Abstract
Background
COVID-19 has been associated with temporary olfactory dysfunction in many infected patients. Calcium plays a great role in the olfaction process with negative feedback for the olfaction transmission. Many reports demonstrated calcium elevation in the nasal secretions with a negative effect on olfaction. Sodium gluconate is a water-soluble salt with a chemical structure that lends to act as a highly efficient chelating agent. It can bind the elevated calcium in the nasal secretions reducing the adverse effects on olfactory function.
Objective
To evaluate the impact of intranasal sodium gluconate on decreasing the rise of nasal calcium and improving the sense of smell in patients with olfactory dysfunction post-COVID-19 infection.
Methods
Fifty patients with a history of confirmed COVID-19 suffering from olfactory dysfunction persisted more than 90 days after severe acute respiratory syndrome-coronavirus-2 negative testing were included in a prospective randomized blinded controlled clinical trial. Patients were divided into 2 equal groups, receiving either 0.9% sodium chloride or 1% sodium gluconate. Olfactory function was assessed before treatment and 1 month later using the Sniffin’ Sticks test. Quantitative analysis of the nasal calcium concentration was performed before treatment and 1 month later using a laboratory-designed screen-printed ion-selective electrode.
Results
After using sodium gluconate, the measured olfactory scores indicated a clinical improvement from anosmia to hyposmia compared to the nonimprovement sodium chloride receiving group. Also, a remarked decrease in the calcium nasal concentration was observed after using sodium gluconate compared to sodium chloride.
Conclusion
Based on the proposed results, sodium gluconate may associate with an improvement of the olfactory dysfunction post-COVID-19 infection.
Keywords: COVID-19, severe acute respiratory syndrome-coronavirus-2, olfaction, sodium gluconate, sodium chloride, chelating agent, calcium, Sniffin' Sticks, anosmia, hyposmia
Introduction
COVID-19 is a multiorgan disease caused by infection with severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). The most common symptoms of COVID-19 infection include fever, cough, and shortness of breath. Other symptoms include fatigue, vomiting, diarrhea, and, less commonly, runny nose, headache, and hemoptysis. Olfactory disturbances are considered a potential early indicator of SARS-CoV-2 infection. This was explained by the high load of coronaviruses in the nasal passages so olfactory neurons were at high risk of damage.1,2 Globally, olfactory dysfunction is one of the most commonly identified disorders, affecting about 20% of adults.3 Although olfactory dysfunction is universal, the defined mechanism is not yet fully understood. In addition, there are few effective treatment protocols. This could be due to the coexistence of neurological and biochemical factors.4
Reports of COVID-19-related olfactory disorders describe a sudden onset of olfactory loss that may occur with or without other symptoms.5 Also, different studies investigated the recovery rate of olfactory function post-COVID-19 infection. Through the first 2 months, 79.5% of patients had olfactory performance recovery and 20.5% of patients did not achieve normal levels of olfactory function.6
Although ions constitute 1% of the nasal secretions, the ion microenvironment of the sensory cleft plays an important role in the transmission of olfactory information from the nasal cavity to the central processing system.7 Disruption of ion levels has been associated with the onset or progression of certain diseases.8 Many reports described the important role of calcium in the olfactory receptor neurons and the mechanism of odor transmission. The calcium in the olfactory receptor neurons is contributed to both odor-induced excitation and intracellular feedback pathways. The effect of the increase in intracellular calcium is negative feedback on various stages of the olfactory transduction mechanism, which is responsible for the downregulation of odor sensitivity such as olfactory adaptation.9–13
Sodium gluconate is a highly efficient chelating agent. It is generally used in industrial, medical, and agricultural applications.14 Sodium gluconate can reduce the adverse effects of different metals by forming a stable chelate metal complex.15 It can bind calcium from the nasal secretions and form a stable complex product, which can associate with an improvement of the olfactory function. Therefore, to investigate the hypothesis that intranasal sodium gluconate improves olfactory dysfunction post-COVID-19, we performed the following prospective randomized blinded controlled clinical trial. To our knowledge, this is the first published clinical study to specifically test the effect of such treatment in patients with olfactory dysfunctions post-COVID-19.
