Summary
A myriad of microbes living together with the host constitutes the microbiota, and the microbiota exerts very diverse functions in the regulation of host physiology. Microbiota regulates cancer initiation, progression, metastasis, and responses to therapy. Here we review known pro-tumorigenic and anti-tumorigenic functions of microbiota, and mechanisms of how microbes can shape tumor microenvironment and affect cancer cells as well as activation and functionality of immune and stromal cells within the tumor. While some of these mechanisms are distal, often distinct members of microbiota travel with and establish colonization with the tumors in the distant organs. We further briefly describe recent findings regarding microbiota composition in metastasis and highlight important future directions and considerations for the manipulation of microbiota for cancer treatment.
Keywords: Microbiome, Tumor microenvironment, Cancer, Tumor progression, Mechanisms, Host-microbiome interactions
Introduction
Microbes are associated with many epithelial surfaces and their continuous interactions with host cells are essential for health and disease [1]. The gut microbial ecosystem is the richest among other microbiomes in the human body, both in terms of number of species and amount of cells. Trillions of microorganisms of many species and taxonomical identities, including bacteria, archaea, viruses, and fungi, inhabit the gastrointestinal tract. These microorganisms play an essential role in maintenance of tissue homeostasis, food digestion and absorption, maintaining the integrity of the mucosal epithelial barriers, protection from pathogens, and exert various essential functions in priming, maturation, tonic stimulation and regulation of response amplitude for the immune system. The quantitative and qualitative characteristics of microbiota as well as its dynamic changes are individual for each person and depend on many endogenous (genetics and polymorphisms) and exogenous (route of delivery during birth, diet, nutrition, lifestyle, history of inflammation and tissue injury, environmental pollutants, and antibiotics and other drug treatments) factors [2]. Abnormal shifts in microbiota composition or localization, named dysbiosis, are commonly associated with and lead to increased epithelial barrier permeability, translocation of bacteria and bacterial products to other organs, as well as changes in inflammation, immune responses, and metabolic characteristics. These active changes in some species of microbiota also lead to micro-ecological changes in other species and their characteristics with competition having positive or negative influences. To date, changes in microbiota were successfully associated with cardiovascular [3], neurological [4], metabolic [5], and inflammatory diseases [6], as well as cancer formation, progression, metastasis, and its resistance or sensitivity to therapy [7,8]. Recently, host-microbiome interactions have become a great area of interest due to the enormous complexity of the microbiome, interpersonal microbiota variability, and the nascent state of the field in terms of the defining underlying mechanisms which connect microbiota with particular changes in host physiology. In cancer, the microbiota has been mechanistically implicated in all stages of tumorigenesis, though changes in microbiota may be a biomarker of malignant transformation which emerges as a secondary phenomenon. Microbiota also plays both a local role inside the tumor, for example, in colorectal or pancreatic cancer [9,10], and a distant role, when gut microbiota affects breast cancer, liver cancer, or sensitivity of distant metastasis to therapies [8,11]. In addition, depending on the context, type and stage of cancer, and qualitative characteristics of the microbiota, these bacteria may play either pro- or anti-tumorigenic roles.
Colorectal cancer (CRC), hepatocellular carcinoma (HCC), and pancreatic cancer are the major prevalent gastrointestinal (GI) cancers. The GI tract is inhabited by the richest and most abundant consortia of microbes when compared to other body sites. As a result, many initial studies connecting the microbiota to cancer were traditionally performed in the context of the gut microbiome and GI cancers [1,2]. This review highlights our current knowledge and understanding of the role of gut microbiota with a specific focus on GI cancer development, prevention, progression, and treatment possibilities. We further discuss possible proven and hypothetical mechanisms of action for microbiota and its products which are instrumental for tumorigenesis. We then discuss potential promises and possible caveats for modulating the microbiota to improve cancer prevention and treatment (Fig. 1).
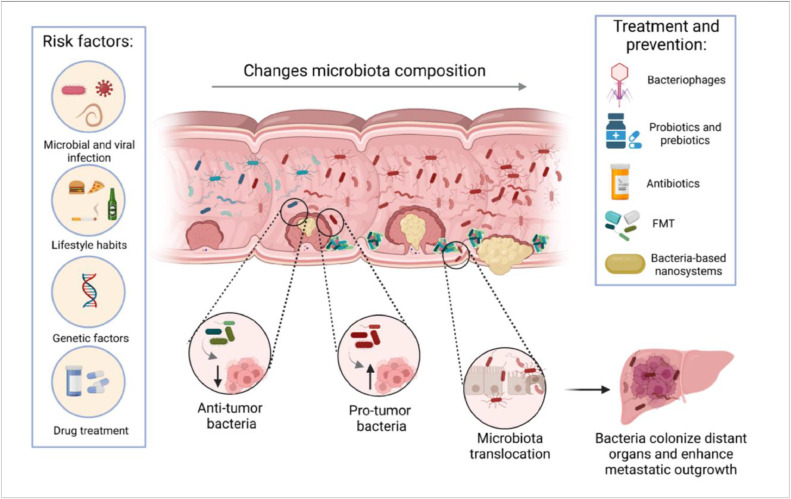
Fig. 1.
Role of gut microbiota during cancer development. Various risk factors, including viral and microbial infections, genetic predisposition, smoking, alcohol consumption, a sedentary lifestyle, obesity, and the use of medications that alter the microbiota, may promote the formation of a primary tumor. Microbiota can facilitate tumor formation via direct mutagenesis, promoting survival of tumor-initiating cells or inducing pro-tumorigenic immune responses. Microbiota further changes during cancer progression, while pro-tumor and anti-tumor bacteria directly compete with each other or with the tumor-specific microecological depletion of protective species and the bloom of pathogenic species. Transformation-induced disruption of the epithelial barrier and depletion of the mucin layer, lead to the possibility of enhanced translocation of the bacteria into the blood or lymphatic system, and the spread of microbes to distant organs and lymph nodes. Defective barriers further aid the formation of bacterial biofilms which may prevent tumor treatment or bacteria eradication. These events have a propensity to facilitate cancer progression and metastasis. Therapies aimed at changing the composition of the microbiota, including fecal microbiota transplantation, probiotics, dietary interventions, and narrow-spectrum antibiotics may be testable strategies. In addition, the use of bacteria-based nanosystems for drug delivery and bacteriophages for modulation of the microbiome may also hold a lot of promise.
Microbial and viral drivers of cancer
Viruses and bacteria may be the primary causes of “infection-driven” cancer. Human papillomaviruses (HPV), Kaposi sarcoma herpesvirus (KSV), Epstein-Barr virus (EBV), Hepatitis B (HBV) and Hepatitis C (HCV) viruses, human immunodeficiency virus-1 (HIV), human T cell lymphotropic virus type 1 (HTLV) associated with skin and cervical cancers, hepatocellular carcinoma, adult T-cell leukemia/lymphoma, and other types of malignancies [12,13] serve as complete carcinogens and the primary drivers of tumorigenesis. These viruses can initiate and promote oncogenesis by directly promoting transformation via viral oncogenes and inhibitors of tumor suppressors causing mutations and epigenetic modifications, inducing chronic inflammation, and regulating infected and neighboring cell proliferation, apoptosis, and senescence [12]. The one bacteria which has undeniable evidence to be an initiator and driver of tumorigenesis is Helicobacter pylori in gastric cancer. Moreover, platyhelminths such as Opisthorchis viverrini, Clonorchis sinensis, and Schistosoma haematobium are associated with cholangiocarcinoma, hepatocellular carcinoma, and bladder cancer [14]. As a result, the International Agency for Research on Cancer (IARC) has classified these infectious agents as Group/Class 1 carcinogens.
However, it has become clear that commensal or “conditionally-pathogenic” bacteria are also involved in tumorigenesis. The normal gut is inhabited by commensal bacteria, which perform protective functions that prevent colonization and invasion by pathogens. Adverse conditions such as excessive diet and medication, alcohol, detergent or emulsifier consumption or ongoing inflammation lead to the gradual replacement of harmless commensal gut microbiota with the outgrowth of pathogenic ones (pathobionts). Recent studies suggest commensal bacteria can potentially contribute to this replacement by becoming pathogenic under host immune stress conditions, which can be caused by the diet [15]. Shifting of microbiota composition increases intestinal permeability resulting in a “leaky” gut, microbiota transplantation, and driving tumorigenesis by several mechanisms described below. Transformation of epithelium can also dictate which bacteria and in what quantities adhere to the host cells or live in close proximity to normal or transformed epithelium. Moreover, cancer cells may become specifically colonized by intracellular bacteria, increasing the ability of these cells to metastasize [16,17].
Changes in microbiota composition during tumor formation
Although the microbiota is typically very diverse, certain bacteria have been identified as predominant commensals of a healthy gut such as Firmicutes and Bacteroidetes including Bacteroides finegoldii, Bacteroides intestinalis, Prevotella copri, Ruminococcus obeum, Lachnobacterium bovis, and others [18]. During tumor formation, the composition of the microbiota changes, possibly starting nearby to the dysplastic epithelium and later on “spilling over” to the luminal compartment. In some cases, these changes in microbiota precede tumor formation and progression [19], and may therefore be suspected as mechanistically involved in early tumorigenesis or be responsible for an increased predisposition to tumor initiation. Pathogenic bacteria, such as Escherichia coli and Bacteroides fragilis (see below), have distinct invasion and biofilm-forming capabilities which may especially manifest in the areas of early transformation. This in turn may further increase the cancer formation potential and be linked to an emerging problem of early-onset colorectal cancers [20,21].
