Abstract
Objective
To analyze the intraoperative neurophysiological monitoring (IONM) data of patients with intraspinal abnormalities undergoing posterior spinal fusion and to determine how intraspinal abnormalities influence IONM results.
Methods
Patients with severe kyphoscoliosis and intraspinal abnormalities who underwent posterior spinal correction and fusion between September 2015 and January 2019 were retrospectively reviewed. Candidate intraspinal abnormalities included Chiari malformation, syringomyelia, split cord malformation, and tethered cord syndrome. Total intravenous anesthesia was administered, and no muscle relaxant or inhalation anesthesia was used for maintenance. IONM data, including somatosensory evoked potentials (SSEP) and motor evoked potentials (MEP), were recorded. The P37 and N50 latencies and amplitude were recorded for SSEP, whereas only the amplitude was recorded for MEP. The possible high‐risk factors for abnormal IONM results were analyzed.
Results
The current study included 87 patients (40 men, 47 women) with an average age of 20.2 ± 10.4 years. The etiologies were neuromuscular in 45 patients, idiopathic in four, and congenital in 38. A total of 136 intraspinal abnormalities were detected, including Chiari malformation in 33 patients, syringomyelia in 55, split‐cord malformation in 25, and tethered cord syndrome in 23. Forty patients had one intraspinal abnormality, whereas 47 patients had two or three intraspinal abnormalities. The monitorabilities were 87.4% and 97.7% for the SSEP and MEP, respectively. SSEP alerts were reported in five patients and MEP alerts in four patients, and new neurological deficits were observed in three patients postoperatively. The sensitivity and specificity were 100% and 97.3% for SSEP, and 100% and 98.8% for MEP, respectively. A significant difference in MEP amplitude between the concave and convex sides was observed, while significantly higher SSEP latency was observed on the concave side in patients with preoperative neurological deficits. There were 52 (59.8%) patients with abnormal IONM data. Preoperative neurological deficits (χ2 = 7.715, p = 0.005) and more than one intraspinal abnormality (χ2 = 9.186, p = 0.004) indicated a higher risk of abnormal IONM data.
Conclusions
IONM can be effectively used in patients with intraspinal abnormalities who undergo posterior spinal fusion. Patients with preoperative neurological deficits and more than one intraspinal abnormality have a higher risk of abnormal IONM monitoring.
Keywords: Intraoperative neurophysiological monitoring, Intraspinal abnormality, Kyphoscoliosis, Motor evoked potential, Somatosensory evoked potenti
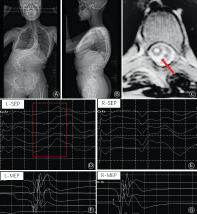
A 14‐year‐old girl with severe scoliosis undergoing posterior spinal correction surgery (A, B). The cervical syrinx (C) was detected at preoperative spinal MRI. The SSEP waveform of left lower extremity failed during surgery (D), while reliable SSEP waveform of right lower extremity and MEP waveforms of bilateral lower extremities were obtained (E, F, G).
Introduction
It is well‐known that severe kyphoscoliosis could be secondary to many etiologies, such as idiopathic, congenital, neuromuscular, degenerative, and iatrogenic etiologies. Currently, posterior spinal correction surgery is regarded as the most common treatment for patients with severe kyphoscoliosis, and satisfactory radiographic and clinical outcomes are expected in most cases. Along with the wide application of posterior spinal fusion, neurological complications have always been regarded as critical concerns 1 , 2 , 3 , especially in patients with coexisting intraspinal abnormalities.
According to previous studies, the incidence of intraspinal abnormalities ranges from 13% to 42.6% in patients with spinal deformity 4 , 5 . To the best of our knowledge, the most common intraspinal abnormalities in patients undergoing spinal correction and fusion surgery are Chiari malformation, syringomyelia, split cord malformation (SCM), and tethered cord syndrome (TCS) 6 , 7 , 8 . Traditionally, prophylactic management of intraspinal abnormalities has been recommended to avoid possible neurological complications. However, recent studies have demonstrated that most patients with kyphoscoliosis can be safely treated without the need for prophylactic neurosurgical intervention for intraspinal abnormalities 9 , 10 , although cautious management with a relatively lower correction rate of deformity is often preferred by spine surgeons.
