Abstract
It is widely believed that substance use disorder (SUD) results from both pre-alterations (vulnerability) and/or post-alterations (drug effects) on cortico-striatal circuits. These circuits are essential for cognitive control, motivation, reward dependent learning, and emotional processing. As such, dysfunctions in cortico-striatal circuits are thought to relate to the core features of SUD, which include compulsive drug use, loss of the ability to control drug intake, and the emergence of negative emotional states (Koob et al. 2010). While the brain circuits underlying SUD have been studied in human patients largely through imaging studies, experiments in animals have allowed researchers to examine the specific cell-types within these circuits to reveal their role in behavior relevant to SUD. Here, we will review imaging studies on cortico-striatal systems that are altered in SUD, and describe animal experiments that relate SUD to specific neural projections and cell types within this circuitry. We will end with a discussion of novel clinical approaches such as deep brain stimulation (DBS), repeated transcranial magnetic stimulation (rTMS), and pharmacological targeting of G protein-coupled receptor (GPCR) heteromers that may provide promising avenues for modulating these circuits to combat SUD in humans.
1. Introduction:
Substance use disorders (SUD) impose a dramatic toll on our society. For instance, cigarette smoking is described by the Center for Disease Control and Prevention as the “leading cause of preventable death in the US, accounting for approximately 1 of every 5 deaths... each year” (CDC 2011). Alcoholism and other drug use account for about another 75,000 deaths annually (Kochanek et al. 2011). SUD also impacts millions of people who are not addicted to drugs, due to crime, poverty, and infectious disease transmission (Leshner 1997). Presently, there is no cure for SUD, and the available treatment options do not reliably help addicts quit. Relapse rates range somewhere between 50% and 90% within the first year of treatment, and for certain drugs (particularly nicotine and opiates), this rate can approach 100% (Davstad et al. 2007, Dawson et al. 2007, Rohsenow et al. 2007, Sullivan et al. 2007, Kaskutas 2009, Gonzales et al. 2010, Powell et al. 2010). On the positive side, a handful of medications do help certain people abstain from drug use (Edens et al. 2010). While these medications do not cure SUD, they demonstrate that drug intake can be managed in certain patients, and that with further research this management may expand to larger populations. While the exact way this management will expand is not clear, recent studies employing novel technologies in both humans and animals have provided clues about novel approaches for treating SUD. In this review, we will discuss 1) imaging studies implicating cortico-striatal transmission in SUD, 2) optogenetic studies investigating cortico-striatal circuitry in drug exposed and addicted animals, and 3) novel clinical approaches for targeting cortico-striatal circuits to treat SUD.
2. Imaging Cortico-striatal Circuitry in SUD
Imaging studies have demonstrated strong links between SUD and dysfunction in cortico-striatal circuitry, and in particular dopamine (DA) function within this circuitry (Kalivas 2004, Volkow et al. 2005, Koob et al. 2008, Ikemoto 2010, Luscher et al. 2011). Positron emission tomography (PET) studies have demonstrated that SUD involves impaired DA receptor function in the striatum, such as decreases in DA D2 receptor (D2R) availability, as well as an associated reduction in baseline glucose metabolism in frontal and temporal cortices (Volkow et al. 1993) (Figure 1). Impairments in D2R function have been demonstrated following drug exposure in animals (Nader et al. 2005), but could also manifest as a pre-existing vulnerability in certain people, such as those with polymorphisms in the gene coding for the D2R (Noble 2000, Le Foll et al. 2009). On a finer temporal scale, function magnetic resonance imaging (fMRI) studies have shown that, while SUD initially affects striatal areas, it also propagates to cortical areas which are involved in attention, memory, motivation, executive function, mood and interoception (Ogawa et al. 1990, Volkow et al. 2014). Other pharmacological PET and fMRI studies demonstrated that enhancing tonic DA signaling through the use of methylphenidate can attenuate limbic brain responses to cocaine cues (Volkow et al. 2010) and normalize fMRI responses during an emotionally salient cognitive task (Li et al. 2010, Goldstein et al. 2011) in cocaine addicted individuals (Volkow et al. 2012).
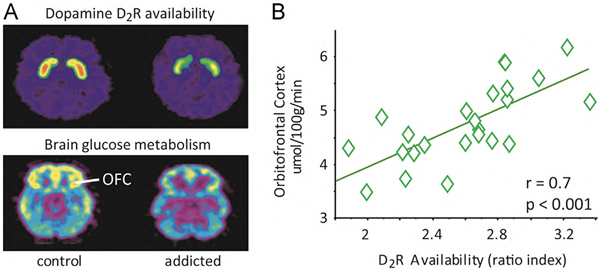
Figure 1.
Association between low DA D2R availability and low cortical glucose metabolism in SUD. A. Examine PET scan images of a control and a SUD patient demonstrating lower 11C-raclopride binding to the D2R (top), and reduced orbitofrontal (OFC) glucose metabolism (bottom). B. D2R availability is positively correlated with OFC metabolism in a group of patients.
More recently, PET and fMRI multimodality studies have documented an association between DA neurotransmission in the striatum and fMRI responses in the default mode network (DMN). The DMN is a collection of brain regions that are activated when an individual is not actively engaged with the world, but is at wakeful rest. These brain regions include the ventral PFC, the precuneus and the angular gyrus (Tomasi et al. 2009, Braskie et al. 2011). While it is unclear how endogenous DA affects the function of the DMN, fMRI studies that used stimulants (e.g., modafinil or methylphenidate) to enhance DA signals, have suggested an association between DA signaling and DMN function (Minzenberg et al. 2011, Tomasi et al. 2011). Specifically, these studies pointed to DA’s possible role in boosting cognitive processing speed in part by reducing interfering activity from DMN. Based on these findings, alterations in striatal DA function, and ensuing dysfunction in cortico-striatal circuits are believed to play a core role in SUD.
2.1. Alterations in cortico-striatal connectivity.
The majority (~90%) of DA in the brain is released in the striatum (Bertler et al. 1959), which has repeatedly been implicated as a major brain site of dysfunction in SUD. Deficits in striatal D2R function have been linked to multiple forms of drug addiction (including cocaine, nicotine, heroin, alcohol, and methamphetamine), and even non-drug consumptive behavior such as obesity (Volkow et al. 2009, Volkow et al. 2013). In addition, SUD has been associated with deficits in DA release in response to cues and events associated with drugs of abuse. One hypothesis for how these deficits in striatal DA function contribute to drug use in SUD is termed the “reward hypo-function” hypothesis. This hypothesis posits that due to dopaminergic impairments, addicts do not receive sufficient levels of DA stimulation from natural rewards or moderate quantities of drugs of abuse, and therefore must seek larger quantities of those drugs to achieve satisfaction. Despite the simple clarity of this hypothesis, and its validity in explaining certain behavioral features of SUD, it is clear that more complicated processing occurs in striatal circuitry of addicts, beyond simply seeking additional DA release.
The striatum is the primary input nucleus of the basal ganglia, a network of brain structures that integrate sensory and motivational information, and use it to guide the selection of goal-directed behaviors. Since the basal ganglia lies at the intersection of the anatomical loops linking many cortical and subcortical structures (Bornstein et al. 2011), it is hardly surprising to find cortical correlates of striatal DArgic deficits. Beyond the deficits mentioned above, recent resting state functional connectivity (RSFC) studies have found that cocaine addicted individuals display evidence of impaired functional connectivity along multiple pathways, including those linking the ventral tegmental area (VTA) and substantia nigra (SN) with the striatum and thalamus (Gu et al. 2010, Tomasi et al. 2010), the two hemispheres (Kelly et al. 2011), and the cortex with the striatum (Hanlon et al. 2011). RSFC studies are particularly useful because, by collecting data at rest, they avoid confounds associated with the performance of any task that requires the subject’s cooperation or motivation. As open access to large RSFC databases begin to successfully integrate datasets from multiple studies, RSFC results will achieve increased statistical power and sensitivity to characterize the connectivity of the human brain and its disruption in SUD (Biswal et al. 2010, Tomasi et al. 2011).
