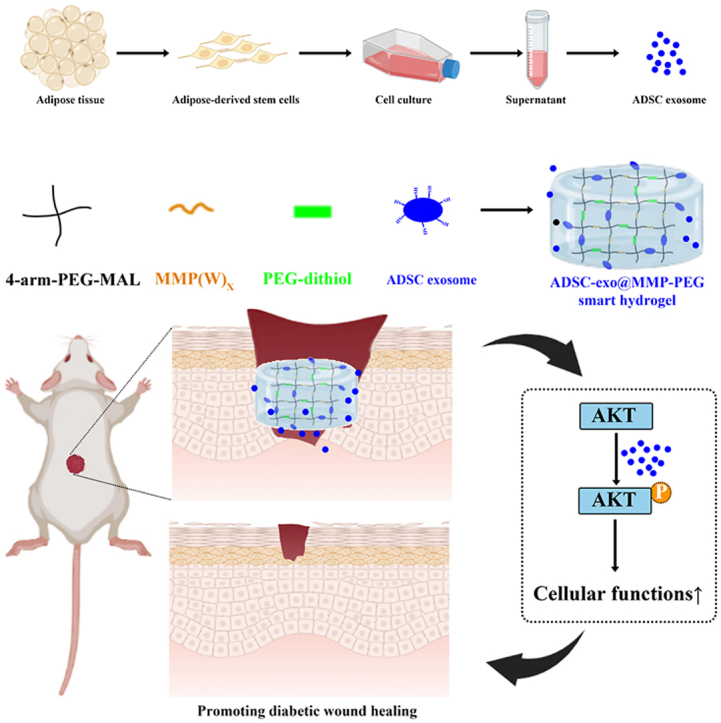
Abstract
Diabetic wound complications are financially costly and difficult to heal in worldwide. Whereas the therapies of diabetic wound, such as wound dressing, endocrine therapy or flap-transplantations, were not satisfied. Based on our previous study of exosome secreted by adipose-derived stem cell (ADSC-exo), we loaded ADSC-exo into the matrix metalloproteinase degradable polyethylene glycol (MMP-PEG) smart hydrogel. Physical and chemical properties of ADSC-exo@MMP-PEG smart hydrogel were tested by scanning electron microscope (SEM), Fourier transform infrared spectroscopy (FTIR), weight loss examination, etc. As the hydrogel degraded in response to MMP, ADSC-exo was released and subsequently enhanced cell function via Akt signaling. Moreover, treatment with ADSC-exo@MMP-PEG smart hydrogel significantly relieved the H2O2-induced oxidative stress, which was widely recognized as a major cause of diabetic wound nonhealing. Similar results were achieved in mice diabetic wound models, in which the ADSC-exo@MMP-PEG treatment group displayed a significantly accelerated wound healing. To summarize, the present smart hydrogel with enzyme-response and exosome-release was proved to be benefit for diabetic wounds healing, which provides a reliable theoretical basis for application of ADSC-exo in treatment of diabetic wounds.
Keywords: Smart hydrogel, Diabetic wound, Exosome, Adipose-derived stem cell
Graphical abstract
Highlights
-
•
Loading ADSC-exo into PEG formed a smart hydrogel.
-
•
The smart hydrogel delivered exosome in response to MMP-2.
-
•
The smart hydrogel promoted diabetic wound healing by optimizing cellular functions.
1. Introduction
Diabetic wound is a chronic complication of diabetes mellitus. Patients with diabetic wounds mainly complain about pain, numbness, skin infection, spontaneous ulcer, gangrene, etc [1,2]. With a sharp rise of patients in diabetes mellitus, more and more chronic diabetic wounds need to be managed in clinical treatment [3]. It has been claimed that a lower limb is amputated every 30 s due to diabetes, and nearly 40% of total expenditure on diabetes is attributable to complications related to the diabetic wounds according to the international diabetes atlas [4]. The treatment of diabetic wounds majors in endocrine therapy, wound dressing and transplantation of flaps or skin [1,5,6]. However, these therapies are unsatisfied and limited in clinic.
Exosome, a kind of extracellular nanoparticles, was in size of 30–150 nm [7,8]. By releasing internal nucleic acids or proteins, exosome can mediate intercellular communication and activate signal pathway, then the physiological processes work normally [9,10]. Studies showed that exosomes secreted by mesenchymal stem cells, fibroblasts or immune cells had abilities of promoting wound healing [11,12]. There are variety of exosome sources, like adipose-derived stem cell (ADSC), bone marrow stem cell, progenitor cell. Compared with other exosome resources, ADSCs are separated from fat tissue, which is abundant in body. Its seems that ADSC-exo is a better choice for exosome-therapy in wound repairing.
The therapeutical effect of exosomes on wound healing was proved, while there are some problems in exosome delivering [[13], [14], [15], [16]]. Traditional exosome-related treatments were performed by injection, single-pass in large-dose or multiple injections in low-dose [17]. However, numbers of exosomes would be wasted due to excessive exosome-concentration when injecting in large-dose. And the operation would be complicated when exosomes were injected multiple times in low-dose [18]. To address these issues above, some biomaterials, such as hydrogels, were applied to load exosomes, which could treat diseases [[19], [20], [21]]. In most studies, exosomes were combined with hydrogels by electrostatic force [[22], [23], [24], [25]]. Whereas the releasing of exosomes wasn't controlled by time, temperature, or other stimuli. Smart hydrogel, with the identities of traditional hydrogel, could react to certain stimulus, e. g. temperature, enzyme or reactive oxygen species (ROS) [[26], [27], [28], [29]]. Some researches indicated that smart hydrogel could heal the wound better due to its stimuli-responsive ability and drug delivery capacity [[29], [30], [31], [32], [33]]. These characteristics make smart hydrogel suitable for loading exosome.
AKT pathway, a signaling related to cell proliferation, differentiation, migration and proteins synthesis, has been proved to be critical in wound repairing [[34], [35], [36]]. In previous study, we found that exosomes from adipose-derived stem cells (ADSC-exo) could promote wound healing by optimizing cellular functions via AKT signaling pathway [37]. In the meantime, it was proved that diabetes altered the activation of AKT pathway negatively, leading to decreased proliferation and delayed wound healing [38]. There also be some evidence that exosome could regulate AKT pathway for adjusting cell functions [39]. Based on these, the healing effect of ADSC-exo on diabetic wounds might be regulated by AKT pathway.
In this research, we designed a smart hydrogel loaded with ADSC-exo, ADSC-exo@MMP-PEG smart hydrogel, which could release exosome by reacting to MMP stimulating, and detected its characterization. With good biocompatibility, the ADSC-exo@MMP-PEG smart hydrogel could accelerate diabetic wound healing by promoting cellular proliferation and migration via AKT signaling pathway.
