Abstract
Background
Acute respiratory distress syndrome (ARDS) is characterized by dysregulated inflammation resulting in hypoxemia and respiratory failure and causes both morbidity and mortality.
Objectives
To describe the clinical profile, outcome, and predictors of mortality in ARDS in children admitted to the Pediatric intensive care unit.
Materials and methods
This is a single-center retrospective study conducted at a tertiary referral hospital in a 12-bed PICU involving children (1 month to 18 years) with ARDS as defined by Pediatric Acute Lung Injury Consensus Conference (PALICC) guidelines, over a period of 5 years (2016–2020). Demographic, clinical, and laboratory details at onset and during PICU stay were collected. Predictors of mortality were compared between survivors and non-survivors.
Results
We identified 89 patients with ARDS. The median age at presentation was 76 months (12–124 months). The most common precipitating factor was pneumonia (66%). The majority of children (35.9%) had moderate ARDS. Overall mortality was 33% with more than half belonging to severe ARDS group (58%). On Kaplan–Meier survival curve analysis, the mean time to death was shorter in the severe ARDS group as compared to other groups. Multiorgan dysfunction was present in 46 (51.6%) of the cases. Non-survivors had higher mean pediatric logistic organ dysfunction (PELOD2) on day 1. PRISM III at admission, worsening trends of ventilator and oxygenation parameters (OI, P/F, MAP, and PEEP) independently predicted mortality after multivariate analysis.
Conclusion
High PRISM score predicts poor outcome, and worsening trends of ventilator and oxygenation parameters (OI, P/F, MAP, and PEEP) are associated with mortality.
How to cite this article
Pujari CG, Lalitha AV, Raj JM, Kavilapurapu A. Epidemiology of Acute Respiratory Distress Syndrome in Pediatric Intensive Care Unit: Single-center Experience. Indian J Crit Care Med 2022;26(8):949–955.
Keywords: Acute hypoxemic respiratory failure, Acute respiratory distress syndrome, Mortality, Pediatric risk of mortality III score
Introduction
Pediatric acute respiratory distress syndrome (PARDS) is defined as a noncardiogenic pulmonary edema characterized by an intense pulmonary inflammatory reaction, developing acutely in 12–48 hours in the context of a severe systemic illness resulting in hypoxemic respiratory failure.1 Pathophysiology in the lung occurs in the setting of dysregulated inflammation, inappropriate activity of leukocytes and platelets, and uncontrolled activation of coagulation pathways.2 In a general PICU population in urban India, PARDS had a prevalence of 9.9% and mortality up to 51.4%.3 Ancillary pharmacological therapies commonly used in clinical practice had low quantity of scientific evidence and their routine use could not be recommended.4
The epidemiology of ARDS, especially in a developing country like India, needs to be defined to assess the burden and factors determining outcome to aid in the management. Hence, we planned this study to describe the clinical phenotype, laboratory characteristics, outcome, and predictors of mortality in children with ARDS admitted to PICU.
Materials and Methods
This was a single-center retrospective study conducted over a period of 5 years (2016–2020) in 12-bed PICU of a tertiary referral hospital. Children between 1 month and 18 years of age with a diagnosis of ARDS were included in the study. We followed PALICC guidelines to define PARDS.5 We excluded children with known, valvular heart diseases or in congestive cardiac failure, chronic kidney diseases with fluid overload states, and those with a PICU stay of less than 6 hours. Clearance of the study with waiver of informed consent was obtained from the Institutional Review Board. Demographic details, clinical details at onset and follow-up, and administered treatment were collected. The severity of illness score was calculated using the Pediatric Risk of Mortality III (PRISM) score.6 For assessing progressive organ dysfunction, the PELOD score was used.7
Duration of mechanical ventilation (DMV), oxygenation parameters [oxygenation index (OI), PaO2/FiO2 (P/F) ratios], length of PICU stay and hospital stay, and outcome of the patient at the time of discharge were captured. Those requiring invasive mechanical ventilation were treated using lung-protective strategies: Plateau pressures (Pplat) of 28 cm of H2O and PEEP of up to 15 cm of H2O or higher in severe ARDS provided the patient was hemodynamically stable, FiO2 to keep saturations >88% and permissive hypercarbia. High-frequency oscillatory ventilation (HFOV) was used as rescue therapy in children with persistent hypoxemia with a high Pplat requirement (>28 cm of H2O) as per protocol.5,8,9 Echocardiography was performed on all patients with ARDS to confirm the non-cardiogenic source of pulmonary edema.
