Key Points
Question
Among patients with transient ischemic attack or ischemic stroke due to symptomatic severe intracranial atherosclerotic stenosis, does performing angioplasty and stenting 3 weeks or more after the index event along with standard medical therapy reduce the risk of stroke or death compared with medical therapy alone?
Findings
In this randomized clinical trial that included 358 patients, the risk of stroke or death within 30 days or stroke in the qualifying artery territory beyond 30 days through 1 year occurred in 8.0% in the percutaneous transluminal angioplasty and stenting group vs 7.2% in the medical therapy alone group, a difference that was not statistically significant.
Meaning
The findings do not support the addition of percutaneous transluminal angioplasty and stenting to medical therapy for the treatment of patients with symptomatic severe intracranial atherosclerotic stenosis.
Abstract
Importance
Prior randomized trials have generally shown harm or no benefit of stenting added to medical therapy for patients with symptomatic severe intracranial atherosclerotic stenosis, but it remains uncertain as to whether refined patient selection and more experienced surgeons might result in improved outcomes.
Objective
To compare stenting plus medical therapy vs medical therapy alone in patients with symptomatic severe intracranial atherosclerotic stenosis.
Design, Setting, and Participants
Multicenter, open-label, randomized, outcome assessor–blinded trial conducted at 8 centers in China. A total of 380 patients with transient ischemic attack or nondisabling, nonperforator (defined as nonbrainstem or non–basal ganglia end artery) territory ischemic stroke attributed to severe intracranial stenosis (70%-99%) and beyond a duration of 3 weeks from the latest ischemic symptom onset were recruited between March 5, 2014, and November 10, 2016, and followed up for 3 years (final follow-up: November 10, 2019).
Interventions
Medical therapy plus stenting (n = 176) or medical therapy alone (n = 182). Medical therapy included dual-antiplatelet therapy for 90 days (single antiplatelet therapy thereafter) and stroke risk factor control.
Main Outcomes and Measures
The primary outcome was a composite of stroke or death within 30 days or stroke in the qualifying artery territory beyond 30 days through 1 year. There were 5 secondary outcomes, including stroke in the qualifying artery territory at 2 years and 3 years as well as mortality at 3 years.
Results
Among 380 patients who were randomized, 358 were confirmed eligible (mean age, 56.3 years; 263 male [73.5%]) and 343 (95.8%) completed the trial. For the stenting plus medical therapy group vs medical therapy alone, no significant difference was found for the primary outcome of risk of stroke or death (8.0% [14/176] vs 7.2% [13/181]; difference, 0.4% [95% CI, −5.0% to 5.9%]; hazard ratio, 1.10 [95% CI, 0.52-2.35]; P = .82). Of the 5 prespecified secondary end points, none showed a significant difference including stroke in the qualifying artery territory at 2 years (9.9% [17/171] vs 9.0% [16/178]; difference, 0.7% [95% CI, −5.4% to 6.7%]; hazard ratio, 1.10 [95% CI, 0.56-2.16]; P = .80) and 3 years (11.3% [19/168] vs 11.2% [19/170]; difference, −0.2% [95% CI, −7.0% to 6.5%]; hazard ratio, 1.00 [95% CI, 0.53-1.90]; P > .99). Mortality at 3 years was 4.4% (7/160) in the stenting plus medical therapy group vs 1.3% (2/159) in the medical therapy alone group (difference, 3.2% [95% CI, −0.5% to 6.9%]; hazard ratio, 3.75 [95% CI, 0.77-18.13]; P = .08).
Conclusions and Relevance
Among patients with transient ischemic attack or ischemic stroke due to symptomatic severe intracranial atherosclerotic stenosis, the addition of percutaneous transluminal angioplasty and stenting to medical therapy, compared with medical therapy alone, resulted in no significant difference in the risk of stroke or death within 30 days or stroke in the qualifying artery territory beyond 30 days through 1 year. The findings do not support the addition of percutaneous transluminal angioplasty and stenting to medical therapy for the treatment of patients with symptomatic severe intracranial atherosclerotic stenosis.
Trial Registration
ClinicalTrials.gov Identifier: NCT01763320
This randomized clinical trial compares the efficacy of percutaneous transluminal angioplasty and stenting plus medical therapy vs medical therapy alone in reducing the risk of stroke or death among patients with symptomatic severe intracranial atherosclerotic stenosis.
Introduction
Stroke was the second leading cause of death worldwide and the leading cause of death in China in 2017.1,2 Intracranial atherosclerotic stenosis accounted for 10% to 15% of ischemic stroke in Western countries,3 and as much as 46.6% in Asia in 2009.4 Patients with intracranial atherosclerotic stenosis were at particularly high risk of recurrent stroke,5,6 prompting the development of percutaneous transluminal angioplasty and stenting.7,8,9,10 However, the Stenting vs Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial was terminated early due to a significantly higher rate of 30-day stroke or death with stenting compared with medical therapy (14.7% vs 5.8%; P = .002).11 Similarly, the Vitesse Intracranial Stent Study for Ischemic Stroke Therapy (VISSIT),12 and a single-center randomized trial in China,13 also showed no benefit of stenting compared with medical therapy.
Subsequently, several prospective, multicenter registries suggested that refined patient selection (eg, excluding patients with perforator [defined as end-arteries arising from basilar artery or middle cerebral artery that vascularize brainstem or basal ganglia, respectively] ischemic events only and requiring a longer time interval from the latest ischemic events) and experienced surgeons may reduce the periprocedural risk of stenting from 14.7% to between 2.0% and 4.3%.14,15,16 A prospective registry (lead-in phase of this trial), in which 100 patients with refined criteria were treated with stenting, reported a rate of 30-day stroke or death of 2.0%.15 The Wingspan Stent System Post Market Surveillance Study (WEAVE) reported a similarly low periprocedural complication rate (2.6%).16 These lower risks of periprocedural complications suggest a potential clinical benefit of stenting that should be tested in a randomized trial.
The China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis (CASSISS) trial was a multicenter, randomized, open-label trial. It evaluated the effect of stenting vs medical therapy alone on mortality and stroke in patients with transient ischemic attack (TIA) or nondisabling ischemic stroke with severe intracranial atherosclerotic stenosis admitted to high-volume medical centers, using refined patient selection.
Methods
Study Design and Oversight
This trial was a multicenter, randomized, open-label, outcome assessor–blinded trial comparing medical therapy alone with medical therapy plus stenting in patients with TIA or nondisabling ischemic stroke with 70% to 99% stenosis of a major intracranial artery. Details of the study protocol have been published previously and provided in Supplement 1.17 A detailed statistical analysis plan is provided in Supplement 2. Written informed consent was obtained from all patients or their legal representatives. The institutional review board of Xuanwu Hospital reviewed and approved the study ([2013]013).
