Abstract
Extracorporeal membrane oxygenation (ECMO) is an artificial lung or heart used to oxygenate and circulate blood. Veno-venous ECMO is most commonly used as an emergency therapy in patients with acute respiratory distress syndrome (ARDS), but it has the potential to be useful in other respiratory-related diseases. We report a case where ECMO successfully allowed for interventional pulmonary procedures in a patient with life-threatening tracheal obstruction secondary to lung cancer, requiring tumor debulking and silicone Y stent placement. The patient was a 44-year-old male who was admitted to the intensive care unit (ICU) for an advanced stage subcarinal tumor invading into the trachea and bilateral main stem bronchi. The tumor was unresectable, and the first attempt to debulk the tumor was terminated due to the risk of complete airway occlusion. With the help of ECMO, the second attempt at tumor debulking was successful, and a Y stent was placed. The patient regained ventilation in both lungs and was transferred out of the ICU on day 2 post-op. The pathology confirmed squamous cell carcinoma, programmed death-ligand 1 (PD-L1) 99%. The patient received immunotherapy after hospital discharge. ECMO has the potential to be useful for patients with severe tracheal obstructions and compromised respiratory systems. For patients with certain types of lung cancer, who are good candidates for novel immunotherapies and targeted therapies, it offers a potential bridging therapy for procedures that would otherwise be too dangerous and should be considered when traditional treatments for these patients fail.
Keywords: Malignant central airway obstruction, Extracorporeal membrane oxygenation (ECMO), Tracheobronchial stent, Immunotherapy