2. Methods & materials
2.1. ADSC-exo extraction and its identification
Adipose-derived stem cells (ADSC) were separated from operation-gained adipose tissues and were cultured in medium for ADSC (Cyagen, USA) with 10% exosome-free fetal bovine serum (FBS). Then ADSCs were starved in DMEM without FBS for 48 h and the supernatant was collected and stored at −80 °C. After removing the cellular debris by centrifugation (8000 g × 30min), ultracentrifugation was then performed to extract exosome (120000 g × 90min, twice). ADSC-exo was separated in PBS and stored at −80 °C. ADSC-exo was identified by transmission electron microscope (TEM) and Western blot analysis.
2.2. Preparation and characterization of ADSC-exo@MMP-PEG smart hydrogel
The raw materials, four-armed polyethylene glycol (PEG) functionalized with maleimide group (4-arm-PEG-MAL), the substrate peptides of matrix metalloproteinase (MMP(W)x), PEG chains functionalized with sulfhydryl group (PEG-SH), and ADSC-exo were mixed in room temperature to gelled into ADSC-exo@MMP-PEG. In brief, 11.05 mg 4-arm-PEG-MAL, 1.75 mg MMP(W)x, 2.21 mg PEG-SH and 50 μg ADSC-exosome were mixed in total 200 μL PBS solutions to form a ADSC-exo@MMP-PEG hydrogel. The MMP(W)x (Mn = 1605 Da) was purchased from GL Biochem (Shanghai) Ltd. The synthesis of PEG-SH and 4-arm-PEG-MAL were shown in previous study [40]. Fourier transform infrared spectroscopy (FTIR), scanning electron microscope (SEM), rheometer and other equipment were used to test the physical and chemical properties and structure. The degradation of smart hydrogel was tested by enzymatic approach (200 ng/mL MMP-2). The degradative ability was assessed by weight loss of the hydrogel (degradative ratio = (Wi–Wx)/Wi × 100%, Wi is the initial weight of dry samples and Wx means the weight of dry samples degraded at day x. The biocompatibility of raw materials and smart hydrogels were confirmed by CCK-8 assay and LIVE/DEAD staining.
2.3. ADSC-exo releasing and uptaking
The exosome releasing property of ADSC-exo@MMP-PEG was tested by protein quantitation via microBCA assay. With the hydrogel with or without 50 μg ADSC-exo dipped in the solutions (1 mL PBS with or without MMP-2), the concentration of protein of the liquid supernatant was tested. Exosome releasing(%) = (Ce– Ch) ∗ 1 mL/50 μg × 100%, Ce is the protein concentration of supernatant of ADSC-exo@MMP-PEG, and Ch is the protein concentration of supernatant of MMP-PEG hydrogel without ADSC-exo. After marked by DiI, a fluorescence probe that can combined with the membrane of exosome [41], DiI-ADSC-exo were used to prepare smart hydrogel, and the degradations of DiI-ADSC-exo@MMP-PEG smart hydrogels were co-cultured with cells. Laser confocal microscopy was adopted for observation of exosome uptaking.
2.4. Proliferation and migration assay in vitro
The proliferation of human dermal fibroblast (HDF), human immortal keratinocyte (HaCaT) and human umbilical vein endothelial cells (HUVEC) were tested by CCK-8 assay and EdU staining. The migration function of HDF, HaCaT and HUVEC were tested by scratch assay or Transwell assay. H2O2 were used to stimulate the condition of high-ROS level caused by diabete mellitus in vitro. The intracellular level of ROS was analyzed by FITC probe.
2.5. Wound healing in vivo
After adaption, C57/BJ6 mice were treated with streptozotocin (STZ) to get diabetic mice. After 2months of STZ treatment, fasting blood glucose of mice was tested to diagnose that whether the mice were in diabetic mellitus. A full-thickness skin wound was operated on the middle-line of back on diabetic mice, the diameter of wounds was 1 cm. And sterile silicone ring was fixed on the wound edge to prevent wound shrinkage automatically. Then the diabetic wounds were dressed with traditional gauze, MMP-PEG hydrogel or ADSC-exo@MMP-PEG hydrogel. The operations were performed aseptically. The wound areas were record with a camera per 7 days. After 2 weeks, tissues around the wound were dissected to further experiments. The re-epithelization and collagen deposition were confirmed by H&E and Masson staining. To analyze the cell proliferation and angiogenesis in wound area, Immunofluorescent staining and Immunochemical staining were performed. Animal experiments were followed the guidelines of Laboratory Animal Center, Huazhong University of Science and Technology.
2.6. Statistical analysis
The experimental data were analyzed by Graph Pad Prism (Version 8.0) and were shown in type of mean ± SE. The differences between two groups were analyzed by Student's T-test, while the differences among 3 or more groups were analyzed by ANOVA analysis.
3. Results & discussion
3.1. Extraction and identification of ADSC-exo
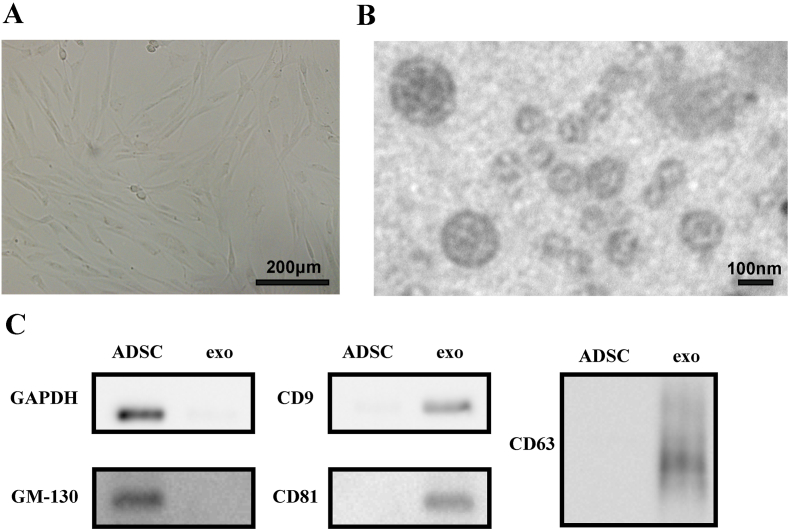
With the above methods, adipose-derived stem cells (ADSC) were separated from fat tissue and were cultured (Fig. 1A). In previous study, we successfully obtained ADSC in high purity with the typical biomarkers (CD73, CD90 and CD44) and pluripotent differentiation [37]. Then the ADSCs were starved in DMEM without fetal bovine serum (FBS) to release exosomes. ADSC-exo were extracted from cellular supernatant by gradient centrifugation and ultracentrifugation. ADSC-exo was then resuspended in PBS in concentration of 1–3 μg/mL. The identification of ADSC-exo was proved by TEM and Western Blot (Fig. 1B&C). Transmission electron microscope (TEM) revealed that ADSC-exo were double membrane and approximately circular. Western blot analysis indicated that markers of exosome, such as CD9, CD63 and CD81, were expressed while GAPDH and GM130 were not expressed in ADSC-exo.
