Abstract
Chimpanzees were used in hepatitis research for over three decades with the aim to identify and develop treatments for the virus, a leading cause of chronic liver disease in humans. We used a dataset of 120 chimpanzees housed at a single institution in Japan, 22 of whom became chronically infected with hepatitis C virus (HCV), to examine whether HCV infection results in a reduced lifespan as reported in humans. Survival analysis showed that HCV carriers experienced a higher mortality risk compared with non-carriers. Although no chimpanzee died from hepatic disease, carriers showed higher gamma-glutamyl transpeptidase (γGTP) levels compared with non-carriers suggesting that HCV infection negatively affected their liver condition. These results provide evidence that special attention is necessary to monitor the long-term condition of ex-biomedical primates.
Keywords: hepatitis, biomedical research, chimpanzee, longevity, mortality, lifespan
1. Background
The hepatitis C virus (HCV) is a leading cause of chronic liver disease, cirrhosis and hepatocellular carcinoma (HCC) affecting an estimated 56 million people, nearly 1% of the world population [1]. Owing to the difficulty of detecting the virus at an acute stage, the pursuit to understand its progression and develop a vaccine and eradication strategy, as well as ethical issues involving human test subjects, scientists turned to an animal model to advance our knowledge about this widespread disease. Since the late 1970s, more than three decades of invasive experiments have been conducted on hundreds of chimpanzees in HCV research [2]. Despite chimpanzees being the closest living relatives of humans, various significant biological inconsistencies in HCV infection and pathology between the two species were discovered, in part due to key differences in gene expression, which contributed to the end of their use as an animal model [2,3]. Nevertheless, research using chimpanzees has contributed to the development of HCV curative therapies [4].
Whereas persistent HCV infection may be as high as 85% in humans, less than half (30–40%) of chimpanzees progress to chronic HCV due to greater viral clearance [2,5]. Additionally, HCV positive chimpanzees show much milder symptoms than infected humans and their pathologies differ in that they do not develop liver fibrosis and cirrhosis as humans do [2]. Only four cases of HCC (one case reported in [6], three cases reported twice in [7,8]) and one case of moderate to severe hepatopathy with pauci-immune rapidly progressive glomerulonephritis [9] have been reported in HCV-infected chimpanzees. No cases of fibrosis have been detected in HCV-infected chimpanzees thus far [6–9]. Various scientific, ethical and practical issues of using these primates in invasive biomedical research exists including the redundancy of their use, their capacity to suffer and experience physical and emotional trauma, as well as the low availability and high cost of maintaining the species in captivity [2,10]. Additionally, direct-acting antiviral agents (DAAs) are now so effective that humans could be used in HCV challenge experiments as a possible route to vaccine development [11].
Biomedical research in Japan began in 1974 and peaked in 1995 when around 139 chimpanzees were being used in experiments, although not all for HCV studies. More than 150 chimpanzees were imported from Africa and used in research across eight institutions. After the Convention of the International Trade in Endangered Species of Wild Fauna and Flora (CITES) was ratified in 1980, importation became illegal except for a brief period in cases where exporting and importing countries reached an agreement. In total, 205 individuals have been biomedical subjects in Japan [12]. In comparison, in 2010 approximately 1000 chimpanzees were in laboratories across the USA, which ended invasive biomedical research using them in 2015 [2,13,14]. Unlike in the USA, invasive biomedical research using chimpanzees in Japan was stopped in 2006 as a result of activism by researchers and the public, and was not the result of a government act. In 2012, the last three individuals being held at a private medical company in Japan were moved to Kumamoto Sanctuary, marking the end of chimpanzees being held at biomedical institutions in Japan [12].
Despite the numerous clinical studies published from the 1980s through the 2000s [2], no follow-up study investigating the long-term effects of HCV infection on the longevity of chimpanzees exists. It is known that chronic HCV infection reduces the lifespan of humans [15,16], but the relationship between HCV infection status and life expectancy in chimpanzees has not yet been examined. This lack of reporting may be due to long-term data being inaccessible by researchers (i.e. held privately by ex-biomedical or no longer existing institutions) or the typically small sample size of past clinical trials. To investigate this, we used long-term data collected on chimpanzees used in biomedical research in Japan to examine whether positive HCV infection has an effect on their lifespan, cause of death and/or liver condition.