Materials and Methods
Study Design
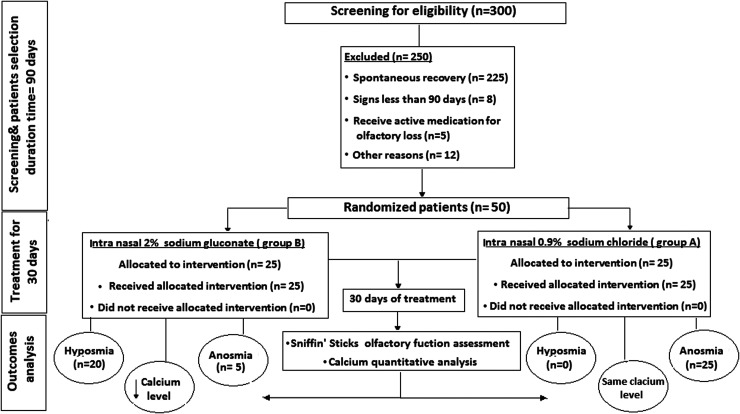
A prospective randomized blinded double controlled clinical trial study was developed in the ENT Department of Damietta Faculty of Medicine, Al Azhar University, Egypt. The study was approved by the Ethical Committee of Damietta Faculty of Medicine, Al Azhar University, Egypt (IRB 00012367-21-01-17). Fifty patients, 31 females, and 19 males, with a history of confirmed COVID-19 infection and olfactory dysfunction, persisted more than 90 days after SARS-CoV-2 negative testing were selected from January 2021 to June 2021. On day 0, participants were randomized to 1 of 2 groups; sodium chloride or sodium gluconate, using unratified block randomization with an equally weighted allocation resulting in 25 patients per group. The block randomization list was developed using a computer-generated randomization plan. The physician and the patients did not know how the group allocations were organized. The record book remained in the hands of the pharmacist team member who did not have any communication with the patients. The described medication was administered accordingly on day 0 or day 1. Complete characterization and examination of the patients were recorded. The flowchart of the proposed study is shown in Figure 1.
Figure 1.
The flow diagram of the proposed study.
Sample Size
Considering that, approximately 79.5% of COVID-19 patients could have complete recovery from the olfactory dysfunction through the first 2 months6 and expecting that sample rejection is up to 10%, it was necessary to screen 300 patients. Thus, 50 patients were included in the study and distributed randomly between the 2 groups in the final analysis for the primary outcome measure.
Inclusion Criteria
To be enrolled in this study the patients had to meet the following inclusion criteria: adults with previous COVID-19 infection confirmed by reverse transcription polymerase chain reaction in nasopharyngeal swabs, recovery from infection confirmed by at least 2 negative nasopharyngeal swabs, clinically confirmed signs of olfactory dysfunction persisted more than 90 days after SARS-CoV-2 negative testing. Only patients with measured olfactory augmented Sniffin' Sticks threshold, discrimination, and identification (TDI) test scores ≤13, which represented only anosmia manifestations, were included in the study.
Exclusion Criteria
The exclusion criteria included: (1) patients with a history of previous olfactory dysfunction related to trauma or surgery; (2) patients with congenital olfactory loss and neurodegenerative diseases; (3) patients with psychiatric or neurological diseases; (4) patients who receive active medication for olfactory dysfunction; (5) patients with a history of adverse reactions to sodium salts; (6) pregnancy; and (7) any patient with current participation in other COVID-19 trials.
Entry into the Study
Before participation in the study, the patients were communicated by one of the members of the study team, who gave detailed explanations of the study, its objective, the possible benefits, and adverse effects. Following comprehensive instructions, the patients must give written consent to participate in the study. After that, the patients received the blinded randomized proposed treatment.
Preparation of the Medication
The Department of Pharmaceutical Analytical Chemistry Faculty of Pharmacy, Al-Azhar University, Cairo Egypt, provided the standard procedures and quality assurance of the medications under the study. The medications were prepared as 0.9% sodium chloride and 1% sodium gluconate in borate buffer solution, pH 8 in the form of an intranasal spray. Considering that the typically used concentration of sodium gluconate in different pharmaceutical formulations was in the range of 0.1% to 1.0%. Also with respect to the sodium gluconate risk profile of the Environmental Protection Agency which considered sodium gluconate to be a safer chemical ingredient under the functional classes of chelating agents and processing aids and additives.15 A 1% sodium gluconate was appropriate and safe to be used as a topical nasal solution. The prepared medications were provided in identical opaque nasal spray bottles which deliver a standardized volume of 0.1 mL for every spray. There was a specific sealed code, organized by the pharmacist responsible for the medications, and was not supplied to the other team members involved in the study. This remained sealed unless there was a need to break the secrecy regarding an allergic reaction presented by any of the patients.