The first patient was enrolled on March 5, 2014, and the last patient on November 10, 2016. Each patient was regularly followed up at 1 month as well as 1, 2, and 3 years after enrollment. The 3-year follow-up for the last enrolled patient was finished on November 10, 2019. Because China is a multiethnic country, ethnicity (Han vs non-Han ethnicity) was assessed in this study and defined by self-report of participants with an open-ended question. The trial was overseen by an independent data and safety monitoring board. The trial did not enroll patients until it was approved by the local ethics committee at each participating site.
Credentialing of Sites
A lead-in phase was conducted for credentialing of surgeons and research sites before the initiation of the trial. From July 2013 to March 2014, 10 candidate sites were contacted, and clinical data from a total of 100 patients with intracranial atherosclerotic stenosis treated with stenting at these sites were collected and evaluated (30-day stroke or death rate: 2%).15 Ultimately, 8 centers that met the following criteria were included: (1) at least 5 cases were performed by each primary surgeon during the lead-in phase; (2) annual volume of intracranial stenting procedures was more than 30 for the past 3 years; and (3) according to the records of the past 3 years, 30-day rate of stroke or death after stenting in the territory of the qualifying artery was lower than 15%. The site selection and monitoring are available in the eMethods in Supplement 3.
Patient Selection
This trial recruited patients with TIA or nondisabling ischemic stroke (modified Rankin Scale score, 0-2) and severe stenosis (degree of stenosis: 70%-99%) of a major intracranial artery supplying the territory of the ischemic event. Conventional angiography was required to confirm the degree of stenosis by Warfarin-Aspirin Symptomatic Intracranial Disease Study (WASID) criteria.18,19 Patients who had a TIA (World Health Organization criteria: acute onset of neurologic deficit, persisting for <24 hours) or an ischemic stroke (persisting >24 hours; confirmed by diffusion-weighted imaging on magnetic resonance imaging [MRI]) within the last 3 weeks, and those who had brainstem or basal ganglia perforator stroke only, were excluded. MRI (or computed tomography if MRI was contraindicated) at recruitment was used to confirm the above criteria. Detailed eligibility criteria are described in the eMethods in Supplement 3.
Randomization and Blinding
Simple 1:1 randomization without block or stratification was performed. Computer-generated random number by an Interactive Voice Response System (Clinical Soft Company) was used for treatment assignment. Eligible patients were randomized in a 1:1 ratio to medical therapy plus stenting vs medical therapy alone. An independent outcome committee and imaging core laboratory determined the primary and secondary outcomes, blinded to treatment assignment.
Trial Treatment
Both groups received the same medical therapy immediately after the randomization, which included aspirin, 100 mg, plus clopidogrel, 75 mg, daily for 90 days (aspirin or clopidogrel alone daily thereafter) and control of stroke risk factors. Risk factor control consisted of control of low-density lipoprotein cholesterol level (target: <2.58 mmol/L [100 mg/dL]) with statins as needed and hypertension (systolic blood pressure <140 mm Hg [<130 mm Hg in the case of patients with diabetes] and diastolic blood pressure <90 mm Hg) with medications as needed based on 2014 American Heart Association/American Stroke Association guidelines.20 Patients randomized to stenting received the stenting with the Wingspan stent (Stryker Neurovascular) within 3 to 5 days after randomization and the procedure was performed under general anesthesia. No loading dose of aspirin or clopidogrel was allowed before the procedure. Details of the procedure and periprocedural care are described in Supplement 1.15
Follow-up and Assessment of Outcome
Clinical follow-up and assessments of patients were conducted via outpatient consultation or by telephone at 1 month, 1 year, 2 years, and 3 years. At each follow-up visit, patients were examined by study physicians who also managed the vascular risk factors. Magnetic resonance angiography or computed tomography was used for routine imaging follow-up. All relevant medical data were recorded into an online database (Tigermed Data Management). An imaging database was established to facilitate central reading by an independent imaging core lab (IsCore Image Corelab).
Outcomes
The primary outcome was a composite clinical outcome that included stroke (World Health Organization criteria: rapidly developed clinical signs of focal [or global] disturbance of cerebral function, lasting more than 24 hours within 30 days after enrollment), death within 30 days after enrollment, or stroke in the territory of qualifying artery beyond 30 days through 1 year. Secondary outcomes included (1) disabling stroke or death after enrollment within 3 years; (2) stroke in the qualifying artery territory within 2 years; (3) stroke in the qualifying artery territory within 3 years; (4) any stroke, TIA, or cardiovascular events within 3 years; and (5) death within 3 years. A post hoc evaluation included assessment of stroke-related and nonstroke-related deaths. Adverse events included an evaluation of disabling stroke, symptomatic intracranial hemorrhage, or death within 1 year as well as evaluation of all-cause death (and causes of death) within 3 years after enrollment. Disabling stroke was defined by any of the following: (1) an mRS score of 3 or more on a scale of 0 to 6, with higher scores indicating greater disability; (2) an increase in at least 1 mRS category from an individual’s prestroke baseline; (3) a score on the composite National Institutes of Health Stroke Scale (NIHSS) of 7 or more on a scale of 0 to 42, with higher scores indicating more severe deficits; or (4) an increase of at least 4 points in the NIHSS score from prestroke baseline (page 23 in Supplement 1).
Sample Size Calculation
The 1-year risk of the primary outcome was assumed to be 18% for medical therapy19 and 7.3% for stenting.21 The estimate of treatment effect was based on the available data at that time.17,21,22 To test the hypothesized 59% relative risk reduction (7.3% vs 18%) with 80% power and an α of .05, a minimum sample size of 151 per group was estimated. The rate of loss to follow-up and early withdrawal was assumed to be 20%, and enlarged the total sample size to 380 (190 per group).
