Key Points
Question
Among patients with large vessel occlusion acute ischemic stroke, does administration of intravenous tirofiban before endovascular thrombectomy improve functional outcomes?
Findings
In this randomized clinical trial that included 948 patients, treatment with tirofiban, compared with placebo, before endovascular thrombectomy resulted in no significant difference in disability severity between groups as measured by the overall distribution of the modified Rankin Scale score at 90 days (adjusted common odds ratio for a lower level of disability, 1.08).
Meaning
The findings do not support use of intravenous tirofiban before endovascular treatment for acute ischemic stroke.
Abstract
Importance
Tirofiban is a highly selective nonpeptide antagonist of glycoprotein IIb/IIIa receptor, which reversibly inhibits platelet aggregation. It remains uncertain whether intravenous tirofiban is effective to improve functional outcomes for patients with large vessel occlusion ischemic stroke undergoing endovascular thrombectomy.
Objective
To assess the efficacy and adverse events of intravenous tirofiban before endovascular thrombectomy for acute ischemic stroke secondary to large vessel occlusion.
Design, Setting, and Participants
This investigator-initiated, randomized, double-blind, placebo-controlled trial was implemented at 55 hospitals in China, enrolling 948 patients with stroke and proximal intracranial large vessel occlusion presenting within 24 hours of time last known well. Recruitment took place between October 10, 2018, and October 31, 2021, with final follow-up on January 15, 2022.
Interventions
Participants received intravenous tirofiban (n = 463) or placebo (n = 485) prior to endovascular thrombectomy.
Main Outcomes and Measures
The primary outcome was disability level at 90 days as measured by overall distribution of the modified Rankin Scale scores from 0 (no symptoms) to 6 (death). The primary safety outcome was the incidence of symptomatic intracranial hemorrhage within 48 hours.
Results
Among 948 patients randomized (mean age, 67 years; 391 [41.2%] women), 948 (100%) completed the trial. The median (IQR) 90-day modified Rankin Scale score in the tirofiban group vs placebo group was 3 (1-4) vs 3 (1-4). The adjusted common odds ratio for a lower level of disability with tirofiban vs placebo was 1.08 (95% CI, 0.86-1.36). Incidence of symptomatic intracranial hemorrhage was 9.7% in the tirofiban group vs 6.4% in the placebo group (difference, 3.3% [95% CI, −0.2% to 6.8%]).
Conclusions and Relevance
Among patients with large vessel occlusion acute ischemic stroke undergoing endovascular thrombectomy, treatment with intravenous tirofiban, compared with placebo, before endovascular therapy resulted in no significant difference in disability severity at 90 days. The findings do not support use of intravenous tirofiban before endovascular thrombectomy for acute ischemic stroke.
Trial Registration
Chinese Clinical Trial Registry Identifier: ChiCTR-IOR-17014167
This clinical trial examines whether intravenous tirofiban could improve disability severity without increasing symptomatic intracranial hemorrhage or mortality in patients with acute ischemic stroke and proximal large vessel occlusions undergoing endovascular treatment.
Introduction
Endovascular treatment has been shown to significantly increase the reperfusion rate and improve the functional outcomes of patients with acute ischemic stroke due to large vessel occlusion.1,2,3,4 However, endovascular thrombectomy has historically failed to yield successful reperfusion in approximately 30% of patients.5 Unsuccessful reperfusion likely arises in part from mechanical thrombectomy devices causing traumatic damage to the vascular endothelium with subendothelial matrix exposure, leading to platelet activation, adhesion, and aggregation and potentially resulting in reocclusion and thromboembolic complications.6,7
Tirofiban, a highly selective nonpeptide platelet glycoprotein IIb/IIIa inhibitor with a relatively short half-life that can reversibly prevent platelet aggregation, has been proven to reduce the risk of thrombotic complications during percutaneous coronary intervention.8,9,10 Given the benefit of treatment of acute coronary syndromes, a growing number of studies have evaluated tirofiban as an adjunctive treatment in patients with large vessel occlusion ischemic stroke undergoing endovascular treatment. However, most of the available data come from small, single-center, retrospective studies with conflicting results.11,12,13,14,15 To date, no randomized clinical trial has assessed the role of tirofiban in endovascular treatment of acute ischemic stroke.
The Endovascular Treatment With vs Without Tirofiban for Patients with Large Vessel Occlusion Stroke (RESCUE BT) trial was conducted to test the hypothesis that intravenous tirofiban could improve disability severity without increasing symptomatic intracranial hemorrhage or mortality in patients with acute ischemic stroke and proximal large vessel occlusions undergoing endovascular treatment within a 24-hour treatment window.
Methods
Trial Design and Oversight
This trial was an investigator-initiated, multicenter, randomized, double-blind, placebo-controlled trial. The trial protocol was approved by the ethics committees of the Xinqiao Hospital, Army Medical University, and all participating centers. The trial protocol and statistical analysis plan are provided in Supplement 1 and Supplement 2 and have been described previously.16 All participating hospitals performed the trial according to the same protocol. Written informed consent was provided by all recruited patients or their legal representatives before randomization. Study adverse events, progress, and overall integrity was monitored by an independent data and safety monitoring board. An independent clinical events committee adjudicated efficacy and safety outcomes, procedure-related complications, and serious adverse events. Tirofiban and the saline placebo were visually identical and were manufactured and provided by Lunan Pharmaceutical Group. eFigure 1 in Supplement 3 shows the overall flow of participants in the trial.
Patients
Study candidates were patients presenting with acute ischemic stroke within 24 hours of time last known well, National Institutes of Health Stroke Scale (NIHSS; range, 0-42; higher scores indicate more severe neurologic deficits) score of 30 or less, Alberta Stroke Program Early CT Score (ASPECTS; range, 0-10; higher scores suggest a smaller infarct core) of 6 or more, and occlusion of the intracranial internal carotid artery or the first or second segment of the middle cerebral artery confirmed by computed tomography angiography (CTA), magnetic resonance angiography (MRA), or digital subtraction angiography. The main exclusion criteria were dual antiplatelet therapy within 1 week of the index stroke or receipt of intravenous thrombolysis after stroke onset. Detailed selection criteria are provided in the eMethods in Supplement 3.
