Abstract
Purpose: This study aimed to verify whether Adjuvant-Induced Arthritis (AIA) and/or Orchiectomy (ORX) modify the expression of the Nox1, Nox2 and Nox4 isoforms, the endothelial function or the structure of rat aortas. Methods: Sixty-three Wistar rats were distributed into four groups: 1) Control; 2) ORX; 3) AIA; 4) Orchiectomy plus to Arthritis-induction (ORX/AIA). Thus, 21 days after the onset of AIA (by intradermal injection of Mycobacterium tuberculosis), the presence of Nox1, Nox2 and Nox4, the acetylcholine (ACh)-induced relaxation and the media layer thickness were assessed in the aorta taken from these animals. Results: The Nox1, Nox2 and Nox4 were immunostained in intima, media and adventitia layers of aortas taken from all studied groups and AIA apparently increased this immunostaining. These modifications of Nox1, Nox2 or Nox4 expression, however, were not confirmed by Western blotting. In addition, neither AIA nor ORX changed the endothelial function, but ORX increased the media layer thickness in the studied aortas. Conclusion: The present study showed weak clues of increased expression of Nox1, Nox2 and Nox4 as a result of AIA, as well as of Nox1 reduction caused by ORX. In addition, the endothelial function was not modified in the aortas of these animals by both AIA and/or ORX. On the other hand, ORX increased significantly the aorta media layer thickness in the studied animals, which was apparently mitigated by AIA.
Keywords: adjuvant-induced arthritis, aorta, histomorphometry, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, orchiectomy, rat
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease, characterized by inflammatory manifestations in articular and periarticular structures. The manifestations of RA go beyond joints, possibly because pro-inflammatory mediators from the affected joints reach the systemic circulation, leading to extra-articular manifestations that have already been described in several organs and systems (1, 2).
People affected by RA are at higher risk of cardiovascular disease since pro-inflammatory mediators can lead to endothelial dysfunction (2, 3). It was also observed endothelial dysfunction in aorta taken from rats submitted to the adjuvant-induced arthritis experimental model (AIA), an experimental model that simulates RA. This process was associated with elevated oxidative stress due to increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase expression as well as the uncoupling of endothelial nitric oxide synthase (eNOS) (4, 5). Reduced acetylcholine (ACh)-induced vasodilation was also observed in the forearm of patients with RA, which indicate endothelial dysfunction. This hemodynamic change occurs in parallel with an increase in the activity of inducible nitric oxide synthase (iNOS) as well as an increase in circulating levels of myeloperoxidase (6). Increments of iNOS in the aorta as well as in renal and cardiac microcirculation, were also observed in DBA / 1J mice submitted to the collagen-induced arthritis (CIA) model (7). Vascular manifestations of arthritis may also be structural, as thickening of the intima and media layers of the carotid artery has been reported in patients with RA (8).
In parallel, reduced circulating testosterone levels have already been demonstrated both in patients with RA (9) and in experimental models of arthritis (10). Testosterone can have immunosuppressive effects and a reduction in the levels of this hormone may be related to the increased severity of autoimmune diseases (11, 12). The effects of testosterone on the cardiocirculatory system, however, are still controversial. In cavernous tissues of rats, testosterone deficiency-induced endothelial dysfunction as a consequence of increased oxidative stress was at least partially related to an increased presence of Nox1 and Nox4 (13). On the other hand, treating culture of vascular smooth muscle cells (VSMC) from mesenteric vascular bed with testosterone augmented oxidative stress, as well as the expression of Nox1, Nox4 and p47phox subunits. Testosterone also increased the migration of these VSMC, suggesting that this hormone may also modulate vascular remodeling (14). Is noteworthy, in this context, that orchiectomy incremented the thickness of the intima-media layer and total wall of rats’ abdominal aorta (15). In addition, treatment with testosterone suppressed the reversion following estrogen replacement of endothelial dysfunction and oxidative stress caused by ovariectomy in rats (16). This effect of testosterone may involve increased expression of the p47phox subunit.
The vascular manifestations of arthritis may differ in characteristics, intensity, as well as in terms of temporal evolution, depending on the studied vascular bed (17, 18). Nevertheless, the effects of arthritis on the endothelial function and on the expression pattern of NADPH oxidase isoforms, as well as on the structure of the aorta, are not yet fully established. There is also little data about the influence of testosterone on the possible manifestations of arthritis on this vascular bed. These gaps deserve to be further investigated as the aorta is an important conductance vessel. Furthermore, in experimental models, the aorta is an artery that is widely used to study endothelial function.