Treatment Regimen Procedures
Participants were divided into 2 equal groups: the group received an intranasal spray of 0.9% sodium chloride (group A) and the group received an intranasal spray of 1% sodium gluconate (group B). Patients received the assigned spray bottle and were counseled to administer the medication 3 sprays for every nostril 3 times daily for 1 month. Patients were instructed to instill the prepared nasal spray solution using a lying position with the head tilted back, which was suggested to improve access to the upper nasal cavity. To reduce variability and confounding of results, a control group was included to eliminate systematic differences between the treated and nontreated groups. Before medication use, an endoscopy examination was done to demonstrate any disease associated with mechanical obstruction and to ensure optimal delivery of the medication. Patients were monitored for side effects throughout the study. Moreover, nasal secretions were also collected from all participants before treatment and 1 month later. Quantitative analysis of the calcium concentration in the nasal secretions was performed using a laboratory-designed screen printed ion selective electrode.
Study Outcomes
Olfactory Function Assessment
The Sniffin' Sticks test (Burghardt®)16 was used to assess the olfactory function by measuring 4 values: threshold value (T), discrimination value (D), identification value (I), and the augmented TDI value.17,18 It was performed before treatment and 1 month later. Odorants were delivered using felt-tip pens carrying a tampon soaked with different concentrations of phenyl ethanol dissolved in propylene glycol. Sixteen concentrations of odorants were developed by a stepwise diluting process. The pen's tip was brought in the front of the patient nose and carefully moved from left to right nostril and backward.16 The threshold score (T) was evaluated using 3 alternative forced choice paradigms where patients were repeatedly presented with triplets of pens and had to assign 1 pen containing an odorous solution from 2 blanks filled with the solvent.19 Starting with the lowest odor concentration, a staircase paradigm was used where 2 subsequent correct identifications of the odorous pen or one incorrect answer marked a so-called turning point and resulted in a decrease or increase, respectively, of concentration in the next triplet. The threshold score (T) was the mean of the last 4 turning points in the staircase, with the final score ranging between 1 and 16 points. The discrimination (D) score was evaluated by introducing 3 alternative forced choice paradigms where patients were repeatedly presented with 2 pens containing the same odorant, while the third pen smelled differently. Patients were asked to discriminate the single pen with a different smell. The score was the sum of correctly identified odors, between 0 and 16 points. The identification score (I) was evaluated by introducing single pens where patients were asked to identify and label the smell, using 4 alternative descriptors for each pen. The total score was the sum of correctly identified pens, thus subjects could score between 0 and 16 points.20
The initial classification of TDI scores defined functional anosmia as a TDI score ≤16.5, normosmia as a TDI score >30.5, and hyposmia as a score between these 2 values.21 An individual increase of more than 6 points was regarded as a clinically significant progression of olfactory function.22
Quantitative Analysis of Calcium in the Nasal Secretions
Nasal secretions were collected from patients before treatment and 1 month later immediately after sneezing. It was collected using a small stainless steel (approximately 10 mm × 5 mm × 2 mm). The stainless steel was clamped on the septum between the nostrils and allowed the fluid to drain into a special 1.5 mL tube. The volume of collected nasal secretions was transferred to different centrifugation tubes after adding 0.5 mL of borate buffer solution. Complete denaturation of proteins was achieved by adding 3 mL of acetonitrile. Shaking for 1 min and centrifugation at 4000 r/min for 30 min was done. The protein-free supernatant was evaporated to dryness, and the residues were diluted with borate buffer solution in 10 mL volumetric flasks. The design of a screen-printed ion selective electrode23 was carried out by the Department of Pharmaceutical Analytical Chemistry, Cairo Faculty of Pharmacy, Al-Azhar University. A specific calcium screen-printed electrode was based on preparing selective sensing material, calcium-tetra phenyl borate, which was specific to recognize calcium cations only. This prepared calcium sensing material was incorporated into the designed screen-printed electrode and used to determine calcium levels in the nasal secretions. This was done before treatment and 1 month later.