Inflammatory bowel diseases (IBD) predispose to colon cancer and are progressively characterized by increased numbers of Proteobacteria and decreased numbers of Firmicutes species [22,23] Shotgun metagenomic sequencing of fecal samples from patients with different stages of CRC and healthy controls revealed that greater percentages of carcinoma and adenoma patients were seen with a significant increase in Bacteroides massiliensis, Bacteroides ovatus, Bacteroides vulgatus, and Escherichia coli [24]. An additional study by Yachida et al. demonstrated that Atopobium parvulum and Actinomyces odontolyticus were significantly increased in multiple polypoid adenomas and intramucosal carcinomas [25]. In other scenarios, an oncogenic transformation leads to metabolic, adhesive, nutrient availability, and microbes competition changes in the niche where both cancer cells and microbes live which induces redistribution and changes in microbiota locally, including microbes associated with the transformed site. Initial transformation and emergence of colonic adenoma lead to tumor-specific accumulation of oral commensal Fusobacterium nucleatum, which is preferentially associated with adenoma but not with normal tissue and further increases its representation with time and coincides with adenoma-to-carcinoma transition [26], [27], [28], [29]. In such scenarios, members of microbiota are not necessarily involved in the initial event of transformation, however as they accumulate they may regulate tumor growth and progression. Enterotoxigenic (ETBF) strains of Bacteroides fragilis, on the other hand, may drive colitis and are increasingly associated with CRC tumors [30,31].
The normal liver is rarely constantly colonized by microbial consortia, however, it is a "filtering" part of the “intestine–liver-body axis” and constantly deals with a flow of microbes and microbial products through the portal system. The emergence of liver diseases such as non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) may lead to temporary or constant liver colonization with different bacteria [32], which in turn would further promote liver disease. In particular, Bacteroides and Clostridia were over-represented in liver tissues of morbidly obese NAFLD patients while Gammaproteobacteria, Alphaproteobacteria, Bacilli, and Deinococci were overrepresented in the non-morbidly obese NAFLD cohort [32]. Additionally, recent studies showed a strong correlation between the composition of the gut microbiota and the progression of liver disease. Gut microbiota in patients with NASH is characterized by increased numbers of Parabacteroides and Allisonella and reduced numbers of Faecalibacterium and Anaerosporobacter [33]. Intestinal microbiota in patients with cirrhosis is enriched in members of the Lachnospiraceae family and species from Veillonellaceae and Streptococcaceae families, such as Streptococcus salivarius, Veillonella parvula, Ruminococcus gnavus, and others [34]. During the formation of HCC, a significant number of microbes can be identified in the tumor-adjacent space and in the tumor itself, while the remaining normal or healthy liver tissue remains relatively free of microbes. The tumor-associated microbiota in HCC typically consists of Bacteroidetes, Firmicutes, and Proteobacteria [35]. Additionally, Ruminococcus gnavus was specifically identified as a component of microbial signature in tumors of HCC patients positive for hepatitis B and/or hepatitis C viruses [35]. Another study on NAFLD-related cirrhosis and HCC patients demonstrated increased numbers of Bacteroides and Ruminococcaceae species and reduced presence of Bifidobacterium in fecal microbiota in the HCC group [36].
The number and diversity of bacteria are also increased in pancreatic adenocarcinoma (PDAC) and most of the tumor-infiltrating bacteria can be typically traced to the intestine as their source [37]. 16S rRNA gene sequencing of PDAC tumor samples revealed that based on taxonomical composition, the enrichment tumoral microbiota likely originates from the duodenum. The most evident increase was observed in the Enterobacteriaceae and Pseudomonadaceae families [37]. Work by Pushalkar et al. demonstrated that PDAC tumors are routinely colonized with Proteobacteria, Bacteroidetes, and Firmicutes [9]. In addition, a “benign” spectrum of pancreatic diseases such as chronic pancreatitis may both change the intestinal microbiome through changes in hormones and digestive enzymes and regulate properties of the pancreas as a “soil” for microbial colonization by bacteria from the intestine. Indeed, Sonika et al. demonstrated that chronic pancreatitis leads to a marked increase in intestinal permeability manifested by a reduced expression of claudin-4, a component of tight junctions which typically “seal” normal epithelial tissue [38]
In addition to bacteria, the intestine is inhabited by viruses and fungi, which also play a role in tumorigenesis. Pathogenic fungi from Malassezia spp. migrate from the gut to the pancreas through the sphincter of Oddi, and promote PDAC [39]. Aykut et al. demonstrated that elimination of fungi protects mice against oncogenic progression [39]. A recent publication showed one particular mechanism of how fungi (mycobiome) can contribute to PDAC development through intratumoral mycobiome-driven secretion of IL-33, which further induces pro-tumorigenic Th2 responses [40]. Interestingly, pancreatic inflammation does not increase fungal infiltration into the pancreas unlike it does for bacteria. Several studies have reported changes in gut mycobiome in CRC and IBD patients. Analysis of fecal shotgun metagenomic sequences of patients with CRC showed an increase in Malasseziomycetes and a decrease in Saccharomycetes and Pneumocystidomycetes [41]. Gao et al. observed fungal dysbiosis in colon polyps and CRC, including an increased Ascomycota/Basidiomycota ratio and an increased presence of Trichosporon and Malassezia [42]. Candida albicans play an important role in inflammation and cancer settings. These fungi produce a cytosolic peptide toxin called candidalysin which directly damages epithelial membrane and activates epithelial immunity [43]. Additional evidence has confirmed the presence of fungal dysbiosis in IBD, including decreased prevalence of Saccharomyces cerevisiae and an increased presence of Candida albicans compared with healthy controls [44]. The potential role(s) of fungi is likely not limited to CRC and PDAC and they may play a role in a variety of cancers.
Anti-tumorigenic effects of microbiota
Disruption of the microbiota composition is strongly associated with the development of colorectal, liver, and pancreatic cancers, and can contribute to their metastasis. However, the microbiota has been shown to play both anti-tumorigenic and pro-tumorigenic roles, depending on its functional taxonomic and spatio-temporal characteristics and stages of tumor development [45,46]. Below we list some of the mechanisms and processes which can enable anti-tumorigenic entities of microbiota.
Modulation of Anti-Tumor Immunity
The gut microbiota can regulate tumor-promoting and tumor-suppressing pathways associated with inflammation and immunity. Germ-free mice which are devoid of microbes have an immune system which is incompletely developed, with aberrant maturation of cells, their representation and spatial organization in the tissue, and altered thresholds for cell activation. This underscores the general requirement of microbiota for tonic stimulation of competent immune responses [47]. In agreement with this observation, various bacteria or bacterial products were shown to potentiate anti-cancer immunity, including the original work by William Coley on “Coley toxins” [48]. Lactobacillus rhamnosus GG is capable of activating dendritic cells and neutrophils and modulating T cell activation, resulting in enhanced anti-tumor effects [49]. Microbiota and microbial products can regulate the production of other cytokines with anti-tumorigenic and immunostimulatory properties, such as IL-12, TNF [50], perhaps acting as stimulators of innate immunity via pathogen-associated molecular patterns (PAMPs). IL-22 can induce tissue repairing activity and protection against infection [51]. Another strong microbiota-driven anti-tumor mechanism could be realized through IFNγ production by immune cells. Certain human gut bacteria, such as Bacteroides dorei, Parabacteroides distasonis, and Paraprevotella xylaniphila can promote IFNγ production by CD8 T cells and anti-tumor immunity in mice [52].
Some of the microbes may share similar antigens with tumor neoantigens and therefore be engaged in “molecular mimicry” of T cell epitopes, influencing activation of anti-tumor immunity [53]. The ability of distinct members of microbiota to influence and augment the efficacy of immunotherapies serves as direct evidence of microbiota modulating immune responses. Several species and families of microbes were found to be correlative with anti-tumor responses in melanoma, kidney, and lung cancer [46,54,55]. A recent study uncovered several “types” of microbiome which correlate with good vs poor prognosis during immunotherapy and suggested that while geographical location and flavors of microbiome may be different, there are specific common denominators of microbiota which drive protective or pro-tumorigenic response [56].
Anti-tumorigenic metabolic effects
Some bacteria-derived metabolites can play a protective role in tumorigenesis. Short-chain fatty acids (SCFA) are produced by commensal bacteria, after high-fiber diet fermentation. They can induce antimicrobial activity in intestinal macrophages and stimulate generation of Treg cells, which reduces inflammatory responses in the intestine [57,58]. SCFA can also regulate cancer cells themselves and components of the tumor microenvironment. SCFA produced by various commensals during fibers fermentation suppress tumor eliciting inflammation [59] linking metabolic action of microbiota with chronic inflammation which otherwise would promote tumorigenesis. Faecalibaculum rodentium and the functionally similar Holdemanella biformis in humans, produced SCFA which inhibited calcineurin and NFATc3 signaling changing protein acetylation and tumor cell proliferation [60]. Nomura et al. demonstrated that in solid cancer patients concentrations of some SCFAs in stool samples were positively associated with anti-PD1 immunotherapy responses [61]. Lactobacillus reuteri and its metabolite, reuterin, reduces proliferation and survival in colon cancer cells by inducing protein oxidation and inhibiting ribosomal biogenesis and protein translation [62]. Finally, microbial metabolite 3-indopropionic acid (IPA) enhances the anti-tumor lytic activity of γδ T cells in a murine model of HCC [63].
