Summary
As sustainable development practitioners have worked to “ensure healthy lives and promote well-being for all” and “conserve life on land and below water”, what progress has been made with win–win interventions that reduce human infectious disease burdens while advancing conservation goals? Using a systematic literature review, we identified 46 proposed solutions, which we then investigated individually using targeted literature reviews. The proposed solutions addressed diverse conservation threats and human infectious diseases, and thus, the proposed interventions varied in scale, costs, and impacts. Some potential solutions had medium-quality to high-quality evidence for previous success in achieving proposed impacts in one or both sectors. However, there were notable evidence gaps within and among solutions, highlighting opportunities for further research and adaptive implementation. Stakeholders seeking win–win interventions can explore this Review and an online database to find and tailor a relevant solution or brainstorm new solutions.
Introduction
Ecosystem degradation can exacerbate infectious diseases that have long plagued humankind or cause novel pathogens to spillover from animals to humans.1, 2, 3, 4, 5, 6 By targeting connections between human infectious disease and the natural world, interventions might “ensure healthy lives and promote well-being for all” and “conserve life on land and below water”—two Sustainable Development Goals (SDGs).7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 For example, putting tick collars on free-ranging dogs might reduce transmission of ticks and tick-borne disease from dogs to people and wildlife.18 Indeed, sustainable development practitioners worldwide are urgently seeking safe and effective cross-sector interventions that might prevent the next pandemic.19
Of course, no single win–win intervention will work in all contexts or solve all problems within complex socioecological systems.20 Interventions that improve some outcomes for human health and ecosystems might even cause collateral impacts in other sectors, creating complex trade-offs among SDGs.16, 21, 22 Tasked with choosing an optimal intervention for any given problem and socioecological context, practitioners need to know about the available intervention options and how they can be compared. In the event that no existing intervention is suitable, practitioners will need to know how to identify and evaluate new intervention options.
Unfortunately, the information needed to identify, implement, and evaluate win–win interventions that prevent or control human infectious diseases tends to be scarce, inconsistent, and unconsolidated.23 For instance, among conservation intervention studies that reported human wellbeing benefits, fewer than 2% considered health-specific outcomes and only a subset of those considered emerging or endemic infectious diseases.24, 25 Furthermore, existing studies are scattered across siloed disciplines that use different research methodologies, measure different outcomes, and publish in different journals. Navigating this dispersed evidence landscape would be prohibitively time consuming for practitioners interested in implementing win–win interventions.
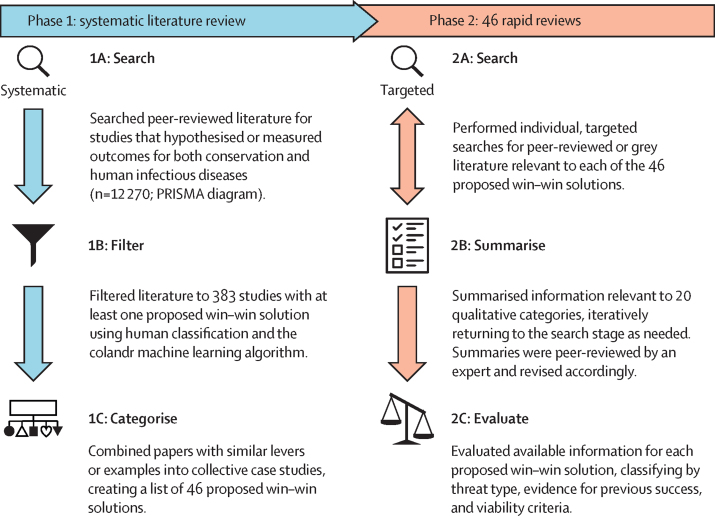
To facilitate timely, evidence-based decision making, we review existing evidence regarding win–win solutions that aim to simultaneously reduce human infectious disease burden and advance conservation goals. We use the terms intervention and solution interchangeably because there are growing policy initiatives for enacting nature-based solutions.8 To find, evaluate, and synthesise evidence from win–win solutions, we used a subject-wide evidence synthesis (figure 1). This two-phase method uses a systematic literature review to identify a landscape of possible interventions and then each intervention is explored using an individual, targeted rapid review.26, 27, 28, 29 This process allowed us to create a menu of 46 proposed solutions ranging from local to international scales, including individual information summaries (figure 2). Stakeholders can explore these examples to find and tailor potential solutions to meet their needs or brainstorm new solutions. Potentially viable solutions that achieve specific goals within resource constraints can be identified and evaluated using general criteria that we derived from synthesising information across the 46 solutions. Finally, we highlight evidence gaps within and among solutions that could be important targets for future research and implementation.
Figure 1.
Subject-wide evidence synthesis
The PRISMA diagram is shown in the appendix (p 14).
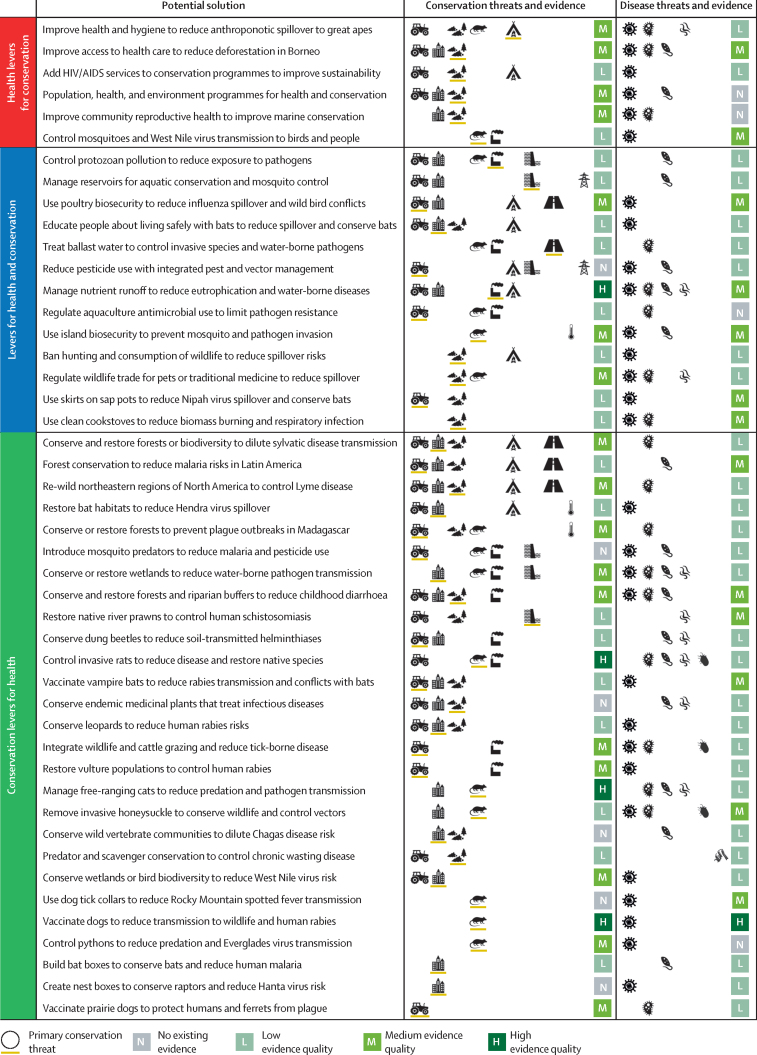
Figure 2.