Such abnormal cortico-striatal connectivity may reflect a general phenomenon that applies to other forms of SUD beyond cocaine users. For example, abnormal functional connectivity between the dorsal prefrontal cortex (DPFC) and striatum predicts impairments in learning and the magnitude of alcohol craving among alcoholics (Park et al. 2010). In addition, decreased RSFC of anterior cingulate cortex (ACC) among chronic heroin users is associated with more drug-cue induced activation (Liu et al. 2011). Consistent with these results, a more recent comparative study of non-using vs using male prisoners (the latter being those who met criteria for SUD on any of several substances) found SUD to be associated with abnormal connectivity between cortical areas (a network of frontal cortical regions including dorsal ACC, DPFC, and frontal operculum) and subcortical areas (ventral striatum) (Motzkin et al. 2014).
In addition to deficits in DA function itself, the picture that is emerging from RSFC studies in SUD is that of a faulty flow of information between the centers that process reward and those that govern cognitive-behavioral control (i.e., the PFC). The frontal cortex is crucial for the orchestration of adaptive behavior because of its preeminent role in shaping cognition, including inhibitory control and decision making among others. Thus, dysfunctions in frontal regions are likely to hamper control over compulsive drug intake (Volkow et al. 2006). Supporting this hypothesis, a recent meta-analysis of functional neuroimaging studies on alcohol, cocaine, methamphetamine, and marijuana users (Tomasi et al. 2013) revealed frontal abnormalities that were consistent with the correlations between striatal D2R reductions and the decreased metabolic activity in ACC, orbitofrontal cortex (OFC) and dorsolateral prefrontal cortex (DLPFC, reviewed in (Tomasi et al. 2013)). The links between SUD and cortico-striatal function are likely to be both a risk factor for, and a consequence of, SUD (Belcher et al., 2014). Animal work (discussed in the next section) has also supported the role of these frontal structures in inhibitory control over drug intake, although it has revealed that this relationship is dynamic, and likely depends on the specifics of the task in which the animal is engaged.
3. Cell type specific manipulations of cortico-striatal circuitry in SUD
While the major brain systems that are altered in SUD have been extensively studied with imaging techniques in humans, modern techniques in animals are allowing the investigation of specific cell types within these circuits with unprecedented control. Two related technologies, optogenetics and chemogenetics, make it possible to selectively stimulate or inhibit particular cell types within a specific brain region by expressing either light-sensitive opsins, or Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) in these cell types (Boyden et al. 2005, Atasoy et al. 2008, Dong et al. 2010). These techniques have been used to examine specific cell types in the structures relevant to SUD, including DA D1R- and D2R-expressing medium spiny neurons (termed D1-MSNs and D2-MSNs) in the striatum, and prefrontal cortical pyramidal neurons.
3.1. Optogenetic studies of the prefrontal cortex.
As described in the imaging studies above, the prefrontal cortex (PFC) is involved in executive control, decision-making and reinforcement learning and valuation (Jentsch et al. 1999, Goldstein et al. 2002, Goldstein et al. 2011). The prelimbic cortex (PLC), which corresponds to the dorsomedial PFC in humans, is critical for instrumental learning and goal-directed behavior (Corbit et al. 2003, Ostlund et al. 2005), and is involved in drug and cue induced drug-seeking and reinstatement (Capriles et al. 2003, Di Pietro et al. 2006, Di Ciano et al. 2007, Zavala et al. 2008). The infralimbic cortex (ILC), which corresponds to the ventromedial PDC in humans, is involved in stimulus-response learning and habitual behavior (Coutureau et al. 2003, Killcross et al. 2003). Optogenetic studies of the PFC have demonstrated that specific circuits within the PFC have different roles in modulating behavior associated with SUD. Importantly, these effects depend on both the specifics of the task and the drug experience of the animal.
Optogenetically inactivating the ILC impaired habit learning (Smith et al. 2012), which could suggest that such a manipulation may prevent the development of compulsive drug seeking in patients with SUD. Indeed, inactivation of ILC inputs to the NAc abolished cocaine induced locomotor sensitization (Pascoli et al. 2012). Similarly, inhibition of ILC neurons during the expression of a cocaine contextual memory impaired recall of recent cocaine memory. Interestingly, activation of ILC neurons during this task facilitated the extinction of remote memory, suggesting a time-dependent switch in the ILC in regards to drug memories (Van den Oever et al. 2013). Photoinhibition of the PLC blunted the cue-induced reinstatement of cocaine-seeking (Stefanik et al. 2013). In apparent contrast, Chen and colleagues demonstrated that the intrinsic excitability of pyramidal neurons in the PLC is decreased following prolonged cocaine self-administration, and that activating these neurons can prevent compulsive cocaine seeking (Chen et al. 2013). While this result may seem at odds with those that suggest that inhibition of the PLC is effective at reducing drug use, the differences may be due to methodological differences (Jasinska et al. 2014). These results suggest that different stimulation parameters may attenuate drug-seeking in different contexts, or in animals with different drug exposure or abstinence histories. In both human and animal studies, it is important to recognize the complexity of neural information processing, and that dysfunction is rarely likely to be the result of homogeneous over-activation or under-activation across a wide range of conditions. Therefore, treatment options aimed at rebalancing these circuits will likely require complex manipulations.
3.2. Cell-type specific manipulations of the striatum.
A major projection of the pre-frontal cortex is to the striatum, where it follows a topographic organization such that different cortical regions preferentially innervate different areas of the striatum (Voorn et al. 2004, Humphries et al. 2010). Cortical afferents combine with those from thalamus, amygdala, and hippocampus to modulate the activity of striatal neurons. Approximately 95% of striatal neurons can be divided into two populations that exhibit distinct neurochemical expression patterns and anatomical projection targets (Albin et al. 1989, Gerfen et al. 1990), as well as differential expression of D1R and D2R. In the dorsal striatum, striatal neurons primarily expressing D1R (D1-MSNs) largely overlap with the “direct pathway” striatal projection, while striatal neurons primarily expressing D2R (D2-MSNs) largely overlap with the “indirect pathway” striatal projection, Figure 2 (Gerfen et al. 1990). D1-MSNs directly project to the internal globus pallidus (iGP) and substantia nigra pars reticulata (SNr), while D2-MSNs project indirectly to the SNr and iGP, by way of the external globus pallidus and subthalamic nucleus. In the ventral striatum (particularly in the shell), approximately 18% of medium spiny neurons express both D1R and D2R, and their projections can innervate both the ventral pallidum and the midbrain (Bertran-Gonzalez et al. 2008, Humphries et al. 2010).
Figure 2.
Basal ganglia circuitry and localization of pre- and postsynaptic adenosine and DA receptor heteromers in the D1-MSNs and D2-MSNs, as possible targets for SUD. D1-MSNs directly connect the striatum with the output structures of the basal ganglia: the internal segment of the globus pallidus (iGP) and substantia nigra pars reticulata (SNpr). D1-MSNs connect with the output structures by relays in the external segment of the globus pallidus (eGP) and the subthalamic nucleus (STN). DA induces a strong thalamo-cortical disinhibition by acting on stimulatory D1R localized in the D1-MSN, which form heteromers with A1R, and on inhibitory postsynaptic D2R localized in the D2-MSN, which form heteromers with A2AR. A2AR forming heteromers with A1 receptors are localized in cortico-striatal glutamatergic terminals that contact D1-MSN.
As D1-MSNs and D2-MSNs express different DA receptors, DA itself can exert different effects on each population. Although DA receptors are complex GPCRs with diverse function, under most conditions activation of the D1R enhances excitability while activation of the D2R reduces excitability. Optogenetic experiments on these neurons provide a frame-work for how striatal DA promotes reinforcing effects of drugs of abuse. Optogenetically activating D1-MSNs is reinforcing, and animals will work for self-stimulation of these neurons (Kravitz et al. 2012). Activating these neurons also enhances the reinforcing value of cocaine (Lobo et al. 2010) and accelerates the development of opioid tolerance (Gaspari et al. 2014). D1-MSNs have also been implicated in cocaine (Bertran-Gonzalez et al. 2008) and opioid reward (Cui et al. 2014). Consistent with these results, inhibiting D1-MSNs activity impairs reward learning and cocaine sensitization (Hikida et al. 2010). As DA itself enhances the excitability of D1-MSNs, DA-dependent reinforcement may depend on signaling through these neurons. The reinforcing properties of drugs of abuse (all of which pharmacologically increase striatal DA) (Di Chiara et al. 1988), may therefore depend on D1-MSNs, which may underlie compulsive drug use seen in SUD.