Currently, intraoperative neurophysiological monitoring (IONM), including somatosensory evoked potentials (SSEP), motor evoked potentials (MEP), and electromyography, have been highly recommended by the Scoliosis Research Society as necessary techniques for safe correction surgery of kyphoscoliosis. The SSEP and MEP reflect the posterior column pathway function and motor pathways of the spinal cord, respectively, together detecting abnormal neurophysiological status and early identification of iatrogenic neurologic impairment. Previous studies 11 , 12 , 13 , 14 implied that coexisting intraspinal abnormalities were associated with a higher incidence of pathologic changes in IONM monitoring results. Based on previous studies, this retrospective study was further designed to: (i) analyze the SSEP and MEP data in patients with intraspinal abnormalities undergoing posterior spinal fusion; (ii) compare the average values of SSEP latency, SSEP amplitude, and MEP amplitude between the concave and convex sides in patients with and without preoperative neurological deficits; and (iii) determine the influence of intraspinal abnormalities on the IONM results.
Materials And Methods
Participants
With the approval of the Clinical Research Ethics Committee of Gulou hospital (No. 2017‐112‐08), patients treated for posterior spinal correction and fusion due to severe kyphoscoliosis between September 2015 and January 2019 at our center were reviewed. This retrospective study included patients aged 12 years or older and with preoperative MRI and CT of the whole spine showing the presence of intraspinal abnormalities, including Chiari malformation, syringomyelia, SCM, or TCS. The participants' muscle strength of both lower extremities should also be no less than grade 3. In addition, patients should have undergone total intravenous anesthesia and intraoperative IONM monitoring. The average values of the SSEP/MEP latency and amplitude of the participants were compared between the concave and convex sides. The influence of intraspinal abnormalities on IONM results was also analyzed. Patients with a previous history of trauma to the brain, spine, or lower extremities were excluded. One‐stage posterior spinal correction and fusion surgery without prophylactic neurosurgical intervention was recommended for patients with an intact or stable neurological status for at least 2 years before correction surgery. In our center, posterior fossa decompression was performed 8–12 months before the correction surgery in patients with Chiari malformation and syringomyelia.
Anesthetics Protocols
The total intravenous anesthesia was successively induced with midazolam (0.06 mg/kg), propofol (2–3 mg/kg), or etomidate (0.2 mg/kg), vecuronium (0.1 mg/kg) or cisatracurium (0.1 mg/kg), and fentanyl (3 μg/kg). Anesthesia was maintained with propofol (80–120 μg/[kg·min]), remifentanil (0.2–1 μg/[kg·min]) and dexmedetomidine (0.2 μg/[kg·h]) infusion. No muscle relaxant or inhalation anesthesia was used for maintenance. The bispectral index was maintained at 40–60.
IONM Examinations
The SSEP and MEP of the concave and convex sides were measured and recorded 15 . SSEP stimulation was delivered to each posterior tibial nerve at the ankle, and SSEP was recorded using a pin electrode at Cz with reference to Fz. The parameters of the SSEP square waves were 0.2 ms duration, 2.1 Hz frequency, 20–40 mA intensity, 100 ms time base, and 30–1000 Hz filter bandwidth. One hundred SSEP were averaged to stimulate each tibial nerve sequentially. MEP was elicited by delivering a brief anodal pulse train between two pairs of pin electrodes over the motor cortex regions C3–C4. Myogenic responses were recorded from the abductor pollicis brevis muscle as a reference and from the bilateral abductor hallucis muscles of the lower extremities. The parameters of MEP stimulation were 6–9 pulses, 50 μs stimulus duration, 2–4 ms inter‐stimulus interval, 200—500 V stimulus intensity, 100 ms time base, and 10–1000 Hz filter band pass. The P37 and N50 latencies and amplitude were recorded for SSEP, and amplitude was recorded for MEP.
Parameters
Successful or Failed IONM Monitoring
SSEP/MEP monitoring is successful if reliable waveforms are obtained at baseline, whereas those without reliable waveforms are defined as failed.