Thus, this study aimed to verify whether AIA and/or orchiectomy (ORX) modify the expression of the Nox1, Nox2 and Nox4 isoforms, the endothelial function, or the structure of rat aortas.
Methods
Animals
Sixty-three Wistar rats were housed in plastic cages (four animals per cage) under controlled temperature (22 ± 2°C) in a 12-h light/dark cycle, with free access to lab chow and filtered water. These animals were distributed in four groups: 1) Control (CTRL): animals that were not submitted to arthritis induction procedure; 2) ORX: animals submitted to orchiectomy but not submitted to arthritis induction procedure; 3) AIA: animals submitted to arthritis induction procedure but not submitted to ORX (SHAM); 4) Orchiectomy in addition to adjuvant-induced-arthritis (ORX+AIA): animals submitted to both ORX and arthritis induction procedure.
Orchiectomy
Under anesthesia with 2,2,2-tribromoethanol (250 mg/kg of body weight, i.p., Sigma-Aldrich, St Louis, MO, USA), both testes were surgically removed through a longitudinal incision made in the scrotum. SHAM animals belonging to Control and AIA groups were submitted to the same surgical procedures, but the testes were preserved. The animals were orchiectomized at 12 weeks of age, since as plasma testosterone levels are considered full around this age (19). Orchiectomy’s effectiveness was confirmed by reductions of the wet weight of testosterone-dependent sexual accessory organs (seminal vesicle and prostate) and circulating testosterone level.
Adjuvant-induced arthritis
Twenty days after the orchiectomy, animals from both AIA and ORX+AIA groups were submitted to intradermal injection of 100 µl emulsion of mineral oil-distilled water (3:1) containing 3.8 mg/ml heat-inactivated Mycobacterium tuberculosis (Difco, Detroit, MI, USA), in the right hind paw, under anesthesia with 2,2,2-tribromoethanol (250 mg/kg, of body weight, i.p.). Animals belonging to Control and ORX groups received mineral oil. The earliest signs of AIA in the contralateral hind paw, characterized by mild erythema and paws’ diameter and weight, were usually observed around 15 days post-immunization. The animals that showed no inflammatory signs on the contralateral paw up to 20 days post-immunization were excluded from the study.
Blood and tissue harvest
Twenty-one days after the onset of AIA in the paw contralateral to the induction, which normally occurred about 15 days after AIA induction, rats were killed by exsanguination under anesthesia with thiopental (Thiopentax®; 10 mg/100 g of body weight, i.p.). Blood samples were collected via inferior cava vena puncture with BD Vacutainer® blood collection tubes (Ref 367820). These harvested blood samples were then centrifuged for 10 min at 4°C (3,500 rpm) to obtain serum, which was stored in a freezer at −80°C until the testosterone determinations. In addition, seminal vesicle and prostate were harvested and weighted.
Serum testosterone
Blood was collected from the vena cava, transferred to BD Vacutainer® tubes, and centrifuged at 3,500 rpm for 10 min at 4°C. Supernatants were recovered and stored at −80°C until the determination of serum testosterone levels. The concentration of testosterone was determined in the serum by chemiluminescence method—Atellica IM Testosterone II (TSTII) Analyzer, Siemens Healthineers, Erlangen, Germany with 7–1,500 ng/dl as the limit of detection. Serum testosterone level was expressed in ng/dl.
Immunohistochemistry
Segments of aortas were fixed in 4% paraformaldehyde phosphate-buffered saline (PBS) at pH 7.2 for 24 h, washed for another 24 h with PBS, dehydrated in alcohol and embedded in the tissue embedding medium (Paraplast Plus® Tissue, McCormick Scientific, Skokie, IL, USA). After drying in an oven at 60°C for 60 min, the 5-µm-thick sections of the aortas were undergoing deparaffinization in xylene, and rehydration in graded ethanol solutions. Next, the sections were heated in Tris-EDTA buffer at pH 9.0 plus Tween-20 for 15 min and were blocked with 3% hydrogen peroxide in methanol for 30 min. To block the non-specific reactions, the slides were incubated in a 3% Molico® milk solution (Nestlé, São Paulo, Brazil) for 1 h. The sections were then immunostained with primary antibodies: rabbit polyclonal anti-Nox1 (Abcam®; ab131088) and rabbit monoclonal anti-Nox4 (Abcam®; ab133303) or rabbit polyclonal anti-Nox2 (Sigma-Aldrich®; 07-024), at a concentration of 1 µl/50 µl in PBS buffer and were incubated overnight at 4°C. After primary antibody incubation, the sections were washed with PBS and then incubated with Polymer N-Histofine RAT Multi (Nichirei Biosciences Inc., Tokyo, Japan) for 30 min at room temperature. Finally, the sections were washed with PBS and incubated for 3 min in 50 µl of diaminobenzidine (DAB) solution containing 2 µl of H2O2. Counterstaining was performed using hematoxylin. The histological fields were photographed using the Olympus DP-25 digital camera attached to the Olympus BX41 microscope (Olympus, Tokyo, Japan).
