ABSTRACT
Pregnancy is an independent risk factor for severe covid-19. Vaccination is the best way to reduce the risk for SARS-CoV-2 infection and limit its morbidity and mortality. The current recommendations from the World Health Organization, Centers for Disease Control and Prevention, and professional organizations are for pregnant, postpartum, and lactating women to receive covid-19 vaccination. Pregnancy specific considerations involve potential effects of vaccination on fetal development, placental transfer of antibodies, and safety of maternal vaccination. Although pregnancy was an exclusion criterion in initial clinical trials of covid-19 vaccines, observational data have been rapidly accumulating and thus far confirm that the benefits of vaccination outweigh the potential risks. This review examines the evidence supporting the effectiveness, immunogenicity, placental transfer, side effects, and perinatal outcomes of maternal covid-19 vaccination. Additionally, it describes factors associated with vaccine hesitancy in pregnancy. Overall, studies monitoring people who have received covid-19 vaccines during pregnancy have not identified any pregnancy specific safety concerns. Additional information on non-mRNA vaccines, vaccination early in pregnancy, and longer term outcomes in infants are needed. To collect this information, vaccination during pregnancy must be prioritized in vaccine research.
Introduction
On 11 March 2020, the World Health Organization declared the SARS-CoV-2 outbreak a worldwide pandemic.1 To date, nearly 400 million cases of SARS-CoV-2 infection have been confirmed globally. Although stating the exact number of cases that have occurred in pregnant patients is difficult, recent statistics suggest that women of reproductive age make up more than 20% of the global population, and approximately 5% of women of reproductive age are pregnant at any given time.2 3 4 Therefore, several million cases of covid-19 during pregnancy are expected to have occurred in the past two years, making SARS-CoV-2 infection one of the most prevalent illnesses to affect this population.
Pregnant patients with symptomatic covid-19 infection are at increased risk for severe disease, with increased rates of hospital admission, intensive care unit (ICU) admission, intubation, and death.5 6 According to a recent systematic review, pregnant patients with covid-19 face higher rates of several adverse maternal outcomes as well as ICU admission (odds ratio 2.61, 95% confidence interval 1.84 to 3.71) and invasive ventilation (2.41, 2.13 to 2.7) compared with non-pregnant women of reproductive age with covid-19, and significantly higher rates of death (6.09, 1.82 to 20.38) compared with pregnant patients without covid-19.7 Given the risks faced by both pregnant patients and neonates, understanding the immunologic response to vaccination in pregnancy is critically important. More than 100 vaccines were tested in clinical trials, with 12 vaccines currently in use.8 Given that pregnancy was an exclusion criterion in early clinical trials of vaccines, obstetric patients and their providers initially were required to make decisions about vaccination with limited data. To help to fill this gap, this review aims to summarize available data on the immunogenicity, effectiveness, adverse effects, and perinatal outcomes of maternal covid-19 vaccination, as well as on vaccine hesitancy during pregnancy. This review is aimed at clinicians caring for pregnant patients and researchers involved in vaccine development or public health.
Sources and selection criteria
We considered studies reporting on adult human populations who received covid-19 vaccination in pregnancy for inclusion in this review. An additional topic of interest was vaccine hesitancy in pregnancy. We did a comprehensive literature search to identify relevant articles. An experienced medical librarian developed and conducted search strategies, with input from the research team. We searched four bibliographic databases between November 2019 and March 2022: EBSCO’s CINAHL (Cumulative Index of Nursing and Allied Health), Embase, MEDLINE via PubMed, and Web of Science Classic Core Collection (Science Citation Index Expanded, Social Sciences Citation Index, Arts and Humanities Citation Index, Conference Proceedings Citation Index-Science, Conference Proceedings Citation Index-Social Sciences and Humanities, Book Citation Index- Science, Book Citation Index-Social Sciences and Humanities, Emerging Sources Citation Index, Current Chemical Reactions, Index Chemicus).
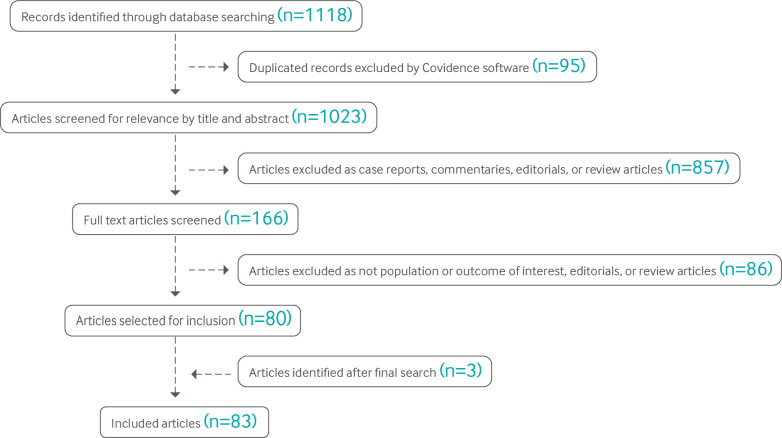
The searches combined controlled vocabulary supplemented with keywords related to the concepts of vaccination (for example, immunization, immunogenicity), pregnancy (for example, gestation, breastfeeding), covid-19 (for example, SARS-CoV-2, coronavirus), empirical methods (for example, prospective trial, cohort study), vaccine hesitancy (for example, uptake, unwillingness), and ethics related to vaccination during pregnancy (for example, therapeutic orphan, patient selection). We included articles written in the English language. We did not review preprints, and we did not include case reports. Searches were carried out on 8 February 2022 and rerun for updates on 8 March 2022 (fig 1).
Fig 1.
Flow diagram of included articles
We identified 45 studies evaluating the use of covid-19 vaccines in pregnancy via our search. Of these studies, 20 investigated immunogenicity, 10 investigated vaccine efficacy, 12 evaluated adverse effects of vaccines, and 26 evaluated perinatal outcomes after prenatal vaccination. We also included two additional studies published after our search given that their large sample size substantially expanded the available data on pregnancy outcomes after covid-19 vaccination. We also added one study on risk of congenital fetal anomalies after the search, as it specifically evaluated the association of covid-19 vaccination during the highest risk period for teratogenicity. Of the 48 included studies, most reported the use of mRNA vaccines (43 included the Pfizer-BioNTech vaccine (BNT162b2); 28 included the Moderna vaccine (mRNA-1273)); only six reported use of the Johnson & Johnson-Janssen vaccine (Ad26.COV2.S), and five reported use of the Oxford-AstraZeneca vaccine (ChAdOx1-S). Some studies included multiple vaccine types, and some did not report the specific vaccine used. One study included two participants who received the Sinovac vaccine (CoronaVac; inactivated), three who received a combination of CoronaVac and Pfizer, and 78 who received Pfizer.9 The paper reported on infection rates during the era of the omicron variant, finding that fully vaccinated pregnant patients had milder illness, but did not break down the results by vaccine type. No other studies identified included inactivated vaccines. Vaccine hesitancy was evaluated in 42 studies. Several studies evaluated more than one outcome, so the total number of studies included in this review is 83.
Incidence/prevalence
Pregnancy is an independent risk factor for severe covid-19.5 Although the absolute risk for severe maternal morbidity and mortality is low, pregnancy remains a risk factor for hospital admission, ICU admission, and need for extracorporeal membrane oxygenation related to covid-19 compared with non-pregnant women.5 Covid-19 in pregnancy has also been associated with an increased risk of stillbirth and maternal death,7 10 11 and comorbidities such as advanced maternal age, obesity, diabetes, and heart disease further increase these risks. Moreover, several cases of intrauterine transmission of SARS-CoV-2 have been carefully documented, although the risk seems to be low.12 Additionally, neonates have been shown to be susceptible to severe illness from SARS-CoV-2 infection,13 so providing protection to the neonate through maternal vaccination is important. Neonates rely on the active placental transfer of maternal IgG for their protection against pathogens during the first six months of life,14 15 so vaccination during pregnancy has the potential to protect both mother and neonate.
Although vaccination with other respiratory viruses such as influenza during pregnancy has been shown to be highly successful and safe,16 17 18 these vaccinations have been the subject of ongoing research in the pregnant population for decades. Despite the clear harm SARS-CoV-2 infection poses to pregnant patients and their neonates, the paucity of initial safety and efficacy data on covid-19 vaccines in pregnancy led countries around the globe to take different positions on whether people should be vaccinated during pregnancy. According to the Covid-19 Maternal Immunization Tracker (COMIT), a website that provides global information on countries’ public health policies regarding recommendations about covid-19 vaccines for pregnant and lactating people,19 106 countries recommend covid-19 vaccination for some or all pregnant or lactating people, whereas 15 countries specifically recommend against vaccination in this population. Given the wide diversity of recommendations worldwide, the prevalence of people vaccinated for covid-19 during pregnancy likely also varies considerably. In the US, all major professional health organizations, including the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal Fetal Medicine (SMFM), have consistently recommended that everyone who is pregnant should be vaccinated against covid-19 since early 202120 21; however, as of April 2022, the US Vaccine Safety Datalink estimated that only 69.4% of pregnant women aged 18-49 years had been fully vaccinated with covid-19 vaccines before or during pregnancy.22
Covid-19 vaccines
We identified four vaccines as having published data on their use in pregnancy: Pfizer-BioNTech, Moderna, Johnson & Johnson-Janssen, and Oxford-AstraZeneca (table 1). The Pfizer-BioNTech and Moderna mRNA vaccines had the most data in pregnancy. The mRNA vaccines work by releasing mRNA that encodes for the SARS-CoV-2 spike protein into cells, allowing the cell’s machinery to produce the spike protein. Once mRNA from the vaccine has been read, it is destroyed. The body’s immune cells then generate a response to the spike protein, creating the antibodies that provide immunity to SARS-CoV-2. The other two vaccines with data in pregnancy, the Oxford-AstraZeneca and Johnson & Johnson-Janssen vaccines, rely on an adenovirus vector to introduce DNA for the SARS-CoV-2 spike protein into the body’s cells. The DNA is first copied into mRNA, and then, as with the mRNA vaccines, the mRNA is used to produce copies of the spike protein, which then stimulate the immune system to create antibodies. Thus far, mRNA vaccines have shown better efficacy in the non-pregnant population than the viral vector vaccines for preventing symptomatic illness—with Pfizer and Moderna vaccines showing 95.0% and 94.1% efficacy, respectively, in the initial clinical trials—as well as severe disease, two weeks after the two dose series.23 25 By contrast, the Johnson & Johnson-Janssen vaccine was 72% effective in preventing symptomatic infection and 85% effective in preventing severe disease in the non-pregnant population,27 and the AstraZeneca vaccine was 70.4% effective after two doses.29 However, some of these differences between vaccines might be due to the variants circulating at the time of the trials.
Table 1.
Covid-19 vaccines with data in pregnancy
| Vaccine | Manufacturer | Vaccine type | Dosing | Ages | Efficacy based on randomized clinical trials | Efficacy against severe covid-19 | Current approval | Developmental and reproductive toxicity studies |
|---|---|---|---|---|---|---|---|---|
| BNT162b2 | Pfizer-BioNTech23 | mRNA: encodes stabilized spike, lipid nanoparticles | 100 µg; 2 doses, 21 days apart | ≥5 y | 95.0% against symptomatic covid-19 | 88.9% | EUA: USA, UK, EU, Canada | Studies in rats showed no adverse effects on female mating performance, fertility, or any ovarian/uterine parameters or effects on embryo-fetal or postnatal survival, growth, or physical development24 |
| mRNA-1273 | Moderna25 | mRNA: encodes stabilized spike, lipid nanoparticles | 30 µg; 2 doses, 28 days apart | ≥18 y | 94.1% against symptomatic covid-19 | 100.0% | EUA: USA, UK, EU, Canada | DART studies in pregnant and lactating female rats did not show any adverse effects at clinically relevant 100 mg dose26 |
| Ad26.CoV2.5 | Johnson & Johnson-Janssen27 | Recombinant replication incompetent human adenovirus vector encoding stabilized SARS-CoV-2 spike protein | 5×1010 viral particles; 1 dose | ≥18 y | 66.1% against moderate to severe-critical covid-19 | 85.4% | EUA: USA, EU, Canada | No adverse effect on fertility, embryo-fetal, or postnatal development when twice human dose was injected in female rabbits 7 days before mating and at gestational days 6 and 20 (early and late gestation)28 |
| ChAdOx1 (AZS1222) | Oxford-AstraZeneca29 | Recombinant replication deficient chimpanzee adenoviral vector encoding SARS-CoV-2 spike protein | 5×1010 viral particles; 2 doses, 4-12 weeks apart | ≥18 y | 70.4% against symptomatic covid-19 | 100.0% | EUA: WHO/Covax, UK, India, Mexico | DART studies have not shown harmful effects of vaccine in pregnant animals and their offspring30 |
DART=developmental and reproductive toxicity; EUA=emergency use authorization.
As pregnancy was an exclusion criterion for these studies, which vaccine is the most effective in the pregnant population is unclear. More information on all vaccine types is needed, as many countries, including India, a country with approximately one sixth of the world’s pregnant population, have no mRNA vaccines available. However, given the high morbidity from SARS-CoV-2 infection in pregnancy, most countries surveyed recommend covid-19 vaccination for some or all pregnant or lactating individuals.19 Most advocate for covid-19 vaccination regardless of previous infection and state that serologic testing, a pregnancy test, or a letter from an obstetric provider should not be required before vaccination. No recommendation for specific timing of vaccination in pregnancy exists; however, given that the primary goal of vaccination is to prevent maternal infection, most countries recommend vaccination regardless of pregnancy trimester. Pregnancy need not be delayed after covid-19 vaccination, as none of the currently available vaccines is a live vaccine.
