Abstract
Background:
Growth of Mycoplasma in genital tract can cause problems such as infertility, pelvic inflammatory disease (PID), and preterm labor. This study was designed to evaluate the role of these bacteria in preterm labor among individuals in Gorgan city which is located in north of Iran.
Methods:
The study included 100 women with complaints of pain in preterm labor before 37 weeks of pregnancy (case group) and 100 women with term labor (control group) who were referred to Shahid Sayyad Shirazi Teaching Hospital in Gorgan city, north of Iran. Vaginal swabs, collected from all of these women, were evaluated for genital Mycoplasma sp. by molecular method using specific primers with polymerization chain reaction (PCR). The comparison of results was done by conducting X 2 and p<0.05 was considered significant.
Results:
Genital Mycoplasma was detected in 78 cases (39%) of 200 vaginal samples. Genital Mycoplasma colonization rates in the preterm and term samples were 60% and 18%, respectively, with relative risk of 2.05 (1.78–2.37) (p=0.001). The proportion of Ureaplasma parvum (44% and 15%), Ureaplasma urealyticum (11%, 3%), and Mycoplasma homins (5%, 0%) was significantly higher in women with preterm birth (PTB) than term labor. No cases of Mycoplasma genitalum were detected in this study.
Conclusion:
There is a significant relationship between presence of genital Mycoplasma in vaginal secretion and the risk of preterm labor.
Keywords: Infertility, Mycoplasma, Preterm labor, Ureaplasma, Vaginal secretion
Introduction
Preterm birth (PTB) is the live birth before 37 completed weeks of gestation since the first day of last menstrual period (LMP) of the woman (1). Approximately, 15 million babies are born preterm annually and this rate is increasing. Preterm birth rates vary from 9% in higher-income countries compared with 12% in lower-income countries (2, 3). In Iran, the overall prevalence of preterm delivery based on the random effects model is estimated to be 10% (95% CI, 9–12). The prevalence of preterm labor varies from 5.4% in Bam to 19.85% in Tehran (4). Preterm newborns still die because of inadequate newborn management in low-income and middle-income countries (5).
Preterm birth is leading to infant’s mortality and morbidity. The main cause of preterm labor is unknown but scientists believe that it is a multi-factorial and complex problem (6). Microbial infection of the placenta, amniotic fluid, vaginal canal, and oral cavity is known to significantly contribute to preterm birth (7). Witt et al. detected Ureaplasma spp. and Mycoplasma spp. in cases of suspected chorioamnionitis using analysis of the maternal and umbilical cord (8). Jang et al. showed that positive culture rate of Ureaplasma in pregnant women admitted with preterm labor was 26.5%; however, they couldn’t detect any cases of Mycoplasma hominis (9).
The meta-analysis of Moridi et al. revealed that the prevalence of Ureaplasma urealyticum was 17.53% in Iran and the prevalence of Mycoplasma genitalium and Mycoplasma hominis was 11.33 and 9.68%, respectively. The rate of Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma urealyticum infection in women with symptoms of genitourinary tract infection was higher than men with genitourinary tract infection (6.46% vs. 5.4, 7.67% vs. 5.88 and 21.04% vs. 12.13%, respectively). As expected, the prevalence of M. genitalium, U. urealyticum, and M. hominis among infertile women (12.73, 19.58 and 10.81%) was higher than fertile women (3%, 10. 85%, and 4. 35%). Similarly, the prevalence of M. hominis and U. urealyticum among infertile men (14 and 21.18%) was higher than fertile men (4 and 3%). Based on this analysis, the rate of U. urealyticum was higher than M. genitalium and M. hominis among infertile men and women compared to the fertile group. The prevalence rate of M. genitalium, M. hominis, and U. urealyticum in central provinces is higher than other parts of Iran (10).