Western blotting
The aortas were homogenized in 200 µl of lysis buffer containing protease inhibitor cocktail (#11697498001, Roche, Basel, Switzerland). Equal amounts of protein (40 µg) were resolved by electrophoresis on 10% polyacrylamide-SDS gels for 80 min at 150 V in a mini-gel device (Mini Protean III, Bio-Rad, Hercules, CA, USA). Then, the proteins were electrically transferred onto a nitrocellulose membrane (Bio-Rad) at 100 V for 90 min. Then, the membrane was stained with Ponceau solution to check the transference effectiveness. The membrane was then incubated in Tris buffered saline (TBS) containing Tris (10 mmol/l), NaCl (150 mmol/l), Tween 20 (0.02%), and skimmed milk (7%) for 1 h to reduce the non-specific binding. Following, the membrane was incubated at 4°C overnight with one of the following primary antibodies: goat polyclonal anti-Nox1 (1:500, sc-5821, Santa Cruz Biotechnology, Dallas, TX, USA), goat polyclonal anti-gp91phox (1:500, sc-5827, Santa Cruz 186 Biotechnology) and rabbit polyclonal anti-Nox4 (1:250, sc-30141, Santa Cruz Biotechnology). Membranes were washed out and incubated with secondary antibodies for 90 min at room temperature. The signals were revealed by chemiluminescence, visualized using a ChemiDoc™ XRS+ (Bio-Rad, Hercules, CA, USA), and quantified by densitometry. Mouse monoclonal anti-β-actin (1:1,000, sc-47778, Santa Cruz Biotechnology) was used as an internal control.
Vascular responsiveness
Thoracic aortas were carefully dissected, cleaned of connective tissues, and cut into rings of 3 mm in length. The rings were placed in organ bath chambers containing Krebs-Henseleit solution (in mmol/l: NaCl 130; KCl 4.7; CaCl2 1.6; KH2PO4 1.2; MgSO4 1.2; NaHCO3 15; glucose 11.1[pH 7,4]), equilibrated with 95% O2 and 5% CO2 and maintained at 37°C. In this environment, rings were kept between two stainless steel stirrups, one fixed and the other connected to an isometric force transducer (Powerlab 8/30 data-acquisition system - AD Instruments, Castle-Hill, NSW, Australia). Before administering drugs, the rings were equilibrated for 60 min at a resting tension of 1.5 g. To assess endothelial integrity, these preparations were pre-contracted with 10−5 mol/l norepinephrine (Sigma-Aldrich) and then 10−5 mol/l acetylcholine (ACh; Sigma-Aldrich) was added to the organ bath.
Aorta rings pre-contracted with 10−5 mol/l norepinephrine were challenged by ACh (10−9 – 10−4 mol/l; Sigma-Aldrich), cumulatively added to the organ bath, and the evoked relaxing responses (g) were recorded. The challenges by ACh occurred both in the absence and presence of 10−4 mol/l apocynin (Sigma-Aldrich), added directly to the organ bath 20 min before. The recorded contractions were used to determine the concentration-response curves and, from these curves, the pEC50 (the negative logarithm of the concentration of an agonist that produces 50% of the maximal possible response) was determined. This parameter was calculated by non-linear regression using Prism 6.0® software (GraphPad Software Corporation, San Diego, CA, USA). The maximal agonist response, i.e., maximal response (Rmax) evoked by the studied agonist was also determined.
Histomorphometry
Segments of aortas were fixed in 2% glutaraldehyde and 4% paraformaldehyde in 0.1 M of Sorensen’s phosphate buffer at pH 7.4 for 24 h. After, the samples were dehydrated in 95% alcohol and embedded in a Leica Historesin Embedding Kit. The 5-µm-thick sections were stained with hematoxylin and eosin. A panoramic digital photomicrograph of the aorta was obtained using the Olympus DP-25 digital camera attached to an Olympus SZX7 stereomicroscope. For each aorta, the thickness of the media layer was measured at four different points using an arbitrary line tool of the Olympus CellSens software. The mean of these measurements, obtained at the four points, was expressed in µm.