Fig. 1.
A) image of ADSC under phase contrast microscope; B) TEM image of ADSC secreted exosome; C) Western blot analysis of markers of ADSC-exo.
3.2. Preparation and characterization of ADSC-exo@MMP-PEG smart hydrogel
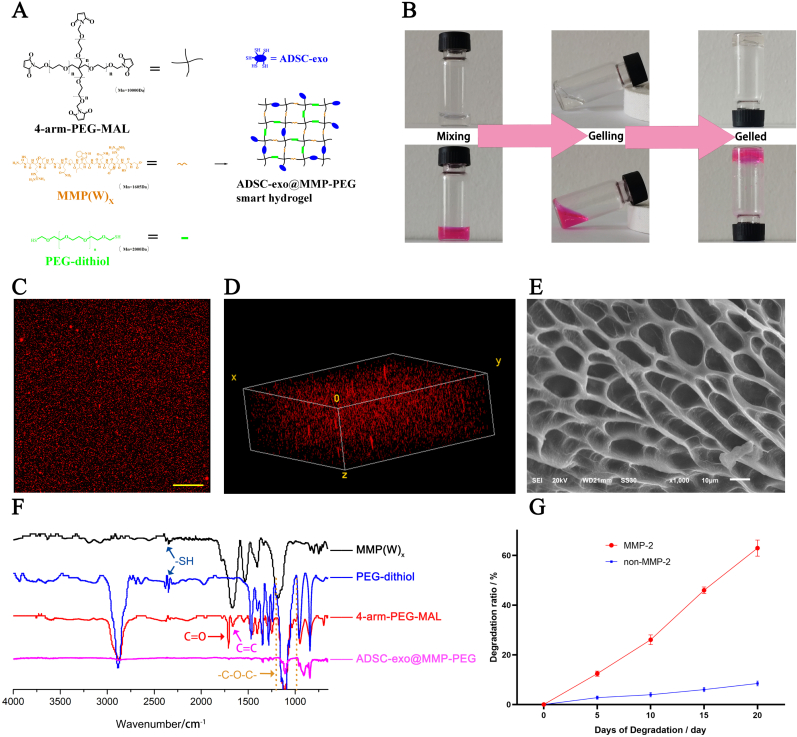
The ADSC-exo@MMP-PEG smart hydrogel was synthesized at room temperature (Fig. 2A). The cross-linking process of PEG hydrogel occurs immediately after the cross-linkers and the cross-linking monomer were in contact and reached the maximum cross-linking in about 5 min (Figure S1). The storage modulus of smart hydrogel is nearly 7400Pa. Michael addition reaction was happened between maleimide group (4-arm-PEG-MAL) and thiol group (PEG-dithiol, MMP degradable peptide and exosome). To visualized the hydrogel, rhodamine was mixed in (Fig. 2B). In order to test the exosome distribution in hydrogel, DiI was applied to label the exosome. As shown in Fig. 2C and D, the ADSC-exo was separated uniformly in the hydrogel. Fig. 2E shows the porous structure of ADSC-exo@MMP-PEG smart hydrogel and pore size was in the range of 10–30 μm. Fig. 2F shows the FTIR spectra of ADSC-exo@MMP-PEG hydrogel. There was a major peak at 2400 cm−1 attributed to –SH group in PEG-dithiol and MMP(W)x. In 4-arm-PEG-MAL, the FTIR spectra exhibited a peak at 1730 cm−1 characteristic of C O, while the absorption peak approach 1600 cm−1 was attributed to C C of maleimide group. After gelation, the peak observed at nearly 1100 cm−1 was related to C–O–C group as a result of Michael addition reaction between thiol groups and maleimide groups. The degradation of smart hydrogel was tested as well (Fig. 2G). The hydrogel was degraded a little in non-enzyme environment (<10%), and was degraded due to the presence of MMP-2. Together, these results confirmed that a smart hydrogel loaded with ADSC-exo and responsive to MMP-2 was synthesis successfully.
Fig. 2.
Preparation and characterization of ADSC-exo@MMP-PEG smart hydrogel. A) The schematic of hydrogel synthesis; B) Visualized gelling photos of smart hydrogel (stained by Rhodamine B); C&D) Plan and 3D construction image of ADSC-exo@MMP-PEG hydrogel, scar bar 100 μm (exosome was labeled by DiI); E) SEM image of hydrogel; F) FTIR spectra of MMP(W)x, PEG dithiol, 4-arm-PEG-MAL and ADSC-exo@MMP-PEG smart hydrogel; G) degradation rate of smart hydrogel with or without MMP-2.
3.3. Biocompatibility of ADSC-exo@MMP-PEG smart hydrogel
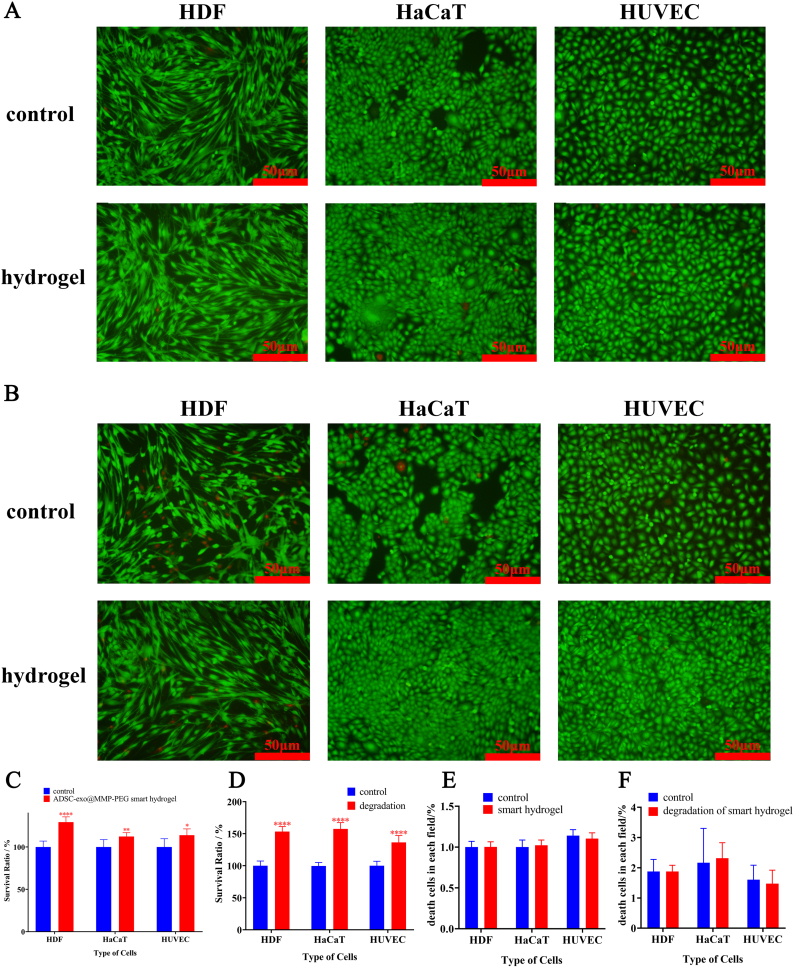
By incubating the cells with smart hydrogel, there was no cytotoxicity in HDF, HaCaT and HUVEC (Fig. 3A&C). The live cells were stained with calcein-AM (green) and dead cells were stained with propidium iodide (red). In order to eliminate the cytotoxic effects during the period of hydrogel-degrading, biocompatibility of degradative products from smart hydrogel was also tested. As shown in Fig. 3B&D, the degradation of smart hydrogel was non-toxic and promoted the proliferation of HDF, HaCaT and HUVEC. It was worth noting that the degradation of smart hydrogel improved the survival rate of both HDF, HaCaT and HUVECs whether in CCK-8 assay or live/dead staining assay. The inflammation and the foreign body response of smart hydrogels were also been performed, which there was no obviously inflammation triggered by the smart hydrogel (Figure S2). We believed that the smart hydrogel was degraded by MMP-2 and released the loaded ADSC-exo in hydrogel, which could promote the proliferation of cells.
