol. 2012;64:452–470. doi: 10.1016/j.ympev.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Zhao Y.P., Fan G.Y., Yin P.P., et al. Resequencing 545 ginkgo genomes across the world reveals the evolutionary history of the living fossil. Nat. Commun. 2019;10:4201. doi: 10.1038/s41467-019-12133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A., Fan W.S., Adams R.P., et al. Phylogenomic evidence for ancient recombination between plastid genomes of the Cupressus-Juniperus-Xanthocyparis complex (Cupressaceae) BMC Evol. Biol. 2018;18:137. doi: 10.1186/s12862-018-1258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.