Statistical analysis
The data normality was verified by the Kolmogorov–Smirnov test with Lilliefors correction and the homogeneity of the variances by the Levene test. The variables with parametric distribution are presented as mean ± standard error of the mean (S.E.M.) whereas the variables without parametric distribution are presented as the median and interquartile range (25th first quartile; 75th third quartile). When the parametric distribution of the data was verified, two-way ANOVA followed by the Tukey Post-Hoc test was used for comparisons between four independent groups. On the other hand, when the nonparametric distribution of the data was verified, the Mann–Whitney test was used for comparisons between two independent groups whereas the Kruskal–Wallis test, followed by the peer-to-peer comparison by the Mann–Whitney test with the Holm–Sidak Post-Hoc correction, was used for comparisons between four independent groups. The significance level adopted was 5% (P-value ≤ 0.05) and the data were analyzed in SPSS software (version 19.0).
Results
Body and organ weights
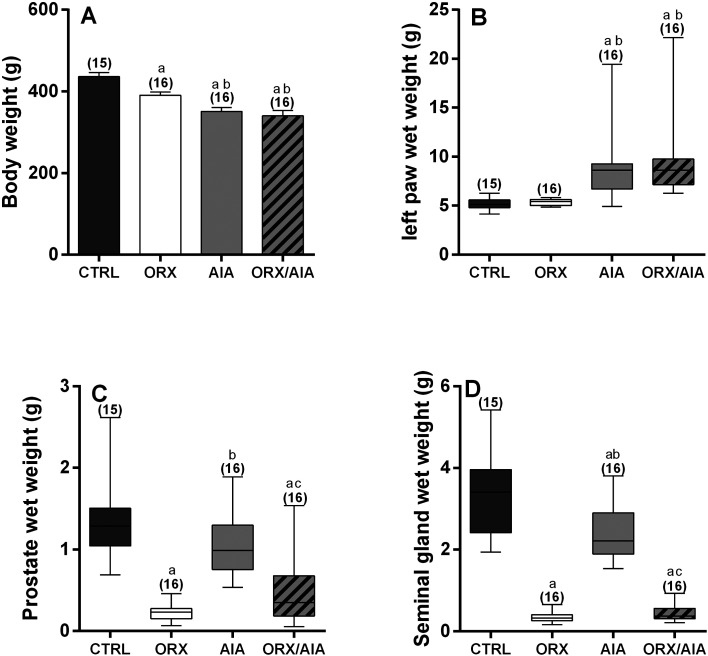
On the day of the organ bath experiments, animals submitted either to ORX or AIA, as well as those submitted to both, ORX and AIA, exhibited lower body weight in comparison to CTRL animals. In addition, animals submitted to both, ORX and AIA, exhibited lower body weight in comparison to those submitted to only ORX. Notably, the body weight of animals AIA and ORX/AIA was not significantly different (Fig. 1A). Moreover, AIA increased the wet weights of the studied animals’ left paws, regardless of ORX (Fig. 1B). On the other hand, there were significant reductions in the wet weight of the prostate and seminal vesicle in all animals submitted to ORX, either with or without AIA. In addition, AIA slightly reduced the prostate and seminal vesicle wet weights, but this reduction reached statistical significance only in the seminal vesicle (Fig. 1C and Fig. 1D).
Fig. 1.
Body mass (A) and wet mass of the left posterior paw (B), prostate (C) and seminal vesicle (D) obtained from animals from CTRL, ORX, AIA and ORX/AIA groups. Parametric data were compared by two-way ANOVA followed by the Tukey Post-Hoc test and values are expressed by mean ± S.E.M. (A). Non-parametric data were compared by the Mann-Whitney test, with critical values of P adjusted by Holm–Sidak post hoc correction and values expressed as median and interquartile range (25th first quartile; 75th third quartile; B–D). In parentheses is the number of samples. Superscript letters indicate statistically significant (P≤0.05) differences (a Compared to CTRL group; b Compared to ORX group; c Compared to AIA group). CTRL: control; ORX: orchiectomy; AIA: adjuvant-induced arthritis; ORX/AIA: orchiectomy in addition to adjuvant-induced arthritis.