IONM Alerts
The criteria for IONM alerts were as follows: (i) SSEP latency increased by >10%; (ii) SSEP amplitude decreased by >50%; or (iii) MEP amplitude decreased by >80%. Interventions such as instrumentation adjustment, positioning adjustment, in situ adjustment, and anesthesia/systemic adjustment would be performed in a timely manner based on the reasons for significant IONM alerts.
IONM Performance
True positive (TP): IONM alerts associated with the corresponding postoperative neurological complications. False positive (FP): IONM alerts without any postoperative iatrogenic neurological complications. True negative (TN): stable IONM without any postoperative iatrogenic neurological complications. False‐negative (FN): stable IONM but with postoperative iatrogenic neurological complications.
Asymmetrical SSEP
The asymmetrical SSEP between the concave and convex sides were defined as an inter‐side latency difference of more than 2.8 ms, amplitude of more than 1μV, or absence of unilateral SSEP wave 16 .
Statistical Analysis
Data were statistically analyzed using the SPSS software (version 19.0; SPSS, Inc., Chicago). Patient demographics are shown as mean ± standard deviation (SD). Differences between the concave and convex sides were investigated using a paired‐sample t‐test. Comparisons between patients with and without abnormal IONM were performed using the chi‐square test. Statistical significance was set at p < 0.05.
Results
General Data
A total of 87 patients (40 men, 47 women; mean age, 20.2 ± 10.4 years) with intraspinal abnormalities were included in the study. The etiologies were neuromuscular in 45 patients, idiopathic in four, and congenital in 38. The apex was thoracic in 42 patients, thoracolumbar in 26, and lumbar in 19. A total of 136 intraspinal abnormalities were detected, including Chiari malformation in 33 patients, syringomyelia in 55 patients, SCM in 25 patients, and TCS in 23 patients. In addition, 40 patients had one intraspinal abnormality, whereas the other 47 had two or more intraspinal abnormalities. Neurological deficits were detected in 55 patients, and neurological intactness was found in 32 patients. Detailed data on the intraspinal abnormalities are presented in Table 1. The average Cobb angle of the main curve was 74.4° ± 31.6° preoperatively. The mean operative time was 207.3 ± 111.4 min, and the average estimated blood loss was 1045.3 ± 644.8 mL. The mean fusion span was 10.7 ± 6.6 levels.
TABLE 1.
Details of intraspinal abnormalities of the patients
| Intraspinal abnormality | Parameters | Values | |
|---|---|---|---|
| Chiari malformation | Grades of tonsillar ectopia | I | 23/33 |
| II | 10/33 | ||
| III | 0/33 | ||
| Distance of tonsillar decent (mm) | 7.2 ± 3.3 | ||
| Syringomyelia | Syrinx location | Cervical | 36/55 |
| Cervical + thoracic | 15/55 | ||
| Thoracic | 4/55 | ||
| Syrinx size | Maximal syrinx/cord ratio | 0.39 ± 0.20 | |
| Syrinx length (no. of vertebrae) | 4.78 ± 3.6 | ||
| SCM | Type I | 4/25 | |
| Type II | 21/25 | ||
| Type III | 0/25 | ||
| TCS | Causes | Fatty filum | 4/23 |
| Lipomeningocele | 3/23 | ||
| SCM | 9/23 | ||
| Epidermoid cyst | 7/23 | ||
Abbreviations: SCM, split cord malformation; TCS, tethered cord syndrome.
IONM Data
Reliable baseline waveforms of bilateral SSEP and MEP were not obtained in 11 and two patients, respectively. The overall monitorabilities were 87.4% (76/87) and 97.7% (85/87) for the SSEP and MEP, respectively. SSEP alerts were reported in five patients, and MEP alerts were reported in four patients. Postoperatively, new neurological deficits were observed in three patients, including unilateral sensory deficits of the lower extremity, bilateral sensory deficits of the lower extremity, and unilateral motor deficits of the lower extremity. The etiologies of the three patients were neuromuscular in one patient and congenital in two, and the curve patterns were thoracic in two patients and thoracolumbar in one. There were three true‐positive cases, two false‐positive cases, 71 true‐negative cases, and zero false‐negative cases for SSEP, whereas there were three, one, 81, and zero, respectively, for MEP. The sensitivity and specificity were 100% (3/3) and 97.3% (71/73), respectively, for SSEP and 100% (3/3) and 98.8% (81/82), respectively, for MEP.