Randomization and Masking
Patients were randomized to the tirofiban or placebo group at a ratio of 1:1. Randomization was performed via a web-based mobile phone app or computer and stratified by baseline NIHSS score (≤17 or >17), occlusion site (the intracranial internal carotid artery or not), and participating center. Fixed block randomization with block sizes of 4 was used. Patients were assigned a random serial number according to the time they were enrolled, and corresponding masked and numbered medications were provided. All trial personnel and patients were unaware of the treatment assignment.
Interventions
All patients received the study drug intravenously within 5 minutes after randomization. The study drug was administrated as a bolus dose of 10 μg/kg, followed by continuous infusion of 0.15 μg/kg/min for up to 24 hours. Patients underwent rapid endovascular treatment. Salvage therapy was defined as failure of primary means of thrombectomy (eg, stent retriever or local aspiration) and use of balloon angioplasty and/or stenting. If the antegrade blood flow could not be maintained after angioplasty and/or stenting, the use of rescue drug was permitted. The rescue drug was available in the medication kits and its dosage and usage was consistent with the study drug. The rescue drug was saline placebo in the tirofiban group and tirofiban in the placebo group.
At the 20th hour after using the study drug, aspirin and/or clopidogrel tablets were administrated orally. Dual antiplatelet therapy with aspirin and clopidogrel was given to patients who underwent angioplasty/stenting. Otherwise, single antiplatelet therapy was given. At the 24th hour, the study drug was stopped. eFigure 2 in Supplement 3 shows the treatment flowchart.
Intravenous heparin was allowed during the thrombectomy procedure. Further, postprocedure use of subcutaneous heparin or low-molecular-weight heparin for deep vein thrombosis prophylaxis was permitted.
Outcomes
The primary outcome was the level of global disability at 90 days, based on the modified Rankin Scale (mRS), with scores ranging from 0 (no symptoms) to 6 (death), and statistically compared between the tirofiban vs placebo groups by calculating an adjusted common odds ratio. Adjudication was based on the central evaluation by 2 mRS-certified neurologists who were blinded to treatment randomization and who reviewed the video or voice recordings elicited using a structured assessment.17 If video or voice recordings were unavailable, outcomes were determined in person by the local investigators, who were also unaware of the treatment assignments.
Secondary clinical efficacy outcomes included the proportion of patients without disability (mRS score of 0 to 1) or who returned to their premorbid mRS score at 90 days (for patients with prestroke mRS score >1), the proportion of patients with functional independence (mRS score of 0 to 2) at 90 days, the proportion of patients who were ambulatory or capable of attending to bodily needs or better (mRS score of 0 to 3), the change of the NIHSS score from baseline to 24 hours and from baseline to 5 to 7 days (or discharge if earlier), and score of the European Quality of Life 5-Dimension 5-level scale (EQ-5D-5L; range, −0.39 to 1; lower scores denote a worse quality of life) at 90 days. Secondary technical efficacy outcomes included the proportions of patients with substantial reperfusion at initial preprocedure catheter angiogram, substantial reperfusion at final angiogram, rescue drug use, and recanalization as assessed by CTA or MRA 48 hours after endovascular treatment. Substantial reperfusion was defined as an expanded Thrombolysis In Cerebral Infarction grade of 2b50 (substantial reperfusion), 2c (near-complete reperfusion), or 3 (complete reperfusion).18 The primary safety outcome was the incidence of symptomatic intracranial hemorrhage assessed according to Heidelberg bleeding classification within 48 hours.19 Other safety variables included incidence of any radiologic intracranial hemorrhage, mortality within 90 days, serious adverse events (eg, acute respiratory failure, large or malignant middle cerebral artery infarction, acute heart failure, hemicraniectomy), and procedure-associated complications.
Sample Size Calculation
Sample size estimations used the distribution of mRS scores among individuals who were treated with mechanical thrombectomy without intravenous thrombolysis in an individual participant-level pooled analysis of the 5 randomized trials of endovascular thrombectomy.5 The proportion of patients with a 90-day mRS score of 0 to 1 was assumed to be 26.0% in the placebo group. Based on pooled published data excluding patients with posterior circulation occlusion stroke,13,15,20,21,22,23,24,25 the study was powered to detect an 8.5% absolute increase to approximately 34.5% in the tirofiban group, corresponding to a treatment effect with a common odds ratio of 1.5 compared with the placebo group. A total sample size of 930 patients (465 patients per group) would provide 90% power to detect a treatment effect with a 2-sided significance level of .05, taking 15% attrition rate into account.
Statistical Analysis
The primary outcome was to be analyzed by means of ordinal logistic regression if the proportional odds assumption was satisfied to generate an adjusted common odds ratio as the primary effect measure. Otherwise, assumption-free ordinal analysis would be used. The proportional odds assumption was verified using the score test. Secondary outcomes were analyzed using a logistic or linear regression model as appropriate. Analyses of the primary and secondary clinical efficacy outcomes were adjusted for age, baseline NIHSS score, baseline ASPECTS, time from last known well to randomization, and occlusion site. Treatment effect modification was investigated in prespecified subgroups based on the above variables and 3 other variables of interest: sex, stroke etiology (large artery atherosclerosis or not), and use of salvage therapy. The Wald χ2 test was used to assess the interaction. The multivariable logistic regression, Kaplan-Meier method, and log-rank test were used to analyze the mortality of the 2 groups. A post hoc mixed-effect model, with site as a random effect, was used to assess study center effects.
Missing values of baseline variables included in the multivariable regression models were imputed with multiple imputation by fully conditional specification regression for continuous variables or by fully conditional specification logistic regression for binary and ordinal variables. In the primary analysis, all patients were analyzed according to their randomization group. No missing data imputation was performed in this trial because there were no missing data for the primary outcome. Patients who received the randomized treatment and did not have major protocol violations were included in the per-protocol analysis. For the as-treated analysis, patients in the placebo group who received the rescue drug (tirofiban) were categorized into the tirofiban group, while patients in the tirofiban group treated with the rescue drug (saline placebo) remained in the tirofiban group. One interim safety analysis comparing the frequencies of symptomatic intracranial hemorrhage between the 2 groups was conducted after 465 patients had been randomized. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. The protocol specified a fixed sequential order of testing that was not prespecified in the statistical analysis plan and was not intended to preclude statistical testing of any of the outcomes. The statistical analysis plan prespecified correction for multiple comparisons for safety outcomes, but this was not performed because the study was not powered for such an assessment. All P values are 2-sided with a significance threshold at .05. Statistical analyses were performed using SAS, version 9.4 (SAS Institute). Figures were drawn using Excel software 2019 (Microsoft). For more information, see the statistical analysis plan provided in Supplement 2.