Conversely, animals will avoid places associated with stimulation of D2-MSNs (Kravitz et al. 2012), and stimulation of D2-MSNs reduces the reinforcing value of cocaine (Lobo et al. 2010), and is generally involved in aversive behavior (Hikida et al. 2010). DREADD-mediated inhibition of D1-MSNs in the NAc diminished behavioral sensitization to amphetamine, with the opposite response seen when D2-MSNs were inhibited (Ferguson et al. 2011). As stimulation of D2-MSNs is aversive, and DA can reduce the excitability of these cells through its actions on the D2R, another function of striatal DA may be to remove an otherwise pervasive aversive state. Finally, recent evidence indicates that striatal interneurons are also involved in DA function, as optogenetically inhibiting cholinergic interneurons in the NAc altered MSN activity and decreased cocaine-induced conditioned place preference, although optogenetic activation of cholinergic interneurons did not modify cocaine preference in this study (Witten et al. 2010). This latter result is somewhat surprising, as optogenetically activating cholinergic interneurons in the NAc strongly increased DA release, although again without reports of modifying behavior (Cachope et al. 2012). In the aggregate, these studies point to opposing roles for D1-MSNs and D2-MSNs in drug reward, and a role for interneurons in modulating the function of these output neurons (Table 1). Considering imaging studies that point to deficits in D2R signaling in SUD, it is possible that the impaired DA signaling through D2Rs reduces the ability of DA to remove this pervasive aversive state, which may relate to the inability of non-drug rewards to create pleasure in addicts. In addition, it is worth nothing that D2-MSN induced punishment is much more transient than D1-MSN induced reinforcement, which may relate to why both positive and negative reinforcement processes can be so persistent in SUD (Wise et al. 2014).
Table 1.
Optogenetic/chemogenetic studies implicating cortico-striatal circuitry in substance use disorder
| Publication | Brain region/circuit | Manipulation | Effect on behavior | |
|---|---|---|---|---|
| Striatum/NAc | Lobo et al., 2010 | NAc | Pairing cocaine with optogenetic activation of D1R-MSNs or D2-MSNs | D1-MSN: ↑cocaine reward D2-MSN: ↓ cocaine reward |
| Witten et al., 2010 | NAc | Optogenetic activation or inhibition of cholinergic interneurons | Inhibition during cocaine exposure: ↓ cocaine CPP Activation: no effect |
|
| Cachope et al., 2012 | NAc | Optogenetic activation of cholinergic interneurons | ↑ NAc DA release | |
| Ferguson et al., 2011 | NAc | Inhibitory DREADD in D1-MSNs or D2-MSNs | D1-MSN: ↓ amphetamine sensitization D2-MSN: ↑ amphetamine sensitization |
|
| Kravitz et al., 2012 | Dorsal striatum | Operant responding for D1- MSNor D2-MSN optogenetic stimulation | D1-MSN: ↑ reinforcement D2-MSN: ↓reinforcement |
|
| Cassataro et al., 2014 | NAc | Inhibitory or excitatory DREADD in NAc neurons | excitation: no effect inhibition: ↓ alcohol consumption |
|
| Gaspari et al., 2014 | NAc | Optogenetic activation of D1-MSNs, D2-MSNs, or RGS9-expressing neurons | D1-MSN: ↑ RGS9–2 levels ↑ morphine tolerance D2-MSN: ↓RGS9–2 levels RGS9: ↑ morphine tolerance |
|
| Prefrontal cortex | Chen et al., 2013 | PLC (dmPFC) | Optogenetic activation or inhibition after cocaine was paired with footshock | Activation: ↓cocaine seeking inhibition: ↑cocaine seeking |
| Stefanik et al., 2013 | PLC (dmPFC) | Optogenetic inhibition | ↓cue-induced reinstatement | |
| Van den Oever et al., 2013 | ILC (vmPFC) | Optogenetic activation or inhibition of pyramidal neurons during expression of cocaine-contextual memory | Activation: ↑ extinction of remote cocaine memory Inhibition: ↓recall of recent cocaine memory |
|
| PFC-to-NAc projections | Stuber et al., 2011 | mPFC-to-NAc projections | Optogenetic activation | No self-stimulation |
| Britt et al., 2012 | mPFC-to-NAc projections | Optogenetic activation | ↑real-time place preference and self-stimulation | |
| Pascoli et al., 2012 | ILC-to-NAc projections | Optogenetic inhibition | ↓cocaine induced locomotor sensitization | |
| Stefanik et al., 2013 | PLC-to-NAc projections | Optogenetic inhibition | ↓cue-induced reinstatement |
3.3. Cortico-striatal projections.
As demonstrated in human imaging studies, brain changes associated with SUD likely depend on connections between the cortex and striatum. The connections between the mPFC and NAc in particular have been suggested to be a final common pathway for eliciting drug seeking (Kalivas et al. 2005) and have been suggested to exert control over drug seeking at different phases during the development of SUD (Everitt et al. 2005). However, optogenetic studies investigating this connection have produced mixed results. While some studies have reported that stimulating the mPFC-NAc projections can support self-stimulation and real time place preference (Britt et al. 2012), others have found no reinforcement from photostimulation of this pathway (Stuber et al. 2011). Both short term (1 day) and long-term (45 days) withdrawal from either contingent or non-contingent cocaine increased release probability of the ILC-to-NAc synapses, although it was greatest in long-term withdrawal from contingent cocaine (Suska et al. 2013). The authors suggest that this reflects an increase in ILC-to-NAc shell glutamatergic synaptic transmission after withdrawal from exposure to cocaine, which could result in more habitual drug seeking. Consistent with this idea, optogenetic inhibition of the ILC-to-NAc shell pathway abolished cocaine-induced locomotor sensitization (Pascoli et al. 2012) while inhibition of the PLC-to-NAc core pathway impaired the reinstatement of cocaine seeking (Stefanik et al. 2013). This suggests that both of these pathways are critical for cocaine-directed behaviors and both may be potential targets for therapeutics. This said, whether the PLC-to-NAc pathway should be stimulated or inhibited may depend on the task, the drug history, or the state of the individual, per the discussion in section 3.2 (Jasinska et al. 2014). In addition, optogenetic stimulation protocols often differ between studies, making direct comparisons difficult (Kravitz et al. 2013). That said, both human imaging and animal studies implicate connections between cortex and striatum in SUD. In the final sections of this review, we will explore two relatively novel approaches for rebalancing cortico-striatal circuits in SUD. These include neuromodulation with DBS and rTMS, and the pharmaceuticals that can act preferentially on specific cell types and circuits through actions on GPCR heteromers.
4. Novel therapeutic approaches for treating SUD: Neuromodulation
While clinical manipulations generally lack the cell-type specific and temporal precision of optogenetic experiments, novel approaches are beginning to close this gap. One approach that has been widely applied to treating movement disorders is DBS, which refers to the implantation of chronic stimulating electrodes in specific structures to modulate their activity. The mechanism of action for DBS is not fully understood, but it likely includes inhibition of the target structure, in addition to distributed effects throughout the brain (Benazzouz et al. 2000, Hammond et al. 2008, Lee et al. 2011). Another less invasive approach to neuromodulation that also shows promise for treating SUD is repeated trans-cranial magnetic stimulation (rTMS).