1. Introduction
In the past few years, new immunotherapies for lung cancer have significantly improved outcomes for patients fighting for lung cancer [1]. Veno-venous extracorporeal membrane oxygenation (VV ECMO) has had the most clinical use in patients with acute respiratory distress syndrome (ARDS) [2]. However, VV ECMO is now being recognized for creating new treatment options in interventional pulmonology with the possibility of longer term survival for patients. It provides critical time for patients with select advanced malignancy to receive novel immunotherapy agents to which patients may have dramatic and long-lasting responses [1]. In this report, we present a case where VV ECMO successfully allowed for the interventional treatment of a patient with an unresectable, invasive malignancy almost completely obstructing the trachea.
2. Case presentation
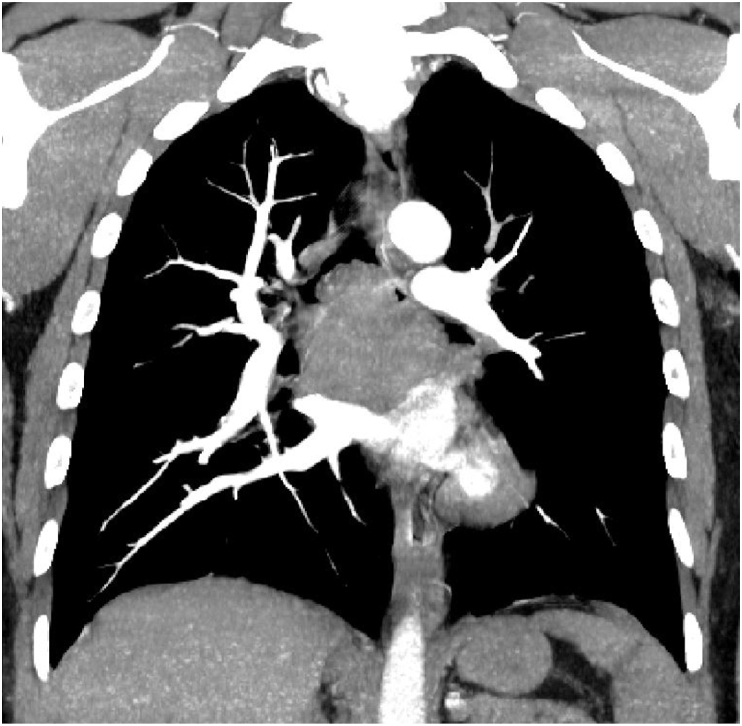
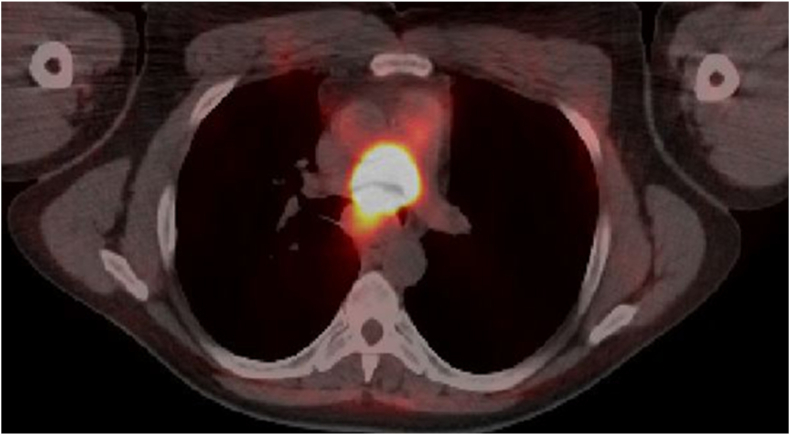
A 44-year-old male presented with a two-week history of cough, hemoptysis, chest pain, solid food dysphagia, and a 7.7-kg weight loss. He had hypertension, dyslipidemia, and a 30 pack year smoking history. On physical examination, the patient had reduced breath sounds in the left lung field. He required 3 liters per minute of supplemental oxygen through a nasal cannula to keep oxygen saturation at 90%. A chest computed tomography (CT) showed that the patient had a 4.7 cm × 6.5 cm subcarinal tracheal mass invading into the carina, lower portion of the trachea, and main stem bronchus (Fig. 1). The positron emission tomography (PET) scan revealed hypermetabolic subcarinal mass (standardized uptake value [SUV] 50.0), gastrohepatic node (SUV 4.0), and focal hypermetabolism in the left paraspinous muscle (SUV 8.1) (Fig. 2). He was admitted into the intensive care unit (ICU) for airway monitoring due to the high risk of complete tracheal obstruction.