Anti-tumorigenic effect by competition with pro-tumor bacteria
Competition between anti-tumor and pro-tumor bacteria for nutrients is one of the mechanisms to maintain homeostasis in the intestine and prevent an outgrowth of potentially pathogenic species. For example, Lactobacillus fermentum altered the composition of gut microbiota by reducing the percentage of Bacteroides [64]. Commensal Enterobacteriaceae confer colonization resistance against Salmonella enteritidis by competing for oxygen [65]. Pathogenic Clostridioides difficile has to compete with commensal bacteria for proline [66]. Iron is the element that plays a crucial role in the growth of commensal and pathogenic bacteria. Some of the potentially pro-tumorigenic bacteria acquire iron by using its binding by strong affinity siderophores, while commensal Bacteroides thetaiotaomicron uses xenosiderophores – siderophores enterobactin and salmochelin produced by members of Enterobacteriaceae [67,68]. Moreover, commensal and pathogenic bacteria may also develop a mutualistic relationship for cooperative growth. Gut colonization by various Bacteroides species results in generation of metabolic byproducts, such as sialic acid, fucose, succinate, indole, and 1,2-propanediol. These metabolites are often used for the cooperative growth of other commensal or pathogenic bacteria [69].
In addition, such competition can drive long-term effects of tissue colonization by bacteria with distinct properties. For example, “bacteria 1” may create a niche for “bacteria 2 and 3” with anti-tumorigenic properties, while outcompeting “bacteria 4” which would otherwise promote further colonization with “bacteria 5 and 6” which would have pro-tumorigenic properties. Studies on anti-tumor vs pro-tumor bacteria competition suggest that under the "right" circumstances, prebiotics and probiotics may be tested or used for normalization of microbiota and deterrence of pro-tumorigenic species.
Pro-tumorigenic effects of microbiota
A pro-tumorigenic effect of microbiota is prominent among many types of cancer and operates via several distinct mechanisms characterized so far. These include DNA damaging/genotoxic effects of bacteria by bacterial genotoxins and bacteria-induced immune responses, such as reactive oxygen species (ROS) and reactive nitrogen intermediates (RNI) production by activated macrophages and neutrophils, microbe-driven alterations in metabolism and production of pro-tumorigenic metabolites by bacteria, induction of pro-tumorigenic chronic inflammation, as well as induction of immunosuppressive programs which impede anti-tumor immunosurveillance (Fig. 2).
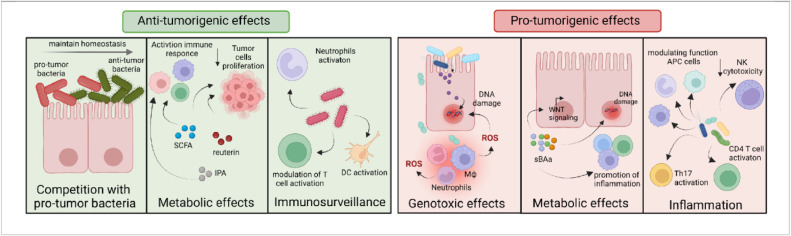
Fig. 2.
Pro- and anti-tumorigenic effects of microbiota. Microbiota plays a dual role in carcinogenesis. Anti-tumorigenic bacteria inhibit tumorigenesis through the following mechanisms. 1. Interbacterial competition for nutrients leads to the depletion of pathogenic bacteria. 2. Anti-cancer bacteria produce metabolites such as IPA, SCFA, and reutein that reduce cancer cell proliferation and immune response activation. 3. Anti-tumor bacteria can activate cytotoxic and helper T lymphocytes and NK cells, thereby inhibiting tumor growth. Pro-tumorigenic bacteria promote tumor development through various mechanisms.1. Pathogenic bacteria can produce toxins directly leading to DNA damage. Additionally, DNA damage can occur indirectly through the production of ROS and RNI. 2. Oncogenic bacteria produce metabolites and their effects are quite varied. The most studied to date are sBAs which can damage DNA, alter Wnt signaling, and induce and perpetuate inflammation. 3. Bacteria are involved with direct induction of inflammation including the production of pro-inflammatory and pro-tumorigenic cytokines, suppression of NK cell and T cell cytotoxicity, and activation of the Th2 and Th17 pro-tumorigenic immune responses.
Genotoxic effects
Genotoxins produced by the gut microbiota exert tumorigenic effects by inducing DNA damage in host cells, resulting in direct mutations as well as alterations in the DNA repair machinery, changes in the expression of key oncogenes or tumor suppressors leading to transformation and oncogenesis by the induction of one or several "hallmarks of cancer" such as excessive proliferation, or reduced cell death, changes in epigenetics, inflammation or senescence [70]. Certain bacteria, such as Bacteroides fragilis, Enterococcus faecalis, Helicobacter hepaticus, and Escherichia coli, can enhance carcinogenesis directly by producing factors that inflict DNA damage in host cells, thereby increasing the chance of oncogenic mutations [31,[71], [72], [73], [74]. Escherichia coli secretes genotoxin colibactin encoded by the pks pathogenicity island which induces double-stranded DNA breaks in eukaryotic cells [73]. During chronic inflammation caused by altered microbiota, inflammatory cells release ROS and RNI, causing DNA damage in normal epithelial cells. Enterococcus faecalis infection induces superoxide production by macrophages, resulting in DNA damage in epithelial cells, called the bystander effect [75]. Other bacteria, such as Fusobacterium nucleatum, can indirectly enhance carcinogenesis through factor FadA production which binds E-cadherin at tight junctions between intratumor epithelial cells and increases the permeability of colonic epithelial barrier [71]. Moreover, Fusobacterium nucleatum has Fap2 protein, binding epithelial cells and immune cells, and promoting persistent local inflammation at the site of transformation and bacterial invasion [76]. Helicobacter hepaticus triggers immune cells to produce nitric oxide/RNI [71]. Oxidative stress induced by altered gut microbiota is shown to be implicated in the formation of HCC [77]. Another mechanism by which microbiota can induce DNA damage is the microbiota-derived metabolite gallic acid, which inhibits the tumor-suppressor activity of p53 in epithelial cells, resulting in defective responses to host DNA damage and mutations facilitating malignant transformation [78].
Pro-tumorigenic metabolic effects
In the gut, the microbiota produces a significant amount of metabolites, however, their function in the context of cancer is still poorly understood due to the nascent state of the field and great interpersonal variability in microbiota, diet, and environmental factors. Diet is one of the factors that modulates the risk of cancer development and the composition of the gut microbiota [79]. Recent studies showed that a high-fat diet and a high-fructose diet increase CRC and HCC progression and the effects of the diet are in part mediated by the effects of microbiota [79], [80], [81], [82]. Secondary bile acids (sBAs) produced by gut microbiota from the bile acids, the end product of cholesterol metabolism in the liver, are important microbiota-derived metabolites. They contribute to immune homeostasis maintenance by modulating colonic FoxP3+ regulatory T (Treg) cells expressing the transcription factor RORγt which promotes tumor progression [83,84]. Another microbial metabolite, deoxycholic acid (DCA), can promote CRC by regulating the Wnt signaling pathway [85]. Lithocholic acid (LCA) can stimulate expression of IL-8, an important inflammation mediator upregulated in the serum of patients with CRC [86].
During liver cirrhosis microbial metabolites can enter the hepatic sinusoids through the portal vein and cause chronic liver inflammation. Primary bile acids increase the hepatic sinusoidal endothelial expression of CXCL16 receptor, which acts as a scavenger receptor on macrophages and can induce recruitment and retention of anti-tumorigenic natural killer (NK) cells. However, numerous studies demonstrated that sBAs are capable to decrease CXCL16 expression, thereby impeding NK-cell mediated immunity [87]. DCA, implicated in CRC tumorigenesis, can also contribute to liver carcinogenesis by promoting DNA damage and inducing release of proinflammatory cytokines by hepatic stellate cells [87,88].
Inflammation
Inflammation, especially chronic, non-resolving inflammation, plays an important role during all stages of tumorigenesis [89]. Microbes are powerful inducers of inflammation and chronic access to microbial products leads to chronic inflammation, whether it is systemic, organ-specific, or local. Chronic inflammation in the target tissue, whether clinical or subclinical, often precedes tumor formation in the same tissue. For example, IBD promotes colon cancer while pancreatitis is a risk factor for PDAC development [13]. Chronic inflammation is characterized by immunosuppressive microenvironment and promotes epigenetic changes, oncogene activation and repression of tumor suppressor action, DNA damage, and oxidative stress [90]. Chronic inflammation also causes perpetuating tissue damage and regeneration, a condition where transformed cells may gain a competitive advantage to grow and substitute bulk of tissue, giving rise to the tumor. While IL-22 has a protective role against pathogens and stimulate tumor proliferation, IL-22 activity may have a different role at different stages of cancer [91] and can also drive nitric oxide-dependent DNA damage [92] or conversely protect against DNA damage in stem cell compartment [93]. Equally important is that inflammatory entities such as cytokines can serve as growth factors for tumor cells to ensure their survival and proliferation [89]. Numerous studies highlight important cross-interactions between gut microbiota, tumor microbiota, and tumor progression in the settings of colorectal, pancreatic, and liver cancers [8,22,36,45,87]. In CRC, the neoplastic transformation is accompanied with the disruption of the integrity of the colonic epithelium due to the loss of the proper cell junction organization, cell hyperproliferation, and a loss of differentiated mucus-producing Goblet cells [89]. This leads to increased crosstalk and interaction of microbes with tumor cells and their microenvironment, inducing transformation site-specific, non-resolving inflammation.