A menu of 46 potential solutions for advancing conservation goals and controlling human infectious diseases
N denotes none (defined as hypotheses and anecdotes), L denotes low (defined as some supporting studies with moderate to major gaps, inconsistency, or low applicability), M denotes medium (defined as several lines of evidence that are mostly consistent and applicable), and H denotes high (defined as diverse, consistent, and highly applicable evidence that leaves little to no uncertainty regarding the outcome).
Widespread and diverse potential solutions
The 46 potential solutions addressed diverse threats, collectively covering all continents (except Antarctica), most major pathogen groups (except fungi), and most conservation threat classes defined by the International Union for Conservation of Nature (IUCN; except geological events and other; figures 3, 4).31 Most solutions addressed multiple threats for health and conservation (eg, multiple pathogen species and multiple IUCN threat classes). The 46 potential solutions also covered numerous intervention types and targets, ranging from vaccinating vampire bats against rabies in Peru32 to establishing sustainable harvesting programmes for medicinal plant species in Tanzania.33 Potential solutions were diverse because the problems that they addressed were diverse.
Most solutions addressed pathogens with environmentally mediated transmission, such as vector-borne diseases and zoonotic diseases transmitted from animals to people (figure 4), mirroring the strong focus on these diseases in the One Health, Planetary Health, and EcoHealth fields.9, 11, 34 For example, WHO and other international organisations support the expansion of training for integrated pest and vector management globally, because these management techniques might reduce total pesticide use and control crop pests and disease vectors, such as mosquitoes. Environmentally mediated diseases such as these have probably been the easiest entry points for cross-sector solutions due to obvious underlying links between human health and ecosystems.
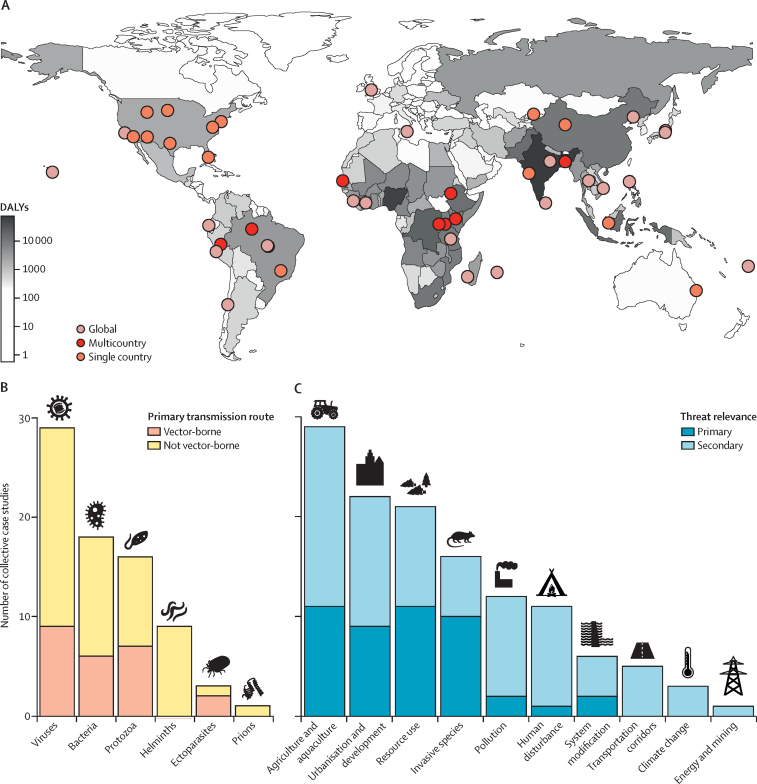
Figure 4.
Solutions are widespread and diverse
(A) The solutions covered all continents (except Antarctica), including countries with high and low burdens of infectious diseases, as measured by total DALYs and reported by WHO in 2018.30 (B) The solutions covered most pathogen taxa (except fungi) and transmission modes. (C) Seven International Union for Conservation of Nature threat classes were considered the primary conservation threat addressed by at least one solution, whereas transportation, climate change, and energy and mining were only secondary conservation threats addressed by any solution. DALYs=disability-adjusted life-years.
Only a few potential solutions addressed diseases without environmentally mediated transmission, such as HIV/AIDS and pneumonia. For example, people with poor health due to HIV/AIDS or other diseases are more likely to use easier and more destructive fishing practices in communities near Lake Victoria in Africa, so treatment and prevention programmes might support both human health and aquatic conservation.35 Focusing on these understudied links between ecosystems and directly transmitted and chronic human diseases might yield additional solutions for advancing conservation and health.
In addition to addressing diverse health threats, the potential solutions also addressed all IUCN conservation threats related to anthropogenic activities (figure 4C). Solutions related to land use change—agriculture and aquaculture (n=29 collective case studies) and urbanisation and development (n=22)—were most common, probably because land use change is a leading driver of biodiversity declines36 and disease spillover.6, 11, 37, 38 Only a few solutions addressed transportation infrastructure, energy and mining, and climate change, and these were never addressed as the primary threat.
We did not include a collective case study in which climate change was the primary conservation threat and global emission reduction was the solution (appendix pp 3–4) because the numerous health and conservation outcomes that could be achieved by global emission reduction9, 10 have yet to be measured. Climate change is expected to become a more urgent threat over time,7 so there is a clear need for actionable targeted solutions related to climate change.
The conservation and health threats targeted by potential solutions spanned from subnational extents within single countries to multiple countries within a continent to global applicability (figure 4A). For example, vaccinating prairie dogs to reduce plague risk for endangered black-footed ferrets and humans applies subnationally in Wyoming, South Dakota, Montana, and Arizona (USA);39 whereas forest conservation to reduce human malaria might be relevant to multiple countries in Latin America, Africa, and Asia.40, 41 The potential solutions included targets in low-income and middle-income countries with high burden of environmentally mediated human infectious diseases and high-income countries with lower infectious disease burden and higher research efforts. Ultimately, one or more potential solutions probably exist for all countries, but the set of relevant solutions that apply to any country could be expanded by future efforts to scale or translate existing solutions to new locations.
There were 27 potential solutions that involved implementing classic conservation interventions that have health benefits (ie, conservation levers for health; figure 2), which are sometimes called nature-based solutions or ecological levers for health.8, 42, 43 One common conservation intervention type was species management, including controlling or eradicating invasive honeysuckle to reduce negative impacts on native vertebrates and reduce vector populations44 and reintroducing native prawns extirpated by dams to help control snails that transmit human schistosomiasis.45 Another common conservation intervention type was land and water management or protection, such as conserving or restoring wetlands to increase biodiversity and reduce water-borne diarrhoeal diseases.46 Together, these 27 potential solutions are the most comprehensive list to date of conservation solutions that might reduce human infectious diseases, an important subsector within the global focus on conservation solutions that improve general human wellbeing.8, 9, 11, 42
Six potential solutions involved public health interventions that have conservation benefits (ie, health levers for conservation). For example, health system strengthening or family planning and reproductive health programmes—including population, health, and environment programmes in many countries47—have reduced illegal logging and deforestation;48, 49 improved coral and mangrove conditions in marine environments;50 and improved community participation in, or approval of, conservation initiatives.51 There were also several solutions that used insect vector control to reduce vector-borne disease risk for people and wildlife.52, 53 These health interventions were often supported by scarce evidence, either because there were not enough resources dedicated to monitoring and evaluation or interventions implemented by the health sector did not quantify ecosystem or conservation outcomes. Therefore, we expect that more health interventions that advance conservation goals could be developed and evaluated through increased collaboration between conservation and health organisations.
