Abstract
Extracorporeal membrane oxygenation (ECMO) is used in critically ill patients with coronavirus disease 2019 (COVID-19) with acute respiratory distress syndrome unresponsive to other interventions. However, a COVID-19 infection may result in a differential tolerance to both medical treatment and ECMO management. The aim of this study was to compare outcomes (mortality, organ failure, circuit complications) in patients on ECMO with and without COVID-19 infection, either by venovenous (VV) or venoarterial (VA) cannulation. This is a multicenter, retrospective analysis of a national database of patients placed on ECMO between May 2020 and January 2022 within the United States. Nine-hundred thirty patients were classified as either Pulmonary (PULM, n = 206), Cardiac (CARD, n = 279) or COVID-19 (COVID, n = 445). Patients were younger in COVID groups: PULM = 48.4 ± 15.8 years versus COVID = 44.9 ± 12.3 years, p = 0.006, and CARD = 57.9 ± 15.4 versus COVID = 46.5 ± 11.8 years, p < 0.001. Total hours on ECMO were greatest for COVID patients with a median support time two-times higher for VV support (365 [101, 657] hours vs 183 [63, 361], p < 0.001), and three times longer for VA support (212 [99, 566] hours vs 70 [17, 159], p < 0.001). Mortality was highest for COVID patients for both cannulation types (VA-70% vs 51% in CARD, p = 0.041, and VV-59% vs PULM-42%, p < 0.001). For VA supported patients hepatic failure was more often seen with COVID patients, while for VV support renal failure was higher. Circuit complications were more frequent in the COVID group as compared to both CARD and PULM with significantly higher circuit change-outs, circuit thromboses and oxygenator failures. Anticoagulation with direct thrombin inhibitors was used more often in COVID compared to both CARD (31% vs 10%, p = 0.002) and PULM (43% vs 15%, p < 0.001) groups. This multicenter observational study has shown that COVID patients on ECMO had higher support times, greater hospital mortality and higher circuit complications, when compared to patients managed for either cardiac or pulmonary lesions.
Keywords: acute respiratory distress syndrome, COVID-19, extracorporeal membrane oxygenation, venoarterial, venovenous, thrombosis
Introduction
The severe acute respiratory distress syndrome seen in Coronavirus Disease 2019 (COVID-19) is associated with perturbations to the inflammatory and coagulation systems. The hyperinflammatory response elicited by COVID-19 may disrupt coagulation leading to thrombosis and multisystem organ failure.1,2
A growing body of literature on the use of extracorporeal membrane oxygenation (ECMO) for severely ill COVID-19 patients shows disparate outcomes that obfuscate guidance on techniques that offer the best chance for improved results.3-6 While the reasons for these differences are unknown, several discrete features associated with COVID-19 infections have appeared. Furthermore, there may exist an evolving increase in mortality related to the phase during the pandemic that patients were placed on ECMO.7,8 While the causes for this decline in survivability are speculative, they are not attributed to increased risk factors prior to ECMO. The Extracorporeal Life Support Organization (ELSO) recommends that anticoagulation management for COVID-19 patients be similar to non-COVID-19 patients, albeit at the higher end of normal.9 However, increased cannula and oxygenator thrombosis at the initiation and during ECMO are reported.10,11 While on ECMO, COVID-19 patients have been shown to display high levels of hypercoagulability and hyperfibrinolysis, which may result in early circuit complications.10,12 Such complications may hinder recovery in these severely ill patients. Furthermore, it is unknown how the multiple new variants of COVID-19 will affect the conduct of ECMO. A recent report has shown that mortality did not differ between COVID-19 patients and those without COVID-19 who were supported on ECMO for acute respiratory distress syndrome (ARDS).13
The purpose of this study was to compare outcomes (including in-hospital mortality circuit change-out, oxygenator failure, circuit thrombosis, and circuit hemolysis) in patients with and without COVID-19 when supported with ECMO.
Methods
This multicenter cohort study was conducted by using a proprietary database of procedures involving extracorporeal circulation (SpecialtyCare Operative Procedural rEgistry, (SCOPE) [https://specialtycareus.com/]. Data were prospectively collected on all adult patients over 18 years of age who were placed on ECMO between May 2020 and January 2022 from 67 centers within the United States across 25 different states. Data captured included patient characteristics, circuit types, cannulation strategies, anticoagulation use and management, hemorrhage requiring allogeneic transfusion, and in-hospital outcomes. Institutional Review Board (IRB) approval and waiver of the need for consent were obtained. The human subjects’ research protocol for this study was reviewed and approved by an independent IRB who monitored the use of data from SCOPE (ADVARRA Center for IRB Intelligence, approval number 012017, 6940 Columbia Gateway Drive, Suite 110, Columbia, Maryland, USA).
The disease states included in each category were as follows: PULM––ARDS, pneumonia, or acute respiratory failure, CARD––acute heart failure, cardiogenic shock, cardiomyopathy, COVID––ARDS, pneumonia, cardiogenic shock, cardiomyopathy, or myocarditis. Patients were included who were supported either by venovenous (VV) or venoarterial (VA) cannulation. All patients were grouped into one of four categories according to indication for ECMO support and ECMO cannulation strategy: PULM-VV, COVID-VV, CARD-VA, and COVID-VA. Patients were excluded from the study if they were transferred from the hospital where ECMO was initiated without knowledge of outcome, or if patients were on ECMO support at the end of the study period. All decisions concerning the initiation and conduct of ECMO were made by the local team and followed protocols at each institution. Circuit complications were recorded and included oxygenator failure and/or pump malfunction that resulted in the circuit being changed-out while on ECMO. Additional complications included circuit hemolysis and circuit thrombosis.
