Abstract
Purpose
To determine the association between rare genetic variants in complement factor H (CFH) and phenotypic features in age-related macular degeneration (AMD) patients from the Coimbra Eye Study (CES).
Methods
AMD patients from the Incidence CES (NCT02748824) underwent ophthalmologic examination and color fundus photography, spectral-domain optical coherence tomography (SD-OCT), fundus autofluorescence, and near-infrared imaging. Multimodal phenotypic characterization was carried out in a centralized reading center. The coding and splice-site regions of the CFH gene were sequenced through single-molecule molecular inversion probe–based next-generation sequencing in association with the EYE-RISK consortium. Variants with minor allele frequency <0.05 resulting in splice-site or protein change were selected. Differences in phenotypic features between carriers and noncarriers were analyzed using generalized estimated equations logistic regression models, considering intereye correlations.
Results
We included 39 eyes of 23 patients carrying rare CFH variants and 284 eyes of 188 noncarriers. Carrier status was associated with having higher drusen burden in the macula in the inner Early Treatment Diabetic Retinopathy Study circle (odds ratio [OR], 5.44 [95% confidence interval {CI}, 1.61–18.37]; P = 0.006), outer circle (OR, 4.37 [95% CI, 1.07–17.77]; P = 0.04), and full grid (OR, 4.82 [95% CI, 1.13–20.52]; P = 0.033). In SD-OCT, a lower total macular volume and lower inner retinal layers’ volume (OR, 0.449 [95% CI, 0.226–0.894]; P = 0.023; OR, 0.496 [95% CI, 0.252–0.979]; P = 0.043) and pigment epithelial detachments (PEDs) (OR, 5.24 [95% CI, 1.08–25.44]; P = 0.04) were associated with carrying a rare CFH variant. Carriers with subretinal drusenoid deposits (SDD) had the rare variant P258L in all cases except one.
Conclusions
We identified in our cohort phenotypic differences between carriers and noncarriers of rare variants in the CFH gene. Carriers had more severe disease, namely superior drusen burden, PEDs, and thinner retinas. The rare variant P258L may be associated with SDD. Carriers are probably at increased risk of progression.
Keywords: age-related macular degeneration, Coimbra Eye Study, rare CFH variants, AMD phenotype, genotype-phenotype associations
Age-related macular degeneration (AMD) is the leading cause of blindness in the older population in industrialized countries, and its prevalence is expected to significantly increase in the future.1,2 Although in the early stages of disease visual loss is commonly not perceived by patients, in late-stage AMD the visual compromise can be profound and irreversible. Thus significant effort is being made to develop strategies capable of halting disease progression and predicting individual risk. Further understanding of the pathophysiology is essential to achieve these goals because AMD is a complex multifactorial disease, influenced by demographic, environmental, and genetic factors.3–7
Several genetic risk variants have been identified in recent years, and a landmark genome-wide association study (GWAS) identified 52 variants at 34 genomic regions as independently associated with AMD (45 common variants and seven rare variants [minor allele frequency <1%]).5 A large risk effect has been reported for common genetic variants located at the CFH and ARMS2/HTRA1 loci.6,8,9 However, Fritsche et al.5 also noted that a significant burden of rare variants was observed in the CFH and CFI, whereas other groups confirmed that rare genetic variants located in these genes conferred a high risk of disease.10–12 To date, more than 100 rare variants are described to be associated with AMD.5,11
The identification of rare variants is important because they can have a strong impact due to high penetrance and may predispose to more severe disease. The CFH gene encodes factor H, which is an inhibitor of the alternative complement pathway, leading to decreased activity and preventing complement overactivation. Compromise of this regulatory function leads to a proinflammatory state that is associated with both AMD development and progression.13,14 Additionally, Triebwasser et al.11 showed that rare variants act in an autosomal dominant manner, in that haploinsufficiency of the cofactor protein (FH) or the necessary protease (Factor I) to inactivate C3b is sufficient to allow this proinflammatory state.
Despite this, there is not much information on the phenotypic characteristics associated with rare variants in AMD.15,16 Furthermore, most of these reports address features based on color fundus photography alone. A better understanding of phenotype-genotype associations with respect to rare variants and based on multimodal imaging could contribute to improving the identification of patients at greater risk of progression to late-stage disease. It would also help in selecting those who could benefit more from targeted therapies inhibiting specific pathways in the complement system or even gene therapy.17–19
The Coimbra Eye Study (CES) is a two-visit epidemiologic population-based study on the prevalence and 6.5-year incidence of AMD in a Portuguese population (NCT01298674, NCT02748824). Subjects who participated in the Incidence study also had blood samples collected for genetic analysis.20–23 We have previously reported on the genetic characterization of this cohort, and we found that rare and low-frequency variants in the CFH gene with damaging effects were more common in AMD cases (Farinha C, et al., unpublished data, 2021). The purpose of this study is to determine the association between the carrier status of rare genetic variants in the CFH gene and the phenotypic features in AMD patients who participated in the Incidence study.
Material and Methods
Study Population and Data Collection
The AMD Incidence Study (NCT027048824) is a single-center population-based study that was conducted in the context of the Coimbra Eye Study, an epidemiologic project for the estimation of AMD prevalence and incidence in a Portuguese population. The Incidence Study was conducted 6.5 years after the Epidemiological Study (NCT01298674), which reported on AMD prevalence. A detailed description of the global study population and recruitment details have been published elsewhere.20–22
In the Incidence Study, the population was extensively characterized, including demographic, clinical, and lifestyle/nutritional information, and blood samples were collected from the participants who consented to further genetic analysis.22,24 Bilateral ophthalmological assessment was performed including multimodal imaging. This multimodal approach included color fundus photography (CFP) of fields 1M, 2, and 3M acquired at 45° (Topcon fundus camera, TRC-NW8; Topcon Corp., Tokyo, Japan), spectral domain optical coherence tomography (SD-OCT), fundus autofluorescence (FAF), and infrared (IR) imaging with Spectralis HRA+OCT (Heidelberg Engineering, Heidelberg, Germany). SD-OCT acquisitions consisted of one EDI Macular Volume Scan (20° × 20°, 49 or 97 B-scans, 16 frames per scan), one radial scan centered in the fovea (20° × 20°, 24 B-scans, 10 frames per scan), and two high-resolution EDI Line Scans (30°, acquired at 0° and 90°, with ≥20 frames each), with signal strength ≥25. Both FAF (488 nm) and IR images were acquired for field 2 at 30° (high resolution with ≥15 frames each).23
Signed informed consent was obtained for all participants. The study adhered to the tenets of the Declaration of Helsinki (2008) and the International Conference on Harmonization–Good Clinical Practice Guideline. The Association for Innovation and Biomedical Research on Light and Image Ethics Committee issued a favorable opinion for the conduction of the study.