Fig. 3.
A) Representative image of biocompatibility of smart hydrogel by live/dead staining assay, live cells were labeled with calcein-AM (green) and dead cells were labeled with propidium iodide (red); B) Representative image of biocompatibility of hydrogel degradations by live/dead staining assay; C&D) Survival rate of cells cultured with hydrogel and its degradation in CCK-8 assay; E&F) Statistical analysis of death cells in each field of Fig. 3A&B.
3.4. Exosome releasing and uptaking
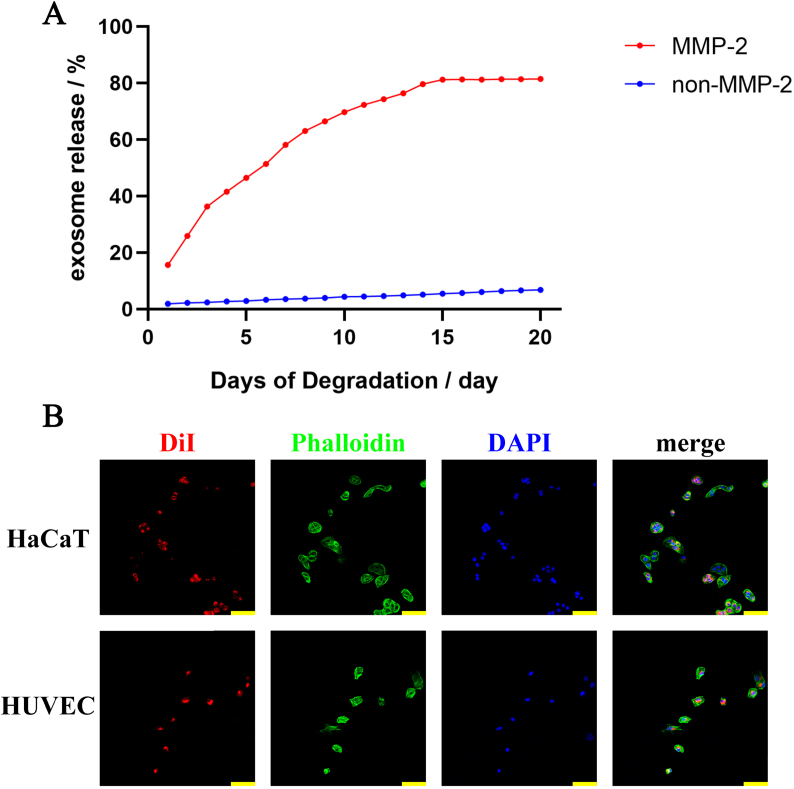
In our previous study, ADSC-exo could promote migration and proliferation of HaCaT and HUVEC by being uptaking into cells. In order to confirm that the exosome could discharge from the hydrogel and the released exosome could be took into cells, some tests were performed. The smart hydrogel was incubated in PBS with or without MMP-2, and the exosome releasing was evaluated by microBCA Protein Assay. Nearly 90% exosome loaded in the smart hydrogel was released in 20 days (Fig. 4A). By labeled with DiI, the exosome could be visualized under laser scanning confocal microscope. After co-cultured with the degradation of ADSC-exo@MMP-PEG smart hydrogel, the red fluorescence was observed in the cytoplasm of cells, meaning that HaCaT and HUVEC could uptake the exosome discharging from smart hydrogel (Fig. 4B).
Fig. 4.
A) Exosome releasing; B) Confocal images of HaCaT and HUVEC incubated with DiI-labeled ADSC-exo released from smart hydrogel, cytoskeleton was labeled with phalloidin in green and nucleus was labeled with DAPI in blue, scar bar 100 μm.
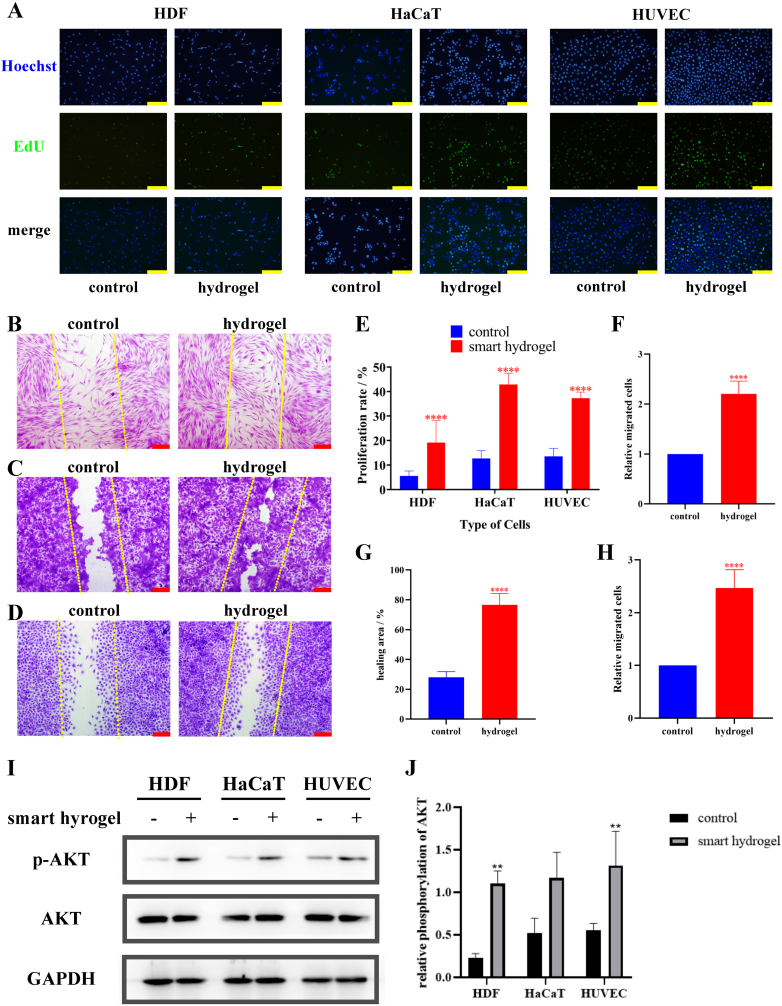
3.5. Promoting proliferation and migration by ADSC-exo@MMP-PEG smart hydrogel via AKT pathway
When cultured with products of smart hydrogel and MMP-2, the proliferation of HDF, HaCaT and HUVEC was examined by EdU Staining. Same to the CCK-8 Assay in Fig. 3A–C, the proliferation of HDF, HaCaT and HUVEC were promoted by smart hydrogel (Fig. 5A). Scratch healing assay revealed that the migration of cells was increased with the effect of ADSC-exo@MMP-PEG hydrogel (Fig. 5B–D). Additionally, Western Blot analysis proved that smart hydrogel could improve the phosphorylation of AKT, a well-known proliferation-associated protein (Fig. 5I). On the basis of these data, ADSC-exo@MMP-PEG smart hydrogel could be degraded by MMP-2 and release exosome, so that the proliferation and migration of cells could be improved via AKT pathway.