Statistical Analysis
All statistical analyses were performed using SPSS v23 statistical software (SPSS, Inc.). The results obtained were tested for normality and parametric or nonparametric tests were used accordingly. Results were expressed as mean ± standard deviation (SD) unless otherwise stated. Differences in frequencies were assessed using Fisher’s exact probability test. An unpaired t-test was used to compare and test the significance of the results of the 2 groups. Statistical significance was assigned when p < .05.
Results
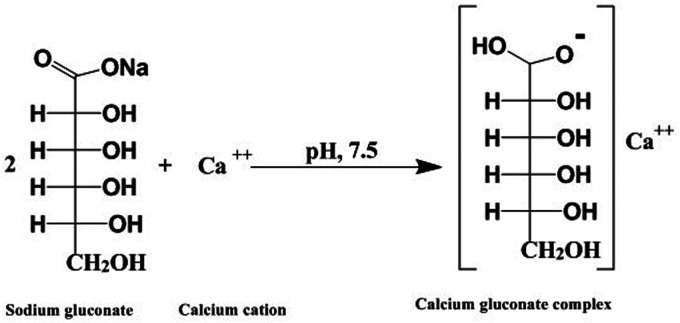
Fifty patients, 31 females and 19 males, were enrolled in the current study. The complete characteristics of the patients are described in Table 1. There was a nonsignificant difference in the frequency of smoking between the sodium chloride group and the sodium gluconate group (3/25 vs. 5/25; p = .70). The results revealed a nonsignificant difference between the sodium chloride group and the sodium gluconate group for the comorbidities, as presented in Table 1. A topical intranasal spray containing sodium gluconate had the ability to chelate calcium in the nasal secretions and form corresponding soluble complex products. The proposed reaction pathway for the reaction of sodium gluconate with calcium is shown in Figure 2.
Table 1.
Patient Demographics and Clinical Data.
| Character | Sodium chloride | Sodium gluconate | p (Fisher exact probability test) |
|---|---|---|---|
| Sample size, n | 25 | 25 | |
| Age (years), mean ± SD | 38.37 ± 8.58 | 39.25 ± 7.23 | |
| Days since symptoms to enrollment, mean ± SD | 95.23 ± 6.24 | 97.37 ± 5.89 | |
| Gender (male/female), n | 9/16 | 10/15 | |
| Smokers (current/never), n | 3/22 | 5/20 | 0.70 |
| Comorbidities, n | |||
| Asthma | 2 | 3 | 1.00 |
| Diabetes | 7 | 8 | 1.00 |
| Hypertension | 5 | 3 | 0.70 |
| Migraine | 0 | 1 | 1.00 |
| Sinusitis | 7 | 8 | 1.00 |
| Current medication | Antihistamine Metformin B-blocker ACE inhibitor Paracetamol |
Antihistamine Glipizide Amlodipine ACE inhibitor Paracetamol |
|
| Previous anosmia treatment | None (n = 10) Topical steroids (n = 10) Systemic + topical steroids (n = 5) |
None (n = 8) Topical steroids (n = 9) Systemic + topical steroids (n = 5) Vitamin A (n = 3) |
Abbreviations: SD, standard deviation: ACE, angiotensin-converting enzyme.
Figure 2.
Proposed pathway for the chemical reaction of sodium gluconate and calcium.