AMD Definitions and Staging
The participants’ imaging examination results were sent to a centralized reading center for grading (Coimbra Ophthalmology Reading Center (CORC), AIBILI, Coimbra, Portugal). All graders were senior medical retina specialists certified by the reading center. CFP image grading was supported by Retmarker AMD Research software (Retmarker SA, Coimbra, Portugal) according to the International Age-Related Macular Epidemiological Study Group Classification, while simultaneously analyzing the corresponding SD-OCT, IR, and FAF images in the Heidelberg Eye Explorer software (version 1.10.4.0) as previously reported.21,23,25,26
The Rotterdam staging system was used to assess the AMD severity status of all included eyes.27 Early AMD was defined by the presence of large (≥125 µm in diameter), soft, indistinct, or reticular drusen only; or of soft distinct (≥63 µm in diameter), indistinct (≥125 µm), or reticular drusen with pigmentary abnormalities, within the macula (3000 µm radius Early Treatment Diabetic Retinopathy Study [ETDRS] grid, centered in the fovea), which corresponds to Rotterdam stages 2a, 2b and 3. Late AMD was defined by the presence of neovascular AMD (nAMD) or geographic atrophy (GA). Neovascular AMD was defined by the presence of any type 1, 2, or 3 macular neovascularization (MNV), associated with features such as intraretinal/subretinal fluid, hemorrhage, fibrosis, or subretinal hyperreflective material. Geographic atrophy was defined as a sharply demarcated area of retinal depigmentation, with a corresponding appearance of complete RPE and outer retina atrophy in OCT, and deep hypoautofluorescence in FAF imaging.28,29 When GA and nAMD coexisted in the same eye, it was categorized as nAMD. Late AMD corresponds to stage 4 in the Rotterdam classification. Both eyes were graded and staged in this manner, but the stage of an individual participant was based on the eye with a more severe status.
AMD Multimodal Grading of Phenotypic Features
The following fundus features were assessed and quantified directly in CFP with Retmarker AMD Software in the total 6 mm ETDRS grid centered in the fovea, and in the central, inner, and outer circles: (1) number, type, and size of drusen; (2) predominant type of drusen and the confluence of drusen; (3) total area occupied by drusen (<10%, 10%–50%, ≥50%) and the cumulative real drusen area − total and in each ETDRS circle; (4) presence of hyperpigmentation and hypopigmentation; (5) presence of GA and neovascular AMD. Grading of these features was confirmed by visualizing the corresponding SD-OCT, FAF, and IR images.
Analysis of the SD-OCT scans concerning the vitreomacular interface, neuroretina, and RPE was performed according to the “European Eye Epidemiology spectral‐domain optical coherence tomography classification of macular diseases for epidemiological studies.”30 Several features were graded including the presence of soft drusen (size, location, confluence, internal core reflectivity), subretinal drusenoid deposits (SDD)/pseudodrusen, hyperreflective foci, intraretinal/subretinal fluid, subretinal hyperreflective material, pigment epithelial detachments (PED), RPE atrophy, and presence of MNV. Quantification in the macula of the total retinal thickness and volume, and layer-by-layer including the RPE/Bruch's membrane layer (which is an indirect measurement of the drusen load), was performed in patients with early AMD (stages 2 and 3) through semi-automatic segmentation as reported previously.31 Eyes in stage 4 were excluded from retinal thickness and volume measurements because of the inherent retinal layer distortion caused by GA or MNV. In brief, the Heidelberg segmentation software displays seven distinct retinal layers (retinal nerve fiber layer, ganglion cell layer, inner plexiform layer, inner nuclear layer, outer plexiform layer, outer nuclear layer, and RPE/Bruch's membrane layer), and also provides information on two additional “combination” layers: (1) the inner retinal layers, extending from the inner limiting membrane to the external limiting membrane; and (2) the outer retinal layers, from external limiting membrane to Bruch's membrane. The subfoveal choroidal thickness was measured manually in both early and late AMD patients with the in-built caliper tool available in the software.31
In FAF and IR imaging the presence of SDD and geographic atrophy were confirmed, and the corresponding areas were measured with the Heidelberg Eye Explorer software in-built tools. The SDD total area was measured by using the manual Heidelberg Eye Explorer region overlay tool to draw the borders of the area of interest, and GA was measured by using the semi-automated RegionFinder software.31,32
Genetic Sequencing and Selection of Carriers/Non-Carriers
Genomic DNA samples obtained from the AMD Incidence study participants were genotyped according to standard procedures in the context of a collaboration with the E3–The European Eye Epidemiology Consortium and the EYE-RISK Consortium. The EYE-RISK genotype assay is designed to genotype 87 single nucleotide polymorphisms, including the 52 independently associated single nucleotide polymorphisms identified by the International AMD Genomics Consortium.5,9 The sequencing of rare variants in the CFH gene was obtained with the EYE-RISK single-molecule molecular inversion probes–based next-generation sequencing (NextSeq500; Illumina, San Diego, CA, USA), as described in detail by de Breuk et al.9 All coding and splice-site regions of the CFH gene were sequenced, and the EYE-RISK carried out the selection and filtering of variants to ensure the quality of the data. Variants with fewer than 40 reads coverage on reference allele and variants with less than 40 reads coverage on alternate allele were changed to missing values. For homozygous reference samples genotype was kept unchanged, even if it did not have 40 reads coverage in alternate alleles. Variants with a minor allele frequency (MAF) > 0.05 were removed from the dataset to retain only rare and low-frequency variants.9
For this analysis AMD patients were eligible for inclusion, whereas participants without AMD were excluded, to compare only between carriers and noncarriers of rare variants in the CFH gene who had the disease. Carriers were AMD patients carrying a rare variant in the CFH gene that results in a splice-site or protein change (nonsynonymous), because these variants are more likely to be pathogenic.9 Patients carrying rare CFH variants with a described protective effect in case-control analyses (Q950H) and with a likely benign effect in functional studies (S890I, T956M, and V1007L) were excluded.15 Noncarriers were the remaining patients not having a rare CFH variant.
Statistical Analysis
General (demographic, environmental, clinical, and genetic) characteristics between carriers and noncarriers were compared, using Mann-Whitney U Test and Pearson's χ2 test for continuous and categorical variables, respectively (significance level was set to 0.05). The genotyped samples and selected rare variants were tested regarding the presence of the above-mentioned phenotypic features obtained with multimodal imaging-based grading. For this purpose, odds ratios (ORs) at 95% confidence interval (CI) was computed for the presence of any CFH rare variant according to the presence of the selected phenotypic features of interest using binary logistic regression models, while adjusting for age, sex, AMD stage, and history of smoking. Generalized estimated equations were used to account for intereye correlations. A nominal significance level was set to 0.05, as correction for multiple comparisons was hampered by small sample size. All statistical analyses were performed using R Statistical Software (v4.0.2; R Core Team 2020).
Results
From the original cohort of 1617 participants in the AMD incidence study, of which 237 (14.7%) were early AMD cases and 28 (1.73%) were late AMD cases, a total of 859 samples, including 218 from AMD patients, were genotyped under the association with the EYE-RISK/E3–The European Eye Epidemiology Consortium. Eyes with spherical equivalent >3.00 diopters or poor image quality hampering grading were excluded. The final cohort in the analysis comprised 323 eyes from 211 AMD patients. Of these, 256 eyes (79.3%) were in stage 2, 41 eyes (12.7%) were in stage 3, and 26 eyes (8%) were in stage 4.
