Abstract
The glucose analog 2-deoxyglucose (2dGlc) inhibits the growth and multicellular development of Myxococcus xanthus. Mutants of M. xanthus resistant to 2dGlc, designated hex mutants, arise at a low spontaneous frequency. Expression of the Escherichia coli glk (glucokinase) gene in M. xanthus hex mutants restores 2dGlc sensitivity, suggesting that these mutants arise upon the loss of a soluble hexokinase function that phosphorylates 2dGlc to form the toxic intermediate, 2-deoxyglucose-6-phosphate. Enzyme assays of M. xanthus extracts reveal a soluble hexokinase (ATP:d-hexose-6-phosphotransferase; EC 2.7.1.1) activity but no phosphotransferase system activities. The hex mutants have lower levels of hexokinase activities than the wild type, and the levels of hexokinase activity exhibited by the hex mutants are inversely correlated with the ability of 2dGlc to inhibit their growth and sporulation. Both 2dGlc and N-acetylglucosamine act as inhibitors of glucose turnover by the M. xanthus hexokinase in vitro, consistent with the finding that glucose and N-acetylglucosamine can antagonize the toxic effects of 2dGlc in vivo.
The myxobacteria, including their best-characterized representative, Myxococcus xanthus, are social prokaryotes that undergo multicellular development. When a large number (>105) of M. xanthus cells are starved for essential nutrients, individual cells within a starved population coordinate their movements to form a fruiting body that supports the differentiation of a subset of vegetative cells into spores. Spores represent only a small minority (1 to 10%) of developing cells after carbon starvation and are more resistant to heat, UV light, and sonication than vegetative cells. Spores ensure the survival of this organism under extreme environmental conditions (17).
Development is a rapid process. Spores mature within fruiting bodies in the short span of 36 h. The cycle leading to spore maturation involves a cascade of gene expression, in which successive subsets of developmental stage-specific promoters are activated (22). Development is accompanied by dramatic changes in the flow of carbon through metabolic pathways. The early stages of development involve the oxidative catabolism of vegetative biopolymers to generate intermediary metabolites. These metabolites fuel a burst of primarily gluconeogenic anabolism during the later stages of development. This gluconeogenic burst begins about 4 to 6 h after starvation, and, in turn, supports the assembly of the spore coat, comprised mainly of polysaccharides (48).
Although many studies have focused on the cascade of gene expression that occurs during development, very little is known about the metabolic changes that accompany this adaptive response. Most of the genes known to be required for development function early in this cycle. In contrast, few genes that function late in development have been identified. Thus, little is known about the genes involved in spore maturation, the regulation of gene expression during late development, or the spatial and temporal coordination of polysaccharide biosynthesis with spore coat assembly (17).
To understand how polysaccharide biosynthesis and spore maturation are coordinated, we are exploring the regulation of the gluconeogenic pathway during development. Previous studies of intermediary metabolism have shown that M. xanthus makes many of the enzymes that catalyze steps in this pathway. These include phosphoenolpyruvate (PEP) carboxykinase (EC 4.1.1.32), glyceraldehyde-3-phosphate dehydrogenase (EC 1.2.1.12), aldolase (EC 7.1.2.7), fructose-1,6-phosphate phosphatase (EC 3.1.3.11), and phosphoglucoisomerase (EC 5.3.1.9), as well as phosphoglucomutase (EC 2.7.5.1) and UDPG pyrophosphorylase (EC 2.7.7.9) (46). Expression of the gluconeogenic enzymes is induced during an alternate developmental pathway, in which cells sporulate in response to high external concentrations of short-chain polyols (10).
The gluconeogenic pathway in M. xanthus is unusual in two respects. The M. xanthus PEP carboxykinase, which catalyzes the first committed step in gluconeogenesis, requires GTP or ITP as a phosphoryl donor (46), like eukaryotic PEP carboxykinases and unlike many of their ATP-dependent prokaryotic counterparts. In addition, the M. xanthus glycolytic pathway, which shares many enzymes with the gluconeogenic pathway, initially appeared incomplete. When extracts were prepared from vegetative M. xanthus cells and assayed for glycolytic activities, neither ATP-dependent hexokinase (EC 2.7.1.1) nor pyruvate kinase (EC 2.4.1.40) activities were detected (46).
Their inability to detect intracellular ATP-dependent hexokinase, pyruvate kinase, and high-affinity glucose permease activities led Watson and Dworkin (46) to speculate that M. xanthus may exclude glucose for some selective advantage. These results also could suggest that the apparently glycolytic activities produced by M. xanthus may have roles only in the anabolic pathway of gluconeogenesis. However, this notion is not satisfying, because M. xanthus makes an ATP-dependent phosphofructokinase (EC 2.7.1.11), which catalyzes a committed step in glycolysis that must be bypassed in the gluconeogenic pathway (46).
During vegetative growth, M. xanthus is predatory and is thought to derive its energy primarily from the oxidative catabolism of proteins released by the lysis of prey organisms by a battery of secreted enzymes. The minimal requirements for the growth of M. xanthus in defined media suggest that proteins comprise the core of its rich diet. M. xanthus is auxotrophic for leucine, isoleucine, and valine, the three branched-chain amino acids, and for methionine or cobalamin. M. xanthus is also a phenylalanine bradytroph, and many nonessential amino acids stimulate its growth (2, 7, 18).
Surprisingly, hexose monosaccharides including glucose (Glc), fructose (Fru), galactose, and glucosamine (2-amino-2-deoxyglucose; GlcN), as well as a variety of their disaccharides, do not stimulate the growth of this obligate aerobe on defined medium. In contrast, acetate and pyruvate, as well as the citric acid cycle intermediates malate and succinate, do stimulate growth (2, 18). Again, these results suggest that M. xanthus cannot transport or phosphorylate Glc efficiently or, at the least, utilize Glc as an energy source. Only a small fraction of label added as [14C]glucose is incorporated into biopolymers with high molecular mass during growth on defined media (18, 46), reinforcing the idea that M. xanthus does not require Glc for growth.
On the other hand, it is clear that simple sugars can play critical roles during M. xanthus development. One subset of simple sugars, including the reduced triose glycerol (8) and the pentoses ribose and xylose (34), elicit the synchronous and rapid conversion of rod-shaped vegetative cells in liquid culture into spherical spores. These short-chain polyols trigger an abbreviated unicellular developmental pathway leading to the formation of spores that are more heat resistant than vegetative cells but less heat resistant than the more structurally complex spores produced during starvation-induced multicellular development (42). Furthermore, addition of the GlcN, but not the closely related hexose Glc or N-acetylglucosamine (2-deoxy-2-N-acetylamino-d-glucose; GlcNAc), to exponentially growing cells at high concentrations elicits cell lysis (29). Therefore, it is inviting to speculate that M. xanthus uses these sugars or their phosphorylated derivatives as signals to trigger pathways that define distinct cell fates during development.
M. xanthus is sensitive to the antibiotic 2-deoxyglucose (2dGlc). In most organisms, the analog 2dGlc is transported by mechanisms that also transport glucose and must be phosphorylated to form 2-deoxyglucose-6-phosphate (2dGlc6P) to exert its toxic effect. Starting with this discovery, we obtained genetic evidence implying that M. xanthus phosphorylates Glc with a soluble hexokinase and have identified this hexokinase activity in vitro.
MATERIALS AND METHODS
Media and chemicals.
CTPM liquid medium (1% Casitone, 10 mM Tris [pH 7.6], 1 mM potassium phosphate [pH 7.5], 5 mM MgSO4) was used for growth of M. xanthus. TPM buffer, used for developmental assays, is CTPM without Casitone. Stocks of monosaccharides used to supplement media were dissolved in water and filter sterilized. Antibiotics, sugars, enzymes, substrates, and other chemicals were from Sigma or Aldrich, with the exception of ATP, which was from Pharmacia. 14C-labeled hexoses used as substrates in the assays for glucose-specific phosphotransferase system (PTS) activities were from ICN or NEN-DuPont. Oligonucleotides used for plasmid construction and mutagenesis were made by Biosource Inc. Restriction endonucleases and DNA-modifying enzymes were from New England Biolabs and were used under recommended conditions.
Bacteria and plasmids.
Escherichia coli JM107 (50) was used for the construction of plasmids by standard methods and for the preparation of plasmid DNA (37). Plasmids were introduced into JM107 by electroporation (43). Derivatives of JM107 with plasmids were grown in LB medium supplemented with ampicillin (100 μg/ml) and/or kanamycin (40 μg/ml). M. xanthus strains are derivatives of the wild-type strain DK1622 (19) or the nonmotile strain DZ1 (3). Strains XS200 and XS201, which carry the hex1 and hex3 mutations, respectively, were selected by plating 108 exponentially grown cells from independent cultures of DK1622 on CTPM medium with 1% 2dGlc. In some experiments, the rates of growth of M. xanthus cultures were monitored by measuring relative light scattering in Klett-Summerson units (KU), using a Klett-Summerson colorimeter (model 800; 640- to 700-nm filter). Light scattering is proportional to the numbers of viable wild-type cells grown in CTPM medium at 32°C over the range of 10 to 120 KU. The assay of viable M. xanthus cells in culture was made by the soft-agar overlay method, in which aliquots of cultures diluted in CTPM medium were resuspended in 3 ml of CTPM soft agar (0.7%) and distributed over the surfaces of CTPM agar (1.5%) plates.