Sepsis was defined as systemic inflammatory response syndrome in the presence of or as a result of suspected or proven infection.10 Steroid exposure was defined as any use of systemic corticosteroids within 14 days of admission.9 The number of ventilator-free days was defined as the number of days after ventilation was discontinued up to study day 28. VFDs = 28–x (if successfully extubated after x days of initiation). VFDs = 0, if the subject is mechanically ventilated for >28 days or dies within 28 days. The number of ICU-free days was calculated by subtracting 28 from the number of days in the ICU.11
Statistical Methods
Categorical data were presented as counts and percentages, and continuous data were presented as medians and interquartile ranges (IQR). Mean was compared by independent t-test for symmetric shape of distribution otherwise median was compared by the Mann–Whitney test. Kaplan–Meier survival curves were estimated for 28 days of mortality, and the survival days were compared between different types of ARDS using a log-rank test. Linear mixed model analysis was carried out to compare the change in the trend of ventilator parameters in the first 5 days of ICU stay between survivors and non-survivors. P-value <0.05 is considered statistically significant. The statistical software R version 4.1.0 (R Core Team, 2021, Vienna, Austria) was used to analyze and visualize the data.
Results
Eighty-nine cases of children with ARDS were analyzed, accounting for 7.8% of PICU admissions during the study period. Most children (32 patients, 35.9%) had moderate to severe ARDS (31 children, 34.8%), with mild ARDS occurring in 26 children (29.2%). Demographics, clinical characteristics, and the etiology of ARDS are shown in Table 1. The median (IQR) age at presentation was 76 months (12–124 months). Forty-six (52%) were girls, and the mean (SD) PRISM III score was 12.87 (4.98). Acute respiratory distress syndrome was diagnosed and stratified within 24 hours of admission. Pneumonia was the commonest cause of ARDS, accounting for 63% of cases, followed by ARDS secondary to sepsis (13.5%), dengue infection (11%), and Rickettsial infection (8.9%). We also had three children with ARDS due to paraquat poisoning. Twenty-five patients (28%) were aged <1 year, 21 (23.59%) were aged 1–5 years, and 43 (48.3%) were aged more than 5 years in our study cohort.
Table 1.
Comparison of demography, laboratory characteristics, and outcome in children with ARDS based on severity
| Characteristic | Total (n = 89) | Mild (n = 26) | Moderate (n = 32) | Severe (n = 31) | p-value |
|---|---|---|---|---|---|
| Age (months) | 60 (12,144) | 64 (31,138) | 54 (12,114) | 84 (12,156) | 0.4883 |
| Males, n (%) | 43 (48.3%) | 13 (50%) | 13 (40%) | 17 (54%) | 0.51 |
| PRISM III | 12.4 (5.08) | 8.57 (3.99) | 12.2 (4.39) | 15.7 (4.26) | 0.0000 |
| Direct lung injury Indirect lung injury Sepsis Dengue Rickettsial fever |
59 (66.2%) 12 (13.5%) 10 (11.24%) 8 (8.9%) |
14 (53.8%) 6 (23%) 2 (7.69%) 4 (15.38%) |
20 (62.5%) 4 (12.5%) 4 (12.5%) 4 (12.5%) |
25 (80.6%) 2 (6.4%) 4 (12.9%) 0 |
0.8 0.42 0.7 0.08 |
| Underlying immunosuppression (N%) | 15 (16.8%) | 3 (11.5%) | 5 (15.6%) | 7 (22.5%) | 0.52 |
| Procalcitonin (ng/mL) | 5.59 (1.37, 27.8) | 5.3 (1.22, 13.9) | 3.09 (1.37, 10) | 15.19 (1.45, 58.5) | 0.555 |