Genital mycoplasma usually gets into uterus and amniotic sac through the vagina-cervix and can cause intrauterine infection especially in the second trimester of pregnancy. Intrauterine infection in 40% of cases would cause preterm labor, which is probably associated with host immune response. Bacterial surface antigens, such as PAMP, can be detected by toll like receptors (TLRs) available in the surface of innate immune system cells and they stimulate immune cells such as macrophages or monocytes. This reaction increases the level of inflammatory cytokines and chemokines such as IL1, IL6, IL8, and TNFα; these factors lead to the production of metalloproteinase and prostaglandins which eventually cause pre-term labor (1, 11, 12). In order to determine the role of genital Mycoplasma in preterm labor, the frequency of four species of genital Mycoplasmas in pregnant women with preterm and term labor was examined and compared in this study.
Methods
Study design and population: The purpose of this retrospective case-control study was to compare female genital tract Mycoplasma colonization in term and preterm births. Sampling was conducted from March 2014 to September 2015 and 200 healthy pregnant women with labor pain who attended referral hospital of Shahid Sayad Shirazi in Gorgan, Iran, participated in this study. Among them, 100 cases underwent term delivery (control group) and 100 underwent preterm delivery (case group). Regarding inclusion criteria, every pregnant woman referring to the hospital with labor pain was included. Exclusion criteria were medical complications such as cancer, kidney, lung, heart and liver diseases, obstetrics problems such as multiple gestations, polyhydramnios, fetal macrosomia myometrium, physical or sexual abuse during the pregnancy, sexual intercourse, using vaginal cream or douching during the last two days, use of tobacco or cigarette and also death or neonatal sepsis in previous pregnancies. The samples were excluded from the study if they did not plan to continue participation. The sample size was determined according to the comparison of two ratios using results of the study of Shahshahan and Hoseini (13). The total number of 200 cases (at least 100 samples per group) were indicated with confidence interval of 95%, a power of 80%, and attrition rate of 10%.
Sample collection: Sampling was conducted through Dacron swab from the lower one-third of vagina, without using moisturizer or disinfectant. Vaginal samples were immediately placed into a collection tube containing physiological serum and transported to the microbiology laboratory.
DNA extraction and PCR: DNA extraction was carried out using Phenol-Chloroform method (14). Extracted nucleic acid was preserved at −20°C until further procedures. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were used for quality control (15).
PCR: Specific primers (Metabion, Germany) which were used for the detection of Mycoplasma or Ureaplasma and genital species including Mycoplasma hominis, Mycoplasma genitalium, Urea-plasma urealyticum, and Ureaplasma parvum are described in table 1.
Table 1.
The sequence of oligonucleotides used for detecting genital Mycoplasma
| Bacteria | Target gene | The sequence of oligonucleotides | Ampliqon size (bp) | Reference |
|---|---|---|---|---|
| Mycoplasma and Ureaplasma genus | 16S rRNA | My-in-f : 5’- GTAATACATAGGTCGCAAGCGTTATC-3’ | (14, 25) | |
| MGSO-2- r: 5’- CACCATCTGTCACTCTGTTAACCTC-3’ | 516 | |||
| UGSO- r: 5’- CACCACCTGTCATATTGTTAACCTC-3’ | 492 | |||
| M. hominis | 16S rRNA | RNAH1-f: 5’- CAATGGCTAATGCCGGATACGC-3’ | (25) | |
| RNAH2-r: 5’- GGTACCGTCAGTCTGCAAT-3’ | 334 | |||
| M. genitalium | 140 kDa adhesion protein | MgPa1-f: 5’- AGTTGATGAAACCTTAACCCCTTGG-3’ | 282 | (25) |
| MgPa3-r: 5’- CCGTTGAGGGGTTTTCCATTTTTGC-3’ | ||||
| U. urealyticum | MB antigen | UMS-125-f: 5’ GTATTTGCAATCTTTATATGTTTTCG-3’ | (18, 25) | |
| UMA226-r : 5’- CAGCTGATGTAAGTGCAGCATTAAATTC-3’ | 448 | |||
| U. parvum | MB antigen | UMS-125-f: 5’- GTATTTGCAATCTTTATATGTTTTCG-3’ | 403 | (18, 25) |
| UMA226-r: 5’-CAGCTGATGTAAGTGCAGCATTAAATTC-3’ |
The PCR protocols were arranged in two sets. First, PCR was carried out to evaluate the Mycoplasma or Ureaplasma in vaginal sample; the procedure was done in a total volume of 50 μl including 1x buffer, magnesium chloride 1.75 mM, 10 pmol of each primer, 1.25 units of Taq polymerase, deoxynucleotide triphosphate (dNTP) 0.2 mM (GeNet Bio, Korea), and 5 μl DNA template. Thermocycler (Eppendorf, Germany) temperature program to identify genus included 45 cycles at 95°C for 30 s, at 55°C for 30 s, at 72°C for a minute, and the final cycle was at 72°C for 7 min.