2. Results
We compiled data on 120 individuals (electronic supplementary material, table S1) from records spanning 1978–2021 (electronic supplementary material, Materials and methods). We focused on a single facility to control for environmental factors as much as possible because pooling individual data from different facilities with varying climates, diets, level of veterinary care and other factors may influence survival rates. Of the 120 chimpanzees included in this study, 64 individuals were experimentally injected with a hepatitis virus and 56 individuals never received an injection (see electronic supplementary material, Materials and methods and table S1). In total, 22 chimpanzees became chronically infected with HCV (i.e. carriers) and 98 were not infected (i.e. non-carriers; table 1). Of the HCV carrier individuals, mean age at infection was 7.1 years (electronic supplementary material, table S2). Mean age at death of carriers was 34.0 years (mean survival duration after infection was 27.4 years), and the mean age of carriers who are still alive was 41.2 years (mean ongoing survival duration after infection is 33.3 years; electronic supplementary material, table S2).
Table 1.
Summary of chimpanzees included in our dataset.
| alive | dead | total | males (dead) | females (dead) | majority cause of death | |
|---|---|---|---|---|---|---|
| non-carrier | 87 | 11 | 98 | 8 | 3 | cardiac disease (8) |
| carrier | 8 | 14 | 22 | 7 | 7 | chronic glomerulonephritis (4) |
| total (non-carrier + carrier) | 95 | 25 | 120 | 15 | 10 |
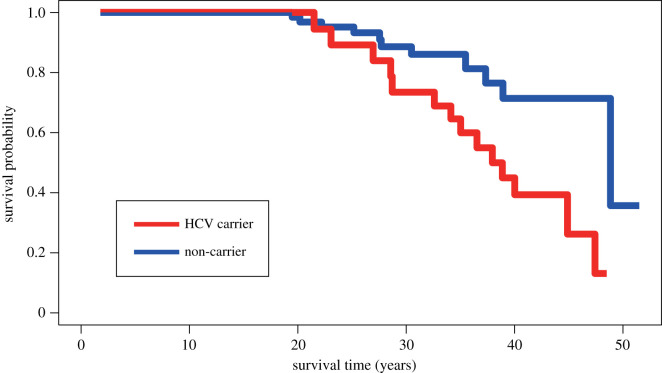
Using Cox proportional hazards regression analysis, we found that the survival patterns of chimpanzees differed by HCV condition (carrier or non-carrier; electronic supplementary material, tables S3–S11). HCV carriers experienced a higher mortality risk over their lifespan compared with non-carriers (figure 1; coefficient = 1.09, z = 2.99, p = 0.009). At age 40, carriers showed survivorship levels of 38.5–45.0% (45.0% at 40.00 years which dropped to 39.3% at 40.02 years), whereas non-carriers experienced a 71.4% survival chance. Median survival time (age at death) was 38.0 years for carriers and 48.9 years for non-carriers; however, the sample size was too small to meaningfully predict survival time. Only one non-carrier (Lennon) survived beyond 48.9 years and many individuals in their 40's were still alive at the time of this study.
Figure 1.
Survival curves of HCV carrier and non-carrier chimpanzees.
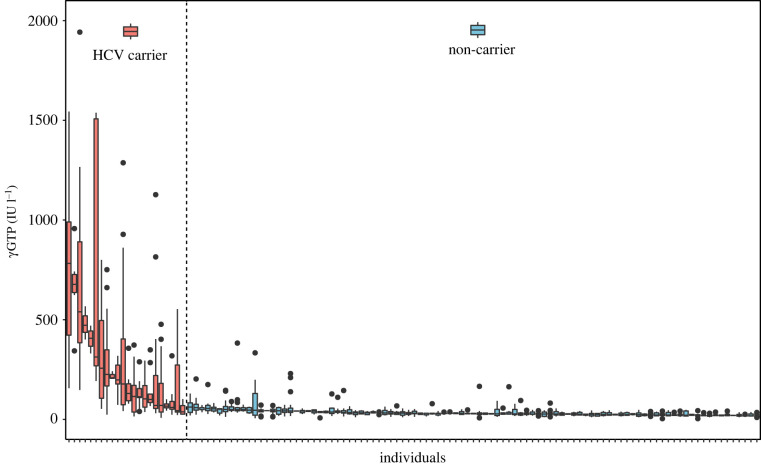
Of the 120 chimpanzees included in our dataset, 25 had died as of 1 January 2022 (table 1; see electronic supplementary material, table S12 for detailed causes of death). Eleven of these deaths were non-carrier individuals and 14 were HCV carriers. Four of 14 carrier deaths were caused by renal (i.e. kidney) disease, whereas no non-carriers died from renal disease (electronic supplementary material, figures S1–S3 and tables S13–S14); the specific cause of death was chronic glomerulonephritis. Eight non-carriers and three carriers died of cardiac disease; one carrier showed significant fibrosis resembling cirrhosis. No individuals died of hepatic disease. Three non-carrier and seven carrier chimpanzees died of other causes of death. For carriers, this included haemorrhagic death due to upper gastrointestinal bleeding (n = 1, liver fibrosis detected), sepsis from Yersinia infection (n = 1), brain haemorrhage (n = 1), tension pneumothorax (n = 1), squamous cell carcinoma (n = 1), strangulation ileus (n = 1) and cerebellar infarction (n = 1). For non-carriers, this included death due to aortic rupture and aneurysm (n = 1), pneumonia (n = 1) and tension pneumothorax (n = 1). Carriers showed higher γGTP levels than non-carriers at the group level (figure 2; LRT: χ2 = 71.6, d.f. = 1, p < 0.001), providing some evidence that HCV infection affects the liver of chimpanzees although it was not the main cause of death. Three of eight currently alive carrier individuals (as of 1 January 2022) showed bleeding tendencies with elevated PIVK-II levels in their blood indicating liver malfunction (electronic supplementary material, figures S4–S7, Supplementary results). Vitamin K is regularly administered to these three individuals, but their condition has not improved.