The primary outcome was in-hospital mortality with secondary outcomes including new onset organ failure (hepatic and renal) and circuit complications that included circuit change-out, oxygenator failure, circuit thrombosis, pump malfunction and circuit hemolysis.
Statistical analysis
Descriptive analysis was stratified by ECMO circuit configuration so that PULM-VV patients were compared to COVID-VV patients, and CARD-VA patients were compared to COVID-VA patients. Values are reported as mean with standard deviation, median with inter-quartile range [IQR], as well as count and percentage as appropriate. Unadjusted group differences by indication within ECMO cannulation strategy were assessed using Welch’s analysis of variance, Kruskal-Wallis rank-sum tests, and chi-square tests for normally-distributed, heavily skewed, and categorical variables, respectively.
In order to assess the effect of each unique combination of indication for support and ECMO cannulation strategy on mortality, a Bayesian mixed effects logistic regression model was estimated. Controls in this model included age, sex, body mass index (BMI), duration of mechanical ventilation support prior to ECMO, anticoagulation strategy, ECMO circuit thrombosis, oxygenator failure, full circuit change-out, renal failure, hepatic failure, total duration of ECMO support, and days between cannulation and the study start date (1 May 2020) to control for possible time trends. Age, BMI, total hours on ECMO, and days since study start date were modeled using restricted cubic splines with five knots distributed symmetrically across the quantiles of each variable, respectively, to allow for flexible estimation of non-linear patterns in continuous variables. A random effect term was included to account for possible correlation in outcomes of patients receiving care at the same hospital. Among the variables included, we observed relatively little missing data; however, BMI was missing for 25% of patients. To minimize the potential for bias we performed our regression analysis using 25 multiply imputed datasets, generated using the chained equations method, the results of which were combined into a unified single set of posterior distributions. All analyses were performed using the R statistical computing environment (version 3.6.1),14 along with the packages ‘compareGroups,’15 ‘rmsb.’16
Results
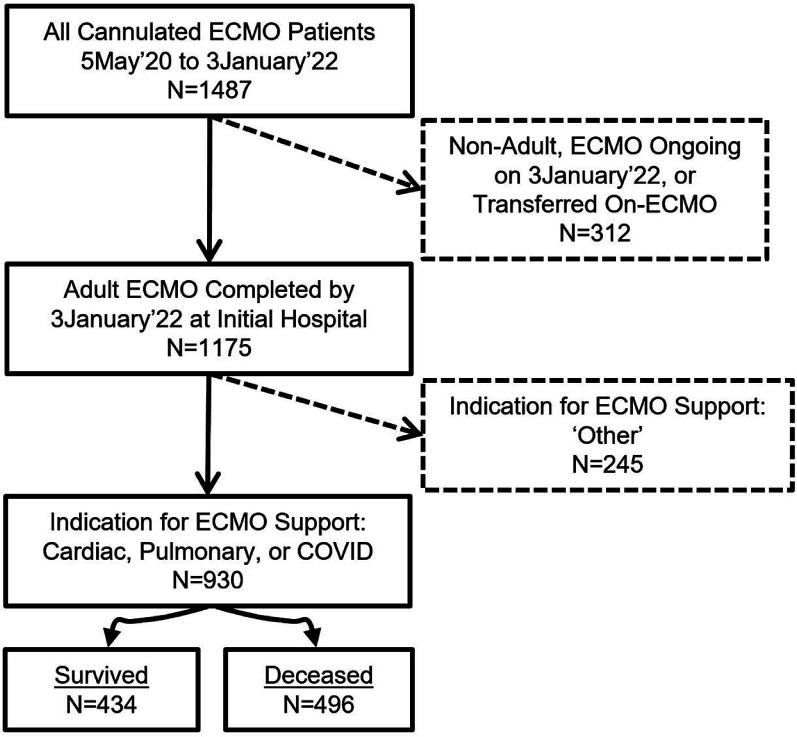
A total of 1,487 patients underwent ECMO during the study period with 930 patients meeting inclusion criteria (Figure 1). There were 434 (47%) survivor patients with survival identified as successful weaning from ECMO without a hospital death, while there were 496 (53%) who died. The COVID group had 445 (48%) patients, while there were 279 (30%) in the CARD group, and 206 (22%) in the PULM group. The oldest patients were in the CARD group and the youngest in the COVID cohorts (Table 1). The primary cannulation strategy was VV in both COVID and PULM patients with VA used among CARD patients and infrequently (n = 37) in those with COVID. Patients in the COVID-VA group had higher median BMI than CARD patients, while BMI was similar in the VV groups. Prior to ECMO, COVID patients had longer durations of mechanical ventilation support for both VV (1.0 [0.0; 4.0] vs 0.0 [0.0; 1.0], p < 0.001, and VA cannulation strategies (1.0 [0.0; 2.0] vs 0.0 [0.0; 0.0], p < 0.001). Median hours on ECMO was significantly longer in the COVID group compared to both PULM (2.0 times) and CARD (3.0 times) groups, p < 0.001. There was a significantly higher use of direct thrombin inhibitors (DTI) for anticoagulation in the COVID group, which included patients who were initially given a DTI for anticoagulation and those that had crossed-over to its use during the ECMO run.