Up to ninety percent of HCCs develop under the conditions of underlying chronic liver inflammation, induction of fibrosis, and subsequent cirrhosis. Several studies have observed significant alterations in the composition of gut microbiota in patients with chronic liver and pancreatic inflammatory diseases, particularly, a reduction in beneficial bacteria and an increase in pathogenic bacteria. A study by Zhang et al demonstrated, that gut microbiome induced CXCL1 expression in hepatocytes through Toll-like receptors 4 (TLR4)-dependent mechanism and an accumulation of CXCR2+ positive polymorphonuclear myeloid-derived suppressor cells (MDSCs), which promote liver cancer via induction of immunosuppression and possibly through other direct mechanisms which include growth factors production and NETosis [94]. Another potentially relevant mechanism is enhanced activation of pro-tumorigenic T cells by tumor-associated microbiota during pancreatic cancer progression, which leads to CD4 T cells producing pro-inflammatory and pro-tumorigenic entities [7]. Enterotoxigenic Bacteroides fragilis, Peptostreptococcus anaerobius, Fusobacterium nucleatum can induce inflammation by activating T helper 17 (Th17) cells, modulating function of MDSCs, tumor-associated macrophages (TAMs), and granulocytic tumor-associated neutrophils, as well as reducing natural killer cells cytotoxicity [45] possibly in many GI cancers.
Another type of tumor elicited inflammation occurs in “non-inflammatory” tumors to support tumor growth and reshape the tumor microenvironment [89]. Of such examples is barrier deterioration induced during CRC initiation by invasion of colonic adenomas by microbial products, resulting in increased production of IL-23 and downstream cytokines such as IL-17 and IL-22 which have a direct pro-tumorigenic effect on growing cancer cells in CRC [95], liver cancer [96] or pancreatic cancer [97].
Gut microbiota translocation and its distant effect on cancer progression
In epithelial barrier tissues including intestine microbiota are physically and functionally separated from host tissues by mechanical and functional epithelial barrier which limits host-microbiota interaction to the remaining extent which is mutualistic in nature [98,99]. The intestinal wall consists of a monolayer of intestinal epithelial cells (IEC) that prevent the penetration of microbes and their products into the submucosal space and contact with inner space of epithelial cells and sentinel immune cells. This single cell layer of cells which are connected and sealed together by tight and adherens junctions, constitutes a physical, chemical, and immunological barrier against intestinal microbiota, their metabolites and bacterial toxins. A healthy intestinal barrier is comprised of several layers of highly organized defense barriers, such as tight junctions, mucosal layer, secreted cytokines, antimicrobial peptides (AMPs), and secretory IgA [100]. Additional protection from bacterial invasion is “gut-vascular” barrier which prevents intestinal microbes from accessing the bloodstream [101].
Bacterial translocation of gut bacteria and their products through the intestinal mucosal barrier into the mesenteric lymph nodes, systemic circulation, or to the distant organs commonly occurs as the result of increased intestinal wall permeability, caused by a variety of insults including local and systemic inflammation, diets, alcohol, dysbiosis, and certain treatments or drugs as well as by tumor formation and growth [80,95,102,103]. For example, dietary emulsifiers interact with the multilayered endogenous mucus secretions that coat the luminal surfaces of the intestinal tract and may compromise the ability of human mucus to prevent contact between microorganisms and epithelial cells [104]. Triclosan is antimicrobial chemical that induces colonic inflammation, promotes colitis, and exacerbates colitis-associated colon cancer in mice [105]. Diminution of mucus and breakdown/remodeling of tight junctions by inflammatory cytokines can cause bacteria invasion and translocation in distant organs such as Enterococcus gallinarum [106]. Formation of biofilms in response to changes in intestinal barrier function helps bacteria to stay in proximity and release microbial products. Mice with colonic biofilms containing Escherichia coli and enterotoxigenic Bacteroides fragilis showed increased DNA damage in colonic epithelium and production of IL-17 which was associated with faster tumor onset, progression and greater mortality [21]. These processes can also be interconnected- for example, preceding chronic inflammation or tumor formation can cause dysbiosis, dietary insults can promote inflammation.
Once bacteria and their products translocate through the epithelial barrier, they can localize in various distant organs by several mechanisms highlighted in Fig. 3. Several studies demonstrate, that pro-tumorigenic bacteria and other components of microbiota can be detected in the pancreas [9,107] and liver [35].
Fig. 3.
Mechanisms of gut microbiota translocation. Several routes contribute to microbial translocation from the gut to distal organs. Bacteria can cross the intestinal barrier as a result of local and systemic inflammation, diet-induced injury, alcohol, infection/food poisoning and dysbiosis, drug treatment, tumor formation, mucus thinning, invasive properties of bacteria, breakdown or remodeling of tight junctions by inflammatory cytokines and formation of biofilms which bring the bacteria into the immediate proximity to the epithelium to be breached. The portal vein is a key vessel connecting the intestine, pancreas, and liver and microbiota can easily enter the portal bloodstream if epithelial barrier is weakened. Alternatively, bacteria can enter the lymphatics and spread to the lymph nodes and organs via lymph flow. The last mechanism is the direct transfer of microbiota from the duodenum to the pancreas through pancreatic ducts.
Microbiota contributes to HCC development [108] and intestine and the liver are connected via the portal vein system, so called the gut-liver axis [109]. Microbial metabolites and molecules travel from the intestine to the liver, where they are normally phagocytosed by Kupffer cells but can also activate local monocytes, neutrophils, or adherent cancer cells (micrometastases). Excessive amounts of immunogenic PAMPs, including bacterial metabolites and toxins produced by intestinal bacteria, such as lipopolysaccharides (LPSs) and bacterial DNA, lead to hepatic inflammation through the activation of pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), C-type lectin receptors (CLRs), galectin 3 and NOD-like receptors (NLRs) [110]. Commensal bacteria might influence liver immunological zonation by activating MyD88-dependent signaling in liver sinusoidal endothelial cells (LSECs), which induce chemokine gradient formation and attraction of myeloid and lymphoid resident immune cells [111]. Exposure of mice with a disturbed intestinal barrier to microbial components led to an increase in the systemic circulation of intestinal microbial factors, which contributed to the formation of HCC [112]. Depletion of microbiota with broad spectrum antibiotics led to a tumor regression in HCC models induced by DEN (diethylnitrosamine) plus carbon tetrachloride (CCl4)treatment; by high-fat diet (HFD) in MUP-uPA mice; or by a combination of 7,12‐dimethylbenzanthracene (DMBA) and HFD [88,113,114].
Bacterial translocation from the intestinal lumen to the pancreas was directly demonstrated by Rohr et al, who gavaged mice withh fluorescently labeled commensal bacteria [115]. The most likely way for this translocation has been suggested to be via the pancreatic duct, which connects pancreas with the duodenum [37]. Bacterial DNA profiles in the pancreas are similar to those in the duodenum tissue [116]. Bacteria are not the only potential pathogens capable to migrate from the gut. Pathogenic fungi can also migrate from the duodenum to the pancreas through sphincter of Oddi, and promote pancreatic ductal adenocarcinoma development [39]. In addition, there is a potential possibility of bacterial translocation through the portal vein and the lymph flow (Fig. 3).
In three independent studies using different genetic models of mice, was shown that ablation of intestinal bacteria is associated with a decrease in tumor growth and activation of the immune response. Sethi et al revealed that after oral treatment with an antibiotic cocktail, the number of IFNγ-producing T cells increased, and the amount of secreted IL-17A and IL-10 decreased [117]. Additionally, Pushalkar et al demonstrated reduced numbers of MDSCs, and increased number of immunogenic “M1-like type” macrophages, Th1 cells, and cytotoxic T cells infiltrating PDAC tumors after microbiota ablation [9]. Thomas et al also established the same effect of antibiotic treatment in the murine pancreatic cancer model [118].
Microbiota and metastasis
Tumors of the GI tract commonly metastasize to regional lymph nodes, liver, and lungs, with the presence of metastatic disease being the biggest adverse factor of poor patient survival. Microbiota plays an initial role in the formation of metastases as well as promotes their growth and resistance to therapy. Time of colonization of metastatic lesions with bacteria may also vary, but at least in some scenarios bacteria may already infect cancer cells in primary tumor [119]. Colon cancer progression induced by the loss of p53 is associated with increased intestinal permeability and formation of an NF-κB-dependent inflammatory microenvironment, which promotes epithelial-mesenchymal transition (EMT) and dissemination of cancer cells to lymph nodes [120]. Modulation of gut microbiota by glycyrrhizic acid prevents high-fat diet-enhanced pre-metastatic niche formation and metastasis to the lungs of breast cancer and melanoma [121].
Bacteria can spread to the metastases in several ways including by penetrating through the epithelial and vascular barriers into the bloodstream or lymphatic system [122] or by invasion/infection of cancer cells or traveling immune cells [16,123] (further illustrated on Fig. 4):
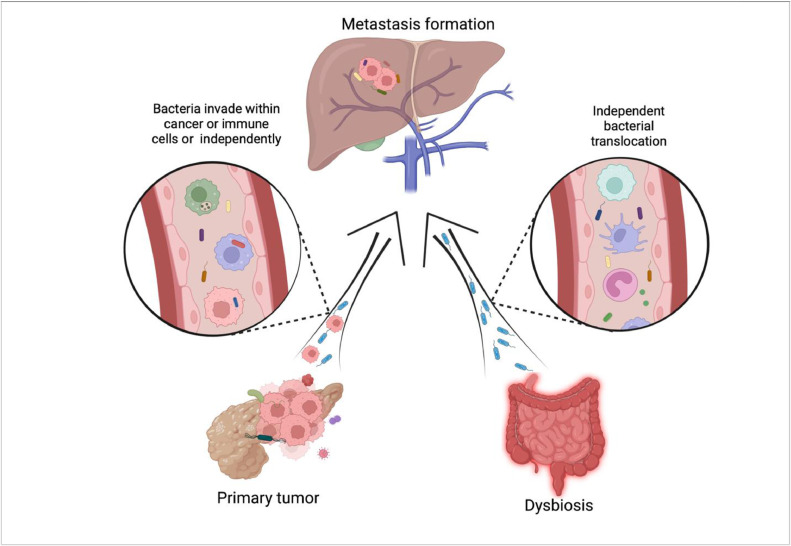
Fig. 4.