For identification of species, in another set of PCR, the multiplex PCR was carried out in a final volume of 50 μl, including 1x buffer, magnesium chloride 3 mM, deoxynucleotide triphosphate (dNTP) 0.2 mM, 1.25 units of single polymerase, 10 pmol of each primer, and finally 5 μl of DNA were extracted. Thermocycler temperature program included 30 cycles at 95°C for 45 s, at 58°C for a min, at 72°C again for a min and the final cycle was at 72°C for 5 min. The PCR product was assessed by electrophoresis on 1.5% agarose gel (Figure 1). Two samples of different PCR products were randomly selected and sent for DNA sequencing (Macrogene Inc., South Korea). Sterilized distilled water without nuclease and Mycoplasma gallisepticum species (Razi Vaccine and Serum Research Institute, Iran) were used as negative and positive control in PCR procedures, respectively.
Figure 1.
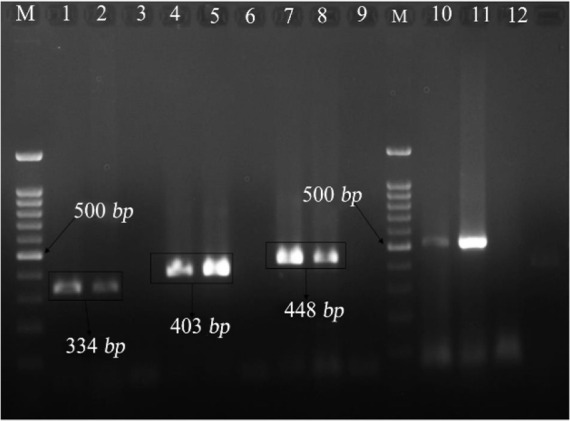
PCR products, electrophoresis gel of vaginal Mycoplasma (M= Marker, 100 bp, lines: 1,2 samples containing Mycoplasma hominis, lines 3, 6, 9, 12: negative control of sterilized distilled water, lines 4, 5: samples containing Ureaplasmaparvum, lines 7, 8: samples containing Ureaplasma urealyticum, and lines 10, 11: samples containing positive Mycoplasma genus
Evaluation of primers’ specificity: In order to evaluate primers’ specificity, a test was conducted on DNA extract of some organisms found in the vaginal ecosystem with the same protocol mentioned above including Trichomonas vaginalis, Herpes Simplex virus, Candida albicans, Staphylococcus aureus, Group B Streptococcus, and E. coli. None of them was amplified and no band was seen on electrophoresis of PCR product.
Data analysis: Data were entered and recorded in SPSS v16 (IMB, USA) and analyzed by using frequency distribution, and X2 test was used to compare the presence of Mycoplasma in both groups. In all cases, p<0.05 was considered statistically significant.
Results
Two-hundred women, 100 cases with term labor and 100 with preterm labor, were evaluated in this study. The age range was 16–35 years old and the mean age was 27±5/7 years. Twenty-two women in each group had the abortion experience (Table 2). Just one woman in the term labor group and no one in the preterm labor group had previous pre-term labor experience. Vaginal bleeding was reported in 20 cases that mostly (15 cases) were in the preterm labor group (p<0.025).
Table 2.