Figure 1.
Patient selection. Examples of procedures in “Other” category include ECCO2R, post-cardiotomy and hypothermic resuscitation. ECMO, extracorporeal membrane oxygenation.
Table 1.
Patient demographic information.
| VV ECMO | VA ECMO | |||||
|---|---|---|---|---|---|---|
| Clinical featurea | Pulmonary (206) | COVID (408) | p-value | Cardiac (279) | COVID (37) | p-value |
| Patient age (years) | 48.4 (15.8) | 44.9 (12.3) | 0.006 | 57.9 (15.4) | 46.5 (11.8) | <0.001 |
| Patient sex | ||||||
| Male | 89 (66.4) | 178 (68.7) | 0.726 | 118 (64.1) | 17 (63.0) | 1.000 |
| Female | 45 (33.6) | 81 (31.3) | 66 (35.9) | 10 (37.0) | ||
| Patient BMI (kg/m2) | 32.2 (27.1; 38.0) | 33.1 (28.6; 39.8) | 0.199 | 29.2 (25.3; 34.7) | 32.8 (28.4; 38.1) | 0.034 |
| Days of ventilator support prior to ECMO | 0.0 (0.0; 1.0) | 1.0 (0.0; 4.0) | <0.001 | 0.0 (0.0; 0.0) | 1.0 (0.0; 2.0) | <0.001 |
| Total hours on ECMO | 182.5 (62.8; 361.2) | 365.0 (100.5; 656.9) | <0.001 | 70.0 (16.5; 158.5) | 212.0 (99.0; 566.0) | <0.001 |
| Anticoagulation method | ||||||
| Heparin | 157 (79.3) | 223 (55.9) | <0.001 | 222 (83.1) | 25 (69.4) | 0.002 |
| DTI | 29 (14.6) | 173 (43.4) | 27 (10.1) | 11 (30.6) | ||
| None | 12 (6.1) | 3 (0.8) | 18 (6.7) | 0 (0.0) | ||
| CVVHD | ||||||
| No | 145 (78.4) | 280 (72.4) | 0.15 | 179 (70.5) | 22 (62.9%) | 0.47 |
| Yes | 40 (21.6) | 107 (27.6) | 75 (29.5) | 13 (37.1%) | ||
BMI, body mass index; CVVHD, continuous venovenous hemodialysis; DTI, direct thrombin inhibitor; ECMO, extracorporeal membrane oxygenation; VA, venoarterial; VV, venovenous.
aValues are given as n (%) for categorical data or median [interquartile range] for continuous data
Unadjusted mortality was greater in COVID patients when compared to both PULM and CARD groups with the highest mortality rate seen in COVID-VA 70% (26) compared to 51% (142) in CARD patients, p = 0.041 (Table 2). For VV patients the PULM mortality was 18% lower than for COVID patients, p < 0.001. Hemorrhagic complications occurred more frequently in both COVID cohorts when compared to PULM and CARD groups. Hepatic failure was encountered more frequently in both COVID groups, which was significantly greater in COVID-VA 26% (9) versus CARD 7% (18), p < 0.002. Renal failure was higher in the COVID-VV 26% (99) than in PULM 16% (31), with a similar pattern among VA patients, albeit with less statistical reliability. The distribution of cause of mortality is shown in Table 3.
Table 2.
Outcomes of extracorporeal membrane oxygenation across study groups.
| Variable | Mortality odds ratio for contrast and 95% credible interval | Posterior probability of increased mortality | Relative explained variation in mortality and 95% credible interval |
|---|---|---|---|
| COVID VV versus pulmonary VV | 2.56 [1.58–4.14] | >99.999% | 13.4% [3.1–23.4] |
| COVID VA versus Cardiac VA | 2.67 [1.1–6.72] | 98.30% | 13.4% [3.1–23.4] |
| Age (61 vs 37) | 3.21 [2.07–5.16] | >99.999% | 24.7% [9.5–35.9] |
| BMI (38 vs 27 kg/m2) | 0.86 [0.6–1.22] | 20.40% | 1.8% [0.2–9.4] |
| Female versus male | 1.78 [1.25–2.44] | >99.999% | 8.9% [0.5–16.3] |
| Mechanical ventilation prior to ECMO (yes vs No) | 0.91 [0.62–1.29] | 30.20% | 0.2% [0–2.9] |
| Anticoagulation (DTI vs heparin) | 1.18 [0.76–1.82] | 76.88% | 0.9% [0–6.1] |
| Anticoagulation (none vs heparin) | 1.41 [0.61–3.15] | 79.33% | 0.9% [0–6.1] |
| Total hours on ECMO (438 vs 41) | 1.03 [0.67–1.59] | 58.23% | 13% [2.9–22.4] |
| Circuit thrombosis (yes vs no) | 1.07 [0.67–1.73] | 60.28% | 0.1% [0–2.7] |
| Oxygenator failure (yes vs No) | 0.91 [0.48–1.75] | 38.25% | 0.1% [0–3.3] |
| Circuit change-out (yes vs no) | 1.07 [0.67–1.68] | 62.33% | 0.1% [0–2.8] |
| Hepatic failure (yes vs no) | 4.38 [2.12–8.47] | >99.999% | 13.7% [3.2–21.1] |
| Renal failure (yes vs No) | 2.98 [1.93–4.48] | >99.999% | 20.1% [5.9–27.9] |
| Time trend (August 21 vs November 20) | 1.15 [0.74–1.77] | 72.38% | 2.5% [0.5–10.8] |
ECMO, extracorporeal membrane oxygenation; VA, venoarterial; VV, venovenous.
