Abstract
Cell membrane-coated nanoparticles (NPs) have attracted growing attention in the field of targeted delivery strategies, which successfully combine the advantages and properties of both cell membranes and synthetic NPs. Stem cell-based delivery systems have the innate targeting capability to tumor tissues, but inappropriate stem cells might promote tumor growth after being injected into the body. Accordingly, it is urgent to explore novel drug delivery systems that might combine the advantages of stem cells and eliminate the possible risks. This review aimed to investigate the stem cell membrane-camouflaged targeted delivery system in tumors. We discussed the underlying mechanisms of stem cell homing to target tumors. Then, the common membrane modification methods well as preparation methods of stem cell membrane coated NPs were concluded. NPs coating the stem cell membranes could obtain the tumor targeting ability, enhanced biocompatibility, and effective drug loading. Furthermore, we investigated the potential clinical applications of mesenchymal stem cells (MSCs) and induced pluripotent stem (iPS) cells membrane-camouflaged targeted delivery systems for anti-tumor therapies, such as chemotherapy, photodynamic therapy, magnetic hyperthermia therapy and imaging, CRISPR-Cas9 gene therapy, and synergistic therapy. Taken together, stem cell membrane-coated NPs hold the tremendous prospect for biomedical applications in tumor therapy.
Keywords: Stem cell, Membrane coated nanoparticles, Stem cell homing, Tumor targeting therapy
Graphical abstract
1. Introduction
Cell-based targeted delivery systems are regarded as a novel delivery strategy owing to low immunogenicity and toxicity, long circulation time, innate targeting capability, and integration of receptors [1]. A variety of cells could function as effective natural vesicles, such as red blood cells, platelets, immune cells, tumor cells, stem cells, and even viruses and bacteria [2]. However, it is well known that several limitations should be resolved in the clinical applications of differentiated cells. It is hard to obtain enough quantities of differentiated cells under natural conditions. Moreover, it is not easy for differentiated cells to be modified or survive in vitro.
Nowadays, mesenchymal stem cells (MSCs), which could be derived from bone marrow (BM-MSCs), umbilical cord (UC-MSCs), and adipose tissue (ATMSCs), are commonly utilized in clinical applications [3]. Induced pluripotent stem (iPS) cells are more attractive than MSCs, since iPS cells could be relatively easily obtained by reprogramming differentiated somatic cells with transcription factors [4]. Taken together, stem cells such as MSCs [2,5] and iPS cells could function as targeted delivery vehicles for stem cell-based tumor therapy owing to the following advantages: (a) Stem cells could be easily isolated and expanded in vitro [6]. More importantly, phenotypes and multilineage potential might be preserved even for over 50 population doublings in vitro [7]; (b) Some drug delivery systems based on stem cells have been established for satisfying anti-tumor strategies [8]; (c) Stem cell-based delivery systems have the innate targeting capability to inflammation or tumor lesions [9]. However, stem cells were found to be entrapped in the lung after intravenous injection, which might result in microembolism because of the size of stem cells [10,11]. Meanwhile, stem cells have the differentiation potential. It should not be ignored that inappropriate stem cells might promote tumor growth after being injected into the body. Accordingly, it holds great promise for exploring novel drug delivery systems, that might combine the advantages of stem cells and eliminate the possible risks.
Cell membrane-coated nanoparticles (NPs) have attracted growing attention in the field of targeted delivery strategies, which successfully combine the advantages and properties of both cell membranes and synthetic NPs [12,13]. Therefore, NPs coating the stem cell membranes could obtain the tumor targeting ability, enhanced biocompatibility, and effective drug loading. Taken together, the stem cell membrane-coated NPs hold the tremendous prospect for a broad range of biomedical applications. Several previous studies have investigated the role of cell membrane-coated nanocarriers for targeted therapy in tumors [14,15]. However, stem cell membrane-based targeted delivery systems in tumors have not been investigated and discussed thoroughly.
In this review, we aimed to investigate the stem cell membrane-camouflaged targeted delivery system in tumors. Two previous studies mainly focus on the roles of the MSC membrane in the diseases of inflammation [16] and arthritis [17]. To thoroughly reveal the stem cell membrane-based delivery system in tumors, we first discussed the underlying mechanisms of stem cell homing to target tumor tissues. Then, the common modification methods of the stem cell membrane, as well as preparation methods of stem cell membrane coated NPs were concluded. Furthermore, we investigated the potential clinical applications of MSCs and iPS cell membrane-camouflaged targeted delivery systems for anti-tumor therapies, such as chemotherapy, photodynamic therapy, magnetic hyperthermia therapy, and imaging, CRISPR-Cas9 gene therapy, and synergistic therapy.
2. Mechanisms of stem cell homing
Karp et al. [18] defined stem cell homing as the arrest of stem cells within the vasculature of a tissue followed by transmigration across the endothelium. Taken together, tumor homing of stem cells includes three processes: getting into the blood system by direct administration or recruitment from bone marrow; rolling, capture, adhesion, and extravasation across epithelial layers into lesions mediated by chemokine gradients; and migrating towards tumor tissues.
Various receptors could express in the newly isolated stem cells, such as CXCR1/4/5/6 and CCR2/7/9/10 [2]. The expression of receptors largely relies on sources of stem cells, culturing processes, and cell age. The mechanisms for stem cell homing could be categorized into the following four types: (a) Macrophage migration inhibitory factor (MIF), whose native receptors are CXCR4, CXCR2, and CD74, plays a vital role in MSC recruitment [19]. (b) CXCR5 is upregulated to improve the homing ability of MSC by crosstalk with CXCL13 in the mice model [20]. (c) CXCR4 is dominant in affecting the tumor-targeting ability of stem cells. Mechanically, chemokine stromal-derived factor 1 (SDF-1) is released by injury or tumor lesions [21], and the crosstalk between SDF-1 (CXCL12) and CXCR4 induced the homing ability of stem cells [22,23]. (d) During the second phase of homing, signals including galectin-1 [24], CD24 [25], CXCL9/16, CCL20/25 [26], and integrins α/β [27] mediate the process of cell rolling, adhesion, capture, and extravasation.
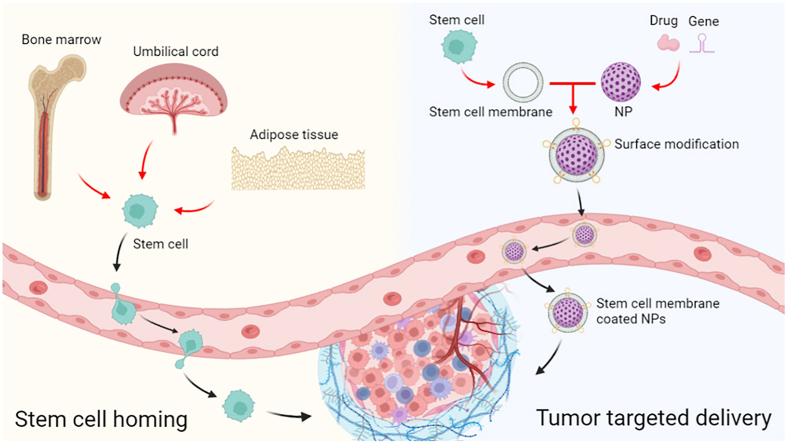
Stem cells could migrate to tumor lesions and have direct interaction with targeted tumor cells, thereby resulting in targeted drug delivery (Fig. 1). In addition, stem cells might also be engineered to highly express the specific key gene or delivery drugs stably. Accordingly, stem cells equipped with homing ability have been regarded as promising candidates for the targeted delivery systems. Because the above ligands and receptors associated with the mechanisms of stem cell homing are mainly expressed on the stem cell membrane, the stem cell membrane can still retain the tumor homing ability.