Finally, 13 potential solutions acted through interventions that were not specific to public health or conservation, but affected both sectors (ie, levers for health and conservation). Many of these were policies regarding the food–energy–water nexus,54 such as regulating protozoan pollution,55 reducing antibiotic use in aquaculture,56 reducing nutrient pollution and eutrophication associated with agriculture,57 and implementing ballast water treatment protocols to prevent invasive pathogens and wildlife from moving among ports.58 There were also outreach, education, or livelihood interventions such as teaching people how to live safely with bats that might be virus reservoirs,59 protecting tree sap collection pots from bat contamination using bamboo skirts,60, 61 and replacing wood-burning stoves with cleaner cookstoves to reduce deforestation and smoke-related pneumonia.62 However, livelihood-focused interventions were rare, so future efforts might discover more interventions that primarily target poverty and inequalities (SDGs 1 and 10) and that have downstream benefits for health and conservation (SDGs 1, 14, and 15).
Examples of each lever type are illustrated in figure 3. The figure illustrates (1) health systems that provide affordable health care in the Indonesian portion of the island of Borneo reduce human disease burden and illegal logging done to pay for health care (health lever); (2) vector control is a public health intervention that might also benefit biodiversity, as in the case of North American birds susceptible to the West Nile virus (health lever); (3) law and policy interventions that ban importation of non-native wildlife reservoirs (eg, pouch rats) prevent spillover to humans and native wildlife (lever for health and conservation); (4) education and outreach empower people to live safely with bats, reducing zoonotic spillover risk (eg, Nipah virus; lever for health and conservation); (5) species management, such as vaccinating or sterilising free-ranging domestic dogs, reduces rabies transmission to humans and African carnivores (conservation lever); and (6) ecosystem management interventions, such as restoring wetland vegetation, reduce the survival of human and wildlife pathogens in the environment while restoring wildlife habitat (conservation lever).
Figure 3.
Six lose–lose scenarios that could be improved with win–win solutions
This figure was commissioned from artist Hiram Henriquez, and all photographs were used under creative commons licences or purchased with commercial licences (ie, iStockphoto). Photographs of palms and sap collection pots were used with permission from Fernando Garcia and Nazmun Nahar.
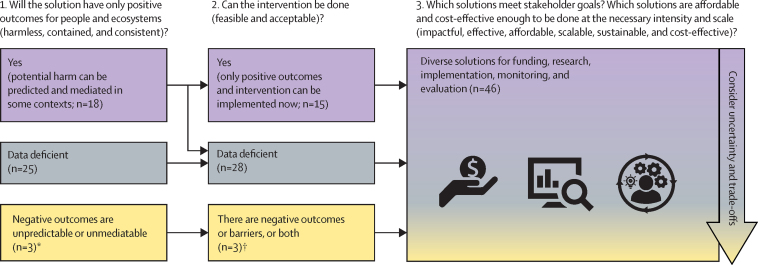
The diversity among potential solutions is promising because “there is no one-size-fits-all approach for One Health implementation”.11 The 46 examples we described here cover many context-specific health and conservation threats; therefore, stakeholders might be able to adapt one of these solutions to meet their needs. In cases where none of the potential solutions are relevant, stakeholders could design new solutions to meet specific goals within their resource constraints. To determine whether any given solution will be viable for a given context, stakeholders can evaluate each solution using the 11 viability criteria described below (harmless, contained, consistent, feasible, acceptable, impactful, effective, affordable, scalable, sustainable, and cost-effective; figure 5; appendix pp 7–8).
Figure 5.
Viable solutions can be identified and evaluated by 11 criteria
We evaluated five criteria to determine whether solutions were harmless, contained, consistent, feasible, and acceptable in predictable contexts given the currently available evidence or whether these evidence were data deficient for the specified criteria. Stakeholders can evaluate six other criteria on the basis of priorities and resource constraints (impactful, effective, affordable, scalable, sustainable, and cost-effective). *Three potential solutions had evidence for trade-offs that were unmitigable or unpredictable and were categorised as not harmless, not consistent, or both. All solutions were categorised as contained or data deficient for the contained criterion. †None of the potential solutions had evidence for unmediatable barriers to implementation (not feasible or not acceptable).
Identifying and minimising trade-offs
To evaluate potential trade-offs caused by a given intervention, we suggest considering three viability criteria (figure 5): (1) harmless solutions are not expected to harm non-target aspects of human wellbeing for some people while attempting to help others; (2) contained solutions are not expected to have negative, collateral effects on non-human targets, or else potential collateral effects could be avoided or fully mitigated; and (3) consistent solutions are expected to have only positive outcomes for their intended conservation and human infectious disease control targets in predictable contexts (ie, no known negative outcomes). Two investigators used these definitions and the available evidence to determine whether each potential solution met, did not meet, or was data deficient for the three criteria (appendix pp 6–7). Data limitations often made it difficult to decide whether a solution involved substantial trade-offs, but existing evidence showed that 18 solutions could be harmless, contained, and consistent under some contexts (figure 5).
Context dependency was common among the 46 potential solutions. For example, introducing invasive predators that consume larval mosquitoes to control malaria has often negatively impacted ecosystems (eg, violating the contained criterion),63 but at least in some contexts (eg, native predators), mosquito predators are expected to only have positive outcomes for ecosystems (ie, meeting the contained criterion). However, for other solutions, we could not identify a mediating context. For example, correlational studies suggest that forest cover and host biodiversity impact competent host abundance in ways that can reduce human Lyme disease risk in North America (also known as the dilution effect).64 Yet in this complex system, forest cover or host biodiversity have also been associated with unexpected amplification of Lyme disease risk;65 thus, how forest conservation or restoration interventions would impact human health remains unclear (ie, this solution violates the consistent criterion). Future research or innovation might identify specific scales and circumstances in which this solution does no harm, but at present, it risks causing unpredictable harm to some people in certain contexts.
Similarly to most solutions, those that address pathogen spillover from wildlife to humans had data gaps regarding trade-offs. For example, wildlife trade is a conservation threat and pathway for spillover from wildlife.66, 67, 68, 69 However, in several African countries, past bans on all wildlife hunting and consumption sometimes created food insecurity, illegal markets, and distrust in health authorities.21, 70, 71, 72 Bans developed in response to the COVID-19 pandemic might be similarly problematic.73, 74 This evidence shows that wildlife trade bans can cause harm when they affect subsistence hunting and consumption, but might be safe and feasible in other specific contexts. For example, many existing national and international wildlife pet trade restrictions aim to conserve wildlife (eg, the Convention on International Trade in Endangered Species), and there is increasing, but not universal, public support for bans or restrictions on luxury commercial wildlife trade in Asia due to the 21st century coronavirus outbreaks.75 If successful, restrictions and bans that target the multibillion-dollar commercial wildlife trade could prevent multitrillion-dollar pandemics.16, 76 However, whether and when these policies will be successful is still unclear, including whether they will favour illegal markets or erode support for conservation. Efforts are urgently needed to identify contexts in which negative impacts are mediated, conservation is advanced, and spillover risks are reduced.
Achieving socially acceptable and feasible solutions
Two criteria can be used to determine whether solutions are immediately achievable (ie, the feasible and acceptable criteria; figure 5). Feasible solutions could be successfully implemented given existing technology and sufficient resources, and socially acceptable solutions are supported by stakeholders who are affected by the intervention or could be made acceptable to stakeholders. For example, the livestock medication diclofenac caused substantial vulture population declines in India, which could have contributed to the increase in free-ranging dogs and human rabies risk.77 Therefore, diclofenac was banned in India and the surrounding nations, an intervention that was achievable because there were acceptable alternative veterinary drugs that could replace diclofenac and these were not toxic to vultures.77 As this example illustrates, some of the 46 potential solutions have already been successfully implemented on national or multinational scales.