| Serum albumin (gm/dL) | 2.6 (2, 3.1) | 2.8 (2.4, 3.4) | 2.2 (2, 2.7) | 2.5 (2, 3.4) | 0.188 |
| VIS 1 | 20 (10, 65.5) | 15 (10, 20) | 25 (10, 60) | 50 (20, 80) | 0.0476 |
| VIS 2 | 20 (30, 65) | 10 (10, 20) | 25 (10, 65) | 30 (10, 100) | 0.1421 |
| VIS 3 | 20 (8, 55) | 6 (5, 20) | 70 (20, 130) | 16 (10, 50) | 0.0606 |
| PELOD–Day 1 | 7 (3, 18) | 3 (2, 7) | 7 (3.5, 1) | 9 (4, 19) | 0.0013 |
| PELOD–Day 2 | 5 (2, 13) | 3 (1, 5) | 7 (3, 17) | 7 (5, 21) | 0.0008 |
| PELOD–Day 3 | 5 (2, 10.5) | 3 (2, 6) | 7 (1, 12.5) | 7 (4, 21) | 0.0144 |
| Mechanical ventilation Non-invasive, n (%) Invasive, n (%) |
28 (31.5%) 65 (73.03%) |
15 (57.7%) 11 (46%) |
10 (31%) 24 (75%) |
3 (9.6%) 30 (93.75%) |
0.021 0.000 |
| RRT | 15 (25.4%) | 3 (21.43%) | 7 (30.4%) | 5 (22.7%) | 0.776 |
| PRBC | 37 (41%) | 13 (50%) | 9 (28.1%) | 15 (48.3%) | 0.154 |
| VAP | 20 (22.47%) | 5 (19.23%) | 5 (15.63%) | 10 (32.2%) | 0.256 |
| 28 ventilator-free day (days) | 18 (0, 23) | 23 (18, 24) | 17.5 (0, 23.5) | 0 (0, 20) | 0.0010 |
| 28 ICU-free day (days) | 16 (0, 23) | 20.5 (15, 22) | 18.5 (0, 25) | 0 (0, 20) | 0.0307 |
| 30-day mortality, n (%) | 30 (33.7%) | 3 (11.5%) | 9 (28.13%) | 18 (58%) | 0.001 |
Values displayed are medians with interquartile ranges unless indicated otherwise; *p-value <0.05 is significant; BUN, blood urea nitrogen; CRP, C-reactive protein; PRBC, packed red blood cell transfusion; PELOD, pediatric logistic organ dysfunction; PRISM, pediatric risk of mortality III; RRT, renal replacement therapy; T WBC, total white blood cell count; VAP, ventilator-associated pneumonia; VIS, vasoactive infusion score
Documented microbial infection with positive source cultures was found in 24 (27%) patients. The blood culture report was positive in 8 (9%), and 16 (18%) had positive endotracheal cultures. The proportion of shock in the present cohort was 62 (54 septic shock and 8 Dengue shock). Fifty-four children had concomitant septic shock. Dengue shock was present in 8 children. Invasive mechanical ventilation was required in 73% (n = 65) for a median duration of 4 days (1–6 days), while the rest of them required non-invasive ventilation. Prone ventilation was performed on 15 children, and HFOV was used in 12 children. Air leak was noted in four cases. Four children failed NIV and subsequently required invasive ventilation. Thirty children received steroids in our cohorts (3 children Paraquat toxicity, 2 children with COVID ARDS, and rest for catecholamine refractory septic shock).
The median duration of PICU stay and hospital stay in the total cohort was 6 days (4–11 days) and 8.5 days (5–18 days), respectively. The mean (SD) PRISM III score was higher in the severe ARDS group [15.7 (4.26), p = 0.000]. The organ dysfunction scores, PELOD on day 1, day 2, and day 3, and the vasoactive infusion score on day 1 were higher in the severe group. Likewise, 90% of children in the severe group required mechanical ventilation as compared to the mild (76.9%) and moderate (65.6%) ARDS groups. Nevertheless, both the median 28 days ventilator-free and the ICU free days in the mild [VFD-23 (18, 24)] group were significantly (p-value <0.05) higher than the moderate [VFD-17.5 (0, 23.5)] and severe [VFD-0 (0, 20), IFD-0 (0, 20)] groups.