Fig. 1.
Schematic illustration of stem cell homing and the stem cell membrane-camouflaged targeted delivery system in tumors.
3. Modification of stem cell membrane
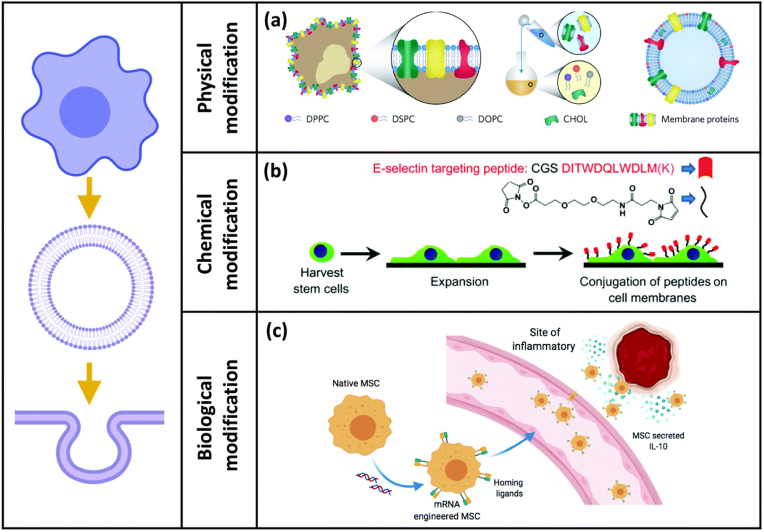
Owing to numerous ligands and receptors on the stem cell membrane, stem cell-based therapy has been widely utilized in different diseases [28]. Accordingly, the surface modification of stem cell membranes might enable the membrane-coated NPs with more specific abilities beyond cell membranes [29]. Three common modification strategies are shown as follows (Fig. 2) [16].
Fig. 2.
Three common methods to modify stem cell membranes [16]. Adapted from Wang et al. (2021). Reprinted with permission from M. Wang, Y. Xin, H. Cao, W. Li, Y. Hua, T.J. Webster, C. Zhang, W. Tang, Z. Liu, Recent advances in mesenchymal stem cell membrane-coated nanoparticles for enhanced drug delivery, Biomater. Sci. 9 (2021) 1088-1103. Copyright 2021 Royal Society of Chemistry.
3.1. Physical modification
Cell membranes are mainly composed of lipids with membrane fluidity. Therefore, lipid-based biomaterials might link to the surface of cell membranes or be coated by cell membranes [30]. Liposomes fused with cell membrane-camouflaged NPs could enhance the inclusion of ligands or drugs within the membrane. For example, liposomes could be fused with enriched leukocyte membrane proteins to develop a new nanovesicle [31,32]. The fused new nanovesicles with transmembrane proteins including CD45, CD47, and PSGL-1, might target inflamed sites effectively and across the inflamed vascular barrier easily [33]. Although physical modification of membrane-camouflaged NPs is convenient and widely applied [34], we should not ignore the great obstacles of insufficient stability and efficiency. Hydrophobic interactions could contribute to the risk of changing the original membranes and serum proteins might influence the insertion efficiency.
3.2. Chemical modification
Because of the thiol and amine residues of membrane-associated proteins and hydroxyl residues of polysaccharides, cell membranes could be utilized for covalent conjugation modifications [35]. For instance, functional amine groups could react with preactivated carboxyl groups and N-hydroxy succinimide (NHS) groups [36], which enable molecules or ligands with those groups might conjugate directly to cell membranes. Similarly, NHS-PEG2-maleimide could conjugate thiol-rich peptides and amine-bearing cell membranes with maleimide and NHS groups, respectively [37]. PEG in this linker has provided enough space for ligand-receptor binding [38], which might enhance cell uptake. Moreover, maleimide-functionalized ligands could conjugate thiol residues on hematopoietic stem cells [39]. Chemical modification is characterized by being more stable, straightforward, and efficient. More importantly, chemical covalent conjugation might not change the membrane permeability and structure.
3.3. Biological modification
Biological modification aims to introduce desired proteins onto cell membranes via genetic technology. The majority of genetic modifications of stem cell membranes are designed to increase the expression of tumor-targeting ligands [40]. For example, CXCR4-overexpressing adipose tissue-derived MSC exhibited better homing to bone marrow [41]. Genetically modified MSC with upregulated CXCR4 expression increased MSC targeting to ischemic lesions [42]. Moreover, mRNA transfection was utilized to genetically modify MSC to target inflammatory sites, which enabled engineered MSC to exhibit improved inflammation targeting ability and deliver immunomodulatory drugs [42].
4. Preparation of stem cell membrane-camouflaged targeted delivery system
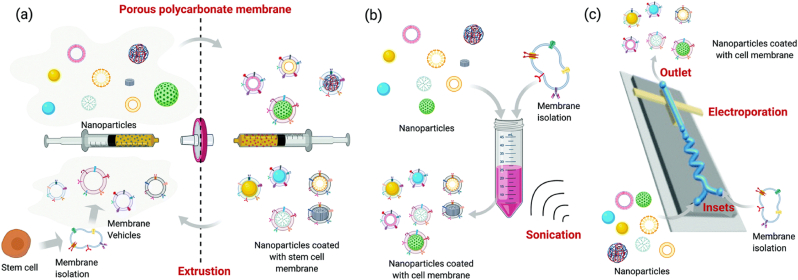
The preparation methods of stem cell membrane-camouflaged nanoparticles mainly included the following strategies (Fig. 3): co-extrusion method, sonication method, microfluidic electroporation method, and electrostatic attraction method [16].
Fig. 3.
Common preparation methods of stem cell membrane coating nanotechnology: (a) physical extrusion method, (b) sonication method, and (c) microfluidic system with electroporation device [16]. Adapted from Wang et al. (2021). Reprinted with permission from M. Wang, Y. Xin, H. Cao, W. Li, Y. Hua, T.J. Webster, C. Zhang, W. Tang, Z. Liu, Recent advances in mesenchymal stem cell membrane-coated nanoparticles for enhanced drug delivery, Biomater. Sci. 9 (2021) 1088-1103. Copyright 2021 Royal Society of Chemistry.
4.1. Co-extrusion method
The most common method to prepare cell membrane-camouflaged NPs is extrusions by an extruder with porous polycarbonate membranes and 100–400 nm pore sizes several times [43]. Mechanically, the mechanical force during the extruding process enables NPs to be coated by the phospholipid bilayers owing to the fluidity of membranes, which could assure the structure and function of membranes. The sizes of cell membrane-camouflaged NPs rely on the pore sizes of an extruder. The advantage of the co-extrusion method is that a uniform distribution of size could be obtained, but it is a time-consuming process.
4.2. Sonication method
During sonication, the membrane structure could be destroyed by ultrasonic waves. Moreover, cell membranes are camouflaged on the surface of NPs by simple co-incubation [44]. Although the sonication method is easy and time-saving, the power, frequency, and duration of sonication could be further improved to maximize fusion efficiency and decrease protein denaturation.
4.3. Microfluidic electroporation method
Microfluidic electroporation is regarded as a new method for cell membrane coating [45,46]. NPs and membranes are infused into the microfluidic chip and then mixed thoroughly. The electrical pulses contribute to the formation of transient pores on membranes and NPs are further coated. The membrane perforation could be either reversible or irreversible. Although the cost of the microfluidic electroporation method is relatively high, it could produce high-quality NPs with complete membrane coating with desirable stability.