Integrative plasmid pAY703 (26) carries the M. xanthus mglBA operon subcloned into kanamycin-resistant (Kmr) vector pBGS18 (41). To express the E. coli glk (glucokinase) gene as part of the mglBA operon, template DNA isolated from E. coli JM107 was amplified with primers GLK1 (5′ AAAGGTACCATGAGGAGGTGTACATGACAAAGTATGCATTAGTCG) and GLK2 (5′ CCCGAATTCTCTAGAGAATGTGACCTAAGGTCTGGCG). The amplified product was cleaved with Acc65I and EcoRI and ligated to the same sites of pAY703 to make pAY1108. Plasmids were introduced into M. xanthus by electroporation (20).
Assays for the multicellular development of M. xanthus.
To initiate development, derivatives of DK1622 were grown to a density of 5 × 108/ml in CTPM medium at 32°C, concentrated by low-speed centrifugation, and resuspended in 0.1× TPM buffer. For standard assays (23), multiple spots (20 μl) were made on TPM (1.5%) agar; plates were incubated at 32°C for 120 h. Sets of five 20-μl spots were harvested after incubation at 50°C for 2 h and scraped together into 1 ml of TPM buffer. Suspensions were sonicated for 15 s at 5 W on a Fisher model 60 Sonic Dismembranator to disperse spores. Serial dilutions of spore suspensions were plated on CTPM plates by the soft-agar overlay method and scored after 72 h at 32°C.
To determine the time(s) after the onset of starvation at which 2dGlc inhibits development, we modified the standard assay. Aliquots (1 ml) of exponentially growing cells suspended in 100 μl of TPM buffer were spotted on the surfaces of nitrocellulose filters (0.025-μm-pore-size type VS; Millipore) that had been sterilized by autoclaving in distilled H2O. Spots were allowed to dry for 2 h at 25°C; then the plates with filters were placed at 32°C. At each 12-h interval after the 2-h initial drying period and throughout the 5-day incubation period, duplicate filters were transferred with sterile forceps from the surfaces of TPM agar plates to TPM agar plates supplemented with 0.1% 2dGlc. Two filters were retained on TPM plates, and not transferred to plates with 2dGlc, to ensure that fruiting body formation and sporulation were not affected by cell contact with the filters. After transfer, filters on plates with 2dGlc were incubated at 32°C to complete the incubation time of 120 h and then incubated at 50°C for 2 h to kill vegetative cells. Filters were quartered by using sterile scissors and placed into a 2-ml microcentrifuge tube containing 1 ml of TPM buffer, and samples were sonicated for 15 s at 5 W. Serial dilutions of each sonicate were plated on CTPM agar plates. Colonies arising from germinated spores were counted after 72 h at 32°C.
To determine the precise time after the initiation of the developmental cycle at which spores mature when cells are allowed to develop on filters, we set up a parallel experiment. Cells were grown, harvested, and spotted onto filters on the surfaces of TPM agar plates with or without 0.1% 2dGlc. At 12-h intervals after the initiation of development, filters were removed, and incubated at 50°C for 2 h to kill vegetative cells prior to assays for heat-resistant spores. The results of this control experiment showed that spores first acquire heat resistance at about 36 h after the initiation of development, as they do when cells are placed directly upon the TPM agar surface.
PTS activity assays.
Wild-type strain DK1622 and its mutant derivatives were grown to a density of 5 × 108 cells/ml in CTPM medium supplemented with 2% of each of the PTS sugars Glc, GlcNAc, Fru, and mannitol (Mtl) at 32°C and concentrated by low-speed centrifugation. Cell pellets were suspended in 1/10 volume 20 mM Tris buffer (pH 8.0)–1 mM EDTA–5 mM 2-mercaptoethanol and disrupted by sonication with four 10-s bursts at 5 W in a Branson W140D Sonifier cell disruptor. Assays for partial PTS activities (35a), for fructose-1-phosphate kinase activity (1a), and for mannitol-1-phosphate dehydrogenase activity (35a) were performed as described elsewhere.
Hexokinase activity assays.
Wild-type strain DK1622 and its mutant derivatives were grown to a density of 5 × 108 cells/ml in CTPM medium at 32°C, concentrated by low-speed centrifugation, and suspended in 1/10 volume TPM buffer at 4°C. Cell extracts were prepared by sonication of cultures in TPM for 10 s at 100 mW in a model 60 Fisher Sonic Dismembranator and then subjected to microcentrifugation at 20,000 × g for 5 min to remove cell debris. Extracts were maintained at 4°C no longer than 4 h prior to assay.
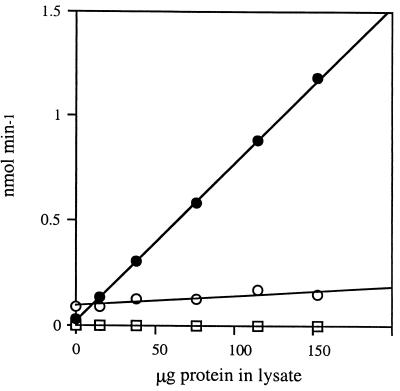
Hexokinase activities were determined by using the coupled reaction of Fraenkel and Horecker (12), with minor modifications. Standard reaction mixtures (1 ml) contained 50 mM Tris (pH 9.1)–10 mM MgCl2 (hexokinase buffer), 20 mM glucose, 10 mM ATP (pH 9.1), 10 to 100 μl of cell extract (0.1 to 2.0 mg of protein), and 500 to 1,000 U of Leuconostoc mesenteroides glucose-6-phosphate (Glc6P) dehydrogenase. (One unit corresponds to 1 nmol of Glc6P min−1 mg−1 Glc6P oxidized to 6-phospho-d-gluconate in the presence of NADP at pH 7.6 at 25°C.) In experiments used to determine apparent values of Km for ATP and Glc and Ki for 2dGlc, the concentrations of these compounds were varied in the standard reaction mixtures. Assays were initiated by the addition of NADP to 200 μM. The initial velocity of NADP reduction (ɛNADPH = 6.22 × 106 cm−2) was measured at 25°C as the change in absorbance at 340 nm at 30-s intervals. Spectrophotometry was done with a Perkin-Elmer Lambda 12 UV/VIS spectrophotometer supported by a Dell OptiPlex XMT590 workstation and the uv-WinLab software package. Initial velocities were found to be linear for at least 5 to 10 min and proportional to the protein concentration of extracts (Fig. 1). Control experiments showed that the concentrations of inhibitors used in the assays that we report did not decrease the rate of the coupled, NADP-dependent oxidation of Glc6P by Glc6P dehydrogenase to make this the rate-limiting step in these assays. Cell supernatants used in enzyme assays were assayed for total protein content by the bicinchoninic acid method (40); reagents for assay were from Pierce. Samples (10 μl) were placed in the wells of a 96-well microtiter plate, and 200 μl of reagent was added. Microtiter plates were incubated at 37°C for 30 min and then absorbance at 540 nm was measured with a Titertech Multiscan MCC340 spectrometer. Protein concentrations were extrapolated from a standard bovine serum albumin curve.
FIG. 1.
Hexokinase activity in extracts from wild-type M. xanthus cells is a linear function of protein concentration. Activities of hexokinase are plotted as a function of total protein in extracts prepared from wild-type M. xanthus cells, are the averages of three determinations, and had standard deviations of <±0.032 nmol min−1. Extracts were incubated in 50 mM Tris–10 mM MgCl2 (pH 9.1) with 500 to 1,000 U of Glc6P dehydrogenase plus Glc (20 mM) and ATP (10 mM) (filled circles), ATP (10 mM) (open circles), or Glc (20 mM) (squares). The observed rates of NADP reduction in the absence of added ATP (squares) are similar in reactions with or without added extract, activity is dependent on the addition of Glc, and the addition of Glc decreases the rate of spontaneous NADP reduction in the complete assay without added extract.
When hexokinase activity is assayed directly by the method of Darrow and Colowick (5), which measures the rate at which protons are generated concomitant with Glc phosphorylation, a competing activity that consumes protons at a rate faster than their liberation is observed. This is true even in extracts prepared from M. xanthus cells expressing E. coli glucokinase. We chose to assay hexokinase activity at pH 9.1 in the coupled assay, because we found that E. coli glucokinase is about twofold more active (and has lower Kms for both ATP and Glc) at pH 9.1 than at pH 7.6, whereas M. xanthus hexokinase has comparable activities at both pH values. Hexokinase activity in M. xanthus extracts is labile. Activity is lost within 48 h if extracts are stored at 4°C and does not survive repeated cycles of freezing at −20°C and thawing. Therefore, we took care to assay extracts immediately after the sonication of cells. This instability may account for the negative results of Watson and Dworkin (46), as may a relatively high background of hexokinase activity present in preparations of Saccharomyces cerevisiae Glc6P dehydrogenase, given that the specific activity of M. xanthus hexokinase is quite low.
RESULTS
2dGlc inhibits the growth of M. xanthus.
Wild-type M. xanthus DK1622 does not form colonies efficiently on medium containing the Glc analog 2dGlc (Table 1). Concentrations of 2dGlc as low as 0.04% (2.4 mM) inhibit the efficiency of plating of wild-type cells by a factor of greater than 105. This result is surprising in light of the previous findings that cell extracts from vegetative M. xanthus cells lack an ATP-dependent glucokinase activity (46) and that less than 2% of label added to exponentially growing cells in the form of [14C]glucose is incorporated into intracellular materials with higher molecular mass (18, 46). In other microbes, 2dGlc is most often transported by mechanisms that facilitate the transport of glucose.