4.1. Deep Brain Stimulation.
Multiple groups have evaluated DBS for treating neuropsychiatric disorders including SUD (Wichmann et al. 2011). A number of case studies, often aimed at relieving other psychiatric symptoms, have reported that DBS to the nucleus accumbens (NAc) can be beneficial for reducing intake of alcohol (Kuhn et al. 2007, Muller et al. 2009, Kuhn et al. 2011), nicotine (Mantione et al. 2010, Zhou et al. 2011), and heroin (Zhou et al. 2011, Valencia-Alfonso et al. 2012). Trials of 3–10 patients also revealed beneficial effects of NAc DBS for treating alcoholism and nicotine abuse (Kuhn et al. 2009, Voges et al. 2013). DBS studies in animals are consistent with these results, while also providing some mechanistic insights. DBS of the NAc reduced alcohol (Knapp et al. 2009, Henderson et al. 2010, Wilden et al. 2014) and cocaine (Vassoler et al. 2008, Vassoler et al. 2013) intake in rats. The study by Wilden et al. (2014) suggested that the relevant mechanism for reduction of intake is inhibition of the NAc, although other studies suggest the opposite (Vassoler et al. 2013). DBS of the PFC has been used to treat depression, chronic pain (Thomas et al. 2009, Boccard et al. 2014), and eating disorders. Although it has not been targeted to treat SUD in humans, optogenetic studies (described above) suggest that neuromodulation of the pre-frontal cortex may also be a promising approach for treating SUD. However, these studies do not provide consistent guidelines for stimulation parameters, which will likely depend on the drug experience and history of the patient, as well as state-dependent effects that are discussed below.
SUD is a complex disorder with symptoms that wax and wane over time. For example, stress and anxiety make addicts (and animals when using models of addiction) more vulnerable to relapse (or reinstatement of drug self-administration). Despite their importance in SUD, these states are often transient, lasting a few hours or days. As such, DBS manipulations may benefit from stimulation paradigms that modulate stimulation parameters in real time, based on the changing needs of the patient. Novel developments in DBS for movement disorders include “closed loop” stimulation, in which recordings from the DBS electrodes are used to optimize the stimulation parameters to best manage symptoms. Such approaches have shown promise in patients with movement disorders (Rosin et al. 2011, Carron et al. 2013, Beuter et al. 2014), and could be beneficial for treating SUD. For instance, as optogenetic work suggests that PFC inhibition can attenuate stress-induced relapse, physiological read-outs of stress (ie: heart rate, blood pressure, galvanic skin response) could inform a DBS stimulator to increase inhibition of the PFC during particularly stressful periods.
4.2. Repeated Transcranial Magnetic Stimulation.
Despite its promise, DBS requires an invasive brain surgery that is not ideal for many SUD patients. An alternative non-invasive neuromodulation technique that has shown promise in treating SUD is rTMS. rTMS refers to the repeated exposure to alternating magnetic fields to modulate brain activity (Burt et al. 2002). In general, low frequency (<1Hz) rTMS is believed to attenuate, while high frequency (>5Hz) is believed to potentiate, synaptic transmission and local brain activity (Lefaucheur 2008). While the direct effects of the magnetic stimulation are limited to cortical areas, rTMS can modulate the activity of deeper structures through cortical projections (Ben-Shachar et al. 1997, Post et al. 2001). Although the literature on the use of rTMS in the treatment of SUD is relatively sparse, it has shown promise for non-invasively modulating neural activity and treating SUD (Barr et al. 2011, Wing et al. 2013). Several studies have reported that high frequency rTMS of the left dorso-lateral prefrontal cortex (l-dlPFC) reduces cravings for tobacco (Eichhammer et al. 2003, Johann et al. 2003, Amiaz et al. 2009, Pripfl et al. 2014). Other studies have indicated that rTMS of the dlPFC reduces craving for cocaine (Camprodon et al. 2007, Politi et al. 2008) and alcohol (Mishra et al. 2010, De Ridder et al. 2011). While reductions in craving are promising, there are few demonstrations of reductions in intake following rTMS. Two studies reported transient reductions in intake of cigarettes (Amiaz et al. 2009) and alcohol (De Ridder et al. 2011), although these reductions were not tracked over long periods. Future work looking at longer-term rTMS, with the goal of reducing intake is necessary to evaluate the promise of turning reductions in craving into reductions in intake.
While the sophistication of neuromodulation is increasing, these techniques inherently lack the cell-type specificity of optogenetics or chemogenetics. As such, some researchers have speculated that optogenetics or DREADD technology will be applied to humans to bring the benefits of cell-type specific manipulations to patients. Researchers are already applying optogenetics to the human retina to combat blindness (Garg et al. 2013, Jacobson et al. 2013), and while applying optogenetics to central brain structures involves additional hurdles, there is no theoretical reason that this is not possible. However, other approaches can take advantage of normal human biology to produce cell-type specific modulation of neural activity in a non-invasive manner. In the next section, we will discuss one promising approach for targeting specific cell types in cortico-striatal circuitry.
5. Targeting specific projections with GPCR heteromer-selective ligands
5.1. Allosteric properties of GPCR oligomers.
Since their discovery, receptors have mostly been considered as single functional units. However, in recent years, a fast growing list of GPCR forming receptor oligomers has emerged (Milligan et al. 2005, Pin et al. 2007, Ferré et al. 2009, Ferré et al. 2014). Receptor oligomers are defined as a macromolecular complexes composed of at least two (functional) receptor units (protomers) with biochemical properties that are demonstrably different from those of its individual components (Ferré et al. 2009). As such, if a specific cell type expresses a unique combination of receptors, it may form unique hetereomeric complexes that could be targeted with pharmaceuticals. A first important concept that arises from the new field of GPCR oligomerization is that the pentameric structure constituted by one GPCR homodimer and one heterotrimeric G protein provides a main functional unit, and oligomeric entities can be viewed as multiples of dimers (Ferré et al., 2014). More specifically, GPCR heteromers are being considered as heterotetramers, formed of two different homodimers, each able to signal with their preferred G protein (Guitart et al., 2004; Navaro et al., 2004).
In such heteromers, each GPCR molecular unit contributes to allosteric modulation of the complex, altering the function or ligand affinity of each GPCR. For example, a ligand binding to one GPCR unit in the complex can lead to changes in the properties of a ligand binding to a different GPCR unit. The best reported example of this phenomenon is the allosteric antagonistic interaction between adenosine A2A receptor (A2AR) agonists on D2R agonists in the A2AR-D2R heteromer (Ferré et al. 1991, Dixon et al. 1997, Kudlacek et al. 2003, Navarro et al., 2014). The A2AR-D2R heteromer is selectively localized in D2-MSNs (Ferré et al. 2007, Azdad et al. 2009, Trifilieff et al. 2011) (Figure 2) and it has been hypothesized that the allosteric interactions between A2AR and D2R agonists within the A2AR-D2R heteromer provide a main mechanism responsible for the behavioral depressant effects of adenosine analogues and for the psychostimulant effects of selective A2AR antagonists and the non-selective adenosine receptor antagonist caffeine, with implications for several neuropsychiatric disorders. In fact, the same mechanism provided the main rationale for the use of A2AR antagonists in Parkinson’s disease (Armentero et al., 2011; Jorg et al., 2014). A compound that selectively activated D2Rs only when bound into A2AR-D2R heteromers could have a higher affinity for D2Rs on D2-MSNs than D2Rs on other cell types in the brain and also be potentially useful as asntiparkinsonian agent. But, based on the important role of D2-MSNs in SUD (described in section 2 and 3.2), D2R agonists or A2AR receptor antagonists with preferential affinity or functional response in A2AR-D2R receptor heteromers could could also constitute a promising approach for preferentially targeting these cells with a systemically delivered pharmaceuticals.