4.4. Electrostatic attraction method
The electrostatic attraction method means that the electrostatic attraction between the negatively charged cell membranes and positively charged NPs could lead to the spontaneous formation of cell membrane-camouflaged NPs. For example, the self-assembly of negatively charged leukocyte membrane-derived vesicles and positively charged silicon NPs was introduced by previous studies [31]. The electrostatic attraction plays a vital role in the fusion of membranes and NPs [47], therefore, the number of charges on the surface of NPs should be controlled [48]. For NPs with a high positive charge, the interactions with vesicles are strong enough to result in an incomplete coating. Among the four methods above, the ratio of NPs and membranes must be strictly modulated to improve the coating efficiency [49].
5. Stem cell membrane-camouflaged targeted delivery system for anti-tumor therapy
5.1. Chemotherapy
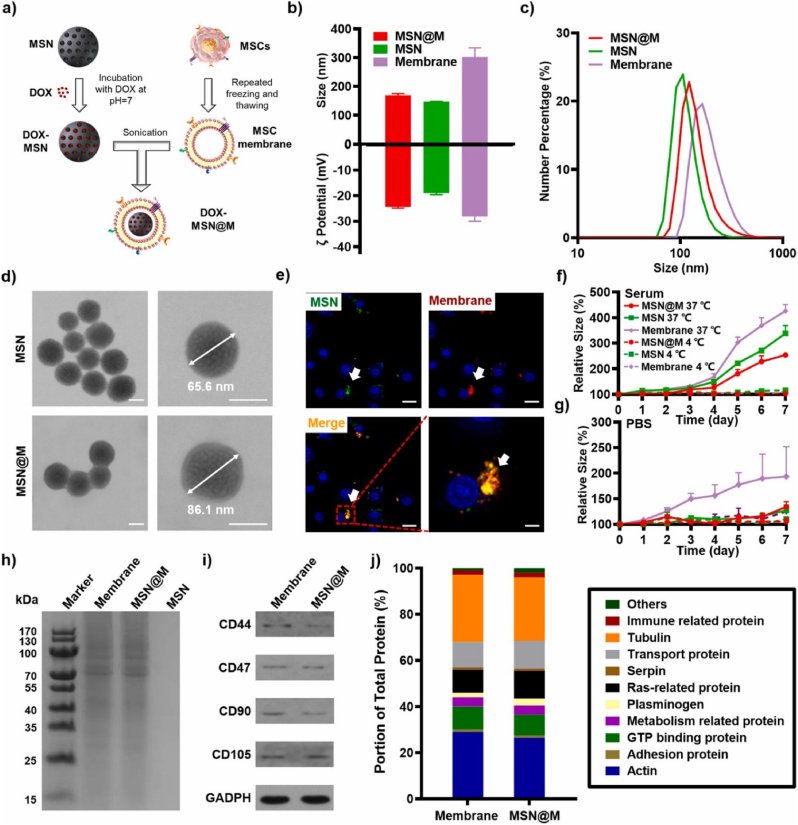
Stem cell membrane-coated NPs might obtain the complicated functions from stem cells to achieve biological interfacing, and preserve the nano size and loading ability of NPs. Accordingly, stem cell membrane-coated NPs have been regarded as a novel targeted drug delivery system for tumors. Nie et al. had reported the MSC membrane-coated PLGA nanovesicle loaded with doxorubicin (DOX). The results indicated that the effective uptake of membrane-coated nanovesicle was several times than that of uncoated PLGA, thereby leading to enhanced anti-tumor efficacy [50]. Similarly, He et al. constructed the MSC membrane-coated gelatin nanogels loaded with DOX. The nanogel was camouflaged by the MSC membrane via a coextrusion procedure. As shown by an electron microscope, the stem cell membrane was tightly camouflaged on the surface of nanogel. This membrane-coated drug delivery nanovesicle has increased storage stability and showed a sustained drug release [51]. Li et al. [52] coated the MSC membrane onto the mesoporous silica nanoparticle (MSN) to form MSN@M (Fig. 4). MSN@M showed a remarkably decreased clearance rate in vitro because of the CD47 marker on the MSC membrane. In HepG2 xenograft mice, MSN@M was observed with stronger tumor targeting and penetration ability and sustained DOX release ability. The results indicated the enhanced anti-tumor effects and decreased side effects of DOX-loaded MSN@M in vivo. To sum up, stem cell membrane-coated NPs might act as novel vehicles for stem cells and provide more approaches for targeted drug delivery for tumors. With the abilities of tumor targeting and penetration, stem cell membrane-coated nanotechnology could largely enhance the anti-tumor effects of chemotherapeutic agents and minimize the adverse effects. It is of great importance in resolving the obstacles of chemoresistance in the clinic [53].
Fig. 4.
Preparation and characterization of MSC membrane-coated DOX-MSN (DOX-MSN@M) as biomimetic vehicles for effective anti-tumor therapy [52]. Adapted from Li et al. (2021). Reprinted from J Control Release, 335, Y.S. Li, H.H. Wu, X.C. Jiang, T.Y. Zhang, Y. Zhou, L.L. Huang, P. Zhi, Y. Tabata, J.Q. Gao, Active stealth and self-positioning biomimetic vehicles achieved effective antitumor therapy, Pages 515-526, Copyright 2021, with permission from Elsevier.
5.2. Photodynamic therapy
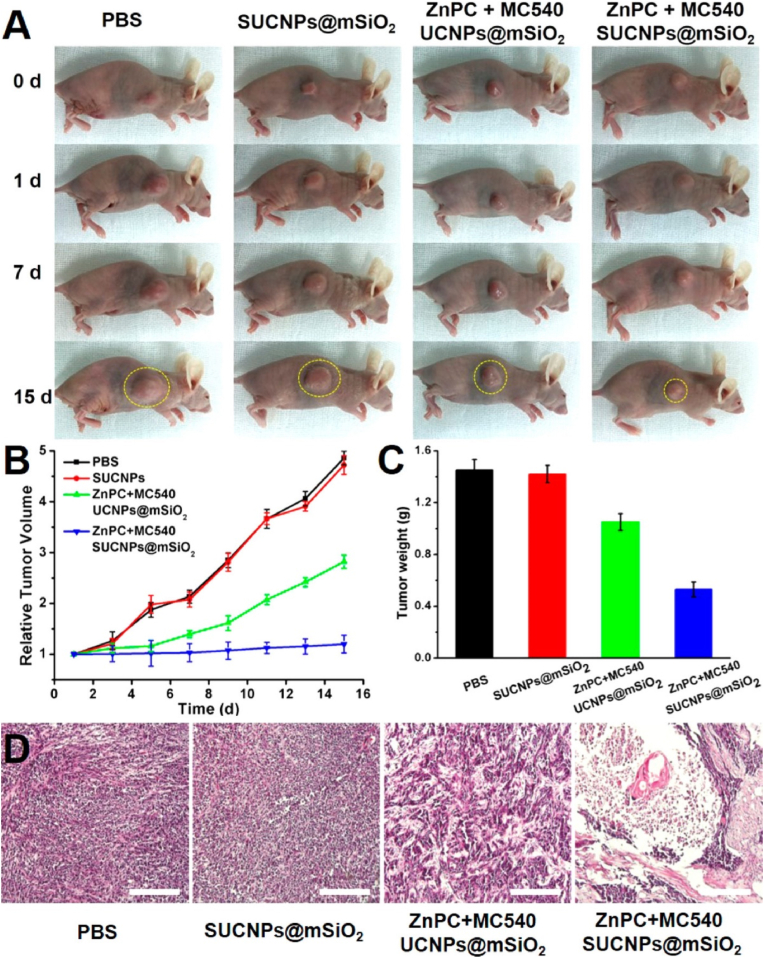
Photodynamic therapy (PDT) mainly depends on reactive oxygen species (ROS) generation mediated by photosensitizer with laser irradiation [54,55]. Gao et al. coated MSC membrane on mesoporous silica and encapsulated β-NaYF4:Yb3+, Er 3+ up-conversion NPs (photosensitizer) that were loaded with ZnPC and MC50 to prepare SUCNPs@mSiO2 [56]. The SUCNPs@mSiO2 showed tumor-targeting ability owing to a higher tumor affinity after MSC membrane coating. The membrane-coated NPs indicated good cytocompatibility and hemocompatibility with normal hepatocyte cells and blood respectively. Accordingly, stem cell membrane-coated NPs could effectively circulate for a longer time and evade the immune system, which increased the accumulation in tumor sites and reduced reticuloendothelial system (RES) clearance. More importantly, under the 980 nm laser, SUCNPs@mSiO2 might effectively inhibit tumors both in vitro and in vivo with PDT therapy (Fig. 5).