Fig. 1.
CT showed subcarinal tumor invading into the carina.
Fig. 2.
PET showed hypermetabolic subcarinal mass.
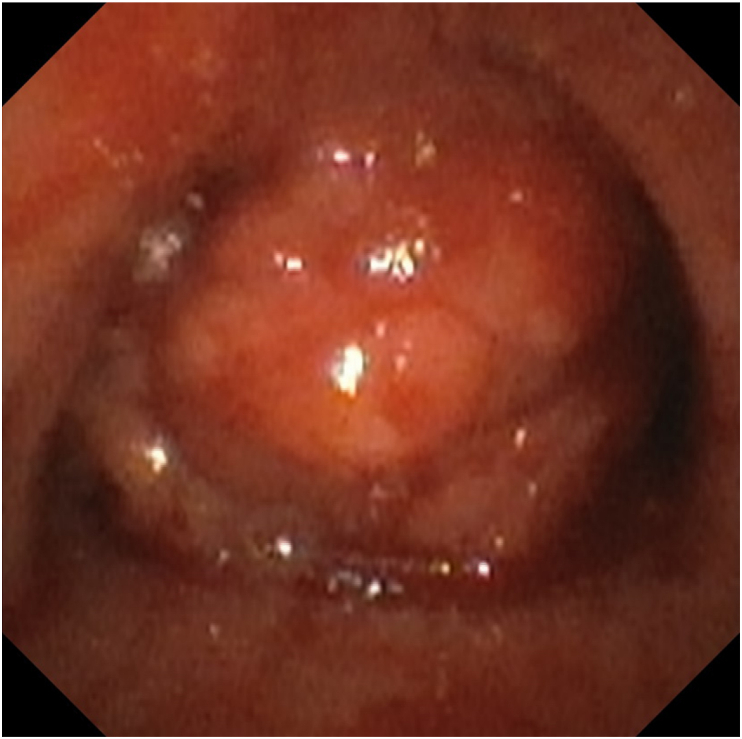
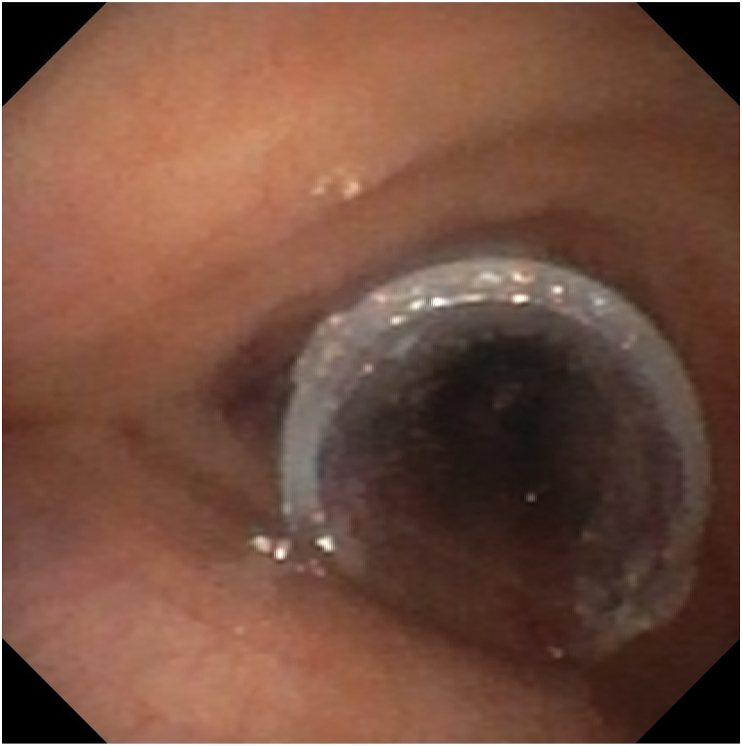
Given the position of the tumor, the patient was not a candidate for tumor resection surgery. Interventional pulmonology was consulted for rigid bronchoscopy and tumor debulking, to help open the airway. A flexible bronchoscopy was performed under general anesthesia to evaluate the airway in the operating room. The tumor completely occluded the left main stem bronchus and caused approximately 95% occlusion of the right main stem bronchus (Fig. 3). Rapid onsite evaluation of the tumor fine needle aspiration smears showed non-small cell lung cancer (NSCLC). Because of the risk of total airway obstruction and life-threatening bleeding, debulking was terminated. The patient was extubated and transferred back to the ICU. After an in-depth discussion of the procedure findings, prognosis, and next-step management options with the interventional pulmonology, oncology, critical care, and ECMO teams, the patient agreed to undergo tumor debulking under ECMO support. Rigid bronchoscopy with tumor debulking and stent placement with VV ECMO support was planned for later the same day.
Fig. 3.
The flexible bronchoscopy showed the tumor right above the carina.
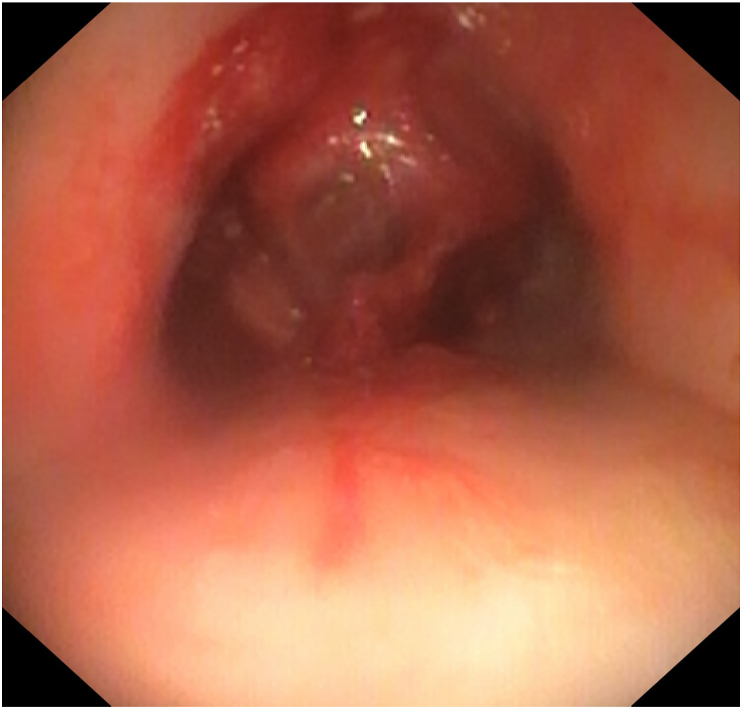
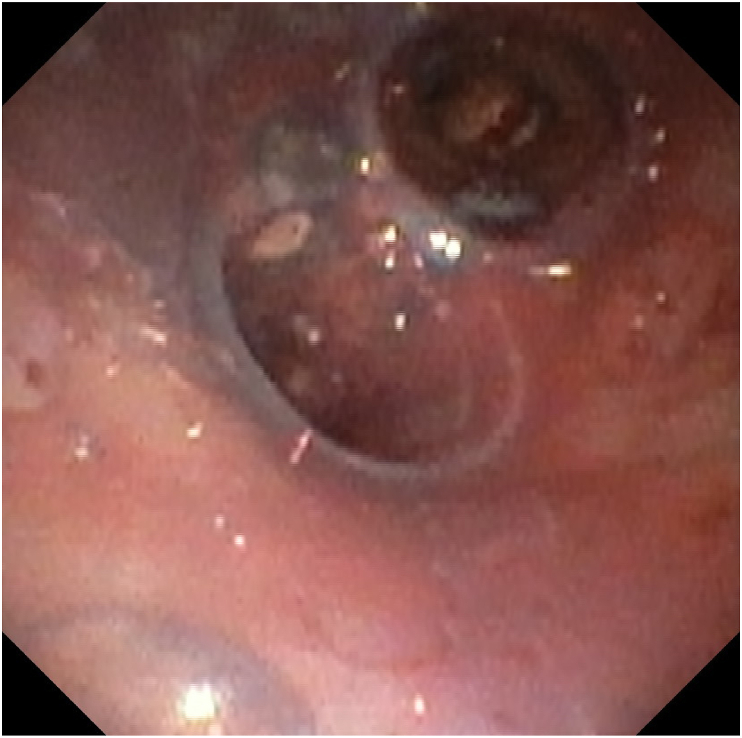
