Abstract
Coronary artery intramural hematoma is a rare complication of percutaneous coronary intervention which develops from intimal tear of coronary artery and propagates by blood accumulation along the medial surface of adjacent segment. Fifty-three-year-old male presented with nonexertional chest pain; he was referred after a positive stress test with+ moderate lateral wall ischemia. Coronary angiography showed 80% lesion in mid-left anterior descending artery (mLAD). Angiogram after angioplasty with 2.0 mm × 15 mm balloon and 3.0 mm × 15 mm drug-eluting-stent demonstrated a new stenotic lesion distal to stented mLAD segment. Subsequently, an overlapping 3.0 mm × 30 mm stent was placed with effective restoration of blood flow through LAD. During percutaneous coronary intervention (PCI), balloon predilatation can result in plaque fracture and stent deployment may cause intimal tear forming intramural hematoma which can lead to post-PCI myocardial infarction necessitating prompt detection by intravascular imaging with intravascular ultrasound and optical coherence tomography. Management is based on individual patient's characteristics and includes medical therapy, angiographic surveillance or repeat PCI.
Keywords: Coronary artery intramural hematoma, Percutaneous coronary intervention, Iatrogenic myocardial infarction, Post-percutaneous coronary intervention myocardial infarction, Coronary angiography, Angiogram
Abbreviations: CIH, Coronary Intramural Hematoma; DES, Drug-eluting stent; ICU, Intensive care unit; IVUS, Intravascular Ultrasound; LVEF, left ventricular ejection fraction; LAD, left anterior descending artery; mLAD, mid segment of left anterior descending artery; PCI, Percutaneous Coronary Intervention; OCT, Optical Coherence Tomography
Introduction
Due to its invasive nature, percutaneous coronary intervention (PCI) has mechanical complications, a rare one is coronary intramural hematoma (CIH) [1]. American College of Cardiology defines it as an “accumulation of blood within the medial space of the coronary artery displacing the internal elastic membrane inward and the external elastic membrane outward, with or without identifiable entry and exit points” [2]. It originates during PCI due to intimal tear leading to dissection into media and radiates by blood accumulation along the medial space of adjacent normal arterial segments due to lack of entry [1]. Here, we present a case of CIH developed during PCI performed for 80% stenosis of left anterior descending artery (LAD).
Case report
Fifty-three-year-old male with medical history of primary hypertension, hyperlipidemia, and prediabetes mellitus presented with new onset nonexertional left sided chest pain for 2 weeks relieved by isosorbide mononitrate and exertional dyspnea on walking 7-8 blocks. He was referred for an elective left heart catheterization after positive stress test. At admission, he had no active chest pain or shortness of breath. He was hypertensive with a blood pressure of 156/98 mmHg. Otherwise, he was vitally stable and physical exam was unremarkable. Labs results for complete blood count, complete metabolic panel, coagulation profile, troponin I, and creatine kinase-myoglobin binding were unremarkable. Electrocardiogram showed normal sinus rhythm. Chest X-ray showed no radiographic evidence of acute pulmonary disease. Echocardiography showed normal LVEF (left ventricular ejection fraction) of 60%-65%. Nuclear stress test was positive for moderate lateral wall ischemia.
Management
Coronary angiography and PCI balloon angioplasty with DES were performed on the day of admission via right femoral artery approach. Coronary angiography showed 80% lesion of mid segment of left anterior descending artery mLAD (Fig. 1A, Video 1), 80% lesion in proximal segment of ramus intermedius (pRI), and luminal irregularities in left circumflex (LCx). Figure 1B and Video 2 illustrates the right coronary angiogram where luminal irregularities in right coronary artery (RCA) can be seen.
Fig. 1.
Diagnostic coronary angiograms. (A) Coronary angiogram showing 80% lesion in mid segment of Left Anterior Descending Artery (white arrow). (B) Angiogram showing luminal irregularities in right coronary artery.
Plan was made to treat the LAD lesion in the same setting. Subsequent PCI and angioplasty were performed. Initially 2.0 mm × 15 mm balloon was inflated and 3.0 mm × 15 mm drug-eluting-stent (DES) was deployed (Fig. 2, Videos 3, 4).
Fig. 2.
Balloon inflation and stent deployment. (A) Angioplasty was performed using 2.0 × 15 mm balloon. Stent can be seen in the left anterior descending artery (white arrow). (B). 2.0 mm × 15 mm balloon inflation and 3.0 × 15 mm drug eluting stent deployment (black arrow).