Statistical Analysis
Efficacy evaluation was conducted in both the full analysis set (FAS) and per-protocol set (PPS), with analysis in FAS as the primary analysis. FAS included all patients who were eligible for the study and completed randomization. PPS included only patients who followed the study treatment protocols and had complete follow-up data. The difference in primary outcome between groups was tested using log-rank test, with the center information (site effect) as a stratification factor. The same test was used to compare the key secondary outcomes including 2-year and 3-year stroke in the qualifying artery territory between the groups. For other secondary outcomes and baseline characteristics, χ2 or Fisher exact tests were used for categorical variables, and t test or Wilcoxon rank test for quantitative variables. Kaplan-Meier curves were used to show the incidence of outcomes over time. The Cox proportional risk model was used to calculate hazard ratio (HR) and its 95% CI adjusting for center effect. The proportional hazards assumption was tested using Schoenfeld residuals, with P < .05 indicating nonproportionality. Competing risk Cox proportional hazard model treating death as a competing risk was used to compare the risk between 2 groups for the following secondary outcomes: 2-year rate of the same-territory stroke, 3-year rate of the same-territory stroke and any stroke, TIA, and cardiovascular events related to stenting or medical therapy within a follow-up of 3 years. Patients lost to follow-up were censored in analyses of survival.
Post hoc analysis included (1) analysis of the individual components of the primary outcome, including 30-day rate of stroke or death, 30-day rate of fatal stroke, and the rate of stroke in the qualifying artery territory beyond 30 days to 1 year; (2) subgroup analysis by the qualifying events, TIA vs ischemic stroke; and (3) sensitivity analysis of HR by using a mixed-effects model with center as a random effect.
All statistical tests were performed by 2-sided tests. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. A 2-sided P value less than .05 was considered statistically significant. All analyses were performed with SAS software, version 9.4 (SAS Institute).
Results
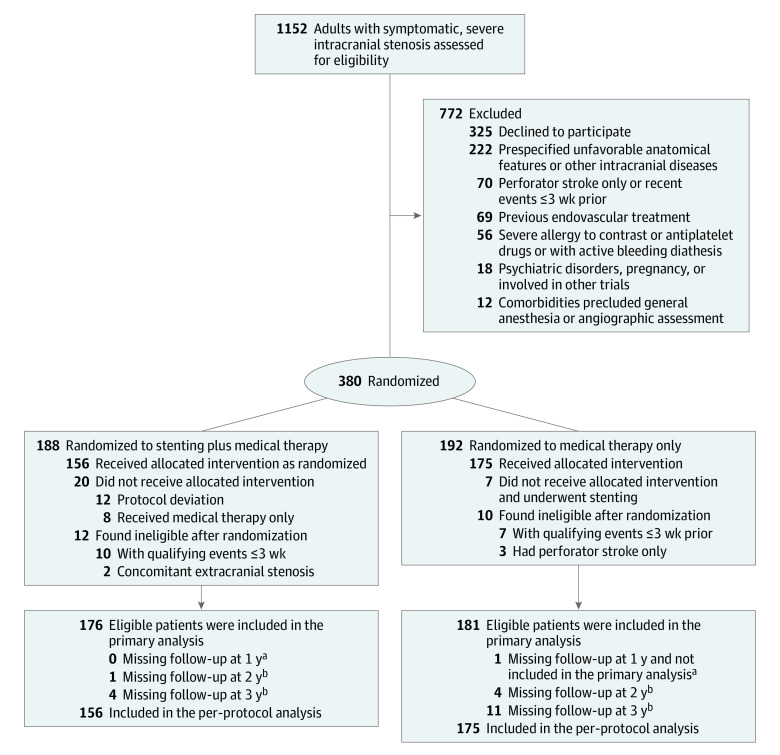
From March 4, 2014, to November 10, 2016, 1152 patients were assessed for trial eligibility at 8 study sites. A total of 380 patients signed informed consent and were enrolled and randomly assigned to the stenting (188 patients) and medical (192 patients) groups (Figure 1). Of the 380 patients, 22 (12 in the stenting and 10 in the medical therapy alone groups) were confirmed ineligible by central adjudication. The remaining 358 patients (176 in the stenting and 182 in the medical therapy alone groups) were included in the FAS for final analysis. A total of 343 patients (95.8%) completed the trial.
Figure 1. Patient Enrollment and Follow-up in the China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis (CASSISS) Trial.
aPrimary outcome was assessed up to 1 year.
bTime points at which some secondary outcomes were assessed.
Seven patients assigned to the medical therapy alone group crossed over to stenting procedures. Among patients assigned to stenting, 8 crossed over to medical therapy only and 12 had either an unsuccessful stenting procedure or a procedure that deviated from the protocol (3 received delayed procedures, 2 did not have it due to failed lesion access, 2 were aborted due to total occlusion, 1 received angioplasty alone, and 4 received nonstudy stents). Thus, a total of 331 patients (156 in the stenting and 175 in the medical therapy alone groups) were included in the PPS for secondary analysis.
The baseline characteristics of patients in the FAS were well balanced between the groups (Table 1). The mean (SD) age was 56.3 (9.6) years, and 73.5% were male. The median time from the latest event to randomization was 35 days. Among all 358 patients, 194 patients (54.2%) presented with index stroke as a qualifying event. The inferred mechanisms of stroke from the brain imaging were artery-to-artery embolism in 115 patients (59.3%), isolated hypoperfusion in 40 (20.6%), and mixed mechanism in 39 (20.1%) (Table 1). The stroke mechanism distribution was balanced between groups (Table 1; eTable 1 in Supplement 3). The measures of all risk factors were similar in both groups at baseline and during follow-up (eTable 2 and eFigure 1 in Supplement 3).
Table 1. Baseline Characteristics of Study Participants.