Fig. 5.
The anti-tumor effects of SUCNPs@mSiO2 with PDT treatment were evaluated in vivo [56]. Adapted from Gao et al. (2016). Reprinted with permission from C. Gao, Z. Lin, Z. Wu, X. Lin, Q. He, Stem-cell-membrane camouflaging on near-infrared photoactivated upconversion nanoarchitectures for in vivo remote-controlled photodynamic therapy, ACS Appl. Mater. Interfaces 8 (2016) 34252-34260. Copyright 2016 American Chemical Society.
5.3. Magnetic hyperthermia therapy and magnetic resonance imaging (MRI)
Chang et al. prepared the superparamagnetic iron oxide (SPIO) NPs coated by adipose-derived MSC membrane that were capable of reducing the immune response. During the preparation process, CD44 on the surface of the MSC membrane was well preserved [57]. This nanovesicle could prevent macrophage recognition and uptake, which enables itself stable in blood circulation. Because of the tumor homing ability and nano size of MSC, the nanovesicle generated dark contrast in T2-weight magnetic resonance imaging (MRI) with dose-dependent. Under an alternating magnetic field, Tramp-C1 prostate cancer cells were significantly inhibited owing to the magnetic hyperthermia therapy induced by this nanovesicle [58]. Similarly, Mu et al. [59] showed siRNA delivery with stem cell membrane-coated magnetic polydopamine (PDA) NPs for imaging-guided photothermal therapy and gene therapy. In their study, Fe3O4@PDA NPs with photothermal effects were utilized for ideal siRNA delivery. MSC membrane camouflaged Fe3O4@PDA-siRNA NPs preserved the photothermal effects and had the ability of MRI. Moreover, this nanovesicle could deliver siRNA against the Plk1 gene and induce the apoptosis of DU145 cells. The combined magnetic hyperthermia therapy and gene therapy indicated enhanced anti-tumor effects in vivo. Accordingly, stem cell membrane-coated NPs have wide clinical applications, including siRNA delivery, magnetic hyperthermia therapy and imaging. To sum up, stem cell membrane-coated nanotechnology showed great potentials for clinical applications in the field of both diagnosis and treatment of tumors.
5.4. CRISPR-Cas9 gene therapy
As known to all, leukemia stem cells (LSCs) might lead to the relapse of acute myeloid leukemia (AML). Therefore, a lipidoid nanoparticle (LNP) loaded Cas9/single guide RNA (sgRNA) [60] ribonucleoprotein (RNP) was designed to target the key gene interleukin-1 receptor accessory protein (IL1RAP) of LSCs. LNP-Cas9 RNP and CXCL12α were further loaded onto the MSC membrane-camouflaged nanofibril scaffolds to induce LSC targeting and increase the retention time of LNP-Cas9 in bone marrow. This study showed that CXCL12α could mediate LSCs migration to the scaffolds, and LNP-Cas9 RNP could edit target gene efficiently. Mechanically, IL1RAP knockout contributed to the inhibition of LSC colony-forming ability and leukemic relapse. To sum up, sustained targeted delivery of Cas9/IL1RAP sgRNA by MSC membrane-coated scaffolds might be a novel therapy for inhibiting LSC growth to treat AML relapse [61].
5.5. Synergistic therapy
Programmed cell death ligand 1 (PD-L1) blockade is widely used in immunotherapy of tumors [62]. Mechanically, PD-L1 siRNA might activate T cells by decreasing PD-L1 expressed on tumor cells [63,64]. However, the anti-tumor effects of PD-1/PD-L1 monotherapy are unsatisfactory [65,66]. DOX might not only result in cell apoptosis but also enhance the antigens release of tumors. In the study of Mu et al. [67], DOX and PD-L1 siRNA were loaded by MSC membrane-coated PDA for synergistic chemo-immunotherapy of prostate cancer (PCa) bone metastases. It showed that the stem cell membrane-camouflaged NPs improved blood circulation and enhanced tumor targeting ability, thereby showing synergistic anti-tumor efficiency.
Photothermal therapy (PTT) has attracted growing attention owing to the use of light radiation and heat generation at the tumor site, which thermal ablation is regarded as a minimally invasive method for tumor therapy [68]. The previous study showed that cell membrane-coated NPs could be a novel biomimetic platform for cancer photothermal therapy [49]. Zhang et al. [69] designed PDA NPs coated by stem cell membranes for synergistic chemo-photothermal therapy of bone tumors. The membrane-camouflaged NPs were utilized to efficiently load the hydrophobic drug 7-ethyl-10-hydroxycamptothecin (SN38). The release of SN38 was triggered by laser irradiation and an acidic stimulus. These synthesized NPs retained an excellent photothermal effect and showed longer blood circulation and effective tumor-targeting ability. Taken together, both in vitro and in vivo experiments indicated the novel synergistic chemo-photothermal therapy of the membrane-camouflaged NPs, which might be a promising therapy for bone tumors.
To sum up, stem membrane-based delivery systems could largely facilitate the clinical applications of synergistic therapy owing to the advantages of high drug loading efficacy and powerful tumor targeting ability.
5.6. Others
Extracellular vesicles (EVs), like exosomes, have drawn a lot of interest because of their potential use in nanomedicine as a drug delivery method [70,71]. With their low immunogenicity, great biocompatibility, and stability against traditional synthetic carriers, EVs derived from stem cells offer many benefits that open up new possibilities for the delivery of therapeutic drugs to target cells. Rezaie et al. summarized the stem cell-derived extracellular vesicles as drug delivery systems for various cancers [72]. Zhou et al. [73] delivered paclitaxel and gemcitabine monophosphate to pancreatic cancer using EVs derived from MSCs. These EVs showed benefits in their homing and piercing powers. Additionally, they demonstrated modest systemic toxicity and anti-matrix characteristics with improved chemoresistance.
Because iPS cells have the great advantage of being relatively easily obtained by reprogramming differentiated somatic cells, the application of iPS cells [74] or iPS-derived stem cells [75] as a drug delivery system for tumors has been widely investigated. It has been reported that human iPS cells could achieve the targeted delivery of gold nanorods and the PDT effect (Fig. 6) [74]. Another study also indicated that mitomycin-treated iPS cells showed good biocompatibility and tumor-targeting ability [76]. Furthermore, mitomycin-treated iPS cells were utilized to deliver MnO2@Ce6 nanoprobes for synergistic PDT and immunotherapy of tumors [77]. In this study, iPS cells function as both a drug delivery system and a source of tumor immune antigens under laser irradiation.
Fig. 6.