The patient received an ECMO cannula in the right internal jugular and the right femoral vein in the operating room. The rigid bronchoscopy with tumor debulking was reattempted and successful with VV ECMO support (Fig. 4). A customized Y stent extending 2.5 cm into the right main stem bronchus and 4.5 cm into the left main stem bronchus was also placed in the trachea and main stem bronchi (Fig. 5, Fig. 6). With the tumor debulked, the residual mass was able to be pushed completely behind the Y stent, which opened all 5 lobes of the lungs. The patient had good ventilation after the procedure and was able to be immediately taken off of ECMO post-operatively. He was monitored in the ICU and treated for post-obstructive pneumonia caused by the tumor. He did not have hemoptysis or a hypoxemic event after the procedure and was transferred out of the ICU on day 2 post-op. The tumor pathology reported squamous cell carcinoma, programmed death-ligand 1 (PD-L1) 99%. He was discharged from the hospital one week later and received immunotherapy with pembrolizumab, a PD-1 inhibitor.
Fig. 4.
Tumor debulking with ECMO support.
Fig. 5.
Y stent was placed (trachea view).
Fig. 6.
Y stent was placed (carina view).
3. Discussion
Lung cancer is one of the most deadly forms of cancer in the United States [3]. Patients diagnosed with lung cancer have an average 5-year survival rate of 15%, with tumor type and stage having the most significant impact on prognosis. Many patients with advanced lung cancer are treated in the ICU for respiratory failure with high mortality. New immunotherapies (e.g., PD-L1 antibodies) and targeted therapies have dramatically improved the prognosis of lung cancer [1,4]. It is critical that we give patients the time they need to receive such treatments.