However, most potential solutions were data deficient for feasibility or social acceptability. For example, two potential solutions involve the effort to broadly re-wild North America with top carnivores to control infectious diseases in wild herbivores and possibly humans.78 These potential solutions face opposition from some stakeholders (eg, ranchers and hunters), and whether these solutions would lead to net reductions in disease risk and whether they can be made socially acceptable in places in which the intervention would impact disease transmission the most remains unclear. Indeed, solutions that involved changing peoples’ lifestyles and cultures were often data deficient for acceptability, which highlights a clear need for social science and implementation research to evaluate cross-sector solution viability, as outlined by the Organisation for Economic Co-operation and Development.79
Impactful and effective solutions achieve stakeholders’ goals
Whether a given solution meets stakeholders’ goals can be evaluated using two criteria (ie, the impactful and effective criteria). Impactful solutions have the potential to meet stakeholders’ quantitative goals (ie, effect magnitude and clinical relevance) and effective solutions can successfully achieve the desired outcomes. For example, building nest boxes to increase local predatory bird populations is proposed to control the rodent species that are reservoirs for hantaviruses.80 This solution is a proposed ecological lever for health.42 However, there is no evidence that nesting sites are scarce so the intervention might only redistribute non-threatened wildlife populations in ways that benefit humans, creating little value for stakeholders with strong conservation priorities (ie, not impactful for conservation). There is also scarce evidence that this solution can successfully reduce human disease burdens (ie, might not be effective for human health). We did not quantify how impactful each potential solution was because there was no common system available to rank impacts given the diverse methods and metrics used across relevant literature. However, we did qualitatively assess how effective each solution was, as evidenced by previous success in achieving proposed goals (figure 6; appendix pp 5–7).
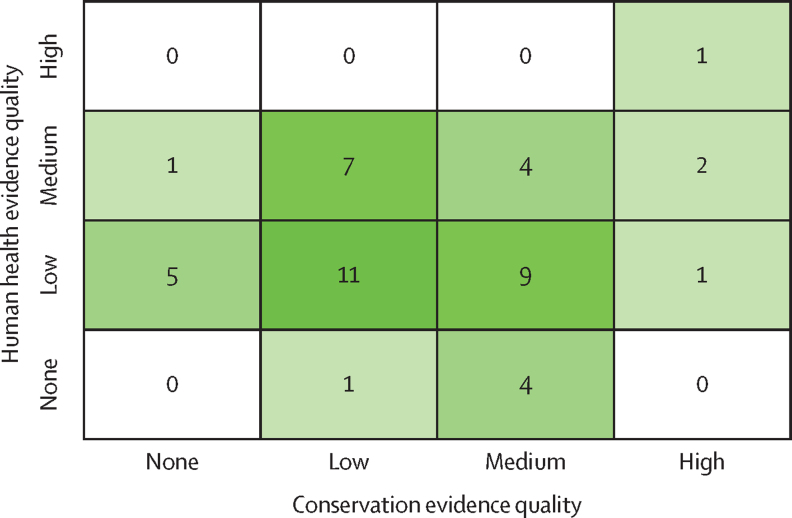
Figure 6.
Most solutions had evidence gaps
Each cell contains the number of solutions that had a given composite evidence quality score on the basis of evidence diversity, consistency, and applicability (none is defined as hypotheses and anecdotes; low quality evidence is defined as some supporting studies with moderate to major gaps, inconsistency, or low applicability; medium is defined as several lines of evidence that are mostly consistent and applicable; and high is defined as diverse, consistent, and highly applicable evidence that leaves little to no uncertainty regarding the outcome). The green colour is used to emphasise that most solutions were supported by low or medium evidence for conservation or health outcomes.
We categorised existing evidence quality for each potential solution using a modified Bridge Collaborative rubric81 with three categories (evidence types and diversity, evidence consistency, and evidence applicability; appendix pp 5–7, 15). We then combined these three categories into one composite score for overall evidence quality for health outcomes and one score for conservation outcomes (Figure 2, Figure 6). No evidence indicated that cases were supported only by hypotheses and anecdotes; low quality evidence indicated that there were few supporting studies with moderate to major evidence gaps, unexplained inconsistency, or low applicability; medium quality indicated that there were several lines of evidence that were mostly consistent and applicable, and inconsistency could be explained; and high quality indicated diverse evidence types, usually including an intervention study, that yielded consistent and applicable results and left little to no uncertainty regarding the outcome. The resulting composite evidence quality scores highlight which solutions have had demonstrable success for health and conservation outcomes and which still have evidence gaps.
There were seven solutions that already had medium to high evidence quality for conservation and human health success (figure 6). For example, vaccinating dogs and wild carnivores to reduce rabies transmission among dogs, wildlife, and people was supported by high evidence quality for both outcomes (including successful intervention programmes).82, 83 Most solutions had higher evidence quality regarding effectiveness for conservation than for health. For example, controlling invasive rats, brushtail possums, and cats can conserve endemic species,84, 85, 86 especially on islands. There are also studies linking invasive species control and human infectious disease burden,87, 88 but the evidence types and diversity are low. Similarly, although forest restoration and conservation have well established benefits to ecosystem structure and function89, 90 and several correlational studies link upstream forest cover to reduced childhood diarrhoea risk downstream,91 none of these are intervention studies. Although evidence for these solutions could still be improved, these examples show that effective cross-sector solutions exist.
There were 17 potential solutions that had low overall evidence quality due to low evidence diversity, applicability, or consistency that was difficult to explain for one or both outcomes. For example, two observational studies92, 93 (scarce evidence diversity) quantified high leopard predation rates on free-ranging domestic dogs, an important disease reservoir for rabies near Mumbai (India).92 Leopard conservation might reduce dog rabies, and thus human rabies risk, but predation or culling of dogs could also counterintuitively increase rabies in dog reservoir populations (ie, potentially not consistent).94 In another example, regulating drawdown rates for water reservoirs created by dams might restore aquatic communities and reduce larval mosquito survival, thus reducing human malaria risk near dams.95 However, the existing evidence did not come from countries with high malaria burden (ie, low applicability). Additionally, integrating wild grazing animals on land parcels used for grazing cattle (frequently treated for ticks) might increase forage availability for wildlife and reduce tick abundance on land parcels,96 which in turn might affect human disease risk, but tick-borne disease incidence in humans has never been measured for this solution (ie, low applicability). As these examples illustrate, many promising solutions had some supporting evidence, but most solutions had notable data gaps (figure 5).
Optimal solutions achieve goals within resource constraints
In addition to goals (ie, impactful and effective criteria), stakeholders also have resource constraints. Resources determine which solutions can be implemented at the necessary scales and intensities to achieve the desired outcomes (eg, the affordable, scalable, and sustainable criteria) and how big the impact will be for a given resource budget (ie, the cost-effective criterion). Various stakeholders might evaluate these four criteria differently because of differing goals, priorities, and resources.
We note that resource costs and affordability are distinct; cost is the resource price tag whereas affordability is the ability to pay. For human infectious diseases, public health intervention cost-effectiveness is usually quantified in disability-adjusted life-years (DALYs) averted per US, Canadian, or Australian dollar but DALYs and cost were rarely reported for the 46 potential solutions. This omission highlights an important area for future research because for most stakeholders, and perhaps especially those interested in human health outcomes, cost-effectiveness and affordability will be the most important considerations when choosing a solution.