| Characteristic | No. (%) | |
|---|---|---|
| Percutaneous transluminal angioplasty and stenting group (n = 176) | Medical therapy alone group (n = 182) | |
| Age, mean (SD), y | 56.7 (9.4) | 55.9 (9.8) |
| Sex | ||
| Male | 128 (72.7) | 135 (74.2) |
| Female | 48 (27.3) | 47 (25.8) |
| Ethnicitya | ||
| Han | 172 (97.7) | 179 (98.4) |
| Non-Han | 4 (2.3) | 3 (1.6) |
| Medical historyb | ||
| Hypertension | 117 (66.5) | 125 (68.7) |
| Diabetes | 57 (32.4) | 44 (24.2) |
| Coronary artery disease | 19 (10.8) | 19 (10.4) |
| Lipid disorder | 18 (10.2) | 21 (11.5) |
| Peripheral artery disease | 0 (0.0) | 1 (0.5) |
| Received antiplatelet therapy prior to latest qualifying event | 49 (27.8) | 48 (26.4) |
| Received statin therapy prior to latest qualifying event | 19 (10.8) | 20 (11.0) |
| Alcohol history | ||
| Former | 25 (14.2) | 22 (12.1) |
| Current | 30 (17.0) | 32 (17.6) |
| Smoking history | ||
| Former | 39 (22.2) | 38 (20.9) |
| Current | 41 (23.3) | 50 (27.5) |
| Qualifying event | ||
| TIAc | 87 (49.4) | 77 (42.3) |
| Stroke | 89 (50.6) | 105 (57.7) |
| Artery-to-artery embolism | 57 (64.0) | 58 (55.2) |
| Isolated hemodynamic compromised | 18 (20.2) | 22 (21.0) |
| Mixed mechanism | 14 (15.7) | 25 (23.8) |
| Time from latest ischemic event to randomization, median (IQR), d | 34.5 (27.0-65.5) | 36.0 (28.0-68.0) |
| TIA | 33.0 (25.0-52.0) | 33.0 (28.0-57.0) |
| Stroke | 38.0 (27.0-75.0) | 40.0 (29.0-72.0) |
| Symptomatic qualifying artery | ||
| Middle cerebral artery (M1) | 65 (36.9) | 79 (43.4) |
| Basilar artery | 50 (28.4) | 52 (28.6) |
| Intracranial vertebral artery | 46 (26.1) | 34 (18.7) |
| Intracranial internal carotid artery | 15 (8.5) | 17 (9.3) |
| Stenosis of symptomatic qualifying arterye | ||
| % Stenosis, median (IQR) | 78.5 (74.1-82.6) | 76.6 (73.2-80.9) |
| Distribution, % stenosis | ||
| 70-79 | 105 (59.7) | 130 (71.4) |
| 80-89 | 65 (36.9) | 46 (25.3) |
| 90-99 | 6 (3.4) | 6 (3.3) |
| NIHSS score, median (IQR)f | 0.0 (0.0-1.0) | 0.0 (0.0-0.0) |
| mRS score, median (IQR)g | 0.0 (0.0-1.0) | 0.0 (0.0-1.0) |
Abbreviations: mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack.
Ethnicity was self-reported.
Medical history was collected at the baseline visit, based on a combination of self-reports from patients, medicated conditions, and laboratory results.
TIA was a clinical diagnosis without imaging.
Isolated hemodynamic compromise refers to strokes with an arterial border zone or “watershed” pattern.
Stenosis was quantified on the basis of a reading of the angiogram by the site interventionist on the criteria of the WASID trial.18
NIHSS score ranges from 0 to 42, with higher scores indicating worse neurologic deficits.
mRS score ranges from 0 to 6, with higher scores indicating worse function deficits (0 indicates no deficit and 6 indicates death).
Primary Outcome
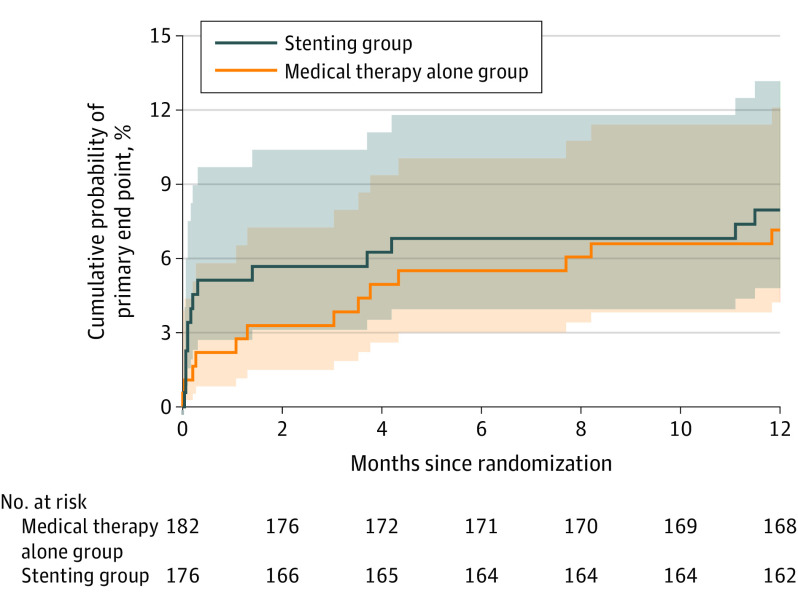
Proportional hazard assumption was tested and met for the primary outcome and all the secondary outcomes in the Cox regression model. The primary outcome, risk of stroke or death within 30 days or stroke in the qualifying artery territory beyond 30 days through 1 year, was not significantly different (stenting: 8.0% [14/176] vs medical: 7.2% [13/181]; difference, 0.4% [95% CI, −5.0% to 5.9%]; HR, 1.10 [95% CI, 0.52-2.35]; P = .82) (Figure 2 and Table 2). The per-protocol analysis yielded a similar result (9.0% [14/156] vs 7.4% [13/175]; HR, 1.23 [95% CI, 0.58-2.62]; P = .59) (eFigure 2 in Supplement 3).
Figure 2. Kaplan-Meier Curves for the Cumulative Probability of the Primary Outcome, According to Treatment Assignment.
The primary outcome was stroke or death within 30 days after enrollment or stroke in the qualifying artery territory beyond 30 days through 1 year. One patient lost to follow-up within 1 year in the control group was treated as censored data. All other patients were followed up to event or 1 year. P = .82 for log-rank testing between the stenting and medical therapy alone groups with center as stratification factor.
Table 2. Primary and Secondary Outcomes.
| No./total (%) | Incidence difference, % (95% CI)b | Hazard ratio (95% CI)b | P valuec | ||
|---|---|---|---|---|---|
| Percutaneous transluminal angioplasty and stenting group (n = 176) | Medical therapy alone group (n = 181)a | ||||
| Components of the primary outcome | 14/176 (8.0) | 13/181 (7.2) | 0.4 (−5.0 to 5.9) | 1.10 (0.52 to 2.35) | .82 |
| Stroke or death within 30 d after enrollmentd | 9/176 (5.1)e | 4/181 (2.2)f | |||
| Stroke in territory of qualifying artery beyond 30 d through 1 yd | 5/176 (2.8) | 9/181 (5.0) | |||
| Secondary outcomes | |||||
| Stroke in the same territory within 2 y | 17/171 (9.9)g | 16/178 (9.0)h | 0.7 (−5.4 to 6.7) | 1.10 (0.56 to 2.16) | .80 |
| Stroke in the same territory within 3 y | 19/168 (11.3)i | 19/170 (11.2)j | −0.2 (−7.0 to 6.5) | 1.00 (0.53 to 1.90) | >.99 |
| Disabling stroke or death within 3 y | 19/168 (11.3)k | 15/166 (9.0)l | 2.0 (−4.6 to 8.6) | 1.28 (0.65 to 2.52) | .49 |
| Any stroke, TIA, cardiovascular events related to stenting or medical therapy within 3 y | 24/169 (14.2)m | 31/172 (18.0)n | −4.1 (−12.0 to 3.7) | 0.76 (0.45 to 1.30) | .31 |
| Death within 3 y | 7/160 (4.4)o,p | 2/159 (1.3)q,r | 3.2 (−0.5 to 6.9) | 3.75 (0.77 to 18.13) | .08 |
| Stroke-related deathd | 4/160 (2.5) | 2/159 (1.3) | |||
| Nonstroke-related deathd | 3/160 (1.9) | 0/159 (0) | |||
Abbreviation: TIA, transient ischemic attack.