Mortality in ARDS
The overall mortality rate in our study population was 33.7%. More than half (58%) died in the severe group (p = 0.001). Multiple organ dysfunction was the cause of death in 27 children, and 3 died due to refractory hypoxemia. On Kaplan–Meier survival curve analysis, the mean time to death was shorter in the severe ARDS group as compared to other groups [Log-rank (Mantel-Cox) – p <0.001] (Fig. 1). On further analysis between the survivors and non-survivors groups, we didn't find any differences with regards to demographic variables and admission laboratory parameters except for the serum creatinine, which was higher in the non-survivors (p = 0.01) (Table 2). Non-survivors had a higher mean PRISM III score (16.5 vs 10.5) and mean PELOD (day 1) (23.5 vs 19.9), which was statistically significant (p <0.05). We observed a higher proportion of children in shock (83.3% vs 62.1%, p = 0.078), the need for dialysis (42.8% vs 15.7%, p = 0.022), and the requirement for steroids (53.3% vs 7.5%, p = 0.005) in non-survivors as compared to survivors. Twenty-seven (90%) in the non-survivor group had multiple organ dysfunctions as compared to only 19 patients (32%) in the survivor group. There was a higher incidence of ventilator-associated pneumonia among survivors (28.81% vs 10%, p-value = 0.044).
Fig. 1.
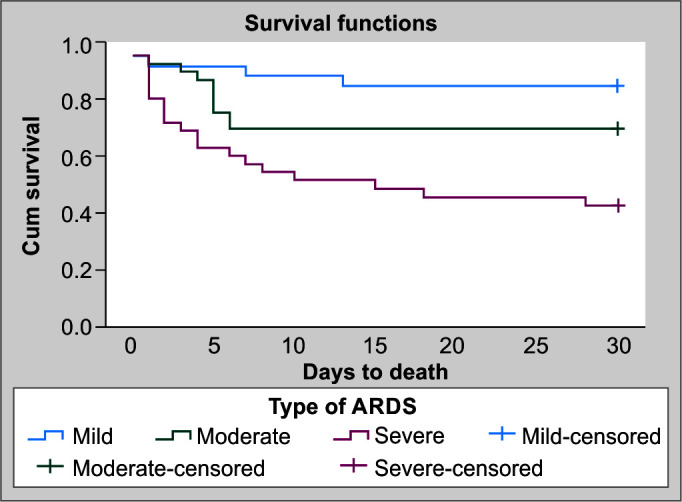
Kaplan–Meier's estimate of survival according to severity of ARDS stratified as mild, moderate, and severe ARDS
Table 2.
Comparison of demography, hemodynamic and laboratory, and other various characteristics between survivors and non-survivors
| Variable | Survivors (n = 59) | Non-survivors (n = 30) | p-value |
|---|---|---|---|
| Age (months) | 60 (12,144) | 72 (12,144) | 0.7 |
| Females, n (%) | 31 (52%) | 15 (50%) | 0.4 |
| #PRISM III, mean (SD) | 10.51 (4.12) | 16.5 (4.17) | 0.0000 |
| ^PELOD 2 score at 24 hours, mean (SD) | 19.9 (4.246818) | 23.5 (3.74) | 0.0004 |
| Serum procalcitonin (ng/mL) | 5.59 (1.37, 15.19) | 4.57 (1.43, 47.6) | 0.9 |
| Serum albumin (gm/dL) | 2.7 (2.2, 3.2) | 2.35 (2, 3) | 0.399 |
| Primary ARDS (N, %) Pneumonia (N, %) Paraquat (N, %) |
40 (67.7%) 40 (67.7%) 0 |
19 (63.3%) 16 (84.2%) 3 (15.8%) |
0.09 0.07 – |
| Secondary ARDS (N, %) Sepsis (N, %) Dengue fever (N, %) Rickettsial fever (N, %) |
7 (36.8%) 6 (31.5%) 6 (31.5%) |
5 (45.4%) 4 (36.3%) 2 (18.1%) |
0.05 0.7 0.035 |
| Underlying immunosuppression (N, %) | 7 (11.8%) | 8 (26.6%) | 0.078 |
| Mode of ventilation at diagnosis Non-invasive (N, %) Invasive (N, %) |
24 (40%) 35 (60%) |
0 30 (100%) |
0.000 0.000 |
| Severity of ARDS (N, %) Mild Moderate Severe |
23 (38%) 23 (38.9%) 13 (22%) |
3 (10%) 9 (30%) 18 (60%) |
0.001 |
| Vasoactive inotrope score Day 1 Day 2 Day 3 |
10 (10, 25) 10 (8, 20) 10 (6, 20) |