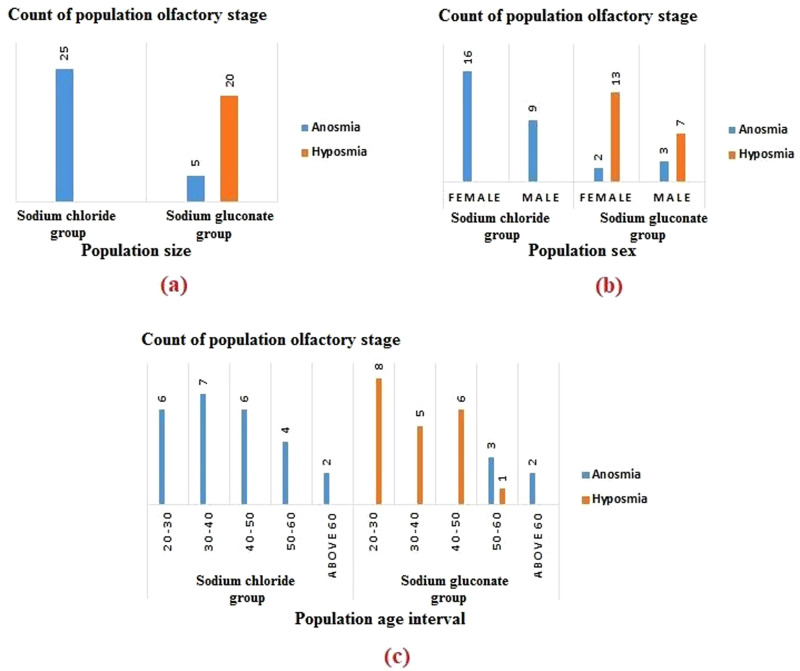
The Sniffin' Sticks test was used to evaluate the olfactory function in all patients before treatment and 1 month later after using sodium chloride and sodium gluconate. T, D, I, and TDI values were evaluated before treatment and 1 month later. Mean T, D, I, and TDI values before treatment and 1 month later are shown in Table 2. The results showed that the mean ± SD of the TDI value before and after receiving sodium chloride was 11.64 ± 1.11 and 11.79 ± 1.01, respectively, Table 2. While, the mean ± SD of the TDI value before and after receiving sodium gluconate was 11.56 ± 1.26 and 16.80 ± 1.85, respectively, Table 2. The change of TDI score (ΔTDI) following treatment with sodium chloride and sodium gluconate was calculated, and the mean ± SD of ΔTDI value for sodium chloride and sodium gluconate is shown in Table 2. In general, TDI values were compared to the reported reference values. Regarding the measured olfactory scores of the sodium gluconate group, generally, 20 patients (80%) showed an improved olfactory function and 5 patients (20%) did not show improvement (Figure 3(a)). The measured female olfactory score of the sodium gluconate group was better than the measured male scores (Figure 3(b)). Also, most patients over 50 years of age did not show olfaction improvement (Figure 3(c)).
Table 2.
Results of the Measured Olfactory Scores and the Measured Nasal Calcium Concentration Levels Pretreatment and Posttreatment.
| Sodium chloride | Sodium gluconate | |||
|---|---|---|---|---|
| Preadministration | Postadministration | Preadministration | Postadministration | |
| T-score, mean ± SD | 2.50 ± 0.56 | 2.62 ± 0.65 | 2.50 ± 0.40 | 3.82 ± 0.968 |
| D-score, mean ± SD | 4.40 ± 0.88 | 4.49 ± 1.21 | 4.39 ± 0.75 | 5.72 ± 1.00 |
| I-score, mean ± SD | 4.74 ± 1.25 | 4.68 ± 0.87 | 4.67 ± 1.22 | 7.26 ± 0.77 |
| TDI score, mean ± SD | 11.64 ± 1.11 | 11.79 ± 1.01 | 11.56 ± 1.26 | 16.80 ± 1.85 |
| Nasal calcium concentration (mM), mean ± SD | 37.64 ± 1.97 | 36.96 ± 1.65 | 37.32 ± 2.15 | 25.32 ± 5.95 |
| Mean ΔTDI ± SD | 0.192 ± 0.326 | 5.24 ± 2.350 | ||
| Mean Δcalcium concentration ± SD | 0.760 ± 0.522 | 12.00 ± 5.400 | ||
Abbreviations: SD, standard deviation; TDI, threshold, discrimination, and identification.
Figure 3.
Population size of the assessed olfaction stage after receiving sodium chloride and sodium gluconate.