Table 3.
Distribution of cause of mortality across groups.
| VV ECMO | p-value | VA ECMO | p-value | |||
|---|---|---|---|---|---|---|
| Cause of mortality, n (%) | Pulmonary (86) | COVID (242) | <0.001 | Cardiac (142) | COVID (26) | <0.001 |
| Multisystem organ failure | 37 (43.0) | 59 (24.4) | 31 (21.8) | 9 (34.6) | ||
| Cardiac arrest | 13 (15.1) | 43 (17.8) | 45 (31.7) | 8 (30.8) | ||
| Sepsis | 8 (9.3) | 15 (6.2) | 3 (2.1) | 2 (7.7) | ||
| Severe brain hypoxia Pre-ECMO | 7 (8.1) | 11 (4.5) | 18 (12.7) | 2 (7.7) | ||
| Hemorrhage | 5 (5.8) | 33 (13.6) | 14 (9.9) | 2 (7.7) | ||
| Respiratory failure | 3 (3.5) | 66 (27.3) | 0 (0.0) | 3 (11.5) | ||
| Circuit thrombosis | 2 (2.3) | 2 (0.8) | 1 (0.7) | 0 (0.0) | ||
| Other | 1 (1.2) | 13 (5.4) | 3 (2.1) | 0 (0.0) | ||
| Unknown cause | 10 (11.6) | 0 (0.0) | 27 (19.0) | 0 (0.0) | ||
ECMO, extracorporeal membrane oxygenation; VA, venoarterial; VV, venovenous.
Mechanical and circuit complications are shown in Table 4. The highest rate of circuit change-outs occurred in COVID patients which was seen more than twice as frequently as in the PULM group and nearly three times higher in CARD patients. The mean number of circuit change-outs was also higher in the COVID groups. Similar trends were seen for oxygenator failures, circuit thromboses, circuit hemolysis, and all-cause hemorrhage.
Table 4.
Complications during extracorporeal membrane oxygenation across study groups.
| VV ECMO | VA ECMO | |||||
|---|---|---|---|---|---|---|
| Complication, n (%) | Pulmonary (206) | COVID (408) | p-value | Cardiac (279) | COVID (37) | p-value |
| Circuit change-out | ||||||
| No | 175 (85.0) | 256 (62.7) | <0.001 | 250 (89.6) | 26 (70.3) | 0.003 |
| Yes | 31 (15.0) | 152 (37.3) | 29 (10.4) | 11 (29.7) | ||
| Number of circuit changes | 0.3 (0.8) | 0.7 (1.2) | <0.001 | 0.1 (0.4) | 0.4 (0.8) | 0.02 |
| Oxygenator failure | ||||||
| No | 193 (93.7) | 327 (80.1) | <0.001 | 276 (98.9) | 30 (81.1) | <0.001 |
| Yes | 13 (6.3) | 81 (19.9) | 3 (1.1) | 7 (18.9) | ||
| Circuit thrombosis | ||||||
| No | 178 (86.4) | 276 (67.6) | <0.001 | 256 (91.8) | 21 (56.8) | <0.001 |
| Yes | 28 (13.6) | 132 (32.4) | 23 (8.2) | 16 (43.2) | ||
| Pump malfunction | ||||||
| No | 205 (99.5) | 402 (98.5) | 0.433 | 278 (99.6) | 36 (97.3) | 0.221 |
| Yes | 1 (0.5) | 6 (1.5) | 1 (0.4) | 1 (2.7) | ||
| Circuit hemolysis | ||||||
| No | 203 (98.5) | 362 (88.7) | <0.001 | 272 (97.5) | 30 (81.1) | <0.001 |
| Yes | 3 (1.5) | 46 (11.3) | 7 (2.5) | 7 (18.9) | ||
VA, venoarterial; VV, venovenous.
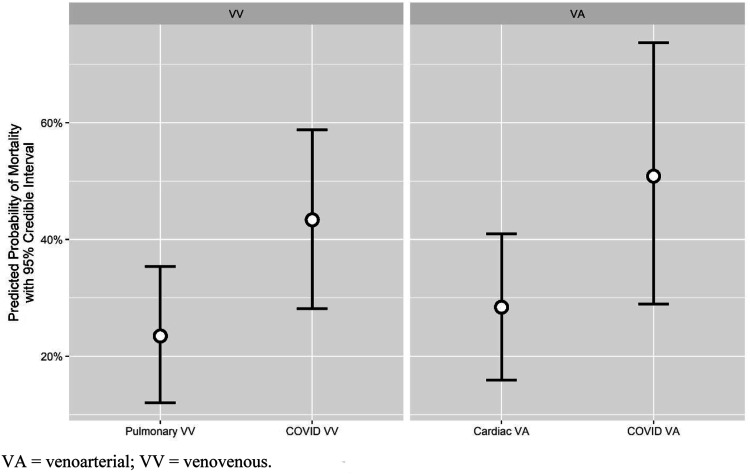
Bayesian logistic regression results are summarized in Table 5 and Figure 2. Having a COVID-19 diagnosis treated with VV ECMO was independently associated with a higher likelihood for mortality relative to PULM-VV (OR: 2.56, 95% Credible Interval: [1.58–4.14], posterior probability of increased mortality: >99.9%). Relative to CARD, COVID-VA had odds of mortality that were 2.67 times greater (95% Credible Interval: [1.10–6.72], posterior probability of increased mortality risk: 98.3%). Age, renal failure, hepatic failure, and female sex were independently associated with increased risk of mortality with posterior probability greater than 95%. The percent of relative explained variation in mortality outcomes attributable to indication by cannulation strategy grouping was 13%, on par with that of hepatic failure (14%) but behind other predictors such as age (25%) and renal failure (20%).