Circulating testosterone levels
The circulating testosterone levels observed in AIA animals [median 134.0 (84.20–245.0) ng/dl; n=16)] were not significantly different from those found in CTRL animals [median 156.8 (117.0–191.1) ng/dl; n=15, P> 0.05 by Mann–Whitney test]. On the other hand, ORX reduced serum testosterone levels below the detection limit in all animals, regardless of the incidence of AIA.
Expression of the Nox1, Nox2 and Nox4 isoforms in aortas
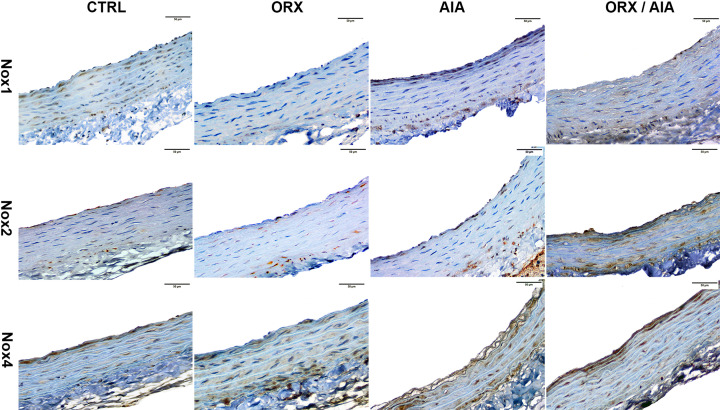
The expression of Nox1, Nox2 and Nox4 was detected by immunohistochemistry in intima, media and adventitia layers of aortas taken from all studied groups (Fig. 2). In CTRL animals, Nox1 was immunostained almost evenly both on the intima and the media, but less on the adventitia. In addition, the Nox2 immunostaining was more evident on the intima as well as on the media, but closer to both lumen or adventitia. The Nox4 in turn was intensely immunostained on the intima and in the smooth muscle layer beneath the intima, whereas this immunostaining occurred uniformly in the other areas of the media layer, and little in the adventitia.
Fig. 2.
Immunohistochemistry for Nox1, Nox2, and Nox 4 of thoracic aorta from CTRL, ORX, AIA and ORX/AIA groups. Nox isoforms-positive labels were observed in nuclei and/or cytoplasm of cells of the aorta layers, with immunoperoxidase staining in brown. Counterstaining with hematoxylin, which stains the background blue. Scale bar=50 µm. CTRL: control; ORX: orchiectomy; AIA: adjuvant-induced arthritis; ORX/AIA: orchiectomy in addition to adjuvant-induced arthritis.
In AIA animals, mainly Nox1 and Nox4 appear to have been immunostained with more intensity compared to CTRL animals. Regarding their distribution pattern, the Nox1 was immunostained more intensely in both intima and adventitia. This immunostaining was much weaker in the media layer, except for the portion closest to the intima. The Nox2, although present in all layers, was more intensely immunostained on the intima as well as on the media layer either beneath the intima or closer to the adventitia. The Nox4 immunostaining was well distributed in all layers, but it was even stronger in both transitions media-intima and media-adventitia.
In ORX animals, Nox1 staining was visibly weaker throughout the histological field in comparison to CTRL animals, being observed with a little more intensity in the media layer, but closer to the adventitia. In these animals, Nox2 stained more intensely on the media, either closer to the lumen or adventitia, but visibly less on both intima and the adventitia. Finally, Nox4 immunostaining was seemingly weaker on the intima, whereas it was stronger in the media-adventitia transition.
In the ORX/AIA group, immunostaining of these proteins was even more visible than in the other groups. The Nox1 was strongly stained in all layers, but mainly in the transition of adventitia-media layers. The Nox2 immunostaining was stronger both in the intima and in the media, mainly closer to the adventitia. The Nox4, in turn, was well colored in all layers, but this immunostaining was stronger in the intima, as well as in the media closer to the intima.