Results
Characteristics of the Patients
From October 10, 2018, to October 31, 2021, a total of 950 patients at 55 hospitals in China underwent randomization. Two patients assigned to the tirofiban group were excluded from all analyses because the legal representative withdrew consent immediately after randomization and received neither study drug nor endovascular treatment. Of 948 patients, 463 were assigned to the tirofiban group and 485 to the placebo group (Figure 1). Fifty-eight patients in the placebo group received rescue drug (tirofiban) therapy and were included in the tirofiban group in the as-treated analysis. There were 99 participants with protocol deviations in the tirofiban group and 100 participants with protocol deviations in the placebo group who were excluded from the per-protocol analysis. No loss to follow-up occurred. eFigure 3 in Supplement 3 shows the number of patients recruited by each center. The median (IQR) age of the 948 patients was 67 (57-74) years and 391 patients (41.2%) were women. Baseline characteristics and the overall workflow were well balanced in both groups (Table 1; eTable 1 in Supplement 3).
Figure 1. Flow of Patients in a Study of the Effect of Intravenous Tirofiban vs Placebo Before Endovascular Thrombectomy .
aOne participant was excluded for metastatic spread of cancer to the brain, 1 was excluded for receiving tirofiban treatment in another hospital, and 1 was excluded for a history of hyperthyroidism that was determined to preclude endovascular treatment.
bRandomization was stratified by baseline National Institutes of Health Stroke Scale score (≤17 or >17), occlusion site (the intracranial internal carotid artery or not), and participating center.
Table 1. Baseline Patient Characteristics in a Study of the Effect of Intravenous Tirofiban vs Placebo Before Endovascular Thrombectomy .
| Characteristic | Group, No. (%) | |
|---|---|---|
| Tirofiban (n = 463) | Placebo (n = 485) | |
| Demographic | ||
| Age, median (IQR), y | 68 (58-74) | 67 (57-75) |
| Men | 263 (56.8) | 294 (60.6) |
| Women | 200 (43.2) | 191 (39.4) |
| Medical historya | ||
| Hypertension | 251 (54.2) | 273 (56.3) |
| Atrial fibrillation | 166 (35.9) | 147 (30.3) |
| Smokingb | 100 (21.6) | 122 (25.2) |
| Diabetes | 99 (21.4) | 105 (21.7) |
| Hyperlipidemia | 74 (16.0) | 58 (12.0) |
| Ischemic stroke | 72 (15.6) | 89 (18.4) |
| Coronary heart disease | 71 (15.3) | 88 (18.1) |
| Prestroke Modified Rankin Scale scorec | ||
| 0 | 431 (93.1) | 435 (89.7) |
| 1 | 20 (4.3) | 38 (7.8) |
| 2 | 11 (2.4) | 10 (2.1) |
| 3 | 0 (0.0) | 2 (0.4) |
| 4 | 1 (0.2) | 0 (0.0) |
| Prestroke antithrombotic therapy | ||
| Oral anticoagulant | 36 (7.8) | 36 (7.4) |
| Single antiplatelet therapy | 33 (7.1) | 42 (8.7) |
| Dual antiplatelet therapyd | 10 (2.2) | 0 (0.0) |
| Stroke etiology | ||
| Cardioembolism | 212 (45.8) | 194 (40.0) |
| Large artery atherosclerosis | 197 (42.6) | 238 (49.1) |
| Unknown | 38 (8.2) | 39 (8.0) |
| Othere | 16 (3.5) | 14 (2.9) |
| Location of the atherosclerotic lesion | ||
| Intracranial | 165/197 (83.8) | 186/238 (78.2) |
| Extracranial | 24/197 (12.2) | 44/238 (18.5) |
| Intracranial and extracranial | 8/197 (4.1) | 8/238 (3.4) |
| Imaging characteristicsf | ||
| ASPECTS score, median (IQR)g | 8 (7-9) | 8 (7-9) |
| Occlusion site | ||
| Intracranial internal carotid artery | 96 (20.7) | 98 (20.2) |
| Middle cerebral artery segment | ||
| M1 | 305 (65.9) | 310 (63.9) |
| M2 | 62 (13.4) | 77 (15.9) |
| Clinical examination at arrival | n = 429 | n = 453 |
| NIHSS score, median (IQR)h | 16 (12-19) | 16 (12-20) |
| Systolic blood pressure, median (IQR), mm Hg | 145 (130-162) | 145 (129-160) |
| Serum glucose, median (IQR), mg/dLi | 124 (103-155) | 124 (104-157) |
| Time from last known well, median (IQR), min | ||
| To randomization | 405 (282-628) | 397 (250-623) |
| To arterial puncture | 400 (272-627) | 398 (246-618) |
| To reperfusion or procedure completion | 490 (340-717) | 481 (314-732) |
| Time from hospital arrival, median (IQR), min | ||
| Confirmation of occlusion site | 55 (34-95) | 54 (34-97) |
| Arterial puncture | 110 (76-151) | 105 (80-150) |
| Start of intravenous study drug | 121 (93-162) | 116 (90-155) |
| Time from arterial puncture to reperfusion or procedure completion, median (IQR), min | 67 (40-102) | 70 (43-110) |
SI conversion factor: To convert glucose values to mmol/L, multiply by 0.0555.
Patient self-report or family report.
Current or within the prior 5 years.
Scores on the modified Rankin Scale of functional disability range from 0 (no symptoms) to 6 (death). The score before stroke onset was evaluated by the site investigator with the use of information obtained from patients (if possible) or their family members.
Ten patients who had received dual antiplatelet therapy before onset were erroneously randomized and assigned to the tirofiban group.