Table 5.
Bayesian logistic mixed effects logistic regression results for mortality on extracorporeal membrane oxygenation.
| VV ECMO | VA ECMO | |||||
|---|---|---|---|---|---|---|
| Outcome measure, n (%) | Pulmonary (206) | COVID (408) | p-value | Cardiac (279) | COVID (37) | p-value |
| Mortality | ||||||
| No | 120 (58.3) | 166 (40.7) | <0.001 | 137 (49.1) | 11 (29.7) | 0.041 |
| Yes | 86 (41.7) | 242 (59.3) | 142 (50.9) | 26 (70.3) | ||
| All cause hemorrhage | ||||||
| No | 138 (67.0) | 212 (52.0) | 0.001 | 168 (60.2) | 14 (37.8) | 0.016 |
| Yes | 68 (33.0) | 196 (48.0) | 111 (39.8) | 23 (62.2) | ||
| Hepatic failure | ||||||
| No | 172 (92.0) | 334 (86.8) | 0.09 | 234 (92.9) | 26 (74.3) | 0.002 |
| Yes | 15 (8.0) | 51 (13.2) | 18 (7.1) | 9 (25.7) | ||
| Renal failure | ||||||
| No | 158 (83.6) | 286 (74.3) | 0.016 | 195 (77.4) | 23 (63.9) | 0.119 |
| Yes | 31 (16.4) | 99 (25.7) | 57 (22.6) | 13 (36.1) | ||
| Infection | ||||||
| No | 185 (89.8) | 331 (81.1) | <0.001 | 274 (98.2) | 28 (75.7) | <0.001 |
| Yes new-onset | 5 (2.4) | 54 (13.2) | 1 (0.4) | 5 (13.5) | ||
| Yes pre-existing | 16 (7.8) | 23 (5.6) | 4 (1.4) | 4 (10.8) | ||
DTI, direct thrombin inhibitor; ECMO, extracorporeal membrane oxygenation; VA, venoarterial; VV, venovenous.
Figure 2.
Model-predicted probability of mortality by indication. VA, venoarterial; VV, venovenous.
Discussion
Infection with the coronavirus SARS-CoV-2 results in a wide range of symptomatology, but in severe cases can lead to pulmonary dysfunction resulting in the need for mechanical respiratory support. While early recommendations for employing ECMO were cautionary due to the vast resources necessary to conduct ECMO, and initial reporting of outcomes less than favorable, later research has shown that ECMO is a viable modality for severe illness.4–6,17–19 The virulent nature of the disease may lead to a variety of biological responses which include a heightened inflammatory response and a hypercoagulable prothrombotic state especially in hospitalized patients and those requiring ECMO.20 While the authors report slightly over 50% of COVID-19 patients had the cause of mortality reported as failure to improve from ARDS, a similar number of patients succumbed to multi-system organ failure, renal failure, central nervous system dysfunction, hemorrhage or sepsis confirming the virulent effect on other organ systems. We listed the causes of mortality in Table 3 and found for VV ECMO the most often reported reason was multisystem organ failure, which reflects the broad range of pathological consequences associated with severe infections. While this was also true for VA support in the COVID group, the main cause for CARD patients was cardiac arrest. Similar to the Jenner et al. study we saw failure to improve from ARDS as a mortality cause in 48% of cases, with multisystem organ failure, hemorrhage, cardiac arrest and sepsis in 47% of the remaining deaths.20 It is likely that COVID-19 induced coagulopathy results from various mitigations that are exacerbated with the use of extracorporeal blood flow contact with non-endothelialized surfaces and the need for systemic anticoagulation.21 While thromboembolic and bleeding events are more frequently seen in COVID-19, it is unknown whether or not this leads to higher mortality.22,23 We found significantly higher levels of both circuit thromboses and hemorrhage in COVID-19 patients as compared to both pulmonary and cardiac cohorts.