TABLE 1.
M. xanthus is sensitive to hexose analogsa
| Hexoseb | Efficiency of platingc
|
||
|---|---|---|---|
| DK1622 | DK1622::pAY703 | DK1622::pAY1108 | |
| None | 1.0 | 1.0 | 1.0 |
| 2% Glc | 0.6 | 0.5 | 0.5 |
| 1% 2dGlc | 3 × 10−6 | 3 × 10−6 | <10−8 |
| 2% MeGlc | 0.9 | 0.9 | 0.8 |
| 2% GlcN | <10−8 | <10−8 | <10−8 |
| 2% GlcNAc | 0.6 | 0.6 | 0.4 |
| 2% Fru | 0.5 | 0.6 | 0.7 |
| 2% Mtl | 0.8 | 0.6 | 0.6 |
Wild-type strain DK1622 and its derivatives with plasmids pAY703 and pAY1108 (expressing the E. coli glk gene) integrated at the mglBA locus were grown to a density of 5 × 108/ml at 32°C in CTPM medium.
Dilutions of each culture were plated on CTPM medium or medium supplemented with each of the hexose analogs. Colonies were counted after 7 days of growth at 32°C.
Calculated as the titer of each culture on a given medium divided by its titer on CTPM medium without added hexose. Numbers are the averages of at least three determinations and varied less than fivefold.
Wild-type M. xanthus cells plate with high efficiencies in the presence of higher concentrations of a variety of other hexoses, including Glc, methyl-α-d-glucopyranoside (MeGlc), GlcNAc, Fru, and mannitol Mtl. However, the growth of M. xanthus is inhibited by at least one other hexose, glucosamine (GlcN). We find, as did Mueller and Dworkin, that wild-type cells do not form colonies on rich medium with 2% GlcN (Table 1). This is consistent with the observation that the addition of GlcN to exponentially growing cells at high concentrations elicits cell lysis (29).
2dGlc, unlike glycerol or GlcN, is not a morphogen.
To address how 2dGlc inhibits growth, we tested whether 2dGlc, like GlcN, might act as a morphogen to induce cell lysis. We also tested whether 2dGlc might behave like another subset of sugars that elicit a different morphogenetic response when added to exponentially growing cells. These sugars, including the reduced triose glycerol (8) and the pentoses ribose and xylose (34), trigger the rapid sporulation of vegetative M. xanthus cells without the concomitant formation of fruiting bodies. The development of M. xanthus cells in response to these short-chain polyols is thought to mimic the later steps in starvation-induced multicellular development for two reasons. A subset of mutants resistant to glycerol-induced sporulation do not undergo multicellular development (9), and the accumulation of intracellular glycerol within starving cells coincides with later steps in starvation-induced development (13).
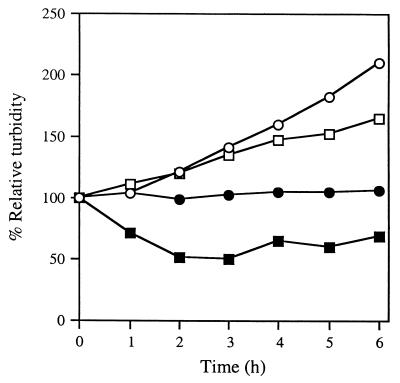
Wild-type cells were grown to exponential density in rich medium, and each of a variety of sugars was added to the growing cells. The growth of the cultures and the microscopic appearances of cells within each culture were monitored at various times after the addition of these sugars. As shown in Fig. 2, when a culture of exponential cells is supplemented to 0.5 M glycerol, these glycerol-treated cells tend to aggregate in liquid suspension as they differentiate into spores. Consequently, the turbidity of a glycerol-treated culture decreases significantly. The majority of cells change from long rods to more refractile spheres within 3 h (Fig. 3B). In contrast, when cells are treated with 2% GlcN, the turbidity of the culture continues to increase, although not so rapidly as that of an untreated culture (Fig. 2). Microscopic examination of the GlcN-treated cells reveals that the majority has lysed after 3 h of treatment, leaving behind empty, rod-shaped husks (Fig. 3D). Presumably, these husks retain the ability to scatter visible light and contribute to the increase in turbidity that accompanies the lysis of GlcN-treated cultures. In contrast, the turbidity of cultures treated with 1% 2dGlc does not change for 6 h after treatment (Fig. 2), and cells within these cultures neither round to form spores nor lyse (Fig. 3C).
FIG. 2.
2dGlc arrests the growth of M. xanthus. A culture of wild-type cells was grown to a density of 5 × 108/ml in CTPM medium at 32°C. At the start of the experiment, equal subcultures were left untreated (open circles) or supplemented to 0.1 M 2dGlc (filled circles), 0.1 M GlcN (open squares), or 0.5 M glycerol (filled squares). The percent relative turbidity (turbidity of each subculture divided by its turbidity at 0 h) is shown; initial values, determined immediately after the addition of supplements, ranged from 90 to 110 KU.
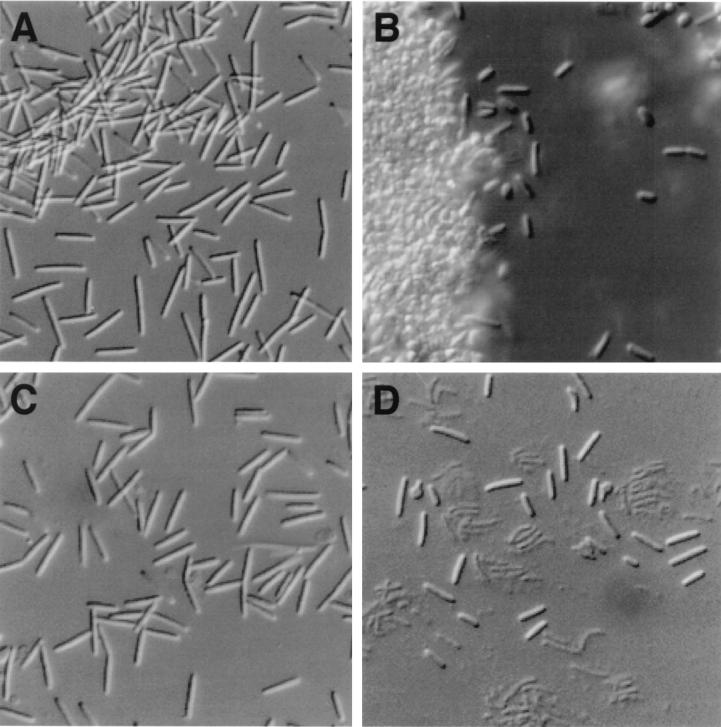
FIG. 3.
2dGlc is not a morphogen. At 3 h after treatment, 1 ml of each culture prepared as described in the legend to Fig. 2 was concentrated by low-speed centrifugation and suspended in 0.1 ml of CTPM medium. Aliquots were examined by using differential interference contrast optics and a 40× objective lens with a Nikon Microphot-FXA microscope (bottom). (A) Untreated cells; B, cells plus 0.5 M glycerol; C, cells plus 1% 2dGlc; D cells plus 2% GlcNAc. Photomicrographs of cells are at the same magnification; the average length of untreated cells is ca. 10 μm.
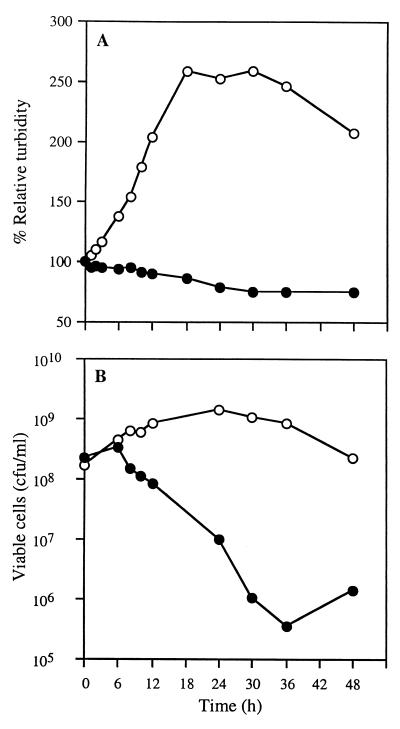
When aliquots of treated cells are plated for viable titers at various times after treatment, we find that glycerol-induced spores can germinate to yield the initial titer of cells from which they arose, consistent with previous results (9). Cells treated with GlcN show a dramatic decrease in titer within 6 h, consistent with the results published by Mueller and Dworkin (29). In contrast, the viable titer of cells after treatment with 1% 2dGlc does not change for 6 h (Fig. 4). After prolonged treatment with 2dGlc, cells round up and eventually lyse.
FIG. 4.
M. xanthus cells die slowly in the presence of 2dGlc. The turbidity (A) and viability (B) of subcultures left untreated (open circles) or treated with 2dGlc as described in the legend to Fig. 2 (filled circles) was monitored over a 48-h time course. Viability was determined by plating serial dilutions of the subcultures on CTPM agar plates. Note that a twofold loss in viability is observed after 12 h of treatment with 2dGlc, corresponding to the period of one doubling in the untreated culture.
Spontaneous mutants of M. xanthus resistant to 2dGlc arise at a frequency indicative of the loss of function.