Despite these strong recommendations for universal vaccination, vaccine hesitancy in the pregnant population remains high. Many people cite concern for fetal/infant wellbeing as a reason to decline vaccination, so more information on perinatal outcomes and transplacental antibody transfer is needed. Efforts are ongoing to fill these gaps, as a recent review of the clinicaltrials.gov website (accessed 23 March 2022) showed that 11 trials investigating covid-19 vaccinations in pregnancy are ongoing, including several prospective observational trials that are evaluating maternal and infant outcomes after covid-19 vaccination in pregnancy. These trials will hopefully provide additional information on the safety and efficacy of these vaccines and on the optimal timing and frequency of vaccination in pregnancy to maximize maternal and neonatal benefit.
Covid-19 vaccines in pregnancy
Vaccination during pregnancy can reduce disease related morbidity and mortality for pregnant people and their infants. Patients are at higher risk of significant morbidity from viral illnesses such as influenza if contracted during pregnancy,31 and other viral infections, such as Zika, rubella, and varicella, can cause serious neonatal morbidity if the primary infection occurs during pregnancy.32 33 34 Other viruses, such as pertussis, are relatively inconsequential for a pregnant person or a fetus, but pertussis infection in a young infant can be fatal. Immunizations have been shown to benefit all three populations—mother, fetus, and neonate—as any vaccine given to the mother can protect the neonate via the transplacental transport of IgG antibodies.14 However, although several vaccines are recommended in pregnancy, the optimal timing of the vaccination varies depending on the pathogen. Some vaccines, such as those for rubella and varicella, are recommended only during the preconception and postpartum period, as live vaccines theoretically could cause congenital infection if given during or just before pregnancy. By contrast, pertussis vaccination is recommended in every pregnancy, optimally between 27 and 36 weeks, to facilitate maximal placental antibody transfer for greatest protection of the neonate. Finally, influenza vaccines are recommended at any time in pregnancy during influenza season, as the priority is to prevent maternal infection. Current recommendations for covid-19 vaccines closely follow those for influenza vaccines—that is, the vaccine should be given as soon as possible in pregnancy for maternal benefit, given the significant increase in morbidity from SARS-CoV-2 infection seen in the pregnant population. Understanding how pregnancy affects response to covid-19 vaccination and subsequent placental antibody transfer is critical to guiding vaccine recommendations for this special population.
Covid-19 vaccine immunogenicity data in pregnancy
Available data on the humoral and functional immune response to covid-19 vaccines in pregnancy are observational. Our review identified 20 studies evaluating the immunogenicity of covid-19 vaccines in pregnancy (table 2).35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Most studies evaluated immunogenicity of the Pfizer-BioNTech vaccine. A few studies did not clearly describe either the vaccine platform or the total number of participants; however, when described, this information is provided in table 2. Multiple studies support a robust maternal antibody response to covid-19 vaccination.40 42 43 44 45 51 54 A cohort study documented humoral immunity in pregnancy, with immunogenicity and reactogenicity similar to those observed in non-pregnant women; vaccine induced antibody titers were equivalent in pregnant and non-pregnant women.42 A few studies have found reduced immunogenicity in pregnancy compared with that observed in non-pregnant vaccinated women.38 35 One study found that pregnant women (n=390) had significantly lower serum SARS-CoV-2 IgG concentrations than non-pregnant women (n=260) (signal to cut-off ratio 27.03 v 34.35; P<0.001).38 A smaller study observed a delay in the evolution of Fc receptor binding and functional antibody responses during pregnancy after initial vaccination; the response improved after the second dose but was still lower than in non-pregnant women.35 An increase in maternal SARS-CoV-2 IgG antibody response with a second dose in pregnancy was also documented by a study that found significantly higher maternal IgG concentrations, week by week, starting two weeks after the first vaccine dose, as well as between the first and second weeks after the second vaccine dose (P<0.005 and P=0.019, respectively).47 Another study also found higher antibody concentrations during pregnancy after two doses compared with one dose of Moderna vaccine.52
Table 2.
Immunogenicity data on covid-19 vaccines in pregnancy
| Author; study design | Vaccine type | Total population | Gestational age at vaccination, weeks | Seropositive maternal blood | Seropositive cord blood |
|---|---|---|---|---|---|
| Atyeo C, 202135; cohort study | Pfizer: 1st dose, n=32; 2nd dose, n=17. Moderna: 1st dose, n=32; 2nd dose, n=19 | Pregnant: 1st dose, n=64; 2nd dose, n=36. Non-pregnant: 1st dose, n=13; 2nd dose, n=14 | 23.2 (range 16.3-32.1) | Fc receptor antibody concentrations were significantly lower in pregnancy v non-pregnant women after 1st dose. Fc receptor antibody concentrations increased significantly after 2nd dose but were still lower than in non-pregnant women | Vaccinated maternal/cord blood pairs (n=8): maternal titers of all antibodies higher than in cord blood; enriched RBD-specific FcyR3a binding in cord |
| Beharier O, 202136; multicenter cohort study | Pfizer | Vaccinated, n=86; infected, n=65; control, n=62 | 34.5 (SD 7.5) | Rise in anti-S and RBD IgG titers within 15 days of 1st dose. Additional risk in anti-S and RBD IgG after 2nd dose. Vaccinated: higher anti-S1 and RBD antibodies. Natural infection: higher anti-S2 and N antibodies | Cord blood IgG S1 and RBD did not differ between vaccinated and natural infection (P=0.70 and P=0.69, respectively). Higher transfer ratios in vaccinated for anti-S1, S2, and RBD IgG v natural infection (P<0.001) |
| Ben-Mayor B, 202237; prospective cohort study | Pfizer: 1st dose, n=19; 2nd dose, n=39 | Vaccinated n=58 | 1st dose 34.538-41; 2nd dose 37.141-43 | Anti-S IgG >50 AU/mL detected in 53 samples | Anti-S IgG >50 AU/mL detected in 51 cord samples. Negative samples (n=7) all 1st dose to delivery interval of <27 days. SARS-CoV-2 IgG antibodies in maternal sera positively correlated with cord blood sera (ρ=0.857; R2 linear=0.719; P<0.001) |
| Bookstein P, 202138; observational case-control study | Pfizer: 2 doses | Pregnant, n=390; non-pregnant, n=260 | Not reported | Pregnant women had significantly lower serum SARS-CoV-2 IgG concentrations than non-pregnant women (P<0.001). Pregnant (n=96): average serum IgG S/CO ratio=27.03. Non-pregnant (n=96): average serum IgG S/CO ratio=34.35 | Not evaluated |
| Citu I, 202239; prospective cohort study | Pfizer; Janssen | Vaccinated, n=227; unvaccinated, n=608; vaccinated without covid-19 history, n=173; unvaccinated without covid-19 history, n=529; non-pregnant, n=227 | Third trimester | Seronegative (n=173) v seropositive (n=54) pregnant patients: median anti-S IgG 0.41 (IQR 0.31-0.45) v 145 (98.2-208.1) U/mL (P<0.001) before vaccination; 1697 (393-2115) v 14 571 (12 628-15 337) U/mL (P <0.001) at 2 months; 1083 (896-1468) v 10 759 (9043-12 571) U/mL (P<0.001) at 4 months. Pregnant (n=173) v non-pregnant (n=173) without history of SARS-CoV-2: anti-S IgG 0.41 (0.31-0.45) v 0.40 (0.32-0.47) U/mL (P=0.46) before vaccination; 1697 (1393-2115) v 1705 (1452-2179) U/mL (P=0.82) at 2 months; 1083 (896-1468) v 1114 (909-1482) U/mL (P=0.35) at 4 months | Not evaluated |
| Collier A, 202140; prospective cohort study | Pfizer, n=11; Moderna, n=19 | Vaccinated, n=103: pregnant, n=30; non-pregnant, n=57. Infected, n=28: pregnant, n=22; non-pregnant, n=6 | 1st trimester, n=5; 2nd trimester, n=15; 3rd trimester, n=10 | Pregnant vaccinated, median RBD IgG 27 601; non-pregnant vaccinated, median RBD IgG 37 839; pregnant infected, median RBD IgG 1321; pregnant vaccinated, median neutralizing titer 910; non-pregnant vaccinated, median neutralizing titer 901; pregnant infected, median neutralizing titer 148 | Vaccinated: 9 paired maternal and infant cord blood samples. Median cord blood RBD IgG titers higher than maternal blood titers (19 873 v 14 953). Median cord neutralization IgG lower than maternal blood (324 vs 1016) |
| Gloeckner S, 202241; observational cohort study | Prime vaccine, AstraZeneca; boost vaccine 12 weeks later, Pfizer or Moderna | Pregnant, n=3; non-pregnant, n=25 | Primary vaccine 21-28 weeks | Spike IgG detected in all samples. ID50 neutralization titers ≥160 in all maternal samples. Increase >1 log10 level after mRNA based boost | Spike IgG detected in all cord samples. ID50 neutralization titers ≥160 in all cord serum samples |
| Gray K, 202142; prospective cohort study | Pregnant: Pfizer, n=41; Moderna, n=43. Non-pregnant: Pfizer, n=8; Moderna, n=8 |
Pregnant, n=84; non-pregnant, n=16 | 1st trimester, n=11; 2nd trimester, n=39; 3rd trimester, n=34 | Robust, comparable IgG across pregnant and non-pregnant (pregnant, median 5.59 (IQR 4.68-5.89); non-pregnant, 5.62 (4.77-5.98); P=0.24). All titers significantly higher than pregnant with previous SARS-CoV-2 infection (P<0.001) | All 10 cord samples were positive for S and RBD IgG. Neutralizing antibody titers lower in umbilical cord than maternal sera; finding did not achieve statistical significance (maternal sera, median 104.7 (IQR 61.2-188.2); cord sera, 52.3 (11.7-69.6); P=0.05) |
| Kashani-Ligumsky L, 202143; cohort study | Pfizer, n=29 | Infected, n=29; vaccinated, n=29; control, n=21 | Not reported | Mean antibody titer 224.7 (SD 64.3) U/mL in vaccinated; 83.7 (91.6) U/mL in infected (P<0.05) | 100% of infected and vaccinated cord blood samples anti-S IgG positive. Mean neonatal antibody titers higher in vaccinated (225 U/mL) v infected (83.7 U/mL) (P<0.05) |
| Kugelman N, 202144; prospective cohort study | Pfizer, n=130 | Vaccinated, n=130 | 24.9 (SD 3.3) | 100% positive for SARS-CoV-2 IgG. Change in maternal antibody level per 1 week increase from 2nd vaccine dose to birth: −10.9% (95% CI −17.2% to −4.2%; P=0.002). Change in maternal antibody level per 1year increase in maternal age: −3.1% (−5.3% to −0.9%; P=0.007) | 100% positive for SARS-CoV-2 IgG; IgG titers 2.6× higher than maternal titers. Change in newborn antibody level per 1 week increase from 2nd vaccine dose to birth: −11.7% (95% CI −19.0% to −3.8%; P=0.005). Change in newborn antibody level per 1 year increase in maternal age: −2.7% (−5.2% to −0.1%, P=0.04) |
| Mithal L, 202145; prospective case series | Pfizer, n=18; Moderna, n=6; unknown, n=4 | Vaccinated, n=27 | 33 (SD 2) | SARS-CoV-2 IgG: 26/27 (97%) | SARS-CoV-2 IgG: 25/28 (89%). Negative cases had 1st dose <3 weeks before delivery. IgG transfer ratio 1.0 (SD 0.6). Increased latency from vaccination to delivery associated with increased transfer ratio (β=0.2, 95% CI 0.1 to 0.2). Second dose before delivery was associated with increased infant IgG levels (β=19.0, 7.1 to 30.8). Latency from vaccination to delivery was associated with increased infant IgG levels (β=2.9, 0.7 to 5.1) |
| Nir O, 202146; prospective cohort study | Pfizer | Fully vaccinated, n=64; unvaccinated and recovered from covid-19, n=11 | 33.5 (SD 3.2) | 100% positive for SARS-CoV-2 IgG. SARS-CoV-2 IgG: 26.1 (IQR 22.0-39.7) in vaccinated v 2.6 (0.9-3.5) in recovered (P<0.001) | 98.3% positive for SARS-CoV-2 IgG. SARS-CoV-2 IgG: 20.2 (12.7-29.0) in vaccinated v 3.27 (0.5-4.6) in recovered (P<0.001) |
| Prabhu M, 202147; cohort study | Pfizer, n=85; Moderna, n=37 | One dose, n=55; two doses, n=67 | Not reported | SARS-CoV-2 IgG: 43.6% after 1 dose; 98.5% after 2 doses. Maternal IgG levels were significantly higher, week by week, starting 2 weeks after first vaccine dose (P=0.005 and 0.019, respectively) | SARS-CoV-2 IgG: 44% after 1 dose; 99% after 2 doses. Placental transfer ratio correlated with number of weeks elapsed since maternal vaccine dose 2 (R=0.8; P=2.6e-15). All but one cord blood samples had detectable IgG antibodies by 4 weeks after vaccine dose 1. One dyad with no transfer of antibodies to neonate was 10 weeks from dose 1 and 6 weeks from dose 2 |
| Rottenstreich, A, 202148; prospective cohort study | Pfizer, n=20 | Fully vaccinated, n=20 | All 3rd trimester | SARS-CoV-2 anti-S and anti-RBD: 100% | SARS-CoV-2 anti-S and anti-RBD: 100%; placental transfer ratios 0.44 (IQR 0.25-0.61) for anti-S IgG and 0.34 (0.27-0.56) for anti-RBD IgG. SARS-CoV-2 anti-S and anti-RBD specific IgG levels in maternal sera were positively correlated with respective cord blood (ρ s=0.72; P<0.001 and ρ s=0.72; P<0.001, respectively). Cord blood titers directly correlated with increasing time from 1st vaccine dose (ρ s=0.71; P=0.001 and ρ s=0.63; P=0.004, respectively) |