An early report from the ELSO registry had shown a discharge rate of 30% of 1,035 supported patients and a mortality rate of 38%.24 This study also reported that a higher hospital ECMO case volume was not associated with a lower mortality rate, which was in contrast to earlier ELSO results.25 A pooled report on 331 ECMO patients with COVID-19 from 10 published studies and registry data (n = 4) reported a 46% mortality rate (95% CI: 34–59%).25 In our study the mortality for COVID-19 patients was higher than that reported by ELSO, but was almost identical to that by Melhuish et al.26 While we did not measure the effect of either hospital or ECMO team level of experience as an outcome predictor, it is not unreasonable to expect that centers with higher volumes would have enhanced results. Such has been shown in a study that evaluated a model of a centralized approach for ECMO service and the use of mobile teams to transport COVID-19 patients to resource-rich hospitals.27 It was demonstrated that high pre-pandemic experience in providing ECMO care was strongly correlated in improving 90-days survival. The outcomes in using ECMO for COVID-19 patients has changed during the pandemic as depicted in our Bayesian logistic mixed effects logistic regression results for mortality (Table 4). While the reasons for this change are unknown, the change in virulence with evolving variants over the course of the pandemic is concerning. We, and others have shown an increase in mortality related to when patients were placed on ECMO and the pandemic phase.7,8,28 While the causes for these fluctuations in mortality are speculative, they may be attributed to a change in patient selection criteria, increased risk factors prior to ECMO and the absence of vaccination in susceptible groups. There have also been changes in treatment strategies that have evolved over the course of the pandemic, which included changes in medication regimens, mechanical ventilation and intubation modalities, the conduct of ECMO,28 as well as the use of vaccinations. Our results differ from those reported by Kurihara and colleagues who reported that there was no difference in survivability when comparing patients placed on ECMO for COVID-19 ARDS with non-COVID-19 ARDS.13 The difference in findings may be related to the smaller number of patients in the COVID-19 cohort limb of their study (19% vs 48%), and the fact that this was a single-center report as compared to our multi-institutional dataset. Furthermore, while the COVID cohorts had significantly longer time on ECMO when this was controlled for in the Bayesian logistic mixed effects logistic regression model it did not result in a strong influence on mortality.
Complications associated with ECMO are common despite technological advances in circuitry and equipment and increased knowledge of patient coagulation management. The decision to change-out a circuit during ECMO is most often related to circuitry thrombosis and a failure to maintain adequate flows or gas exchange.29,30 Lubnow et al. reported on mechanical complications during VV ECMO over a 5 year period and found that patients who required circuit change-outs had longer durations of ECMO support, longer total ventilation times, longer ICU stays, and were more likely to develop renal failure.31 The majority of circuit complications that occur are related to thrombus formation which results in reduced oxygenator gas transfer capabilities.20,31 While the Lubnow study predated the COVID-19 pandemic, those authors also reported a similar finding to the present study with both pulmonary and COVID-19 patients with primary lung failure having the highest rates of circuit change-outs. In a small single-center study there were significantly more thrombotic circuit events occurring during ECMO in COVID-19 patients compared to a non-COVID-19 group, with a lower targeted anticoagulation rate used more often in the non-COVID-19 group.10 While there were no differences in age or BMI between groups this is in contrast to our findings where COVID-19 patients were younger than pulmonary and cardiac patients, and larger in the VA cohort. We had previously identified age as having a large negative association with survival in COVID-19 patients who required ECMO, while BMI was not so associated.32 The circuit change-out rate in the current study was higher for COVID-19 patients both for venovenous support (37.3% vs 15.0%) and for venoarterial supported patients (29.7% vs 10.4%). The duration of support on ECMO has been shown to be associated with both bleeding and thrombotic complications,33 as was seen in the present study. In a study on VV ECMO that compared COVID-19 patients to those presenting with influenza, there were similar thrombosis rates between groups, but oxygenator failures were higher in patients with COVID-19 (20%) than influenza (0.0%).34 Oxygenator failures during ECMO are influenced by the fiber material (polypropylene failing at a rate as high as 91%,35 but all oxygenators used in the present study were made of polymethylpentene and are less prone to failure.
The prothrombotic state elicited by COVID-19 involves a number of intrinsic mechanisms centered both on inflammatory and coagulation processes.36 The heterogeneous response seen with COVID-19 is related to the condition of the patient at the time of infection, with age, male sex, the presence of comorbidities, and other factors affecting severity.1 Several have reported that critically ill COVID-19 patients have a higher degree of both venous thromboembolism and arterial thromboses, which are refractory to different anticoagulation regimens.12,37,38 In a recent multicenter study increased D-dimer levels were shown to be an independent risk factor for death in COVID-19 patients while in the intensive care unit.39 Bhagat and colleagues have shown that severe hypoxia induces tissue factor expression and increases thrombotic risk, which may help explain why patients requiring ECMO are especially vulnerable to circuit thrombotic complications.40 Coagulation management remains a formidable challenge for COVID-19 patients requiring a case-specific approach with modifications to standardized protocols occurring more frequently than other ECMO indications.12 There is concern that the thromboprophylaxis achieved with unfractionated heparin may be insufficient in COVID-19 patients and that the use of a DTI may result in improved anticoagulation control. In a large single-center study comparing bivalirudin and unfractionated heparin during adult ECMO, patients in the bivalirudin arm had significantly less thrombus formation, a lower occurrence of bleeding events, fewer allogeneic transfusions and lower circuit complications.41 A similar single-center study found that the use of argatroban resulted in a quicker attainment of therapeutic anticoagulation than unfractionated heparin, but there were no differences in bleeding or thrombotic complications between anticoagulants.42 While the present study did show a preferential use of DTI (bivalirudin or argatroban) for COVID-19 patients (37.5% use vs 12.0% and 5.8% for both the pulmonary and cardiac groups respectively), circuit complications and thrombus were still found more frequently during ECMO when they were used. This differs from the previous study that showed fewer overall complications, and may reflect the virulent nature of COVID-19 on obviating any benefit of alternative coagulation regimens. More recently it has been shown that COVID-19 patients require higher levels of bivalirudin to achieve and maintain anticoagulation then those presenting with ARDS.43 Unfortunately we did not have access to the pharmacologic characterization of the DTI administered and do not know if a higher dosing protocol was used. Also, we did not differentiate between the initial use of a DTI for anticoagulation or the cross-over from heparin to a DTI, and cannot determine the benefit of using these agents.