The sensitivity of M. xanthus to 2dGlc suggests that M. xanthus can phosphorylate this hexose analog. Sensitivity to 2dGlc in many other microbes requires both the transport of this glucose analog and its phosphorylation to form the toxic intermediate, 2dGlc6P. This can occur by one of two different general mechanisms: group translocation, in which the transport of hexoses is coupled with their phosphorylation, or a combination of active transport and phosphorylation by a soluble hexokinase. For example, E. coli can use the PTS, dependent on the ptsI, ptsH, and crr genes, to simultaneously transport 2dGlc and phosphorylate this substrate, using PEP as the phosphoryl donor (31). Many other gram-negative and gram-positive bacteria have similar PTSs that translocate both Glc and its toxic analog 2dGlc (33, 35).
When microbes do not couple 2dGlc translocation with its phosphorylation, sensitivity to 2dGlc depends on the combination of an independent transport mechanism and a soluble hexokinase activity. Such is the case for both Streptomyces coelicolor (1) and S. cerevisiae (25). Most mutations that confer resistance to 2dGlc in these organisms inactivate the genes encoding hexokinase. Mutations that confer resistance to 2dGlc in yeast also are found in a gene required for the transport of both Glc and 2dGlc (30). Both known mechanisms by which microbes are sensitive to 2dGlc involve the transport and phosphorylation of 2dGlc by proteins that transport and phosphorylate a subset of other hexoses. This subset often includes Glc.
Because many gram-negative bacteria have one or more PTSs capable of 2dGlc group translocation, initially it was inviting to speculate the M. xanthus might also have a PTS. This hypothesis would neatly explain the previous failure to find both ATP-dependent hexokinase and pyruvate kinase activities made by M. xanthus (46); both PTS activities are coupled and most often PEP dependent. Therefore, we assayed extracts prepared from M. xanthus cells grown in the presence of each of the four PTS sugars Glc, GlcNAc, Fru, and Mtl for PTS activities. However, we did not detect PEP- or ATP-dependent PTS activities with any of these four hexoses, MeGlc, or 2dGlc as the labeled substrate. In addition, we could detect neither fructose-1-phosphate kinase activity characteristic of the Fru PTS or mannitol-1-phosphate dehydrogenase activity characteristic of the Mtl PTS. These negative results suggested that, like S. coelicolor (1), M. xanthus has independent mechanisms of 2dGlc (and Glc) transport and phosphorylation.
To confirm this hypothesis, we began with a genetic approach, selecting and characterizing mutants of M. xanthus resistant to 2dGlc. When wild-type strain DK1622 is plated on rich medium supplemented to 1% 2dGlc, it forms colonies with an efficiency of 3 × 10−6 (Table 1). This is about 10-fold higher than the frequency observed for spontaneous mutations that inactivate the uraA gene of M. xanthus (21). This high frequency of spontaneous mutation suggests that resistance to 2dGlc is due to the loss of function, and mutations that confer resistance inactivate one or more target genes.
Colonies formed by mutants resistant to 2dGlc have two distinct morphologies on rich medium with 2dGlc. Under restrictive conditions, most colonies are large with diffuse, spreading edges, whereas fewer colonies are smaller (less motile) and have sharp, well-defined edges. Under permissive conditions (rich medium without 2dGlc), both types of mutants form large, spreading colonies with the same morphology as the wild type. We isolated and characterized two independent mutants of the wild-type strain, carrying hex1 and hex3 mutations, which form larger and smaller colonies, respectively, under restrictive conditions. These mutants form colonies on rich medium supplemented with 1% 2dGlc with similar efficiencies as on medium without 2dGlc (Table 2).
TABLE 2.
Expression of E. coli glk restores sensitivity to 2dGlc-resistant mutants of M. xanthusa
| Plasmid (genotype) | Efficiencies of platingb
|
||
|---|---|---|---|
| Wild type | hex1 | hex3 | |
| None | 3 × 10−6 | 4 | 0.9 |
| pAY703 (mglBA) | 3 × 10−6 | 1 | 0.8 |
| pAY1108 (mglBA-glk) | <10−8 | 7 × 10−5 | 2 × 10−5 |
Wild-type strain DK1622 and its 2dGlc-resistant hex1 and hex mutant derivatives with no integrated plasmids or with plasmids pAY703 and pAY1108, were grown to a density of 5 × 108/ml at 32°C in CTPM medium. Dilutions of each culture were plated on CTPM medium and medium supplemented with 1% 2dGlc. Colonies were counted after 7 days of growth at 32°C.
Calculated as the titer of each culture on CTPM medium with 1% 2dGlc divided by its titer on CTPM medium without 2dGlc. Numbers are the averages of at least two determinations and varied less than fivefold.
Expression of the E. coli glk (glucokinase) gene in mutants of M. xanthus resistant to 2dGlc restores sensitivity to 2dGlc.
If M. xanthus, like E. coli, has a PTS for the coupled transport and phosphorylation of 2dGlc, then we would expect that expression of a soluble hexokinase in such mutants should not restore sensitivity to 2dGlc. For example, mutations in E. coli that inactivate the group translocation system for Glc confer resistance to 2dGlc yet retain the soluble hexokinase activity made by the glk gene (4). Alternatively, if M. xanthus, like S. coelicolor or S. cerevisiae, has independent glucose transport and hexokinase functions, then mutants of M. xanthus resistant to 2dGlc should carry mutations that inactivate a gene, hex, encoding a soluble hexokinase. The expression of a heterologous glucokinase function in these mutants, such as the product of the E. coli glk gene, should restore sensitivity to 2dGlc. For example, when a plasmid with an active glkA gene is introduced into a mutant strain of S. coelicolor resistant to 2dGlc, it restores sensitivity to 2dGlc (45). Therefore, we constructed derivatives of wild-type M. xanthus DK1622, and its mutant derivatives resistant to 2dGlc that express the E. coli glk gene, and examined their phenotypes.
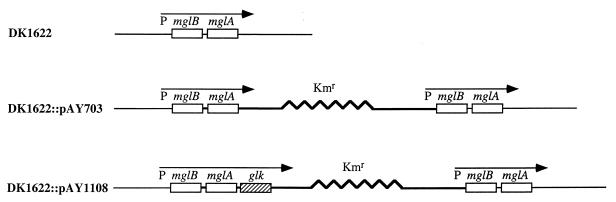
The E. coli glk (glucokinase) gene (28) was amplified by PCR and cloned distal to the mglA gene in the constitutively expressed mglBA operon (16) carried by plasmid vector pAY703 (26). Plasmid pAY703 and its otherwise isogenic derivative with glk, pAY1108, have a Kmr determinant and an origin that supports autonomous replication in E. coli but not in M. xanthus. After electroporation, these plasmids can integrate into the circular M. xanthus chromosome by homologous recombination between the plasmid and chromosome within the region of homology shared by the two elements, the mglBA operon. Integration results in the formation of Kmr merodiploid strains with two copies of the mglBA operon that sandwich nonhomologous plasmid DNA (Fig. 5).
FIG. 5.
Plasmids pAY703 and pAY1108. The structures of the chromosomal mgl locus are shown for wild-type M. xanthus and for recombinant merodiploid strains with integrated plasmid vectors pAY703 and pAY1108. Recombinant strains with plasmid pAY1108 express the E. coli glk (glucokinase) gene from the constitutive mglBA promoter. The level of glucokinase expression in M. xanthus is the same as its basal level of expression in E. coli (28).
A Kmr derivative of DK1622 with integrated plasmid pAY1108 should express the E. coli glk gene as part of the mglBA operon. We have shown that similar derivatives of plasmid pAY703 express the myxophage Mx8 mox (26) and int (36) genes, as well as the M. xanthus sglK gene (47). Kmr electroporants of host DK1622 carrying plasmid pAY1108 were obtained with the same efficiency after electroporation as with parental plasmid pAY703. Also, after purification, we find that strain DK1622::pAY1108 has the same doubling time as strain DK1622::pAY703 in rich medium at 32°C.
A comparison of the abilities of strains DK1622, DK1622::pAY703, and DK1622::pAY1108 to form colonies in the presence of glucose and several glucose analogs is shown in Table 1. All three strains form colonies with similar efficiencies on rich medium supplemented with each of the hexoses, with one exception. Whereas wild-type strain DK1622 and strain DK1622::pAY703 plate with low but detectable efficiencies (3 × 10−6) on medium with 1% 2dGlc, strain DK1622::pAY1108 does not form colonies with a measurable efficiency (<10−8). Expression of the E. coli glk gene in wild-type M. xanthus results in a gain of function and confers an increased level of sensitivity to 2dGlc upon the wild-type strain.
The addition of the glk gene to the wild-type M. xanthus genetic background prevents mutation to 2dGlc resistance in a single step. The simplest hypothesis to account for this finding is that glk is expressed in M. xanthus, and the functions of glk and of the target (hex) for 2dGlc resistance mutations in the wild-type M. xanthus genetic background are similar. Consistent with this hypothesis, derivatives of the mutant hex1 and hex3 strains carrying pAY1108 (mglBA-glk) regain sensitivity to 2dGlc, whereas their otherwise isogenic controls with plasmid pAY703 (mglBA) remain resistant to 2dGlc (Table 2). These two lines of genetic evidence suggest that the hex mutations confer resistance to 2dGlc due to the loss of function and that this function is a soluble hexokinase. A derivative of the wild-type strain expressing both hex and glk cannot acquire resistance in a single mutational step, whereas both the wild-type strain (expressing hex alone) and the complemented mutant strains (expressing glk alone) can (Table 2).