Microbiota in tumor progression and metastasis. The formation of metastases is a complex process that simultaneously affects several organs and circulatory systems. Bacterial translocation probably may occur as early as during pre-metastatic niche formation, at the same time with the colonization of the secondary organ by cancer cells or follow later in time by “re-infection” of growing metastasis with microbes. In the figure, we depicted the source of bacteria during the formation of metastases in the liver. Bacteria can get into the site of metastasis from the primary tumor independently through the blood and lymph flow and as part of the tumor or phagocytic immune cells. Leaky intestines are another source of bacteria in the liver. Dysbiosis is a frequent accompaniment of oncological diseases, leading to a weakening of contacts between epithelial cells and the spreading of bacteria to the site of metastasis.
The premetastatic niche in the liver is an altered pro-tumorigenic inflammatory microenvironment that attracts cancer cells, facilitating their extravasation, colonization, survival and outgrowth. Tumors are characterized by the presence of an intratumoral microbiota that influences tumorigenesis [16]. In addition, the composition of the microbiota of the primary colorectal tumors and hepatic metastasis is the same: they are colonized by identical bacteria such as Fusobacterium nucleatum, Bacteroides fragilis, and Prevotella spp [119]. A recent study demonstrated the important role of bacteria in the formation of the premetastatic niche where, Escherichia coli disrupts the gut-vascular barrier, and disseminates to the liver via portal vein, where bacterium promotes the recruitment of innate immune cells and the formation of an inflammatory pro-metastatic environment [115]. Treatment with broad-spectrum antibiotics showed reduction of tumor growth in liver metastasis models of pancreatic and colon cancers [117]. Fusobacterium nucleatum can also promote CRC lung metastasis, as demonstrated by Chen et al in their in vivo model [124]. Like in primary tumors, some bacteria in metastatic sites can stimulate antitumoral immunity, while others can promote immunosuppression and pro-tumorigenic inflammation. Lastly, bacterial products can play role in metastasis formation, e.g. LPS, produced by Escherichia coli can increase secretion of cathepsin K, which mediates “M2-like” polarization of macrophages and promotes metastasis in colorectal cancer [125].
Targeting microbiota to enhance anti-cancer therapies
Chemotherapy remains a mainstream treatment option in cancer but unfortunately, in many cases a resistance ensues in most of the tumors. Interestingly, resistance or sensitivity to chemotherapy correlates with the composition of intestinal or intratumoral microbiota. For example, the treatment with gemcitabine in PDAC patients loses its efficacy if particular tumor is enriched with Gammaproteobacteria [37]. Fusobacterium nucleatum promotes chemoresistance of CRC metastasis activating autophagy and augmenting survival of cancer cells [126]. On the other hand, effectiveness of chemotherapy may also decrease upon microbiota depletion in various non-GI cancers, such as lung cancer and melanoma [127]. Germ-free or antibiotic-treated tumor bearing mice do not properly respond to immunotherapeutic treatment with intratumoral CpG oligodeoxynucleotides, because these commensal microbes are essential to stimulate tumor-associated myeloid cells to produce inflammatory cytokines for anti-tumor effects [50].
Microbiota and mycobiota also differentially regulate the sensitivity of cancer to radiotherapy, acting through a variety of mechanisms. Shiao et al demonstrated that removing bacteria reduced the effectiveness of radiotherapy, while removing fungi, on the contrary, increased the effectiveness in breast cancer and melanoma [128]. Commensal bacteria support anti-tumor T cell responses while commensal fungi drive pro-tumorigenic macrophage action following RT in a Dectin-1-dependent manner [128].
In the past few years there also has been increasing evidence that microbiota influences the effectiveness of most advanced immunotherapies, including immune checkpoint inhibition therapies (ICI). For example, pancreatic cancer had a better response to PD-1 therapy in the absence of microbiota [9], while use of antibiotics specific to certain gut bacteria in a mouse model of HCC increased proportion of natural killer T cells (NKT) cells, which led to tumor size reduction [87]. These results suggest that each type of cancer and each patient has a distinct profile of the gut and tumor microbiota, that can play different roles during cancer development and therapy. The presence of certain bacteria can enhance response to therapy. Thus, several studies have demonstrated that Akkermansia muciniphila, Bacteroides fragilis, Enterococcus, and some Bifidobacterium strains can enhance the anti-tumor efficacy of ICI treatment [54,[129], [130], [131]. Mager et al demonstrated that ICI increases the permeability of the intestine, as a result of which the serum concentration of the microbial metabolite inosine, produced by the Bifidobacterium pseudolongum, increased. Inosine together with proinflammatory stimuli strongly enhanced the antitumor capacity of T cells in multiple tumor settings, including colorectal cancer, bladder cancer, and melanoma [132]. Additionally, in pre-clinical murine models Andrews et al determined that treatment with combined immune checkpoint blockade (CICB) in melanoma patients leads to toxicity by changing microbiota profiles [133].
Relatively little is known about the therapeutic potential of soluble microbial molecules and metabolites to modulate the effectiveness of cellular cancer immunotherapy. Luu et al demonstrated that SCFAs pentanoate and butyrate enhance the anti-tumor activity of cytotoxic T lymphocytes (CTLs) and chimeric antigen receptor (CAR) T cells through metabolic and epigenetic reprogramming in syngeneic murine melanoma and pancreatic cancer models [134]. Furthermore, a defined commensal consortium of 11 bacterial strains is capable of robustly inducing IFNγ-producing CD8 T cells in the intestine in murine tumor models of CRC and melanoma. This study demonstrated that a mixture of human low-abundant commensals was able to enhance therapeutic efficacy of ICI [52].
Another approach to improve cancer treatment by utilizing microbiota is the delivery of drugs directly to the destination tissue using targeted bacteria-based nanosystems, such as EnGeneIC Dream Vector (EDV). Sagnella et al demonstrated that tumor-targeting EDV nanocells function as an immunotherapeutic compound by delivering a cytotoxic payload in conjunction with activation of the immune system [135]. Some species of native or engineered bacteria may in turn serve as a drug, reducing metastasis, for example, Salmonella YB1, an engineered oxygen-sensitive strain, potently inhibits metastasis of a broad range of cancers [136]. Clostridium sporogenes can be engineered to produce smaller amounts of branched SCFAs by these bacteria, thereby potentially affecting immune and tumor responses [137]. Special Microbiome Derived Metabolism (MDM)-screening study aiming to characterize the ability of the human gut microbiome to metabolize various small molecule drugs [138]. Another approach to manipulate the function and the representation of bacteria is the use of bacteriophages to effectively and specifically reduce target bacteria population(s) in the intestine and protect from an invasive bacteria-exacerbated colorectal cancer [139]. Phage-guided modulation of the Fusobacterium nucleatum significantly augments the efficiency of first-line chemotherapy treatments of CRC [140].
Microbiota and cancer prevention
An attractive but yet understudied possibility of microbiota-based prevention of the malignant transformation is based on several key approaches, such as microbiota transplantation, including fecal transfer or “pill”, treatment with probiotics or prebiotics, use of narrow spectrum antibiotics more selective against pathogenic species, adherence to diet and healthy lifestyle. The main goals of these strategies are to reduce the number of pathogenic bacteria in the intestine and replace them with the true commensal ones, to restore the intestinal epithelial barrier, reduce the translocation of bacteria and their components, and prevent proinflammatory immunosuppression.
Antibiotic treatment, although proven to be an effective way to reduce tumor growth in mouse models, is still difficult to replicate its success in clinical practice due to interpersonal variability and possible complications, such as outgrowth of antibiotic-resistant flora or bloom of pathogens such as Clostridioides difficile. In addition, long-term use of antibiotics also leads to the depletion of potentially beneficial commensal intestinal microbiota. Several studies demonstrated that continuous use of antibiotics is associated with increased risk of colon cancer [141]. However, several clinical studies for treatment of liver cirrhosis using norfloxacin and rifaximin have been concluded with enough safety and efficacy [142,143]. Another promising approach is the use of selective antibiotics to kill certain pathogenic bacteria, such as ones responsible for synthesis of DCA, which promotes HCC [88]. Probiotics are collections of bacteria used for re-shaping the microbiota. They act through a variety of mechanisms including the suppression of colonization by pathogenic bacteria, modulating immunity, and improving the function of the intestinal barrier. Probiotics are effective in mouse models and clinical studies, but their long-term effectiveness remains controversial. Sometimes, the usage of probiotics can lead to increased risk of bacterial translocation and systemic invasion, as well as antimicrobial resistance by the potential transfer of resistant genes to the resident microbiota [144]. Prebiotics, such as dietary components, also can shape and instruct the evolution of microbiota composition. Inulin attenuated the growth of subcutaneously transplanted MC38 colon cancer tumors in syngeneic wild type mice [145]. Diet and alcohol modulate cancer risk, to a certain extent via modification of the metabolic activity of the microbiota. High cholesterol diet leads to progression of HCC by inducing alteration of gut microbiota and metabolites [146]. Western-type diet is capable to lead to loss of gut microbiota diversity, a bloom of Escherichia coli, and an altered metabolic profile with butyrate depletion in pancreatitis mouse model [147].