A total of 90 unique splice-site or protein change rare CFH variants were genotyped in the 859 samples from AMD cases and controls. Of these, and after excluding one patient with a variant with described benign effect (c.2669G>T p.Ser890Ile), 15 rare variants were present in our total population, and 11 were found in AMD patients and included in the analysis.15
Our final cohort in analysis thus included 39 eyes of 23 carriers (AMD patients having at least one of these 11 rare variants [mean ± SD age, 73.1 ± 6.5 years; 60.9% female]) and 284 eyes of 188 noncarriers (AMD patients not having at least one of these variants [mean ± SD age, 75.0 ± 7.5 years; 61.2% female]). Demographic and environmental characteristics were well balanced between carriers and noncarriers (Table 1).
Table 1.
Demographic and Clinical Characteristics of AMD Patients—Carriers Versus Noncarriers
| Characteristic | Non-Carriers, N = 188*(284 Eyes) | Carriers, N = 23*(39 Eyes) | P Value† |
|---|---|---|---|
| Age | 75.0 (7.5) | 73.1 (6.5) | 0.26 |
| Gender | 0.98 | ||
| Male | 73.0/188.0 (38.8%) | 9.0/23.0 (39.1%) | |
| Female | 115.0/188.0 (61.2%) | 14.0/23.0 (60.9%) | |
| Smoking | 0.41 | ||
| Non-smoker | 151.0/186.0 (81.2%) | 20.0/23.0 (87.0%) | |
| Ex-smoker | 31.0/186.0 (16.7%) | 2.0/23.0 (8.7%) | |
| Smoker | 4.0/186.0 (2.2%) | 1.0/23.0 (4.3%) | |
| Familiar history of AMD | 0.30 | ||
| No | 172.0/188.0 (91.5%) | 19.0/23.0 (82.6%) | |
| Doesn't know | 14.0/188.0 (7.4%) | 4.0/23.0 (17.4%) | |
| Yes | 2.0/188.0 (1.1%) | 0.0/23.0 (0.0%) | |
| Diabetes | 0.25 | ||
| No | 165.0/188.0 (87.8%) | 18.0/23.0 (78.3%) | |
| Doesn't know | 4.0/188.0 (2.1%) | 0.0/23.0 (0.0%) | |
| Yes | 19.0/188.0 (10.1%) | 5.0/23.0 (21.7%) | |
| Arterial hypertension | 0.36 | ||
| No | 75.0/188.0 (39.9%) | 11.0/23.0 (47.8%) | |
| Doesn't know | 4.0/188.0 (2.1%) | 1.0/23.0 (4.3%) | |
| Yes | 109.0/188.0 (58.0%) | 11.0/23.0 (47.8%) | |
| Dyslipidemia | >0.99 | ||
| No | 151.0/188.0 (80.3%) | 19.0/23.0 (82.6%) | |
| Doesn't know | 27.0/188.0 (14.4%) | 3.0/23.0 (13.0%) | |
| Yes | 10.0/188.0 (5.3%) | 1.0/23.0 (4.3%) | |
| BMI | 27.8 (4.9) | 28.3 (4.1) | 0.47 |
| AMD stage - worst eye | 0.83 | ||
| 2 | 142.0/188.0 (75.6%) | 18.0/23.0 (78.2%) | |
| 3 | 29.0/188.0 (15.4%) | 2.0/23.0 (8.7%) | |
| 4 | 17.0/188.0 (9.0%) | 3.0/23.0 (13.0%) | |
| Major common risk variants MAF‡ | |||
| ARMS2 rs10490924, T | 76/376 (20.2) | 8/46 (17.4) | 0.56 |
| ARMS2/HTRA1 rs3750846, C | 75/376 (19.9) | 7/46 (15.2) | 0.29 |
| CFH rs570618, T | 130/374 (34.8) | 11/46 (23.9) | 0.34 |
| CFH rs10922109, A | 134/374 (35.8) | 14/46 (30.4) | 0.77 |
| C2/CFB/SKIV2L rs429608, A | 28/370 (7.6) | 5/46 (10.9) | 0.21 |
| C3 rs2230199, C | 64/376 (17.0) | 8/46 (17.4) | 0.36 |
Mean (SD); n/N (%).
Wilcoxon rank sum test; Pearson's χ2 test; Fisher's exact test.
No. of minor alleles/total No. of alleles (%).
Regarding common major risk variants for AMD, we found slight differences in MAF distribution between carriers and noncarriers, although these were non-significant (Table 1).9 The major risk variants ARMS2 rs10490924, ARMS2/HTRA1 rs3750846, and CFH rs570618 had a lower MAF in carriers compared to non-carriers, while the C3 rs2230199 risk variant was well balanced between groups. Moreover, In non-carriers all 4 major risk variants had a lower MAF than that reported in AMD patients from larger populations such as from the EYE-RISK and International AMD Genomics Consortium (Supplementary Table S1).5,9 The MAF of the protective variant CFH rs10922109 was lower in carriers compared to non-carriers, but similar between the latter and AMD patients from these larger cohorts.5,9 The C2/CFB/SKIV2L rs429608 protective variant had a higher MAF in carriers.
The rare CFH variants found in both AMD cases and non-AMD cases from the CES are presented in Table 2, and for completeness, all sequenced variants are presented in Supplementary Table S2. The most frequent rare variant found in AMD patients was CFH rs768526062 (Pro258Leu). Also, variants known to have a functional effect such as CFH rs757785149 (Arg53Cys) were present.
Table 2.