Three additional results support this explanation. First, the mutant hex1 and hex3 strains carrying pAY1108 (mglBA-glk) were plated on rich (CTPM) medium with 1% 2dGlc, and 104 independent 2dGlc-resistant mutants arising from each strain were tested for a Kmr phenotype. Among the 2dGlc-resistant colonies arising from the hex1::pAY1108 and hex3::pAY1108 strains, 104 of 104 (100%) and 52 of 104 (50%), respectively, were found to have acquired a Kms phenotype. These Kms segregants most likely arise due to the loss of integrated plasmid pAY1108 DNA, after homologous recombination within the merodiploid mgl locus promotes excision of the integrated plasmid, and a subset of daughter cells do not inherit the excised plasmid. Second, we isolated a spontaneous Kms segregant from strain DK1622::pAY1108 that had lost its integrated plasmid. This segregant, like its wild-type grandparent, forms colonies on medium with 2dGlc with an efficiency of 3 × 10−6. Third, the multiple mutant, nonmotile M. xanthus strain DZ1 (3), with a different genetic background, carries a uncharacterized mutation (hex2) that confers resistance to 2dGlc. Again, the introduction of plasmid pAY1108 (mglBA-glk), but not plasmid pAY703 (mglBA), into this strain restores sensitivity to 2dGlc.
The sensitivity of M. xanthus to 2dGlc can be antagonized by Glc and GlcNAc but not by Fru or Mtl.
If M. xanthus can transport 2dGlc and, like other microbes, phosphorylates 2dGlc to make the toxic metabolite 2dGlc6P, then a subset of hexoses may antagonize this sensitivity to 2dGlc, by competing with 2dGlc as alternate substrates for phosphorylation or by inhibiting hexokinase by another mechanism. Therefore, we measured the efficiency of plating of wild-type strain DK1622 on medium containing 0.1% (6 mM) 2dGlc and various concentrations of potential hexose antagonists. Wild-type strain DK1622 plates with the low efficiency of about 3 × 10−6 on medium containing 0.1% (ca. 6 mM) 2dGlc alone. However, the addition of Glc or GlcNAc to 2% (ca. 100 mM) permits this strain to form colonies with high efficiencies in the presence of 2dGlc (Table 3). In contrast, the addition of either Fru or Mtl to 2% does not antagonize 2dGlc. Because production of E. coli glucokinase in hex mutant strains of M. xanthus restores sensitivity to 2dGlc, the hex mutant strains retain the ability to transport 2dGlc. In addition, both Glc and GlcNAc are transported by M. xanthus. From these data, however, we cannot assess whether this transport mechanism is active or passive.
TABLE 3.
Glc and GlcNAc can antagonize the sensitivity of wild-type M. xanthus cells to 2dGlc
| Supplement(s)a | Efficiency of platingb |
|---|---|
| None | 1.0 |
| 0.1% 2dGlc | 3 × 10−6 |
| 0.1% 2dGlc + 2% Glc | 0.4 |
| 0.1% 2dGlc + 2% GlcNAc | 0.4 |
| 0.1% 2dGlc + 2% Fru | 2 × 10−6 |
| 0.1% 2dGlc + 2% Mtl | 2 × 10−6 |
Wild-type strain DK1622 was grown to a density of 5 × 108/ml at 32°C in CTPM medium. Dilutions of each culture were plated on CTPM medium or medium supplemented with each of the combinations of hexose analogs. Colonies were counted after 7 days of growth at 32°C.
Calculated as the titer of each culture on a given medium divided by its titer on CTPM medium without added hexoses. Numbers are the averages of at least three determinations and varied less than fivefold.
On the other hand, it is not surprising that M. xanthus transports these hexoses. M. xanthus is known to transport a variety of saccharides, including galactose, β-galactosides, and sucrose. When the E. coli galK gene is expressed in M. xanthus, growth becomes sensitive to both 2-deoxygalactose and galactose (44), indicating that galactose is transported but not phosphorylated efficiently by M. xanthus. The E. coli lacZ gene produces active β-galactosidase activity in M. xanthus, and cells transport the β-galactoside 5-chloro-4-bromo-3-indolyl-β-d-galactopyranoside even in the absence of a functional lacY permease (6). M. xanthus strains expressing E. coli lacZ also are sensitive to o-nitrophenyl-β-d-galactoside (unpublished results). M. xanthus cells that express the foreign Bacillus subtilis sacBR genes also acquire sensitivity to sucrose (49).
Both the fruiting body morphogenesis and sporogenesis of M. xanthus in response to starvation are inhibited by 2dGlc.
The later stages of the M. xanthus developmental cycle involve dramatic changes in carbohydrate metabolism. Late in development, a burst of gluconeogenic anabolism fuels the assembly of the spore coat, comprised primarily of polysaccharides. Because the toxic metabolite 2dGlc6P may inhibit enzymes common to the glycolytic and gluconeogenic pathways, and the pathway(s) that this metabolite inhibits may be required for development, we also examined whether 2dGlc inhibits the multicellular development of M. xanthus.
Cultures of wild-type and hex mutant strains were grown to exponential density in rich medium, concentrated by centrifugation, and spotted on starvation (TPM) medium with various added concentrations of 2dGlc. After 120 h, all three strains were found to form fruiting bodies on TPM medium without 2dGlc (Fig. 6) (4). On TPM medium with as little as 0.05% 2dGlc, only the hex1 mutant strain forms fruiting bodies. Under these conditions, the wild-type strain forms no aggregates, and the hex3 mutant strain forms poorly defined, translucent aggregates. Higher concentrations of 2dGlc (>0.5%) inhibit the fruiting body morphogenesis of even the hex1 mutant (data not shown).
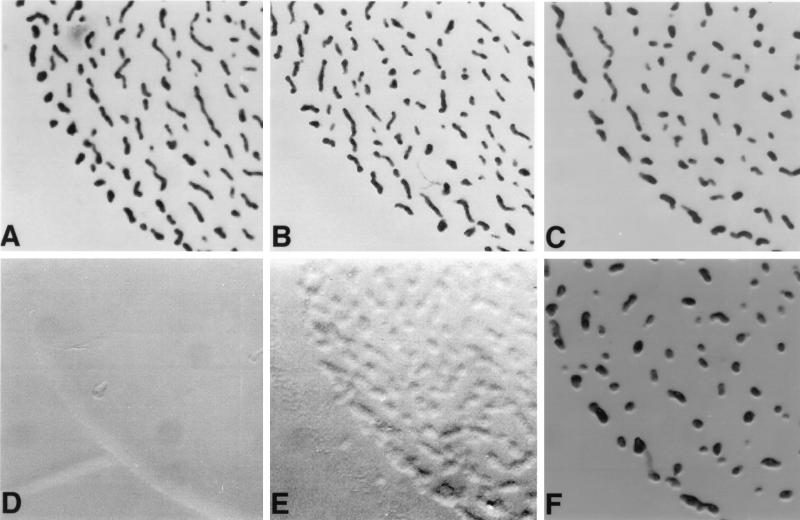
FIG. 6.
2dGlc impairs fruiting body morphogenesis during the development of M. xanthus. The morphologies of developing wild-type and hex3 and hex1 mutant cells were photographed with a Nikon SMZ-U stereomicroscope at a magnification of ×15 on TPM medium and TPM medium supplemented with 0.05% 2dGlc 120 h after the onset of starvation. (A) Wild type on TPM; (B) hex3 on TPM; (C) hex1 on TPM; (D) wild type on TPM plus 2dGlc; (E) hex3 on TPM plus 2dGlc; (F) hex1 on TPM plus 2dGlc.
Cells that had undergone starvation for 120 h were recovered from plates, resuspended in TPM buffer, incubated at 50°C to kill vegetative cells, and assayed for the presence of heat-resistant spores. All three strains form a wild-type complement of heat-resistant spores on starvation agar without 2dGlc (Table 4). Because the hex mutations result in a loss of function, it is likely that the hex gene is not essential for development, because hex mutants both fruit and sporulate. Table 4 also shows that at concentrations of both 0.5 and 2%, 2dGlc inhibits the formation of heat-resistant spores by wild-type and hex3 mutant cells but not by hex1 mutant cells. Thus, hex1 mutant cells can sporulate in the presence of 2% 2dGlc, even though fruiting is impaired under these conditions.
TABLE 4.
2dGlc inhibits the ability of M. xanthus to produce heat-resistant spores in response to starvationa
| Supplementb | Efficiency of sporulationc
|
||
|---|---|---|---|
| hex+ | hex1 | hex3 | |
| 0.5% 2dGlc | 0.00002 | 40 | 0.00001 |
| 2.0% 2dGlc | 0.000001 | 100 | 0.00002 |
| 0.5% GlcN | 0.02 | 0.05 | 0.1 |
| 2.0% GlcN | 0.000002 | 0.000001 | 0.000004 |
Wild-type strain DK1622 (hex+) and its mutant derivatives (hex1 and hex3) were grown to a density of 5 × 108 cells/ml in CTPM medium at 32°C, concentrated 10-fold by low-speed centrifugation, and suspended in TPM buffer.
Suspended cells were spotted onto starvation agar supplemented with the indicated concentrations of hexoses and incubated at 32°C for 120 h, prior to assay for heat-resistant spores, as described in Materials and Methods.
Percentage of heat-resistant spores relative to titers of heat-resistant spores that arose after development on TPM agar without supplements. Values are averages of at least three determinations and varied less than fivefold. For each of the starting strains, DK1622 (wild type), XS200 (hex1), and XS201 (hex3), the titers of heat-resistant spores per 5 × 108 cells starved on TPM medium were 1.9 × 107 (3.8%), 2.3 × 107 (4.6%), and 1.1 × 107 (2.2%), respectively.