Fig. 5.
A) EdU staining of cells treated with PBS or smart hydrogel, the proliferating and dividing cells were stained in green, scar bar 100 μm; B-D) scratch assay of HDF, HaCaT and HUVECs, cells were stained with crystal violet, the original scratch was labeled by yellow dotted lines, scar bar 200 μm; E-H) Statistical analysis of A-D, ∗∗∗∗p < 0.001 vs. control group. I) Western blot analysis of phosphorylation of AKT in cells treated with smart hydrogel; J) Statistical analysis of Fig. 5I, ∗p < 0.05 vs. control, ∗∗p < 0.01 vs. control.
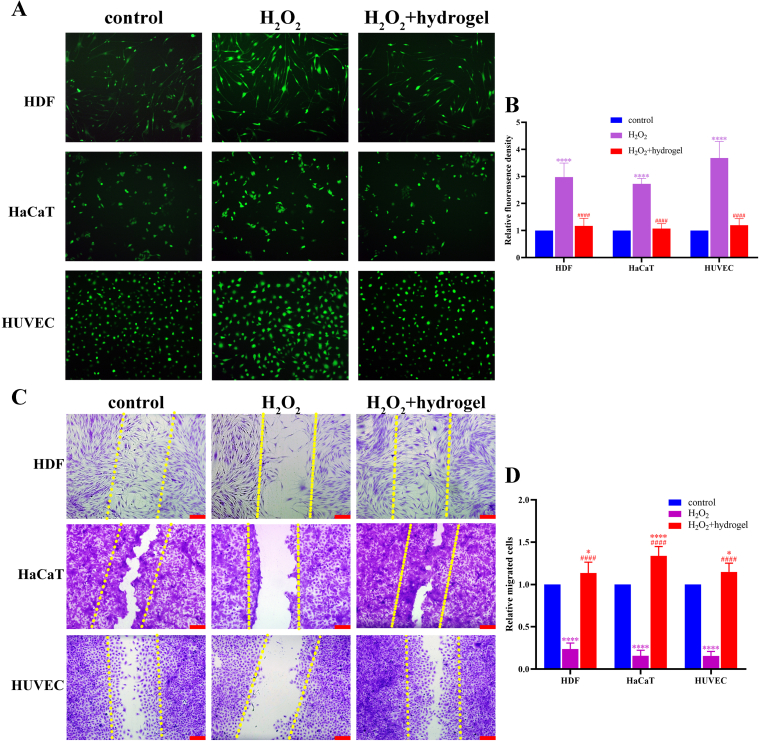
3.6. Reducing ROS in cells and reversing damaged cell migration by smart hydrogel
It is confirmed that oxidative stress plays an important role in non-healing wounds of diabetes. Endothelial cells in diabetic wounds may be damaged due to the over-loaded reactive oxygen species (ROS). Dermal fibroblast and keratinocyte could be impaired by ROS as well, which prolong the period of wound healing. The ROS-level of cells was elevated caused by H2O2, and it was lower significantly within the effect of ADSC-exo@MMP-PEG smart hydrogel (Fig. 6A&B). Fig. 6C&D reveals that the migration of HDF, HaCaT and HUVEC could be damaged by H2O2 and this weakened migration of cells was rescued by smart hydrogel. This anti-ROS property may contribute to the pro-healing ability of smart hydrogel in diabetic wounds.
Fig. 6.
A) Level of ROS in HDF, HaCaT and HUVECs, ROS was imaging in green by FITC probe; B) Statistical analysis of A, ∗∗∗∗p < 0.001 vs. control, ####p < 0.001 vs. H2O2 group; C&D) Scratch assay of HDF, HaCaT and HUVECs and statistical analysis, scar bar 200 μm, ∗p < 0.05 vs. control, ####p < 0.001 vs. control, ####p < 0.001 vs. H2O2 group.
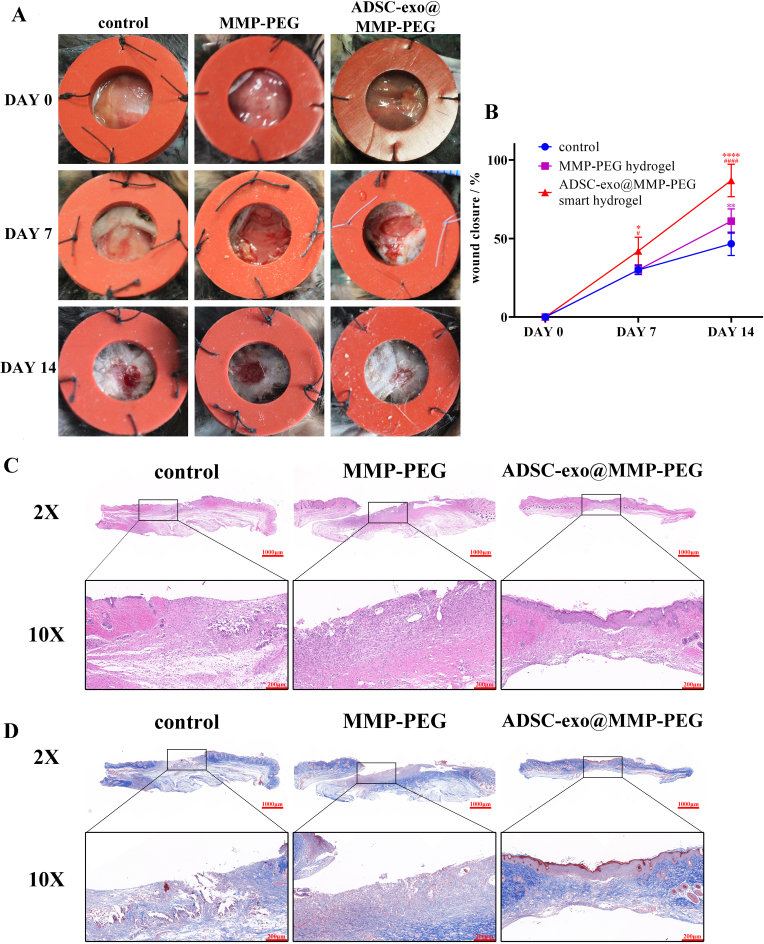
3.7. ADSC-exo@MMP-PEG accelerates wound repairing
Streptozocin-induced diabetic mice were used to assess the wound healing ability of smart hydrogel. The healing areas of wounds were recorded at 0, 7 and 14 days after the full-thickness skin damages were made. Compared to the control group, both MMP-PEG hydrogel and ADSC-exo@MMP-PEG smart hydrogel could accelerate diabetic wound repairing (Fig. 7A&B). The healing efficiency of ADSC-exo@MMP-PEG smart hydrogel was much higher than MMP-PEG hydrogel. Apart from evaluating wound healing efficiency under direct vision, some biopsy was performed to observe the regeneration of wound area. In Fig. 7C&D, smart hydrogel promoted the re-epithelialization and collagen deposition. It is notable that some cutaneous appendages were regrew in the healing area of wounds treated with ADSC-exo@MMP-PEG smart hydrogel.