CFH Rare Variants Identified in the CES Cohort
| Gene | Position GRCh37 (hg19) | REF | ALT | ID | Nucleotide Change | Protein Change | Maf CES | Variants (n) | MAC Cases | MAC Controls | Maf Cases | Maf Controls |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CFH | 196642206 | C | T | rs757785149 | C157T | R53C*,§,‡ | 0.000612 | 1 | 1 | 0 | 0.002 | 0.000 |
| CFH | 196646659 | G | T | rs777300338 | G481T | A161S† | 0.000612 | 1 | 1 | 0 | 0.002 | 0.000 |
| CFH | 196648794 | A | G | rs774239374 | A661G | I221V*,§ | 0.000612 | 1 | 0 | 1 | 0.000 | 0.001 |
| CFH | 196648906 | C | T | rs768526062 | C773T | P258L† | 0.011409 | 17 | 13 | 4 | 0.033 | 0.004 |
| CFH | 196658607 | G | A | rs371192606 | G1022A | R341H† | 0.000612 | 1 | 1 | 0 | 0.002 | 0.000 |
| CFH | 196658733 | T | C | rs762389370 | T1148C | V383A | 0.000612 | 1 | 0 | 1 | 0.000 | 0.001 |
| CFH | 196684751 | T | A | rs147403664 | T1548A | N516K† | 0.000614 | 1 | 1 | 0 | 0.002 | 0.000 |
| CFH | 196684825 | A | G | 1:196684825:A:G | A1622G | E541G | 0.000612 | 1 | 1 | 0 | 0.002 | 0.000 |
| CFH | 196694418 | A | G | 1:196694418:A:G | A1864G | I622V† | 0.001224 | 2 | 1 | 1 | 0.002 | 0.001 |
| CFH | 196695985 | C | A | rs763441589 | C2151A | F717L† | 0.000612 | 1 | 1 | 0 | 0.002 | 0.000 |
| CFH | 196706659 | C | A | rs114743644 | C2651A | S884Y† | 0.000612 | 1 | 0 | 1 | 0.000 | 0.001 |
| CFH | 196706677 | G | T | rs515299 | G2669T | S890I†,|| | 0.0153 | 25 | 9 | 16 | 0.021 | 0.013 |
| CFH | 196711052 | G | C | rs201816520 | G3004C | G1002R† | 0.000612 | 1 | 1 | 0 | 0.002 | 0.000 |
| CFH | 196712596 | A | T | rs35274867 | A3148T | N1050Y*,‡ | 0.019584 | 32 | 3 | 29 | 0.007 | 0.024 |
| CFH | 196712624 | T | C | rs35343172 | T3176C | I1059T† | 0.000612 | 1 | 1 | 0 | 0.002 | 0.000 |
| 196716415 | T | A | 1:196716415:T:A | T3668A | L1223Q | 0.000612 | 1 | 0 | 1 | 0.000 | 0.001 |
Variants reported to be significantly associated with AMD in one or more AMD case-control cohorts.
Variants found in one or more studies.
Variants with a functional effect on the protein or change in systemic levels.10
Risk-conferring variants in GWAS.5
Variant removed from the analyzed dataset due to having a described protective effect in case-control analyses or a likely begin effect in functional studies.
Associations With Phenotypic Features
Regarding the phenotypic features analyzed with multimodal imaging in AMD patients, associations were found with carrying rare variants in the CFH gene.
The risk of carrying at least one of these variants increased with a larger drusen area in the inner ETDRS circle (OR, 3.22 [95% CI, 1.18–8.78]; P = 0.022) and higher percentual coverage of the ETDRS grid by drusen in color fundus photography. Specifically, having a 10% to 50% area of the ETDRS grid occupied by drusen in the inner circle (OR, 5.44 [95%CI, 1.61–18.37]; P = 0.006), the outer ETDRS circle (OR, 4.37 [95%CI, 1.07–17.77]; P = 0.04), and in the full ETDRS grid (OR, 4.82 [95%CI, 1.13–20.52]; P = 0.033), but not in the central fovea.
In SD-OCT phenotypic analysis we found that having a higher macular retinal volume appears to decrease the risk of having a rare variant (OR, 0.449 [95% CI, 0.226–0.894]; P = 0.023), and the same was true for having a higher volume of all combined inner retinal layers (OR, 0.496 [95% CI, 0.252–0.979]; P = 0.043). The presence of pigment epithelial detachments in OCT was predictive of having a rare variant (OR, 5.24 [95% CI, 1.08–25.44]; P = 0.04). A trend in the same direction was found regarding the presence of hyperreflective foci (OR, 2.61 [95% CI, 0.88–7.71]; P = 0.08).
Despite not reaching statistical significance, hard drusen were more common in noncarriers, and intermediate and large drusen were more common in carriers. Plus, carriers had on average thinner choroids (208.7 ± 83.8 µm vs. 228.3 ± 87.7 µm, P = 0.15) and larger retinal areas affected by SDD (7.89 ± 16.8 mm2 vs. 4.64 ± 10.10 mm2, P = 0.13). An interesting finding was that in carriers of the most frequent rare variant (CFH rs768526062, Pro258Leu), 46.2% (n = 6) had SDD in both eyes, and in most cases affecting an extensive retinal area. In fact, and except for only one case, all carriers with SDD shared this rare variant in our cohort.
Regarding late AMD, both GA and MNV were more common in carriers, but this was more striking for MNV (10.26% in carriers vs. 2.82% in noncarriers); however, it was not statistically significant. The associations between all assessed phenotypic features and carrier status are shown in Table 3. Exemplificative multimodal images of the main phenotypic characteristics of carriers are presented in Figures 1 and 2.
Table 3.
Phenotypic Characterization of Carriers Versus Noncarriers of Rare CFH Variants
| Phenotypic Characteristics | Noncarriers (n = 284 Eyes) | Carriers (n = 39 Eyes) | OR (95% CI) | P Value |
|---|---|---|---|---|
| Area covered by drusen (in ETDRS grid), mm2 | ||||
| Central subfield | 0.028 (0.063) | 0.048 (0.084) | NA | 0.16 |
| Inner circle | 0.15 (0.30) | 0.36 (0.56) | 3.22 [1.18–8.78] | 0.022 |
| Outer circle | 0.54 (0.98) | 0.96 (1.25) | 1.34 [0.93–1.94] | 0.11 |
| % Area occupied by drusen in ETDRS grid (all subfields) | ||||
| <10% | 275 (96.8) | 33 (84.6) | 1.0 | Ref |
| 10%–50% | 9 (3.2) | 6 (15.4) | 4.82 [1.13–20.52] | 0.033 |
| ≥50% | 0 | 0 | NA | NA |
| % Area occupied by drusen–Central field | ||||
| 0%–10% | 266 (93.66) | 33 (84.62) | Ref | |
| 10%–50% | 18(6.34) | 6 (15.38) | 3.28 [0.83-12.97] | 0.091 |
| ≥50% | 0 | 0 | NA | NA |