One participant randomized to the medical therapy alone group was not included due to missing outcome data. See Figure 1.
Adjusted for site effect.
Log-rank test adjusted for site effect.
Post hoc analysis.
There were 5 ischemic stroke and 4 hemorrhagic strokes. Of the 4 symptomatic hemorrhagic strokes, 1 was periprocedural subarachnoid hemorrhage immediately after percutaneous transluminal angioplasty and stenting (probably related to guidewire perforation); 1 was periprocedural parenchymal and subdural brain hemorrhage evident immediately after percutaneous transluminal angioplasty and stenting (probably related to guidewire perforation); 1 was cerebellar and occipital hemorrhage that occurred 3 days after percutaneous transluminal angioplasty and stenting (probably related to reperfusion); and 1 was subarachnoid hemorrhage within 24 hours after percutaneous transluminal angioplasty and stenting (probably related to reperfusion). A total of 2 of these hemorrhages were fatal (1 developed massive cerebral infarction and brain hernia, and 1 had parenchymal brain hemorrhage), and 2 were nondisabling (1 cerebellar and occipital hemorrhage and 1 subarachnoid hemorrhage).
There were 4 ischemic strokes and 0 hemorrhagic strokes. Of the 4 ischemic strokes, 2 were disabling, 2 were nondisabling, and none were fatal.
One missing follow-up and 4 died.
Four missing follow-up and 0 died.
Four missing follow-up and 4 died.
Eleven missing follow-up and 1 died.
Eight missing follow-up, including 4 with primary outcomes (but no disabling stroke or death).
Sixteen missing follow-up, including 5 with primary outcomes (but no disabling stroke or death).
Four missing follow-up and 3 died.
Ten missing follow-up and 0 died.
Sixteen missing follow-up, including 12 with primary outcomes.
The causes of death in the percutaneous transluminal angioplasty and stenting group were as follows: brain hemorrhage (n = 2), ischemic stroke (n = 2), sudden cardiac arrest (n = 1), intrahepatic cholangiocarcinoma (n = 1), and aortic artery aneurysm (n = 1).
Twenty-three missing follow-up, including 12 with primary outcomes.
The causes of death in the medical management group were as follows: ischemic stroke (n = 1) and brain hemorrhage (n = 1).
Secondary Outcomes
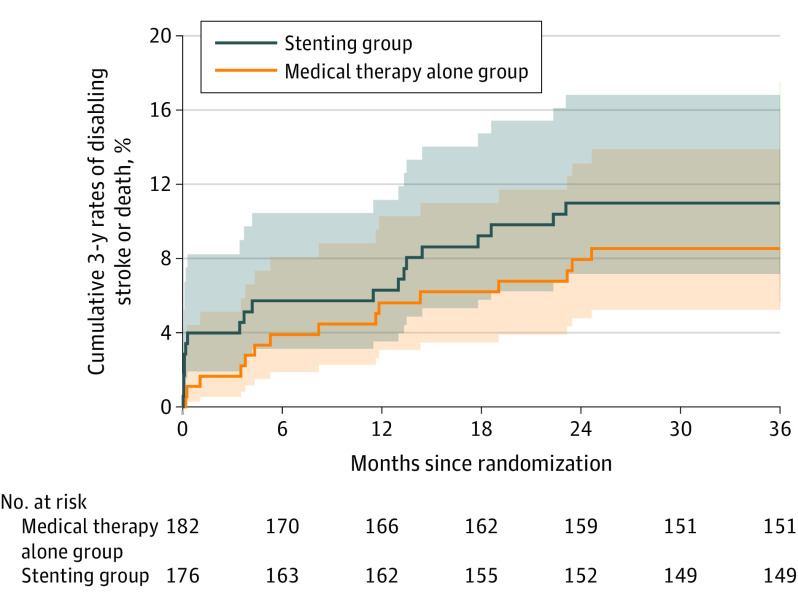
No significant difference was found between groups for the 2-year risk of stroke in the qualifying artery territory (9.9% [17/171] in the stenting group vs 9.0% [16/178] in the medical therapy alone group; difference, 0.7% [95% CI, −5.4% to 6.7%]; HR, 1.10 [95% CI, 0.56-2.16]; P = .80), 3-year risk of stroke in the qualifying artery territory (11.3% [19/168] vs 11.2% [19/170]; difference, −0.2% [95% CI, −7.0% to 6.5%]; HR, 1.00 [95% CI, 0.53-1.90]; P > .99) (Table 2), and the cumulative 3-year risk of disabling stroke or death (11.3% [19/168] vs 9.0% [15/166]; difference, 2.0% [95% CI, −4.6% to 8.6%]; HR, 1.28 [95% CI, 0.65-2.52]; P = .49) (Figure 3). There was no significant difference in the rate of 3-year risk of death (4.4% [7/160] vs 1.3% [2/159]; difference, 3.2% [95% CI, −0.5% to 6.9%]; HR, 3.75 [95% CI, 0.77-18.13]; P = .08) or cumulative 3-year risk of any stroke, TIA, or cardiovascular events (14.2% [24/169] vs18.0% [31/172]; difference, −4.1% [95% CI, −12.0% to 3.7%]; HR, 0.76 [95% CI, 0.45-1.30]; P = .31) between the groups (eFigure 3 in Supplement 3).
Figure 3. Kaplan-Meier Curves for the Secondary Outcome of Cumulative 3-Year Rates of Disabling Stroke or Death, According to Treatment Assignment.
The median time of observation was 36.0 months (IQR, 36.0-36.0) for the stenting group and 36.0 months (IQR, 36.0-36.0) for the medical therapy alone group. P = .49 for log-rank testing between stenting group and medical therapy alone group with center as stratification factor.