5.2. GPCR heteromers for targeting circuits involved in SUD.
The proof of concept of using GPCR heteromers to dissect distinct subpopulations of receptors came from experiments that compared the effects of several A2AR antagonists for their ability to produce locomotor activation or to block glutamate release induced by cortical stimulation (Orru et al. 2011). Locomotor activation depends on postsynaptic A2AR, which form heteromers with D2R on D2-MSNs. Blockade of presynaptic cortico-striatal neurotransmission depends on presynaptic A2AR which form heteromers with adenosine A1 receptors (A1Rs) localized in terminals of cortical neurons (Ciruela et al. 2006, Quiroz et al. 2009) (Figure 2). Therefore, assays of locomotor activation and cortico-striatal neurotransmission can reveal whether post-synaptic A2AR-D2R heteromers or presynaptic A2AR-A1R heteromers are preferentially activated. Based on their potency for blocking striatal glutamate release and potency for inducing locomotor activation in rats, two A2AR antagonists, SCH-442416 and KW-6002, were found to have preferential pre or post-synaptic activities, respectively (Orru et al. 2011). Parallel experiments in transfected cells demonstrated that the pre- and postsynaptic effects of these A2AR antagonists depend on their differential affinity for binding to A2AR heteromers. SCH-442416 bound with much less affinity to A2AR when co-expressed with D2R than with A1R. KW-6002 showed the best relative affinity for A2AR co-expressed with D2R. The expected differences in affinity of SCH-442416 for A2AR in the presence and absence of D2R have been reproduced in striatal tissue from wild-type mice and conditional striatal D2R knock-out mice (results in preparation). The relative affinities for the different receptor heteromers may explain the behavioral actions of these compounds.
The possibility of targeting A1R-A2AR heteromers was also used to identify an important contributor to the reinforcing effects of cannabinoids (Justinova et al. 2011, Justinova et al. 2014). A paradoxical result reported that the A2AR antagonist MSX-3 decreases THC and anandamide self-administration in squirrel monkeys at a relatively low dose, while a three-fold higher dose produced the opposite effect (Justinova et al. 2011). Based on results obtained in rats (Orru et al. 2011), it was hypothesized that the different dose-dependent effects of MSX-3 could be related to a slightly selective presynaptic effect at lower doses with an overriding postsynaptic effect at larger doses. This hypothesis was confirmed by testing the effects of SCH-442416 and KW-6002 (Justinova et al. 2014). SCH-442416 produced a significant shift to the right of the THC self-administration dose-response curves, consistent with antagonism of the reinforcing effects of THC. On the other hand, KW-6002 produced a significant shift to the left, consistent with potentiation of the reinforcing effects of THC. These results show that selectively blocking presynaptic A2AR could provide a pharmacological approach to the treatment of marijuana dependence, and underscore cortico-striatal glutamatergic neurotransmission as a possible main mechanism involved in the rewarding effects of THC. At a more general level, these results also show that while the concept of using GPCR heteromers to target specific cell types is relatively new, it is a promising approach for targeting specific cell types to modulate specific symptoms of SUD.
6. Conclusion
SUD is associated with alterations throughout the brain, including cortical and striatal circuits. Imaging studies in humans have demonstrated multiple alterations in these circuits, and animal studies are beginning to unravel the function of specific neuron types in these circuits with unprecedented precision. Ultimately, there is a need for new therapies that target these cells and ameliorate the symptoms of SUD. Based on data from human and animal work, new brain stimulation approaches are starting to make headway in targeting these structures in addicted patients. Novel pharmacological development involving GPCR heteromer-selective ligands represents another approach for targeting these circuits and alleviating the symptoms of SUD. While both brain stimulation and GPCR heteromer technologies are in their infancy, we believe that they represent promising new approaches for treating SUD.
Acknowledgements
Work supported by the intramural funds of NIDA, NIAAA and NIDDKD
References
- Albin RL, Young AB and Penney JB (1989). “The functional anatomy of basal ganglia disorders.” Trends Neurosci 12(10): 366–375. [DOI] [PubMed] [Google Scholar]
- Amiaz R, Levy D, Vainiger D, Grunhaus L. and Zangen A. (2009). “Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption.” Addiction 104(4): 653–660. [DOI] [PubMed] [Google Scholar]
- Armentero MT, Pinna A, Ferre S, Lanciego JL, Muller CE and Franco R. (2011). “Past, present and future of A(2A) adenosine receptor antagonists in the therapy of Parkinson’s disease.” Pharmacol Ther 132(3): 280–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH and Sternson SM (2008). “A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping.” J Neurosci 28(28): 7025–7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azdad K, Gall D, Woods AS, Ledent C, Ferré S. and Schiffmann SN (2009). “DA D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization.” Neuropsychopharmacology 34(4): 972–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Wing VC, George TP, Fitzgerald PB and Daskalakis ZJ (2011). “Repetitive transcranial magnetic stimulation and drug addiction.” Int Rev Psychiatry 23(5): 454–466. [DOI] [PubMed] [Google Scholar]
- Belcher AM, Volkow ND, Moeller FG and Ferré S. (2014). “Personality traits and vulnerability or resilience to substance use disorders.” Trends Cogn Sci 18(4): 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar D, Belmaker RH, Grisaru N. and Klein E. (1997). “Transcranial magnetic stimulation induces alterations in brain monoamines.” J Neural Transm 104(2–3): 191–197. [DOI] [PubMed] [Google Scholar]
- Benazzouz A. and Hallett M. (2000). “Mechanism of action of deep brain stimulation.” Neurology 55(12 Suppl 6): S13–16. [PubMed] [Google Scholar]
- Bertler A. and Rosengren E. (1959). “On the distribution in brain of monoamines and of enzymes responsible for their formation.” Experientia 15: 382–384. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E. and Girault JA (2008). “Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol.” J Neurosci 28(22): 5671–5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuter A, Lefaucheur JP and Modolo J. (2014). “Closed-loop cortical neuromodulation in Parkinson’s disease: An alternative to deep brain stimulation?” Clin Neurophysiol 125(5): 874–885. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX and Milham MP (2010). “Toward discovery science of human brain function.” Proc Natl Acad Sci U S A 107(10): 4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccard SG, Pereira EA, Moir L, Van Hartevelt TJ, Kringelbach ML, FitzGerald JJ, Baker IW, Green AL and Aziz TZ (2014). “Deep brain stimulation of the anterior cingulate cortex: targeting the affective component of chronic pain.” Neuroreport 25(2): 83–88. [DOI] [PubMed] [Google Scholar]
- Bornstein AM and Daw ND (2011). “Multiplicity of control in the basal ganglia: computational roles of striatal subregions.” Curr Opin Neurobiol 21(3): 374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G. and Deisseroth K. (2005). “Millisecond-timescale, genetically targeted optical control of neural activity.” Nat Neurosci 8(9): 1263–1268. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Landau SM, Wilcox CE, Taylor SD, O’Neil JP, Baker SL, Madison CM and Jagust WJ (2011). “Correlations of striatal dopamine synthesis with default network deactivations during working memory in younger adults.” Hum Brain Mapp 32(6): 947–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA and Bonci A. (2012). “Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens.” Neuron 76(4): 790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt T, Lisanby SH and Sackeim HA (2002). “Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis.” Int J Neuropsychopharmacol 5(1): 73–103. [DOI] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, Lovinger DM and Cheer JF (2012). “Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing.” Cell Rep 2(1): 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camprodon JA, Martinez-Raga J, Alonso-Alonso M, Shih MC and Pascual-Leone A. (2007). “One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving.” Drug Alcohol Depend 86(1): 91–94. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE and Stewart J. (2003). “A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats.” Psychopharmacology (Berl) 168(1–2): 66–74. [DOI] [PubMed] [Google Scholar]
- Carron R, Chaillet A, Filipchuk A, Pasillas-Lepine W. and Hammond C. (2013). “Closing the loop of deep brain stimulation.” Front Syst Neurosci 7: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2011). “Adult Cigarette Smoking in the United States: Current Estimate.” Retrieved August 16, 2011, from http://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm.