| % Area occupied by drusen–Inner circle | ||||
| 0%–10% | 273 (96.1) | 32 (82.1) | 1.0 | REF |
| 10%–50% | 11 (3.9) | 7 (17.8) | 5.44 [1.61–18.37] | 0.006 |
| ≥50% | 0 | 0 | NA | NA |
| % Area occupied by drusen–Outer circle | ||||
| 0%–10% | 274 (96.5) | 33 (84.6) | 1.0 | Ref. |
| 10%–50% | 10 (3.5) | 6 (15.4) | 4.37 [1.07–17.77] | 0.040 |
| ≥50% | 0 | 0 | NA | NA |
| Predominant drusen type within ETDRS grid | ||||
| Absent | 28 (10.18) | 4 (10.81) | ref | |
| Hard drusen | 114 (52.36) | 15 (40.54) | 0.60 [0.11–3.22] | 0.55 |
| Intermediate drusen | 83 (30.18) | 15 (40.54) | 1.15 [0.21–6.22] | 0.87 |
| Large drusen | 20 (7.27) | 3 (8.11) | 0.98 [0.13–7.88] | 0.99 |
| Hyperpigmentation (CFP) | ||||
| No | 232 (81.7) | 32 (82.0) | ref | |
| Yes | 52 (18.3) | 7 (17.9) | 1.20 [0.16–9.19] | 0.86 |
| Hypopigmentation (CFP) | ||||
| No | 253 (89.1) | 34 (87.2) | ref | |
| Yes | 31 (10.9) | 5 (12.8) | 1.32 [0.25–6.86] | 0.74 |
| Presence of SDD (FAF+IR+OCT) | ||||
| No | 203 (71.48) | 27 (69.23) | ref | |
| Yes | 81 (28.52) | 12 (30.77) | 1.43 [0.48–4.27] | 0.52 |
| Total area of SDD (FAF), mm2 | 4.64 ± 10.1 | 7.89 ± 16.8 | 1.03 [0.99–1.08] | 0.128 |
| Retinal thickness in central subfield (OCT), µm | 278.4 ± 34.9 | 273.0 ± 26.8 | 0.996 [0.98–1.01] | 0.58 |
| Volume | ||||
| Overall Retina (OCT), mm3 | 8.40 ± 0.50 | 8.29 ± 0.32 | 0.449 [0.226–0.894] | 0.023 |
| IRL (OCT), mm3 | 6.17 ± 0.51 | 6.05 ± 0.36 | 0.496 [0.252–0.979] | 0.043 |
| ORL (OCT), mm3 | 2.22 ± 0.09 | 2.20 ± 0.10 | 0.033 [0.0005–2.26] | 0.114 |
| RPE-Bruch layer (OCT), mm3 | 0.39 ± 0.05 | 0.38 ± 0.05 | NA | 0.88 |
| Subfoveal choroidal thickness (OCT), µm | 228.3 ± 87.7 | 208.7 ± 83.8 | 0.995 [0.99–1.00] | 0.145 |
| Pigment epithelial detachment (OCT) | ||||
| No | 275 (96.83) | 34 (87.18) | Ref | |
| Yes | 9 (3.17) | 5 (12.82) | 5.24 [1.08–25.44] | 0.04 |
| Hyperreflective foci (OCT) | ||||
| No | 239 (84.15) | 28 (71.79) | Ref | |
| Yes | 45 (15.85) | 11 (28.21) | 2.61 [0.88–7.71] | 0.083 |
| MNV (OCT) | ||||
| No | 276 (97.18) | 35 (89.74) | Ref | |
| Yes | 8 (2.82) | 4 (10.26) | 6.08 [0.48–76.82] | 0.16 |
| Geographic atrophy/(CFP+FAF+IR+OCT) | ||||
| No | 251 (93.66) | 35 (92.11) | Ref | |
| Yes | 17 (6.34) | 3 (7.89) | 0.57 [0.07–1.08] | 0.52 |
ORL, outer retinal layers; IRL, inner retinal layers.
Generalized estimated equation logistic regression analysis, adjusted by AMD stage, age, sex, and smoking (non-smokers vs smokers/ex-smokers).
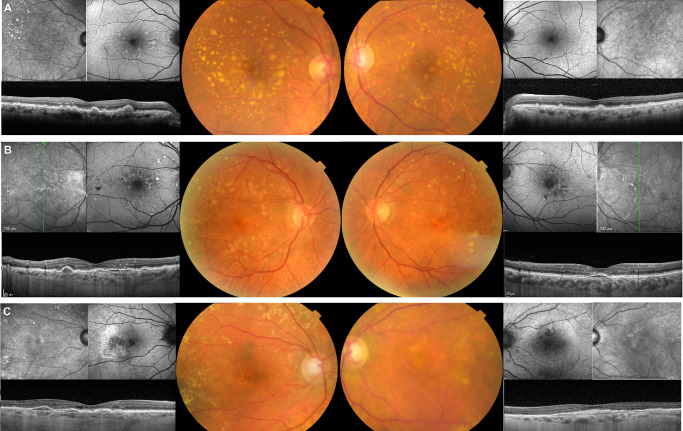
Figure 1.
Exemplificative images of fundus features of carriers. (A) Female, 68 years old (yo) (CFH rs757785149; Arg53Cys) with extensive soft drusen both inside the ETDRS grid and outside extending beyond the vascular arcades, and crystalline drusen temporal to the macula. There is a high degree of phenotypic symmetry between both eyes. (B) Male, 68yo (CFH rs371192606; Arg341His) with large, soft, confluent drusen mainly located in the outer ETDRS grid circle, and extending to the vascular arcades and nasal peripapillary area, along with hypo and hyperpigmentation in the central macula. The OCT reveals shallow and heterogeneously hyporreflective PEDs under the fovea in both eyes, but no intraretinal or subretinal fluid. The symmetry of all pathologic changes in multimodal imaging is striking. (C) Female, 80yo (CFH 1:196694418:A:G; Ile622Leu) with large soft, confluent drusen mainly located in the outer ETDRS grid circle and temporal to the macula. They extend outside the vascular arcades and to the nasal peripapillary area. There is hypopigmentation and hyperpigmentation in the central macula in both eyes. The OCT shows PED under the fovea in both eyes, in the right eye with intraretinal fluid (type 1 MNV), and the left eye without fluid (probably quiescent MNV).
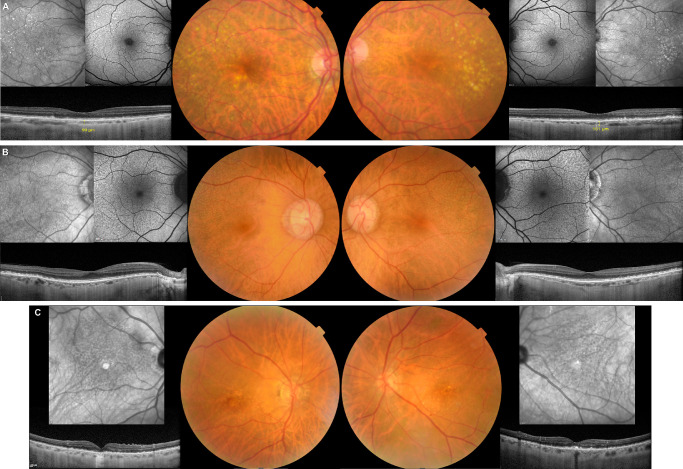
Figure 2.
Exemplificative multimodal images of CFH rs768526062 (Pro258Leu) carriers. (A) Female, 72yo with soft drusen mainly clustering in the temporal macula and SDD in parafoveal, nasal, and superior distribution in both eyes. The choroid is very thin (99 micra in right eye and 101 micra in left eye, subfoveal). (B) Female, 68yo with extensive SDD in both eyes affecting the posterior pole, except for the fovea, and extending to the vascular arcades. (C) Male, 81yo with SDD in both eyes affecting the posterior pole. There is foveal geographic atrophy in the right eye and soft drusen with hyperpigmentary changes in the fovea of the left eye.
Discussion
In our study, we identified phenotypic differences between carriers and noncarriers of rare CFH variants in AMD patients through multimodal imaging. Carriers presented with more severe disease, including superior drusen burden in the macula, more PEDs of any cause, and thinner retinas, especially at the level of the inner retinal layers, independently of AMD stage. Our findings agree with previous studies reporting a significant association between having rare variants in the CFH gene and increased drusen load. However, the finding of carriers also having thinner retinas, namely thinner inner retinal layers as quantified by SD-OCT, is described here for the first time to the best of our knowledge.13,15,16 Our results also suggest that the AMD phenotype characterized by thinner choroid and SDD seems to be more common in carriers of rare CFH variants, namely the association of SDD with the P258L variant, as well as having MNV in late stages, although these differences did not reach statistical significance in our analyzed population.