The concentration of calcium in the nasal secretions was quantitatively analyzed in all patients. A laboratory-designed screen-printed calcium-selective electrode20 was designed for potentiometric quantitative analysis of calcium. Electromotive force values were measured over a calcium concentration range of 100 to 0.001 mM. A calibration plot was constructed relating the electromotive force values versus the negative logarithmic value of the calcium concentration. The designed electrode exhibited a near Nernstian slope of 28.23 mV/decade with a detection limit of 0.0001 mM in the dynamic range of 100 to 0.001 mM. The electrode was highly selective to calcium determination even in the presence of sodium, potassium, and magnesium as interfering cations. The calcium concentration of the nasal secretions was successfully determined using the developed electrode. The mean value of calcium concentration before treatment and 1 month later is shown in Table 2. The change of the measured nasal calcium concentration (Δ calcium concentration) following treatment with sodium chloride and sodium gluconate was calculated, and the mean ± SD of Δ calcium concentration level for sodium chloride and sodium gluconate is shown in Table 2. Calcium level was decreased in the patients treated with sodium gluconate. In general, the obtained results indicated nonimprovement in patients receiving sodium chloride. On the other hand, there was a significant improvement in patients receiving sodium gluconate with clinical change from anosmia to hyposmia.
To test whether using intranasal sodium gluconate resulted in a significant improvement of the olfactory function in comparison to the sodium chloride group, the change in the olfactory scores (ΔTDI) and the change of the measured nasal calcium concentration (Δ calcium concentration) of the patients treated with sodium gluconate were compared to the calculated values of sodium chloride group using unpaired t-test. Results are shown in Table 3. The largest difference between sodium chloride and sodium gluconate groups was indicated by the significant data output. The results of patients receiving intranasal sodium gluconate revealed a significant difference with a clinical improvement in the olfactory performance from anosmia to the hyposmia olfaction stage. Sodium gluconate was generally well tolerated. Nasal discharge was the commonest side effect seen. However, mild burning sensations in either the nose or throat were also reported.
Table 3.
Unpaired t-Results for Statistical Comparing the Change of TDI Scores and the Change of Nasal Calcium Concentrations Between the 2 Groups.
| Parameter | Change in TDI scores statistical assessment results | Change in nasal calcium concentration statistical assessment results |
|---|---|---|
| t | 10.6374* | 10.3578* |
| df | 24 | 24 |
| p | <.0001* | <.001* |
Abbreviations: TDI, threshold, discrimination, and identification.
Statistical significance is indicated by *.
Discussion
Human strains of coronavirus have been shown to enter the central nervous system via the olfactory neuroepithelium and replicate from the olfactory bulb. Among the various neurological manifestations of COVID-19, olfactory dysfunction has been observed in many patients.24,25
Calcium inhibits olfactory neuron transduction, through negative feedback inhibition, making the cyclic nucleotide-gated cation channel less active and inducing phosphorylation of adenylyl cyclase to decrease.9–13,26 Thus, topical intranasal application of a calcium chelating agent as sodium gluconate leads to a decrease in extracellular calcium concentration. This can cause calcium decrease in the olfactory mucus and improve olfaction by reducing calcium-mediated inhibition in olfactory neuron signal transduction.
Previous studies have investigated the use of intranasal sodium citrate as a calcium sequestering agent to reduce free calcium and improve olfactory function. The first study included 57 patients with different causes of hyposmia, and there was a significant improvement in the identification scores of participants with postviral hyposmia, following sodium citrate treatment.27 Furthermore, prospective clinical study testing sodium citrate in 49 patients with postinfectious olfactory impairment was done. The effect size did not reach clinical significance although there was a statistically significant improvement in TDI scores following treatment with intranasal sodium citrate.28
This current prospective randomized blinded controlled trial was developed to test the intranasal use of sodium gluconate for the treatment of olfactory dysfunction post-COVID-19. Sodium gluconate is a chelating agent that can form strong complexes with divalent and trivalent cations. In an aqueous solution, sodium gluconate can form a complex product with calcium by displacing water molecules in the solvation sphere of the cations through their hydroxyl groups. The presence of carboxyl groups also increases the ability to form complexes. The chelation process is mainly influenced by the pH and the presence of other competing cations.14,15 At a pH of 8, sodium gluconate selectively forms a calcium gluconate complex even in the presence of sodium, potassium, or magnesium cations. This results in a decrease in calcium concentration in the nasal secretions. The current results indicate that decreasing calcium in the nasal secretion by sodium gluconate is associated with an improvement in olfactory function.