For some of the proposed solutions, the potential conservation and health impacts would likely be too small for most stakeholders to justify the cost. For example, invasive python control in Florida reduces predation pressures on native vertebrates and might reduce human exposure to the vector-borne Everglades virus.97 However, python eradication is costly and has not been achieved using existing resources, and maintaining continuous python control efforts at current intensities might not be feasible indefinitely (ie, potentially not sustainable). From a public health perspective, a cheaper and more direct public health or medical intervention might be preferred to python control. However, local stakeholders in Florida might value the small human health co-benefits from python control, even if their main goal is a potentially large conservation impact. As this example illustrates, sometimes a small impact in one sector (health or conservation) can be valued because it accompanies a large impact in another sector or fully addresses a local problem.
Quantifying the net value associated with all positive impacts in all sectors for a given intervention is difficult. However, identifying these potential impacts explicitly can help stakeholders to compare multisector interventions.16, 21, 22 Ultimately, for any given cross-sector problem, collaborations among stakeholders, economists, social scientists, and implementation scientists might be needed to determine which solution is optimal.
Evidence-based management under uncertainty
Data-limited solutions that appear safe and feasible could be ideal for immediate research and adaptive implementation. However, strict adaptive implementation requires that multiple interventions are implemented simultaneously and compared, and approaches are subsequently modified according to what works best.98 This approach is often infeasible in conservation and public health programmes,98 and might be even more difficult for multisector solutions, leaving many data gaps unaddressed. When adaptive implementation is not possible, there might be other ways to fill in data gaps using safe implementation, such as by comparing different programmes that monitor, evaluate, and share outcomes. For example, multiple programmes are improving hygiene or health care for people who work or tour in great ape conservation areas, which could increase human health and reduce pathogen spillover from humans to apes.99 Comparing outcomes across these programmes might provide new insights for the Best Practice Guidelines for Health Monitoring and Disease Control in Great Ape Populations created by the IUCN.100 As evidence accumulates for this solution and others, uncertainty will decline. Data gaps are still likely to remain prominent in the near future, but action despite uncertainty will already be familiar for most public health and conservation practitioners.9, 10
Conclusions
The growing Planetary Health field emphasises the links between human wellbeing and ecosystem integrity, but there has been scarce guidance for how to leverage these relationships to implement viable win–win solutions that specifically reduce human infectious disease burden. Here, we identified 46 such potential solutions. We found that proposed solutions address diverse, context-dependent, and dynamic threats with cross-sector interventions that are equally diverse. Numerous solutions had the potential to be safe and feasible under predictable contexts and some were supported by medium and high quality evidence of success. Some solutions had the potential for large human health or conservation impacts, such as forest conservation projects and health system strengthening initiatives. Others had small effects but might still be highly valued by local stakeholders. Synergies such as these might be pivotal for achieving the soon-to-be-revised Sustainable Development Goals.16, 21, 22, 101
Although promising, all the proposed solutions had some evidence gaps and, collectively, they did not cover all possible health and conservation threats. Evidence regarding conservation and health impacts (quantitative outcomes) and intervention cost-effectiveness and affordability were especially scarce, highlighting priorities for future research. Currently, these data gaps within and among solutions complicate decision making. More evidence will accumulate when stakeholders invest in research, adaptive implementation, monitoring, and evaluation for existing approaches. New solutions will also be created, filling in existing gaps or addressing new problems. Until then, the viability criteria described here can be used to compare and update the evidence database for potential solutions, differentiating the solutions that do not work from those that successfully and cost-effectively advance health and conservation.
Search strategy and selection criteria
To find and synthesise evidence among proposed solutions, we used a subject-wide evidence synthesis, a two-phase approach for identifying and assessing a broad suite of interventions supported by heterogeneous evidence (Shackelford and colleagues, 2019; Sutherland and colleagues, 2018). In the first phase, we performed a systematic literature review of peer-reviewed papers and book chapters, which we used to identify solutions that have been proposed to reduce human infectious disease burdens and advance conservation goals (appendix pp 2–4). In the second phase, we performed targeted rapid reviews of peer-reviewed and grey literature (Grant and Booth, 2009), iteratively revising evidence summaries for each proposed solution (appendix pp 4–5). Finally, we used these evidence summaries to categorise information for each solution, making it easier to synthesise and compare.
To create a list of proposed win–win solutions (phase 1), we systematically reviewed publications in Thomson Reuters Web of Science and PubMed (n=12 270 papers), including records published between database inception and March 14, 2018. We performed the search using 167 English terms regarding conservation, ecology, infectious disease, and human populations (adapted from McKinnon and colleagues, 2016; appendix pp 2–4, 14). We identified 617 papers containing hypothesised or measured outcomes for conservation and human infectious diseases—excluding papers that did not discuss proposed outcomes for one or both sectors—by using a combination of researcher classification and machine learning to sort records by relevance (Cheng and colleagues, 2018). During subsequent full-text analysis, we removed any records that did not suggest at least one proposed win–win solution (eg, papers about trade-offs in which environmental degradation improves health). We then used full-text analysis of the final list of 383 records to group those pertaining to the same win–win solutions into collective case studies (appendix p 4), which resulted in a list of 46 proposed solutions.
Each solution was then individually reviewed by one or two investigators (phase 2: targeted rapid reviews), who used keyword searches to find additional peer-reviewed publications and grey literature. These rapid reviews were not systematic because they did not examine all published literature—a task that would not be possible for 46 interventions. Instead, investigators specifically sought publications relevant to 20 information categories, determined a priori, and summarised all information in a standardised format (appendix pp 4–5). A single lead investigator reviewed all collective case study summaries to ensure consistency and then each summary was reviewed by an external expert (appendix p 5). Based on feedback from external experts, investigators iteratively searched for more information and revised the collective case study summary until the investigator and lead investigator deemed the review complete.
After finishing the collective case study summary, each investigator used a list of qualitative variables defined a priori to categorise information consistently for comparison across case studies. Variables included geographical location, conservation threat, infectious disease threat, mechanism or lever type, evidence for conservation and human infectious disease outcomes, and 11 criteria that we identified as indicative of viable solutions (harmless, contained, consistent, feasible, acceptable, impactful, effective, affordable, scalable, sustainable, and cost-effective; appendix pp 7–8). The investigators’ designations were confirmed by the lead and one or two other investigators to ensure consistency. Any discrepancies between how different people categorised information were discussed until consensus was reached. Finally, we synthesised information across the 46 solutions to describe their diversity and evidence gaps.
Data sharing
The 46 evidence summaries are publicly available in a searchable database.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We thank the researchers and practitioners who provided friendly reviews of the 46 evidence summaries. This research was conducted by the Ecological Levers for Health working group supported by the Science for Nature and People Partnership, a collaboration of The Nature Conservancy, the Wildlife Conservation Society, and the National Center for Ecological Analysis and Synthesis at the University of California. KDL was supported by the US Geological Survey Ecosystems Mission Area. CLW was supported by a research fellowship from the Alfred P Sloan Foundation, an award from the National Science Foundation Division of Geosciences Biological Oceanography Program (OCE-1829509), and an innovation award from the University of Washington Innovation Imperative. AJM was supported by a National Science Foundation postdoctoral research fellowship in biology (1611767) and the Fogarty International Center (DEB-2011147). NN was supported by the Bing fellowship in honor of Paul Ehrlich and the Stanford Data Science Scholars programme. TESM is a Simons Foundation fellow of the Life Sciences Research Foundation. AJP was supported by a Queensland Government Accelerate postdoctoral research fellowship and the DARPA PREEMPT programme Cooperative Agreement (D18AC00031). AJL was supported by a James and Nancy Kelso fellowship through the Stanford Interdisciplinary Graduate Fellowship programme at Stanford University. IJJ was supported by a National Science Foundation graduate research fellowship (1656518). GADL and SHS were partly supported by the National Science Foundation (ICER-2024383 and DEB-2011179) and the Bill & Melinda Gates Foundation (OPP1114050). Any use of trade, product, or firm names in this Review is only for descriptive purposes and does not imply endorsement by the US Government.