Comparison between Concave and Convex Sides
The average values of the SSEP latency, SSEP amplitude, and MEP amplitude in patients with and without preoperative neurological deficits are shown in Table 2. The comparison analysis demonstrated a significant difference in MEP amplitude between the concave and convex sides. In the 55 patients with preoperative neurological deficits, significantly higher SSEP latency was observed on the concave side. Notably, asymmetrical SSEP between the concave and convex sides were found in 53.9% (41/76) of patients.
TABLE 2.
Comparison between concave and convex sides of SSEP and MEP parameters
| Patients | Parameters | Concave side | Convex side | Concave side vs convex side | |
|---|---|---|---|---|---|
| With pre‐op neurological deficit (n = 55) | SSEP | P37 latency (ms) | 37.8 ± 3.8 | 36.4 ± 3.0 |
t = 3.582 p = 0.001 |
| N50 latency (ms) | 46.1 ± 4.2 | 44.7 ± 3.3 |
t = 3.326 p = 0.002 |
||
| Amplitude (μV) | 1.8 ± 1.1 | 1.9 ± 1.0 |
t = −0.568 p = 0.573 |
||
| MEP | Amplitude (μV) | 416.4 ± 321.6 | 644.9 ± 794.3 |
t = −2.577 p = 0.015 |
|
| Without pre‐op neurological deficit (n = 32) | SSEP | P37 latency (ms) | 36.3 ± 3.1 | 36.8 ± 4.3 |
t = −0.768 p = 0.451 |
| N50 latency (ms) | 45.3 ± 4.1 | 45.9 ± 5.2 |
t = −0.582 p = 0.567 |
||
| Amplitude (μV) | 1.8 ± 0.9 | 2.2 ± 1.6 |
t = −1.508 p = 0.147 |
||
| MEP | Amplitude (μV) | 454.8 ± 339.4 | 897.4 ± 1205.7 |
t = −2.344 p = 0.026 |
|
Abbreviations: MEP, motor evoked potentials; SSEP, somatosensory evoked potentials.
Risk Factors for Abnormal IONM
There were 52 (59.8%) patients with abnormal IONM data (SSEP monitoring not obtained in 11 patients, asymmetrical SSEP in 39 patients, asymmetrical SSEP with MEP monitoring not obtained in two patients), with Chiari malformation observed in 18 patients, syringomyelia in 35, SCM in 18, and TCS in 17 (χ2 = 2.955, p = 0.399). The chi‐square test showed that preoperative neurological deficits and more than one intraspinal abnormality indicated a higher risk of abnormal IONM data (χ2 = 7.715, p = 0.005; χ2 = 9.186, p = 0.004, Table 3). A demonstration case is shown in Figure 1.
TABLE 3.
Risk factors for abnormal IONM data
| Parameters | Number of patients | p | |
|---|---|---|---|
| Age | ≤18 years | 29 | χ2 = 2.179 |
| >18 years | 23 | p = 0.105 | |
| Etiologies | Neuromuscular | 28 |
χ2 = 2.133 p = 0.344 |
| Idiopathic | 1 | ||
| Congenital | 23 | ||
| Location of apex | Thoracic | 25 |
χ2 = 0.138 p = 0.933 |
| Thoracolumbar | 15 | ||
| Lumbar | 12 | ||
| Cobb angle of main curve | ≤90° | 22 |
χ2 = 0.003 p = 0.567 |
| >90° | 30 | ||
| Pre‐op neurological deficit | With | 39 |
χ2 = 7.716 p = 0.005 |
| Without | 13 | ||
| Intraspinal abnormalities | Chiari malformation | 18 |
χ2 = 2.955 p = 0.399 |
| syringomyelia | 35 | ||
| SCM | 18 | ||
| TCS | 17 | ||
| 1 | 17 |
χ2 = 9.184 p = 0.004 |
|
| >1 | 35 | ||
Abbreviations: IONM, intra‐operative neurophysiological monitoring; SCM, split cord malformation; TCS, tethered cord syndrome.