Schematic illustration of human iPS cells for targeted delivery of gold nanorods and enhanced photothermal therapy for tumors [74]. Adapted from Liu et al. (2016). Reprinted with permission from Y. Liu, M. Yang, J. Zhang, X. Zhi, C. Li, C. Zhang, F. Pan, K. Wang, Y. Yang, J. Martinez de la Fuentea, D. Cui, Human induced pluripotent stem cells for tumor targeted delivery of gold nanorods and enhanced photothermal therapy, ACS Nano 10 (2016) 2375-2385. Copyright 2016 American Chemical Society. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
However, it is observed that iPS cells could widely distribute in different organs, thereby leading to unintended side effects [75]. To solve this problem, stem cell death should occur in the entire body after tumor therapy is achieved. Zhu et al. engineered neural stem cells (NSCs) derived from iPS, which showed no organ toxicity and exhibited anti-tumor effects in a pH-dependent manner [78]. With the development of membrane-coated technology, it is more convenient and safer to utilize iPS cell membranes to camouflage NPs. In this way, iPS cell membranes could not only inherit membrane functionalities from iPS cells but also avoid the above side effects in the entire body.
6. Conclusion and future perspectives
Cell membrane-coated NPs have attracted growing attention in the field of targeted delivery strategies. For example, Zhang et al. concluded various biomimetic cell membrane-camouflaged nanoplatforms for cancer-targeted drug delivery [79]. Our review aimed to thoroughly reveal the stem cell membrane-based delivery system in tumors. Firstly, the underlying mechanisms of stem cell homing to target tumor tissues were highlighted. Moreover, the common modification methods of stem cell membrane along with preparation methods of stem cell membrane coated NPs were concluded for the appropriate choice of future research works. Most importantly, we discussed the potential clinical applications of MSCs and iPS cell membrane-camouflaged targeted delivery systems for anti-tumor therapies, such as chemotherapy, photodynamic therapy, magnetic hyperthermia therapy, imaging, CRISPR-Cas9 gene therapy, and synergistic therapy (Table 1). In the future, the applications of this delivery system should be further investigated in other anti-tumor therapies, such as tumor blockade therapy [80] and anti-tumor immune therapy [81]. To sum up, stem cell membrane-coated NPs hold the tremendous prospect for biomedical applications in tumor therapy.
Table 1.
Stem cell membrane-camouflaged targeted delivery system in tumors.
| Stem cell type | Nanoparticle | Tumor type | Cell line | Applications and limitations | Citation |
|---|---|---|---|---|---|
| Chemotherapy | |||||
| Human umbilical cord-derived MSC | PLGA-encapsulated DOX | Liver cancer | MHCC97H cell | (1) improve the tumor-targeting and accumulation of the nanoparticles; (2) enhance the tumor-killing efficacy of chemotherapy; (3) the great potential applications in anti-liver cancer therapy; (4) the scarcity of source cells is limited | 50 |
| MSC | Gelatin nanogels loaded with DOX | Cervical cancer | HeLa cell | (1) effectively evade clearance of the immune system; (2) enhance the tumor-targeting properties and anti-tumor chemotherapy efficacy; (3) the scarcity of source cells is limited | 51 |
| SD rat bone marrow-derived MSC | MSN core loaded with DOX | HepG2 cell | (1) show stronger tumor targeting and penetration ability; (2) effectively inhibit the growth of tumors and decrease the side effects of treatment by decreasing the exposure of other tissues to DOX | 52 | |
| Photodynamic therapy | |||||
| Human and rat bone marrow-derived MSC | UCNPs@mSiO2 | Cervical cancer | HeLa cell | (1) the long circulation and tumor-targeting capability; (2) produce remote-controlled photodynamic therapy in vivo; (3) the potential application for deep-tissue cancer treatment; (4) the scarcity of source cells is limited | 56 |
| Magnetic hyperthermia therapy and magnetic resonance imaging | |||||
| Human adipose-derived MSC | SPIO | Prostate cancer | Tramp-C1 cell | magnetic hyperthermia therapy and magnetic resonance imaging | 57 |
| Human umbilical cord-derived MSC | Fe3O4@PDA-siRNA | Prostate cancer | DU145 cell | the synergistic combination of magnetic photothermal treatment and gene silencing therapy | 59 |
| CRISPR-Cas9 gene therapy | |||||
| Human bone marrow-derived MSC | LNP-Cas9 RNP and CXCL12α | Acute myeloid leukemia | Leukemia stem cell | (1) sustained local delivery of Cas9/IL1RAP sgRNA; (2) provide an effective gene strategy for tumor | 61 |
| Synergistic therapy | |||||
| Human umbilical cord-derived MSC | PDA encapsulating SN38 | Bone tumor | MG63 cell | (1) exhibit lower nonspecific macrophage uptake, longer retention in blood, and more effective accumulation at tumor sites; (2) produce synergistic chemo-photothermal therapy; (3) the scarcity of source cells is limited | 69 |
| Human umbilical cord-derived MSC | PDA carrying DOX and PD-L1 siRNA | PCa bone metastases | PC-3 prostate cancer cell | (1) effectively enhance blood retention and improve accumulation at tumor sites; (2) excellent performance in synergistic chemoimmunotherapy; (3) the potential application for PCa bone metastasis treatment; (4) the scarcity of source cells is limited | 67 |
MSC: mesenchymal stem cell; DOX: doxorubicin; MSN: mesoporous silica nanoparticle; UCNPs@mSiO2: mesoporous silica coated upconversion nanoparticles; SPIO: superparamagnetic iron oxide; PDA: polydopamine; LNP: lipidoid nanoparticle; SN38: 7-ethyl-10-hydroxycamptothecin; PD-L1: programmed cell death ligand 1; PCa: prostate cancer.
Taken together, stem cells membrane-mediated delivery systems have the following advantages: (a) Stem cells could be easily isolated and expanded in vitro. More importantly, phenotypes and multilineage potential might be preserved even for over 50 population doublings in vitro; (b) Stem cell-based delivery systems have the innate targeting capability to inflammation or tumor lesions; (c) Drug delivery systems based on stem cells have already been established for anti-tumor strategies, which might build a solid foundation for stem cells membrane-mediated delivery systems. However, there are also several obstacles as follows: (a) Although MSC could be collected from various sources including bone marrow, umbilical cord, and placenta, the number of cells is limited. Therefore, the scarcity of source cells is an important challenge for MSC membrane-coated nanotechnology; (b) As for iPS cells, they have the great advantage of being relatively easily obtained by reprogramming differentiated somatic cells. However, the high cost of reprogramming may restrict the clinical application of iPS cell membranes. Accordingly, several obstacles should be resolved before the success of the clinical application of stem cell membrane camouflaged nanotechnology. The membrane preparation methods should be ameliorated to obtain higher production, reduce cost and maintain membrane properties. To improve the membrane capabilities, hybrid membrane-camouflaged nanovesicles might be a better choice to inherit hybrid functionalities from different membranes. For instance, Jiang et al. designed erythrocyte-cancer hybrid membrane-coated NPs for enhancing anti-tumor therapy efficacy [82]. Moreover, it is necessary to reveal the functionality of individual receptors on the membrane to have a comprehensive understanding of this biomimetic nanotechnology.
Funding
This study is supported by the China Postdoctoral Science Foundation (2021M701335).
Data availability statement
All data generated or analyzed during this study are available from the corresponding author.