The first genetic studies in AMD mainly focused on common variants in the population through GWAS. A major GWAS has established 52 genetic risk variants to be strongly associated with AMD: 45 common plus 7 rare variants.5 Genetic causality in a disease can be further explored by the identification of protein-altering variants in coding regions. These variants might be rare in the population, and several studies thus focused on their discovery by sequencing genes in AMD loci. In these studies, rare variants were found to be individually associated with AMD. These variants have mainly been identified in genes involved in the complement pathway: CFH, CFI, C3, and C9.11,12,33 In the CES we previously described 12 variants to be associated with AMD. Eleven of these were common variants, whereas one noncoding variant in the CFH gene (rs35292876) was a rare variant. Furthermore, we also found that rare or low-frequency variants in the CFH gene with a predicted damaging effect were more common in AMD cases (Farinha C, et al., unpublished data, 2021).
Rare genetic variants located in the CFH gene are among the variants that confer the highest risk for AMD.10 Because of their high penetrance and strong effect size, these variants may account for familial clustering of AMD and lead to more severe disease.14 Despite this, few studies exploring genotype-phenotype associations considering only rare variants are available.15,16 Thus our objective in this report was to explore the presence of rare variants in the CFH gene and their relationship with the phenotypic features of our AMD patients.
Ferrara et al.13 focused on the rare CFH variant rs121913059 (Arg1210Cys), the strongest genetic risk variant of AMD in North-American populations and showed that the typical phenotype was characterized by extensive, voluminous, and confluent soft-drusen accumulation in the macula but also throughout the fundus. There was also a higher risk of developing late AMD, namely geographic atrophy. Wagner et al.16 further reported that four highly penetrant rare CFH variants were strongly associated with advanced AMD, a higher frequency of drusen, earlier age of disease onset, and phenotypic symmetry. Kersten et al.15 evaluated the phenotypic effect of rare CFH variants cumulatively, and they reported in their work that patients with an extensive drusen area, drusen with crystalline appearance, and drusen nasal to the optic disc were more likely to have at least one rare variant in the CFH gene. In our study, we too identified phenotypic differences between carriers and noncarriers of rare CFH variants in a cumulative analysis. In the same way as these previous reports, AMD patients who were carriers presented with more severe disease in our study, including superior drusen burden in the macula, both in the perifovea and parafovea. This apparent difference was found by quantifying the real drusen area derived from the sum of each druse, as well as by measuring the percentage of the ETDRS grid and respective rings covered by drusen.
Regarding common variants, several studies addressed the clinical and phenotypic implications of individual variants. Dietzel et al.34 showed that common variants in the CFH, ABCA1, and ARMS2 genes were related to the presence and progression of drusen burden in early AMD. Another study by Seddon et al.35 measured drusen burden through SD-OCT quantification and found that variants in CFH and ARMS2/HTRA1 were independently associated with an increase in both drusen volume and area in eyes with early and intermediate AMD. Thee et al.36 recently found in a large cohort from the E3/EYE-RISK consortium that the ARMS2/HTRA1 locus was highly associated with intermediate AMD features, including a five times higher risk of a large macular area covered by drusen, and a six times higher risk of SDD, and with late AMD at a younger age. The same group reported, however, that phenotypic risk differences between ARMS2/HTRA1 and complement genes were found only for MNV and not for other AMD lesions, because they overlapped significantly.36 In our study, we found no significant difference in the MAF distribution of major common AMD risk variants between the carrier and non-carrier groups. However, there were some relevant findings because the MAF of these risk variants (ARMS2 rs10490924, ARMS2/HTRA1 rs3750846, CFH rs570618, C3 rs2230199) were not only inferior in carriers compared to noncarriers, but also inferior or similar in noncarriers when compared to AMD patients from larger populations.5,9 Kersten et al.15 reported similar findings as the frequency of common genetic variants in CFH and ARMS2 were inferior in carriers compared with noncarriers in their study. They suggested that carriers of rare CFH variants are less burdened by common AMD risk variants and that their AMD risk and associated phenotypic features are thus attributable to the rare variants. This relevant finding seems to be supported by our study. Regarding the inferior MAF of major risk variants in AMD patients from the CES compared to other cohorts, this was already described in our previous report and is probably related to populational differences (Farinha C, et al., unpublished data, 2021). Plus, our sample is relatively small and originally from a limited geographic area. Our AMD cases seem not only less burdened by common genetic major risk variants, but carriers of CFH rare variants are even less.
When further exploring other phenotypic features in multimodal imaging, we found that in SD-OCT analysis a lower macular volume was associated with carrier status, and this was also true when considering only the inner retinal layers but not the outer retinal layers where drusen are located. Quantitative SD-OCT-derived information on all segmented retinal layers is here presented for the first time in association with rare CFH variants and suggests that carriers present with thinning of the retina and mainly of the inner neuronal retina. This could be due to neurodegeneration in the context of more severe disease and caused or enhanced by genetic factors, independently of AMD stage. The association of PEDs and hyperreflective foci graded in OCT with the carrier status also points towards a more severe phenotype and perhaps increased risk of disease progression, related to more pathogenic CFH rare variants.
Our group previously reported that in early AMD patients from the CES the presence of SDD was associated with both thinner neuroretinal layers and thinner choroids.31 Subretinal drusenoid deposits are a distinct feature of AMD and a known risk factor for advanced disease. However, their pathophysiological mechanism remains unclear.37 Spaide38 demonstrated in this respect that one difference between drusen and SDD was the significantly thinner underlying choroid of the latter, but despite the phenotypic differences, genetic data yielded conflicting results, and the total genetic risk score for SDD did not differ significantly from that seen for drusen in AMD. Other studies analyzed possible genetic associations, and a post hoc analysis of the Comparison of AMD Treatment Trials observed that common risk variants ARMS2 rs10490924 and HTRA1 rs11200638 were associated with an increased risk of SDD, while CFH rs1061170 was associated with a lower risk.37 Bonyadi et al.39 also found a stronger contribution of ARMS2 rs10490924 in comparison with CFH genotypes in AMD with SDD versus without. Dutheil et al.,40 however, found that in participants of the ALIENOR study the risk variants ARMS2 rs10490924, LIPC rs10468017, and CFH rs1061170 were all associated with incident SDD. When analyzing our data, the distribution of common variants was not different between groups, including the ARMS2 rs10490924. Regarding rare variants, to the best of our knowledge, there isn't yet any report addressing the associations between the presence and extension of SDD and the presence of rare variants in the CFH gene in AMD patients. Saksens et al.14 observed a higher familial occurrence of AMD and an earlier age at onset in the carriers of the rare genetic variants CFI rs141853578 (Gly119Arg), C3 rs147859257 (Lys155Gln), and C9 rs34882957 (Pro167Ser), but no association to the presence of SDD. In our study, we found that carriers of at least one rare CFH variant had larger areas of retinal involvement by SDD, and carriers also had on average thinner choroids, besides thinner retinas as discussed above. We acknowledge that associations with SDD and choroidal thickness did not reach statistical significance, but this can be attributed to the relatively small sample size of our study. Interestingly, we also found that for the rare variant CFH rs768526062 (Pro258Leu), which was the most common in our carriers’ group, almost half of the carriers had an SDD phenotype, alone or in combination with drusen, and most strikingly, virtually all eyes from carriers who also had SDD shared this rare variant. We speculate that there could be a role for rare and more pathogenic CFH variants in the development of SDD and associated increased risk of disease progression, and we propose that this finding should be further explored in larger studies on genotype-phenotype correlation.