Fig. 1.
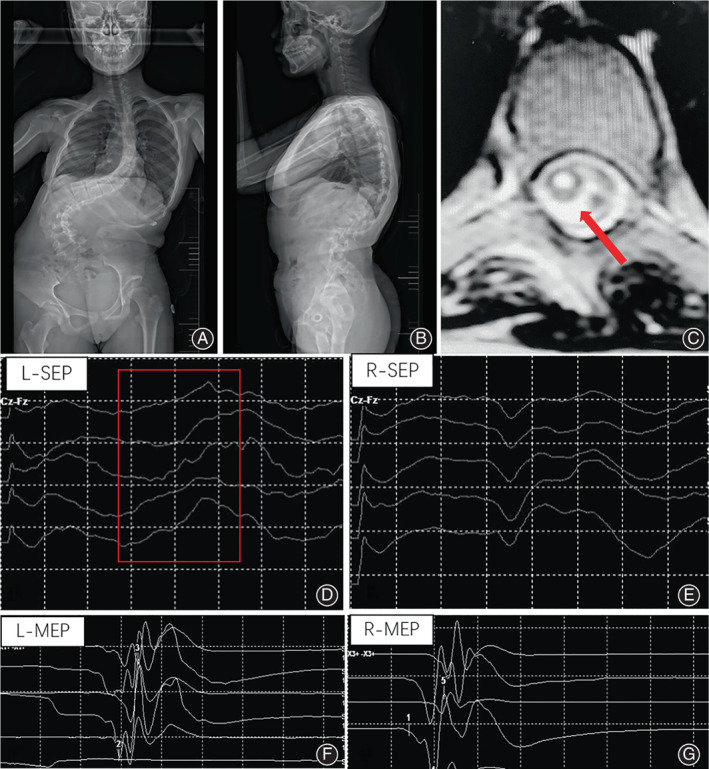
A 14‐year‐old girl with severe scoliosis undergoing posterior spinal correction surgery (A, B). The cervical syrinx (C) was detected at preoperative spinal MRI. The SSEP waveform of the left lower extremity failed during surgery (D), whereas reliable SSEP waveform of the right lower extremity and MEP waveforms of the bilateral lower extremities were obtained (E, F, G)
Discussion
The association between intraspinal abnormality and neurophysiological monitoring has been documented in the literature 8 , 11 , 12 , 17 . Morioka et al. 11 and Moncho et al. 12 reported that altered SSEP could be present in patients with mild spinal cord dysfunction, serving as a valuable predictor of the presence of syringomyelia. Cheng et al. 8 demonstrated that idiopathic scoliosis patients with abnormal SSEP had a higher risk of tonsillar ectopia than those with normal SSEP results. In addition, the SSEP results could be used to assess the deterioration of neurological function and timing of neurosurgery 18 , 19 . Spontaneously, patients with coexisting intraspinal abnormalities undergoing spinal correction surgery might have less reliable IONM results and accompanying higher risks of neurological complications, even though coexisting intraspinal abnormalities in patients with normal or stable neurological status have been proven to be safe for correction surgery 14 . The current study revealed that the monitorabilities were 87.4% and 97.7% for SSEP and MEP, respectively, which is consistent with previous studies 20 , 21 , 22 . A total of three false‐positive cases were identified, and the sensitivities and specificities were 100% and 97.3% for SSEP and 100% and 98.8% for MEP, respectively. Therefore, IONM can be effectively applied to patients with intraspinal abnormalities undergoing posterior correction and fusion.
Interferences after IONM Alerts
In total, SSEP and MEP alerts were reported in five and four patients during surgery, respectively. Based on our clinical experience, several key interferences should be successively and promptly performed after IONM alerts. First, the mean arterial pressure should be increased (≥80 mm Hg), and high‐risk surgical procedures should be ceased. Second, possible misplacement of pedicle screws, compression or traction of the spinal cord, and over‐correction of deformity should be confirmed and excluded individually. In particular, the levels of intraspinal abnormalities if present in fusion regions should be carefully checked. Third, if iatrogenic neurological deficits are highly suspected, a wake‐up test should be performed, and methylprednisolone is still recommended. Generally, surgical procedures can be continued after significant recovery of the IONM waveforms. In the current study, new neurological deficits were observed postoperatively in three patients who recovered during follow‐up. Therefore, promising neurological improvements can be expected with timely and appropriate management of IONM alerts.