Editorial note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
CLW, IJJ, KDL, SRH, and SHS conceptualised and designed the study. AJP, AJL, AJM, APD, CLW, IJJ, KDL, MEH, NN, SHO, SRH, and SHS designed the methods. AJP, AJL, AG, AJM, AEL, AMK, CLW, CL, DCGM, GADL, IJJ, JLF, JCB, JVR, KJF, KDL, LHK, MW, MEH, NN, SHO, SRH, SHS, and TESM contributed case studies or performed experiments. SRH conducted the formal data analysis. AJP, AJL, AJM, CLW, IJJ, KJF, NN, SHO, SRH, and SHS curated the data. AJP, AJL, AJM, CLW, CL, IJJ, JCB, KDL, LHK, NN, SHO, SRH, and SHS drafted figures or directed data visualisation. KDL, SRH, and SHS provided oversight and leadership for the study. SRH and SHS managed and coordinated research planning and execution. DLC, KDL, and SHS acquired financial support for the project. All authors wrote and revised the manuscript.
Supplementary Material
References
- 1.Karesh WB, Dobson A, Lloyd-Smith JO, et al. Ecology of zoonoses: natural and unnatural histories. Lancet. 2012;380:1936–1945. doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White RJ, Razgour O. Emerging zoonotic diseases originating in mammals: a systematic review of effects of anthropogenic land-use change. Mammal Rev. 2020;50:336–352. doi: 10.1111/mam.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones BA, Grace D, Kock R, et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc Natl Acad Sci USA. 2013;110:8399–8404. doi: 10.1073/pnas.1208059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faust CL, McCallum HI, Bloomfield LSP, et al. Pathogen spillover during land conversion. Ecol Lett. 2018;21:471–483. doi: 10.1111/ele.12904. [DOI] [PubMed] [Google Scholar]
- 7.IPBES Global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. 2019. https://ipbes.net/news/ipbes-global-assessment-summary-policymakers-pdf
- 8.Cohen-Shacham E, Walters G, Janzen C, Maginnis S. Nature-based solutions to address global societal challenges. 2016. [DOI]
- 9.Whitmee S, Haines A, Beyrer C, et al. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation-Lancet Commission on planetary health. Lancet. 2015;386:1973–2028. doi: 10.1016/S0140-6736(15)60901-1. [DOI] [PubMed] [Google Scholar]
- 10.UN Environment Global environment outlook. 2019. http://www.unenvironment.org/resources/global-environment-outlook-6
- 11.Berthe FCJ, Bouley T, Karesh WB, et al. Operational framework for strengthening human, animal and environmental public health systems at their interface. 2018. http://documents.worldbank.org/curated/en/703711517234402168/Operational-framework-for-strengthening-human-animal-and-environmental-public-health-systems-at-their-interface
- 12.UN Transforming our world: the 2030 agenda for sustainable development. 2015. https://sdgs.un.org/2030agenda
- 13.Dye C. Expanded health systems for sustainable development. Science. 2018;359:1337–1339. doi: 10.1126/science.aaq1081. [DOI] [PubMed] [Google Scholar]
- 14.Naidoo R, Fisher B. Reset Sustainable Development Goals for a pandemic world. Nature. 2020;583:198–201. doi: 10.1038/d41586-020-01999-x. [DOI] [PubMed] [Google Scholar]
- 15.WHO WHO manifesto for a healthy recovery from COVID-19. 2020. https://www.who.int/news-room/feature-stories/detail/who-manifesto-for-a-healthy-recovery-from-covid-19
- 16.IPBES Workshop report on biodiversity and pandemics of the Intergovernmental Platform on Biodiversity and Ecosystem Services (IPBES) 2020. https://zenodo.org/record/4158500#.YrcjGHbMI2x
- 17.Roche B, Garchitorena A, Guégan JF, et al. Was the COVID-19 pandemic avoidable? A call for a “solution-oriented” approach in pathogen evolutionary ecology to prevent future outbreaks. Ecol Lett. 2020;23:1557–1560. doi: 10.1111/ele.13586. [DOI] [PubMed] [Google Scholar]
- 18.Drexler N, Miller M, Gerding J, et al. Community-based control of the brown dog tick in a region with high rates of Rocky Mountain spotted fever, 2012-2013. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roche B, Lebarbenchon C, Gauthier-Clerc M, et al. Water-borne transmission drives avian influenza dynamics in wild birds: the case of the 2005-2006 epidemics in the Camargue area. Infect Genet Evol. 2009;9:800–805. doi: 10.1016/j.meegid.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Ostrom E. A diagnostic approach for going beyond panaceas. Proc Natl Acad Sci USA. 2007;104:15181–15187. doi: 10.1073/pnas.0702288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pooley S, Fa JE, Nasi R. No conservation silver lining to Ebola. Conserv Biol. 2015;29:965–967. doi: 10.1111/cobi.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hopkins SR, Sokolow SH, Buck JC, et al. How to identify win–win interventions that benefit human health and conservation. Nat Sustain. 2020;4:298–304. [Google Scholar]
- 23.Kilpatrick AM, Salkeld DJ, Titcomb G, Hahn MB. Conservation of biodiversity as a strategy for improving human health and well-being. Philos Trans R Soc Lond B Biol Sci. 2017;372 doi: 10.1098/rstb.2016.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKinnon MC, Cheng SH, Garside R, Masuda YJ, Miller DC. Sustainability: map the evidence. Nature. 2015;528:185–187. doi: 10.1038/528185a. [DOI] [PubMed] [Google Scholar]
- 25.McKinnon MC, Cheng SH, Dupre S, et al. What are the effects of nature conservation on human well-being? A systematic map of empirical evidence from developing countries. Environ Evid. 2016;5:8. [Google Scholar]
- 26.Shackelford GE, Kelsey R, Sutherland WJ, et al. Evidence synthesis as the basis for decision analysis: a method of selecting the best agricultural practices for multiple ecosystem services. Front Sustain Food Syst. 2019;3:83. [Google Scholar]
- 27.Sutherland WJ, Wordley CFR. A fresh approach to evidence synthesis. Nature. 2018;558:364–366. doi: 10.1038/d41586-018-05472-8. [DOI] [PubMed] [Google Scholar]
- 28.Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 29.Cheng SH, Augustin C, Bethel A, et al. Using machine learning to advance synthesis and use of conservation and environmental evidence. Conserv Biol. 2018;32:762–764. doi: 10.1111/cobi.13117. [DOI] [PubMed] [Google Scholar]
- 30.WHO Global health estimates 2016: disease burden by cause, age, sex, by country and by region, 2000–2016. 2018. https://www.who.int/data/global-health-estimates
- 31.IUCN Threats classification scheme (version 3.2) 2018. https://www.iucnredlist.org/resources/threat-classification-scheme
- 32.Streicker DG, Recuenco S, Valderrama W, et al. Ecological and anthropogenic drivers of rabies exposure in vampire bats: implications for transmission and control. Proc Biol Sci. 2012;279:3384–3392. doi: 10.1098/rspb.2012.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Syampungani S, Chirwa PW, Akinnifesi FK, Sileshi G, Ajayi OC. The miombo woodlands at the cross roads: potential threats, sustainable livelihoods, policy gaps and challenges. Nat Resour Forum. 2009;33:150–159. [Google Scholar]
- 34.Zinsstag J, Schelling E, Waltner-Toews D, Tanner M. From “one medicine” to “one health” and systemic approaches to health and well-being. Prev Vet Med. 2011;101:148–156. doi: 10.1016/j.prevetmed.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiorella KJ, Milner EM, Salmen CR, et al. Human health alters the sustainability of fishing practices in east Africa. Proc Natl Acad Sci USA. 2017;114:4171–4176. doi: 10.1073/pnas.1613260114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maxwell SL, Fuller RA, Brooks TM, Watson JEM. Biodiversity: the ravages of guns, nets and bulldozers. Nature. 2016;536:143–145. doi: 10.1038/536143a. [DOI] [PubMed] [Google Scholar]