Two neurologists blinded to treatment randomization adjudicated stroke etiology based on the Trial of ORG 10172 in Acute Stroke Treatment and assessed using site information, clinical findings, and brain imaging. Other causes included small vessel occlusion, nonatherosclerotic vasculopathies, hypercoagulable states, and hematologic disorders.
Imaging characteristics were assessed by the imaging core laboratory.
The Alberta Stroke Program Early Computed Tomography Score (ASPECTS) is an imaging measure of the extent of ischemic stroke. Scores range from 0 to 10, with higher scores indicating a smaller infarct core. Listed are values for the core laboratory assessment.
Scores on National Institutes of Health Stroke Scale (NIHSS) range from 0 to 42, with lower scores indicating less severe neurologic deficits.
Glucose levels ranging from 70 to 140 mg/dL are defined as normal.
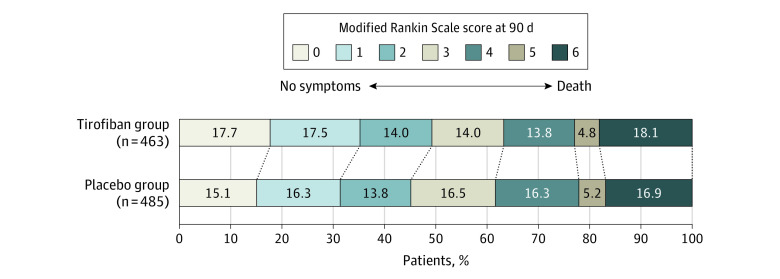
Primary Outcome
The median (IQR) 90-day mRS score was 3 (1-4) in the tirofiban group and 3 (1-4) in the placebo group, and the proportional odds assumption was satisfied (P = .76). The adjusted common odds ratio for a favorable shift to a lower mRS score at 90 days comparing tirofiban with placebo was 1.08 (95% CI, 0.86-1.36; P = .50) (Table 2 and Figure 2). The per-protocol and as-treated analyses also showed no significant between-group difference for the primary outcome (eTables 2 and 3 in Supplement 3). The distribution of mRS scores for the per-protocol and as-treated populations are shown in the eFigures 4 and 5 in Supplement 3. The post hoc mixed-effect modeling indicated that the study center effects were significant (eTables 4 and 5 in Supplement 3). A post hoc sensitivity analysis of the primary outcome that additionally included adjustment for study center resulted in an adjusted common odds ratio of 1.08 (95% CI, 0.86-1.35; P = .51).
Table 2. Efficacy and Safety Outcomes in a Study of the Effect of Intravenous Tirofiban vs Placebo Before Endovascular Thrombectomy.
| Outcome | Group, No. (%) | Unadjusted difference (95% CI) | OR (95% CI) | P valuea | ||
|---|---|---|---|---|---|---|
| Tirofiban (n = 463) | Placebo (n = 485) | Unadjusted | Adjustedb | |||
| Primary efficacy outcome, median (IQR) | ||||||
| Modified Rankin Scale score at 90 dc | 3 (1 to 4) | 3 (1 to 4) | 0 (0 to 0) | 1.11 (0.89 to 1.39)d | 1.08 (0.86 to 1.36)d | .50 |
| Secondary clinical efficacy outcomes | ||||||
| Modified Rankin Scale score at 90 d | ||||||
| 0 to 1 or return to premorbid score | 168 (36.3) | 157 (32.4) | 3.9 (−2.1 to 10.0) | 1.19 (0.91 to 1.56) | 1.21 (0.91 to 1.62) | .20 |
| 0 to 2 | 228 (49.2) | 219 (45.2) | 4.1 (−2.3 to 10.4) | 1.18 (0.91 to 1.52) | 1.21 (0.92 to 1.59) | .18 |
| 0 to 3 | 293 (63.3) | 299 (61.7) | 1.6 (−4.5 to 7.8) | 1.07 (0.82 to 1.40) | 1.09 (0.82 to 1.45) | .57 |
| NIHSS score, median (IQR), change from baselinee | β Coefficient (95% CI) | |||||
| Unadjusted | Adjusted | |||||
| 24 h after randomization | −2 (−6 to 2) | −2 (−6 to 2) | 0 (−1 to 1) | 0.36 (−0.77 to 1.49) | 0.33 (−0.79 to 1.44) | .56 |
| 5-7 d after randomization or at early discharge | −5 (−10 to 0) | −5 (−10 to 1) | 0 (−1 to 1) | 0.46 (−1.00 to 1.92) | 0.39 (−1.03 to 1.82) | .59 |
| EQ-5D-5L score at 90 d, median (IQR)f | 0.71 (0.17 to 0.96) | 0.66 (0.12 to 0.96) | 0 (0 to 0.05) | 0.02 (−0.03 to 0.07) | 0.02 (−0.02 to 0.07) | .30 |
| Secondary technical efficacy outcomes | OR (95% CI) | |||||
| Unadjusted | Adjusted | |||||
| Substantial reperfusion (eTICI 2b50-3) on initial DSA prior to endovascular treatmentg | 0 | 3 (0.6) | −0.6 (−1.3 to 0.1) | NA | NA | NA |
| Substantial reperfusion (eTICI 2b50-3) at final angiogramh | 427 (92.2) | 439 (90.5) | 1.7 (−1.9 to 5.3) | 1.24 (0.79 to 1.97) | 1.23 (0.78 to 1.96) | .38 |
| Rescue drug usei | 38 (8.2) | 58 (12.0) | −3.8 (−7.6 to 0.1) | 0.66 (0.43 to 1.01) | 0.63 (0.41 to 0.97) | .04 |
| Recanalization on follow-up CTA or MRA within 48 hj | 299/335 (89.3) | 322/369 (87.3) | 2.0 (−2.8 to 6.7) | 1.21 (0.77 to 1.93) | 1.24 (0.78 to 1.98) | .37 |
| Primary safety outcome | ||||||
| Symptomatic intracranial hemorrhage within 48 hk | 45/462 (9.7) | 31/483 (6.4) | 3.3 (−0.2 to 6.8) | 1.57 (0.98 to 2.55) | 1.56 (0.97 to 2.56) | .07 |
| Secondary safety outcomesl | ||||||
| Any radiologic intracranial hemorrhagem | 161/462 (34.9) | 135/483 (28.0) | 6.9 (1.0 to 12.8) | 1.37 (1.05 to 1.82) | 1.40 (1.06 to 1.86) | .02 |
| Mortality at 90 d | 84 (18.1) | 82 (16.9) | 1.2 (−3.6 to 6.1) | 1.09 (0.80 to 1.52) | 1.09 (0.77 to 1.55) | .63 |
Abbreviations: CTA, computed tomography angiography; DSA, digital subtraction angiography; MRA, magnetic resonance angiography; NA, not applicable; OR, odds ratio.