Study limitations
There are several limitations to this study. There have been several Coronavirus variants over the course of the pandemic with associated changes in virulence which may have influenced outcomes and mortality rates. While it is impossible to discern which infections in the COVID-19 population were caused by specific variants it is not unreasonable to expect an influence based upon these mutations. Nevertheless, the patient population all came from the start of the pandemic through January 2022 to establish continuity in observations. While the data collection system utilized for the registry contains definitions for each of the measured fields, and training on documenting data was made, there may have been some misinterpretation by the clinicians loading the information. Although we maintained a parallel COVID-19 database where additional variables are contained (pre-ECMO patient risk factors and medication treatments to reduce respiratory dysfunction, and discharge information), this information was not systematically documented for patients in the non-COVID-19 groups so was not available for comparison. While completeness of data entry was assured by software coding, and validation steps made to assure accuracy, we cannot rule out that submission errors may have occurred. While data were collected in a prospective manner it is nonetheless non-randomized. Differences in practice patterns across sites do exist, and although we attempted to minimize bias through the use of multiple imputation and mixed-effects logistic regression, we realize that unmeasured confounding is still present.
Conclusions
The application of ECMO has been shown to be an effective management technique in patients with refractory respiratory failure as a result of COVID-19. The present study using registry data has shown that COVID-19 patients who required ECMO are both at an increased risk for mortality and have more circuit complications than patients on support for either general pulmonary or cardiac failure. Continued research to better understand the factors associated with these differences will help guide distinct management opportunities to improve outcomes.
Acknowledgements
The authors wish to thank the numerous clinicians across the multiple hospitals in this study, who are involved in the management of patients requiring ECMO support, and particularly in those patients with COVID-19 who demand the highest level of attention, while presenting with an easily transmissible disease.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Alfred H Stammers https://orcid.org/0000-0001-5057-3526
Linda B Mongero https://orcid.org/0000-0002-2780-8961
References
- 1.Miesbach W, Makris M. COVID-19: Coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin Appl Thromb Hemost 2020; 26: 1076029620938149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yusuff H, Zochios V, Brodie D. Thrombosis and coagulopathy in COVID-19 patients requiring extracorporeal membrane oxygenation. ASAIO J 2020; 66: 844–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kon ZN, Smith DE, Chang SH, et al. Extracorporeal membrane oxygenation support in severe COVID-19. Ann Thorac Surg 2021; 111: 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs JP, Stammers AH, St. Louis JD, et al. Multi-institutional analysis of 100 consecutive patients with Covid-19 and severe pulmonary compromise treated with extracorporeal membrane oxygenation: outcomes and trends over time. ASAIO J 2021; 67: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs JP, Stammers AH, St. Louis JD, et al. Multi-institutional analysis of 200 COVID-19 patients treated with ECMO: outcomes and trends. Ann Thorac Surg 2021; 113: 1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall CA, Jacobs JP, Stammers AH, et al. Multi-institutional analysis of 505 patients with Coronavirus Disease-2019 supported with extracorporeal membrane oxygenation: predictors of survival. Ann Thorac Surg. 2022; 114: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international extracorporeal life support organization registry. Lancet 2021; 398: 1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broman LM, Eksborg S, Lo Coco V, et al. EuroECMO COVID-19 Working Group; Euro-ELSO Steering Committee: extracorporeal membrane oxygenation for COVID-19 during first and second waves. Lancet Respir Med 2021; 9: e80–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badulak J, Antonini MV, Stead CM, et al. ELSO COVID-19 working group members. Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the extracorporeal life support organization. ASAIO J 2021; 67: 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bemtgen X, Zotzmann V, Benk C, et al. Thrombotic circuit complications during venovenous extracorporeal membrane oxygenation in COVID-19. J Thromb Thrombolysis 2021; 51: 301–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyls C, Huette P, Abou-Arab O, et al. Extracorporeal membrane oxygenation for COVID-19-associated severe acute respiratory distress syndrome and risk of thrombosis. Br J Anaesth 2020; 125: e260–e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Z, Sun L, Li B, et al. Anticoagulation management in severe coronavirus disease 2019 patients on extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth 2021; 35: 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurihara C, Manerikar A, Gao CA, et al. Outcomes after extracorporeal membrane oxygenation support in COVID-19 and non-COVID-19 patients. Artif Organs 2021; 46, 688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.R Core Team . R A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2019. [Google Scholar]
- 15.Subirana I, Sanz H, Vila J. Building bivariate tables: the ‘compareGroups’ package for R. J Statist Soft 2014; 57: 1–16. [Google Scholar]
- 16.van Buuren S, Groothuis-Oudshoorn K. ‘mice’: multivariate imputation by chained equations in R. J Statist Soft 2011; 45: 1–67. [Google Scholar]