In the United States, an estimated 80,000 patients per year are treated for a central airway obstruction caused by a malignancy [5]. Managing the airway is particularly difficult in these patients, who require immediate intervention if large tumors invade into the airway and obstruct the trachea. The best option for patients with an unresectable tracheal obstruction secondary to lung cancer is the placement of a stent with or without tumor debulking, which immediately opens the airway [6]. Stent placement depends upon the nature of the central airway obstruction. In our case, obstruction was mixed (both extrinsic compression and intrinsic obstruction) and necessitated stent placement after tumor debulking.
Rigid bronchoscopy is required for tumor debulking and stent placement [7]. Rigid bronchoscopy can become high risk for patients with severe tracheal obstructions because of the possibility for further occlusion of the airway during the procedure. Even minor bleeding or clotting can convert a 90% critical narrowing to a complete airway obstruction. For these patients, a better way to proceed with the treatment is with an external oxygenation source, as mechanical ventilation would be ineffective for complete airway obstruction. VV ECMO offers this possibility because it can oxygenate and recirculate the blood without assistance from the lungs. In VV ECMO, a cannula inserted into the femoral vein drains blood from the venous circulation and oxygenates the blood before returning it to circulation through a second cannula located at the right internal jugular vein [8]. VV ECMO has had some success in ARDS and is considered a rescue therapy for patients with severe ARDS when mechanical ventilation fails [9]. A trial comparing VV ECMO with traditional mechanical ventilation in patients with severe respiratory failure found that 63% of VV ECMO patients had a 6-month survival without disabilities compared with 47% of patients on mechanical ventilation [10]. Furthermore, there have been reports of successful VV ECMO therapy in the management of central airway obstructions related to tracheal stenosis and hemoptysis, indicating that VV ECMO therapy may have broader applications in pulmonary pathogenesis [11,12].
Y stents are used for tracheal obstructions that originate near the carina. The stent's shape allows for the stabilization of both the right and left primary bronchi. Y stents are safe, provide rapid relief of patient symptoms, and allow time for the treatment of the underlying condition [13,14]. Since Y stents require rigid bronchoscopy, patients who have high risk of severe hypoxemia during the procedures might not be candidates for stent placement. VV ECMO can act as a bridging therapy in these cases. Y stent placement supported by VV ECMO therapy is rare, but it has been reported for tracheal tears and tracheobronchomalacia [15,16]. Our center reported another case in which Y stent placement combined with VV ECMO was successful in treating a central airway obstruction caused by advanced stage cancer involving the trachea.
For our patient, VV ECMO allowed for interventional pulmonary therapy that would have otherwise been too risky. There are only rare reports of ECMO used in combination with either the airway tumor debulking or stent placement, but our patient was able to undergo both procedures while on VV ECMO with favorable outcomes. Furthermore, there has been one additional case that also reported success with a similar treatment strategy for an airway obstructing lung cancer [17]. This finding is encouraging as there are currently few treatment options for patients with severe tracheal obstructions secondary to tumors. ECMO-supported tumor debulking and stent placement is a viable and life-saving treatment strategy for patients with these conditions. With the development of immunotherapy and targeted therapy, it may also increase the long-term survival in selected lung cancer patients.
It is our opinion that such cases should be carefully selected with multidisciplinary discussion between the interventional pulmonology, oncology, ECMO, ICU, and anesthesia teams prior to treating any applicable cases. Factors such as ECMO availability, expertise, cost, and appropriateness of case selection are key to optimizing outcomes in these situations. Additionally, we believe that a database with relevant ECMO cases should be kept for future reference. While ECMO was useful in our patient's treatment, it should not be considered the only way to deal with a malignant central airway obstruction, as a majority of cases can be treated successfully without it.
4. Conclusion
The potential applications of VV ECMO may be useful for patients with advanced stages of lung cancer. In patients with life-threatening tracheal obstructions and inadequate ventilation, ECMO can act as a bridging therapy for necessary procedures. Our case uniquely reflected the use of ECMO in bronchoscopic interventions for a patient with a severe tracheal obstructing malignancy with high positive PD-L1 expression, providing the patient with time to receive novel immunotherapy. We encourage physicians to consider ECMO support for carefully selected severe tracheal obstruction cases, as it has the potential to improve treatment outcomes for these patients.