P values pertain to adjusted common odds ratio, odds ratio, and β coefficient.
Values were adjusted for age, baseline National Institutes of Health Stroke Scale (NIHSS) score, baseline Alberta Stroke Program Early CT Score (ASPECTS), occlusion site, and time from last known well to randomization.
Scores on the modified Rankin Scale of functional disability range from 0 (no symptoms) to 6 (death). The score was evaluated centrally by 2 modified Rankin Scale–certified neurologists who were blinded to treatment allocation and who reviewed the video or voice recordings elicited using a structured assessment.
This value is common odds ratio, which was estimated from an ordinal logistic-regression model and indicates the odds of improvement of 1 point on the modified Rankin Scale, with a common odds ratio greater than 1 favoring tirofiban treatment.
Scores on NIHSS range from 0 to 42, with lower scores denoting less severe neurologic deficits. The β coefficient was adjusted for age, baseline ASPECTS, time from last known well to randomization, occlusion site, and study centers using a multivariable linear regression model.
Scores on the European Quality Five-Dimension Five-Level Self-Report Questionnaire (EQ-5D-5L) range from −0.39 to 1, with higher scores indicating a better quality of life; 0 is the value of a health state equivalent to death.
The expanded Thrombolysis In Cerebral Infarction (eTICI) reperfusion grading system is a 6-point scale: 0 indicates no reperfusion noted; 1, reduction in thrombus without filling of distal arterial branches; 2a, reperfusion of <50% of the territory; 2b, a reperfusion of ≥50% of the territory; 2c, near-complete perfusion with distal slow flow or presence of small cortical emboli; and 3, complete reperfusion. Successful reperfusion before endovascular treatment was defined as an eTICI grade of 2b, 2c, or 3 on the first intracranial angiogram.
The eTICI grade was determined at the final angiogram. A complete list of eTICI grades is provided in eTable 1 in Supplement 3.
The rescue drug was administered intravenously when the antegrade blood flow could not be maintained after angioplasty and/or stenting. The rescue drug was saline placebo in the tirofiban group and tirofiban in the placebo group.
Data for follow-up CTA or MRA were not available for 244 patients (128 in the tirofiban group and 116 in the placebo group). Vessel patency was adjudicated at the imaging core laboratory.
Intracranial hemorrhage was adjudicated by a clinical events committee. Symptomatic intracranial hemorrhage was assessed according to the Heidelberg criteria.19 Data were not available for 3 patients (1 in the tirofiban group and 2 in the placebo group).
Additional safety outcomes are shown in eTable 6 in Supplement 3.
Data were not available for 3 patients (1 in the tirofiban group and 2 in the placebo group). Subtypes of radiologic intracranial hemorrhage are shown in eTable 7 in Supplement 3.
Figure 2. Distribution of Global Disability at 90 Days Based on the Modified Rankin Scale Score.
Scores on the modified Rankin Scale for patients in the tirofiban group and the placebo group are shown according to randomization. Scores on the modified Rankin Scale of functional disability range from 0 (no symptoms) to 6 (death). The score was evaluated centrally by 2 modified Rankin Scale–certified neurologists who were blinded to treatment randomization and who reviewed the video or voice recordings elicited using a structured assessment.
Secondary Outcomes
Prespecified secondary outcomes are shown in Table 2. For all 6 of the secondary clinical efficacy outcomes, no statistically significant difference was noted. For example, the percentage of patients without disability (mRS score of 0-1) or who returned to their premorbid mRS score was 36.3% for the tirofiban group and 32.4% for the placebo group (difference, 3.9% [95% CI, −2.1% to 10%]; adjusted odds ratio, 1.21 [95% CI, 0.91-1.62]). Among the technical efficacy outcomes, the proportion of patients receiving the rescue drug was lower in the tirofiban group than the placebo group (8.4% vs 12.0%; difference, −3.8% [95% CI, −7.6% to 0.1%]; adjusted odds ratio, 0.63 [95% CI, 0.41-0.97]; P = .04). For the 3 remaining technical efficacy outcomes, no statistically significant difference was noted. For example, substantial reperfusion (expanded Thrombolysis In Cerebral Infarction grade 2b50 to 3) at final angiogram was observed in 92.2% of patients in the tirofiban group and 90.5% in the placebo group (difference, 1.7% [95% CI, −1.9% to 5.3%]; adjusted odds ratio, 1.23 [95% CI, 0.78-1.96]). eTables 2 and 3 in Supplement 3 show the secondary functional and technical efficacy outcomes in the per-protocol and as-treated analyses.
Safety Outcomes
Safety outcomes are shown in Table 2 and eTable 6 and eFigure 6 in Supplement 3. No significant difference was detected in the incidence of symptomatic intracranial hemorrhage between the groups (9.7% vs 6.4%; difference, 3.3% [95% CI, −0.2% to 6.8%]; adjusted odds ratio, 1.56 [95% CI, 0.97-2.56]). However, the incidence of any radiologic intracranial hemorrhage was significantly higher in the tirofiban group than in the placebo group (34.9% vs 28.0%; difference, 6.9% [95% CI, 1.0%-12.8%]; adjusted odds ratio, 1.40 [95% CI, 1.06-1.86]; P = .02). Rates of radiologic intracranial hemorrhage subtypes are shown in eTable 7 in Supplement 3. Ninety-day mortality was 18.1% with tirofiban and 16.9% with placebo (difference, 1.2% [95% CI, −3.6% to 6.1%]; adjusted odds ratio, 1.09 [95% CI, 0.77-1.55]; P = .63). eTables 8 and 9 in Supplement 3 show the safety outcomes in the per-protocol and as-treated populations.