| Rottenstreich A, 202249; cohort study | Pfizer, n=171 | First dose at 27-31 weeks, “early 3rd trimester,” (n=83); first dose at 32-36 weeks, “late 3rd trimester,” (n=88) | All 3rd trimester | 100% SARS-CoV-2 anti-S and anti-RBD. Median anti-S specific and anti-RBD specific IgG at time of delivery were lower in those vaccinated in early 3rd trimester v late 3rd trimester | 100% SARS-CoV-2 anti-S and anti-RBD. Anti-RBD specific IgG concentrations in neonatal sera were higher after early v late 3rd trimester vaccination (median 9620 (IQR 5131-15 332) AU/mL v 6697 (3157-14 731) AU/mL; P 0.02). Median antibody placental transfer ratios were increased after early v late 3rd trimester immunization (anti-S ratio 1.3 (IQR 1.1-1.6) v 0.9 (0.6-1.1); anti-RBD specific ratio 2.3 (1.7-3.0) v 0.7 (0.5-1.2); P<0.001). Neutralizing antibodies placental transfer ratio was greater after early v late third trimester immunization (median 1.9 (1.7-2.5) v 0.8 (0.5-1.1); P<0.001). Neutralizing antibodies placental transfer ratio positively associated with longer duration from vaccination (r=0.77; P<0.001) |
| Rottenstreich M, 202250; prospective cohort study | Pfizer | Vaccinated, n=402 | 1st trimester, n=90; 2nd trimester, n=124; 3rd trimester, n=188 | 1st trimester: anti-S IgG 76 AU/mL; anti-RBG IgG 478 AU/mL. 2nd trimester: anti-S IgG 126 AU/mL; anti-RBG IgG 1263 AU/mL. 3rd trimester: anti-S IgG 240 AU/mL; anti-RBG IgG 5855 AU/mL. Vaccine in 1st trimester with booster dose in 3rd trimester: anti-S IgG 1665 AU/mL; anti- RBG IgG:20 946 AU/mL | All 402 neonates positive for anti-S and anti-RBG IgG. 1st trimester: anti-S IgG 126 AU/mL; anti-RBG IgG 1140 AU/mL. 2nd trimester: anti-S IgG 204 AU/mL; anti-RBG IgG 8038AU/mL. 3rd trimester: anti-S IgG 255 AU/mL; anti-RBG IgG 8038 AU/mL. 3rd trimester, 27-31 weeks: anti-RBG IgG 6811 AU/mL; transfer ratio 2.4. 3rd trimester, 32-36 weeks: anti-RBG IgG 9516 AU/mL; transfer ratio 0.8 (P<.0001). Vaccine in 1st trimester with booster dose in 3rd trimester: anti-S IgG 528 AU/mL; anti- RBG IgG 4225 AU/mL |
| Shanes E, 202151; cohort study | Pfizer, n=49; Moderna, n=25; mRNA unknown type, n=9 | Vaccinated, n=84; unvaccinated, n=116 | All 3rd trimester | Vaccinated, n=52: anti-SARS-CoV-2 IgG 22.8 (SD 14.5). Unvaccinated, n=116: anti-SARS-CoV-2 IgG 0.04 (0.05). P<0.001 | Not evaluated |
| Shen C, 202252; prospective cohort study | Moderna, n=29 | Vaccinated: 1 dose, n=4; 2 doses, n=25 | 1st dose, 28.45 (SD 2.64); 2nd dose, 33.31 (2.13) | Wild type variant: SARS-CoV-2 neutralizing IgG 40.32% after 1 dose; 97.46% after 2 doses. Delta variant: SARS-CoV-2 neutralizing IgG 4.01% after 1 dose; 80.49% after 2 doses | Wild type variant: SARS-CoV-2 neutralizing IgG 43.33% after 1 dose; 97.37% after 2 doses. Delta variant: SARS-CoV-2 neutralizing IgG 1.44% after 1 dose; 66.25% after 2 doses. Wildtype variant: cord to maternal ratio 1.07 after 1 dose; 0.99 after 2 doses. Delta variant: cord to maternal ratio 0.92 after 1 dose; 0.90 after 2 doses |
| Trostle M, 202153; prospective cohort study | Pfizer, n=26; Moderna, n=10 | Vaccinated, n=36 | 1st trimester, n=2; 2nd trimester, n=30; 3rd trimester, n=4 | Not evaluated | 100% SARS-CoV-2 anti-S; 34 titers >250U/mL; 2 with titers <250 U/mL were both vaccinated >20 weeks before delivery |
| Yang YJ, 202154; retrospective cohort study | Pfizer, n=1025; Moderna, n=301; Janssen, n=33 | Vaccinated, n=1359 | First dose: pre-pregnancy, n=38; 1st trimester, n=193; 2nd trimester, n=699; 3rd trimester, n=429 | Anti S IgG in all women—pre-pregnancy: Pfizer 3.7, Moderna 4.8, Janssen NA; 1st trimester: Pfizer 3.9, Moderna 4.8, Janssen 3.6; 2nd trimester: Pfizer 4.8, Moderna 5.7, Janssen 3.0; 3rd trimester: Pfizer 6.2, Moderna 6.6, Janssen 2.5. Anti-S IgG in fully vaccinated women (≥14 days after final dose)—pre-pregnancy: Pfizer 3.7, Moderna 4.8, Janssen NA; 1st trimester: Pfizer 3.9, Moderna 4.8, Janssen 3.6; 2nd trimester: Pfizer 4.8, Moderna 5.7, Janssen 3.0; 3rd trimester: Pfizer 6.4, Moderna 7.1, Janssen 2.5 | Cord blood anti-S IgG (maternal titers) in those with no history of infection: pre-pregnancy 4.5 (SD 4.1); 1st trimester 4.7 (4.2); 2nd trimester 5.5 (4.9); 3rd trimester (1 dose) 2.5 (3.4); 3rd trimester (2 doses) 4.1 (5.3); 3rd trimester (fully vaccinated) 6.3 (6.2). Booster received in 3rd trimester (no infection): pregnancy 8.1 (8.3); 1st trimester 7.1 (8.3). History of infection: pre-pregnancy 8.5 (8.9); 1st trimester 6.7 (6.4); 2nd trimester 6.9 (6.4); 3rd trimester (1 dose) 5.7 (6.4); 3rd trimester (2 doses) 6.9 (7.5); 3rd trimester (fully vaccinated) 7.4 (7.4) |
CI=confidence interval; IQR=interquartile range; N=nucleocapsid; NA=not applicable; RBD=receptor binding domain; S=spike; S/CO=signal to cut-off ratio; SD=standard deviation.
Two studies evaluated the maternal immunogenicity after a third trimester booster vaccination.49 54 A prospective cohort study found considerable antibody waning throughout pregnancy in those vaccinated in the first trimester, with a boosting effect of a third vaccine dose.49 Booster dose administration was associated with significantly increased maternal and neonatal antibody concentrations compared with those who completed the two dose vaccine series in the first trimester without a booster dose (P<0.001). In a retrospective study of 1359 pregnant women, completion of the vaccination course, history of SARS-CoV-2 infection, and a third trimester booster dose were associated with the highest maternal and umbilical cord antibody concentrations.54 These immunogenicity studies highlight the importance of adherence to full vaccination recommendations in pregnancy and additionally support the benefit of a booster dose in pregnancy to optimize immunity.
Several studies documented a more robust maternal antibody response to vaccination than to previous infection.36 39 40 42 43 46 One found significantly higher SARS-CoV-2 IgG concentrations in maternal serum and cord blood among fully vaccinated women (n=64) (P<0.001) compared with 11 previously infected pregnant women.46 Vaccination during pregnancy may also provide protection for the newborn. We identified 16 studies that documented presence of SARS-CoV-2 antibodies in cord blood after maternal vaccination.35 36 40 41 42 43 44 45 46 47 48 49 51 52 53 Additionally, several studies have shown a positive correlation between maternal serum and cord blood antibody concentrations, which is generally described as the placental transfer ratio.36 40 45 46 48 49 52 With regard to timing of antibody production, one study found maternal antibody production as early as five days after a first vaccine dose and transplacental transfer as early as 16 days after a first dose.47 Some variability exists, however, and lack of transfer was seen in one neonate who was delivered six weeks after the second dose. Another study found that the three of 28 cord samples that were IgG negative all came from deliveries with less than three weeks between the time of first dose and delivery.45 Maternal IgG concentrations and placental IgG transfer ratios increased over time, suggesting that time between vaccination and birth may be an important factor.
The degree of maternal protection via placental transfer of antibodies likely depends on maternal antibody concentration, which is related to timing of vaccination and delivery. In one study, early third trimester (27-31 weeks) vaccination was associated with higher neonatal anti-SARS-CoV-2 antibody and serum neutralizing activity. This may support early third trimester as the optimal timing for a booster vaccination to optimize maternal to fetal antibody transfer for neonatal protection.49 In general, transplacental antibody transfer starts in the second trimester; however, transfer is most efficient in the third trimester.55 56
More studies of factors affecting placental antibody transfer are needed, along with better understanding of the degree of protection provided. Although we identified 20 studies evaluating the immunogenicity of covid-19 vaccines in pregnancy, they were primarily on mRNA vaccines. These limited data highlight the importance of fully understanding the immunology of pregnancy to develop evidence based, pregnancy specific vaccine recommendations. Overall, the immunogenicity data in pregnancy illustrate a robust immune response to vaccination and maternal transfer of antibodies to the neonate via cord blood.
Covid-19 vaccines in prevention of maternal and infant infection
We identified nine articles evaluating risk of maternal SARS-CoV-2 infection after maternal vaccination.9 57 58 59 60 61 62 63 64 The data overwhelming support maternal vaccination as being effective at reducing the risk for infection and severe illness. A large national prospective cohort study in Scotland of 18 399 vaccinated and 126 149 unvaccinated pregnant women found that 77.4% (3833/4950; 95% confidence interval 76.2% to 78.6%) of SARS-CoV-2 infections, 90.9% (748/823; 88.7% to 92.7%) of SARS-CoV-2 associated hospital admissions, and 98% (102/104; 92.5% to 99.7%) of SARS-CoV-2 associated critical care admission occurred in those who were unvaccinated.63 In Israel, a large observational cohort of 10 861 vaccinated pregnant women matched to 10 861 unvaccinated pregnant controls found an estimated vaccine effectiveness from seven through 56 days after the second dose of 96% (95% confidence interval 89% to 100%) for any documented infection, 97% (91% to 100%) for infections with documented symptoms, and 89% (43% to 100%) for covid-19 related hospital admission.60
These results reflect effectiveness mainly against the original SARS-CoV-2 reference strain and the B.1.1.7 (alpha) variant, as these were the dominant strains circulating during the study period. This is similar to the vaccine effectiveness estimated in the general population in Israel. A retrospective cohort study in the US of 1332 vaccinated pregnant patients and 8760 incompletely vaccinated or unvaccinated pregnant patients found that vaccinated patients had lower odds of severe covid-19, defined as SpO2 <94% on room air, PaO2/FiO2 ratio <300 mm Hg, respiratory rate >30 breaths per minute, or lung infiltrates >50%, or critical covid-19, defined as respiratory failure, septic shock, or multiple organ failure (0.08% v 0.66%; adjusted odds ratio 0.10, 95% confidence interval 0.01 to 0.49) and covid-19 of any severity (1.1% v 3.3%; 0.31, 0.17 to 0.51).62 These and the other smaller studies show an association between maternal SARS-CoV-2 vaccination and lower odds of covid-19 illness. Overall, covid-19 vaccines have been found to be highly effective in preventing severe illness, hospital admission, and death; these data support similar effectiveness in pregnancy.
Additionally, one study evaluated the effectiveness of maternal vaccination during pregnancy against covid-19 related hospital admission in infants during the first six months of life.65 Of 176 infants admitted with covid-19, 16% of their mothers had been vaccinated compared with 32% of 203 infants admitted without covid-19 (P<0.01). Effectiveness of maternal vaccination during pregnancy against covid-19 related hospital admission in infants aged <6 months was found to be 61% (31% to 78%). Effectiveness of a two dose covid-19 vaccination series was 32% (–43% to 68%) in the first 20 weeks of pregnancy and 80% (55% to 91%) after 21 weeks through 14 days before delivery. This gestational age breakdown has wide confidence intervals and should be interpreted with caution. Overall, completion of a two dose mRNA covid-19 vaccination series during pregnancy seems to reduce covid-19 related hospital admissions among infants aged <6 months, but the duration of clinical protection remains uncertain. A smaller study evaluating antibody response in infants found that most infants (98%; 48/49) had detectable anti-S IgG antibodies at 2 months of life after maternal vaccination and 57% (16/28) still had detectable concentrations at 6 months.66 This was compared with only 8% (1/12) with detectable antibodies after SARS-CoV-2 infection during pregnancy. The concentrations of antibodies needed to provide protection in infants is unknown, so clinical data in correlation with titer information are needed. However, given the burden of SARS-CoV-2 in infants and that covid-19 vaccines are not planned for infants <6 months, these findings provide support that babies receive protective benefits from maternal vaccination.