Authors’ contributions
Xin Huang: Conceptualization, Investigation, Methodology, Writing – original draft, Software, Supervision, Writing – review & editing. Weiyue Zhang: Funding acquisition, Writing – review & editing, Software, Supervision, Writing – review & editing. All authors have given final approval for this version of the manuscript to be published.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Thanuja M.Y., Anupama C., Ranganath S.H. Bioengineered cellular and cell membrane-derived vehicles for actively targeted drug delivery: so near and yet so far. Adv. Drug Deliv. Rev. 2018;132:57–80. doi: 10.1016/j.addr.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Wu H.H., Zhou Y., Tabata Y., Gao J.Q. Mesenchymal stem cell-based drug delivery strategy: from cells to biomimetic. J. Contr. Release. 2019;294:102–113. doi: 10.1016/j.jconrel.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Hass R., Kasper C., Böhm S., Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Stuckey D.W., Shah K. Stem cell-based therapies for cancer treatment: separating hope from hype. Nat. Rev. Cancer. 2014;14:683–691. doi: 10.1038/nrc3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Compte M., Cuesta A.M., Sánchez-Martín D., Alonso-Camino V., Vicario J.L., Sanz L., Alvarez-Vallina L. Tumor immunotherapy using gene-modified human mesenchymal stem cells loaded into synthetic extracellular matrix scaffolds. Stem Cell. 2009;27:753–760. doi: 10.1634/stemcells.2008-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.In 't Anker P.S., Scherjon S.A., Kleijburg-van der Keur C., de Groot-Swings G.M., Claas F.H., Fibbe W.E., Kanhai H.H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cell. 2004;22:1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 8.Hu Y.L., Fu Y.H., Tabata Y., Gao J.Q. Mesenchymal stem cells: a promising targeted-delivery vehicle in cancer gene therapy. J. Contr. Release. 2010;147:154–162. doi: 10.1016/j.jconrel.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Hall B., Andreeff M., Marini F. The participation of mesenchymal stem cells in tumor stroma formation and their application as targeted-gene delivery vehicles. Handb. Exp. Pharmacol. 2007;180:263–283. doi: 10.1007/978-3-540-68976-8_12. [DOI] [PubMed] [Google Scholar]
- 10.Zhang T.Y., Huang B., Yuan Z.Y., Hu Y.L., Tabata Y., Gao J.Q. Gene recombinant bone marrow mesenchymal stem cells as a tumor-targeted suicide gene delivery vehicle in pulmonary metastasis therapy using non-viral transfection. Nanomedicine. 2014;10:257–267. doi: 10.1016/j.nano.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Zhang T.Y., Huang B., Wu H.B., Wu J.H., Li L.M., Li Y.X., Hu Y.L., Han M., Shen Y.Q., Tabata Y., Gao J.Q. Synergistic effects of co-administration of suicide gene expressing mesenchymal stem cells and prodrug-encapsulated liposome on aggressive lung melanoma metastases in mice. J. Contr. Release. 2015;209:260–271. doi: 10.1016/j.jconrel.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Fang R.H., Kroll A.V., Gao W., Zhang L. Cell membrane coating nanotechnology. Adv. Mater. 2018;30 doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dash P., Piras A.M., Dash M. Cell membrane coated nanocarriers - an efficient biomimetic platform for targeted therapy. J. Contr. Release. 2020;327:546–570. doi: 10.1016/j.jconrel.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Xu C.H., Ye P.J., Zhou Y.C., He D.X., Wei H., Yu C.Y. Cell membrane-camouflaged nanoparticles as drug carriers for cancer therapy. Acta Biomater. 2020;105:1–14. doi: 10.1016/j.actbio.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 15.Li S., Liu J., Sun M., Wang J., Wang C., Sun Y. Cell membrane-camouflaged nanocarriers for cancer diagnostic and therapeutic. Front. Pharmacol. 2020;11:24. doi: 10.3389/fphar.2020.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M., Xin Y., Cao H., Li W., Hua Y., Webster T.J., Zhang C., Tang W., Liu Z. Recent advances in mesenchymal stem cell membrane-coated nanoparticles for enhanced drug delivery. Biomater. Sci. 2021;9:1088–1103. doi: 10.1039/d0bm01164a. [DOI] [PubMed] [Google Scholar]
- 17.Shin M.J., Park J.Y., Lee D.H., Khang D. Stem cell mimicking nanoencapsulation for targeting arthritis. Int. J. Nanomed. 2021;16:8485–8507. doi: 10.2147/IJN.S334298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karp J.M., Leng Teo G.S. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Santos L.L., Fan H., Hall P., Ngo D., Mackay C.R., Fingerle-Rowson G., Bucala R., Hickey M.J., Morand E.F. Macrophage migration inhibitory factor regulates neutrophil chemotactic responses in inflammatory arthritis in mice. Arthritis Rheum. 2011;63:960–970. doi: 10.1002/art.30203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X., Huang W., Chen X., Lian Y., Wang J., Cai C., Huang L., Wang T., Ren J., Xiang A.P. CXCR5-Overexpressing mesenchymal stromal cells exhibit enhanced homing and can decrease contact hypersensitivity. Mol. Ther. 2017;25:1434–1447. doi: 10.1016/j.ymthe.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naderi-Meshkin H., Bahrami A.R., Bidkhori H.R., Mirahmadi M., Ahmadiankia N. Strategies to improve homing of mesenchymal stem cells for greater efficacy in stem cell therapy. Cell Biol. Int. 2015;39:23–34. doi: 10.1002/cbin.10378. [DOI] [PubMed] [Google Scholar]
- 22.Cheng Z., Ou L., Zhou X., Li F., Jia X., Zhang Y., Liu X., Li Y., Ward C.A., Melo L.G., Kong D. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol. Ther. 2008;16:571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 23.Brenner S., Whiting-Theobald N., Kawai T., Linton G.F., Rudikoff A.G., Choi U., Ryser M.F., Murphy P.M., Sechler J.M., Malech H.L. CXCR4-transgene expression significantly improves marrow engraftment of cultured hematopoietic stem cells. Stem Cell. 2004;22:1128–1133. doi: 10.1634/stemcells.2003-0196. [DOI] [PubMed] [Google Scholar]
- 24.Suila H., Hirvonen T., Kotovuori A., Ritamo I., Kerkelä E., Anderson H., Natunen S., Tuimala J., Laitinen S., Nystedt J., Räbinä J., Valmu L. Human umbilical cord blood-derived mesenchymal stromal cells display a novel interaction between P-selectin and galectin-1. Scand. J. Immunol. 2014;80:12–21. doi: 10.1111/sji.12179. [DOI] [PubMed] [Google Scholar]
- 25.Nitzsche F., Müller C., Lukomska B., Jolkkonen J., Deten A., Boltze J. Concise review: MSC adhesion cascade-insights into homing and transendothelial migration. Stem Cell. 2017;35:1446–1460. doi: 10.1002/stem.2614. [DOI] [PubMed] [Google Scholar]
- 26.Chamberlain G., Smith H., Rainger G.E., Middleton J. Mesenchymal stem cells exhibit firm adhesion, crawling, spreading and transmigration across aortic endothelial cells: effects of chemokines and shear. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamberlain G., Fox J., Ashton B., Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cell. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 28.Trounson A., McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Mager M.D., LaPointe V., Stevens M.M. Exploring and exploiting chemistry at the cell surface. Nat. Chem. 2011;3:582–589. doi: 10.1038/nchem.1090. [DOI] [PubMed] [Google Scholar]