As a control for this experiment, we also tested whether the sporulation of wild-type and hex mutant cells is sensitive to GlcN. As shown in Table 4, the development of all three strains is inhibited by GlcN. Unlike their response to 2dGlc, however, the development of all three strains suffers to similar extents in the presence of different concentrations of this hexose.
Cells in two of the colonies formed by the rare, heat-resistant spores made by wild-type cells in the presence of 2% 2dGlc were purified and found to plate with high efficiencies on rich medium with 2dGlc. Thus, the selection for the ability to produce heat-resistant spores in the presence of 2dGlc also yields mutants resistant to 2dGlc.
Both early and late development are inhibited by 2dGlc.
The sporulation of M. xanthus in response to starvation involves the catabolism of vegetative biopolymers and the anabolism of resting biopolymers, including the spore coat polysaccharides which protect the resting cell against harsh environmental conditions. From a metabolic point of view, many of the mutations that confer defects in the early stages of M. xanthus development appear to be blocked in pathways of amino acid catabolism and act during the first 6 h after the onset of starvation. Presumably, these mutations result in a decrease of the flow of carbon into and through catabolic pathways that can feed gluconeogenesis. In contrast, the later stages of development appear to be involved primarily in gluconeogenic anabolism or in spore coat assembly, which is fueled by gluconeogenesis (17).
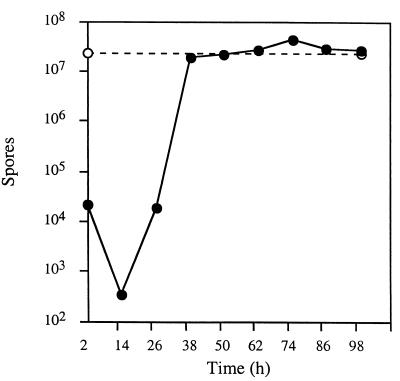
Because we have found that 2dGlc blocks the development of wild-type cells, we determined at which times during development at which 2dGlc exerts its inhibitory activity. We might expect, for example, that if 2dGlc6P inhibits glycolysis and glycolysis is required only early in development, that developing cells would be refractory to 2dGlc after early developmental stages have been completed. We initiated the development of wild-type cells on starvation medium without 2dGlc and, at various times after the initiation of development, transferred developing cells to starvation medium with 0.1% 2dGlc. Cells were allowed to develop for a total time of 120 h under a combination of permissive and restrictive conditions, and spore production was assayed. We find that if cells are transferred to starvation medium with 2dGlc within the first 26 h of development, even after fruiting bodies have formed, 2dGlc inhibits the production of heat-resistant spores by a factor of at least 1,000-fold (Fig. 7). In contrast, cells transferred to medium with 2dGlc at 38 h or more after the initiation of development produce a full complement of heat-resistant spores. This time coincides with the first appearance of mature, heat-resistant spores on medium without 2dGlc. Thus, 2dGlc blocks the development of wild-type cells throughout the developmental cycle.
FIG. 7.
Sporulation is inhibited by 2dGlc throughout the entire developmental cycle. Wild-type cells were grown to exponential density in rich medium, concentrated, spotted on nitrocellulose filters placed on the surfaces of plates with starvation agar, and incubated at 32°C. At the indicated times after the initiation of development, filters were removed from TPM plates and placed onto TPM plates supplemented with 0.1% 2dGlc, and incubation was continued at 32°C. Development on TPM buffer and then on TPM buffer with 2dGlc was allowed to proceed for a total duration of 120 h; then filters were heat treated and assayed for the presence of spores as described in Materials and Methods. Closed circles, spore titers of developing cells transferred from TPM agar to TPM agar with 2dGlc; open circles, spore titers of developing cells maintained on TPM agar.
The hex mutations decrease the specific activity of a soluble hexokinase from M. xanthus.
To show that the hex mutations decrease the specific activity of a soluble hexokinase, we prepared extracts from wild-type cells and assayed these extracts for glucose- and ATP-dependent hexokinase activity, using the coupled assay of Fraenkel and Horecker (12). In this assay, hexokinase activity is measured as the rate of reduction of NADP concomitant with the oxidation of Glc6P, the product of hexokinase, by Glc6P dehydrogenase. As shown in Table 5, extracts prepared from wild-type M. xanthus cells have considerable hexokinase activity. A similar level of activity is present in extracts made from the derivative of wild type with integrated plasmid vector pAY703 (mglBA). Extracts prepared from the derivative of the wild type with plasmid pAY1108 (mglBA-glk) have an approximately 40-fold-higher level of activity. This result confirms that the E. coli glk gene is expressed in M. xanthus. In support of this claim, we find that the apparent Km for ATP of this activity at pH 9.1 in our assays is about 2 mM (data not shown). This value is comparable to that of the remarkably high Km (3.8 mM) that E. coli glucokinase has for this substrate in assays at pH 7.65 (28). In contrast, we find that the M. xanthus hexokinase from wild-type cells has an apparent Km of 200 ± 100 μM for ATP.
TABLE 5.
The E. coli glk gene is expressed in M. xanthus
| Straina | Relevant genotype | Glucokinase activity (U)b |
|---|---|---|
| DK1622 | hex+ | 9.2 |
| DK1622::pAY703 | hex+ | 8.2 |
| DK1622::pAY1108 | hex+ glk+ | 310 |
| XS200 | hex1 | 1.3 |
| XS200::pAY703 | hex1 | 1.4 |
| XS200::pAY1108 | hex1 glk+ | 300 |
| XS201 | hex3 | 2.8 |
| XS201::pAY703 | hex3 | 2.7 |
| XS201::pAY1108 | hex3 glk+ | 510 |
| DK1622::pAY1108*c | hex+ | 9.5 |
Wild-type strain DK1622 and its mutant derivatives were grown to a density of 5 × 108 cells/ml in CTPM medium at 32°C, concentrated 10-fold by low-speed centrifugation, and suspended in TPM buffer.
Cell extracts were prepared by sonication and centrifugation to remove cellular debris, and the supernatants were assayed for E. coli glucokinase activity as described in Materials and Methods. Activities (nanomoles minute−1 milligram−1) are the averages of three determinations and varied less than 25%.
Spontaneous Kms segregant of strain DK1622::pAY1108 that has lost the integrated pAY1108 (mglBA-glk) plasmid.
Extracts made from the 2dGlc-resistant mutants, with the hex1 and hex3 mutations, have lower levels of hexokinase activity than the wild type (Table 5). The hex1 mutant, as well as its derivative with plasmid pAY703, expresses about 15% of the level of wild-type activity, whereas the hex3 mutant and its derivative with pAY703 express about 30% of the wild-type level. Both mutants with plasmid pAY1108 express much higher levels of activity, presumably because they express the E. coli glk gene from the constitutive mgl promoter. No hexokinase activity is detectable in these extracts if glucose is omitted from the assay (Fig. 1).
There is a simple correlation between (i) the specific activities of hexokinase activity present in the wild-type and mutant hex1 and hex3 extracts and (ii) the behavior of these strains during growth and sporulation in the presence of 2dGlc (Tables 2 and 4). The wild type, with the highest hexokinase activity, is sensitive to 2dGlc during both growth and sporulation. The hex3 mutant, with intermediate hexokinase activity, forms small nonmotile colonies upon growth and cannot sporulate with 2dGlc present. The hex1 mutant, with the lowest activity, can grow and sporulate well in the presence of 2dGlc. This correspondence between phenotype in vivo and hexokinase activity in vitro shows that resistance to 2dGlc in M. xanthus is due to a decreased level of hexokinase activity.
Both 2dGlc and GlcNAc, but neither Fru nor Mtl, inhibit the turnover of Glc by M. xanthus hexokinase.
Although 2dGlc inhibits the growth of wild-type M. xanthus cells, the hexoses Glc and GlcNAc can antagonize this toxic effect of 2dGlc in vivo (Table 3). If Glc and GlcNAc act as antagonists of 2dGlc by inhibiting its phosphorylation by hexokinase in vivo, then both 2dGlc and GlcNAc should be inhibitors of Glc turnover in the reaction catalyzed by hexokinase in vitro. Therefore, we tested whether 2dGlc and GlcNAc, as well as other hexoses, could inhibit the phosphorylation of Glc in extracts prepared from wild-type cells. In these assays, Glc was added to 240 μM (ca. 1.5 × Km), and inhibitors were added to 0.2% (1 to 1.5 mM). Each hexose tested as a potential inhibitor could decrease measured activity by acting either as an inhibitor of hexokinase that is not turned over or as an alternative substrate, because the phosphorylated products of these potential antagonists are not substrates for Glc6P dehydrogenase in our coupled assay. As shown in Table 6, both 2dGlc and GlcNAc, but neither Fru nor Mtl, antagonize the rate of glucose turnover by M. xanthus hexokinase. Curiously, GlcNAc is a more potent antagonist of activity, suggesting that GlcNAc or a derivative metabolite may be an alternative substrate for M. xanthus hexokinase. In addition, the results in Table 6 show that GlcN also inhibits the phosphorylation of Glc by hexokinase; it too may be an alternate substrate for this enzyme. Like most known hexokinase activities, the M. xanthus enzyme is refractile to α-methyl-d-glucopyranoside.
TABLE 6.