The frequency of clinical signs and symptoms in women with term and preterm birth
| Maternal factors | Labor type | ||
|---|---|---|---|
|
| |||
| Term | Preterm | p-value | |
| Abortion experience | 22 | 22 | 1 |
| Unwanted pregnancy | 11 | 9 | 0.65 |
| Preterm labor experience | 1 | 0 | 0.56 |
| Vaginal bleeding | 5 | 15 | 0.025 * |
| Cerclage | 0 | 4 | 0.102 |
| Fever | 0 | 1 | 0.56 |
| Tachycardia | 1 | 2 | 0.56 |
| Vaginal discharge | 4 | 8 | 0.248 |
The difference is statistically significant
Seventy-eight women (39%) were infected with genital Mycoplasmas including 18 cases (18%) in term labor and 60 samples (60%) in preterm labor groups and the difference was statistically significant (p=0.00001).
The frequency of Mycoplasma hominis, Urea-plasma urealyticum, and Ureaplasma parvum was 5 (2.5%), 14 (7%), and 59 (29.5%) cases, respectively (Table 3). There weren’t any cases of Mycoplasma genitalium in vaginal samples. The frequency of all species of genital Mycoplasmas in preterm labor group was higher than women with term labor (p<0.05).
Table 3.
The frequency of genital Mycoplasma in vaginal secretion of women with term and preterm birth
| Mycoplasma types (Number) | Labor | ||
|---|---|---|---|
|
| |||
| Term (100 samples) | Preterm (100 samples) | p-value | |
| Mycoplasma hominis (5*) | 0 | 5 (5%) | 0.059 |
| Ureaplasma urealyticum (14) | 3 (3%) | 11 (11%) | 0.033 |
| Ureaplasma parvum (69) | 15 (15%) | 44 (44%) | 0.0001 |
| Mycoplasma genitalium (0) | 0 | 0 | 1 |
| Total | 18 | 60 | 0.00001 |
Simultaneous infection with two or more different species was not seen. Mycoplasma hominis was detected only in preterm labor cases. The relative risk of preterm labor in women with Mycoplasma hominis, Ureaplasma urealyticum, and Ureaplasma parvum with 95% confidence level was 2.05 (1.78–2.37), 2.43 (0.884–6.69) and 2.37 (1.5–3.47), respectively.
As shown in table 4, there were no statistical differences between the clinical signs and symptoms of women who had genital Mycoplasma and others without this infection.
Table 4.
Maternal clinical signs and symptoms in pregnant women infected/non-infected with genital Mycoplasma
| Maternal clinical signs and symptoms (number) | Genital Mycoplasmas (number) | ||
|---|---|---|---|
|
| |||
| Detected (78 cases) | Absent (122 cases) | p-value | |
| Cerclage (4) | 3(3.8%) | 1(0.8%) | 0.31 |
| Fever (1) | 1(1.3%) | 0 | 0.56 |
| Multiple pregnancies (3) | 1(1.3%) | 2(2.4%) | 0.56 |
| Polyhydramnios (1) | 1(1.3%) | 0 | 0.56 |
| Vaginal secretion (12) | 6(7.75) | 6(4.9%) | 1 |
| Tachycardia (3) | 0 | 3(2.4%) | 0.18 |
| Myoma (1) | 0 | 1(0.8%) | 0.56 |
Discussion
PTB is a multifactorial disorder and its relationship to pyrogenic bacterial infection, bacterial vaginosis, and periodontal disorder has been considered (1). The importance of genital Mycoplasma in this regard is questionable (16). Some believe that access of genital Mycoplasma to the placenta or the chorioamniotic membranes can induce gestation, due to the rupture of membranes by increasing the inflammatory reactions, secretory products, ammonia and urea production from the Mycoplasma and Ureaplasma, respectively (15), extracellular enzyme or toxins production such as phospholipase, or by the enhancement of the uterine contraction (17). Mazor et al. evaluated the relationship between Ureaplasma sp. and preterm labor by culturing amniotic liquid, and other researchers confirmed this by placenta culturing; in fact, the endocervical colonization of the bacteria had a significant relationship with preterm birth, but there are conflicting results about the importance of genital mycoplasma presence in vaginal secretion and its relation to PTB (18).