- 37.Plowright RK, Parrish CR, McCallum H, et al. Pathways to zoonotic spillover. Nat Rev Microbiol. 2017;15:502–510. doi: 10.1038/nrmicro.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loh EH, Zambrana-Torrelio C, Olival KJ, et al. Targeting transmission pathways for emerging zoonotic disease surveillance and control. Vector Borne Zoonotic Dis. 2015;15:432–437. doi: 10.1089/vbz.2013.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbott RC, Osorio JE, Bunck CM, Rocke TE. Sylvatic plague vaccine: a new tool for conservation of threatened and endangered species? EcoHealth. 2012;9:243–250. doi: 10.1007/s10393-012-0783-5. [DOI] [PubMed] [Google Scholar]
- 40.Guerra CA, Snow RW, Hay SI. A global assessment of closed forests, deforestation and malaria risk. Ann Trop Med Parasitol. 2006;100:189–204. doi: 10.1179/136485906X91512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tucker Lima JM, Vittor A, Rifai S, Valle D. Does deforestation promote or inhibit malaria transmission in the Amazon? A systematic literature review and critical appraisal of current evidence. Philos Trans R Soc Lond B Biol Sci. 2017;372 doi: 10.1098/rstb.2016.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sokolow SH, Nova N, Pepin KM, et al. Ecological interventions to prevent and manage zoonotic pathogen spillover. Philos Trans R Soc Lond B Biol Sci. 2019;374 doi: 10.1098/rstb.2018.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seddon N, Smith A, Smith P, et al. Getting the message right on nature-based solutions to climate change. Global Change Biology. 2021;27:1518–1546. doi: 10.1111/gcb.15513. [DOI] [PubMed] [Google Scholar]
- 44.Allan BF, Dutra HP, Goessling LS, et al. Invasive honeysuckle eradication reduces tick-borne disease risk by altering host dynamics. Proc Natl Acad Sci USA. 2010;107:18523–18527. doi: 10.1073/pnas.1008362107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sokolow SH, Huttinger E, Jouanard N, et al. Reduced transmission of human schistosomiasis after restoration of a native river prawn that preys on the snail intermediate host. Proc Natl Acad Sci USA. 2015;112:9650–9655. doi: 10.1073/pnas.1502651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Martis G, Mulas B, Malavasi V, Marignani M. Can artificial ecosystems enhance local biodiversity? The case of a constructed wetland in a Mediterranean urban context. Environ Manage. 2016;57:1088–1097. doi: 10.1007/s00267-016-0668-4. [DOI] [PubMed] [Google Scholar]
- 47.Yavinsky R, Lamere C, Patterson K, Bremner J. The impact of population, health, and environment projects. 2015. https://pdf.usaid.gov/pdf_docs/PA00MGJP.pdf
- 48.Webb K. Planetary health in the tropics: how community health-care doubles as a conservation tool. Lancet Glob Health. 2018;6(suppl 2):28. [Google Scholar]
- 49.Jones IJ, MacDonald AJ, Hopkins SR, et al. Improving rural health care reduces illegal logging and conserves carbon in a tropical forest. Proc Natl Acad Sci USA. 2020;117:28515–28524. doi: 10.1073/pnas.2009240117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D'Agnes L, D'Agnes H, Schwartz JB, Amarillo ML, Castro J. Integrated management of coastal resources and human health yields added value: a comparative study in Palawan (Philippines) Environ Conserv. 2010;37:398–409. [Google Scholar]
- 51.Harris A, Mohan V, Flanagan M, Hill R. Integrating family planning service provision into community-based marine conservation. Oryx. 2012;46:179–186. [Google Scholar]
- 52.Carney RM, Husted S, Jean C, Glaser CA, Kramer VL. Efficacy of aerial spraying of mosquito adulticide in reducing incidence of West Nile virus, California, 2005. Emerg Infect Dis. 2008;14:747–754. doi: 10.3201/eid1405.071347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wheeler S, Barker CM, Fang Y, et al. Differential impact of West Nile virus on California birds. Condor. 2009;111:1–20. doi: 10.1525/cond.2009.080013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D'Odorico P, Davis KF, Rosa L, et al. The global food-energy-water nexus. Rev Geophys. 2018;56:456–531. [Google Scholar]
- 55.Fayer R, Dubey JP, Lindsay DS. Zoonotic protozoa: from land to sea. Trends Parasitol. 2004;20:531–536. doi: 10.1016/j.pt.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Cabello FC, Godfrey HP, Tomova A, et al. Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial resistance and to animal and human health. Environ Microbiol. 2013;15:1917–1942. doi: 10.1111/1462-2920.12134. [DOI] [PubMed] [Google Scholar]
- 57.Galloway JN, Townsend AR, Erisman JW, et al. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science. 2008;320:889–892. doi: 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- 58.Hess-Erga O-K, Moreno-Andrés J, Enger Ø, Vadstein O. Microorganisms in ballast water: disinfection, community dynamics, and implications for management. Sci Total Environ. 2019;657:704–716. doi: 10.1016/j.scitotenv.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Lu H, McComas KA, Buttke DE, Roh S, Wild MA. A one health message about bats increases intentions to follow public health guidance on bat rabies. PLoS One. 2016;11 doi: 10.1371/journal.pone.0156205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khan SU, Gurley ES, Hossain MJ, Nahar N, Sharker MA, Luby SP. A randomized controlled trial of interventions to impede date palm sap contamination by bats to prevent Nipah virus transmission in Bangladesh. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nahar N, Paul RC, Sultana R, et al. A controlled trial to reduce the risk of human Nipah virus exposure in Bangladesh. EcoHealth. 2017;14:501–517. doi: 10.1007/s10393-017-1267-4. [DOI] [PubMed] [Google Scholar]
- 62.Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M, Bruce N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ. 2008;86:390–398C. doi: 10.2471/BLT.07.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azevedo-Santos VM, Vitule JRS, Pelicice FM, García-Berthou E, Simberloff D. Nonnative fish to control Aedes mosquitoes: a controversial, harmful tool. Bioscience. 2017;67:84–90. [Google Scholar]