Some limitations should be addressed in our study. First, despite being originally an epidemiological population-based study, for genetic analysis, it has a small cohort, and the population is originally from a single location in Portugal. Second, we focused on rare variants in the CFH gene alone, but both common and rare variants in other genes of the complement pathway and other biological pathways also influence phenotype in AMD (Fig. 3). In addition, the CFH variants were assessed cumulatively, making phenotypic associations to all individual variants not possible. However, given that they are rare, single associations would not be feasible to establish in our cohort. Assessing their conjoined effect still revealed an association to more severe disease status, so they seem to share or overlap phenotypic characteristics. Pursuing a similar cumulative-based approach in other genes for rare variants would be of interest in future studies. Rare variants in other complement genes were, however, too few to analyze in our study. Another important limitation is that an analysis of the identified rare variants in family members from carriers would be most relevant to pursue to better characterize their pathogenic role and to better establish genotype-phenotype correlations. However, this was not possible due to the design of the epidemiological study on which this report is based. Still, we found that patients carrying these rare CFH variants were phenotypically different when compared to noncarriers, and because the phenotype was more severe, the overall effect of these variants is probably pathogenic. It would also be of interest to quantify the serum levels of FH in carriers, and functional studies are important to pursue to confirm our findings. We also recognize that nominal significance levels are provided in phenotypic analysis, as corrected significance was not possible to achieve, likely due to the small sample size. Finally, we only assessed drusen burden in the macula, but studies evaluating extramacular and peripheral retina would be important to further expand the phenotype-genotype correlation in AMD.41 Nevertheless, our study is one of the few genetic studies addressing the effect of rare variants in the complement pathway in AMD phenotype through extensive multimodal imaging characterization, and the associations to the carrier status here documented for the first time strengthen our results. Furthermore, as part of the EYE-RISK project, our results are based on a comprehensive genotype assay recently validated in European populations.
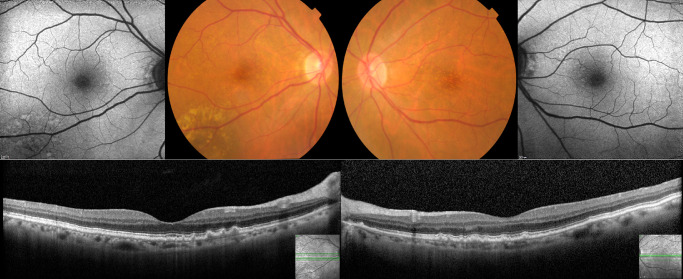
Figure 3.
Female, 74yo (rare variant CFH rs35274867; Asn1050Tyr) with cuticular drusen in the fovea and nasal parafovea. Besides the rare variant, this patient harbors multiple common variants, including three other CFH variants (rs10922109, rs1410996, rs3753394) and three risk-conferring variants in ARHGAP21 (rs12357257), NPLOC4_TSPAN10 (rs6565597) and SLC16A8 (rs8135665).
In summary, we identified phenotypic differences between carriers and noncarriers of rare CFH variants in AMD patients. Carriers presented with more severe disease, including superior drusen burden in the macula, PEDs, hyperreflective foci, and thinner retinas, mainly at the level of the inner retinal layers. Our results also suggest that exudative late AMD and the phenotype of SDD with thin choroid seem to be more prevalent in carriers, which was especially true for those carrying the variant P258L. These patients might be at increased risk of progression, and identification of such features can help in the selection of those who could benefit from genetic investigation. This can be especially relevant if complement-targeted therapies and genetic-based therapies are to be pursued in the future.
Supplementary Material
Acknowledgments
Supported by Novartis.
Disclosure: C. Farinha, Novartis (C), Bayer (C); P. Barreto, None; R. Coimbra, None; A. Iutis, None; M.L. Cachulo, Bayer (C), Novartis (C); J. Cunha-Vaz, Precision Ocular Ltd (C), Roche (C), Carl Zeiss Meditec (C), AlimeraSciences (C), Allergan (C), Bayer (C), Gene Signal (C), Novartis (C), Pfizer (C), Sanofi-Aventis (C), Vifor Pharma (C); Y.T.E. Lechanteur, Novartis (C), Bayer (C); C.B. Hoyng, Bayer (C), Novartis (C), Horus Pharma Abbvie (C), Horama (C), Astherna (F); R. Silva, Bayer (C), Alcon (C), Thea (C), Novartis (C), Alimera Sciences (C), Allergan (C)
References
- 1. Colijn JM, Buitendijk GHS, Prokofyeva E, et al.. Prevalence of age-related macular degeneration in Europe: the past and the future. Ophthalmology. 2017; 124: 1753–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wong WL, Su X, Li X, et al.. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health. 2014; 2(2): e106–e116. [DOI] [PubMed] [Google Scholar]
- 3. Lambert NG, ElShelmani H, Singh MK, et al.. Risk factors and biomarkers of age-related macular degeneration. Prog Retin Eye Res. 2016; 54: 64–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fritsche LG, Fariss RN, Stambolian D, Abecasis GR, Curcio CA, Swaroop A.. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet. 2014; 15: 151–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fritsche LG, Igl W, Bailey JN, et al.. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016; 48: 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colijn JM, Meester-Smoor M, Verzijden T, et al.. Genetic risk, lifestyle, and age-related macular degeneration in Europe: The EYE-RISK Consortium. Ophthalmology. 2021; 128: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 7. Merle BMJ, Colijn JM, Cougnard-Gregoire A, et al.. Mediterranean diet and incidence of advanced age-related macular degeneration: the EYE-RISK Consortium. Ophthalmology. 2019; 126: 381–390. [DOI] [PubMed] [Google Scholar]
- 8. Kersten E, Paun CC, Schellevis RL, et al.. Systemic and ocular fluid compounds as potential biomarkers in age-related macular degeneration. Surv Ophthalmol. 2018; 63: 9–39. [DOI] [PubMed] [Google Scholar]
- 9. de Breuk A, Acar IE, Kersten E, et al.. Development of a genotype assay for age-related macular degeneration: the EYE-RISK Consortium. Ophthalmology. 2021; 128: 1604–1617. [DOI] [PubMed] [Google Scholar]
- 10. Geerlings MJ, de Jong EK, den Hollander AI.. The complement system in age-related macular degeneration: A review of rare genetic variants and implications for personalized treatment. Mol Immunol. 2017; 84: 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Triebwasser MP, Roberson ED, Yu Y, et al.. Rare variants in the functional domains of complement factor H are associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015; 56: 6873–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seddon JM, Yu Y, Miller EC, et al.. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet. 2013; 45: 1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferrara D, Seddon JM.. Phenotypic characterization of complement factor H R1210C rare genetic variant in age-related macular degeneration. JAMA Ophthalmol. 2015; 133: 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saksens NT, Geerlings MJ, Bakker B, et al.. Rare genetic variants associated with development of age-related macular degeneration. JAMA Ophthalmol. 2016; 134: 287–293. [DOI] [PubMed] [Google Scholar]