Subgroup Analyses
Subgroup analyses for the primary end point are presented in Figure 3. In the analysis by stroke etiology, although there was a more favorable point estimate for tirofiban among the large artery atherosclerosis subgroup but not the non–large artery atherosclerosis subgroup, results of the test for interaction were not statistically significant (adjusted common odds ratio for less mRS disability, 1.40 [95% CI, 1.00-1.97; P = .049] vs 0.84 [95% CI, 0.62-1.15; P = .28]; P for interaction = .09). Clinical efficacy, technical efficacy, and safety outcomes of the large artery atherosclerosis subgroup are shown in eTables 10 and 11 in Supplement 3.
Figure 3. Heterogeneity of Treatment Effect for Less Disability Among Prespecified Subgroups.
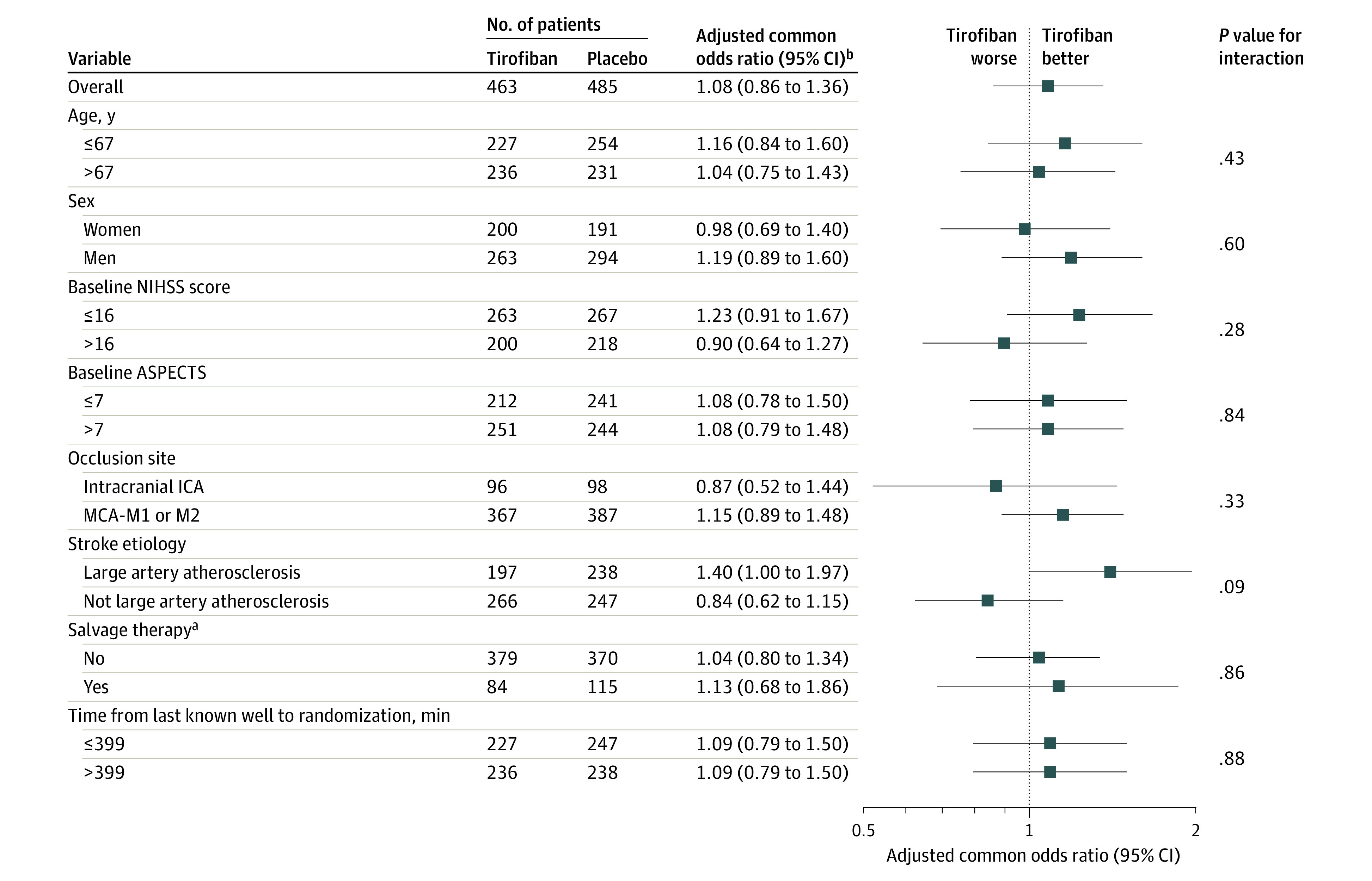
The forest plot displays effect variation across 8 prespecified subgroups for the adjusted common odds ratio of less disability at 90 days. A lower modified Rankin Scale (mRS) score indicates less disability. The thresholds for age, baseline National Institutes of Health Stroke Scale score, baseline Alberta Stroke Program Early CT Score (ASPECTS), and onset to randomization time were chosen at the median. MCA-M1 and M2 indicate the first and second segments of the middle cerebral artery, respectively.
aSalvage therapy was defined as failure of primary means of thrombectomy (eg, stent-retriever or local aspiration) and use of balloon angioplasty and/or stenting.
bThe outcome presented was defined as the adjusted common odds ratio for a favorable shift to a lower mRS score at 90 days. Adjusted odds ratios are used for the testing of statistical significance.
Discussion
In this multicenter randomized clinical trial conducted in China, intravenous tirofiban administered prior to endovascular thrombectomy did not significantly improve the distribution of 90-day disability among patients with acute ischemic stroke due to anterior circulation large vessel occlusion.