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW and Bonci A. (2013). “Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking.” Nature 496(7445): 359–362. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferré S. and Franco R. (2006). “Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers.” J Neurosci 26(7): 2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH and Balleine BW (2003). “The role of prelimbic cortex in instrumental conditioning.” Behav Brain Res 146(1–2): 145–157. [DOI] [PubMed] [Google Scholar]
- Coutureau E. and Killcross S. (2003). “Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats.” Behav Brain Res 146(1–2): 167–174. [DOI] [PubMed] [Google Scholar]
- Cui Y, Ostlund SB, James AS, Park CS, Ge W, Roberts KW, Mittal N, Murphy NP, Cepeda C, Kieffer BL, Levine MS, Jentsch JD, Walwyn WM, Sun YE, Evans CJ, Maidment NT and Yang XW (2014). “Targeted expression of mu-opioid receptors in a subset of striatal direct-pathway neurons restores opiate reward.” Nat Neurosci 17(2): 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davstad I, Stenbacka M, Leifman A, Beck O, Korkmaz S. and Romelsjo A. (2007). “Patterns of illicit drug use and retention in a methadone program: a longitudinal study.” J Opioid Manag 3(1): 27–34. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB and Grant BF (2007). “Rates and correlates of relapse among individuals in remission from DSM-IV alcohol dependence: a 3-year follow-up.” Alcohol Clin Exp Res 31(12): 2036–2045. [DOI] [PubMed] [Google Scholar]
- De Ridder D, Vanneste S, Kovacs S, Sunaert S. and Dom G. (2011). “Transient alcohol craving suppression by rTMS of dorsal anterior cingulate: an fMRI and LORETA EEG study.” Neurosci Lett 496(1): 5–10. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. and Imperato A. (1988). “Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats.” Proc Natl Acad Sci U S A 85(14): 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Benham-Hermetz J, Fogg AP and Osborne GE (2007). “Role of the prelimbic cortex in the acquisition, re-acquisition or persistence of responding for a drug-paired conditioned reinforcer.” Neuroscience 150(2): 291–298. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD and Kantak KM (2006). “Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats.” Eur J Neurosci 24(11): 3285–3298. [DOI] [PubMed] [Google Scholar]
- Diaz-Cabiale Z, Hurd Y, Guidolin D, Finnman UB, Zoli M, Agnati LF, Vanderhaeghen JJ, Fuxe K. and Ferré S. (2001). “Adenosine A2A agonist CGS 21680 decreases the affinity of dopamine D2 receptors for dopamine in human striatum.” Neuroreport 12(9): 1831–1834. [DOI] [PubMed] [Google Scholar]
- Dixon AK, Widdowson L. and Richardson PJ (1997). “Desensitisation of the adenosine A1 receptor by the A2A receptor in the rat striatum.” J Neurochem 69(1): 315–321. [DOI] [PubMed] [Google Scholar]
- Dong S, Rogan SC and Roth BL (2010). “Directed molecular evolution of DREADDs: a generic approach to creating next-generation RASSLs.” Nat Protoc 5(3): 561–573. [DOI] [PubMed] [Google Scholar]
- Edens E, Massa A. and Petrakis I. (2010). “Novel pharmacological approaches to drug abuse treatment.” Curr Top Behav Neurosci 3: 343–386. [DOI] [PubMed] [Google Scholar]
- Eichhammer P, Johann M, Kharraz A, Binder H, Pittrow D, Wodarz N. and Hajak G. (2003). “High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking “ J Clin Psychiatry 64(8): 951–953. [DOI] [PubMed] [Google Scholar]
- Everitt BJ and Robbins TW (2005). “Neural systems of reinforcement for drug addiction: from actions to habits to compulsion.” Nat Neurosci 8(11): 1481–1489. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL and Neumaier JF (2011). “Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization.” Nat Neurosci 14(1): 22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, Fuxe K, George SR, Javitch JA, Lohse MJ, Mackie K, Milligan G, Pfleger KD, Pin JP, Volkow ND, Waldhoer M, Woods AS and Franco R. (2009). “Building a new conceptual framework for receptor heteromers.” Nat Chem Biol 5(3): 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Casado V, Devi LA, Filizola M, Jockers R, Lohse MJ, Milligan G, Pin JP and Guitart X. (2014). “G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives.” Pharmacol Rev 66(2): 413–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Woods AS, Lluis C. and Franco R. (2007). “Functional relevance of neurotransmitter receptor heteromers in the central nervous system.” Trends Neurosci 30(9): 440–446. [DOI] [PubMed] [Google Scholar]
- Ferré S, von Euler G, Johansson B, Fredholm BB and Fuxe K. (1991). “Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes.” Proc Natl Acad Sci U S A 88(16): 7238–7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg SJ and Federman J. (2013). “Optogenetics, visual prosthesis and electrostimulation for retinal dystrophies.” Curr Opin Ophthalmol 24(5): 407–414. [DOI] [PubMed] [Google Scholar]
- Gaspari S, Papachatzaki MM, Koo JW, Carr FB, Tsimpanouli ME, Stergiou E, Bagot RC, Ferguson D, Mouzon E, Chakravarty S, Deisseroth K, Lobo MK and Zachariou V. (2014). “Nucleus Accumbens-Specific Interventions in RGS9–2 Activity Modulate Responses to Morphine.” Neuropsychopharmacology. [DOI] [PMC free article] [PubMed]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr. and Sibley DR (1990). “D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons.” Science 250(4986): 1429–1432. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ and Volkow ND (2002). “Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex.” Am J Psychiatry 159(10): 1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ and Volkow ND (2011). “Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications.” Nat Rev Neurosci 12(11): 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ and Volkow ND (2011). “Oral methylphenidate normalizes cingulate activity and decreases impulsivity in cocaine addiction during an emotionally salient cognitive task.” Neuropsychopharmacology 36(1): 366–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Jorenby DE, Brandon TH, Arteaga C. and Lee TC (2010). “Immediate versus delayed quitting and rates of relapse among smokers treated successfully with varenicline, bupropion SR or placebo.” Addiction 105(11): 2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA and Yang Y. (2010). “Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity.” Neuroimage 53(2): 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart X, Navarro G, Moreno E, Yano H, Cai NS, Sanchez-Soto M, Kumar-Barodia S, Naidu Y, Mallol J, Cortes A, lluis C, Canela EI, Casado V, McCormick PJ and Ferré S. (2014). Functional selectivity of allosteric interactions within GPCR oligomers: the dopamine D1-D3 receptor heterotetramer. Mol. Pharmacol 86: 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Ammari R, Bioulac B. and Garcia L. (2008). “Latest view on the mechanism of action of deep brain stimulation.” Mov Disord 23(15): 2111–2121. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Stapleton JR, Laurienti PJ and Porrino LJ (2011). “The association between frontal-striatal connectivity and sensorimotor control in cocaine users.” Drug Alcohol Depend 115(3): 240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson MB, Green AI, Bradford PS, Chau DT, Roberts DW and Leiter JC (2010). “Deep brain stimulation of the nucleus accumbens reduces alcohol intake in alcohol-preferring rats.” Neurosurg Focus 29(2): E12. [DOI] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K. and Nakanishi S. (2010). “Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior.” Neuron 66(6): 896–907. [DOI] [PubMed] [Google Scholar]
- Humphries MD and Prescott TJ (2010). “The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward.” Prog Neurobiol 90(4): 385–417. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. (2010). “Brain reward circuitry beyond the mesolimbic DA system: a neurobiological theory.” Neurosci Biobehav Rev 35(2): 129–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Sumaroka A, Luo X. and Cideciyan AV (2013). “Retinal optogenetic therapies: clinical criteria for candidacy.” Clin Genet 84(2): 175–182. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Chen BT, Bonci A. and Stein EA (2014). “Dorsal medial prefrontal cortex (MPFC) circuitry in rodent models of cocaine use: implications for drug addiction therapies.” Addict Biol. [DOI] [PMC free article] [PubMed]
- Jentsch JD and Taylor JR (1999). “Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli.” Psychopharmacology (Berl) 146(4): 373–390. [DOI] [PubMed] [Google Scholar]
- Johann M, Wiegand R, Kharraz A, Bobbe G, Sommer G, Hajak G, Wodarz N. and Eichhammer P. (2003). “[Transcranial magnetic stimulation for nicotine dependence].” Psychiatr Prax 30 Suppl 2: S129–131. [PubMed] [Google Scholar]