62.5 (30, 80) 72.5 (30, 109) 60 (48, 130) |
0.000 0.000 0.0004 |
| Cumulative fluid overload, mL/kg (std. error) Day 1 Day 2 Day 3 |
28.80 (9.6) 44.18 (9.6) 56.24 (9.6) |
37.00 (13.3) 58.9 (14.14) 72.7 (14.74) |
0.003 0.000 0.000 |
| RRT (N, %) | 6 (15.7%) | 9 (42.8%) | 0.022 |
| PRBC (N, %) | 23 (38.9%) | 14 (46.6%) | 0.48 |
| Steroid used (N, %) | 14 (23.7%) | 16 (53.3%) | 0.005 |
| Multiorgan dysfunction (N, %) | 19 (32.2%) | 27 (90%) | 0.002 |
| Proportion with shock (N, %) | 37 (62.71%) | 25 (83.3%) | 0.078 |
| Ventilator-associated pneumonia (N, %) | 17 (28.81%) | 3 (10%) | 0.044 |
| Duration of PICU (days) | 8 (5, 13) | 4 (2, 6) | 0.0000 |
| Duration of hospital stay (days) | 15 (8, 20) | 4.5 (2, 7) | 0.0000 |
Values displayed are medians with interquartile ranges unless indicated otherwise. Categorical variables were compared using Chi-square or extended Fisher exact test where appropriate. Continuous variables compared using Mann–Whitney U test; *p-value <0.05 is significant; BUN, blood urea nitrogen; CRP, C-reactive protein; IQR, interquartile range; PELOD, pediatric logistic organ dysfunction score; PRBC, packed red blood cell component; PRISM, pediatric risk of mortality; RRT, renal replacement therapy; TWBC, total white blood cell count
A linear mixed-model analysis was used to compare the change in trend of mean ventilator parameters [Positive End Expiratory Pressure (cm H2O), Oxygen Index (OI), and P/F ratios in the first 5 days of ICU stay between survivors and non-survivors]. We noticed an exponential increase in mean PEEP, OI, and P/F ratio over 5 days with a significant p-value (0.0000) between the groups, as depicted in Figure 2. Furthermore, the cumulative fluid balance (mL/kg) from day 1 to day 5 between the two groups was also found to be significant in sub-group analysis. The trend of median fluid accumulation between the groups progressively increased after the second day of PARDS onset, finally reaching the median cumulative fluid on day 5 of 63 mL/kg and 92.6 mL/kg in survivors and non-survivors, respectively (p-value = 0.000).
Figs 2A to C.
Ventilator parameters between survivors and non-survivors. (A) Oxygen index; (B) Positive end-expiratory pressure (cm H2O), and (C) PaO2/FiO2
By univariate analysis, the following factors were associated with mortality: PRISM III score (OR, 1.13; 95% CI, 1.3–1.5), severe ARDS (OR, 10.6; 95% CI, 2.6–42.9), steroid use (OR, 3.6; 95% CI, 1.44–9.35), mechanical ventilation (OR, 19.8; 95% CI, 2.5–156), and RRT (OR, 4; 95% CI, 1.1–13.6). After we applied the multivariate analysis, the PRISM III score (OR, 1.2; 95% CI, 1.03–1.5), P/F ratio (OR, 0.94; 95% CI, 0.94–0.95), and oxygen index (OR, 2.54; 95% CI, 2.08–3.1) at diagnosis were found to be independent predictors of mortality Table 3.
Table 3.