The phosphorylation of glucose by M. xanthus hexokinase is inhibited by 2dGlc, GlcNAc, and GlcN
Hexokinase activity was assayed in lysates from wild-type M. xanthus DK1622 in the presence of 10 mM ATP, 240 μM Glc, and 0.2% (wt/vol) each potential antagonist, using the Glc6P dehydrogenase-dependent coupled assay as described in Materials and Methods.
Activities (averages of three determinations) varied less than 15% and are expressed as fractions of the activity in the absence of added potential inhibitor.
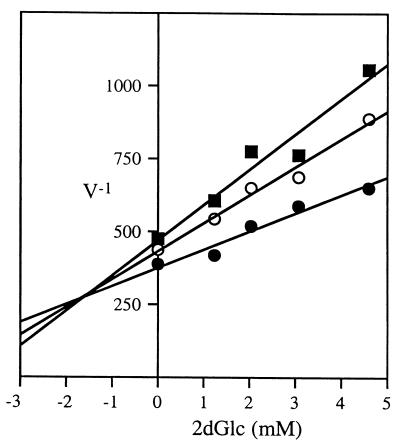
The Dixon plot shown in Fig. 8 reveals that 2dGlc, as expected, acts as an inhibitor of Glc in our assay. Its kinetics of inhibition are simply consistent with its predicted role as an alternate substrate whose phosphorylated product cannot be oxidized by Glc6P dehydrogenase. The initial velocity measured in the hexokinase assay is plotted in Fig. 8 as a function of inhibitor (2dGlc) concentration at several different concentrations of substrate (Glc). Because the plots at all values of substrate concentration converge at a single value of -Ki corresponding to 1/Vmax for the enzyme, inhibition is either competitive or of the linear mixed type (39). Although competitive inhibition is the simpler model, we cannot as yet exclude the possibility that both 2dGlc and Glc can bind hexokinase simultaneously to form an inactive ternary complex.
FIG. 8.
Competitive inhibition of M. xanthus hexokinase by 2dGlc. Extracts (100 μl) made from M. xanthus wild-type strain DK1622 were incubated in 50 mM Tris–10 mM MgCl2 (pH 9.1) with Glc (160 μM [squares], 200 μM [open circles], or 300 μM [filled circles]), ATP (10 mM), 500 to 1,000 U of Glc6P dehydrogenase, and Glc6P at concentrations of 0, 1.2, 2.0, 3.0, and 4.6 mM. Results from a representative experiment are plotted as 1/V (absorbance at 340 nm × 105 min−1)−1 versus [I], the concentration (millimolar) of the inhibitor 2dGlc. Plots are linear least-square fits to the data.
In extracts made from wild-type M. xanthus cells, we find hexokinase to have an apparent Km of 170 ± 50 μM for glucose and an apparent Ki of 2 ± 1 mM for 2dGlc. Remarkably, this apparent Ki that we measure for 2dGlc in vitro is in close agreement with the concentration of 2dGlc (2.5 mM) required to inhibit the formation of colonies by M. xanthus in vivo. However, we must be cautious about our interpretation of these kinetic constants, because they were determined with whole-cell lysates that may include enzymes or metabolites that adversely affect their accuracy. More accurate measurements of these kinetic constants and of the substrate specificity of M. xanthus hexokinase await its purification.
DISCUSSION
We have provided both indirect genetic and corroborating, direct biochemical evidence that M. xanthus makes a hexokinase that can catalyze the first step in its glycolytic pathway. Currently, we refer to this enzyme as a hexokinase, not a glucokinase, because it may phosphorylate substrates in addition to Glc, including GlcN. Studies are in progress to purify this enzyme by classical methods to test this directly and to clone the hex structural gene by using reverse genetics. The isolation and sequence of the hex gene will set the stage for the high-level expression of its product and for the purification of sufficient amounts of hexokinase for detailed kinetic and mechanistic studies. Because hex mutants, which make lower levels of hexokinase activity, are resistant to 2dGlc, we are also attempting to clone the hex gene by complementation. That is, integrative plasmids with M. xanthus inserts that restore 2dGlc sensitivity to hex mutants may carry the hex structural gene, if the hex mutations reduce the activity of the hexokinase structural gene and not a positive regulatory gene.
The main role of hexokinase in M. xanthus metabolism remains unknown. Clearly, it is not the utilization of polysaccharides as carbon and energy sources, as for the cellulose-degrading myxobacterial species Polyangium (Sorangium) cellulosum (38). Hexokinase may play a key role in the utilization of spore coat polysaccharides during spore germination. McBride and Zusman (27) have shown that a large fraction of the carbon in mature spores is present as trehalose, which is catabolized during germination. Although the hexokinase-deficient hex mutants make viable spores (which germinate), these mutants retain partial, residual hexokinase activities (Table 5) that may suffice for trehalose degradation. These residual activities in part may be due to the presence of a second hexokinase, a hypothesis that we are now testing. The closely related species Myxococcus coralloides has been shown to make two distinct hexokinase activities (15).
The target and mechanism of 2dGlc toxicity in M. xanthus and many other organisms also remains unknown. Lee and Cerami (24) have shown that the accumulation of Glc6P in Hfr strains of E. coli that carry a plasmid target for mutation results in a higher mutation rate. They speculated that the accumulation of adducts formed by Glc6P and other reducing sugars with DNA may account for this increased mutation rate. On the basis of their results, one might imagine that 2dGlc6P accumulation may be toxic to M. xanthus because it leads to DNA damage. However, this explanation is not satisfying for two reasons. First, unlike the effects of other DNA-damaging agents, the response of M. xanthus to toxic levels of 2dGlc is reversible for several generations (Fig. 2) and does not result in an increased frequency of mutation (unpublished results). Indeed, we now know that the conditions Lee and Cerami (24) used to elicit the accumulation of Glc6P in stationary-phase E. coli cells are likely to induce a hypermutable state, in which events dependent on an F factor lead to an increased rate of mutation (11, 14, 32). Consistent with this interpretation, the majority of mutations that they observed were plasmid rearrangements presumably catalyzed by mobile elements, such as those residing on the integrated F episome.
Our data indicate that 2dGlc must be phosphorylated to form 2dGlc6P in order to act as an antibiotic in M. xanthus, as in other microbes. Preliminary results suggest that the accumulation of 2dGlc6P leads to the depletion of intracellular ATP in M. xanthus, a consequence that may account simply for the profound inhibitory effects of 2dGlc on both the growth and development of wild-type cells. Therefore, we will focus our future studies on potential enzyme targets of 2dGlc6P to understand the mechanism of its toxicity at a physiological level. Our evidence that M. xanthus makes a hexokinase calls into question the prior claim that this organism has an incomplete glycolytic pathway (46). Currently, we are searching for its putatively missing pyruvate kinase activity. If we find that M. xanthus has a complete glycolytic pathway, however, we still must contend with a regulatory puzzle. Glc does not stimulate the growth of this obligate aerobe on defined medium whereas acetate does, leading us to suspect that the regulation of carbon flow through the M. xanthus glycolytic pathway during vegetative growth is governed by a mechanism that is novel among gram-negative bacteria. On the other hand, this is not surprising, given that M. xanthus has relegated several pentoses and hexoses to the roles of signals that can trigger pathways of cellular differentiation during development.
ACKNOWLEDGMENTS
We thank Martin Dworkin for asking us to investigate why M. xanthus might not make a hexokinase activity, Patricia L. Hartzell for help with microscopy and critical assessment of the manuscript, and Vincent Magrini for help with protein assays.
This work was supported initially by National Institutes of Health grants GM53392 to P.Y. and AI14176 to M.H.S. and subsequently by National Science Foundation grant MCB 9808848 to P.Y.