COVID-19 vaccines and perinatal outcomes
On the basis of 26 studies describing perinatal outcomes after covid-19 vaccination in pregnancy, the overall rates of adverse perinatal outcomes were not increased after maternal vaccination (table 3). The rates of preterm birth, fetal growth restriction, cesarean delivery, and neonatal intensive care unit (NICU) admission varied across studies, which is likely explained by different patient populations; however, in the studies with a comparison group of unvaccinated pregnant patients, the rates were not significantly increased. Some challenges in evaluating perinatal outcomes after exposure during pregnancy include understanding the baseline risk of the outcome, the timing of the exposure in pregnancy related to the outcome, and the necessary sample size needed to detect a difference. Additionally, common sources of bias in maternal vaccination observational studies including healthy vaccinee bias and confounding by indication, immortal time bias, and cohort truncation must be considered.79
Table 3.
Perinatal outcome data with covid-19 vaccines in pregnancy
| Author; country; study design | Vaccine type | Population with delivery outcomes | Perinatal outcomes |
|---|---|---|---|
| Kharbanda E, 202167; USA; case-control surveillance study | Pfizer, n=8267; Moderna, n=6313; Janssen, n=528 | Pregnant vaccinated, n=15 108; pregnant unvaccinated, n=90 338 | SAB: 1128/13 160 (8.6%) SAB occurred within 28 days of vaccination; 20 139/250 994 (8.0%) of ongoing pregnancies occurred within 28 days of vaccination. Among women with SAB, odds of covid-19 vaccine exposure were not increased in previous 28 days compared with women with ongoing pregnancies (AOR 1.02, 95% CI 0.96 to 10.8) |
| Zauche L, 202168; USA; cohort study | Pfizer, n=1294; Moderna, n=1162 | Vaccinated, n=2456 | Cumulative SAB risk from 6 to <20 weeks was 14.1% (95% CI 12.1% to 16.1%). With direct maternal age standardization, SAB risk was 12.8% (10.8% to14.8%). Cumulative risk of SAB increased with maternal age. Compared with data from two historical cohorts that represent lower and upper ranges of SAB risk, cumulative risks of spontaneous abortion were within expected risk range |
| Magnus M, 202169; Norway; case-control study | Pfizer, n=790; Modern, n=137; AstraZeneca, n=76 | Vaccinated, n=958; unvaccinated, n=13 613 | SAB: AOR for vaccinated/unvaccinated 0.81 (95% CI 0.69 to 0.95) for vaccination in the previous 5 weeks |
| Trostle M, 202170; USA, case series | Pfizer, n=332; Moderna, n=92 | Vaccinated, n=424; delivered liveborn infant, n=85 | SAB 6.5%; preterm birth 5.9%; FGR/SGA 12.2%; cesarean delivery 35.3%; fetal anomaly 1.2%; stillbirth 0%; NICU admission 15.3%; any antenatal complication 23.5% |
| Rottensteich M, 202250; Israel; Multicenter retrospective database study | Pfizer, n=712 | Two doses, n=712; unvaccinated, n=1063 | Preterm birth: vaccinated 1%; unvaccinated 0.9% (P=0.93). FGR/SGA: vaccinated 11.4%; unvaccinated 9.2% (P=0.14). Cesarean delivery: vaccinated 15.6%; unvaccinated 10.8% (P<0.01). Stillbirth: vaccinated 0.7%; unvaccinated 0.5% (P=0.52). NICU admission: vaccinated 4.1%; unvaccinated 4.5% (P=0.65). Composite adverse maternal outcome: vaccinated 24.2%; unvaccinated 23.6% (AOR 0.8, 95% CI 0.61 to 1.03; P=0.79). Composite adverse neonatal outcome: vaccinated 7.9%; unvaccinated 11.4% (AOR 0.5, 0.36 to 0.74; P=0.02) |
| Shimabukuro T, 202171; USA; V-safe Surveillance System and VAERS Pregnancy Registry | Pfizer: 1 dose, n=9052; 2 doses, n=6638. Moderna: 1 dose, n=7930; 2 doses, n=5635 | n=827 with completed pregnancy outcome | Preterm birth 9.4%; FGR/SGA 3.2%; major congenital anomaly 2.2%; SAB 12.6%; stillbirth 0.1%; neonatal death 0% |
| Theiler R, 202164; USA; cohort study | Pfizer, n=127; Moderna, n=12; Janssen, n=1 | Vaccinated, n=140; unvaccinated, n=1862 | Preterm birth: vaccinated 9.3%; unvaccinated 8.5% (P=0.70). FGR/SGA: vaccinated 7.9%; unvaccinated 6.5% (P=0.53). Cesarean delivery: vaccinated 31.4%; unvaccinated 29.8% (P=0.65). Stillbirth: vaccinated 0%; unvaccinated 0.3% (P=1.0). NICU admission: vaccinated 0.07%; unvaccinated 0.6% (P=0.58) (for >1day, >37weeks, >2500 g). Any antenatal complication: vaccinated 5%; unvaccinated 4.9% (P=0.95) |
| Dick A, 202272; Israel; retrospective cohort study | Pfizer or Moderna | Vaccinated, n=2305; unvaccinated, n=3313 | Preterm birth: vaccinated 5.5%; unvaccinated 6.2% (P=0.31). Only significant finding: 2nd trimester vaccination had increased risk of preterm birth compared with unvaccinated counterparts (8.1% v 6.2%; P<0.001). FGR/SGA: vaccinated 6.2%; unvaccinated 7.0% (P=0.2). Cesarean delivery: vaccinated 15.5%; unvaccinated 16.0%. Stillbirth: vaccinated 0.87%; unvaccinated 1.0% |
| Lipkind H, 202273; USA; observational retrospective cohort study | Pfizer, n=5478; Moderna, n=4162; Janssen, n=424 | Vaccinated, n=10 064; unvaccinated n=36 015 | Preterm birth: vaccinated 4.9%; unvaccinated 7.0% (AHR 0.91, 95% CI 0.82 to 1.01; P=0.06). FGR/SGA: vaccinated 8.2%; unvaccinated 8.2% (AHR 0.95, 0.87 to 1.03; P=0.24) |
| Goldshtein I, 202261; Israel; cohort study | Pfizer | Infants of unvaccinated mothers, n=7591; infants of vaccinated mothers, n=16 697 | Preterm birth: vaccinated 4.4%; unvaccinated 4.1% (RR 0.95, 95% CI 0.83 to 1.10). FGR/SGA: vaccinated 6.6%; unvaccinated 6.7% (RR 0.97, 0.87 to 1.08). Fetal anomaly: first trimester vaccination—unvaccinated, n=3584; vaccinated, n=2134; risk of any congenital malformations—RR 0.69, 0.44 to 1.04); risk for major heart malformations was lower among exposed group (RR 0.46, 0.24 to 0.82) |
| Citu I, 202239; Romania; prospective cohort study | Pfizer; Janssen | Vaccinated, n=173; unvaccinated, n=529 | Preterm birth: vaccinated 8.1%; unvaccinated 6.9% (P=0.63). FGR/SGA: vaccinated 3.4%; unvaccinated 4.9% (P=0.43). Cesarean delivery: vaccinated 11.5%; unvaccinated 13.0% (P=0.61) |
| Bookstein P, 202138; Israel; observational case-control study | Pfizer | Vaccinated, n=57 | Preterm birth 0%; FGR/SGA 5.3%; cesarean delivery 17.6%; stillbirth/neonatal death 0%; NICU admission 3.5%; hypertensive disorder of pregnancy 1.8% |
| Beharier O, 202136; Israel; multicenter cohort study | Pfizer | Vaccinated, n=92; infected, n=74; control, n=66; delivery outcomes, n=92 | Preterm birth: vaccinated 7.6%; infected 10.5%; control 4.3% (NS). NICU admission: vaccinated 4.3%; infected 2.7%; control 1.6% (NS) |
| Magnus M, 202274; Norway, Sweden; registry based retrospective cohort study | Pfizer, n=20 424; Moderna, n=7607; AstraZeneca, n=475 | Vaccinated, n=28 506; unvaccinated, n=129 015 | Preterm birth: vaccinated 6.2 v unvaccinated 4.9 per 10 000 pregnancy days at risk (AHR 0.98, 95% CI 0.91 to 1.05). Similar for 2nd and 3rd trimester vaccination, with 1 or 2 doses, and for both mRNA vaccines. SGA: vaccinated 7.8%; unvaccinated 8.5% (AOR 0.97, 0.90 to 1.04). Stillbirth: vaccinated 2.1 v unvaccinated 2.4 per 100 000 pregnancy days at risk (AHR 0.86, 0.63 to 1.17). NICU admission: vaccinated 8.5% v unvaccinated 8.5% (AOR 0.97, 0.86 to 1.10) |
| Gray K, 202142; prospective cohort study | Pfizer, n=41; Moderna, n=43 | Vaccinated, n=13 | Preterm birth 8%; FGR 0%; cesarean delivery 23%; NICU admission 15% |
| Morgan J, 202262; USA; retrospective cohort study | Pfizer, n=883; Moderna, n=382; Janssen, n=67 | Vaccinated, n=1332; incompletely vaccinated or unvaccinated, n=8760 | Stillbirth: vaccinated 0%; unvaccinated 0.07% (OR 0, 95% CI 0 to 4.73). Maternal death: vaccinated 0%; unvaccinated 0.01% (OR 0, 0 to 651) |
| Aslam J, 202275; Pakistan; Observational study | Not described | Pregnant in high dependency unit and ICU, n=33; vaccinated, n=6; unvaccinated, n=27 | Stillbirth: n=16, all unvaccinated. Neonatal death: n=3, all unvaccinated. Maternal death: n=22, all unvaccinated |
| Wainstock T, 202176; Israel; Retrospective cohort study | Pfizer, n=913 | Vaccinated, n=913; unvaccinated, n=3486 | FGR/SGA: vaccinated 2.8%; unvaccinated 3.8% (OR 0.75, 95% CI 0.49 to 1.15). Cesarean delivery: vaccinated 19.9%; unvaccinated 17.2% (OR 1.19, 0.99 to 1.44) |
| Blakeway H, 202257; UK; retrospective cohort study | Pfizer, n=109; Moderna, n=18; AstraZeneca, n=13 | Vaccinated, n=133; unvaccinated, n=399 | FGR/SGA: vaccinated 12%; unvaccinated 12% (P>1.0). Cesarean delivery: vaccinated 30.8%; unvaccinated 34.1% (P=0.49). Stillbirth: vaccinated 0%; unvaccinated 0.2% (P=NE). NICU admission: vaccinated: 5.3%; unvaccinated 5.0% (P=0.93). Fetal abnormality: vaccinated 2.2%; unvaccinated 2.5% (P=0.87) |
| Bleicher I, 202158; Israel; prospective cohort study—interim analysis of broader observations study—questionnaire | Pfizer, n=202 | Vaccinated, n=202; unvaccinated, n=124 | FGR/SGA: vaccinated 1.5%; unvaccinated 0% (P=0.29). Fetal anomaly: vaccinated 4.5%; unvaccinated 4.8% (P=1.0). Composite pregnancy complications: vaccinated 16%; unvaccinated 20% (P=0.37) |
| Ruderman R, 202277; USA; cohort study | Not described | Vaccinated within teratogenic window, n=1149; vaccinated outside teratogenic window, n=1473; unvaccinated, n=534 | Congenital anomalies: vaccinated within teratogenic window—30 days before conception to 14 weeks 48/1149 (4.2%); 2-10 weeks 34/840 (4.0%); vaccinated outside teratogenic window—30 days before conception to 14weeks 61/1473 (4.1%); 2-10 weeks 75/1782 (4.2%); unvaccinated or vaccinated outside teratogenic window—30days before conception to 14 weeks 88/2007 (4.4%); 2-10 weeks 102/2316 (4.4%); odds ratios between unvaccinated or vaccinated outside teratogenic window and vaccinated within teratogenic window non-significant; odds ratios between vaccinated outside teratogenic window and vaccinated within teratogenic window non-significant |
| Fell D, 202278; Canada; retrospective cohort study | Pfizer, n=18 101; Moderna, n=4507; other, n=52 | Vaccinated, n=22 660; vaccinated after pregnancy, n=44 815; unvaccinated, n=30 115 | Cesarean delivery: vaccinated 30.8%; vaccinated after pregnancy 32.2% (ARR 0.92, 95% CI 0.89 to 0.95); unvaccinated 28.5%. NICU admission: vaccinated 11.0%; vaccinated after pregnancy 13.3% (ARR 0.85, 0.80 to 0.90); unvaccinated 12.8% (ARR 0.92, 0.87 to 0.97) |
| Nir O, 202146; Israel; prospective cohort study | Pfizer | Fully vaccinated, n=64; unvaccinated and recovered from covid-19, n=11 | Cesarean delivery: vaccinated 36%; recovered 27.3% |
| Rottensteich A, 202249; Israel; cohort study | Pfizer | First dose at 27-31 weeks, “early 3rd trimester,” n=83; first dose at 32-36 weeks, “late 3rd trimester,” n=88 | Cesarean delivery: vaccinated early 3rd trimester 7.2%; late 3rd trimester 8.0% |
| Kashani-Ligumsky L, 202143; Israel; cohort study | Pfizer | Infected, n=29; vaccinated, n=29; control, n=21 | Vaginal delivery: vaccinated 89.7%; infected 82.8%; control 85.7% (P=0.86) |
| Shanes F, 202151; USA; cohort study | Pfizer, n=49; Moderna, n=25; mRNA unknown type, n=9 | Vaccinated, n=84; unvaccinated, n=116 | Vaginal delivery: vaccinated 79%; unvaccinated 65% (P=0.04). No difference in decidual arteriopathy, fetal vascular malperfusion, or low or high grade chronic villisitis |
AHR=adjusted hazard ratio; AOR=adjusted odds ratio; ARR, adjusted risk ratio; CI, confidence interval; FGR=fetal growth restriction; HR=hazard ratio; NE=not estimatable; NICU=neonatal intensive care unit; NS=not significant; OR=odds ratio; SAB=spontaneous abortion; SGA=small for gestational age; RR=risk ratio.