Statement of ethics
Written informed consent was obtained.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
All data used to support the findings of this study are available from the corresponding author upon request.
Declaration of competing interest
There are no conflicts of interest to disclose from the authors.
References
- 1.Reck M., et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50% J. Clin. Oncol. 2021;39(21):2339–2349. doi: 10.1200/jco.21.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaheen A., et al. Veno-venous extracorporeal membrane oxygenation (V V ECMO): indications, preprocedural considerations, and technique. J. Card. Surg. 2016;31(4):248–252. doi: 10.1111/jocs.12690. [DOI] [PubMed] [Google Scholar]
- 3.Mattiuzzi C., Lippi G. Current cancer epidemiology. J Epidemiol Glob Health. 2019;9(4):217–222. doi: 10.2991/jegh.k.191008.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz-Cordero R., Devine W.P. Targeted therapy and checkpoint immunotherapy in lung cancer. Surg Pathol Clin. 2020;13(1):17–33. doi: 10.1016/j.path.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Oberg C., Folch E., Santacruz J.F. Management of malignant airway obstruction. AME Medical Journal. 2018;3 https://amj.amegroups.com/article/view/4747 [Google Scholar]
- 6.Tjahjono R., Chin R.Y., Flynn P. Tracheobronchial stents in palliative care: a case series and literature review. BMJ Support. Palliat. Care. 2018;8(3):335–339. doi: 10.1136/bmjspcare-2018-001522. [DOI] [PubMed] [Google Scholar]
- 7.Batra H., Yarmus L. Indications and complications of rigid bronchoscopy. Expet Rev. Respir. Med. 2018;12(6):509–520. doi: 10.1080/17476348.2018.1473037. [DOI] [PubMed] [Google Scholar]
- 8.Jayaraman A.L., et al. Cannulation strategies in adult veno-arterial and veno-venous extracorporeal membrane oxygenation: techniques, limitations, and special considerations. Ann. Card Anaesth. 2017;20(Supplement):S11–s18. doi: 10.4103/0971-9784.197791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiumello D., Brioni M. Severe hypoxemia: which strategy to choose. Crit. Care. 2016;20(1):132. doi: 10.1186/s13054-016-1304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peek G.J., et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. doi: 10.1016/s0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 11.Natt B., et al. The use of extracorporeal membrane oxygenation in the bronchoscopic management of critical upper airway obstruction. J Bronchology Interv Pulmonol. 2017;24(1):e12–e14. doi: 10.1097/lbr.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 12.Stiff A., Harrison R., Palatnik A. Case report of massive hemoptysis in pregnancy requiring veno-venous extracorporeal membrane oxygenation. J. Obstet. Gynaecol. Res. 2019;45(12):2452–2455. doi: 10.1111/jog.14110. [DOI] [PubMed] [Google Scholar]
- 13.Gompelmann D., et al. Self-expanding Y stents in the treatment of central airway stenosis: a retrospective analysis. Ther. Adv. Respir. Dis. 2013;7(5):255–263. doi: 10.1177/1753465813489766. [DOI] [PubMed] [Google Scholar]
- 14.Madan K., et al. A multicenter experience with the placement of self-expanding metallic tracheobronchial Y stents. J Bronchology Interv Pulmonol. 2016;23(1):29–38. doi: 10.1097/lbr.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 15.Mohd Esa N.Y., et al. Silicone Y-stent insertion under extracorporeal membrane oxygenation (ECMO) in a patient with tracheal tear. BMJ Case Rep. 2020;13(12) doi: 10.1136/bcr-2020-236414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George T.J., et al. Respiratory support with venovenous extracorporeal membrane oxygenation during stenting of tracheobronchomalacia. Ann. Thorac. Surg. 2012;94(5):1736–1737. doi: 10.1016/j.athoracsur.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitazawa S., et al. Successful use of extracorporeal membrane oxygenation for airway-obstructing lung adenocarcinoma. Thorac Cancer. 2020;11(10):3024–3028. doi: 10.1111/1759-7714.13623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used to support the findings of this study are available from the corresponding author upon request.