In the overall trial population, the lack of functional outcome benefit with intravenous tirofiban at 3 months reflected that tirofiban did not improve outcomes of endovascular treatment. The number of passes with thrombectomy devices, time from arterial puncture to successful reperfusion, and rate of final substantial reperfusion did not differ between the treatment groups. In this trial, the rate of substantial reperfusion in the placebo group was 90.5%, which is higher than the 71% achieved in the first 5 randomized trials of stent retrievers.5 This difference likely reflects interval advances in endovascular treatment techniques and, as a result, there was limited room for additional improvement. In addition, tirofiban did not statistically significantly reduce reocclusion rate as anticipated. A contributing factor to absence of effect on reocclusion may have been that a substantial proportion of patients were treated with aspiration devices that apply suction force to the proximal face of the clot rather than stent retrievers that exert a radial force on the vessel wall to capture the clot and may more often damage the endothelium and result in platelet activation.26
Intracranial hemorrhage is one of the most common adverse events after endovascular treatment in large vessel occlusion stroke. The overall rate of radiographic intracranial hemorrhage in this trial was consistent with those of previous studies, which reported rates of 22% to 49%.12,13 In patients receiving tirofiban, a significantly increased risk of radiographic intracranial hemorrhage was found compared with patients receiving placebo, which might affect clinical outcomes. Symptomatic intracranial hemorrhage is the most worrisome type of intracranial hemorrhage and independently predicts an unfavorable prognosis.11 Patients in the tirofiban group also had a numerically higher incidence of symptomatic intracranial hemorrhage, although the difference was not statistically significant after adjusting for confounding factors. The safety profile of tirofiban was consistent with a previous observational study that showed an increased risk of symptomatic intracranial hemorrhage associated with tirofiban treatment.12 Nevertheless, no significant difference was observed in mortality between the 2 treatment groups.
In subgroup analyses, the point estimates for tirofiban vs placebo raise the possibility that tirofiban might be associated with lower disability level among patients with stroke due to large artery atherosclerosis, although the test for interaction did not reach statistical significance. This could reflect the absence of a difference between the subgroups, but may also reflect inadequate study power to assess interactions. Observational studies have suggested that intravenous tirofiban was associated with substantial reperfusion rates and favorable functional outcomes among patients with large artery atherosclerosis strokes.13,14,15 Particularly in Asian populations, large artery atherosclerosis stroke is often due to intracranial, rather than extracranial, atherosclerosis so that the target occlusion is comprised of both in situ atherosclerotic plaque and supervening thrombus. For these occlusions, mechanical thrombectomy will only remove the thrombosis component. The persisting atherosclerotic lesion has an irregular and disrupted surface exposed to rapidly flowing blood, precipitating platelet activation and re-thrombosis that may be responsive to tirofiban. In addition, treatment of the persisting atherosclerotic lesion often required rescue angioplasty with or without stenting. Platelet-mediated thrombotic reocclusions occur more often after angioplasty and stenting procedures. The current study’s hypothesis-generating finding of potential benefit of tirofiban in patients with ischemic stroke due to large artery atherosclerosis may merit a future confirmatory trial confined to this population.
A strength of this study is that it used a large-scale, placebo-controlled, double-blind design, which mitigates the potential for subjective bias of investigators and patients to influence study results. Additional strengths include that the trial did not use an upper age limit for enrollment, recruited patients within a relatively broad treatment window of 24 hours, and had little missing data.
Limitations
This study has several limitations. First, enrollment in the later 6- to 24-hour time window was based on ASPECTS scores on noncontrast CT scans. CT perfusion assessment of ischemic core and penumbral volume was not mandatory, because most participating hospitals are not equipped with automated CT perfusion analysis software. This is not consistent with the current standards for patient selection in the extended therapeutic window. However, studies have shown that there is no significant difference in the accuracy of ASPECTS score on noncontrast CT and CT perfusion imaging in predicting the lesion volume of acute ischemic stroke,27,28 and noncontrast CT selection seems to lead to similar outcomes to CT perfusion in patients treated within the 6- to 24-hour window.29 The overall percentage of patients achieving functional independence at 3 months in the current trial was 47.2%, which is generally consistent with that of trials using advanced multimodal imaging to screen patients for thrombectomy.2,4 Therefore, it is considered reasonable and more pragmatic to use ASPECTS as the parenchymal imaging inclusion criterion. Second, this trial was designed to enroll patients with large vessel occlusions and thus the generalization of the study results to patients without stroke due to large vessel occlusion is limited. An ongoing randomized trial of tirofiban treatment in non–large vessel occlusive stroke (RESCUE BT 2 [ChiCTR2000029502]) may shed additional light on this issue. Third, because all patients enrolled in this trial were from China, the generalizability of the trial results is limited owing to the significantly higher prevalence of intracranial atherosclerotic disease in Asian populations than in non-Asian populations.
Conclusions
Among patients with large vessel occlusion acute ischemic stroke undergoing endovascular thrombectomy, treatment with intravenous tirofiban, compared with placebo, before endovascular therapy resulted in no significant difference in disability severity at 90 days. The findings do not support use of intravenous tirofiban before endovascular thrombectomy for acute ischemic stroke.