Difference between the Concave and Convex Sides
Shi et al. 15 retrospectively compared SSEP and MEP results between neurologically asymptomatic Chiari malformation‐associated scoliosis and idiopathic scoliosis patients, showing similar absolute values of IONM results. They also demonstrated that the coexistence of syringomyelia in Chiari malformation‐associated scoliosis resulted in a lower SSEP amplitude. In addition, previous studies reported that the incidence of asymmetrical SSEP between the concave and convex sides was14.6%–31.0% for idiopathic scoliosis patients and 31.7%–42% for Chiari malformation‐associated scoliosis patients 8 , 12 , 15 . In the current study, the inter‐side latency difference of more than 2.8 ms, amplitude of more than 1 μV, or absence of unilateral wave was defined as the asymmetrical SSEP between the concave and convex sides. A total of 53.9% patients were detected with asymmetrical SSEP, which was relatively higher than that reported in previous studies. Therefore, asymmetrical IONM results seem to be more prevalent in patients with intraspinal abnormalities, and IONM monitors should be fully aware of this common phenomenon.
Notably, Shi et al. 15 demonstrated a significant difference in the SSEP amplitude between Chiari malformation‐associated scoliosis patients with and without syringomyelia. In addition to the fact that the SSEP latency and MEP amplitude showed significant differences between the concave and convex sides in the current study, we speculated that all IONM parameters could be influenced by intraspinal abnormalities, depending on the etiology. However, the mechanisms underlying this deviation should be investigated in depth in future studies.
Risk Factors for Abnormal IONM
Although IONM monitoring could be effectively applied in patients with intraspinal abnormalities, there were still 59.8% patients with abnormal IONM data. The comparative analysis demonstrated no significant difference in terms of age, etiologies, curve pattern, or Cobb angle of the main curve between patients with and without abnormal IONM results. The incidence of intraspinal abnormalities in Chiari malformation, syringomyelia, SCM, and TCS also seemed comparable (p = 0.399). Preoperative neurological deficits and the number of co‐existing intraspinal abnormalities were proven to be independently correlated with a higher risk of abnormal IONM monitoring (p = 0.005 and p = 0.004). Therefore, careful assessment of possible intraspinal abnormalities is highly recommended, and preoperative IONM testing should be routinely performed in suspicious cohorts.
Limitations
The potential weaknesses of the current study were its retrospective nature, the relatively small sample size, and the exclusion of patients younger than 12 years of age. The impact of different intraspinal abnormalities on IONM data was not compared, and this should be evaluated further. The inevitable differences in the details of the anesthetic scheme might have disturbed the results because of the retrospective review. In our center, posterior fossa decompression before spinal correction surgery was performed for patients with Chiari malformation‐associated scoliosis with syringomyelia, which should have a beneficial influence on IONM results. However, the precise impact of posterior fossa decompression on monitoring in this cohort remains unclear and should be further evaluated in detail. Nevertheless, the current study demonstrated that, although intraspinal abnormalities indicated a higher incidence of abnormal monitoring, IONM could still be effectively applied during posterior spinal fusion surgery in patients with intraspinal abnormalities. Neurological deficits and more than one intraspinal abnormality detected preoperatively indicated a higher risk of abnormal IONM monitoring.
Conclusion
IONM could be effectively applied during posterior spinal fusion surgery in patients with intraspinal abnormalities, and the overall monitoring rate was 87.4% for SSEP and 97.7% for MEP. Patients with intraspinal abnormalities had a higher incidence of asymmetrical SSEP and a lower MEP amplitude on the concave side. Preoperatively, neurological deficits and more than one intraspinal abnormality tended to be correlated with a higher risk of abnormal IONM monitoring. Careful assessment of possible neurological deficits and intraspinal abnormalities is highly recommended, and preoperative IONM tests should be performed in patients with identified intraspinal abnormalities.