Fecal microbiota transplantation (FMT), which is based on the transfer of healthy microbiota from a healthy donor to a sick or high-risk recipient is another effective approach to eliminate dysbiosis and “normalize” or “reset” normal microbiota. The advantage of this method is that its effects can be long lasting, and the disadvantage is the possible transmission of unrecognized pathogens. Transplantation of bacteria from CRC patients has been shown to increase carcinogenesis in mice [148,149]. Recolonization of antibiotic-treated or germ-free mice before tumor onset with fecal microbiota from genetically engineered mice with late-stage tumors resulted in tumor progression in the recipient mice [9]. The stools of patients with severe alcoholic hepatitis increased the susceptibility to chronic alcoholic liver disease in mice and possibly HCC predisposition [150]. All these examples demonstrate a potential for microbiota normalization via FMT to prevent the development or progression of cancer. In addition, it has been shown that FMT can influence immunotherapy by ICI treatment [151,152].
While there is no universal strategy yet to prevent tumorigenesis, further insights into integrated approaches can potentially help patients to reduce the pathogenic effect of microbiota in cancer and other diseases by influencing and expanding normal microbiota counterparts.
Conclusions
The influence of microbiota on the development of malignant neoplasms is very diverse. The interrelationships between the gut microbiota and the tumor microenvironment remain largely unexplored, but the disruption in the microbiota-host relationship is a key event, leading to tumor progression. The composition of the tumor and gut microbiota can be used as a biomarker for disease progression or risk for cancer development. Altering the gut microbiota with new approaches holds a promise for substantial therapeutic benefit to improve therapeutic responses. In addition, new technologies for the genetic modification of bacterial properties and the specific targeted drugs using bacteria-based payloads give us hope for improving patient survival.
Author Contribution
E.A.I. outlined and drafted the review. E.A.I. and S.I.G. wrote the review. E.A.I. conceptualized and prepared the Figures.
Declarations of Competing Interest
None.
Acknowledgements
We thank Dan Kamen and Michael Gallagher for critical reading of this review. We apologize to colleagues whose important original work could not be cited due to the citation limits. Figures were prepared using BioRender platform. The work was supported by NIH R01CA227629 and CA218133 and partially supported by Cedars-Sinai Cancer to S.I.G.
Editor: Prof Alnawaz Rehemtulla
References
- 1.Daniel N, Lecuyer E, Chassaing B. Host/microbiota interactions in health and diseases-time for mucosal microbiology! Mucosal Immunol. 2021;14(5):1006–1016. doi: 10.1038/s41385-021-00383-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 3.Fatkhullina AR, Peshkova IO, Dzutsev A, Aghayev T, McCulloch JA, Thovarai V, et al. An Interleukin-23-interleukin-22 axis regulates intestinal microbial homeostasis to protect from diet-induced atherosclerosis. Immunity. 2018;49(5) doi: 10.1016/j.immuni.2018.09.011. 943-57 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agirman G, Yu KB, Hsiao EY. Signaling inflammation across the gut-brain axis. Science. 2021;374(6571):1087–1092. doi: 10.1126/science.abi6087. [DOI] [PubMed] [Google Scholar]
- 5.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 6.Li X, He C, Li N, Ding L, Chen H, Wan J, et al. The interplay between the gut microbiota and NLRP3 activation affects the severity of acute pancreatitis in mice. Gut Microbes. 2020;11(6):1774–1789. doi: 10.1080/19490976.2020.1770042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178(4) doi: 10.1016/j.cell.2019.07.008. 795-806 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones RM, Neish AS. Gut microbiota in intestinal and liver disease. Annu Rev Pathol. 2021;16:251–275. doi: 10.1146/annurev-pathol-030320-095722. [DOI] [PubMed] [Google Scholar]
- 9.Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018;8(4):403–416. doi: 10.1158/2159-8290.CD-17-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16(11):690–704. doi: 10.1038/s41575-019-0209-8. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Xia Y, Sun J. Breast and gut microbiome in health and cancer. Genes Dis. 2021;8(5):581–589. doi: 10.1016/j.gendis.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zella D, Gallo RC. Viruses and bacteria associated with cancer: an overview. Viruses. 2021;13(6) doi: 10.3390/v13061039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cullin N, Azevedo Antunes C, Straussman R, Stein-Thoeringer CK, Elinav E. Microbiome and cancer. Cancer Cell. 2021;39(10):1317–1341. doi: 10.1016/j.ccell.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Proenca JT, Barral DC, Gordo I. Commensal-to-pathogen transition: One-single transposon insertion results in two pathoadaptive traits in Escherichia coli -macrophage interaction. Sci Rep. 2017;7(1):4504. doi: 10.1038/s41598-017-04081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368(6494):973–980. doi: 10.1126/science.aay9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu A, Yao B, Dong T, Chen Y, Yao J, Liu Y, et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. 2022;185(8) doi: 10.1016/j.cell.2022.02.027. 1356-72 e26. [DOI] [PubMed] [Google Scholar]
- 18.Weir TL, Manter DK, Sheflin AM, Barnett BA, Heuberger AL, Ryan EP. Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS One. 2013;8(8):e70803. doi: 10.1371/journal.pone.0070803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagnaire A, Nadel B, Raoult D, Neefjes J, Gorvel JP. Collateral damage: insights into bacterial mechanisms that predispose host cells to cancer. Nat Rev Microbiol. 2017;15(2):109–128. doi: 10.1038/nrmicro.2016.171. [DOI] [PubMed] [Google Scholar]
- 20.Johnson CH, Dejea CM, Edler D, Hoang LT, Santidrian AF, Felding BH, et al. Metabolism links bacterial biofilms and colon carcinogenesis. Cell Metab. 2015;21(6):891–897. doi: 10.1016/j.cmet.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 2018;359(6375):592–597. doi: 10.1126/science.aah3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng MY, Inohara N, Nunez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10(1):18–26. doi: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37(1):47–55. doi: 10.1007/s00281-014-0454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Q, Liang S, Jia H, Stadlmayr A, Tang L, Lan Z, et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. 2015;6:6528. doi: 10.1038/ncomms7528. [DOI] [PubMed] [Google Scholar]
- 25.Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat Med. 2019;25(6):968–976. doi: 10.1038/s41591-019-0458-7. [DOI] [PubMed] [Google Scholar]
- 26.Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42(2):344–355. doi: 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22(2):292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Jiao N, Zhu R, Zhang Y, Wu D, Wang AJ, et al. Identification of microbial markers across populations in early detection of colorectal cancer. Nat Commun. 2021;12(1):3063. doi: 10.1038/s41467-021-23265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15(9):1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung L, Orberg ET, Geis AL, Chan JL, Fu K, DeStefano Shields CE, et al. Bacteroides fragilis toxin coordinates a pro-carcinogenic inflammatory cascade via targeting of colonic epithelial cells. Cell Host Microbe. 2018;23(3):421. doi: 10.1016/j.chom.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sookoian S, Salatino A, Castano GO, Landa MS, Fijalkowky C, Garaycoechea M, et al. Intrahepatic bacterial metataxonomic signature in non-alcoholic fatty liver disease. Gut. 2020;69(8):1483–1491. doi: 10.1136/gutjnl-2019-318811. [DOI] [PubMed] [Google Scholar]
- 33.Wong VW, Tse CH, Lam TT, Wong GL, Chim AM, Chu WC, et al. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis–a longitudinal study. PLoS One. 2013;8(4):e62885. doi: 10.1371/journal.pone.0062885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513(7516):59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 35.Komiyama S, Yamada T, Takemura N, Kokudo N, Hase K, Kawamura YI. Profiling of tumour-associated microbiota in human hepatocellular carcinoma. Sci Rep. 2021;11(1):10589. doi: 10.1038/s41598-021-89963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. 2019;69(1):107–120. doi: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- 37.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357(6356):1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonika U, Goswami P, Thakur B, Yadav R, Das P, Ahuja V, et al. Mechanism of increased intestinal permeability in acute pancreatitis: alteration in tight junction proteins. J Clin Gastroenterol. 2017;51(5):461–466. doi: 10.1097/MCG.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 39.Aykut B, Pushalkar S, Chen R, Li Q, Abengozar R, Kim JI, et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019;574(7777):264–267. doi: 10.1038/s41586-019-1608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alam A, Levanduski E, Denz P, Villavicencio HS, Bhatta M, Alhorebi L, et al. Fungal mycobiome drives IL-33 secretion and type 2 immunity in pancreatic cancer. Cancer Cell. 2022;40(2) doi: 10.1016/j.ccell.2022.01.003. 153-67 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coker OO, Nakatsu G, Dai RZ, Wu WKK, Wong SH, Ng SC, et al. Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut. 2019;68(4):654–662. doi: 10.1136/gutjnl-2018-317178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao R, Kong C, Li H, Huang L, Qu X, Qin N, et al. Dysbiosis signature of mycobiota in colon polyp and colorectal cancer. Eur J Clin Microbiol Infect Dis. 2017;36(12):2457–2468. doi: 10.1007/s10096-017-3085-6. [DOI] [PubMed] [Google Scholar]
- 43.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532(7597):64–68. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, et al. Fungal microbiota dysbiosis in IBD. Gut. 2017;66(6):1039–1048. doi: 10.1136/gutjnl-2015-310746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng WY, Wu CY, Yu J. The role of gut microbiota in cancer treatment: friend or foe? Gut. 2020;69(10):1867–1876. doi: 10.1136/gutjnl-2020-321153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zitvogel L, Daillere R, Roberti MP, Routy B, Kroemer G. Anticancer effects of the microbiome and its products. Nat Rev Microbiol. 2017;15(8):465–478. doi: 10.1038/nrmicro.2017.44. [DOI] [PubMed] [Google Scholar]