Figure 2.
γGTP values (IU l–1) for individual chimpanzees (HCV carriers in red and non-carriers in blue). Boxplot shows the median, interquartile-range and outliers.
3. Discussion/conclusion
Chronic HCV infection in humans often progresses to chronic liver disease, including cirrhosis and HCC, and results in a shortened life expectancy [1,15–17]. Here, we showed that reflecting findings from human studies, chimpanzees who were chronically infected with HCV experienced a reduced lifespan compared with non-infected individuals. No cause of death among the carrier chimpanzees in this study was determined to be hepatic (i.e. liver) disease. However, the majority of carriers showed consistently higher γGTP levels than non-carriers, suggesting that HCV infection affects the liver of chimpanzees to a certain extent without being the main cause of death. Three HCV carriers at the Texas Biomedical Research Institute (TBRI) in the USA were diagnosed with HCC, suggesting that infection has a negative long-term effect on the liver [7]. Additionally, four of the 14 HCV carriers in this study died from renal (i.e. kidney) disease. Renal pathology is not uncommon among ageing chimpanzees and was identified in 59 of 191 individuals examined at TBRI [9]; however, as no non-carriers died from renal disease in this study, it suggests that HCV infection may have negatively influenced kidney function. Future studies with a larger number of individuals are necessary to expose whether or not a link between chronic HCV infection and renal disease exists.
Chimpanzees who become HCV carriers do not die immediately after infection, but years later after a slow progression of disease. Hepatitis C in humans has become a nearly curable disease due to medical progress [18], but no cure for HCV-infected chimpanzees has yet been established. Carrier chimpanzees at Kumamoto Sanctuary receive special care including continuous drug administration to suppress infection side-effects and reduce disease risk; however, the condition of some individuals is not improving. The long-term effects of HCV infection in chimpanzees remain poorly understood, despite hundreds of individuals having been used as animal models in biomedical research around the world for decades. We have shown that positive HCV infection has a negative influence on the long-term health of chimpanzees, including their liver condition as well as a shortened lifespan, and thus we provide evidence that special attention is required to monitor the lifelong condition of these ex-biomedical primates currently housed at various facilities including sanctuaries and zoos around the world. This might include examining the liver and kidney condition of HCV carriers on a more regular basis than non-carriers and attempting treatment using recently developed DAAs, which are currently too financially costly to widely administer, or other therapies. As biomedical research using chimpanzees has already come to an end, the next crucial step will be to increase international collaboration and information exchange to determine how to provide the best care to these retired biomedical chimpanzees.
4. Methods
(a) . Subjects and housing facility
We used long-term individual data from a facility founded in 1978 in Kyushu, Japan by a pharmaceutical company named Sanwa Kagaku Kenkyusho Co. Ltd, which was renamed Chimpanzee Sanctuary Uto in 2007 and later became Kumamoto Sanctuary in 2011 (electronic supplementary material, table S1). As institutions including zoos and research centres across Japan have varying husbandry and veterinary practices which could affect the mortality and longevity rates of animals, we focused on this single facility (henceforth, Sanwa-CSU-KS).
(b) . Data collation
Carrier status of HCV was confirmed by the presence of HCV-RNA in the blood. First, a HCV-antibody test was performed. If an individual showed abnormal levels, they were further tested to confirm whether their blood contained HCV-RNA. Individuals with a negative HCV-antibody result were considered non-carriers.