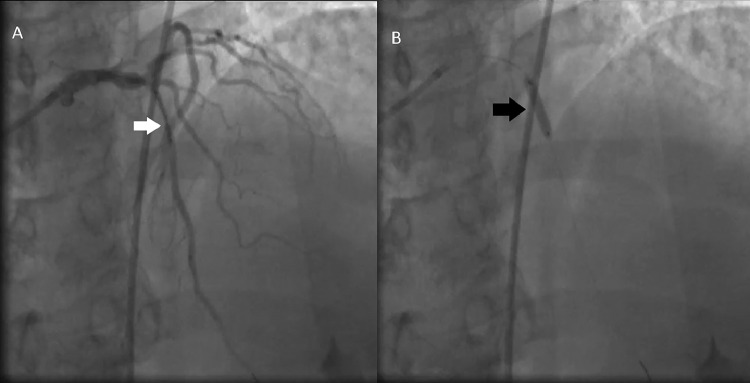
On subsequent angiogram, we noticed newly appearing severely stenotic lesion with minimal blood flow distal to mLAD segment with freshly placed stent (Fig. 3A, Video 5). The progression of this lesion can be seen in the angiogram demonstrated in Figure 3B and Video 6. Now the differential diagnoses were coronary artery perforation, coronary dissection or intramural hematoma formation.
Fig. 3.
Development of coronary intramural hematoma. (A) Angiogram showing newly appeared severely stenotic lesion (white arrow) distal to the freshly placed stent in left anterior descending artery. B. This sequence shows progression of the lesion known as coronary intramural hematoma (black arrow).
Afterwards, 2.50 mm × 30 mm balloon was inserted and the lesion was ballooned (Fig. 4A, Video 7). Subsequent angiogram after balloon inflation didn't illustrate any improvement in the stenosis as seen in Figure 4B and Video 8 which confirmed our suspicion for hematoma as there was no improvement in coronary blood flow after second angioplasty.
Fig. 4.
New balloon insertion and failure to restore blood flow. (A) 2.50 mm × 30 mm balloon was inserted over the same wire and the lesion was ballooned (white arrow). (B) Subsequent angiogram after repeat balloon inflation did not show any improvement in the flow (black arrow).
It is crucial to deploy another overlapping stent to avoid propagation of the intramural hematoma in the involved coronary artery further distally. The entire new lesion was stented using 3.0 mm × 30 mm DES (Fig. 5A, Video 9) and subsequent angiogram showed successful placement of the stent and effective restoration of the coronary blood flow through LAD (Fig. 5B, Video 10). This proves the importance of measuring the length of stenotic segment and carefully calibrating the length of balloon and stent. Also, timely detection and management of hematoma is crucial before it expands further distally.
Fig. 5.
Repeat angioplasty and effective restoration of blood flow. (A) The entire new lesion was stented using 3.0 × 30 mm stent (white arrow). (B) Angiogram illustrates the successful placement of 3.0 × 30 mm DES and effective restoration of the coronary blood flow through LAD (black arrow).
Patient was observed for 24 hours in Intensive Care Unit (ICU) for post-PCI monitoring. Patient remained vitally stable with unremarkable labs except troponin I of 0.22 which down-trended on repeat labs. Patient was discharged with follow up for PCI of the Ramus Intermedius.
Discussion
PCI is a promising procedure which has been successfully performed for decades for emergent and elective management of coronary artery disease. However, due to the interventional nature of this procedure it has its mechanical complications. We presented a case of a rare complication, intramural hematoma of the coronary artery. During PCI, balloon predilatation can result in plaque fracture [3], the stiffer atherosclerotic plaque resists circumferential expansion more than normal adjacent arterial wall leading to coronary arterial wall dissection forming coronary intramural hematoma [4]. Stent deployment may cause intimal tear at the transition point between rigid stent edges and adjacent unstented arterial wall [5], causing blood accumulation along the medial surface of normal segment of the arterial wall forming intramural hematoma [1,6]. These post-PCI intramural hematomas can cause significant compression of the normal arterial segment leading to myocardial ischemia [1]. Hence, timely detection and management of the hematoma is vital to prevent myocardial injury and effectively restore the normal coronary blood flow.
Coronary intramural hematomas can be detected by intravascular imaging with IVUS (intravascular ultrasound) and OCT (optical coherence tomography) [2]. Intramural hematomas appear as homogenous, hyperechoic with distinct echo lucent zones, and crescent shaped areas by IVUS [7]. By OCT, they appear as low-signal area with low light attenuation present at tunica media lined by adventitia peripherally [8]. OCT can also outline some of the independent predictors of dissection including presence of plaque at the stent edges, calcification angle, and fibrous cap thickness [8]. In our case, we were able to detect the intramural hematoma by angiography, deploy a new stent overlapping with previously placed stent promptly and restore coronary blood flow. But, because of angiography limitations, if it isn't detected during PCI, it can lead to post-PCI MI. Therefore, poststenting IVUS or OCT is crucial in suspected cases of intramural hematoma.
Management of post-PCI intramural hematoma remains controversial and there hasn't been general agreement on the ideal management [9]. Treatment options include medical therapy, angiographic surveillance and repeat PCI. If performed at a later time, stenting of the stenotic segment which developed due to hematoma can cause expansion of the hematoma longitudinally and also increases the risk of re-stenosis and stent thrombosis [9]. Forceful injection of contrast media during OCT can also cause further expansion of hematoma [9]. Therefore, the treatment needs to be selected based on patient's characteristics including the severity of symptoms.