A large registry based study of births in Sweden and Norway (28 506 vaccinated; 129 015 unvaccinated) found no significant increased risk of adverse pregnancy outcomes including preterm birth, stillbirth, small for gestational age, or NICU admission in people vaccinated against SARS-CoV-2 during pregnancy.74 The results were similar for vaccinations during the second or third trimester, with one or two doses of vaccine, and with different mRNA vaccine types. A large (>10 000 people vaccinated during pregnancy) US based multisite retrospective cohort with a diverse population and comprehensive data on vaccination did not find an increase in preterm birth or small for gestational age birth.73 Additionally, a large (913 vaccinated; 3486 unvaccinated) retrospective cohort in Israel found no differences in pregnancy, delivery, or newborn complications, including gestational age at delivery, incidence of small for gestational age birth, and newborn respiratory complications.76
Another important perinatal outcome of interest after maternal vaccination is risk of spontaneous abortion. Studies evaluating this risk should ideally include a control group and document timing of maternal vaccination. We identified four studies on risk of spontaneous abortion after covid-19 vaccinations in pregnancy.67 68 69 70 One used the Vaccine Safety Datalink to analyze the odds of receiving a covid-19 vaccine in the 28 days before a spontaneous abortion.67 It found that pregnancies ending in a spontaneous abortion did not have an increased odds of exposure to a covid-19 vaccination in the previous 28 days compared with ongoing pregnancies (adjusted odds ratio 1.02, 0.96 to 1.08). Results were consistent for Moderna and Pfizer vaccines and by gestational age group. Additionally, a case-control study from Norwegian registries of 13 956 women with ongoing pregnancies (958 vaccinated) found adjusted odds ratios of 0.91 (0.75 to 1.10) for covid-19 vaccination in the previous three weeks following a spontaneous abortion and 0.81 (0.69 to 0.95) for vaccination in the previous five weeks, showing no evidence of an increased risk for early pregnancy loss after covid-19 vaccination.69
Six studies evaluated the risk of fetal anomalies after maternal vaccination (table 3).57 58 61 70 71 77 A large Israeli study of 16 738 infants prenatally exposed to the Pfizer vaccine compared with 7452 unexposed infants did a subgroup analysis among newborns exposed to first trimester vaccination (n=2021) versus unexposed newborns (n=3580) and found no difference in congenital anomalies (risk ratio 0.69, 95% confidence interval 0.44 to 1.04).61 Additionally, the risk for major heart malformations was lower among the exposed group (risk ratio 0.46, 0.24 to 0.82). Given the importance of timing in pregnancy and risk of fetal anomalies, a large cohort study evaluating the association of covid-19 vaccination during early pregnancy with risk of congenital fetal anomalies identified an anomaly in 27 (5.1%) of 534 unvaccinated people versus 109 (4.2%) of 2622 people who received at least one dose of vaccine (P=0.35).77 Importantly, after control for potential confounders such as hemoglobin A1c level in the first trimester and age at delivery, vaccination within the highest risk period for teratogenicity was not associated with presence of congenital anomalies identified by ultrasonography (adjusted odds ratio 1.05, 0.72 to 1.54).
We identified nine studies evaluating the risk of stillbirth after covid-19 vaccination.38 49 62 64 70 71 72 74 75 None found an increased risk; however, given the rarity of stillbirth as an event, large studies will be needed to evaluate this risk adequately. In the largest study (n=28 506), no difference was seen in risk of stillbirth after vaccination (2.1 v 1.4 per 100 000 pregnancy days at risk in vaccinated compared with unvaccinated; adjusted hazard ratio 0.86, 0.63 to 1.17).74 The small number of stillbirths meant that the study could not explore the risk of stillbirth according to vaccination by pregnancy trimester, number of doses, or vaccine type. A recent systematic review and meta-analysis including five of these studies found that the risk of stillbirth was significantly lower in those vaccinated in pregnancy by 15% (pooled odds ratio 0.85, 0.73 to 0.99).80
Twelve studies evaluated the risk of preterm birth after maternal covid-19 vaccination, and the data are reassuring.36 38 39 42 50 61 64 70 71 72 73 74 One retrospective study of the Pfizer vaccine in Israel found the overall rate of preterm birth to be 5.5% in the vaccinated group compared with 6.2% in the unvaccinated group (P=0.31); however, people vaccinated in the second trimester of pregnancy (n=964) were more likely to have a preterm birth than those vaccinated in the third trimester (n=1329) (8.1% v 6.2%; P<0.001).72 This association persisted after adjustment for potential confounders (adjusted odds ratio 1.49, 1.11 to 2.01). The authors note that no preterm births were seen within two weeks after second trimester vaccination and that most of the preterm births were late preterm, suggesting that unmeasured confounding might have contributed to the results. A larger retrospective study in the US did not find an increased risk of preterm birth with maternal vaccination (4.9% v 7.0%; adjusted hazard ratio 0.91, 0.82 to 1.01).73 Additionally, no increased risk of preterm birth was seen in the very large cohort study in Norway and Sweden, including no difference between second and third trimester vaccination.74
Nine studies evaluated NICU admission rates after maternal vaccination, and none identified an increased risk.36 38 42 50 57 64 70 74 78 The largest study found that overall rates of adverse newborn outcomes were lower among those born to people vaccinated during pregnancy versus those vaccinated afterwards, including lower NICU admission (11.0% v 13.3%; adjusted risk ratio 0.85, 0.80 to 0.90).78 Additionally, compared with the 30 115 mothers who were never vaccinated, no significantly increased risks of any outcomes were seen in those vaccinated during pregnancy, and NICU admission (11.0% v 12.8%; adjusted risk ratio 0.92, 0.87 to 0.97) was significantly lower among those who were vaccinated during pregnancy.
On the basis of review of available data, no increased risk of adverse pregnancy outcomes was observed among people vaccinated against SARS-CoV-2 during pregnancy, supporting recommendations for vaccination of pregnant patients against SARS-CoV-2. Vaccine safety concerns have been a significant barrier to vaccine acceptance in pregnancy; thus, highlighting the amount of accumulated data is important.
Covid-19 vaccine side effects in pregnancy
We identified 12 studies evaluating adverse effects after maternal covid-19 vaccination.37 38 39 40 42 44 55 58 71 81 82 83 In general, symptoms after covid-19 vaccination are usually mild to moderate and occur within the first three days after vaccination. Most occur the day after vaccination and resolve within one to two days. The second dose is associated with more frequent and severe symptoms. The vaccine side effect profile in pregnancy seems to be similar to that in non-pregnant people, with pain at the injection site, fatigue, headache, and myalgia being the most frequent symptoms.37 38 42 55 71 Although routine prophylaxis with acetaminophen is not recommended in some countries, pregnant patients should take acetaminophen if they experience fever after vaccination. Severe allergic reactions are rare following covid-19 vaccination in non-pregnant patients (4.7 per million for Pfizer-BioNTech and 2.5 per million for Moderna). Anaphylaxis following vaccination in pregnancy should be managed the same as in non-pregnant patients.21
A large prospective cohort study including 7809 pregnant people found that covid-19 vaccines were well tolerated among people who were pregnant, lactating, or planning pregnancy. Odds of several reactions were decreased among people who were pregnant, such as fever after Pfizer dose 2 (odds ratio 0.44, 0.38 to 0.52; P<.001) and after Moderna dose 2 (0.48, 0.40 to 0.57; P<0.001) compared with those who were neither pregnant nor lactating.55 The frequency of complaints after vaccination was similar to that observed in non-pregnant patients, and reports of adverse outcomes were infrequent. Additionally, a prospective study of 83 vaccinated pregnant women who were age matched with 166 vaccinated female non-pregnant controls found the frequency of complaints following vaccine administration did not differ between pregnant and non-pregnant patients (18.1% v 16.9%; P=0.20).82 However, pregnant patients were more likely to report fever (4.8% v 0.6%; P=0.04) and gastrointestinal symptoms (4.8% v 0%; P=0.01). This could reflect differences in the physiologic responses to the vaccines in pregnancy. One study used data from the “v-safe after vaccination health checker” surveillance system, the v-safe pregnancy registry, and the Vaccine Adverse Event Reporting System (VAERS) to characterize the initial safety of mRNA covid-19 vaccines in pregnancy.71 Local and systemic reactogenicity was compared between people who identified as pregnant and non-pregnant women. The study found small differences in reporting frequency between pregnant people and non-pregnant women observed for specific reactions (injection site pain was reported more frequently in pregnancy, and other systemic reactions were reported more frequently among non-pregnant women), but the overall reactogenicity profile was similar. None of the available data indicate obvious increased adverse effects or safety concerns with use of covid-19 vaccines in pregnancy.
Covid-19 vaccine hesitancy in pregnancy
The search identified 42 articles evaluating vaccine hesitancy in pregnancy in the English language from around the world. Table 4 outlines the studies with either positive or negative associations with willingness or decision for vaccination in pregnancy; three additional studies did not fit these criteria but discussed factors associated with vaccine uptake.115 116 117 Factors positively associated with vaccine willingness/uptake in pregnancy tended to be the same around the globe, including older maternal age, higher education, previous influenza vaccine uptake, higher level of trust in the healthcare system, increased perceived risk of covid-19, fertility treatments, urban living, and higher socioeconomic status (table 4). Factors associated with a lower likelihood of vaccination during pregnancy included younger age, lower education/socioeconomic status, and lack of adherence to influenza vaccination recommendations. Pregnancy itself was found to be negatively associated with vaccine acceptance in several studies when a non-pregnant cohort was used for comparison.
Table 4.