- 17.Yang X, Cai S, Luo Y, et al. Extracorporeal membrane oxygenation for coronavirus disease 2019-induced acute respiratory distress syndrome: a multicenter descriptive study. Crit Care Med 2020; 48: 1289–1295. [DOI] [PubMed] [Google Scholar]
- 18.MacLaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA 2020; 323: 1245–1246. [DOI] [PubMed] [Google Scholar]
- 19.Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): pooled analysis of early reports. J Crit Care 2020; 58: 27–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenner WJ, Gorog DA. Incidence of thrombotic complications in COVID-19: on behalf of ICODE: the international COVID-19 thrombosis biomarkers colloquium. J Thromb Thrombolysis 2021; 52: 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sniderman J, Monagle P, Annich GM, et al. Hematologic concerns in extracorporeal membrane oxygenation. Res Pract Thromb Haemost 2020; 4: 455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helms J, Tacquard C, Severac F, et al. CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020; 46: 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durak K, Kersten A, Grottke O, et al. Thromboembolic and bleeding events in COVID-19 patients receiving extracorporeal membrane oxygenation. Thorac Cardiovasc Surg 2021; 69: 526–536. [DOI] [PubMed] [Google Scholar]
- 24.Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet 2020; 396: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbaro RP, Odetola FO, Kidwell KM, et al. Association of hospital level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med 2015; 191: 894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melhuish TM, Vlok R, Thang C, et al. Outcomes of extracorporeal membrane oxygenation support for patients with COVID-19: a pooled analysis of 331 cases. Am J Emerg Med 2021; 39: 245–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebreton G, Schmidt M, Ponnaiah M, et al. Extracorporeal membrane oxygenation network organization and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med 2021; 9: 851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs JP, Stammers AH, St. Louis J, et al. Variation in survival in patients with COVID-19 supported with ECMO: A multi-institutional analysis of 594 consecutive COVID-19 patients supported with ECMO across 49 hospitals within 21 States. J Thorac Cardiovasc Surg 2022; S0022-5223(22) 00526–8. DOI: 10.1016/j.jtcvs.2022.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel B, Arcaro M, Chatterjee S. Bedside troubleshooting during venovenous extracorporeal membrane oxygenation (ECMO). J Thorac Dis 2019; 11(Suppl 14): S1698–S1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagler B, Hermann A, Robak O, et al. Incidence and etiology of system exchanges in patients receiving extracorporeal membrane oxygenation. ASAIO J 2021; 67: 776–784. [DOI] [PubMed] [Google Scholar]
- 31.Lubnow M, Philipp A, Foltan M, et al. Technical complications during veno-venous extracorporeal membrane oxygenation and their relevance predicting a system-exchange--retrospective analysis of 265 cases. PLoS One 2014; 9: e112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mongero LB, Stammers AH, Tesdahl EA, et al. The use of extracorporeal membrane oxygenation in Covid-19 patients with severe cardiorespiratory failure: the influence of obesity on outcomes. J Extra Corpor Technol 2021; 53: 293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nunez JI, Gosling AF, O’Gara B, et al. Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: an ELSO registry analysis. Intensive Care Med 2022; 48: 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cousin N, Bourel C, Carpentier D, et al. Lille Intensive Care COVID-19 Group. SARS-CoV-2 versus influenza-associated acute respiratory distress syndrome requiring veno-venous extracorporeal membrane oxygenation support. ASAIO J 2021; 67: 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daniel JM, Bernard PA, Skinner SC, et al. Hollow fiber oxygenator composition has a significant impact on failure rates in neonates on extracorporeal membrane oxygenation: a retrospective analysis. J Pediatr Intensive Care. 2018; 7: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kowalewski M, Fina D, Słomka A, et al. COVID-19 and ECMO: the interplay between coagulation and inflammation-a narrative review. Crit Care 2020; 24: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trigonis RA, Holt DB, Yuan R, et al. Incidence of venous thromboembolism in critically ill coronavirus disease 2019 patients receiving prophylactic anticoagulation. Crit Care Med 2020; 48: e805–e808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020; 191: 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Short SAP, Gupta S, Brenner SK, et al. Stop-Covid Investigators. D-dimer and death in critically ill patients with coronavirus disease 2019. Crit Care Med 2021; 49: e500–e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhagat S, Biswas I, Ahmed R, et al. Hypoxia induced up-regulation of tissue factor is mediated through extracellular RNA activated toll-like receptor 3-activated protein 1 signaling. Blood Cell Mol Dis 2020; 84: 102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivosecchi RM, Arakelians AR, Ryan J, et al. Comparison of anticoagulation strategies in patients requiring venovenous extracorporeal membrane oxygenation: heparin versus bivalirudin. Crit Care Med 2021; 49: 1129–1136. [DOI] [PubMed] [Google Scholar]
- 42.Sattler LA, Boster JM, Ivins-O’Keefe KM, et al. Argatroban for anticoagulation in patients requiring venovenous extracorporeal membrane oxygenation in Coronavirus Disease 2019. Crit Care Explor 2021; 3: e0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trigonis R, Smith N, Porter S, et al. Efficacy of bivalirudin for therapeutic anticoagulation in COVID-19 patients requiring ECMO support. J Cardiothorac Vasc Anesth 2022; 36: 414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]