AUTHORS' CONTRIBUTION TO THIS WORK
1. Junyin Qiu: the conception and design of the study; analysis and interpretation of data; revising it critically for important intellectual content; final approval of the version to be submitted.
2. Wanyou Liu: acquisition of data; analysis and interpretation of data; drafting the article; revising the article critically for important intellectual content.
3. Benlong Shi: the conception and design of the study; acquisition of data; revising the article critically for important intellectual content.
4. Yang Li: acquisition of data; analysis and interpretation of data; drafting the article; revising the article critically for important intellectual content.
5. Huang Yan: acquisition of data; revising the article.
6. Zezhang Zhu: the conception and design of the study; acquisition of data; revising the article critically for important intellectual content.
7. Zhen Liu: acquisition of data; analysis and interpretation of data.
8. Xu Sun: acquisition of data; analysis and interpretation of data.
9. Yong Qiu: the conception and design of the study; acquisition of data.
Highest academic degrees for all authors. Bachelor of Medicine for Junyin Qiu. MM for Wanyou Liu. MD for Yang Li, Benlong Shi, Huang Yan, Zezhang Zhu, Zhen Liu, Xu Sun, Yong Qiu.
Departmental and institutional affiliations for all authors. Division of Spine Surgery, Department of Orthopedic Surgery, Nanjing Drum Tower Hospital, the Affiliated Drum Tower Hospital of Nanjing University Medical School, Zhongshan Road 321, Nanjing 210008, China
Conflict of Interest
The authors have no conflict of interest to declare.
This study was approved by the University Institutional Review Board.
Junyin Qiu and Wanyou Liu contributed equally to this study and share first authorship.
References
- 1. Hamilton DK, Smith JS, Sansur CA, Glassman SD, Ames CP, Berven SH, et al. Rates of new neurological deficit associated with spine surgery based on 108,419 procedures: a report of the scoliosis research society morbidity and mortality committee. Spine. 2011;36:1218–28. [DOI] [PubMed] [Google Scholar]
- 2. MacEwen GD, Bunnell WP, Sriram K. Acute neurological complications in the treatment of scoliosis. A report of the Scoliosis Research Society. J Bone Joint Surg Am. 1975;57:404–8. [PubMed] [Google Scholar]
- 3. Wang S, Li C, Guo L, Hu H, Jiao Y, Shen J, et al. Survivals of the intraoperative motor‐evoked potentials response in pediatric patients undergoing spinal deformity correction surgery: what are the neurologic outcomes of surgery? Spine. 2019;44:E950–6. [DOI] [PubMed] [Google Scholar]
- 4. Zhang Y, Xie J, Wang Y, Bi N, Li T, Zhang J, et al. Intraspinal neural axis abnormalities in severe spinal deformity: a 10‐year MRI review. Eur Spine J. 2019;28:421–5. [DOI] [PubMed] [Google Scholar]
- 5. Mariscal G, Nuñez JH, Bhatia S, Marsh R, Barrios C, Domenech‐Fernández P. Frequency and characteristics of congenital intraspinal abnormalities in a cohort of 128 patients with congenital scoliosis. J Craniovertebral Junction Spine. 2019;10:229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rajasekaran S, Kamath V, Kiran R, Shetty AP. Intraspinal anomalies in scoliosis: an MRI analysis of 177 consecutive scoliosis patients. Indian J Orthop. 2010;44:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shen J, Wang Z, Liu J, Xue X, Qiu G. Abnormalities associated with congenital scoliosis: a retrospective study of 226 Chinese surgical cases. Spine. 2013;38:814–8. [DOI] [PubMed] [Google Scholar]
- 8. Cheng JC, Guo X, Sher AH, et al. Correlation between curve severity, somatosensory evoked potentials, and magnetic resonance imaging in adolescent idiopathic scoliosis. Spine. 1999;24:1679–84. [DOI] [PubMed] [Google Scholar]