- Jorg M, Scammells PJ and Capuano B. (2014). The dopamine D2 and adenosine A2A receptors: past, present and future trends for the treatment of Parkinson’s disease. Curr Med Chem 21(27): 3188–3210. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Ferré S, Redhi GH, Mascia P, Stroik J, Quarta D, Yasar S, Muller CE, Franco R. and Goldberg SR (2011). “Reinforcing and neurochemical effects of cannabinoid CB1 receptor agonists, but not cocaine, are altered by an adenosine A2A receptor antagonist.” Addict Biol 16(3): 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Redhi GH, Goldberg SR and Ferré S. (2014). “Differential Effects of Presynaptic versus Postsynaptic Adenosine A2A Receptor Blockade on Delta9-Tetrahydrocannabinol (THC) Self-Administration in Squirrel Monkeys.” J Neurosci 34(19): 6480–6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW (2004). “Glutamate systems in cocaine addiction.” Curr Opin Pharmacol 4(1): 23–29. [DOI] [PubMed] [Google Scholar]
- Kalivas PW and Volkow ND (2005). “The neural basis of addiction: a pathology of motivation and choice.” Am J Psychiatry 162(8): 1403–1413. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA (2009). “Alcoholics anonymous effectiveness: faith meets science.” J Addict Dis 28(2): 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, Imperati D, Garavan H, Rotrosen J, Castellanos FX and Milham MP (2011). “Reduced interhemispheric resting state functional connectivity in cocaine addiction.” Biol Psychiatry 69(7): 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killcross S. and Coutureau E. (2003). “Coordination of actions and habits in the medial prefrontal cortex of rats.” Cereb Cortex 13(4): 400–408. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Tozier L, Pak A, Ciraulo DA and Kornetsky C. (2009). “Deep brain stimulation of the nucleus accumbens reduces ethanol consumption in rats.” Pharmacol Biochem Behav 92(3): 474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek KD, Xu J, Murphy SL, Miniño AM and Kung H-C (2011). “Deaths: Preliminary Data for 2009.” Natl Vital Stat Rep 59(4): 1–51. [PubMed] [Google Scholar]
- Koob GF and Le Moal M. (2008). “Addiction and the brain antireward system.” Annu Rev Psychol 59: 29–53. [DOI] [PubMed] [Google Scholar]
- Koob GF and Volkow ND (2010). “Neurocircuitry of addiction.” Neuropsychopharmacology 35(1): 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV and Bonci A. (2013). “Optogenetics, physiology, and emotions.” Front Behav Neurosci 7: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD and Kreitzer AC (2012). “Distinct roles for direct and indirect pathway striatal neurons in reinforcement.” Nat Neurosci 15(6): 816–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudlacek O, Just H, Korkhov VM, Vartian N, Klinger M, Pankevych H, Yang Q, Nanoff C, Freissmuth M. and Boehm S. (2003). “The human D2 dopamine receptor synergizes with the A2A adenosine receptor to stimulate adenylyl cyclase in PC12 cells.” Neuropsychopharmacology 28(7): 1317–1327. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Bauer R, Pohl S, Lenartz D, Huff W, Kim EH, Klosterkoetter J. and Sturm V. (2009). “Observations on unaided smoking cessation after deep brain stimulation of the nucleus accumbens.” Eur Addict Res 15(4): 196–201. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Grundler TO, Bauer R, Huff W, Fischer AG, Lenartz D, Maarouf M, Buhrle C, Klosterkotter J, Ullsperger M. and Sturm V. (2011). “Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring.” Addict Biol 16(4): 620–623. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Lenartz D, Huff W, Lee S, Koulousakis A, Klosterkoetter J. and Sturm V. (2007). “Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications?” J Neurol Neurosurg Psychiatry 78(10): 1152–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Gallo A, Le Strat Y, Lu L. and Gorwood P. (2009). “Genetics of dopamine receptors and drug addiction: a comprehensive review.” Behav Pharmacol 20(1): 1–17. [DOI] [PubMed] [Google Scholar]
- Lee KH, Chang SY, Jang DP, Kim I, Goerss S, Van Gompel J, Min P, Arora K, Marsh M, Hwang SC, Kimble CJ, Garris P, Blaha C. and Bennet KE (2011). “Emerging techniques for elucidating mechanism of action of deep brain stimulation.” Conf Proc IEEE Eng Med Biol Soc 2011: 677–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP (2008). “Principles of therapeutic use of transcranial and epidural cortical stimulation.” Clin Neurophysiol 119(10): 2179–2184. [DOI] [PubMed] [Google Scholar]
- Leshner AI (1997). “Addiction is a brain disease, and it matters.” Science 278(5335): 45–47. [DOI] [PubMed] [Google Scholar]
- Li CS, Morgan PT, Matuskey D, Abdelghany O, Luo X, Chang JL, Rounsaville BJ, Ding YS and Malison RT (2010). “Biological markers of the effects of intravenous methylphenidate on improving inhibitory control in cocaine-dependent patients.” Proc Natl Acad Sci U S A 107(32): 14455–14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Qin W, Yuan K, Li J, Wang W, Li Q, Wang Y, Sun J, von Deneen KM, Liu Y. and Tian J. (2011). “Interaction between dysfunctional connectivity at rest and heroin cues-induced brain responses in male abstinent heroin-dependent individuals.” PLoS One 6(10): e23098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH and Nestler EJ (2010). “Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward.” Science 330(6002): 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C. and Malenka RC (2011). “Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling.” Neuron 69(4): 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantione M, van de Brink W, Schuurman PR and Denys D. (2010). “Smoking cessation and weight loss after chronic deep brain stimulation of the nucleus accumbens: therapeutic and research implications: case report.” Neurosurgery 66(1): E218; discussion E218. [DOI] [PubMed] [Google Scholar]
- Milligan G. and Bouvier M. (2005). “Methods to monitor the quaternary structure of G protein-coupled receptors.” FEBS J 272(12): 2914–2925. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Yoon JH and Carter CS (2011). “Modafinil modulation of the default mode network.” Psychopharmacology (Berl) 215(1): 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BR, Nizamie SH, Das B. and Praharaj SK (2010). “Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study.” Addiction 105(1): 49–55. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Baskin-Sommers A, Newman JP, Kiehl KA and Koenigs M. (2014). “Neural correlates of substance abuse: Reduced functional connectivity between areas underlying reward and cognitive control.” Hum Brain Mapp. [DOI] [PMC free article] [PubMed]
- Muller UJ, Sturm V, Voges J, Heinze HJ, Galazky I, Heldmann M, Scheich H. and Bogerts B. (2009). “Successful treatment of chronic resistant alcoholism by deep brain stimulation of nucleus accumbens: first experience with three cases.” Pharmacopsychiatry 42(6): 288–291. [DOI] [PubMed] [Google Scholar]
- Nader MA and Czoty PW (2005). “PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation.” Am J Psychiatry 162(8): 1473–1482. [DOI] [PubMed] [Google Scholar]
- Navarro G, Aguinaga D, Moreno E, Hradsky J, Reddy PP, Cortes A, Mallol J, Casado V, Mikhaylova M, Kreutz MR, lluis C, Canela EI, McCormick PJ, Ferré S. (2014). Intracellular calcium levels determine differential modulation of allosteric interactions within G protein-coupled receptor heteromers. Chem Biol 10.1016/j.chembiol.2014.10.004 [DOI] [PMC free article] [PubMed]
- Noble EP (2000). “Addiction and its reward process through polymorphisms of the D2 dopamine receptor gene: a review.” Eur Psychiatry 15(2): 79–89. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR and Tank DW (1990). “Brain magnetic resonance imaging with contrast dependent on blood oxygenation.” Proc Natl Acad Sci U S A 87(24): 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orru M, Bakesova J, Brugarolas M, Quiroz C, Beaumont V, Goldberg SR, Lluis C, Cortes A, Franco R, Casado V, Canela EI and Ferré S. (2011). “Striatal pre- and postsynaptic profile of adenosine A(2A) receptor antagonists.” PLoS One 6(1): e16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB and Balleine BW (2005). “Lesions of medial prefrontal cortex disrupt the acquisition but not the expression of goal-directed learning.” J Neurosci 25(34): 7763–7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J. and Heinz A. (2010). “Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence.” J Neurosci 30(22): 7749–7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Turiault M. and Luscher C. (2012). “Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour.” Nature 481(7379): 71–75. [DOI] [PubMed] [Google Scholar]