Callahana et al. in two cohort study groups compared the maternal vaginal microbiota in PTB and pregnant women who have had a full-term birth. They found the association between the vaginal microbiota and PTB in both cohort groups but the association between Mycoplasma sp. and PTB was only found among Caucasian pregnant women (16). Their finding is in contrast to some other studies. Romero et al. found that there was no statistically significant difference in vaginal micro-biota of pregnant women who subsequently have spontaneous preterm birth and those with a normal delivery at term (19).
The results in this study showed that the presence of Mycoplasma or Ureaplasma in vaginal secretions during pregnancy or childbirth could significantly increase the risk of preterm labor. This relation was more obvious in the case of Ureaplasma parvum. This finding is in line to the results of Rittenschober-Böhmet et al.’s and Kataoka et al.’s study as they considered the probable role of Ureaplasma parvum in PTB and even in abortion (20, 21).
It was found that the prevalence of Ureaplasma parvum in pregnant women was more than U. urealyticum which confirms the findings of Bartkeviciene et al.’s results (22).
Only five cases of Mycoplasma hominis were isolated from vaginal secretions. All of them were isolated from women that experienced the preterm labor as highlighted in the Nguyen et al.’s and Doyle et al.’s studies (23, 24).
In this study, it was impossible to evaluate the presence of other genera of bacteria in vaginal secretion synchronously with genital Mycoplasma. For this reason, our findings could not present the exact image of the vaginal ecosystem in two groups and this is one of the limitations of our study.
On the other hand, it seems that emphasizing the presence of one or more organisms in vaginal discharge alone cannot predict the PTB incidence in women. Counts and diversity of bacteria, their ability to access the uterus, vagina, or systemic inflammation induction, and the interaction with the immune system all influence on the PTB.
Conclusion
This study showed that the presence of Mycoplasma or Ureaplasma in vaginal secretions increased the risk of preterm labor about two times. This event was related to Ureaplasma parvum, Ureaplasma urealyticum, and Mycoplasma hominis but the study could not confirm the role of Mycoplasma genitalium in preterm labor.
Footnotes
Conflict of Interest
Authors declare no conflict of interests.
References
- 1.Terzic M, Aimagambetova G, Terzic S, Radunovic M, Bapayeva G, Laganà AS. Periodontal pathogens and preterm birth: current knowledge and further interventions. Pathogens. 2021;10(6):730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.F G, lK C, Bloom S, Spong C, Dash J, Hofman B. Williams Obstetrics. 25th ed. United States of America: McGraw-Hill Education, 2018; 2018. 1344 p. [Google Scholar]
- 3.World Health Organization . Preterm birth [Internet]. Geneva: WHO. 2018. Available: https://www.who.int/news-room/fact-sheets/detail/preterm-birth. [Google Scholar]
- 4.Sharifi N, Khazaeian S, Pakzad R, Fathnezhad Kazemi A, Chehreh H. Investigating the prevalence of preterm birth in Iranian population: a systematic review and meta-analysis. J Caring Sci. 2017;6(4): 371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muhe LM, McClure EM, Nigussie AK, Mekasha A, Worku B, Worku A, et al. Major causes of death in preterm infants in selected hospitals in Ethiopia (SIP): a prospective, cross-sectional, observational study. Lancet Glob Health. 2019;7(8):e1130–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.You YA, Yoo JY, Kwon EJ, Kim YJ. Blood microbial communities during pregnancy are associated with preterm birth. Front Microbiol. 