Fig. 7.
Effect of smart hydrogel on diabetic wounds. A) Gross view of diabetic wound treated with gauze, MMP-PEG hydrogel or ADSC-exo@MMP-PEG smart hydrogel; B) Measurement shown in A, ∗p < 0.05 vs. control, ∗∗p < 0.01 vs. control, ∗∗∗∗p < 0.001 vs. control, #p < 0.05 vs. MMP-PEG hydrogel group, ####p < 0.001 vs. MMP-PEG hydrogel group; C&D) H&E and Masson staining of wound sections at 14 days post wounding.
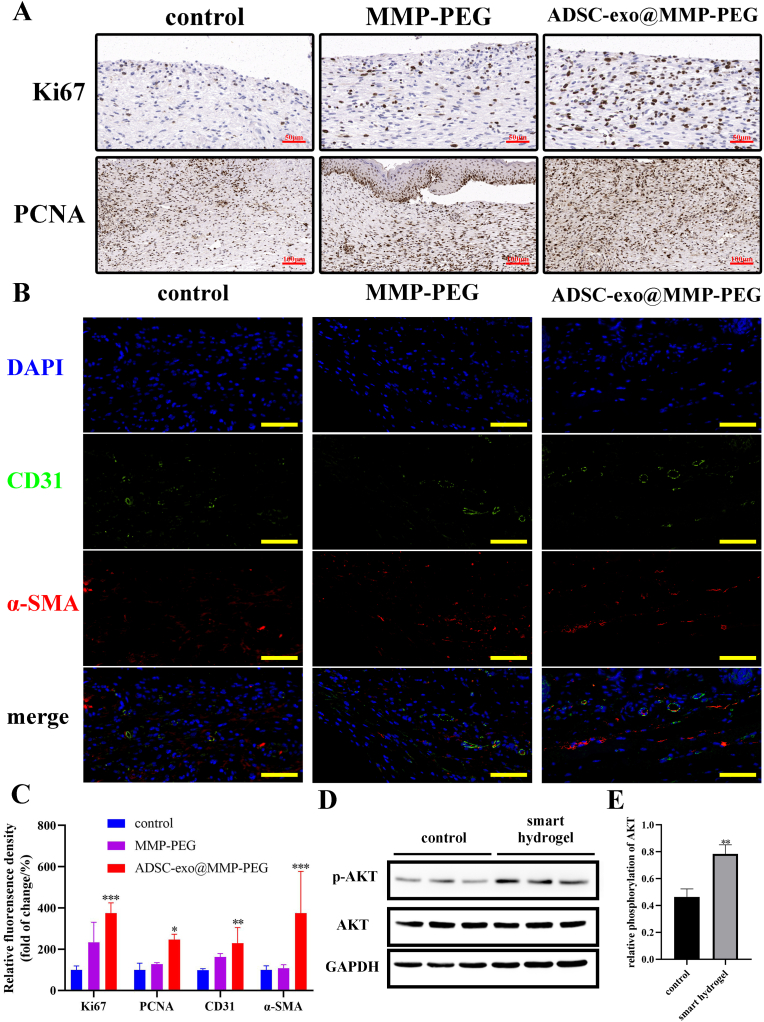
In the previous study of our research group, ADSC-exo could promote proliferation in wound area. To test whether the cells in diabetic wounds treated with smart hydrogel were proliferated, immunohistochemical analysis of Ki67 and PCNA were performed. It is obvious that cells in mitosis (Ki67 stained) and proliferation (PCNA stained) in wounds covered with smart hydrogel were much more than the wound treated with gauze (Fig. 8A). In addition, immunofluorescence staining of CD31 and α-SMA revealed that the smart hydrogel could promote angiogenesis in diabetic wounds (Fig. 8B). Western blot analysis of tissue protein obtained from wound area was performed as well. The phosphorylation of AKT in wound tissue was enhanced same as the results in vitro (Fig. 8D&E).
Fig. 8.
A) Immunohistochemical staining of Ki67 (scar bar 50 μm) and PCNA (scar bar 100 μm); B) Immunofluorescence staining of CD31 (green) and α-SMA (red), nucleus was stained blue with DAPI, scar bar 40 μm; C) Relative fluorescence of A&B; D) Western blot analysis of tissue from wound area; E) Relative phosphorylation of AKT, ∗p < 0.05 vs. control, ∗∗p < 0.01 vs. control, ∗∗∗p < 0.005 vs. control.
4. Discussion
In past decades, stem cells therapies have been widely studied in tissue regeneration and repair. While limited success has been achieved in clinical application apert from stem cell therapies on hematological diseases, because lack of efficacy and safety [42]. Recently, several studies showed that exosomes can be employed as small molecules for treatment [43]. And our previous study showed that the ADSC-exo could accelerate the wound repairing via by optimizing cellular functions via AKT and ERK pathways [37]. However, the application of exosome is limited by injection with multi-time and exosome storage and releasing [44]. In this study, we improved the delivery of ADSC-exo by loading the exosome into a smart hydrogel.
Among the biomaterials, PEG and its deviants were widely applied in tissue engineering due to its great biocompatibility, which could be degraded and manufacture a scaffold for cell migration in wound repairing [45,46]. While the traditional PEG hydrogel could not delivery small molecules according to the specific stimuli. As the biomaterial develops, there is an urgent need for smart wound-healing hydrogels that can response to the stimuli (such as temperature, pH, glucose, enzyme, etc.) [26,47]. MMPs are peptidase family which degrades extracellular matrix that involved in tissue remolding [48]. And it's reported that the over-expressed MMP may be a reason of delayed wound healing [49,50]. In this research, we also introduced a smart hydrogel which could release ADSC-exo with the stimulus of MMP in wound area.