- 15. Kersten E, Geerlings MJ, den Hollander AI, et al.. Phenotype characteristics of patients with age-related macular degeneration carrying a rare variant in the complement factor H gene. JAMA Ophthalmol. 2017; 135: 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wagner EK, Raychaudhuri S, Villalonga MB, et al.. Mapping rare, deleterious mutations in factor H: association with early onset, drusen burden, and lower antigenic levels in familial AMD. Sci Rep. 2016; 6: 31531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Jong S, Gagliardi G, Garanto A, et al.. Implications of genetic variation in the complement system in age-related macular degeneration. Prog Retin Eye Res. 2021; 84: 100952. [DOI] [PubMed] [Google Scholar]
- 18. Sitnilska V, Kersten E, Altay L, et al.. Major predictive factors for progression of early to late age-related macular degeneration. Ophthalmologica. 2020; 243: 444–452. [DOI] [PubMed] [Google Scholar]
- 19. Heesterbeek TJ, Lores-Motta L, Hoyng CB, Lechanteur YTE, den Hollander AI.. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol Opt. 2020; 40: 140–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cachulo Mda L, Lobo C, Figueira J, et al.. Prevalence of age-related macular degeneration in Portugal: the Coimbra Eye Study—Report 1. Ophthalmologica. 2015; 233(3-4): 119–127. [DOI] [PubMed] [Google Scholar]
- 21. Cachulo Mda L, Lains I, Lobo C, et al.. Age-related macular degeneration in Portugal: prevalence and risk factors in a coastal and an inland town. The Coimbra Eye Study—Report 2. Acta Ophthalmol. 2016; 94(6): e442–e453. [DOI] [PubMed] [Google Scholar]
- 22. Farinha CVL, Cachulo ML, Alves D, et al.. Incidence of age-related macular degeneration in the central region of Portugal: the Coimbra Eye Study—Report 5. Ophthalmic Res. 2019; 61: 226–235. [DOI] [PubMed] [Google Scholar]
- 23. Farinha C, Cachulo ML, Coimbra R, et al.. Age-related macular degeneration staging by color fundus photography vs. multimodal imaging-epidemiological implications (The Coimbra Eye Study-Report 6). J Clin Med. 2020; 9: 1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nunes S, Alves D, Barreto P, et al.. Adherence to a Mediterranean diet and its association with age-related macular degeneration. The Coimbra Eye Study-Report 4. Nutrition. 2018; 51-52: 6–12. [DOI] [PubMed] [Google Scholar]
- 25. Bird AC, Bressler NM, Bressler SB, et al.. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995; 39: 367–374. [DOI] [PubMed] [Google Scholar]
- 26. Marques JP, Costa M, Melo P, et al.. Ocular risk factors for exudative AMD: a novel semiautomated grading system. ISRN Ophthalmology. 2013; 2013: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Leeuwen R KC, Vingerling JR, Hofman A, de Jong PT. The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam study. Arch Ophthalmol. 2003; 121: 519–526. [DOI] [PubMed] [Google Scholar]
- 28. Sadda SR, Guymer R, Holz FG, et al.. Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of Atrophy Report 3. Ophthalmology. 2018; 125: 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spaide RF, Jaffe GJ, Sarraf D, et al.. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology. 2020; 127: 616–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gattoussi S, Buitendijk GHS, Peto T, et al.. The European Eye Epidemiology spectral-domain optical coherence tomography classification of macular diseases for epidemiological studies. Acta Ophthalmol. 2019; 97: 364–371. [DOI] [PubMed] [Google Scholar]
- 31. Farinha C, Silva AL, Coimbra R, et al.. Retinal layer thicknesses and neurodegeneration in early age-related macular degeneration: insights from the Coimbra Eye Study. Graefes Arch Clin Exp Ophthalmol. 2021; 259: 2545–2557. [DOI] [PubMed] [Google Scholar]
- 32. Panthier CQG, Puche N, Le Tien V, et al.. Evaluation of semiautomated measurement of geographic atrophy in age-related macular degeneration by fundus autofluorescence in clinical setting. Retina. 2014; 34: 576–582. [DOI] [PubMed] [Google Scholar]
- 33. Corominas J, Colijn JM, Geerlings MJ, et al.. Whole-exome sequencing in age-related macular degeneration identifies rare variants in COL8A1, a component of Bruch's membrane. Ophthalmology. 2018; 125: 1433–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dietzel M, Pauleikhoff D, Arning A, et al.. The contribution of genetic factors to phenotype and progression of drusen in early age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2014; 252: 1273–1281. [DOI] [PubMed] [Google Scholar]
- 35. Seddon JM, Dossett JP, Widjajahakim R, Rosner B.. Association between perifoveal Drusen burden determined by OCT and genetic risk in early and intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2019; 60: 4469–4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thee EF, Colijn JM, Cougnard-Gregoire A, et al.. The phenotypic course of age-related macular degeneration for ARMS2/HTRA1: the EYE-RISK Consortium. Ophthalmology. 2022; 129: 752–764. [DOI] [PubMed] [Google Scholar]
- 37. Lin LY, Zhou Q, Hagstrom S, et al.. Association of single-nucleotide polymorphisms in age-related macular degeneration with pseudodrusen: secondary analysis of data from the comparison of AMD treatments trials. JAMA Ophthalmol. 2018; 136: 682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spaide R. Improving the age-related macular degeneration construct: a new classification system. Retina. 2018; 38: 891–899. [DOI] [PubMed] [Google Scholar]
- 39. Jabbarpoor Bonyadi MH, Yaseri M, Nikkhah H, Bonyadi M, Soheilian M. Association of risk genotypes of ARMS2/LOC387715 A69S and CFH Y402H with age-related macular degeneration with and without reticular pseudodrusen: a meta-analysis. Acta Ophthalmol. 2018; 96(2): e105–e110. [DOI] [PubMed] [Google Scholar]
- 40. Dutheil C, Le Goff M, Cougnard-Gregoire A, et al.. Incidence and risk factors of reticular pseudodrusen using multimodal imaging. JAMA Ophthalmol. 2020; 138: 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seddon JM, Reynolds R, Rosner B.. Peripheral retinal drusen and reticular pigment: association with CFHY402H and CFHrs1410996 genotypes in family and twin studies. Invest Ophthalmol Vis Sci. 2009; 50: 586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.