Multivariate analysis of variables with adjusted odds ratio predicting mortality
| Variable | Adjusted odds ratio (95% CI) | p-value |
|---|---|---|
| Severe ARDS | 7.6 (0.59–98) | 0.119 |
| Steroid use | 0.9 (0.19–4.9) | 0.96 |
| RRT | 4.2 (0.76–23) | 0.09 |
| MODS | 0.47 (0.08–2.70) | 0.4 |
| PRISM III | 1.2 (1.03–1.5) | 0.021* |
| P/F ratio | 0.94 (0.92–0.95) | 0.000* |
| OI | 2.54 (2.08–3.0) | 0.000* |
| MAP | 1.7 (1.4–2.1) | 0.000* |
*p-value <0.05 is significant;
CI, confidence interval; MAP, mean airway pressure; MODS, multiorgan dysfunction syndrome; OI, oxygenation index; P/F, PaO2/fractional oxygen concentration in inspired air; PRBC, packed red blood cell; PRISM, pediatric risk of mortality; RRT, renal replacement therapy
Discussion
Although there is mounting emphasis on early recognition and management of ARDS in children, only a few descriptive and epidemiological studies on pediatric ARDS in developing countries have been published.12 The prevalence of ARDS in this study is 7.8%, which is lower than the prevalence of 9.75% in earlier pediatric investigations conducted by Gupta et al.3 Parvathaneni et al. observed a prevalence of 5.8% using PALICC criteria.13 The decreased prevalence in our study could be due to treatment of milder cases in a high-dependency facility, and data could not be captured. We observed higher number of moderate (35.9%) to severe ARDS (34.8%). Lopez-Fernandez et al. found that severe ARDS cases outnumbered moderate ARDS cases (44.5% vs 36%).14 In our analysis, pneumonia was the most common cause of ARDS (63%), which is consistent with Wong et al.15 and Lopez-Fernandez et al.14 In our group, sepsis was the second most prevalent cause of ARDS (13.5%). Our results are similar to the study done by Yadav et al. in 2019.16 However, the study by Singh et al. in adult population admitted to surgical ICU showed sepsis as leading cause of ARDS.17 There is lack of evidence for prevalence of sepsis-induced ARDS, but in the limited number of previous pediatric data, the prevalence ranged from 13 to 32%.18,19 Interestingly, dengue and rickettsial infections accounted for 20% of the etiologies in our study group. It is not uncommon for tropical infections to cause ARDS. The study by Bhadade et al.12 from Mumbai in adult ARDS observed tropical infections contributing to 53% of the study population with Malaria and Leptospirosis being the commonest etiologies.
Only a few studies have reported data on NIV usage in pediatric ARDS without clear guidelines for NIV use based on ARDS severity. In our study, NIV was used in 31% of the children with ARDS, and 93% of the children had mild or moderate ARDS. NIV use is higher in this study than in previous pediatric ARDS trials, where it ranged from 8.5 to 21.5%.20–22
Mortality in children due to ARDS is lower compared to adult ARDS, which has been reported as high as 46%.21 In the present study, the mortality was 33% similar to the pediatric study by Khilnani et al. (28%).23 Meta-analysis by Wong et al.24 reported a 24% pooled mortality using a random-effects model. However, a study by Gupta et al.3 observed a higher mortality of 51.4%. Marginally better outcomes in our study could be due to the usage of lung-protective strategies in ARDS.
More than half of non-survivors had severe ARDS compared to survivors (60% vs 22%), reinforcing the fact that the more severe the disease, the higher the risk of mortality. Similarly, Dowell et al.25 also reported higher mortality in children with severe ARDS (49%). In terms of risk factors for mortality, we highlighted a significant association of a higher PRISM III, PELOD, vasoactive scores, multiple organ dysfunction, and worsening trends of ventilator and oxygen parameters. Multiple organ dysfunction and PELOD score were mortality predictors on univariate analysis, consistent with previous studies by Lopez et al.,14 where 40% of non-survivor had multisystem organ failure. The study by Wong et al.24 in PARDS also reported multiorgan dysfunction in 91% of their non-survivor cohort. Furthermore, a higher vasopressor score (VIS) was a predictor of mortality in the current study. Similarly, the study by Yehya et al.26 consisting of 624 children with ARDS reported association between higher VIS and mortality. On multivariate analysis, we observed higher PRISM III scores in non-survivors, which is consistent with other studies.25,27,28
In the present study, worsening trends of ventilator and oxygenation parameters (OI, P/F, MAP, and PEEP) were associated with mortality by linear mixed-model analysis. Trachsel et al.27 studied 131 children with acute hypoxic respiratory failure and multiple logistic analyses of serial measurements showed that from the second day onwards, the predictive power of OI remained intact, with a steadily increasing risk of dying with worsening OI. Similarly, Khemani et al.22 concluded improved gradation of mortality risk with OI by 6 hours after PARDS diagnosis. Children with severe ARDS had lesser VFD and IFD compared to other groups.22
Thirty patients from our cohort received steroids with no significant difference in mortality. A pilot study by Drago et al.9 in 35 children with ARDS found no difference in mortality with steroid therapy. Pediatric Acute Lung Injury Consensus Conference recommends against corticosteroids as routine therapy in PARDS pending further studies.