Covid-19 vaccine hesitancy in pregnancy
| Author; study design | Population | Positive association with vaccination or willingness to be vaccinated | Negative association with vaccination or willingness to be vaccinated |
|---|---|---|---|
| United Kingdom | |||
| Blakeway H, 202257; retrospective cohort study | Pregnant vaccinated, n=140; pregnant unvaccinated, n=1188 | Pre-pregnancy diabetes | Younger women; non-white ethnicity; lower socioeconomic background |
| Anderson E, 202184; qualitative interview study | Pregnant, n=31 | - | Concern vaccine riskier than covid |
| United States | |||
| Sznajder K, 202285; secondary analysis | Pregnant, n=196 | Received influenza vaccine in previous year; full time employment; feeling overloaded | |
| Battarbee A, 202286; cross sectional survey | Pregnant, n=915 | Received influenza vaccine in previous season | Hispanic and Black women |
| Keifer M, 202287; cross sectional survey | Pregnant, n=435; postpartum, n=21 | Higher level of education; older age; Asian race; reporting a friend or family member who received covid-19 vaccine; planning or had Tdap vaccine; receipt of influenza vaccine in current year; concern about covid-19; discussing vaccine with obstetric provider | Non-Hispanic Black; young age; public health insurance; tobacco use in pregnancy; any drug use; parity |
| Wang T, 202188; cross sectional cohort; electronic survey | Healthcare workers, n=83; pregnant, n=20; lactating, n=19; would like to become pregnant soon, n=47 | Increased perceived risk of covid; agreed covid-19 vaccine was safe and effective in pregnancy | Pregnancy; concern for fertility |
| Huddleston H, 202289; national prospective cohort study (ASPIRE) | Pregnant, n=2506 | Higher income; higher education; living in metropolitan area; worry about covid-19; being counseled about vaccination by provider | Black race; being counseled by provider not to vaccinate |
| Levy A, 202190; survey study | Pregnant, n=662 | Trust in information received about vaccinations | Younger age; Black or African-American race; Hispanic ethnicity; less education; declining influenza vaccine; concern for fetal safety |
| Razzaghi H, 202191; Vaccine Safety Datalink (VSD) | Pregnant, n=135 968 | Older age; Asian women | Black women; Hispanic women |
| Sutton D, 202192; online survey | Respondents, n=1012; pregnant, n=656; lactating, n=122 | - | Pregnancy; non-white race; Spanish speaking |
| Theiler R, 202164; cohort study | Pregnant; vaccinated, n=140; unvaccinated, n=1862 | Older age; higher maternal education; non-smoker; fertility treatment; lower gravity | - |
| Israel | |||
| Rottensteich M, 202250; multicenter retrospective database study | Pregnant; vaccinated, n=712; unvaccinated, n=1063 | Older age; fertility treatment; previous cesarean delivery; previous miscarriage | - |
| Bleicher I, 202158; prospective observational study | Pregnant, n=313 | Flu vaccine in current or previous year; medical employee | Concern about lack of safety data in pregnancy; fear of short and long term side effects; history of covid infection; no comorbidities |
| Saleh O, 202293; online survey | Women 6 months before or after giving birth, n=410; pregnant, n=293; post partum, n=117 | Jewish religion; academic education; employment; urban | - |
| Taubman-Ben-Ari O, 202294; online survey | Jewish pregnant women, n=187; Arab pregnant women, n=673 | Lower levels of psychological distress in Arab women | - |
| Wainstock T, 202176; retrospective cohort study | Pregnant; vaccinated, n=913; unvaccinated, n=3486 | Older age; fertility treatment; sufficient prenatal care; higher socioeconomic position | - |
| Ethiopia | |||
| Mose A, 202195; cross sectional survey | Pregnant, n=396 | Maternal age 34-41; educational status; good knowledge; good practice with covid-19 guidelines | - |
| Hailemariam S, 202196; cross sectional survey | Pregnant, n=412 | Urban residence; secondary and higher education; compliance with covid-19 guidelines; good perception toward covid vaccine | - |
| Taye E, 202297; cross sectional survey | Pregnant, n=360; post partum, n=159 | Urban residence; favorable attitude toward covid vaccine; worried about covid-19 disease | - |
| France | |||
| Egloff C, 202298; cross sectional survey | Pregnant, n=664 | Older; higher education; multiparty; having discussed vaccination with a care giver; acceptance of influenza vaccine | - |
| Deruelle P, 202199; online survey | n=1416; obstetrician/gynecologist, n=749; midwife, n=598; general practitioner, n=69 | Being an obstetrician; working in a group; usually offering flu vaccine; wanting to be vaccinated | - |
| Italy | |||
| Carbone L, 2021100; multicenter cross sectional study | Pregnant, n=119; post partum, n=23 | - | Pregnancy |
| Mappa I, 2021101; prospective observational study | Pregnant, n=161 | - | Lower education; lower employment level |
| 16 countries | |||
| Skjefte M, 2021102; cross sectional survey | Pregnant, n=5294; non-pregnant mothers, n=12 562 | Mexico; India; confidence in vaccine safety and efficacy; importance of vaccines/mass vaccination in their own country; worry about covid-19; trust of public health/science; compliance with mask guidelines | United States; Australia; Russia; younger age; lower income; lower education not married; no health insurance |
| Australia | |||
| Bradfield Z, 2021103; national cross sectional online study | Doctors, n=58; midwives, n=391; midwifery students, n=78; women, n=326 | Doctors | Midwives |
| China | |||
| Tao L, 2021104; multicenter cross sectional study | Pregnant, n=1392 | Younger age; western region; low level of education; late pregnancy; high knowledge score on covid-19; high level of perceived susceptibility; low level of perceived barriers; high level of perceived benefit; high level of perceived cues to action | - |
| Czech Republic | |||
| Riad A, 2021105; cross sectional survey | Pregnant, n=278; lactating, n=84 | Third trimester > 1st trimester; older age; higher education; trust in industry and healthcare professionals | - |
| Germany | |||
| Schaal N, 2021106; cross sectional survey | Pregnant, n=1043; lactating, n=1296 | Anxiety about getting infected | - |
| Greece | |||
| Daskalakis G, 2021107; electronic survey | Obstetricians, n=504; midwifes, n=214; pediatricians, n=176; other healthcare providers, n=332 | - | Non-medical, non-midwife/nurse professional, non-involvement in higher education; lack of adherence for vaccinations of pregnant women against flu/pertussis; healthcare provider not vaccinated |
| Ireland | |||
| Geoghegan S, 2021108; survey | Pregnant, n=300 | Later gestational age; age 30-35 | - |
| Japan | |||
| Hosokawa Y, 2022109; cross sectional survey | Pregnant, n=1791 | Lack of trust in government | |
| Poland, Ukraine | |||
| Januszek S, 2022110; cross sectional survey | Pregnant in Poland, n=150; pregnant in Ukraine, n=150 | Medical consultation; Polish > Ukrainian | Fear of harming fetus; complications in pregnancy |
| Qatar | |||
| Mohan S, 2021111; nationwide online cross sectional study | Pregnant or lactating, n=341 | - | Concern about vaccine safety; worry about vaccine problems; feeling natural immunity is better/safer |
| Romania | |||
| Citu I, 2022112; cross sectional survey | Pregnant, n=184; non-pregnant, n=161 | - | Not being afraid of covid; below average income; trusting rumors in social media; not believing in covid virus or vaccines; vaccination non-believer |
| Saudi Arabia | |||
| Samannodi M, 2021113; cross sectional survey | Pregnant or planning pregnancy, n=214; other women, n=217 | - | Pregnancy or planning pregnancy |
| Switzerland | |||
| Stuckelberger S, 2021114; cross sectional survey | Pregnant, n=515; lactating n=1036 | Age >40; higher education; influenza vaccine in previous year; third trimester; having obstetrician as primary healthcare practitioner | - |
Data from Vaccine Safety Datalink, a collaboration between the Centers for Disease Control and Prevention (CDC) and multiple integrated health systems, analyzed receipt of at least one dose of any covid-19 vaccine in 135 968 pregnant women between 14 December 2020 and 8 May 2021.91 Vaccination increased with age, with highest rates of at least one dose observed among women aged 35-49 years (22.7%) and lowest rates among those aged 18-24 years (5.5%). Receipt of at least one dose was highest among Asian women (24.7%), followed by white (19.7%) women, and lowest among Black (6.0%) and Hispanic (11.9%) women. One study found that the impact of education and influenza vaccination history on vaccine acceptance varied depending on race, suggesting that different public health messaging might be appropriate for Black or African-American women because of their lived experiences with systemic racism and mistrust in the healthcare system.90
Another emerging theme from the data was the importance of counseling from healthcare providers. Previous data show that the recommendation and offer of the influenza vaccine by a healthcare provider are critical for uptake in pregnancy.118 Several studies found that a provider’s recommendation was positively associated with covid-19 vaccination.87 89 98 110 A large nationwide prospective study in the US was used to characterize vaccination rates and acceptance during pregnancy in the first six months of vaccine rollout.89 Among 2506 pregnant respondents, 57.4% had received one dose of covid-19 vaccination in pregnancy. In an adjusted model, the predictors of lower odds of vaccination included Black race (21.8%) compared with white race (58.2%) (P<0.001); being counseled about vaccination by a provider was a strong predictor of getting vaccinated (69.4%) compared with no counseling (38.5%) (P<0.001). The best methods to provide educational resources to providers and to ensure that covid-19 vaccination recommendations are routinely discussed with obstetric patients should be a research priority. Ensuring that providers have up to date information on the maternal and neonatal safety of covid-19 vaccines in pregnancy is critical, as this is needed to help guide their counseling.
In a large multi-country study of 17 871 women, including 5282 who were pregnant, conducted before vaccines were available, 2747 (52.0%) pregnant women and 9214 (73.4%) non-pregnant women indicated an intention to receive a covid-19 vaccine.102 The top three reasons why pregnant women would decline vaccination even if the vaccine were safe and free were that they did not want to expose their developing baby to any possible harmful side effects (65.9%), they were concerned that approval of the vaccine would be rushed for political reasons (44.9%), and they would like to see more safety and effectiveness data during pregnancy (48.8%). Vaccine acceptance was lowest in Russia, the US, and Australia. The strongest predictors of vaccine acceptance included confidence in vaccine safety or effectiveness, worrying about covid-19, belief in the importance of vaccines to their own country, compliance with mask guidelines, trust of public health agencies/health science, and attitudes toward routine vaccines.
Over the past year, substantial data have been generated on covid-19 vaccines in pregnancy. Concerns for fetal/neonatal safety due to lack of data on safety of the vaccines in pregnancy are often cited as justification for declining the vaccine during pregnancy. The inclusion of pregnant people in vaccine research is of crucial importance to help to increase knowledge for this population.
Guidelines
WHO states that pregnant women can receive covid-19 vaccines and, if not already vaccinated, should have access to WHO Emergency Use Listing approved vaccines, as the benefits of vaccination during pregnancy outweigh potential risks.119 WHO recommends the Johnson & Johnson-Janssen, Moderna, Novavax, Oxford-AstraZeneca, and Pfizer-BioNTech vaccines and permits Sinopharm, BIBP-CorV, Sinovac CoronaVac, and Bharat Biotech Covaxin. In the UK, the Royal College of Obstetricians and Gynaecologists (RCOG) and Joint Committee on Vaccination and Immunisation (JCVI) state that: “COVID-19 vaccines are strongly recommended in pregnancy.”120 The RCOG prefers that pregnant women be offered the Pfizer-BioNTech or Moderna mRNA vaccines, where available, given the greater amount of data on this vaccine type and that current data have not raised any safety concerns. However, women who have already had one dose of the Oxford-AstraZeneca vaccine are advised to complete vaccination with a second dose of the same vaccine.
In the US, the CDC, the ACOG, and the SMFM all recommend that people who are pregnant get vaccinated and stay up to date with their covid-19 vaccines, including getting a booster shot.20 21 121 Overall, the CDC states a preference for mRNA covid-19 vaccines over the Johnson & Johnson-Janssen vaccine for primary and booster vaccination; however, the latter vaccine may be considered in certain situations such as an allergic reaction, limited access to mRNA vaccines, or patient preference; this includes during pregnancy.
As of 12 March 2022, China’s position is that covid-19 vaccination during pregnancy is not recommended, and vaccination while lactating is recommended for some or all.19 In India, with approximately 25 million births annually, the currently available covid-19 vaccines are Covishield and Covaxin.19 At present, the recommendations from the Ministry of Health and Family Welfare, Government of India, state that pregnancy and lactation are contraindications to covid-19 vaccinations.122 However, the Federation of Obstetric and Gynaecological Societies of India’s position statement on covid-19 vaccination for pregnant and breastfeeding women states that protection from covid-19 vaccination should extend to pregnant and lactating women, given that the benefits seem to far outweigh any theoretical and remote risks of vaccination.123 The International Society of Infectious Diseases in Obstetrics and Gynaecology advises policy makers and societies to prioritize pregnant women to receive vaccination against SARS-CoV-2 and favors the mRNA vaccines until further safety information becomes available.124
Conclusions
Covid-19 vaccination is the safest and most effective way for people who are pregnant to protect themselves and their babies against severe covid-19 disease. Available data do not support an increased risk of adverse outcomes following covid-19 vaccination in pregnancy, so vaccination should be recommended as the benefits of vaccination during pregnancy seem to outweigh any potential risks. The immunogenicity of vaccinations in pregnancy seems to be similar to that in the non-pregnant population; however, the optimal timing of vaccination in pregnancy for neonatal/infant benefit remains uncertain. Additional information on non-mRNA vaccines, vaccination early in pregnancy, and longer term infant outcomes are also needed. Given that pregnant people are at increased risks for severe complications from covid-19, increasing the data and knowledge surrounding vaccination in this population is important to help to reduce vaccine hesitancy. Along with more data, directed personal vaccine counseling by obstetric providers to pregnant patients may also improve vaccination rates. To meet all these goals, pregnant people around the world must be prioritized in covid-19 vaccine research.
Research questions.
Should the covid-19 vaccine schedule in pregnancy be different from that in non-pregnant people? Does an optimal gestational timing of covid-19 vaccination in pregnancy exist?
What are the degree and extent of neonatal/infant protection with maternal covid-19 vaccination in pregnancy?
What are the most effective strategies to reduce vaccine hesitancy in pregnancy?
Do perinatal outcomes differ among the various covid-19 vaccines?
Patient involvement.
We provided an initial draft of the manuscript and outline of the key points of the paper to a patient who chose to be vaccinated in pregnancy and one who opted to wait until post partum. They both expressed concern about unknown fetal risks with receiving a novel vaccine in pregnancy. The patient who declined vaccination stated that she did not want to be responsible for choosing anything that might increase the risk for adverse perinatal outcomes for her baby and she would proceed with vaccination only once pregnancy specific data were available. The patient who got vaccinated and also boosted in pregnancy reported that a key factor in her decision was previous history with always receiving recommended vaccinations before covid-19 and trusting medicine at baseline. Both patients emphasized the importance of receiving direct vaccine counseling about their specific pregnancy from their obstetric healthcare provider. On the basis of these observations, we modified the manuscript to ensure a focus on perinatal outcomes and vaccine hesitancy and also to acknowledge the importance of counseling by the obstetric provider on covid-19 vaccination in pregnancy.
Acknowledgments
We thank Sharon L Leslie, nursing informationist, Woodruff Health Sciences Center Library, Emory University, Atlanta, GA, USA, for developing and conducting the literature search strategy.
Contributors: All authors defined the intellectual content, conducted the literature research, acquired data, and participated in preparing, editing, and critically reviewing the manuscript. DJJ is the guarantor.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare: MLB and CMD receive NIH grant funding for DMID 21-0004, an observational, prospective cohort study of the immunogenicity and safety of SARS-CoV-2 vaccines administered during pregnancy or post partum and evaluation of antibody transfer and durability in infants.
Provenance and peer review: Commissioned; externally peer reviewed.
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
References
- 1. Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed 2020;91:157-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins University Coronavirus Research Center. COVID-19 Dashboard. https://coronavirus.jhu.edu/map.html.
- 3.Elflein J. Population of women aged 15-49 in the U.S. and wordwide in the 2013 and 2025. 2019. https://www.statista.com/statistics/654630/female-population-aged-15-49-us-worldwide.
- 4.Curtin S, Abma J. 2010 Pregnancy Rates Among U.S. Women. 2015. https://www.cdc.gov/nchs/data/hestat/pregnancy/2010_pregnancy_rates.html.
- 5. Zambrano LD, Ellington S, Strid P, et al. CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team . Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1641-7. 10.15585/mmwr.mm6944e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Badr DA, Mattern J, Carlin A, et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case-control study with propensity score matching. Am J Obstet Gynecol 2020;223:764-8. 10.1016/j.ajog.2020.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allotey J, Stallings E, Bonet M, et al. for PregCOV-19 Living Systematic Review Consortium . Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The New York Times. Coronavirus Vaccine Tracker. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html.