Conclusion
Coronary intramural hematoma is a rare but grave complication of PCI which can induce myocardial ischemia and infarction post-intervention. Thus, when CIH is suspected, it is vital to diagnose it with IVUS or OCT for its immediate detection and management. Treatment options differ based on the time of detection of CIH and individual patient's characteristics.
Patient consent
Informed consent was obtained from the subject involved in the study. The well-being of the subject takes precedence over the interests of science and society. No human experimentation was involved.
Video 1: Left Diagnostic Coronary Angiogram. Left Anterior Descending Artery with 80% lesion in mid-segment can be seen in this coronary angiogram.
Video 2: Right Diagnostic Coronary Angiogram. Right coronary artery luminal irregularities are illustrated in this angiogram.
Video 3: Balloon inflation. PCI with 2.0 mm × 15 mm balloon and 3.0×15 mm DES is depicted in this angiogram, DES can be viewed in the LAD.
Video 4: Balloon Inflation and stent placement. This angiogram characterizes inflation of 2.0 mm × 15 mm balloon and placement of 3.0 mm × 15 mm DES.
Video 5: Development of Coronary Intramural Hematoma. Angiogram representing minimal blood flow distal to the freshly placed stent in LAD due to coronary intramural hematoma formation.
Video 6: Progression of Coronary Intramural Hematoma. Further distal progression of coronary intramural hematoma can be observed.
Video 7: New balloon insertion. A new balloon with a size of 2.50 mm × 30 mm balloon was inserted over the same wire.
Video 8: Failure to restore blood flow. The inflation of the new 2.50 mm × 30 mm balloon was unsuccessful in restoring blood flow.
Video 9: Repeat angioplasty. Repeat angioplasty was performed with insertion and deployment of 3.0 × 30 mm DES.
Video 10: Successful restoration of blood flow. With the effective placement of 3.0 × 30 mm DES, blood flow through the stenotic segment of LAD formed due to coronary intramural hematoma was successfully restored.
Footnotes
Competing Interests: All authors declare no conflict of interest.
Disclosure: The authors have nothing to disclose.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Acknowledgment: I appreciate the input from Dr. Dishang Bhavsar and Dr. Vishal Dhulipala for the case report and thank them for their guidance. They have given full permission to be co-authors for this project.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2022.07.001.
Appendix. Supplementary materials
References
- 1.Maehara A, Mintz GS, Bui AB, et al. Incidence, morphology, angiographic findings, and outcomes of intramural hematomas after percutaneous coronary interventions. Circulation. 2002;105:2037–2042. doi: 10.1161/01.CIR.0000015503.04751.BD. [DOI] [PubMed] [Google Scholar]
- 2.Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus document on standards for acquisition, measurement and reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478–1492. doi: 10.1016/S0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 3.van der Lugt A, Gussenhoven EJ, von Birgelen C, Tai JA, Pieterman H. Failure of intravascular ultrasound to predict dissection after balloon angioplasty by using plaque characteristics. Am Heart J. 1997;134:1075–1081. doi: 10.1016/S0002-8703(97)70028-0. [DOI] [PubMed] [Google Scholar]
- 4.Lee RT, Kamm RD. Vascular mechanics for the cardiologist. J Am Coll Cardiol. 1994;23:1289–1295. doi: 10.1016/0735-1097(94)90369-7. [DOI] [PubMed] [Google Scholar]
- 5.Sheris SJ, Canos MR, Weissman NJ. Natural history of intravascular ultrasound-detected edge dissections from coronary stent deployment. Am Heart J. 2000;139:59–63. doi: 10.1016/S0002-8703(00)90309-0. [DOI] [PubMed] [Google Scholar]
- 6.Waller BF. The eccentric coronary atherosclerotic plaque: morphologic observations and clinical relevance. Clin Cardiol. 1989;12:14–20. doi: 10.1002/clc.4960120103. [DOI] [PubMed] [Google Scholar]
- 7.Werner G, Diedrich J, Kreuzer H. Sonographic and angiographic features of intramural hematoma as a cause of failed coronary angioplasty. J Invasive Cardiol. 1996;8:208–214. [PubMed] [Google Scholar]
- 8.Chamié D, Bezerra HG, Attizzani GF, et al. Incidence, predictors, morphological characteristics, and clinical outcomes of stent edge dissections detected by optical coherence tomography. JACC Cardiovasc Interv. 2013;6:800–813. doi: 10.1016/j.jcin.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Moidy M, Al Kindi F. Coronary intramural hematoma: challenges in diagnosis and management. Heart Views. 2019;20:17–20. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_15_19. 10.4103%2FHEARTVIEWS.HEARTVIEWS_15_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.