Despite the limitations of a retrospective investigation, our data on prevalence, epidemiology, and risk factors associated with mortality adds on to the slim body of knowledge on PARDS from developing countries. However, data on adjunct therapies such as proning and HFOV could not be analyzed due to small number. Moreover, the study is conducted at a large, academic, tertiary care hospital and, therefore, may not be generalizable to all intensive care settings.
Conclusion
Acute respiratory distress syndrome is not uncommon in the PICU and can present with varying severity, resulting in significant mortality and morbidity. Pneumonia was the commonest cause of ARDS, followed by sepsis, dengue infection, and rickettsial infection. Severe ARDS has the worst outcome, higher PRISM III score, and worsening trends in ventilator and oxygenation parameters were associated with mortality. Further multicenter studies from the Indian subcontinent will help in a better understanding of epidemiology, management, and outcome of PARDS.
Orcid
Chandrakant G Pujari https://orcid.org/0000-0002-0485-5802
Lalitha AV https://orcid.org/0000-0002-6168-1677
John Michael Raj https://orcid.org/0000-0002-1526-7432
Ananya Kavilapurapu https://orcid.org/0000-0002-6696-0173
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Sapru A, Flori H, Quasney MW, et al. Pathobiology of acute respiratory distress syndrome. Pediatr Crit Care Med. 2015;16(5 Suppl 1):S6–S22. doi: 10.1097/PCC.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 2.Meduri GU, Annane D, Chrousos GP, Marik PE, Sinclair SE. Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest. 2009;136(6):1631–1643. doi: 10.1378/chest.08-2408. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Sankar J, Lodha R, Kabra SK. Comparison of prevalence and outcomes of pediatric acute respiratory distress syndrome using Pediatric Acute Lung Injury Consensus Conference Criteria and Berlin Definition. Front Pediatr. 2018;6:93. doi: 10.3389/fped.2018.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamburro RF, Kneyber MCJ, Pediatric Acute Lung Injury Consensus Conference Group. Pulmonary specific ancillary treatment for pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5 Suppl 1):S61–S72. doi: 10.1097/PCC.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 5.The Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5):428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit Care Med. 1996;24(5):743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Leteurtre S, Duhamel A, Salleron J, Grandbastein B, Lacroix J, Leclerc F, et al. PELOD-2: an update of the pediatric logistic organ dysfunction score. Crit Care Med. 2013;41(7):1761–1773. doi: 10.1097/CCM.0b013e31828a2bbd. [DOI] [PubMed] [Google Scholar]
- 8.Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 9.Drago BB, Kimura D, Rovnaghi CR, Schwingshackl A, Rayburn M, Meduri GU, et al. Double-blind, placebo-controlled pilot randomized trial of methylprednisolone infusion in pediatric acute respiratory distress syndrome. Pediatr Crit Care Med. 2015;16(3):e74–e81. doi: 10.1097/PCC.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 11.Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200(7):828–836. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhadade RR, de Souza RA, Harde MJ, Khot A. Clinical characteristics and outcomes of patients with acute lung injury and ARDS. J Postgrad Med. 2011;57(4):286–290. doi: 10.4103/0022-3859.90077. [DOI] [PubMed] [Google Scholar]
- 13.Parvathaneni K, Belani S, Leung D, Newth CJ, Khemani RG. Evaluating the performance of the pediatric acute lung injury consensus conference definition of acute respiratory distress syndrome. Pediatr Crit Care Med. 2017;18(1):17–25. doi: 10.1097/PCC.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 14.López-Fernández Y, Martínez-de Azagra A, de la Oliva P, Modesto V, Sánchez JI, Parrilla J, et al. Pediatric Acute Lung Injury Epidemiology and Natural History study: incidence and outcome of the acute respiratory distress syndrome in children. Crit Care Med. 2012;40(12):3238–3245. doi: 10.1097/CCM.0b013e318260caa3. [DOI] [PubMed] [Google Scholar]