It is recommended that a high-precision evaluation method be selected to test the performance of the olfactory function. Sniffin' Sticks test consists of 3 subtests, T, D, and I, for each of which a score of up to 16 points can be obtained. The expanded TDI score is calculated by summing the scores of the 3 subtests. The results obtained showed that treatment with intranasal sodium gluconate produced a significant improvement in olfactory performance, which was reflected in the calculated olfactory test scores.
It is also recommended to determine the concentration of calcium in the nasal secretions. Electrochemical quantitative analysis of calcium using a screen-printed selective electrode offers the advantages of rapid response time and high selectivity for calcium. Moreover, it can be used with a small volume of nasal secretion.23 The developed electrode was used to quantify the calcium concentration before and after the described treatment. The results obtained showed a remarked decrease in calcium concentration in the patients who received intranasal sodium gluconate. This can be explained by the chelation of the calcium and the formation of the calcium gluconate complex product.
The current results indicated an association between the decrease of calcium concentration in the nasal secretions by the sodium gluconate and the increase in the measured olfactory assessment score with a subsequent improvement in clinical olfactory performance.
This study has several limitations. The major limitation is the small sample size, which is predisposed to an underpowered analysis. Also, the need for additional studies is recommended to confirm the association between the changes in nasal calcium concentration and olfactory function. Furthermore, additional work is necessary to test various dosing regimens with an evaluation of the pharmacokinetics of the proposed treatment. Actually, it will be important to extend this study to more studied populations and record observations over 1 month. Despite the significant result of patients receiving intranasal sodium gluconate, the final TDI value was still low and only clinical improvement was provided from anosmia to hyposmia olfaction stage.
Conclusion
In this paper, the effect of intranasal sodium gluconate to decrease elevated nasal calcium concentration in patients with olfactory dysfunction post-COVID-19 infection was demonstrated. After using sodium gluconate compared to sodium chloride, a clinical improvement from anosmia to hyposmia with a decrease in the nasal calcium concentration was observed. Based on the results of this study intranasal sodium gluconate can represent a useful therapy for persisted olfactory dysfunction post-COVID-19 infection.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ahmed H. Abdelazim https://orcid.org/0000-0002-8907-3497
References
- 1.Felsenstein S, Herbert JA, McNamara PS, et al. COVID-19: immunology and treatment options. Clin Immunol. 2020;215:108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidari F, Karimi E, Firouzifar M, et al. Anosmia as a prominent symptom of COVID-19 infection. Rhinology. 2020;58(3):302–303. [DOI] [PubMed] [Google Scholar]
- 3.Brämerson A, Johansson L, Ek L, et al. Prevalence of olfactory dysfunction: the Skövde population-based study. Laryngoscope. 2004;114(4):733–737. [DOI] [PubMed] [Google Scholar]
- 4.Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385(6618):725–729. [DOI] [PubMed] [Google Scholar]
- 5.Eliezer M, Hautefort C, Hamel A-L, et al. Sudden and complete olfactory loss of function as a possible symptom of COVID-19. JAMA otolaryngology–Head & Neck Surgery. 2020;146(7):674–675. [DOI] [PubMed] [Google Scholar]
- 6.Lechien JR, Journe F, Hans S, et al. Severity of anosmia as an early symptom of COVID-19 infection may predict lasting loss of smell. Front Med (Lausanne). 2020;24(7):582802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam K, Conley DB, Liu K, et al. Effect of ionic compositions in nasal irrigations on human olfactory thresholds. Laryngoscope. 2015;125(2):E50–E56. [DOI] [PubMed] [Google Scholar]
- 8.Sauer G, Richter C-P, Klinke R. Sodium, potassium, chloride and calcium concentrations measured in pigeon perilymph and endolymph. Hear Res. 1999;129(1-2):1–6. [DOI] [PubMed] [Google Scholar]