Post Hoc Outcomes and Analyses
A post hoc analysis of the primary outcome using a mixed-effects model with center as a random effect yielded an HR of 1.11 (95% CI, 0.52-2.36) (eTable 3 in Supplement 3). Considering the components of the primary outcome, the 30-day rate of stroke or death was 5.1% (9/176) in the stenting group and 2.2% (4/181) in the medical therapy alone group (Table 2). In the stenting group, there were 5 ischemic strokes within 30 days (5 were ultimately disabling, 0 were fatal) and 4 hemorrhagic strokes within 30 days (0 were ultimately disabling, 2 were fatal). In the medical therapy alone group, there were 4 ischemic strokes within 30 days (2 were ultimately disabling, 0 were fatal) and 0 hemorrhagic strokes within 30 days (eTable 4 in Supplement 3). The rate of stroke in the qualifying artery territory beyond 30 days to 1 year was 2.8% (5/176) in the stenting group and 5.0% (9/181) in the medical therapy alone group (Table 2). Subgroup analysis by the qualifying events showed the rate of primary outcome in patients qualified with ischemic stroke was 10.1% (9/89) in the stenting group and 8.6% (9/105) in the medical therapy alone group. For patients qualified with TIA, the rate of primary outcome was 5.7% (5/87) in the stenting group and 5.3% (4/76) in the medical therapy alone group (eTable 5 in Supplement 3).
Adverse Events
In the stenting group, 5 patients (2.8%) had disabling stroke, 4 (2.3%) had symptomatic intracranial hemorrhage, and 2 (1.1%) died of stroke within 30 days. In the medical therapy alone group, 2 patients (1.1%) had disabling ischemic stroke within 30 days. At 3 years of follow-up, 4.4% (7/160) and 1.3% (2/159) patients died in the stenting and medical therapy alone groups, respectively (Table 2).
Discussion
This multicenter, randomized, open-label trial in patients presenting with TIA or nondisabling ischemic stroke and severe intracranial atherosclerotic stenosis demonstrated that the addition of stenting to medical therapy, compared with medical therapy alone, resulted in no significant difference in the risk of subsequent stroke or death within 30 days or stroke in the qualifying artery territory beyond 30 days through 1 year. The results on all prespecified secondary outcomes also showed no significant difference.
Despite efforts to reduce perioperative complication rates by vetting of surgeons and sites and refining patient selection, the findings nonetheless demonstrated no clinical benefit from the addition of stenting to medical therapy for the treatment of patients with symptomatic severe intracranial atherosclerotic stenosis. The results of this study, together with that from previous trials,11,12,13 support the recent American Academy of Neurology Practice Advisory regarding stroke prevention in symptomatic large artery intracranial atherosclerosis,23 which recommends aggressive medical therapy rather than stenting for patients with symptomatic severe intracranial atherosclerotic stenosis.
Compared with previous randomized trials,11,12,13 the cumulative 1-year risk of stroke or death in both the stenting and medical therapy alone groups was much lower. The primary reason for the difference is likely attributable to the exclusion of patients with ischemic symptoms within 3 weeks of study enrollment. These patients were likely at the highest risk for 30-day stroke or death. All previous studies enrolled patients without the requirement on the interval between disease onset and enrollment. Other possible reasons include differences in age or ethnicity. The mean age was 56 years in the present study vs 60 years in SAMMPRIS and 62 years in VISSIT. The study populations of SAMMPRIS and VISSIT were predominantly Black and White, and the present study population were mainly Chinese Hans.
The 30-day event rate in the stenting group was much higher in both the SAMMPRIS and VISSIT studies, leading to early stopping in both. In the present study, the 30-day event rate was also numerically higher in the stenting group. While a large portion of the procedural risk reduction observed in the present study may be attributable to patient selection, other factors are likely involved as well. First, the present study selected high-volume clinical sites and used a lead-in phase to credential surgeons and to ensure their experience with stenting. A recent prospective registry in high-volume centers (>100 cases each year) showed similar 30-day rate of stroke or death (4.3%).14 The association of lower risk of complications with higher-volume centers was also shown in SAMMPRIS,24 the WEAVE registry,16 and the National Institutes of Health registry,25 suggesting the importance of experience in performing intracranial stenting procedures.26 Differences in periprocedural care could also be a key factor that distinguishes high-volume centers. Second, patient selection also likely decreases periprocedural risk. All patients in the present study underwent MRI or computed tomography at the time of screening, and those with perforator stroke alone without artery-to-artery embolism or distal hypoperfusion were excluded. This exclusion criterion may have reduced the risk of perforator occlusion related to the stenting procedure. The SAMMPRIS trial had 22.8% of patients recruited with perforator stroke only.27 A post hoc analysis of SAMMPRIS data showed that most periprocedural strokes in the stenting group were perforator strokes (15 of 21).28,29 Third, timing was also shown to be associated with safety outcomes of stenting.30 Recent studies have indicated stenting within a time interval of 3 weeks may confer higher procedural risk.31,32 The present study enrolled patients with a time from most recent event to stenting of more than 3 weeks (median time, 35 days), which was significantly longer than that for SAMMPRIS (median time, 7 days)11 and VISSIT (median time, 9 days).12 A higher risk of complication for early stenting might be related to plaque detachment and/or reperfusion injury,11,25,33 which was less pronounced with extended intervals.
One of the factors in the observed lack of superiority of stenting over medical therapy may be related to the nonnegligible periprocedural complications. The rate of 30-day symptomatic intracranial hemorrhage was numerically higher in the stenting than the medical therapy alone group (2.3% [4/176] vs 0% [0/182]; eTable 4 in Supplement 3). In the stenting group, the risk of hemorrhage may be related to guidewire perforation during the stenting procedure. In addition to the device limitations, the endovascular approach for treatment of intracranial stenosis is technically challenging and involves navigating the tortuous nature of intracranial vasculature and through diseased small vessels that can disturb the atherosclerotic plaque during advancement. Furthermore, periprocedural management (eg, maintaining a goal systolic blood pressure to avoid hyperperfusion after a procedure)14,15,16 may also be a target for future improvement in safety of stenting in intracranial atherosclerotic stenosis because there were 2 reperfusion hemorrhages after the procedure in the stenting group.