2019;10:1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witt RG, Blair L, Frascoli M, Rosen MJ, Nguyen QH, Bercovici S, et al. Detection of microbial cell-free DNA in maternal and umbilical cord plasma in patients with chorioamnionitis using next generation sequencing. PloS One. 2020;15(4):e0231239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang YS, Min JW, Kim YS. Positive culture rate and antimicrobial susceptibilities of Mycoplasma hominis and Ureaplasma urealyticum. Obstet Gynecol Sci. 2019;62(2):127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moridi K, Hemmaty M, Azimian A, Fallah MH, Abyaneh HK, Ghazvini K. Epidemiology of genital infections caused by Mycoplasma hominis, M. genitalium and Ureaplasma urealyticum in Iran; a systematic review and meta-analysis study (2000–2019). BMC Public Health. 2020; 20(1):1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavlidis I, Spiller OB, Demarco GS, MacPherson H, Howie SE, Norman JE, et al. Cervical epithelial damage promotes Ureaplasma parvum ascending infection, intrauterine inflammation and preterm birth induction in mice. Nat Commun. 2020;11(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ericson JE, Laughon MM. Chorioamnionitis: implications for the neonate. Clin Perinatol. 2015;42(1):155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahshahan Z, Hoseini N. Investigating prevalence of mycoplasma and ureaplasma infection in pregnant women with preterm labor. Iran J Obstet Gynecol Infertil. 2012;15(10):8–13. [Google Scholar]
- 14.Yoshida T, Maeda SI, Deguchi T, Miyazawa T, Ishiko H. Rapid detection of Mycoplasma genitalium, Mycoplasma hominis, Ureaplasma parvum, and Ureaplasma urealyticum organisms in genitourinary samples by PCR-microtiter plate hybridization assay. J Clin Microbiol. 2003;41(5):1850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed ST. Antigenic ureaplasma variation and adaptation to ovine complement response following experimental intrauterine infection [dissertation]. [United Kingdom]: Cardiff University; 2015. 266 p. [Google Scholar]
- 16.Callahan BJ, DiGiulio DB, Goltsman DSA, Sun CL, Costello EK, Jeganathan P, et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc Natl Acad Sci USA. 2017;114(37): 9966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyoshi Y, Suga S, Sugimi S, Kurata N, Yamashita H, Yasuhi I. Vaginal ureaplasma urealyticum or Mycoplasma hominis and preterm delivery in women with threatened preterm labor. J Matern Fetal Neonatal Med. 2022:35(5):878–83. [DOI] [PubMed] [Google Scholar]
- 18.Mazor M, Chaim W, Horowitz S, Leiberman J, Glezerman M. Successful treatment of preterm labour by eradication of Ureaplasma urealyticum with erythromycin. Arch Gynecol Obstet. 1993;253(4): 215–8. [DOI] [PubMed] [Google Scholar]
- 19.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Bieda J, et al. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome. 2014;2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kataoka S, Yamada T, Chou K, Nishida R, Morikawa M, Minami M, et al. Association between preterm birth and vaginal colonization by mycoplasmas in early pregnancy. J Clin Microbiol. 2006;44(1):51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rittenschober-Böhm J, Waldhoer T, Schulz SM, Stihsen B, Pimpel B, Goeral K, et al. First trimester vaginal Ureaplasma biovar colonization and pre-term birth: results of a prospective multicenter study. Neonatology. 2018;113(1):1–6. [DOI] [PubMed] [Google Scholar]
- 22.Bartkeviciene D, Opolskiene G, Bartkeviciute A, Arlauskiene A, Lauzikiene D, Zakareviciene J, et al. The impact of Ureaplasma infections on pregnancy complications. Libyan J Med. 2020;15(1): 1812821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen DP, Gerber S, Hohlfeld P, Sandrine G, Witkin SS. Mycoplasma hominis in mid-trimester amniotic fluid: relation to pregnancy outcome. J Perinat Med. 2004;32(4):323–6. [DOI] [PubMed] [Google Scholar]
- 24.Doyle RM, Alber DG, Jones HE, Harris K, Fitzgerald F, Peebles D, et al. Term and preterm labour are associated with distinct microbial community structures in placental membranes which are independent of mode of delivery. Placenta. 2014;35(12):1099–101. [DOI] [PubMed] [Google Scholar]
- 25.Hosny AEDM, El-Khayat W, Kashef MT, Fakhry MN. Association between preterm labor and genitourinary tract infections caused by Trichomonas vaginalis, Mycoplasma hominis, Gram-negative bacilli, and coryneforms. J Chin Med Assoc. 2017; 80(9):575–81. [DOI] [PubMed] [Google Scholar]