- 9.Panagiotopoulos G, Naxakis S, Papavasiliou A, et al. Decreasing nasal mucus Ca improves hyposmia. Rhinology. 2005;43(2):130–134. [PubMed] [Google Scholar]
- 10.Leinders-Zufall T, Greer CA, Shepherd GM, et al. Imaging odor-induced calcium transients in single olfactory cilia: specificity of activation and role in transduction. J Neurosci. 1998;18(15):5630–5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zufall F, Leinders-Zufall T, Greer CA. Amplification of odor-induced Ca2+ transients by store-operated Ca2+ release and its role in olfactory signal transduction. J Neurophysiol. 2000;83(1):501–512. [DOI] [PubMed] [Google Scholar]
- 12.Reisert J, Bauer PJ, Yau K-W, et al. The Ca-activated Cl channel and its control in rat olfactory receptor neurons. J Gen Physiol. 2003;122(3):349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balasubramanian S, Lynch J, Barry P. Calcium-dependent modulation of the agonist affinity of the mammalian olfactory cyclic nucleotide-gated channel by calmodulin and a novel endogenous factor. J Membr Biol. 1996;152(1):13–23. [DOI] [PubMed] [Google Scholar]
- 14.Phadungath C, Metzger L. Effect of sodium gluconate on the solubility of calcium lactate. J Dairy Sci. 2011;94(10):4843–4849. [DOI] [PubMed] [Google Scholar]
- 15.Fiume MM, Bergfeld WF, Belsito DV, et al. Safety assessment of monosaccharides, disaccharides, and related ingredients as used in cosmetics. Int J Toxicol. 2019;38(1_suppl):5S–38S. [DOI] [PubMed] [Google Scholar]
- 16.Hummel T, Sekinger B, Wolf SR, et al. ‘Sniffin’sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22(1):39–52. [DOI] [PubMed] [Google Scholar]
- 17.Rumeau C, Nguyen D, Jankowski R. How to assess olfactory performance with the Sniffin' Sticks test®. Eur Ann Otorhinolaryngol, Head Neck Dis. 2016;133(3):203–206. [DOI] [PubMed]
- 18.Lötsch J, Reichmann H, Hummel T. Different odor tests contribute differently to the evaluation of olfactory loss. Chem Senses. 2008;33(1):17–21. [DOI] [PubMed] [Google Scholar]
- 19.Croy I, Lange K, Krone F, et al. Comparison between odor thresholds for phenyl ethyl alcohol and butanol. Chem Senses. 2009;34(6):523–527. [DOI] [PubMed] [Google Scholar]
- 20.Oleszkiewicz A, Schriever V, Croy I, et al. Updated Sniffin’Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Oto-Rhino-Laryngol. 2019;276(3):719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rumeau C, Nguyen D, Jankowski R. How to assess olfactory performance with the Sniffin’Sticks test®. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133(3):203–206. [DOI] [PubMed] [Google Scholar]
- 22.Reden J, Lill K, Zahnert T, et al. Olfactory function in patients with postinfectious and posttraumatic smell disorders before and after treatment with vitamin A: a double-blind, placebo-controlled, randomized clinical trial. Laryngoscope. 2012;122(9):1906–1909. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Li Y-T, Li D-W, et al. Recent developments and applications of screen-printed electrodes in environmental assays—A review. Anal Chim Acta. 2012;734:31–44. [DOI] [PubMed] [Google Scholar]
- 24.Han AY, Mukdad L, Long JL, et al. Anosmia in COVID-19: mechanisms and significance. Chem Senses. 2020;45(6):423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lechien JR, Hopkins C, Saussez S. Sniffing out the evidence; it’s now time for public health bodies recognize the link between COVID-19 and smell and taste disturbance. Rhinology. 2020;58(4):402–403. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Kang H, Jin HJ, et al. Can sodium citrate effectively improve olfactory function in non-conductive olfactory dysfunction? Korean J Otorhinolaryngol-Head Neck Surg. 2019;62(2):75–81. [Google Scholar]
- 27.Whitcroft K, Merkonidis C, Cuevas M, et al. Intranasal sodium citrate solution improves olfaction in post-viral hyposmia. Rhinology. 2016;54(4):368–374. [DOI] [PubMed] [Google Scholar]
- 28.Whitcroft K, Ezzat M, Cuevas M, et al. The effect of intranasal sodium citrate on olfaction in post-infectious loss: results from a prospective, placebo-controlled trial in 49 patients. Clin Otolaryngol. 2017;42(3):557–563. [DOI] [PubMed] [Google Scholar]