REFERENCES
- 1.Angell S, Schwarz E, Bibb M J. The glucose kinase gene of Streptomyces coelicolor A3(2): its nucleotide sequence, transcriptional analysis and role in glucose repression. Mol Microbiol. 1992;6:2833–2844. doi: 10.1111/j.1365-2958.1992.tb01463.x. [DOI] [PubMed] [Google Scholar]
- 1a.Baumann P, Baumann L. Catabolism of D-fructose and D-ribose by Pseudomonas doudoroffii. I. Physiological studies and mutant analysis. Arch Microbiol. 1975;105:225–240. doi: 10.1007/BF00447141. [DOI] [PubMed] [Google Scholar]
- 2.Bretscher A P, Kaiser A D. Nutrition of Myxococcus xanthus, a fruiting myxobacterium. J Bacteriol. 1978;133:763–768. doi: 10.1128/jb.133.2.763-768.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campos J M, Geisselsoder J, Zusman D R. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978;119:167–178. doi: 10.1016/0022-2836(78)90431-x. [DOI] [PubMed] [Google Scholar]
- 4.Curtis S J, Epstein W. Phosphorylation of d-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975;122:1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darrow R A, Colowick S P. Hexokinase from baker’s yeast. Methods Enzymol. 1962;5:226–235. [Google Scholar]
- 6.Downard J S, Zusman D R. Differential expression of protein S genes during Myxococcus xanthus development. J Bacteriol. 1985;161:1146–1155. doi: 10.1128/jb.161.3.1146-1155.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dworkin M. Nutritional requirements for vegetative growth of Myxococcus xanthus. J Bacteriol. 1962;84:250–257. doi: 10.1128/jb.84.2.250-257.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dworkin M, Gibson S M. A system for studying microbial morphogenesis: rapid formation of microcysts in Myxococcus xanthus. Science. 1964;146:243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- 9.Dworkin M, Sadler W. Induction of cellular morphogenesis in Myxococcus xanthus. I. General description. J Bacteriol. 1966;91:1516–1519. doi: 10.1128/jb.91.4.1516-1519.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filer D, Kindler S H, Rosenberg E. Myxospore coat synthesis in Myxococcus xanthus: enzymes associated with uridine 5′-diphosphate-N-acetylgalactosamine formation during myxospore development. J Bacteriol. 1977;131:745–750. doi: 10.1128/jb.131.3.745-750.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster P L, Trimarchi J M. Conjugation is not required for adaptive reversion of an episomal frameshift mutation in Escherichia coli. J Bacteriol. 1995;177:6670–6671. doi: 10.1128/jb.177.22.6670-6671.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraenkel D G, Horecker B L. Pathways of d-glucose metabolism in Salmonella typhimurium. A study of a mutant lacking phosphoglucose isomerase. J Biol Chem. 1964;239:2765–2771. [PubMed] [Google Scholar]
- 13.Frasch S C, Dworkin M. Increases in the intracellular concentration of glycerol during development in Myxococcus xanthus. FEMS Microbiol Lett. 1994;122:321–325. [PubMed] [Google Scholar]
- 14.Galitski T, Roth J R. Evidence that F plasmid transfer replication underlies apparent adaptive mutation. Science. 1995;268:421–423. doi: 10.1126/science.7716546. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez F, Fernandez-Vivas A, Arias J M, Montoya E. Polyphosphate glucokinase and ATP glucokinase activities in Myxococcus coralloides D. Arch Microbiol. 1990;154:438–442. [Google Scholar]
- 16.Hartzell P, Kaiser D. Upstream gene of the mgl operon controls the level of MglA protein in Myxococcus xanthus. J Bacteriol. 1991;173:7625–7635. doi: 10.1128/jb.173.23.7625-7635.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartzell P L, Youderian P. Genetics of gliding motility and development in Myxococcus xanthus. Arch Microbiol. 1995;164:309–323. doi: 10.1007/BF02529977. [DOI] [PubMed] [Google Scholar]
- 18.Hemphill H E, Zahler S A. Nutrition of Myxococcus xanthus FBa and some of its auxotrophic mutants. J Bacteriol. 1968;95:1011–1017. doi: 10.1128/jb.95.3.1011-1017.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci USA. 1979;76:5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashefi K, Hartzell P L. Genetic suppression and phenotypic masking of a Myxococcus xanthus frzF-defect. Mol Microbiol. 1995;15:483–494. doi: 10.1111/j.1365-2958.1995.tb02262.x. [DOI] [PubMed] [Google Scholar]
- 21.Kimsey H H, Kaiser D. Targeted disruption of the Myxococcus xanthus orotidine 5′-monophosphate decarboxylase gene: effects on growth and fruiting-body development. J Bacteriol. 1991;173:6790–6797. doi: 10.1128/jb.173.21.6790-6797.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroos L, Kuspa A, Kaiser D. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev Biol. 1986;117:252–266. doi: 10.1016/0012-1606(86)90368-4. [DOI] [PubMed] [Google Scholar]
- 23.Kroos L, Kuspa A, Kaiser D. Defects in fruiting body development caused by Tn5 lac insertions in Myxococcus xanthus. J Bacteriol. 1990;172:484–487. doi: 10.1128/jb.172.1.484-487.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee A T, Cerami A. Elevated glucose 6-phosphate levels are associated with plasmid mutations in vivo. Proc Natl Acad Sci USA. 1987;84:8311–8314. doi: 10.1073/pnas.84.23.8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lobo Z, Maitra P K. Resistance to 2-deoxyglucose in yeast: a direct selection of mutants lacking glucose-phosphorylating enzymes. Mol Gen Genet. 1977;157:297–300. doi: 10.1007/BF00268666. [DOI] [PubMed] [Google Scholar]
- 26.Magrini V, Salmi D, Thomas D, Herbert S K, Hartzell P L, Youderian P. Temperate Myxococcus xanthus phage Mx8 encodes a DNA adenine methylase, Mox. J Bacteriol. 1997;179:4254–4263. doi: 10.1128/jb.179.13.4254-4263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McBride M J, Zusman D R. Trehalose accumulation in vegetative cells and spores of Myxococcus xanthus. J Bacteriol. 1989;171:6383–6386. doi: 10.1128/jb.171.11.6383-6386.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer D, Schneider-Fresenius C, Horlacher R, Peist R, Boos W. Molecular characterization of glucokinase from Escherichia coli K-12. J Bacteriol. 1997;179:1298–1306. doi: 10.1128/jb.179.4.1298-1306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller C, Dworkin M. Effects of glucosamine on lysis, glycerol formation, and sporulation in Myxococcus xanthus. J Bacteriol. 1991;173:7164–7175. doi: 10.1128/jb.173.22.7164-7175.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novak S, D’Amore T, Stewart G G. 2-Deoxy-d-glucose resistant yeast with altered sugar transport activity. FEBS Lett. 1990;269:202–204. doi: 10.1016/0014-5793(90)81154-g. [DOI] [PubMed] [Google Scholar]
- 31.Postma P W, Lengeler J W, Jacobson G R. Phosphoenolpyruvate: carbohydrate phosphotransferase systems. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: molecular and cellular biology. Washington, D.C: ASM Press; 1996. pp. 1149–1174. [Google Scholar]
- 32.Radicella J P, Park P U, Fox M S. Adaptive mutation in Escherichia coli: a role for conjugation. Science. 1995;268:418–420. doi: 10.1126/science.7716545. [DOI] [PubMed] [Google Scholar]
- 33.Reizer J, Saier M H J, Deutscher J, Grenier F, Thompson J, Hengstenberg W. The phosphoenolpyruvate: sugar phosphotransferase system in gram-positive bacteria: properties, mechanism, and regulation. Crit Rev Microbiol. 1988;15:297–338. doi: 10.3109/10408418809104461. [DOI] [PubMed] [Google Scholar]
- 34.Sadler W, Dworkin M. Induction of cellular morphogenesis in Myxococcus xanthus. II. Macromolecular synthesis and mechanism of inducer action. J Bacteriol. 1966;91:1520–1525. doi: 10.1128/jb.91.4.1520-1525.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saier M H, Jr, Reizer J. The bacterial phosphotransferase system: new frontiers 30 years later. Mol Microbiol. 1994;13:755–764. doi: 10.1111/j.1365-2958.1994.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 35a.Saier M H, Jr, Simoni R D, Roseman S. Sugar transport. Properties of mutant bacteria defective in proteins of the phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1976;251:6584–6597. [PubMed] [Google Scholar]
- 36.Salmi D, Magrini V, Hartzell P L, Youderian P. Genetic determinants of immunity and integration of temperate Myxococcus xanthus phage Mx8. J Bacteriol. 1998;180:614–621. doi: 10.1128/jb.180.3.614-621.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Sarao R, McCurdy H D, Passador S. Enzymes of the intermediary carbohydrate metabolism of Polyangium cellulosum. Can J Microbiol. 1985;31:1142–1146. [Google Scholar]
- 39.Segel I H. Biochemical calculations. New York, N.Y: John Wiley & Sons; 1976. [Google Scholar]
- 40.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 41.Spratt B G, Hedge P J, te Heesen S, Edelman A, Broome-Smith J K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41:337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]
- 42.Sudo S Z, Dworkin M. Resistance of vegetative cells and microcysts of Myxococcus xanthus. J Bacteriol. 1969;98:883–887. doi: 10.1128/jb.98.3.883-887.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taketo A. DNA transfection of Escherichia coli by electroporation. Biochim Biophys Acta. 1988;949:318–324. doi: 10.1016/0167-4781(88)90158-3. [DOI] [PubMed] [Google Scholar]
- 44.Ueki T, Inouye S, Inouye M. Positive-negative KG cassettes for construction of multi-gene deletions using a single drug marker. Gene. 1996;183:153–157. doi: 10.1016/s0378-1119(96)00546-x. [DOI] [PubMed] [Google Scholar]
- 45.van Wezel G P, Bibb M J. A novel plasmid vector that uses the glucose kinase gene (glkA) for the positive selection of stable gene disruptants in Streptomyces. Gene. 1996;182:229–230. doi: 10.1016/s0378-1119(96)00563-x. [DOI] [PubMed] [Google Scholar]
- 46.Watson B F, Dworkin M. Comparative intermediary metabolism of vegetative cells and microcysts of Myxococcus xanthus. J Bacteriol. 1968;96:1465–1473. doi: 10.1128/jb.96.5.1465-1473.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weimer R M, Creighton C, Stassinopoulos A, Youderian P, Hartzell P L. A chaperone in the HSP70 family controls the production of extracellular fibrils in Myxococcus xanthus. J Bacteriol. 1998;180:5357–5368. doi: 10.1128/jb.180.20.5357-5368.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White D. Myxospore of Myxococcus xanthus. In: Gerhardt P, Costilow R N, Sadoff H S, editors. Spores IV. Washington, D.C: American Society for Microbiology; 1975. pp. 44–51. [Google Scholar]
- 49.Wu S S, Kaiser D. Markerless deletions of pil genes in Myxococcus xanthus generated by counterselection with the Bacillus subtilis sacB gene. J Bacteriol. 1996;178:5817–5821. doi: 10.1128/jb.178.19.5817-5821.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]