- Pin JP, Neubig R, Bouvier M, Devi L, Filizola M, Javitch JA, Lohse MJ, Milligan G, Palczewski K, Parmentier M. and Spedding M. (2007). “International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers.” Pharmacol Rev 59(1): 5–13. [DOI] [PubMed] [Google Scholar]
- Politi E, Fauci E, Santoro A. and Smeraldi E. (2008). “Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving.” Am J Addict 17(4): 345–346. [DOI] [PubMed] [Google Scholar]
- Post A. and Keck ME (2001). “Transcranial magnetic stimulation as a therapeutic tool in psychiatry: what do we know about the neurobiological mechanisms?” J Psychiatr Res 35(4): 193–215. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawkins L, West R. and Pickering A. (2010). “Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors.” Psychopharmacology (Berl) 212(4): 537–549. [DOI] [PubMed] [Google Scholar]
- Pripfl J, Tomova L, Riecansky I. and Lamm C. (2014). “Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power.” Brain Stimul 7(2): 226–233. [DOI] [PubMed] [Google Scholar]
- Quiroz C, Lujan R, Uchigashima M, Simoes AP, Lerner TN, Borycz J, Kachroo A, Canas PM, Orru M, Schwarzschild MA, Rosin DL, Kreitzer AC, Cunha RA, Watanabe M. and Ferré S. (2009). “Key modulatory role of presynaptic adenosine A2A receptors in cortical neurotransmission to the striatal direct pathway.” ScientificWorldJournal 9: 1321–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Martin RA, Eaton CA and Monti PM (2007). “Cocaine craving as a predictor of treatment attrition and outcomes after residential treatment for cocaine dependence.” J Stud Alcohol Drugs 68(5): 641–648. [DOI] [PubMed] [Google Scholar]
- Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z, Vaadia E. and Bergman H. (2011). “Closed-loop deep brain stimulation is superior in ameliorating parkinsonism.” Neuron 72(2): 370–384. [DOI] [PubMed] [Google Scholar]
- Smith KS, Virkud A, Deisseroth K. and Graybiel AM (2012). “Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex.” Proc Natl Acad Sci U S A 109(46): 18932–18937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW and LaLumiere RT (2013). “Optogenetic inhibition of cocaine seeking in rats.” Addict Biol 18(1): 50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K. and Bonci A. (2011). “Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking.” Nature 475(7356): 377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MA, Garawi F, Bisaga A, Comer SD, Carpenter K, Raby WN, Anen SJ, Brooks AC, Jiang H, Akerele E. and Nunes EV (2007). “Management of relapse in naltrexone maintenance for heroin dependence.” Drug Alcohol Depend 91(2–3): 289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suska A, Lee BR, Huang YH, Dong Y. and Schluter OM (2013). “Selective presynaptic enhancement of the prefrontal cortex to nucleus accumbens pathway by cocaine.” Proc Natl Acad Sci U S A 110(2): 713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L, Bledsoe JM, Stead M, Sandroni P, Gorman D. and Lee KH (2009). “Motor cortex and deep brain stimulation for the treatment of intractable neuropathic face pain.” Curr Neurol Neurosci Rep 9(2): 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D. and Volkow ND (2011). “Association between functional connectivity hubs and brain networks.” Cereb Cortex 21(9): 2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D. and Volkow ND (2013). “Striatocortical pathway dysfunction in addiction and obesity: differences and similarities.” Crit Rev Biochem Mol Biol 48(1): 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang GJ, Wang R, Telang F, Caparelli EC, Wong C, Jayne M. and Fowler JS (2011). “Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls.” Neuroimage 54(4): 3101–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, Woicik PA, Telang F. and Goldstein RZ (2010). “Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers.” PLoS One 5(5): e10815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Telang F, Wang GJ, Chang L, Ernst T. and Fowler JS (2009). “Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention.” PLoS One 4(6): e6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Rives ML, Urizar E, Piskorowski RA, Vishwasrao HD, Castrillon J, Schmauss C, Slattman M, Gullberg M. and Javitch JA (2011). “Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum.” Biotechniques 51(2): 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia-Alfonso CE, Luigjes J, Smolders R, Cohen MX, Levar N, Mazaheri A, van den Munckhof P, Schuurman PR, van den Brink W. and Denys D. (2012). “Effective deep brain stimulation in heroin addiction: a case report with complementary intracranial electroencephalogram.” Biol Psychiatry 71(8): e35–37. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Rotaru DC, Heinsbroek JA, Gouwenberg Y, Deisseroth K, Stuber GD, Mansvelder HD and Smit AB (2013). “Ventromedial prefrontal cortex pyramidal cells have a temporal dynamic role in recall and extinction of cocaine-associated memory.” J Neurosci 33(46): 18225–18233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Schmidt HD, Gerard ME, Famous KR, Ciraulo DA, Kornetsky C, Knapp CM and Pierce RC (2008). “Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats.” J Neurosci 28(35): 8735–8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Hopkins TJ, Guercio LA, Espallergues J, Berton O, Schmidt HD and Pierce RC (2013). “Deep brain stimulation of the nucleus accumbens shell attenuates cocaine reinstatement through local and antidromic activation.” J Neurosci 33(36): 14446–14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voges J, Muller U, Bogerts B, Munte T. and Heinze HJ (2013). “Deep brain stimulation surgery for alcohol addiction.” World Neurosurg 80(3–4): S28 e21–31. [DOI] [PubMed] [Google Scholar]
- Volkow N. and Li TK (2005). “The neuroscience of addiction.” Nat Neurosci 8(11): 1429–1430. [DOI] [PubMed] [Google Scholar]
- Volkow ND and Baler RD (2014). “Addiction science: Uncovering neurobiological complexity.” Neuropharmacology 76 Pt B: 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R. and Telang F. (2009). “Imaging dopamine’s role in drug abuse and addiction.” Neuropharmacology 56 Suppl 1: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL and Wolf AP (1993). “Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers.” Synapse 14(2): 169–177. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein R, Alexoff D. and Thanos PK (2006). “High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors.” Arch Gen Psychiatry 63(9): 999–1008. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS and Tomasi D. (2012). “Addiction circuitry in the human brain.” Annu Rev Pharmacol Toxicol 52: 321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D. and Baler RD (2013). “Obesity and addiction: neurobiological overlaps.” Obes Rev 14(1): 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Telang F, Fowler JS, Pradhan K, Jayne M, Logan J, Goldstein RZ, Alia-Klein N. and Wong C. (2010). “Methylphenidate attenuates limbic brain inhibition after cocaine-cues exposure in cocaine abusers.” PLoS One 5(7): e11509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW and Pennartz CM (2004). “Putting a spin on the dorsal-ventral divide of the striatum.” Trends Neurosci 27(8): 468–474. [DOI] [PubMed] [Google Scholar]
- Wichmann T. and Delong MR (2011). “Deep-Brain Stimulation for Basal Ganglia Disorders.” Basal Ganglia 1(2): 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden JA, Qing KY, Hauser SR, McBride WJ, Irazoqui PP and Rodd ZA (2014). “Reduced ethanol consumption by alcohol-preferring (P) rats following pharmacological silencing and deep brain stimulation of the nucleus accumbens shell.” J Neurosurg 120(4): 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing VC, Barr MS, Wass CE, Lipsman N, Lozano AM, Daskalakis ZJ and George TP (2013). “Brain stimulation methods to treat tobacco addiction.” Brain Stimul 6(3): 221–230. [DOI] [PubMed] [Google Scholar]
- Wise RA and Koob GF (2014). “The development and maintenance of drug addiction.” Neuropsychopharmacology 39(2): 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C. and Deisseroth K. (2010). “Cholinergic interneurons control local circuit activity and cocaine conditioning.” Science 330(6011): 1677–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Osredkar T, Joyce JN and Neisewander JL (2008). “Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking behavior.” Synapse 62(6): 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Xu J. and Jiang J. (2011). “Deep brain stimulation of nucleus accumbens on heroin-seeking behaviors: a case report.” Biol Psychiatry 69(11): e41–42. [DOI] [PubMed] [Google Scholar]