Individuals were included in the life table and analyses only if their carrier or non-carrier status was known from their blood test. We excluded individuals who had no blood test result. We included only the period of time that each individual was housed at Sanwa-CSU-KS. For carrier individuals, the ‘start age’ is the age at which they were transferred to Sanwa-CSU-KS or the age they were infected (if they were born at Sanwa-CSU-KS). If an individual had multiple transfers into and out of Sanwa-CSU-KS, the start age was determined as the date that individual last entered Sanwa-CSU-KS and previous entries were neglected. All wild-born individuals (except for Remu) were injected with HCV somewhere other than Sanwa-CSU-KS (e.g. Tokyo University). Remu was infected with HCV accidentally after moving to Sanwa-CSU-KS when a contaminated needle was used at a physical check-up. Remu was also the youngest (start age) carrier chimpanzee, at 650 days old. It is not possible to compare the survival of carriers and non-carriers before 650 days of age because there was no carrier individual younger than this, thus the start age of non-carriers was determined as 650 days. In all cases, ‘end age’ is the age at which an individual either (i) died, for those who died at Sanwa-CSU-KS, (ii) moved out of Sanwa-CSU-KS (death after leaving Sanwa-CSU-KS is considered a lost to follow-up [LTF] case and is therefore neglected), or (iii) is 1 January 2022 for individuals who are still alive (see electronic supplementary material, Materials and methods).
(c) . Survival analysis
We conducted survival analyses [19] using the survival R package (R version 4.0.5; [20]) to describe the survival patterns of HCV carrier and non-carrier chimpanzees and test for differences between the two conditions. We used Cox proportional hazards regression to test whether survival curves differed by sex and HCV condition (carrier or non-carrier), and their interaction (see electronic supplementary material, Materials and methods).
(d) . Gamma-glutamyl transpeptidase
In order to evaluate the effect of HCV infection on hepatitis function, we selected γGTP as an index. Blood samples were collected at routine physical health checks conducted every 1–5 years depending on the condition of each individual. Data were not used when an individual was considered to be sick or unwell at the time of collection. We modelled the value of γGTP as a generalized linear mixed model (GLMM) using the gamma distribution and 1/μ link function. The predictor variable was the HCV condition (carrier versus non-carrier). We included individual ID as a random intercept. We used the ‘anova’ function in the car package [20] to compare this model to a random effect only model using a likelihood ratio test (LRT). All data and model script are provided in the electronic supplementary materials, Materials and methods, Supplementary results, Data S2 and Data S3.
Acknowledgements
We thank the caretakers and staff of Sanwa-CSU-KS. We would also like to thank Steve Ross, who passed away on 20 April 2022; our previous conversations and collaboration with him in part inspired this study.
Contributor Information
Satoshi Hirata, Email: hirata.satoshi.8z@kyoto-u.ac.jp.
Kristin Havercamp, Email: havercamp.kristinann.6r@kyoto-u.ac.jp.
Ethics
This study is an analysis of existing data on life-history and physical check-up results of ex-biomedical subject chimpanzees (in the past); it did not involve the direct care of chimpanzees, and we did not perform any experiments. This study (i.e. analyses of existing data) has been approved by the animal experimentation committee of the Wildlife Research Center of Kyoto University (no. WRC-2020-KS002A).
Data accessibility
Data are provided in the electronic supplementary material [21].
Authors' contributions
S.H.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft, writing—review and editing; K.H.: conceptualization, investigation, project administration, writing—original draft, writing—review and editing; Y.Y.: conceptualization, investigation, methodology, visualization, writing—review and editing; T.U.: conceptualization, data curation, investigation, project administration, supervision, validation, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This study was supported by Great Ape Information Network of National BioResource Project, Japan.