Trial protocol
Statistical analysis plan
eMethods
eFigures
eTables
eReferences
Nonauthor collaborators
Data sharing statement
References
- 1.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 2.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 3.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 4.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 6.Heo JH, Lee KY, Kim SH, Kim DI. Immediate reocclusion following a successful thrombolysis in acute stroke: a pilot study. Neurology. 2003;60(10):1684-1687. doi: 10.1212/01.WNL.0000063323.23493.98 [DOI] [PubMed] [Google Scholar]
- 7.Power S, Matouk C, Casaubon LK, et al. Vessel wall magnetic resonance imaging in acute ischemic stroke: effects of embolism and mechanical thrombectomy on the arterial wall. Stroke. 2014;45(8):2330-2334. doi: 10.1161/STROKEAHA.114.005618 [DOI] [PubMed] [Google Scholar]
- 8.Cannon CP, Weintraub WS, Demopoulos LA, et al. ; TACTICS (Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy)—Thrombolysis in Myocardial Infarction 18 Investigators . Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344(25):1879-1887. doi: 10.1056/NEJM200106213442501 [DOI] [PubMed] [Google Scholar]
- 9.Van’t Hof AW, Ten Berg J, Heestermans T, et al. ; Ongoing Tirofiban In Myocardial infarction Evaluation (On-TIME) 2 study group . Prehospital initiation of tirofiban in patients with ST-elevation myocardial infarction undergoing primary angioplasty (On-TIME 2): a multicentre, double-blind, randomised controlled trial. Lancet. 2008;372(9638):537-546. doi: 10.1016/S0140-6736(08)61235-0 [DOI] [PubMed] [Google Scholar]
- 10.Cura FA, Bhatt DL, Lincoff AM, et al. Pronounced benefit of coronary stenting and adjunctive platelet glycoprotein IIb/IIIa inhibition in complex atherosclerotic lesions. Circulation. 2000;102(1):28-34. doi: 10.1161/01.CIR.102.1.28 [DOI] [PubMed] [Google Scholar]
- 11.Kellert L, Hametner C, Rohde S, et al. Endovascular stroke therapy: tirofiban is associated with risk of fatal intracerebral hemorrhage and poor outcome. Stroke. 2013;44(5):1453-1455. doi: 10.1161/STROKEAHA.111.000502 [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Yin C, Yang J, Jiang L, Parsons MW, Lin L. Endovascular thrombectomy. Stroke. 2018;49(11):2783-2785. doi: 10.1161/STROKEAHA.118.022919 [DOI] [PubMed] [Google Scholar]
- 13.Zhao W, Che R, Shang S, et al. Low-dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke. 2017;48(12):3289-3294. doi: 10.1161/STROKEAHA.117.019193 [DOI] [PubMed] [Google Scholar]
- 14.Baek BH, Yoon W, Lee YY, Kim SK, Kim J-T, Park MS. Intravenous tirofiban infusion after angioplasty and stenting in intracranial atherosclerotic stenosis-related stroke. Stroke. 2021;52(5):1601-1608. doi: 10.1161/STROKEAHA.120.033551 [DOI] [PubMed] [Google Scholar]
- 15.Pan X, Zheng D, Zheng Y, et al. Safety and efficacy of tirofiban combined with endovascular treatment in acute ischaemic stroke. Eur J Neurol. 2019;26(8):1105-1110. doi: 10.1111/ene.13946 [DOI] [PubMed] [Google Scholar]
- 16.Qiu Z, Li F, Sang H, et al. Endovascular treatment with versus without tirofiban for stroke patients with large vessel occlusion: the multicenter, randomized, placebo-controlled, double-blind RESCUE BT study protocol. Int J Stroke. Published online January 27, 2022.doi: 10.1177/17474930211069510 [DOI] [PubMed] [Google Scholar]
- 17.Patel N, Rao VA, Heilman-Espinoza ER, Lai R, Quesada RA, Flint AC. Simple and reliable determination of the modified Rankin Scale score in neurosurgical and neurological patients: the mRS-9Q. Neurosurgery. 2012;71(5):971-975. doi: 10.1227/NEU.0b013e31826a8a56 [DOI] [PubMed] [Google Scholar]
- 18.Liebeskind DS, Bracard S, Guillemin F, et al. ; HERMES Collaborators . eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019;11(5):433-438. doi: 10.1136/neurintsurg-2018-014127 [DOI] [PubMed] [Google Scholar]
- 19.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg Bleeding Classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981-2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 20.Zhao H, Zhang J, Gu D, et al. Tirofiban facilitates the reperfusion process during endovascular thrombectomy in ICAS. Exp Ther Med. 2017;14(4):3314-3318. doi: 10.3892/etm.2017.4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun C, Li X, Zhao Z, et al. Safety and efficacy of tirofiban combined with mechanical thrombectomy depend on ischemic stroke etiology. Front Neurol. Published online October 29, 2019. doi: 10.3389/fneur.2019.01100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi TY, Chen WH, Wu YM, et al. Special endovascular treatment for acute large artery occlusion resulting from atherosclerotic disease. World Neurosurg. 2017;103:65-72. doi: 10.1016/j.wneu.2017.03.108 [DOI] [PubMed] [Google Scholar]
- 23.Lee JS, Hong JM, Lee KS, Suh HI, Choi JW, Kim SY. Primary stent retrieval for acute intracranial large artery occlusion due to atherosclerotic disease. J Stroke. 2016;18(1):96-101. doi: 10.5853/jos.2015.01347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JW, Jeon P, Kim G-M, Bang OY, Byun HS, Kim KH. Local intraarterial tirofiban after formation of anterograde flow in patients with acute ischemic stroke: preliminary experience and short term follow-up results. Clin Neurol Neurosurg. 2012;114(10):1316-1319. doi: 10.1016/j.clineuro.2012.04.022 [DOI] [PubMed] [Google Scholar]
- 25.Mangiafico S, Cellerini M, Nencini P, Gensini G, Inzitari D. Intravenous tirofiban with intra-arterial urokinase and mechanical thrombolysis in stroke: preliminary experience in 11 cases. Stroke. 2005;36(10):2154-2158. doi: 10.1161/01.STR.0000181751.06736.64 [DOI] [PubMed] [Google Scholar]
- 26.Peschillo S, Diana F, Berge J, Missori P. A comparison of acute vascular damage caused by ADAPT versus a stent retriever device after thrombectomy in acute ischemic stroke: a histological and ultrastructural study in an animal model. J Neurointerv Surg. 2017;9(8):743-749. doi: 10.1136/neurintsurg-2016-012533 [DOI] [PubMed] [Google Scholar]
- 27.Demeestere J, Garcia-Esperon C, Garcia-Bermejo P, et al. Evaluation of hyperacute infarct volume using ASPECTS and brain CT perfusion core volume. Neurology. 2017;88(24):2248-2253. doi: 10.1212/WNL.0000000000004028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouslama M, Barreira CM, Haussen DC, et al. Endovascular reperfusion outcomes in patients with a stroke and low ASPECTS is highly dependent on baseline infarct volumes. J Neurointerv Surg. 2022;14(2):117-121. doi: 10.1136/neurintsurg-2020-017184 [DOI] [PubMed] [Google Scholar]
- 29.Nogueira RG, Haussen DC, Liebeskind D, et al. ; Trevo Registry and DAWN Trial Investigators . Stroke imaging selection modality and endovascular therapy outcomes in the early and extended time windows. Stroke. 2021;52(2):491-497. doi: 10.1161/STROKEAHA.120.031685 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eMethods
eFigures
eTables
eReferences
Nonauthor collaborators
Data sharing statement