Another factor contributing to the lack of observed benefit with stenting was the lower rate of ischemic stroke risk in the medical therapy alone group. During the 3-year follow-up, the rates of the outcome were low in both groups and not significantly different between the 2 groups. These data imply that even if the periprocedural risk in the stenting group could be reduced to as low as the 30-day rate in the medical therapy alone group, stenting still may not provide long-term benefit over medical therapy. As mentioned above, the low event rate in the medical therapy alone group is likely related to a longer time interval after last symptom onset to randomization (median, 35 days in this trial vs 7 days in SAMMPRIS11 vs 15 days in VISSIT).12 In 3 studies, most strokes occurred within a relatively short interval after initial onset, with no further ischemic events in the second or third year.11,19,34 In the MyRIAD (Mechanisms of Early Recurrence in Intracranial Atherosclerotic Disease) study that included patients with symptomatic intracranial atherosclerotic disease (50%-99% stenosis), 5 of 9 same-territory ischemic strokes (55.6%) occurred within the first 6- to 8-week follow-up visit.34 Previous randomized trials seemed to follow this trend.11,19,33
Limitations
This study has several limitations. First, this trial did not evaluate angioplasty alone or other devices (eg, drug-coated balloon, drug-eluting stent, other self-expandable stents, or a combination) that are currently used off-label to treat patients with intracranial atherosclerotic stenosis.35,36,37,38 Second, this trial applied routine history, the NIHSS and mRS scoring, and brain imaging when necessary to identify a recurrent stroke during follow-up, but didn’t use the Questionnaire for Verifying Stroke-Free Status, which may cause potential missing information in follow-up case ascertainment. However, because the study used central independent adjudication, the bias is expected to be small and may affect both groups similarly.
Third, because the study was conducted only in centers in China, its generalizability to other populations outside of China is uncertain. Fourth, the patients were treated from 2014 to 2016, so it is uncertain whether or how the results apply to current stroke care, given the changes in management that have occurred over that time. Fifth, the medical management used in this trial may not have been as aggressive as that used in previous trials and the management in this trial may differ from what is considered standard of care in some countries. Sixth, the enrolled population had a lower risk of events than was anticipated in the power calculations. Therefore, the study findings apply specifically to the study population included in this study and the results may not be applicable to patients with intracranial stenosis with higher risk for events.
Conclusions
Among patients with TIA or ischemic stroke due to symptomatic severe intracranial atherosclerotic stenosis, the addition of percutaneous transluminal angioplasty and stenting to medical therapy, compared with medical therapy alone, resulted in no significant difference in the risk of stroke or death within 30 days or stroke in the qualifying artery territory beyond 30 days through 1 year. The findings do not support the addition of percutaneous transluminal angioplasty and stenting to medical therapy for the treatment of patients with symptomatic severe intracranial atherosclerotic stenosis.
Trial Protocol
Statistical Analysis Plan
eMethods
eFigure 1. Three-Year Time Trends of Risk Factor Control in Medical and Stenting Group
eFigure 2. Primary Outcome: Kaplan–Meier Curves for the Cumulative Probability of the Primary End Point, According to Treatment Assignment (Per-Protocol Analysis)
eFigure 3. Secondary Outcome: Kaplan–Meier Curves for the Cumulative 3-Year Rate of Any Stroke, TIA, or Cardiovascular Events, According to Treatment Assignment (Full Analysis Set Population)
eTable 1. Baseline Infarcts Patterns Among Patients With Qualifying Event as Stroke: Anterior vs Posterior Circulation
eTable 2. Measures of Risk Factors at Baseline and Each Visit Within 3-Year Follow-up
eTable 3. Post Hoc Analysis of Primary and Secondary Outcomes
eTable 4. Adverse Events Analysis (Full Analysis Set population)
eTable 5. Subgroup Analysis of Primary and Secondary Outcomes by the Qualifying Events (TIA Versus Ischemic Stroke
Nonauthor Collaborators. CASSISS Trial Investigators
Data Sharing Statement
References
- 1.GBD 2019 Stroke Collaborators . Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795-820. doi: 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145-1158. doi: 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111(10):1327-1331. doi: 10.1161/01.CIR.0000157736.19739.D0 [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Zhao X, Liu L, et al. ; CICAS Study Group . Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. 2014;45(3):663-669. doi: 10.1161/STROKEAHA.113.003508 [DOI] [PubMed] [Google Scholar]
- 5.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease. Stroke. 2008;39(8):2396-2399. doi: 10.1161/STROKEAHA.107.505776 [DOI] [PubMed] [Google Scholar]
- 6.Arenillas JF. Intracranial atherosclerosis: current concepts. Stroke. 2011;42(1)(suppl):S20-S23. [DOI] [PubMed] [Google Scholar]
- 7.Bose A, Hartmann M, Henkes H, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke. 2007;38(5):1531-1537. doi: 10.1161/STROKEAHA.106.477711 [DOI] [PubMed] [Google Scholar]
- 8.Zaidat OO, Klucznik R, Alexander MJ, et al. ; NIH Multi-center Wingspan Intracranial Stent Registry Study Group . The NIH registry on use of the Wingspan stent for symptomatic 70-99% intracranial arterial stenosis. Neurology. 2008;70(17):1518-1524. doi: 10.1212/01.wnl.0000306308.08229.a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiorella D, Levy EI, Turk AS, et al. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. 2007;38(3):881-887. doi: 10.1161/01.STR.0000257963.65728.e8 [DOI] [PubMed] [Google Scholar]
- 10.Kurre W, Berkefeld J, Brassel F, et al. ; INTRASTENT Study Group . In-hospital complication rates after stent treatment of 388 symptomatic intracranial stenoses. Stroke. 2010;41(3):494-498. doi: 10.1161/STROKEAHA.109.568063 [DOI] [PubMed] [Google Scholar]
- 11.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. ; SAMMPRIS Trial Investigators . Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365(11):993-1003. doi: 10.1056/NEJMoa1105335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaidat OO, Fitzsimmons BF, Woodward BK, et al. ; VISSIT Trial Investigators . Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis. JAMA. 2015;313(12):1240-1248. doi: 10.1001/jama.2015.1693 [DOI] [PubMed] [Google Scholar]
- 13.Miao Z, Jiang L, Wu H, et al. Randomized controlled trial of symptomatic middle cerebral artery stenosis. Stroke. 2012;43(12):3284-3290. doi: 10.1161/STROKEAHA.112.662270 [DOI] [PubMed] [Google Scholar]