The main process of wound healing includes hemostasis, inflammation, tissue formation and remodeling [51]. And the cell proliferation, migration and angiogenesis of keratinocytes, fibroblast and endothelial cells are happened in this period. In diabetic wound, the impaired wound healing is often associated with overloaded ROS level due to diabetes mellitus, which could damage the cell functions and trigger wound-healing deficiency. Several studies have showed that by regulating the ROS level in the lesion location may be a solution for disease treatments [52,53]. Therefore, in this research we tested the effects of ADSC-exo@MMP-PEG smart hydrogel on ROS eliminating as well as the effects on cell proliferation, migration and angiogenesis both in vivo and in vitro. Consistently with other studies of exosome-treating wounds, the smart hydrogel could reduce ROS level and promote the both proliferation and migration of HDF, HaCaT and HUVECs.
Finally, it is clearly that the smart hydrogel accelerated wound healing in the diabetic wound mice model. The phases of wound healing in this study focused on new tissue regeneration rather than remodeling phase, because that the tissue remodeling happens 3–6 weeks after injury, while the life of ADSC-exo in mice only last for 15 days [37]. However, it is important to carry on research in longer observation for further study of long-term implication of the smart hydrogel on diabetic wound remodeling.
5. Conclusion
In this study, we synthesis a smart hydrogel loaded with ADSC-exo. The loaded exosome could be released from hydrogel and be took up into cells for further use. Then the enzymatical degradation of ADSC-exo@MMP-PEG smart hydrogel could promote proliferation and migration of HDF, HaCaT and HUVECs via enhancing the phosphorylation of AKT. The smart hydrogel could decrease the ROS in cells, as well as eliminate the adverse impact on cell migration caused by oxidative stress. In vivo study showed that ADSC-exo@MMP-PEG could accelerate diabetic wound healing by promoting re-epithelialization, collagen deposition, cell proliferation and neovascularization. This study suggests that ADSC-exo@MMP-PEG smart hydrogel set a new strategy for enhancing cell functions and promoting diabetic wound repairing.
Credit author statement
Tao Jiang: Term, Methodology, Software, Investigation, Data Curation, Writing – Original Draft, Conceptualization. Siju Liu: Methodology, Software, Investigation, Data Curation, Writing – Original Draft. Zihan Wu: Formal analysis, Data Curation. Qianyun Li: Validation. Sen Ren: Methodology. Jing Chen: Visualization. Xiang Xu: Resources, Supervision. Cheng Wang: Software. Cuifen Lu: Term, Methodology, Writing - Review & Editing, Project administration. Xiaofan Yang: Methodology, Writing - Review & Editing, Project administration. Zhenbing Chen: Term, Methodology, Writing - Review & Editing, Project administration, Funding acquisition, Supervision, Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the support from the National Natural Science Foundation of China (grant number 81974289, 82172221), the Key Research and Development Program of Hubei Province (grant number 2020BCB031).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2022.100365.
Contributor Information
Cuifen Lu, Email: lucf@hubu.edu.cn.
Xiaofan Yang, Email: 2017xh0119@hust.edu.cn.
Zhenbing Chen, Email: zbchen@hust.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Armstrong D.G., Boulton A.J.M., Bus S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017;376(24):2367–2375. doi: 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- 2.Wong S.L., et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 2015;21(7):815–819. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang P., et al. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis (dagger) Ann. Med. 2017;49(2):106–116. doi: 10.1080/07853890.2016.1231932. [DOI] [PubMed] [Google Scholar]
- 4.Fedration, I.D., IDF Diabetes Atlas (tenth ed.). 2021.
- 5.Lebrun E., Tomic-Canic M., Kirsner R.S. The role of surgical debridement in healing of diabetic foot ulcers. Wound Repair Regen. 2010;18(5):433–438. doi: 10.1111/j.1524-475X.2010.00619.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y., et al. Inflammation-responsive drug-loaded hydrogels with sequential hemostasis, antibacterial, and anti-inflammatory behavior for chronically infected diabetic wound treatment. ACS Appl. Mater. Interfaces. 2021;13(28):33584–33599. doi: 10.1021/acsami.1c09889. [DOI] [PubMed] [Google Scholar]
- 7.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;(6478):367. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shao H., et al. New technologies for analysis of extracellular vesicles. Chem. Rev. 2018;118(4):1917–1950. doi: 10.1021/acs.chemrev.7b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson S.W., Nguyen J. Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. J. Contr. Release. 2016;228:179–190. doi: 10.1016/j.jconrel.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 10.Chang W., Wang J. Exosomes and their noncoding RNA cargo are emerging as new modulators for diabetes mellitus. Cells. 2019;8(8) doi: 10.3390/cells8080853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rani S., Ritter T. The exosome - a naturally secreted nanoparticle and its application to wound healing. Adv. Mater. 2016;28(27):5542–5552. doi: 10.1002/adma.201504009. [DOI] [PubMed] [Google Scholar]
- 12.Kim H., et al. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv. Sci. 2019;6(20) doi: 10.1002/advs.201900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geiger A., Walker A., Nissen E. Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochem. Biophys. Res. Commun. 2015;467(2):303–309. doi: 10.1016/j.bbrc.2015.09.166. [DOI] [PubMed] [Google Scholar]
- 14.Wang J., et al. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J. Nanobiotechnol. 2021;19(1):202. doi: 10.1186/s12951-021-00942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Y., et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J. Nanobiotechnol. 2021;19(1):150. doi: 10.1186/s12951-021-00894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tutuianu R., et al. Human mesenchymal stromal cell-derived exosomes promote in vitro wound healing by modulating the biological properties of skin keratinocytes and fibroblasts and stimulating angiogenesis. Int. J. Mol. Sci. 2021;22(12) doi: 10.3390/ijms22126239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X., et al. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9(13):4430–4438. doi: 10.1039/c7nr00352h. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y., et al. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020;15:6917–6934. doi: 10.2147/IJN.S264498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C., et al. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater. Sci. 2019;8(1):313–324. doi: 10.1039/c9bm01207a. [DOI] [PubMed] [Google Scholar]
- 20.Wang C.G., et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration (vol 9, pg 65, 2019) Theranostics. 2021;11(20):10174–10175. doi: 10.7150/thno.68432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi E.W., et al. Exosomes from human adipose-derived stem cells promote proliferation and migration of skin fibroblasts. Exp. Dermatol. 2018;27(10):1170–1172. doi: 10.1111/exd.13451. [DOI] [PubMed] [Google Scholar]