- 47.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154–158. [PMC free article] [PubMed] [Google Scholar]
- 49.Cai S, Kandasamy M, Rahmat JN, Tham SM, Bay BH, Lee YK, et al. Lactobacillus rhamnosus GG activation of dendritic cells and neutrophils depends on the dose and time of exposure. J Immunol Res. 2016;2016 doi: 10.1155/2016/7402760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arshad T, Mansur F, Palek R, Manzoor S, Liska V. A double edged sword role of interleukin-22 in wound healing and tissue regeneration. Front Immunol. 2020;11:2148. doi: 10.3389/fimmu.2020.02148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565(7741):600–605. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- 53.Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and anticancer immunosurveillance. Cell. 2016;165(2):276–287. doi: 10.1016/j.cell.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Routy B, Gopalakrishnan V, Daillere R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol. 2018;15(6):382–396. doi: 10.1038/s41571-018-0006-2. [DOI] [PubMed] [Google Scholar]
- 56.McCulloch JA, Davar D, Rodrigues RR, Badger JH, Fang JR, Cole AM, et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat Med. 2022;28(3):545–556. doi: 10.1038/s41591-022-01698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulthess J, Pandey S, Capitani M, Rue-Albrecht KC, Arnold I, Franchini F, et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 2019;50(2) doi: 10.1016/j.immuni.2018.12.018. 432-45 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vijay A, Kouraki A, Gohir S, Turnbull J, Kelly A, Chapman V, et al. The anti-inflammatory effect of bacterial short chain fatty acids is partially mediated by endocannabinoids. Gut Microbes. 2021;13(1) doi: 10.1080/19490976.2021.1997559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zagato E, Pozzi C, Bertocchi A, Schioppa T, Saccheri F, Guglietta S, et al. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat Microbiol. 2020;5(3):511–524. doi: 10.1038/s41564-019-0649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nomura M, Nagatomo R, Doi K, Shimizu J, Baba K, Saito T, et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bell HN, Rebernick RJ, Goyert J, Singhal R, Kuljanin M, Kerk SA, et al. Reuterin in the healthy gut microbiome suppresses colorectal cancer growth through altering redox balance. Cancer Cell. 2022;40(2) doi: 10.1016/j.ccell.2021.12.001. 185-200 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han J, Zhang S, Xu Y, Pang Y, Zhang X, Hu Y, et al. Beneficial effect of antibiotics and microbial metabolites on expanded Vdelta2Vgamma9 T cells in hepatocellular carcinoma immunotherapy. Front Immunol. 2020;11:1380. doi: 10.3389/fimmu.2020.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chou YC, Ho PY, Chen WJ, Wu SH, Pan MH. Lactobacillus fermentum V3 ameliorates colitis-associated tumorigenesis by modulating the gut microbiome. Am J Cancer Res. 2020;10(4):1170–1181. [PMC free article] [PubMed] [Google Scholar]
- 65.Litvak Y, Mon KKZ, Nguyen H, Chanthavixay G, Liou M, Velazquez EM, et al. Commensal enterobacteriaceae protect against salmonella colonization through oxygen competition. Cell Host Microbe. 2019;25(1) doi: 10.1016/j.chom.2018.12.003. 128-39 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopez CA, McNeely TP, Nurmakova K, Beavers WN, Skaar EP. Clostridioides difficile proline fermentation in response to commensal clostridia. Anaerobe. 2020;63 doi: 10.1016/j.anaerobe.2020.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu W, Winter MG, Spiga L, Hughes ER, Chanin R, Mulgaonkar A, et al. Xenosiderophore utilization promotes bacteroides thetaiotaomicron resilience during colitis. Cell Host Microbe. 2020;27(3) doi: 10.1016/j.chom.2020.01.010. 376-88 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Golonka R, Yeoh BS, Vijay-Kumar M. The iron tug-of-war between bacterial siderophores and innate immunity. J Innate Immun. 2019;11(3):249–262. doi: 10.1159/000494627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo Y, Kitamoto S, Kamada N. Microbial adaptation to the healthy and inflamed gut environments. Gut Microbes. 2020;12(1) doi: 10.1080/19490976.2020.1857505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 71.Irrazabal T, Belcheva A, Girardin SE, Martin A, Philpott DJ. The multifaceted role of the intestinal microbiota in colon cancer. Mol Cell. 2014;54(2):309–320. doi: 10.1016/j.molcel.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 72.Wilson MR, Jiang Y, Villalta PW, Stornetta A, Boudreau PD, Carra A, et al. The human gut bacterial genotoxin colibactin alkylates DNA. Science. 2019;363(6428) doi: 10.1126/science.aar7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pleguezuelos-Manzano C, Puschhof J, Rosendahl Huber A, van Hoeck A, Wood HM, Nomburg J, et al. Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nature. 2020;580(7802):269–273. doi: 10.1038/s41586-020-2080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol. 2016;17(3):230–240. doi: 10.1038/ni.3384. [DOI] [PubMed] [Google Scholar]
- 75.de Almeida CV, Taddei A, Amedei A. The controversial role of Enterococcus faecalis in colorectal cancer. Therap Adv Gastroenterol. 2018;11 doi: 10.1177/1756284818783606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slade DJ. New roles for fusobacterium nucleatum in cancer: target the bacteria, host, or both? Trends Cancer. 2021;7(3):185–187. doi: 10.1016/j.trecan.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 77.Borrelli A, Bonelli P, Tuccillo FM, Goldfine ID, Evans JL, Buonaguro FM, et al. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: current and innovative therapeutic approaches. Redox Biol. 2018;15:467–479. doi: 10.1016/j.redox.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kadosh E, Snir-Alkalay I, Venkatachalam A, May S, Lasry A, Elyada E, et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature. 2020;586(7827):133–138. doi: 10.1038/s41586-020-2541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schulz MD, Atay C, Heringer J, Romrig FK, Schwitalla S, Aydin B, et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature. 2014;514(7523):508–512. doi: 10.1038/nature13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goncalves MD, Lu C, Tutnauer J, Hartman TE, Hwang SK, Murphy CJ, et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science. 2019;363(6433):1345–1349. doi: 10.1126/science.aat8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dewdney B, Alanazy M, Gillman R, Walker S, Wankell M, Qiao L, et al. The effects of fructose and metabolic inhibition on hepatocellular carcinoma. Sci Rep. 2020;10(1):16769. doi: 10.1038/s41598-020-73653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang J, Wei H, Zhou Y, Szeto CH, Li C, Lin Y, et al. High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology. 2022;162(1) doi: 10.1053/j.gastro.2021.08.041. 135-49 e2. [DOI] [PubMed] [Google Scholar]
- 83.Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, et al. Microbial bile acid metabolites modulate gut RORgamma(+) regulatory T cell homeostasis. Nature. 2020;577(7790):410–415. doi: 10.1038/s41586-019-1865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature. 2020;581(7809):475–479. doi: 10.1038/s41586-020-2193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zou S, Fang L, Lee MH. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol Rep (Oxf) 2018;6(1):1–12. doi: 10.1093/gastro/gox031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nguyen TT, Lian S, Ung TT, Xia Y, Han JY, Jung YD. Lithocholic acid stimulates IL-8 expression in human colorectal cancer cells via activation of Erk1/2 MAPK and suppression of STAT3 activity. J Cell Biochem. 2017;118(9):2958–2967. doi: 10.1002/jcb.25955. [DOI] [PubMed] [Google Scholar]
- 87.Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360(6391) doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 89.Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491(7423):259–263. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang C, Gong G, Sheh A, Muthupalani S, Bryant EM, Puglisi DA, et al. Interleukin-22 drives nitric oxide-dependent DNA damage and dysplasia in a murine model of colitis-associated cancer. Mucosal Immunol. 2017;10(6):1504–1517. doi: 10.1038/mi.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gronke K, Hernandez PP, Zimmermann J, Klose CSN, Kofoed-Branzk M, Guendel F, et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature. 2019;566(7743):249–253. doi: 10.1038/s41586-019-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Q, Ma C, Duan Y, Heinrich B, Rosato U, Diggs LP, et al. Gut microbiome directs hepatocytes to recruit MDSCs and promote cholangiocarcinoma. Cancer Discov. 2021;11(5):1248–1267. doi: 10.1158/2159-8290.CD-20-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491(7423):254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma S, Cheng Q, Cai Y, Gong H, Wu Y, Yu X, et al. IL-17A produced by gammadelta T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 2014;74(7):1969–1982. doi: 10.1158/0008-5472.CAN-13-2534. [DOI] [PubMed] [Google Scholar]
- 97.McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell. 2014;25(5):621–637. doi: 10.1016/j.ccr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takiishi T, Fenero CIM, Camara NOS. Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers. 2017;5(4) doi: 10.1080/21688370.2017.1373208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. 2013;13(11):790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bakshani CR, Morales-Garcia AL, Althaus M, Wilcox MD, Pearson JP, Bythell JC, et al. Evolutionary conservation of the antimicrobial function of mucus: a first defence against infection. NPJ Biofilms Microbiomes. 2018;4:14. doi: 10.1038/s41522-018-0057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350(6262):830–834. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]