- 15.Wong JJ-M, Loh TF, Testoni D, Yeo JG, Mok YH, Lee JH. Epidemiology of pediatric acute respiratory distress syndrome in Singapore: risk factors and predictive respiratory indices for mortality. Front Pediatr. 2014;2:78. doi: 10.3389/fped.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yadav B, Bansal A, Jayashree M. Clinical profile and predictors of outcome of pediatric acute respiratory distress syndrome in a PICU: a prospective observational study. Pediatr Crit Care Med. 2019;20(6):e263–e273. doi: 10.1097/PCC.0000000000001924. [DOI] [PubMed] [Google Scholar]
- 17.Singh G, Gladdy G, Chandy TT, Sen N. Incidence and outcome of acute lung injury and acute respiratory distress syndrome in the surgical intensive care unit. Indian J Crit Care Med. 2014;18(10):659–665. doi: 10.4103/0972-5229.142175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171(9):995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 19.Erickson S, Schibler A, Numa A, Nuthall G, Yung M, Pascoe E, et al. Paediatric Study Group; Australian and New Zealand Intensive Care Society. Acute lung injury in pediatric intensive care in Australia and New Zealand: a prospective, multicenter, observational study. Pediatr Crit Care Med. 2007;8(4):317–323. doi: 10.1097/01.PCC.0000269408.64179.FF. [DOI] [PubMed] [Google Scholar]
- 20.Santschi M, Jouvet P, Leclerc F, Gauvin F, Newth CJ, Carroll CL, et al. Acute lung injury in children: therapeutic practice and feasibility of international clinical trials. Pediatr Crit Care Med. 2010;11(6):681–689. doi: 10.1097/PCC.0b013e3181d904c0. [DOI] [PubMed] [Google Scholar]
- 21.Bellani G, Laffey JG, Pham T, Madotto F, Fan E, Brochard L, et al. Noninvasive ventilation of patients with acute respiratory distress syndrome. Insights from the LUNG SAFE Study. Am J Respir Crit Care Med. 2017;195(1):67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 22.Khemani RG, Smith L, Lopez-Fernandez YM, Kwok J, Morzov R, Klein MJ, et al. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med. 2019;7(2):115–128. doi: 10.1016/S2213-2600(18)30344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khilnani P, Pao M, Singhal D, Jain R, Bakshi A, Uttam R. Effect of low tidal volumes vs conventional tidal volumes on outcomes of acute respiratory distress syndrome in critically ill children. Indian J Crit Care Med. 2005;9(4):195. doi: 10.4103/0972-5229.19758. [DOI] [Google Scholar]
- 24.Wong JJ-M, Jit M, Sultana R, Mok YH, Yeo JG, Koh JWJC, et al. Mortality in pediatric acute respiratory distress syndrome: a systematic review and meta-analysis. J Intens Care Med. 2019;34(7):563–571. doi: 10.1177/0885066617705109. [DOI] [PubMed] [Google Scholar]
- 25.Dowell JC, Parvathaneni K, Thomas NJ, Khemani RG, Yehya N. Epidemiology of cause of death in pediatric acute respiratory distress syndrome. Crit Care Med. 2018;46(11):1811–1819. doi: 10.1097/CCM.0000000000003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yehya N, Harhay MO, Klein MJ, Shein SL, Piñeres-Olave BE, Izquierdo L, et al. Predicting mortality in children with pediatric acute respiratory distress syndrome: a pediatric acute respiratory distress syndrome incidence and epidemiology study. Crit Care Med. 2020;48(6):e514–e522. doi: 10.1097/CCM.0000000000004345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trachsel D, McCrindle BW, Nakagawa S, Bohn D. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2005;172(2):206–211. doi: 10.1164/rccm.200405-625OC. [DOI] [PubMed] [Google Scholar]
- 28.Prasertsan P, Anuntaseree W, Ruangnapa K, Saelim K, Geater A. Severity and mortality predictors of pediatric acute respiratory distress syndrome according to the Pediatric Acute Lung Injury Consensus Conference Definition. Pediatr Crit Care Med. 2019;20(10):e464–e472. doi: 10.1097/PCC.0000000000002055. [DOI] [PubMed] [Google Scholar]