- 22.Wang M., et al. Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano. 2019;13(9):10279–10293. doi: 10.1021/acsnano.9b03656. [DOI] [PubMed] [Google Scholar]
- 23.Wang C., et al. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater. Sci. 2020;8(1):313–324. doi: 10.1039/c9bm01207a. [DOI] [PubMed] [Google Scholar]
- 24.Shiekh P.A., Singh A., Kumar A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials. 2020;249 doi: 10.1016/j.biomaterials.2020.120020. [DOI] [PubMed] [Google Scholar]
- 25.Shafei S., et al. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: an in vivo study. J. Biomed. Mater. Res. 2020;108(3):545–556. doi: 10.1002/jbm.a.36835. [DOI] [PubMed] [Google Scholar]
- 26.Lavrador P., Gaspar V.M., Mano J.F. Stimuli-responsive nanocarriers for delivery of bone therapeutics - barriers and progresses. J. Contr. Release. 2018;273:51–67. doi: 10.1016/j.jconrel.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang N., et al. pH/ROS dual-responsive polymer-drug-based nanocarriers: click-reaction preparation and fluorescence imaging-guided chemotherapy and photodynamic therapy. ACS Appl. Bio Mater. 2021;4(8):6294–6303. doi: 10.1021/acsabm.1c00569. [DOI] [PubMed] [Google Scholar]
- 28.Shi J., et al. Novel CD44-targeting and pH/redox-dual-stimuli-responsive core–shell nanoparticles loading triptolide combats breast cancer growth and lung metastasis. J. Nanobiotechnol. 2021;19(1):188. doi: 10.1186/s12951-021-00934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu S., et al. Tea polyphenol modified, photothermal responsive and ROS generative black phosphorus quantum dots as nanoplatforms for promoting MRSA infected wounds healing in diabetic rats. J. Nanobiotechnol. 2021;19(1):362. doi: 10.1186/s12951-021-01106-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Y., et al. A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J. Contr. Release. 2022;341:147–165. doi: 10.1016/j.jconrel.2021.11.027. [DOI] [PubMed] [Google Scholar]
- 31.Yang X., et al. Multiple stimuli-responsive MXene-based hydrogel as intelligent drug delivery carriers for deep chronic wound healing. Small. 2021 doi: 10.1002/smll.202104368. n/a(n/a) [DOI] [PubMed] [Google Scholar]
- 32.Yuan R., et al. Biomechanical motion-activated endogenous wound healing through LBL self-powered nanocomposite repairer with pH-responsive anti-inflammatory effect. Small. 2021;17(50) doi: 10.1002/smll.202103997. [DOI] [PubMed] [Google Scholar]
- 33.An Z., et al. Injectable thioketal-containing hydrogel dressing accelerates skin wound healing with the incorporation of reactive oxygen species scavenging and growth factor release. Biomater. Sci. 2022;10(1):100–113. doi: 10.1039/d1bm01179k. [DOI] [PubMed] [Google Scholar]
- 34.Jere S.W., Houreld N.N., Abrahamse H. Role of the PI3K/AKT (mTOR and GSK3β) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. 2019;50:52–59. doi: 10.1016/j.cytogfr.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Wang J., et al. Extracellular vesicles from HIF-1alpha-Overexpressing adipose-derived stem cells restore diabetic wounds through accelerated fibroblast proliferation and migration. Int. J. Nanomed. 2021;16:7943–7957. doi: 10.2147/IJN.S335438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X., et al. SETD2 epidermal deficiency promotes cutaneous wound healing via activation of AKT/mTOR Signalling. Cell Prolif. 2021;54(6) doi: 10.1111/cpr.13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren S., et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019;10(1) doi: 10.1186/s13287-019-1152-x. 47-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H.-L., et al. Lucidone promotes the cutaneous wound healing process via activation of the PI3K/AKT, wnt/β-catenin and NF-κB signaling pathways. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864(1):151–168. doi: 10.1016/j.bbamcr.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 39.Pang H., et al. Exosomes derived from colon cancer cells and plasma of colon cancer patients promote migration of SW480 cells through Akt/mTOR pathway. Pathol. Res. Pract. 2021;222 doi: 10.1016/j.prp.2021.153454. [DOI] [PubMed] [Google Scholar]
- 40.Yu J., et al. Synthesis and characterization of MMP degradable and maleimide cross-linked PEG hydrogels for tissue engineering scaffolds. Polym. Degrad. Stabil. 2016;133:312–320. [Google Scholar]
- 41.Hood J.L., et al. Paracrine induction of endothelium by tumor exosomes. Lab. Invest. 2009;89(11):1317–1328. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trounson A., McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 43.S E.L.A., et al. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z.G., Buller B., Chopp M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019;15(4):193–203. doi: 10.1038/s41582-018-0126-4. [DOI] [PubMed] [Google Scholar]
- 45.Sargeant T.D., et al. An in situ forming collagen-PEG hydrogel for tissue regeneration. Acta Biomater. 2012;8(1):124–132. doi: 10.1016/j.actbio.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 46.Xu Q., et al. A hybrid injectable hydrogel from hyperbranched PEG macromer as a stem cell delivery and retention platform for diabetic wound healing. Acta Biomater. 2018;75:63–74. doi: 10.1016/j.actbio.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Y., et al. Incorporation of ROS-responsive substance P-loaded zeolite imidazolate framework-8 nanoparticles into a Ca(2+)-cross-linked alginate/pectin hydrogel for wound dressing applications. Int. J. Nanomed. 2020;15:333–346. doi: 10.2147/IJN.S225197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jobin P.G., Butler G.S., Overall C.M. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864(11 Pt A):2043–2055. doi: 10.1016/j.bbamcr.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 49.Sonamuthu J., et al. MMP-9 responsive dipeptide-tempted natural protein hydrogel-based wound dressings for accelerated healing action of infected diabetic wound. Int. J. Biol. Macromol. 2020;153:1058–1069. doi: 10.1016/j.ijbiomac.2019.10.236. [DOI] [PubMed] [Google Scholar]
- 50.Li N., et al. Efficiency and safety of beta-CD-(d3)7 as siRNA carrier for decreasing matrix metalloproteinase-9 expression and improving wound healing in diabetic rats. ACS Appl. Mater. Interfaces. 2017;9(20):17417–17426. doi: 10.1021/acsami.7b02809. [DOI] [PubMed] [Google Scholar]
- 51.Gurtner G.C., et al. Wound repair and regeneration. Nature. 2008;453(7193):314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 52.He Z., et al. Reactive oxygen species (ROS): utilizing injectable antioxidative hydrogels and ROS-producing therapies to manage the double-edged sword. J. Mater. Chem. B. 2021;9(32):6326–6346. doi: 10.1039/d1tb00728a. [DOI] [PubMed] [Google Scholar]
- 53.Xu Q., et al. Versatile hyperbranched poly(beta-hydrazide ester) macromers as injectable Antioxidative hydrogels. ACS Appl. Mater. Interfaces. 2018;10(46):39494–39504. doi: 10.1021/acsami.8b15006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.