- 102.Cao Y, Wu K, Mehta R, Drew DA, Song M, Lochhead P, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67(4):672–678. doi: 10.1136/gutjnl-2016-313413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68(8):1516–1526. doi: 10.1136/gutjnl-2019-318427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miclotte L, De Paepe K, Rymenans L, Callewaert C, Raes J, Rajkovic A, et al. Dietary emulsifiers alter composition and activity of the human gut microbiota in vitro, irrespective of chemical or natural emulsifier origin. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.577474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang H, Wang W, Romano KA, Gu M, Sanidad KZ, Kim D, et al. A common antimicrobial additive increases colonic inflammation and colitis-associated colon tumorigenesis in mice. Sci Transl Med. 2018;10(443) doi: 10.1126/scitranslmed.aan4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359(6380):1156–1161. doi: 10.1126/science.aar7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McAllister F, Khan MAW, Helmink B, Wargo JA. The tumor microbiome in pancreatic cancer: bacteria and beyond. Cancer Cell. 2019;36(6):577–579. doi: 10.1016/j.ccell.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 108.Schwabe RF, Greten TF. Gut microbiome in HCC - mechanisms, diagnosis and therapy. J Hepatol. 2020;72(2):230–238. doi: 10.1016/j.jhep.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 109.Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14(9):527–539. doi: 10.1038/nrgastro.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wiest R, Albillos A, Trauner M, Bajaj JS, Jalan R. Targeting the gut-liver axis in liver disease. J Hepatol. 2017;67(5):1084–1103. doi: 10.1016/j.jhep.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 111.Gola A, Dorrington MG, Speranza E, Sala C, Shih RM, Radtke AJ, et al. Commensal-driven immune zonation of the liver promotes host defence. Nature. 2021;589(7840):131–136. doi: 10.1038/s41586-020-2977-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rohr MW, Narasimhulu CA, Rudeski-Rohr TA, Parthasarathy S. Negative effects of a high-fat diet on intestinal permeability: a review. Adv Nutr. 2020;11(1):77–91. doi: 10.1093/advances/nmz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. 2017;551(7680):340–345. doi: 10.1038/nature24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21(4):504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bertocchi A, Carloni S, Ravenda PS, Bertalot G, Spadoni I, Lo Cascio A, et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell. 2021;39(5) doi: 10.1016/j.ccell.2021.03.004. 708-24 e11. [DOI] [PubMed] [Google Scholar]
- 116.Del Castillo E, Meier R, Chung M, Koestler DC, Chen T, Paster BJ, et al. The microbiomes of pancreatic and duodenum tissue overlap and are highly subject specific but differ between pancreatic cancer and noncancer subjects. Cancer Epidemiol Biomarkers Prev. 2019;28(2):370–383. doi: 10.1158/1055-9965.EPI-18-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sethi V, Kurtom S, Tarique M, Lavania S, Malchiodi Z, Hellmund L, et al. Gut microbiota promotes tumor growth in mice by modulating immune response. Gastroenterology. 2018;155(1) doi: 10.1053/j.gastro.2018.04.001. 33-7 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thomas RM, Gharaibeh RZ, Gauthier J, Beveridge M, Pope JL, Guijarro MV, et al. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis. 2018;39(8):1068–1078. doi: 10.1093/carcin/bgy073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358(6369):1443–1448. doi: 10.1126/science.aal5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schwitalla S, Ziegler PK, Horst D, Becker V, Kerle I, Begus-Nahrmann Y, et al. Loss of p53 in enterocytes generates an inflammatory microenvironment enabling invasion and lymph node metastasis of carcinogen-induced colorectal tumors. Cancer Cell. 2013;23(1):93–106. doi: 10.1016/j.ccr.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 121.Qiu M, Huang K, Liu Y, Yang Y, Tang H, Liu X, et al. Modulation of intestinal microbiota by glycyrrhizic acid prevents high-fat diet-enhanced pre-metastatic niche formation and metastasis. Mucosal Immunol. 2019;12(4):945–957. doi: 10.1038/s41385-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 122.Siggins MK, Lynskey NN, Lamb LE, Johnson LA, Huse KK, Pearson M, et al. Extracellular bacterial lymphatic metastasis drives Streptococcus pyogenes systemic infection. Nat Commun. 2020;11(1):4697. doi: 10.1038/s41467-020-18454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A, et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature. 2013;494(7435):116–120. doi: 10.1038/nature11809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen S, Su T, Zhang Y, Lee A, He J, Ge Q, et al. Fusobacterium nucleatum promotes colorectal cancer metastasis by modulating KRT7-AS/KRT7. Gut Microbes. 2020;11(3):511–525. doi: 10.1080/19490976.2019.1695494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li R, Zhou R, Wang H, Li W, Pan M, Yao X, et al. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019;26(11):2447–2463. doi: 10.1038/s41418-019-0312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170(3) doi: 10.1016/j.cell.2017.07.008. 548-63 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hakozaki T, Richard C, Elkrief A, Hosomi Y, Benlaifaoui M, Mimpen I, et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol Res. 2020;8(10):1243–1250. doi: 10.1158/2326-6066.CIR-20-0196. [DOI] [PubMed] [Google Scholar]
- 128.Shiao SL, Kershaw KM, Limon JJ, You S, Yoon J, Ko EY, et al. Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell. 2021;39(9) doi: 10.1016/j.ccell.2021.07.002. 1202-13 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 130.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Griffin ME, Espinosa J, Becker JL, Luo JD, Carroll TS, Jha JK, et al. Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science. 2021;373(6558):1040–1046. doi: 10.1126/science.abc9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369(6510):1481–1489. doi: 10.1126/science.abc3421. [DOI] [PubMed] [Google Scholar]
- 133.Andrews MC, Duong CPM, Gopalakrishnan V, Iebba V, Chen WS, Derosa L, et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat Med. 2021;27(8):1432–1441. doi: 10.1038/s41591-021-01406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Luu M, Riester Z, Baldrich A, Reichardt N, Yuille S, Busetti A, et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat Commun. 2021;12(1):4077. doi: 10.1038/s41467-021-24331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sagnella SM, Yang L, Stubbs GE, Boslem E, Martino-Echarri E, Smolarczyk K, et al. Cyto-immuno-therapy for cancer: a pathway elicited by tumor-targeted, cytotoxic drug-packaged bacterially derived nanocells. Cancer Cell. 2020;37(3) doi: 10.1016/j.ccell.2020.02.001. 354-70 e7. [DOI] [PubMed] [Google Scholar]
- 136.Lin Q, Rong L, Jia X, Li R, Yu B, Hu J, et al. IFN-gamma-dependent NK cell activation is essential to metastasis suppression by engineered Salmonella. Nat Commun. 2021;12(1):2537. doi: 10.1038/s41467-021-22755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guo CJ, Allen BM, Hiam KJ, Dodd D, Van Treuren W, Higginbottom S, et al. Depletion of microbiome-derived molecules in the host using Clostridium genetics. Science. 2019;366(6471) doi: 10.1126/science.aav1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Javdan B, Lopez JG, Chankhamjon P, Lee YJ, Hull R, Wu Q, et al. Personalized mapping of drug metabolism by the human gut microbiome. Cell. 2020;181(7) doi: 10.1016/j.cell.2020.05.001. 1661-79 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gogokhia L, Buhrke K, Bell R, Hoffman B, Brown DG, Hanke-Gogokhia C, et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe. 2019;25(2) doi: 10.1016/j.chom.2019.01.008. 285-99 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zheng DW, Dong X, Pan P, Chen KW, Fan JX, Cheng SX, et al. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat Biomed Eng. 2019;3(9):717–728. doi: 10.1038/s41551-019-0423-2. [DOI] [PubMed] [Google Scholar]
- 141.Lu SSM, Mohammed Z, Haggstrom C, Myte R, Lindquist E, Gylfe A, et al. Antibiotics use and subsequent risk of colorectal cancer: a swedish nationwide population-based study. J Natl Cancer Inst. 2021 doi: 10.1093/jnci/djab125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28(3):450–455. doi: 10.1111/jgh.12070. [DOI] [PubMed] [Google Scholar]
- 143.Fernandez J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133(3):818–824. doi: 10.1053/j.gastro.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 144.Hassan H, Rompola M, Glaser AW, Kinsey SE, Phillips RS. Systematic review and meta-analysis investigating the efficacy and safety of probiotics in people with cancer. Support Care Cancer. 2018;26(8):2503–2509. doi: 10.1007/s00520-018-4216-z. [DOI] [PubMed] [Google Scholar]
- 145.Li Y, Elmen L, Segota I, Xian Y, Tinoco R, Feng Y, et al. Prebiotic-induced anti-tumor immunity attenuates tumor growth. Cell Rep. 2020;30(6) doi: 10.1016/j.celrep.2020.01.035. 1753-66 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang X, Coker OO, Chu ES, Fu K, Lau HCH, Wang YX, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70(4):761–774. doi: 10.1136/gutjnl-2019-319664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.van den Berg FF, van Dalen D, Hyoju SK, van Santvoort HC, Besselink MG, Wiersinga WJ, et al. Western-type diet influences mortality from necrotising pancreatitis and demonstrates a central role for butyrate. Gut. 2021;70(5):915–927. doi: 10.1136/gutjnl-2019-320430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology. 2017;153(6) doi: 10.1053/j.gastro.2017.08.022. 1621-33 e6. [DOI] [PubMed] [Google Scholar]
- 149.Li L, Li X, Zhong W, Yang M, Xu M, Sun Y, et al. Gut microbiota from colorectal cancer patients enhances the progression of intestinal adenoma in Apc(min/+) mice. EBioMedicine. 2019;48:301–315. doi: 10.1016/j.ebiom.2019.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Llopis M, Cassard AM, Wrzosek L, Boschat L, Bruneau A, Ferrere G, et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016;65(5):830–839. doi: 10.1136/gutjnl-2015-310585. [DOI] [PubMed] [Google Scholar]
- 151.Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371(6529):602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 152.Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371(6529):595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]