- 14.Miao Z, Zhang Y, Shuai J, et al. ; Study Group of Registry Study of Stenting for Symptomatic Intracranial Artery Stenosis in China . Thirty-day outcome of a multicenter registry study of stenting for symptomatic intracranial artery stenosis in China. Stroke. 2015;46(10):2822-2829. doi: 10.1161/STROKEAHA.115.010549 [DOI] [PubMed] [Google Scholar]
- 15.Gao P, Wang D, Zhao Z, et al. Multicenter prospective trial of stent placement in patients with symptomatic high-grade intracranial stenosis. AJNR Am J Neuroradiol. 2016;37(7):1275-1280. doi: 10.3174/ajnr.A4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander MJ, Zauner A, Chaloupka JC, et al. ; WEAVE Trial Sites and Interventionalists . WEAVE trial. Stroke. 2019;50(4):889-894. doi: 10.1161/STROKEAHA.118.023996 [DOI] [PubMed] [Google Scholar]
- 17.Gao P, Zhao Z, Wang D, et al. China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis (CASSISS). Interv Neuroradiol. 2015;21(2):196-204. doi: 10.1177/1591019915581778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21(4):643-646. [PMC free article] [PubMed] [Google Scholar]
- 19.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. ; Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators . Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352(13):1305-1316. doi: 10.1056/NEJMoa043033 [DOI] [PubMed] [Google Scholar]
- 20.Kernan WN, Ovbiagele B, Black HR, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236. doi: 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 21.Jiang WJ, Yu W, Du B, Gao F, Cui LY. Outcome of patients with ≥70% symptomatic intracranial stenosis after Wingspan stenting. Stroke. 2011;42(7):1971-1975. doi: 10.1161/STROKEAHA.110.595926 [DOI] [PubMed] [Google Scholar]
- 22.Tang CW, Chang FC, Chern CM, Lee YC, Hu HH, Lee IH. Stenting versus medical treatment for severe symptomatic intracranial stenosis. AJNR Am J Neuroradiol. 2011;32(5):911-916. doi: 10.3174/ajnr.A2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turan TN, Zaidat OO, Gronseth GS, et al. Stroke prevention in symptomatic large artery intracranial atherosclerosis practice advisory. Neurology. 2022;98(12):486-498. doi: 10.1212/WNL.0000000000200030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derdeyn CP, Fiorella D, Lynn MJ, et al. ; SAMMPRIS Trial Investigators . Impact of operator and site experience on outcomes after angioplasty and stenting in the SAMMPRIS trial. J Neurointerv Surg. 2013;5(6):528-533. doi: 10.1136/neurintsurg-2012-010504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nahab F, Lynn MJ, Kasner SE, et al. ; NIH Multicenter Wingspan Intracranial Stent Registry Study Group . Risk factors associated with major cerebrovascular complications after intracranial stenting. Neurology. 2009;72(23):2014-2019. doi: 10.1212/01.wnl.0b013e3181a1863c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu SC, Leung TW, Lee KT, Wong LK. Learning curve of Wingspan stenting for intracranial atherosclerosis. J Neurointerv Surg. 2014;6(3):212-218. doi: 10.1136/neurintsurg-2012-010593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wabnitz AM, Derdeyn CP, Fiorella DJ, et al. ; SAMMPRIS Investigators . Hemodynamic markers in the anterior circulation as predictors of recurrent stroke in patients with intracranial stenosis. Stroke. Published December 11, 2018. doi: 10.1161/STROKEAHA.118.020840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derdeyn CP, Fiorella D, Lynn MJ, et al. ; Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis Trial Investigators . Mechanisms of stroke after intracranial angioplasty and stenting in the SAMMPRIS trial. Neurosurgery. 2013;72(5):777-795. doi: 10.1227/NEU.0b013e318286fdc8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abou-Chebl A. Intracranial stenting with Wingspan: still awaiting a safe landing. Stroke. 2011;42(7):1809-1811. doi: 10.1161/STROKEAHA.111.620229 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Sun Y, Li X, et al. Early versus delayed stenting for intracranial atherosclerotic artery stenosis with ischemic stroke. J Neurointerv Surg. 2020;12(3):274-278. doi: 10.1136/neurintsurg-2019-015035 [DOI] [PubMed] [Google Scholar]
- 31.Yu Y, Wang T, Yang K, et al. Timing and outcomes of intracranial stenting in the post-SAMMPRIS era. Front Neurol. 2021;12:637632. doi: 10.3389/fneur.2021.637632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang T, Luo J, Wang X, et al. Endovascular therapy versus medical treatment for symptomatic intracranial artery stenosis. Stroke. 2021;52(2):e53-e54. doi: 10.1161/STROKEAHA.120.032988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. ; Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis Trial Investigators . Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS). Lancet. 2014;383(9914):333-341. doi: 10.1016/S0140-6736(13)62038-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romano JG, Prabhakaran S, Nizam A, et al. ; MyRIAD Investigators . Infarct recurrence in intracranial atherosclerosis. J Stroke Cerebrovasc Dis. 2021;30(2):105504. doi: 10.1016/j.jstrokecerebrovasdis.2020.105504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dumont TM, Sonig A, Mokin M, et al. Submaximal angioplasty for symptomatic intracranial atherosclerosis. J Neurosurg. 2016;125(4):964-971. doi: 10.3171/2015.8.JNS15791 [DOI] [PubMed] [Google Scholar]
- 36.Remonda L, Diepers M, Berberat J, et al. Drug-coated balloon treatment in symptomatic intracranial high grade stenosis. Clin Neuroradiol. 2021;31(1):45-49. doi: 10.1007/s00062-020-00936-9 [DOI] [PubMed] [Google Scholar]
- 37.Jia B, Zhang X, Ma N, et al. ; NOVA Trial Investigators . Comparison of drug-eluting stent with bare-metal stent in patients with symptomatic high-grade intracranial atherosclerotic stenosis. JAMA Neurol. 2022;79(2):176-184. doi: 10.1001/jamaneurol.2021.4804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Zhang X, Zhang J, et al. Drug-coated balloon dilation compared with conventional stenting angioplasty for intracranial atherosclerotic disease. Neurosurgery. 2020;87(5):992-998. doi: 10.1093/neuros/nyaa191 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eMethods
eFigure 1. Three-Year Time Trends of Risk Factor Control in Medical and Stenting Group
eFigure 2. Primary Outcome: Kaplan–Meier Curves for the Cumulative Probability of the Primary End Point, According to Treatment Assignment (Per-Protocol Analysis)
eFigure 3. Secondary Outcome: Kaplan–Meier Curves for the Cumulative 3-Year Rate of Any Stroke, TIA, or Cardiovascular Events, According to Treatment Assignment (Full Analysis Set Population)
eTable 1. Baseline Infarcts Patterns Among Patients With Qualifying Event as Stroke: Anterior vs Posterior Circulation
eTable 2. Measures of Risk Factors at Baseline and Each Visit Within 3-Year Follow-up
eTable 3. Post Hoc Analysis of Primary and Secondary Outcomes
eTable 4. Adverse Events Analysis (Full Analysis Set population)
eTable 5. Subgroup Analysis of Primary and Secondary Outcomes by the Qualifying Events (TIA Versus Ischemic Stroke
Nonauthor Collaborators. CASSISS Trial Investigators
Data Sharing Statement