- 30.Saha S., Anilkumar A.A., Mayor S. GPI-anchored protein organization and dynamics at the cell surface. J. Lipid Res. 2016;57:159–175. doi: 10.1194/jlr.R062885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parodi A., Quattrocchi N., van de Ven A.L., Chiappini C., Evangelopoulos M., Martinez J.O., Brown B.S., Khaled S.Z., Yazdi I.K., Enzo M.V., Isenhart L., Ferrari M., Tasciotti E. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotechnol. 2013;8:61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hymel D., Peterson B.R. Synthetic cell surface receptors for delivery of therapeutics and probes. Adv. Drug Deliv. Rev. 2012;64:797–810. doi: 10.1016/j.addr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanks J.E., Moll T., Eytner R., Vestweber D. Stimulation of P-selectin glycoprotein ligand-1 on mouse neutrophils activates beta 2-integrin mediated cell attachment to ICAM-1. Eur. J. Immunol. 1998;28:433–443. doi: 10.1002/(SICI)1521-4141(199802)28:02<433::AID-IMMU433>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 34.Dawson K.A., Yan Y. Drug delivery: leukocyte-like carriers. Nat. Mater. 2016;15:935–936. doi: 10.1038/nmat4737. [DOI] [PubMed] [Google Scholar]
- 35.Sletten E.M., Bertozzi C.R. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew Chem. Int. Ed. Engl. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie D., Smyth C.A., Eckstein C., Bilbao G., Mays J., Eckhoff D.E., Contreras J.L. Cytoprotection of PEG-modified adult porcine pancreatic islets for improved xenotransplantation. Biomaterials. 2005;26:403–412. doi: 10.1016/j.biomaterials.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 37.Cheng H., Byrska-Bishop M., Zhang C.T., Kastrup C.J., Hwang N.S., Tai A.K., Lee W.W., Xu X., Nahrendorf M., Langer R., Anderson D.G. Stem cell membrane engineering for cell rolling using peptide conjugation and tuning of cell-selectin interaction kinetics. Biomaterials. 2012;33:5004–5012. doi: 10.1016/j.biomaterials.2012.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abstiens K., Gregoritza M., Goepferich A.M. Ligand density and linker length are critical factors for multivalent nanoparticle-receptor interactions. ACS Appl. Mater. Interfaces. 2019;11:1311–1320. doi: 10.1021/acsami.8b18843. [DOI] [PubMed] [Google Scholar]
- 39.Huang B., Abraham W.D., Zheng Y., Bustamante López S.C., Luo S.S., Irvine D.J. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa5447. 291ra294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kell D.B., Swainston N., Pir P., Oliver S.G. Membrane transporter engineering in industrial biotechnology and whole cell biocatalysis. Trends Biotechnol. 2015;33:237–246. doi: 10.1016/j.tibtech.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Bobis-Wozowicz S., Miekus K., Wybieralska E., Jarocha D., Zawisz A., Madeja Z., Majka M. Genetically modified adipose tissue-derived mesenchymal stem cells overexpressing CXCR4 display increased motility, invasiveness, and homing to bone marrow of NOD/SCID mice. Exp. Hematol. 2011;39:686–696. doi: 10.1016/j.exphem.2011.03.004. e684. [DOI] [PubMed] [Google Scholar]
- 42.Ryser M.F., Ugarte F., Thieme S., Bornhäuser M., Roesen-Wolff A., Brenner S. mRNA transfection of CXCR4-GFP fusion--simply generated by PCR-results in efficient migration of primary human mesenchymal stem cells. Tissue Eng. C Methods. 2008;14:179–184. doi: 10.1089/ten.tec.2007.0359. [DOI] [PubMed] [Google Scholar]
- 43.Hu C.M., Zhang L., Aryal S., Cheung C., Fang R.H., Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. U. S. A. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bang K.H., Na Y.G., Huh H.W., Hwang S.J., Kim M.S., Kim M., Lee H.K., Cho C.W. The delivery strategy of paclitaxel nanostructured lipid carrier coated with platelet membrane. Cancers. 2019;11 doi: 10.3390/cancers11060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao L., Cai B., Bu L.L., Liao Q.Q., Guo S.S., Zhao X.Z., Dong W.F., Liu W. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano. 2017;11:3496–3505. doi: 10.1021/acsnano.7b00133. [DOI] [PubMed] [Google Scholar]
- 46.Rao L., Bu L.L., Ma L., Wang W., Liu H., Wan D., Liu J.F., Li A., Guo S.S., Zhang L., Zhang W.F., Zhao X.Z., Sun Z.J., Liu W. Platelet-facilitated photothermal therapy of head and neck squamous cell carcinoma. Angew Chem. Int. Ed. Engl. 2018;57:986–991. doi: 10.1002/anie.201709457. [DOI] [PubMed] [Google Scholar]
- 47.van Weerd J., Karperien M., Jonkheijm P. Supported lipid bilayers for the generation of dynamic cell-material interfaces. Adv. Healthcare Mat. 2015;4:2743–2779. doi: 10.1002/adhm.201500398. [DOI] [PubMed] [Google Scholar]
- 48.Wang X., Wang X., Bai X., Yan L., Liu T., Wang M., Song Y., Hu G., Gu Z., Miao Q., Chen C. Nanoparticle ligand exchange and its effects at the nanoparticle-cell membrane interface. Nano Lett. 2019;19:8–18. doi: 10.1021/acs.nanolett.8b02638. [DOI] [PubMed] [Google Scholar]
- 49.Wu M., Le W., Mei T., Wang Y., Chen B., Liu Z., Xue C. Cell membrane camouflaged nanoparticles: a new biomimetic platform for cancer photothermal therapy. Int. J. Nanomed. 2019;14:4431–4448. doi: 10.2147/IJN.S200284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang N., Ding Y., Zhang Y., Wang B., Zhao X., Cheng K., Huang Y., Taleb M., Zhao J., Dong W.F., Zhang L., Nie G. Surface functionalization of polymeric nanoparticles with umbilical cord-derived mesenchymal stem cell membrane for tumor-targeted therapy. ACS Appl. Mater. Interfaces. 2018;10:22963–22973. doi: 10.1021/acsami.8b05363. [DOI] [PubMed] [Google Scholar]
- 51.Gao C., Lin Z., Jurado-Sánchez B., Lin X., Wu Z., He Q. Stem cell membrane-coated nanogels for highly efficient in vivo tumor targeted drug delivery. Small. 2016;12:4056–4062. doi: 10.1002/smll.201600624. [DOI] [PubMed] [Google Scholar]
- 52.Li Y.S., Wu H.H., Jiang X.C., Zhang T.Y., Zhou Y., Huang L.L., Zhi P., Tabata Y., Gao J.Q. Active stealth and self-positioning biomimetic vehicles achieved effective antitumor therapy. J. Contr. Release. 2021;335:515–526. doi: 10.1016/j.jconrel.2021.05.031. [DOI] [PubMed] [Google Scholar]
- 53.Lepeltier E., Rijo P., Rizzolio F., Popovtzer R., Petrikaite V., Assaraf Y.G., Passirani C. Nanomedicine to target multidrug resistant tumors. Drug Resist. Updates. 2020;52 doi: 10.1016/j.drup.2020.100704. [DOI] [PubMed] [Google Scholar]
- 54.Huang X., Chen J., Wu W., Yang W., Zhong B., Qing X., Shao Z. Delivery of MutT homolog 1 inhibitor by functionalized graphene oxide nanoparticles for enhanced chemo-photodynamic therapy triggers cell death in osteosarcoma. Acta Biomater. 2020;109:229–243. doi: 10.1016/j.actbio.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Kwiatkowski S., Knap B., Przystupski D., Saczko J., Kędzierska E., Knap-Czop K., Kotlińska J., Michel O., Kotowski K., Kulbacka J. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018;106:1098–1107. doi: 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 56.Gao C., Lin Z., Wu Z., Lin X., He Q. Stem-cell-membrane camouflaging on near-infrared photoactivated upconversion nanoarchitectures for in vivo remote-controlled photodynamic therapy. ACS Appl. Mater. Interfaces. 2016;8:34252–34260. doi: 10.1021/acsami.6b12865. [DOI] [PubMed] [Google Scholar]