- 9. Birol Ilter P, Prasad S, Berkkan M, et al. Clinical severity of SARS-CoV-2 infection among vaccinated and unvaccinated pregnancies during the Omicron wave. Ultrasound Obstet Gynecol 2022;59:560-2. 10.1002/uog.24893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ 2021;193:E540-8. 10.1503/cmaj.202604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Villar J, Ariff S, Gunier RB, et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr 2021;175:817-26. 10.1001/jamapediatrics.2021.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun 2020;11:3572. 10.1038/s41467-020-17436-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 Among Children in China. Pediatrics 2020;145:e20200702. 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 14. Gonçalves G, Cutts FT, Hills M, Rebelo-Andrade H, Trigo FA, Barros H. Transplacental transfer of measles and total IgG. Epidemiol Infect 1999;122:273-9. 10.1017/S0950268899002046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Munoz FM, Bond NH, Maccato M, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA 2014;311:1760-9. 10.1001/jama.2014.3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Albrecht M, Arck PC. Vertically Transferred Immunity in Neonates: Mothers, Mechanisms and Mediators. Front Immunol 2020;11:555. 10.3389/fimmu.2020.00555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tessier E, Campbell H, Ribeiro S, et al. Impact of Extending the Timing of Maternal Pertussis Vaccination on Hospitalized Infant Pertussis in England, 2014-2018. Clin Infect Dis 2021;73:e2502-8. 10.1093/cid/ciaa836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Swamy GK, Heine RP. Vaccinations for pregnant women. Obstet Gynecol 2015;125:212-26. 10.1097/AOG.0000000000000581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berman Institute of Bioethics & Center for Immunization Research, Johns Hopkins University. Covid-19 Maternal Immunization Tracker (COMIT). www.comitglobal.org.
- 20.American College of Obstetricians and Gynecologists. COVID-19 Vaccination Considerations for Obstetric–Gynecologic Care. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19.
- 21.Society for Maternal-Fetal Medicine. SMFM Statement: SARS-CoV-2 Vaccination in Pregnancy. 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2591/SMFM_Vaccine_Statement_12-1-20_(final).pdf.
- 22.Centers for Disease Control and Prevention. Vaccine Safety Datalink. https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html.
- 23. Polack FP, Thomas SJ, Kitchin N, et al. C4591001 Clinical Trial Group . Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020;383:2603-15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bowman CJ, Bouressam M, Campion SN, et al. Lack of effects on female fertility and prenatal and postnatal offspring development in rats with BNT162b2, a mRNA-based COVID-19 vaccine. Reprod Toxicol 2021;103:28-35. 10.1016/j.reprotox.2021.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baden LR, El Sahly HM, Essink B, et al. COVE Study Group . Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 2021;384:403-16. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Southwestern Vaccine Science UT, Review Committee. Scientific Review of Moderna COVID-19 Vaccine (mRNA-1273). 2020. https://www.utsouthwestern.edu/covid-19/assets/moderna-review.pdf.
- 27. Sadoff J, Gray G, Vandebosch A, et al. ENSEMBLE Study Group . Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med 2021;384:2187-201. 10.1056/NEJMoa2101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Food and Drug Administration. Emergency Use Authorization (EUA) of the Janssen COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19). 2022. https://www.fda.gov/media/146304/download.
- 29. Voysey M, Clemens SAC, Madhi SA, et al. Oxford COVID Vaccine Trial Group . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99-111. 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stebbings R, Maguire S, Armour G, et al. Developmental and reproductive safety of AZD1222 (ChAdOx1 nCoV-19) in mice. Reprod Toxicol 2021;104:134-42. 10.1016/j.reprotox.2021.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol 2011;205:10-8. 10.1016/j.ajog.2010.12.033 [DOI] [PubMed] [Google Scholar]
- 32. Freitas DA, Souza-Santos R, Carvalho LMA, et al. Congenital Zika syndrome: A systematic review. PLoS One 2020;15:e0242367. 10.1371/journal.pone.0242367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lamont RF, Sobel JD, Carrington D, et al. Varicella-zoster virus (chickenpox) infection in pregnancy. BJOG 2011;118:1155-62. 10.1111/j.1471-0528.2011.02983.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yazigi A, De Pecoulas AE, Vauloup-Fellous C, Grangeot-Keros L, Ayoubi JM, Picone O. Fetal and neonatal abnormalities due to congenital rubella syndrome: a review of literature. J Matern Fetal Neonatal Med 2017;30:274-8. 10.3109/14767058.2016.1169526 [DOI] [PubMed] [Google Scholar]
- 35. Atyeo C, DeRiso EA, Davis C, et al. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci Transl Med 2021;13:eabi8631. 10.1126/scitranslmed.abi8631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beharier O, Plitman Mayo R, Raz T, et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest 2021;131:e154834. 10.1172/JCI154834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ben-Mayor Bashi T, Amikam U, Ashwal E, et al. The association of maternal SARS-CoV-2 vaccination-to-delivery interval and the levels of maternal and cord blood antibodies. Int J Gynaecol Obstet 2022;156:436-43. 10.1002/ijgo.14014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bookstein Peretz S, Regev N, Novick L, et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol 2021;58:450-6. 10.1002/uog.23729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Citu IM, Citu C, Gorun F, et al. Immunogenicity Following Administration of BNT162b2 and Ad26.COV2.S COVID-19 Vaccines in the Pregnant Population during the Third Trimester. Viruses 2022;14:307. 10.3390/v14020307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA 2021;325:2370-80. 10.1001/jama.2021.7563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gloeckner S, Hornung F, Heimann Y, et al. Newborns’ passive humoral SARS-CoV-2 immunity following heterologous vaccination of the mother during pregnancy. Am J Obstet Gynecol 2022;226:261-2. 10.1016/j.ajog.2021.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol 2021;225:303.e1-17. 10.1016/j.ajog.2021.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kashani-Ligumsky L, Lopian M, Cohen R, et al. Titers of SARS CoV-2 antibodies in cord blood of neonates whose mothers contracted SARS CoV-2 (COVID-19) during pregnancy and in those whose mothers were vaccinated with mRNA to SARS CoV-2 during pregnancy. J Perinatol 2021;41:2621-4. 10.1038/s41372-021-01216-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kugelman N, Nahshon C, Shaked-Mishan P, et al. Maternal and Neonatal SARS-CoV-2 Immunoglobulin G Antibody Levels at Delivery After Receipt of the BNT162b2 Messenger RNA COVID-19 Vaccine During the Second Trimester of Pregnancy. JAMA Pediatr 2022;176:290-5. 10.1001/jamapediatrics.2021.5683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mithal LB, Otero S, Shanes ED, Goldstein JA, Miller ES. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am J Obstet Gynecol 2021;225:192-4. 10.1016/j.ajog.2021.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nir O, Schwartz A, Toussia-Cohen S, et al. Maternal-neonatal transfer of SARS-CoV-2 immunoglobulin G antibodies among parturient women treated with BNT162b2 messenger RNA vaccine during pregnancy. Am J Obstet Gynecol MFM 2022;4:100492. 10.1016/j.ajogmf.2021.100492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Prabhu M, Murphy EA, Sukhu AC, et al. Antibody Response to Coronavirus Disease 2019 (COVID-19) Messenger RNA Vaccination in Pregnant Women and Transplacental Passage Into Cord Blood. Obstet Gynecol 2021;138:278-80. 10.1097/AOG.0000000000004438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rottenstreich A, Zarbiv G, Oiknine-Djian E, Zigron R, Wolf DG, Porat S. Efficient Maternofetal Transplacental Transfer of Anti- Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Antibodies After Antenatal SARS-CoV-2 BNT162b2 Messenger RNA Vaccination. Clin Infect Dis 2021;73:1909-12. 10.1093/cid/ciab266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. The effect of gestational age at BNT162b2 mRNA vaccination on maternal and neonatal SARS-CoV-2 antibody levels. Clin Infect Dis 2022;ciac135. 10.1093/cid/ciac135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rottenstreich M, Sela HY, Rotem R, Kadish E, Wiener-Well Y, Grisaru-Granovsky S. Covid-19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG 2022;129:248-55. 10.1111/1471-0528.16941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shanes ED, Otero S, Mithal LB, Mupanomunda CA, Miller ES, Goldstein JA. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccination in Pregnancy: Measures of Immunity and Placental Histopathology. Obstet Gynecol 2021;138:281-3. 10.1097/AOG.0000000000004457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shen CJ, Fu YC, Lin YP, et al. Evaluation of Transplacental Antibody Transfer in SARS-CoV-2-Immunized Pregnant Women. Vaccines (Basel) 2022;10:101. 10.3390/vaccines10010101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Trostle ME, Aguero-Rosenfeld ME, Roman AS, Lighter JL. High antibody levels in cord blood from pregnant women vaccinated against COVID-19. Am J Obstet Gynecol MFM 2021;3:100481. 10.1016/j.ajogmf.2021.100481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang YJ, Murphy EA, Singh S, et al. Association of Gestational Age at Coronavirus Disease 2019 (COVID-19) Vaccination, History of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection, and a Vaccine Booster Dose With Maternal and Umbilical Cord Antibody Levels at Delivery. Obstet Gynecol 2022;139:373-80. 10.1097/AOG.0000000000004693 [DOI] [PubMed] [Google Scholar]
- 55. Kachikis A, Englund JA, Singleton M, Covelli I, Drake AL, Eckert LO. Short-term Reactions Among Pregnant and Lactating Individuals in the First Wave of the COVID-19 Vaccine Rollout. JAMA Netw Open 2021;4:e2121310. 10.1001/jamanetworkopen.2021.21310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maertens K, Orije MRP, Van Damme P, Leuridan E. Vaccination during pregnancy: current and possible future recommendations. Eur J Pediatr 2020;179:235-42. 10.1007/s00431-019-03563-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Blakeway H, Prasad S, Kalafat E, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol 2022;226:236.e1-14. 10.1016/j.ajog.2021.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bleicher I, Kadour-Peero E, Sagi-Dain L, Sagi S. Early exploration of COVID-19 vaccination safety and effectiveness during pregnancy: interim descriptive data from a prospective observational study. Vaccine 2021;39:6535-8. 10.1016/j.vaccine.2021.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Butt AA, Chemaitelly H, Al Khal A, et al. SARS-CoV-2 vaccine effectiveness in preventing confirmed infection in pregnant women. J Clin Invest 2021;131:e153662. 10.1172/JCI153662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dagan N, Barda N, Biron-Shental T, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med 2021;27:1693-5. 10.1038/s41591-021-01490-8 [DOI] [PubMed] [Google Scholar]
- 61. Goldshtein I, Steinberg DM, Kuint J, et al. Association of BNT162b2 COVID-19 Vaccination During Pregnancy With Neonatal and Early Infant Outcomes. JAMA Pediatr 2022;176:470-7. 10.1001/jamapediatrics.2022.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morgan JA, Biggio JRJ, Jr, Martin JK, et al. Maternal Outcomes After Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Vaccinated Compared With Unvaccinated Pregnant Patients. Obstet Gynecol 2022;139:107-9. 10.1097/AOG.0000000000004621 [DOI] [PubMed] [Google Scholar]
- 63. Stock SJ, Carruthers J, Calvert C, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med 2022;28:504-12. 10.1038/s41591-021-01666-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM 2021;3:100467. 10.1016/j.ajogmf.2021.100467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Halasa NB, Olson SM, Staat MA, et al. Overcoming COVID-19 Investigators. Overcoming COVID-19 Network . Effectiveness of Maternal Vaccination with mRNA COVID-19 Vaccine During Pregnancy Against COVID-19-Associated Hospitalization in Infants Aged <6 Months - 17 States, July 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022;71:264-70. 10.15585/mmwr.mm7107e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shook LL, Atyeo CG, Yonker LM, et al. Durability of Anti-Spike Antibodies in Infants After Maternal COVID-19 Vaccination or Natural Infection. JAMA 2022;327:1087-9. 10.1001/jama.2022.1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kharbanda EO, Haapala J, DeSilva M, et al. Spontaneous Abortion Following COVID-19 Vaccination During Pregnancy. JAMA 2021;326:1629-31. 10.1001/jama.2021.15494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zauche LH, Wallace B, Smoots AN, et al. CDC v-safe Covid-19 Pregnancy Registry Team . Receipt of mRNA Covid-19 Vaccines and Risk of Spontaneous Abortion. N Engl J Med 2021;385:1533-5. 10.1056/NEJMc2113891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, Håberg SE. Covid-19 Vaccination during Pregnancy and First-Trimester Miscarriage. N Engl J Med 2021;385:2008-10. 10.1056/NEJMc2114466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Trostle ME, Limaye MA, Avtushka V, Lighter JL, Penfield CA, Roman AS. COVID-19 vaccination in pregnancy: early experience from a single institution. Am J Obstet Gynecol MFM 2021;3:100464. 10.1016/j.ajogmf.2021.100464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shimabukuro TT, Kim SY, Myers TR, et al. CDC v-safe COVID-19 Pregnancy Registry Team . Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. N Engl J Med 2021;384:2273-82. 10.1056/NEJMoa2104983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dick A, Rosenbloom JI, Gutman-Ido E, Lessans N, Cahen-Peretz A, Chill HH. Safety of SARS-CoV-2 vaccination during pregnancy- obstetric outcomes from a large cohort study. BMC Pregnancy Childbirth 2022;22:166. 10.1186/s12884-022-04505-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lipkind HS, Vazquez-Benitez G, DeSilva M, et al. Receipt of COVID-19 Vaccine During Pregnancy and Preterm or Small-for-Gestational-Age at Birth - Eight Integrated Health Care Organizations, United States, December 15, 2020-July 22, 2021. MMWR Morb Mortal Wkly Rep 2022;71:26-30. 10.15585/mmwr.mm7101e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Magnus MC, Örtqvist AK, Dahlqwist E, et al. Association of SARS-CoV-2 Vaccination During Pregnancy With Pregnancy Outcomes. JAMA 2022;327:1469-77. 10.1001/jama.2022.3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aslam J, Masroor M, Mehmood QUA, Arshad M, Jabeen S, Mushtaq MA. Maternal Mortality with <em>SARS-COV-2</em> during its 4th Wave in Pakistan: The Vaccine Paradox and Pregnancy. J Coll Physicians Surg Pak 2022;32:119-21. 10.29271/jcpsp.2022.01.119 [DOI] [PubMed] [Google Scholar]