- 64.Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 65.Salkeld DJ, Padgett KA, Jones JH. A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol Lett. 2013;16:679–686. doi: 10.1111/ele.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Masuzawa T, Okamoto Y, Une Y, et al. Leptospirosis in squirrels imported from United States to Japan. Emerg Infect Dis. 2006;12:1153–1155. doi: 10.3201/eid1207.060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith KM, Zambrana-Torrelio C, White A, et al. Summarizing US wildlife trade with an eye toward assessing the risk of infectious disease introduction. EcoHealth. 2017;14:29–39. doi: 10.1007/s10393-017-1211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martel A, Blooi M, Adriaensen C, et al. Wildlife disease. Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science. 2014;346:630–631. doi: 10.1126/science.1258268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJ, Collen B. Defaunation in the Anthropocene. Science. 2014;345:401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 70.Bonwitt J, Dawson M, Kandeh M, et al. Unintended consequences of the ‘bushmeat ban’ in west Africa during the 2013–2016 Ebola virus disease epidemic. Soc Sci Med. 2018;200:166–173. doi: 10.1016/j.socscimed.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 71.Golden CD, Fernald LCH, Brashares JS, Rasolofoniaina BJR, Kremen C. Benefits of wildlife consumption to child nutrition in a biodiversity hotspot. Proc Natl Acad Sci USA. 2011;108:19653–19656. doi: 10.1073/pnas.1112586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brashares JS, Arcese P, Sam MK, Coppolillo PB, Sinclair AR, Balmford A. Bushmeat hunting, wildlife declines, and fish supply in west Africa. Science. 2004;306:1180–1183. doi: 10.1126/science.1102425. [DOI] [PubMed] [Google Scholar]
- 73.Eskew EA, Carlson CJ. Overselling wildlife trade bans will not bolster conservation or pandemic preparedness. Lancet Planet Health. 2020;4:e215–e216. doi: 10.1016/S2542-5196(20)30123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seytre B. Erroneous communication messages on COVID-19 in Africa. Am J Trop Med Hyg. 2020;103:587–589. doi: 10.4269/ajtmh.20-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang L, Yin F. Wildlife consumption and conservation awareness in China: a long way to go. Biodivers Conserv. 2014;23:2371–2381. doi: 10.1007/s10531-008-9358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walzer C. COVID-19 and the curse of piecemeal perspectives. Front Vet Sci. 2020;7 doi: 10.3389/fvets.2020.582983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Markandya A, Taylor T, Longo A, Murty MN, Murty S, Dhavala K. Counting the cost of vulture decline—an appraisal of the human health and other benefits of vultures in India. Ecol Econ. 2008;67:194–204. [Google Scholar]
- 78.Martin J-L, Chamaillé-Jammes S, Waller DM. Deer, wolves, and people: costs, benefits and challenges of living together. Biol Rev Camb Philos Soc. 2020;95:782–801. doi: 10.1111/brv.12587. [DOI] [PubMed] [Google Scholar]
- 79.Organisation for Economic Co-operation and Development Addressing societal challenges using transdisciplinary research. 2020. https://www.oecd-ilibrary.org/science-and-technology/addressing-societal-challenges-using-transdisciplinary-research_0ca0ca45-en
- 80.Muñoz-Pedreros A, Gil C, Yáñez J, Rau JR. Raptor habitat management and its implication on the biological control of the Hantavirus. Eur J Wildl Res. 2010;56:703–715. [Google Scholar]
- 81.Tallis H, Kreis K, Olander L, et al. Aligning evidence generation and use across health, development, and environment. Curr Opin Environ Sustain. 2019;39:81–93. [Google Scholar]
- 82.Cleaveland S, Kaare M, Tiringa P, Mlengeya T, Barrat J. A dog rabies vaccination campaign in rural Africa: impact on the incidence of dog rabies and human dog-bite injuries. Vaccine. 2003;21:1965–1973. doi: 10.1016/s0264-410x(02)00778-8. [DOI] [PubMed] [Google Scholar]
- 83.Randall DA, Marino J, Haydon DT, et al. An integrated disease management strategy for the control of rabies in Ethiopian wolves. Biol Conserv. 2006;131:151–162. [Google Scholar]
- 84.Keitt BS, Tershy BR. Cat eradication significantly decreases shearwater mortality. Anim Conserv. 2003;6:307–308. [Google Scholar]
- 85.Pender RJ, Shiels AB, Bialic-Murphy L, Mosher SM. Large-scale rodent control reduces pre- and post-dispersal seed predation of the endangered Hawaiian lobeliad, Cyanea superba subsp. superba (Campanulaceae) Biol Invasions. 2013;15:213–223. [Google Scholar]
- 86.Tait P, Saunders C, Nugent G, Rutherford P. Valuing conservation benefits of disease control in wildlife: a choice experiment approach to bovine tuberculosis management in New Zealand's native forests. J Environ Manage. 2017;189:142–149. doi: 10.1016/j.jenvman.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 87.de Wit LA, Croll DA, Tershy B, et al. Potential public health benefits from cat eradications on islands. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barnett SA. Rat control in a plague outbreak in Malta. J Hyg (Lond) 1948;46:10–18. doi: 10.1017/s0022172400036019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daigneault AJ, Eppink FV, Lee WG. A national riparian restoration programme in New Zealand: is it value for money? J Environ Manage. 2017;187:166–177. doi: 10.1016/j.jenvman.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 90.Chazdon RL. Beyond deforestation: restoring forests and ecosystem services on degraded lands. Science. 2008;320:1458–1460. doi: 10.1126/science.1155365. [DOI] [PubMed] [Google Scholar]
- 91.Herrera D, Ellis A, Fisher B, et al. Upstream watershed condition predicts rural children's health across 35 developing countries. Nat Commun. 2017;8:811. doi: 10.1038/s41467-017-00775-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Braczkowski AR, O'Bryan CJ, Stringer MJ, Watson JEM, Possingham HP, Beyer HL. Leopards provide public health benefits in Mumbai, India. Front Ecol Environ. 2018;16:176–182. [Google Scholar]
- 93.Butler JRA, du Toit JT, Bingham J, et al. Free-ranging domestic dogs (Canis familiaris) as predators and prey in rural Zimbabwe: threats of competition and disease to large wild carnivores. Biol Conserv. 2004;115:369–378. [Google Scholar]
- 94.Morters MK, Restif O, Hampson K, Cleaveland S, Wood JL, Conlan AJ. Evidence-based control of canine rabies: a critical review of population density reduction. J Anim Ecol. 2013;82:6–14. doi: 10.1111/j.1365-2656.2012.02033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kibret S, Wilson GG, Ryder D, Tekie H, Petros B. Can water-level management reduce malaria mosquito abundance around large dams in sub-Saharan Africa? PLoS One. 2018;13 doi: 10.1371/journal.pone.0196064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kiernan JD, Moyle PB, Crain PK. Restoring native fish assemblages to a regulated California stream using the natural flow regime concept. Ecol Appl. 2012;22:1472–1482. doi: 10.1890/11-0480.1. [DOI] [PubMed] [Google Scholar]
- 97.Hoyer IJ, Blosser EM, Acevedo C, Thompson AC, Reeves LE, Burkett-Cadena ND. Mammal decline, linked to invasive Burmese python, shifts host use of vector mosquito towards reservoir hosts of a zoonotic disease. Biol Lett. 2017;13 doi: 10.1098/rsbl.2017.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Westgate MJ, Likens GE, Lindenmayer DB. Adaptive management of biological systems: a review. Biol Conserv. 2013;158:128–139. [Google Scholar]
- 99.The Mountain Gorilla Veterinary Project 2002 Employee Health Group Risk of disease transmission between conservation personnel and the mountain gorillas: results from an employee health program in Rwanda. EcoHealth. 2004;1:351–361. [Google Scholar]
- 100.Gilardi KVK, Gillespie TR, Leendertz FH, et al. Best practice guidelines for health monitoring and disease control in great ape populations. 2015. [DOI]
- 101.Nature Time to revise the Sustainable Development Goals. Nature. 2020;583:331–332. doi: 10.1038/d41586-020-02002-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The 46 evidence summaries are publicly available in a searchable database.