- 9. Hui H, Tao HR, Jiang XF, Fan HB, Yan M, Luo ZJ. Safety and efficacy of 1‐stage surgical treatment of congenital spinal deformity associated with split spinal cord malformation. Spine. 2012;37:2104–13. [DOI] [PubMed] [Google Scholar]
- 10. Shen J, Zhang J, Feng F, Wang Y, Qiu G, Li Z. Corrective surgery for congenital scoliosis associated with split cord malformation: it may be safe to leave diastematomyelia untreated in patients with intact or stable neurological status. J Bone Joint Surg Am. 2016;98:926–36. [DOI] [PubMed] [Google Scholar]
- 11. Morioka T, Kurita‐Tashima S, Fujii K, Nakagaki H, Kato M, Fukui M. Somatosensory and spinal evoked potentials in patients with cervical syringomyelia. Neurosurgery. 1992;30:218–22. [DOI] [PubMed] [Google Scholar]
- 12. Moncho D, Poca MA, Minoves T, Ferré A, Rahnama K, Sahuquillo J. Brainstem auditory and somatosensory evoked potentials in relation to clinical and neuroimaging findings in Chiari type 1 malformation. J Clin Neurophysiol. 2015;32:130–8. [DOI] [PubMed] [Google Scholar]
- 13. Chen X, Sterio D, Ming X, Para DD, Butusova M, Tong T, et al. Success rate of motor evoked potentials for intraoperative neurophysiologic monitoring: effects of age, lesion location, and preoperative neurologic deficits. J Clin Neurophysiol. 2007;24:281–5. [DOI] [PubMed] [Google Scholar]
- 14. Zhao Q, Shi B, Sun X, Liu Z, Su H, Li Y, et al. Do untreated intraspinal anomalies in congenital scoliosis impact the safety and efficacy of spinal correction surgery? A retrospective case‐control study. J Neurosurg Spine. 2019;31:40–5. [DOI] [PubMed] [Google Scholar]
- 15. Shi B, Qiu J, Xu L, Li Y, Jiang D, Xia S, et al. Somatosensory and motor evoked potentials during correction surgery of scoliosis in neurologically asymptomatic Chiari malformation‐associated scoliosis: a comparison with idiopathic scoliosis. Clin Neurol Neurosurg. 2020;191:105689. [DOI] [PubMed] [Google Scholar]
- 16. Chen Z, Qiu Y, Ma W, Qian B, Zhu Z. Comparison of somatosensory evoked potentials between adolescent idiopathic scoliosis and congenital scoliosis without neural axis abnormalities. Spine J. 2014;14:1095–8. [DOI] [PubMed] [Google Scholar]
- 17. Wang S, Zhuang Q, Zhang J, Tian Y, Zhao H, Wang Y, et al. Intra‐operative MEP monitoring can work well in the patients with neural axis abnormality. Eur Spine J. 2016;25:3194–200. [DOI] [PubMed] [Google Scholar]
- 18. Anderson RC, Dowling KC, Feldstein NA, et al. Chiari I malformation: potential role for intraoperative electrophysiologic monitoring. J Clin Neurophysiol. 2003;20:65–72. [DOI] [PubMed] [Google Scholar]
- 19. Zamel K, Galloway G, Kosnik EJ, Raslan M, Adeli A. Intraoperative neurophysiologic monitoring in 80 patients with Chiari I malformation: role of duraplasty. J Clin Neurophysiol. 2009;26:70–5. [DOI] [PubMed] [Google Scholar]
- 20. Ashkenaze D, Mudiyam R, Boachie‐Adjei O, Gilbert C. Efficacy of spinal cord monitoring in neuromuscular scoliosis. Spine. 1993;18:1627–33. [DOI] [PubMed] [Google Scholar]
- 21. Hammett TC, Boreham B, Quraishi NA, Mehdian SMH. Intraoperative spinal cord monitoring during the surgical correction of scoliosis due to cerebral palsy and other neuromuscular disorders. Eur Spine J. 2013;22(Suppl 1):S38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang S, Zhang J, Tian Y, Shen J, Zhao Y, Zhao H, et al. Intraoperative motor evoked potential monitoring to patients with preoperative spinal deficits: judging its feasibility and analyzing the significance of rapid signal loss. Spine J. 2017;17:777–83. [DOI] [PubMed] [Google Scholar]