- 76. Wainstock T, Yoles I, Sergienko R, Sheiner E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine 2021;39:6037-40. 10.1016/j.vaccine.2021.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ruderman RS, Mormol J, Trawick E, et al. Association of COVID-19 Vaccination During Early Pregnancy With Risk of Congenital Fetal Anomalies. JAMA Pediatr 2022;176:717-9. 10.1001/jamapediatrics.2022.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fell DB, Dhinsa T, Alton GD, et al. Association of COVID-19 Vaccination in Pregnancy With Adverse Peripartum Outcomes. JAMA 2022;327:1478-87. 10.1001/jama.2022.4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kharbanda EO, Vazquez-Benitez G. COVID-19 mRNA Vaccines During Pregnancy: New Evidence to Help Address Vaccine Hesitancy. JAMA 2022;327:1451-3. 10.1001/jama.2022.2459 [DOI] [PubMed] [Google Scholar]
- 80. Prasad S, Kalafat E, Blakeway H, et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun 2022;13:2414. 10.1038/s41467-022-30052-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kadali RAK, Janagama R, Peruru SR, et al. Adverse effects of COVID-19 messenger RNA vaccines among pregnant women: a cross-sectional study on healthcare workers with detailed self-reported symptoms. Am J Obstet Gynecol 2021;225:458-60. 10.1016/j.ajog.2021.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nakahara A, Biggio JR, Elmayan A, Williams FB. Safety-Related Outcomes of Novel mRNA COVID-19 Vaccines in Pregnancy. Am J Perinatol 2022. 10.1055/a-1745-1168 [DOI] [PubMed] [Google Scholar]
- 83. Zdanowski W, Markiewicz A, Zdanowska N, Lipińska J, Waśniewski T. Tolerability of the BNT162b2 COVID-19 Vaccine during Pregnancy among Polish Healthcare Professionals. Vaccines (Basel) 2022;10:200. 10.3390/vaccines10020200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Anderson E, Brigden A, Davies A, Shepherd E, Ingram J. Maternal vaccines during the Covid-19 pandemic:A qualitative interview study with UK pregnant women. Midwifery 2021;100:103062. 10.1016/j.midw.2021.103062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sznajder KK, Kjerulff KH, Wang M, Hwang W, Ramirez SI, Gandhi CK. Covid-19 vaccine acceptance and associated factors among pregnant women in Pennsylvania 2020. Prev Med Rep 2022;26:101713. 10.1016/j.pmedr.2022.101713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Battarbee AN, Stockwell MS, Varner M, et al. Attitudes Toward COVID-19 Illness and COVID-19 Vaccination among Pregnant Women: A Cross-Sectional Multicenter Study during August-December 2020. Am J Perinatol 2022;39:75-83. 10.1055/s-0041-1735878 [DOI] [PubMed] [Google Scholar]
- 87. Kiefer MK, Mehl R, Costantine MM, et al. Characteristics and perceptions associated with COVID-19 vaccination hesitancy among pregnant and postpartum individuals: A cross-sectional study. BJOG 2022;129:1342-51. 10.1111/1471-0528.17110 [DOI] [PubMed] [Google Scholar]
- 88. Wang T, Krishnamurti T, Bernard M, Lopa S, Quinn B, Simhan H. Perceptions and knowledge of COVID-19 vaccine safety and efficacy among vaccinated and non-vaccinated obstetric healthcare workers. Behav Med 2022;1-13. 10.1080/08964289.2021.2023456 [DOI] [PubMed] [Google Scholar]
- 89. Huddleston HG, Jaswa EG, Lindquist KJ, et al. COVID-19 vaccination patterns and attitudes among American pregnant individuals. Am J Obstet Gynecol MFM 2022;4:100507. 10.1016/j.ajogmf.2021.100507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Levy AT, Singh S, Riley LE, Prabhu M. Acceptance of COVID-19 vaccination in pregnancy: a survey study. Am J Obstet Gynecol MFM 2021;3:100399. 10.1016/j.ajogmf.2021.100399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Razzaghi H, Meghani M, Pingali C, et al. COVID-19 Vaccination Coverage Among Pregnant Women During Pregnancy - Eight Integrated Health Care Organizations, United States, December 14, 2020-May 8, 2021. MMWR Morb Mortal Wkly Rep 2021;70:895-9. 10.15585/mmwr.mm7024e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sutton D, D’Alton M, Zhang Y, et al. COVID-19 vaccine acceptance among pregnant, breastfeeding, and nonpregnant reproductive-aged women. Am J Obstet Gynecol MFM 2021;3:100403. 10.1016/j.ajogmf.2021.100403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Saleh OA, Halperin O. Influenza virus vaccine compliance among pregnant women during the COVID-19 pandemic (pre-vaccine era) in Israel and future intention to uptake BNT162b2 mRNA COVID-19 vaccine. Vaccine 2022;40:2099-106. 10.1016/j.vaccine.2022.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Taubman-Ben-Ari O, Weiss E, Abu-Sharkia S, Khalaf E. A comparison of COVID-19 vaccination status among pregnant Israeli Jewish and Arab women and psychological distress among the Arab women. Nurs Health Sci 2022;24:360-7. 10.1111/nhs.12929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mose A, Yeshaneh A. COVID-19 Vaccine Acceptance and Its Associated Factors Among Pregnant Women Attending Antenatal Care Clinic in Southwest Ethiopia: Institutional-Based Cross-Sectional Study. Int J Gen Med 2021;14:2385-95. 10.2147/IJGM.S314346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hailemariam S, Mekonnen B, Shifera N, et al. Predictors of pregnant women’s intention to vaccinate against coronavirus disease 2019: A facility-based cross-sectional study in southwest Ethiopia. SAGE Open Med 2021;9:20503121211038454. 10.1177/20503121211038454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Taye EB, Taye ZW, Muche HA, Tsega NT, Haile TT, Tiguh AE. COVID-19 vaccine acceptance and associated factors among women attending antenatal and postnatal cares in Central Gondar Zone public hospitals, Northwest Ethiopia. Clin Epidemiol Glob Health 2022;14:100993. 10.1016/j.cegh.2022.100993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Egloff C, Couffignal C, Cordier AG, et al. Pregnant women’s perceptions of the COVID-19 vaccine: A French survey. PLoS One 2022;17:e0263512. 10.1371/journal.pone.0263512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Deruelle P, Couffignal C, Sibiude J, et al. Prenatal care providers’ perceptions of the SARS-Cov-2 vaccine for themselves and for pregnant women. PLoS One 2021;16:e0256080. 10.1371/journal.pone.0256080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Carbone L, Mappa I, Sirico A, et al. Pregnant women’s perspectives on severe acute respiratory syndrome coronavirus 2 vaccine. Am J Obstet Gynecol MFM 2021;3:100352. 10.1016/j.ajogmf.2021.100352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mappa I, Luviso M, Distefano FA, Carbone L, Maruotti GM, Rizzo G. Women perception of SARS-CoV-2 vaccination during pregnancy and subsequent maternal anxiety: a prospective observational study. J Matern Fetal Neonatal Med 2021;1-4. 10.1080/14767058.2021.1910672 [DOI] [PubMed] [Google Scholar]
- 102. Skjefte M, Ngirbabul M, Akeju O, et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol 2021;36:197-211. 10.1007/s10654-021-00728-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bradfield Z, Wynter K, Hauck Y, et al. COVID-19 vaccination perceptions and intentions of maternity care consumers and providers in Australia. PLoS One 2021;16:e0260049. 10.1371/journal.pone.0260049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tao L, Wang R, Han N, et al. Acceptance of a COVID-19 vaccine and associated factors among pregnant women in China: a multi-center cross-sectional study based on health belief model. Hum Vaccin Immunother 2021;17:2378-88. 10.1080/21645515.2021.1892432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Riad A, Jouzová A, Üstün B, et al. COVID-19 Vaccine Acceptance of Pregnant and Lactating Women (PLW) in Czechia: An Analytical Cross-Sectional Study. Int J Environ Res Public Health 2021;18:13373. 10.3390/ijerph182413373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Schaal NK, Zöllkau J, Hepp P, Fehm T, Hagenbeck C. Pregnant and breastfeeding women’s attitudes and fears regarding the COVID-19 vaccination. Arch Gynecol Obstet 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Daskalakis G, Pergialiotis V, Antsaklis P, Theodora M, Papageorgiou D, Rodolakis A. Healthcare workers’ attitudes about vaccination of pregnant women and those wishing to become pregnant. J Perinat Med 2021;50:363-6. 10.1515/jpm-2021-0536 [DOI] [PubMed] [Google Scholar]
- 108. Geoghegan S, Stephens LC, Feemster KA, Drew RJ, Eogan M, Butler KM. “This choice does not just affect me.” Attitudes of pregnant women toward COVID-19 vaccines: a mixed-methods study. Hum Vaccin Immunother 2021;17:3371-6. 10.1080/21645515.2021.1924018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hosokawa Y, Okawa S, Hori A, et al. The Prevalence of COVID-19 Vaccination and Vaccine Hesitancy in Pregnant Women: An Internet-based Cross-sectional Study in Japan. J Epidemiol 2022;32:188-94. 10.2188/jea.JE20210458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Januszek S, Siwiec N, Januszek R, et al. Approach of Pregnant Women from Poland and the Ukraine to COVID-19 Vaccination-The Role of Medical Consultation. Vaccines (Basel) 2022;10:255. 10.3390/vaccines10020255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mohan S, Reagu S, Lindow S, Alabdulla M. COVID-19 vaccine hesitancy in perinatal women: a cross sectional survey. J Perinat Med 2021;49:678-85. 10.1515/jpm-2021-0069 [DOI] [PubMed] [Google Scholar]
- 112. Citu IM, Citu C, Gorun F, et al. Determinants of COVID-19 Vaccination Hesitancy among Romanian Pregnant Women. Vaccines (Basel) 2022;10:275. 10.3390/vaccines10020275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Samannodi M. COVID-19 Vaccine Acceptability Among Women Who are Pregnant or Planning for Pregnancy in Saudi Arabia: A Cross-Sectional Study. Patient Prefer Adherence 2021;15:2609-18. 10.2147/PPA.S338932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Stuckelberger S, Favre G, Ceulemans M, et al. SARS-CoV-2 Vaccine Willingness among Pregnant and Breastfeeding Women during the First Pandemic Wave: A Cross-Sectional Study in Switzerland. Viruses 2021;13:1199. 10.3390/v13071199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hirshberg JS, Huysman BC, Oakes MC, et al. Offering onsite COVID-19 vaccination to high-risk obstetrical patients: initial findings. Am J Obstet Gynecol MFM 2021;3:100478. 10.1016/j.ajogmf.2021.100478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Perez MJ, Paul R, Hirshberg JS, et al. Administration of the Coronavirus Disease 2019 (COVID-19) Vaccine to Hospitalized Postpartum Patients. Obstet Gynecol 2021;138:885-7. 10.1097/AOG.0000000000004590 [DOI] [PubMed] [Google Scholar]
- 117. Perez MJ, Paul R, Raghuraman N, et al. Characterizing initial COVID-19 vaccine attitudes among pregnancy-capable healthcare workers. Am J Obstet Gynecol MFM 2022;4:100557. 10.1016/j.ajogmf.2021.100557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rasmussen SA, Jamieson DJ. Influenza and Pregnancy: No Time for Complacency. Obstet Gynecol 2019;133:23-6. 10.1097/AOG.0000000000003040 [DOI] [PubMed] [Google Scholar]
- 119.World Health Organization. Questions and Answers: COVID-19 vaccines and pregnancy. https://www.who.int/publications/i/item/WHO-2019-nCoV-FAQ-Pregnancy-Vaccines-2022.1.
- 120.Royal College of Obstetricians & Gynaecologists. COVID-19 vaccines, pregnancy and breastfeeding FAQs. https://www.rcog.org.uk/guidance/coronavirus-covid-19-pregnancy-and-women-s-health/vaccination/covid-19-vaccines-pregnancy-and-breastfeeding-faqs/.
- 121.Centers for Disease Control. COVID-19 vaccines while pregnant or breastfeeding. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html.
- 122.Ministry of Health and Family Welfare, Government of India. Frequently Asked Questions, Vaccine. https://www.mohfw.gov.in/covid_vaccination/vaccination/faqs.html.
- 123.Federation of Obstetric and Gynecological Societies of India. FOGSI Position Statement: COVID Vaccination for Pregnant and Breastfeeding Women. https://www.fogsi.org/wp-content/uploads/covid19/fogsi-statement-on-covid-vaccination-in-pregnancy-and-bf.pdf.
- 124. Donders GGG, Grinceviciene S, Haldre K, et al. On The Behalf Of The Covid-Isidog Guideline Group . ISIDOG Consensus Guidelines on COVID-19 Vaccination for Women before, during and after Pregnancy. J Clin Med 2021;10:2902. 10.3390/jcm10132902 [DOI] [PMC free article] [PubMed] [Google Scholar]