- 57.Yan Y., Zuo X., Wei D. Concise review: emerging role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem Cells Transl Med. 2015;4:1033–1043. doi: 10.5966/sctm.2015-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lai P.-Y., Huang R.-Y., Lin S.-Y., Lin Y.-H., Chang C.-W. Biomimetic stem cell membrane-camouflaged iron oxide nanoparticles for theranostic applications. RSC Adv. 2015;5:98222–98230. [Google Scholar]
- 59.Mu X., Li J., Yan S., Zhang H., Zhang W., Zhang F., Jiang J. siRNA delivery with stem cell membrane-coated magnetic nanoparticles for imaging-guided photothermal therapy and gene therapy. ACS Biomater. Sci. Eng. 2018;4:3895–3905. doi: 10.1021/acsbiomaterials.8b00858. [DOI] [PubMed] [Google Scholar]
- 60.Sahel D.K., Mittal A., Chitkara D. CRISPR/Cas system for genome editing: progress and prospects as a therapeutic tool. J. Pharmacol. Exp. Therapeut. 2019;370:725–735. doi: 10.1124/jpet.119.257287. [DOI] [PubMed] [Google Scholar]
- 61.Ho T.C., Kim H.S., Chen Y., Li Y., LaMere M.W., Chen C., Wang H., Gong J., Palumbo C.D., Ashton J.M., Kim H.W., Xu Q., Becker M.W., Leong K.W. Scaffold-mediated CRISPR-Cas9 delivery system for acute myeloid leukemia therapy. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abg3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun C., Mezzadra R., Schumacher T.N. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cha J.H., Chan L.C., Li C.W., Hsu J.L., Hung M.C. Mechanisms controlling PD-L1 expression in cancer. Mol. Cell. 2019;76:359–370. doi: 10.1016/j.molcel.2019.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yi M., Niu M., Xu L., Luo S., Wu K. Regulation of PD-L1 expression in the tumor microenvironment. J. Hematol. Oncol. 2021;14:10. doi: 10.1186/s13045-020-01027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yi M., Zheng X., Niu M., Zhu S., Ge H., Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol. Cancer. 2022;21:28. doi: 10.1186/s12943-021-01489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu D., Wang S., Bindeman W. Clinical applications of PD-L1 bioassays for cancer immunotherapy. J. Hematol. Oncol. 2017;10:110. doi: 10.1186/s13045-017-0479-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mu X., Zhang M., Wei A., Yin F., Wang Y., Hu K., Jiang J. Doxorubicin and PD-L1 siRNA co-delivery with stem cell membrane-coated polydopamine nanoparticles for the targeted chemoimmunotherapy of PCa bone metastases. Nanoscale. 2021;13:8998–9008. doi: 10.1039/d0nr08024a. [DOI] [PubMed] [Google Scholar]
- 68.Alamdari S.G., Amini M., Jalilzadeh N., Baradaran B., Mohammadzadeh R., Mokhtarzadeh A., Oroojalian F. Recent advances in nanoparticle-based photothermal therapy for breast cancer. J. Contr. Release. 2022;349:269–303. doi: 10.1016/j.jconrel.2022.06.050. [DOI] [PubMed] [Google Scholar]
- 69.Zhang M., Zhang F., Liu T., Shao P., Duan L., Yan J., Mu X., Jiang J. Polydopamine nanoparticles camouflaged by stem cell membranes for synergistic chemo-photothermal therapy of malignant bone tumors. Int. J. Nanomed. 2020;15:10183–10197. doi: 10.2147/IJN.S282931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang X., Wu W., Jing D., Yang L., Guo H., Wang L., Zhang W., Pu F., Shao Z. Engineered exosome as targeted lncRNA MEG3 delivery vehicles for osteosarcoma therapy. J. Contr. Release. 2022;343:107–117. doi: 10.1016/j.jconrel.2022.01.026. [DOI] [PubMed] [Google Scholar]
- 71.Yang L., Huang X., Guo H., Wang L., Yang W., Wu W., Jing D., Shao Z. Exosomes as efficient nanocarriers in osteosarcoma: biological functions and potential clinical applications. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.737314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rezaie J., Nejati V., Mahmoodi M., Ahmadi M. Mesenchymal stem cells derived extracellular vesicles: a promising nanomedicine for drug delivery system. Biochem. Pharmacol. 2022;203 doi: 10.1016/j.bcp.2022.115167. [DOI] [PubMed] [Google Scholar]
- 73.Zhou Y., Zhou W., Chen X., Wang Q., Li C., Chen Q., Zhang Y., Lu Y., Ding X., Jiang C. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm. Sin. B. 2020;10:1563–1575. doi: 10.1016/j.apsb.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y., Yang M., Zhang J., Zhi X., Li C., Zhang C., Pan F., Wang K., Yang Y., Martinez de la Fuentea J., Cui D. Human induced pluripotent stem cells for tumor targeted delivery of gold nanorods and enhanced photothermal therapy. ACS Nano. 2016;10:2375–2385. doi: 10.1021/acsnano.5b07172. [DOI] [PubMed] [Google Scholar]
- 75.Yang J., Lam D.H., Goh S.S., Lee E.X., Zhao Y., Tay F.C., Chen C., Du S., Balasundaram G., Shahbazi M., Tham C.K., Ng W.H., Toh H.C., Wang S. Tumor tropism of intravenously injected human-induced pluripotent stem cell-derived neural stem cells and their gene therapy application in a metastatic breast cancer model. Stem Cell. 2012;30:1021–1029. doi: 10.1002/stem.1051. [DOI] [PubMed] [Google Scholar]
- 76.Yang M., Liu Y., Hou W., Zhi X., Zhang C., Jiang X., Pan F., Yang Y., Ni J., Cui D. Mitomycin C-treated human-induced pluripotent stem cells as a safe delivery system of gold nanorods for targeted photothermal therapy of gastric cancer. Nanoscale. 2017;9:334–340. doi: 10.1039/c6nr06851k. [DOI] [PubMed] [Google Scholar]
- 77.Liu Y., Yang J., Liu B., Cao W., Zhang J., Yang Y., Ma L., de la Fuente J.M., Song J., Ni J., Zhang C., Cui D. Human iPS cells loaded with MnO(2)-based nanoprobes for photodynamic and simultaneous enhanced immunotherapy against cancer. Nano-Micro Lett. 2020;12:127. doi: 10.1007/s40820-020-00452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu D., Lam D.H., Purwanti Y.I., Goh S.L., Wu C., Zeng J., Fan W., Wang S. Systemic delivery of fusogenic membrane glycoprotein-expressing neural stem cells to selectively kill tumor cells. Mol. Ther. 2013;21:1621–1630. doi: 10.1038/mt.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang H., Dong S., Li Z., Feng X., Xu W., Tulinao C.M.S., Jiang Y., Ding J. Biointerface engineering nanoplatforms for cancer-targeted drug delivery. Asian J. Pharm. Sci. 2020;15:397–415. doi: 10.1016/j.ajps.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang Z., Liu Y., Shi R., Feng X., Xu W., Zhuang X., Ding J., Chen X. Versatile polymer-initiating biomineralization for tumor blockade therapy. Adv. Mater. 2022;34 doi: 10.1002/adma.202110094. [DOI] [PubMed] [Google Scholar]
- 81.Li Z., Xu W., Yang J., Wang J., Wang J., Zhu G., Li D., Ding J., Sun T. A tumor microenvironments-adapted polypeptide hydrogel/nanogel composite boosts antitumor molecularly targeted inhibition and immunoactivation. Adv. Mater. 2022;34 doi: 10.1002/adma.202200449. [DOI] [PubMed] [Google Scholar]
- 82.Jiang Q., Liu Y., Guo R., Yao X., Sung S., Pang Z., Yang W. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials. 2019;192:292–308. doi: 10.1016/j.biomaterials.2018.11.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are available from the corresponding author.