References
- 1.The Polaris Observatory HCV Collaborators. 2022. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol. Hepatol. 7, 396-415. ( 10.1016/S2468-1253(21)00472-6) [DOI] [PubMed] [Google Scholar]
- 2.Bailey J. 2010. An assessment of the use of chimpanzees in hepatitis C research past, present and future: 1. Validity of the chimpanzee model. Altern Lab Anim. 38, 387-418. ( 10.1177/026119291003800501) [DOI] [PubMed] [Google Scholar]
- 3.Bailey J. 2011. Lessons from chimpanzee-based research on human disease: the implications of genetic differences. Altern Lab Anim. 39, 527-540. ( 10.1177/026119291103900608) [DOI] [PubMed] [Google Scholar]
- 4.Lanford RE, Walker CM, Lemon SM. 2017. The chimpanzee model of viral hepatitis: advances in understanding the immune response and treatment of viral hepatitis. ILAR J. 58, 172-189. ( 10.1093/ilar/ilx028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houghton M, Abrignani S. 2005. Prospects for a vaccine against the hepatitis C virus. Nature 436, 961-966. ( 10.1038/nature04081) [DOI] [PubMed] [Google Scholar]
- 6.Muchmore E, Popper H, Peterson DA, Miller MF, Lieberman HM. 1988. Non-A, non-B hepatitis-related hepatocellular carcinoma in a chimpanzee. J. Med. Primatol. 17, 235-246. ( 10.1111/j.1600-0684.1988.tb00386.x) [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Laurence H, Owston MA, Sharp RM, Williams P, Lanford RE, Hubbard GB, Dick EJ Jr. 2017. Natural pathology of the captive chimpanzee (Pan troglodytes): a 35-year review. J. Med. Primatol. 46, 271-290. ( 10.1111/jmp.12277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurence H, Kumar S, Owston MA, Lanford RE, Hubbard GB, Dick EJ Jr. 2017. Natural mortality and cause of death analysis of the captive chimpanzee (Pan troglodytes): a 35-year review. J. Med. Primatol. 46, 106-115. ( 10.1111/jmp.12267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neidig LE, Owston MA, Ball E, Dick EJ Jr. 2016. Pauci-immune glomerulonephritis in a captive chimpanzee (Pan troglodytes), and a review of spontaneous cases in animals. J. Med. Primatol. 45, 336-341. ( 10.1111/jmp.12233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight A. 2007. The poor contribution of chimpanzee experiments to biomedical progress. J. Appl. Anim. Welf. Sci. 10, 281-308. ( 10.1080/10888700701555501) [DOI] [PubMed] [Google Scholar]
- 11.Liang TJ, Feld JJ, Cox AL, Rice CM. 2021. Controlled human infection model—fast track to HCV vaccine? N Engl. J. Med. 385, 1235-1240. ( 10.1056/NEJMsb2109093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirata S, Morimura N, Watanuki K, Ross SR. 2020. The establishment of sanctuaries for former laboratory chimpanzees: challenges, successes, and cross-cultural context. In Chimpanzees in context (eds Hopper LM, Ross SR), pp. 208-230. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 13.Kaiser J. 2015. An end to US chimp research. Science 350, 1013. ( 10.1126/science.350.6264.1013) [DOI] [PubMed] [Google Scholar]
- 14.Grimm D. 2017. Chimps in waiting. Science 356, 1114-1117. ( 10.1126/science.356.6343.1114) [DOI] [PubMed] [Google Scholar]
- 15.Alavi M, Law MG, Grebely J, Thein HH, Walter S, Amin J, Dore GJ. 2014. Lower life expectancy among people with an HCV notification: a population-based linkage study. J. Viral Hepat. 21, e10-e18. ( 10.1111/jvh.12245) [DOI] [PubMed] [Google Scholar]
- 16.van der Meer AJ, Wedemeyer H, Feld JJ, Dufour JF, Zeuzem S, Hansen BE, Janssen HL. 2014. Life expectancy in patients with chronic HCV infection and cirrhosis compared with a general population. JAMA 312, 1927-1928. ( 10.1001/jama.2014.12627) [DOI] [PubMed] [Google Scholar]
- 17.Saito I, et al. 1990. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc. Natl Acad. Sci. USA 87, 6547-6549. ( 10.1073/pnas.87.17.6547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estes JD, Wong SW, Brenchley JM. 2018. Nonhuman primate models of human viral infections. Nat. Rev. Immunol. 18, 390-404. ( 10.1038/s41577-018-0005-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleinbaum DG, Klein M. 2005. Competing risks survival analysis. In Survival analysis: a self-learning text (ed. Kleinbaum DG), pp. 391-461. New York, NY: Springer. [Google Scholar]
- 20.Fox J, Weisberg S. 2011. Multivariate linear models in R. In An R companion to applied regression (eds Fox J, Weisberg S), pp. 1-31. Thousand Oaks, CA: SAGE Publications. [Google Scholar]
- 21.Hirata S, Havercamp K, Yamanashi Y, Udono T. 2022. Hepatitis C virus infection reduces the lifespan of chimpanzees used in biomedical research. Figshare. ( 10.6084/m9.figshare.c.6125219) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are provided in the electronic supplementary material [21].