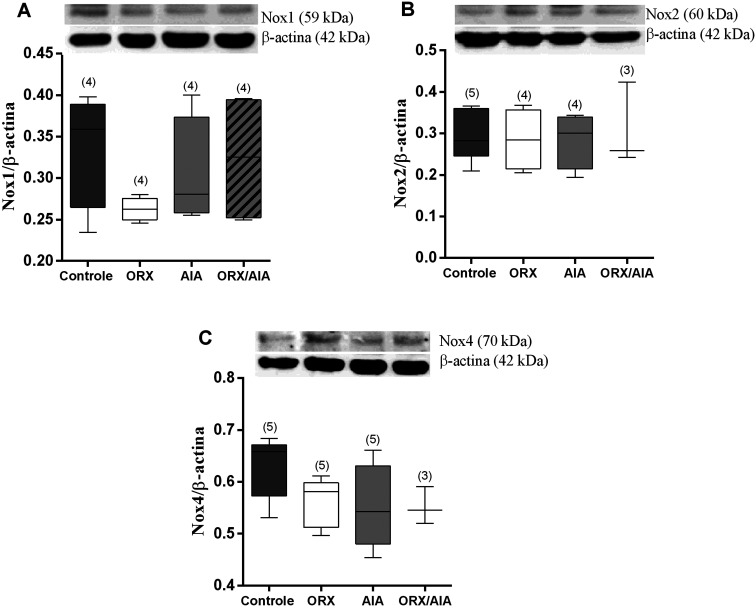
The quantification of these proteins by Western blotting showed only a mild reduction of Nox1 in the ORX group compared to the CTRL group, which was not statistically significant. In addition, no observable changes occurred in the expression of both Nox2 and Nox4 in consequence of AIA or ORX (Fig. 3).
Fig. 3.
Protein expression of the enzymes NADPH oxidases, isoforms Nox1 (A), Nox2 (B) and Nox4 (C) in relation to β-actin, determined in isolated thoracic aortas of animals from CTRL, ORX, AIA and ORX/AIA groups and densitometric quantification of the corresponding bands (Bars). Values expressed as median and interquartile range (25th first quartile; 75th third quartile). In parentheses is the number of samples. Comparisons between groups by Kruskal–Wallis’ test. CTRL: control; ORX: orchiectomy; AIA: adjuvant-induced arthritis; ORX/AIA: orchiectomy in addition to adjuvant-induced arthritis.
ACh-induced aorta relaxation
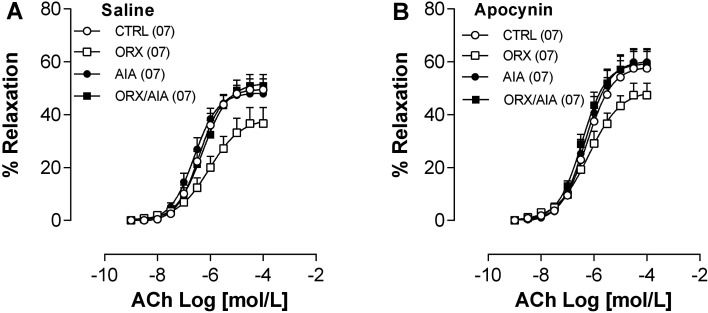
The results obtained show that AIA did not modify the relaxation of the aorta induced by ACh in the animals studied. On the other hand, ACh-induced aortic relaxation was slightly smaller in ORX compared to CTRL animals, although not statistically significant. This apparent reduction in ACh-induced response was not observed in ORX/AIA animals. In the presence of apocynin, ACh responses were slightly enhanced, but the slightly attenuated ACh response profile persisted in the ORX group (Fig. 4; Table 1).
Fig. 4.
Concentration-response curves for acetylcholine (ACh) determined in isolated preparations of the thoracic aorta, obtained from animals belonging to the CTRL, ORX, AIA or ORX/AIA groups, in the absence (A) or presence of 10−4 mol/l apocynin (B). Relaxation was expressed as the percent of the pre-contraction induced by 10−5 mol/l norepinephrine. Points represent mean ± S.E.M. In parentheses, the number of samples. CTRL: control; ORX: orchiectomy; AIA: adjuvant-induced arthritis; ORX/AIA: orchiectomy in addition to adjuvant-induced arthritis.
Table 1. Values of Rmax (% of Phe-induced contraction) and pEC50 obtained in aortas challenged by acetylcholine.
| Rmax | pEC50 | |||
|---|---|---|---|---|
| Saline | Apocynin | Saline | Apocynin | |
| CTRL | 50.54 ± 4.09 | 42.46 ± 6.38 | 6.40 ± 0.18 | 6.21 ± 0.13 |
| ORX | 63.39 ± 6.13 | 52.62 ± 4.55 | 5.99 ± 0.23 | 6.17 ± 0.16 |
| AIA | 52.08 ± 1.99 | 40.07 ± 5.02 | 6.54 ± 0.13 | 6.28 ± 0.09 |
| ORX+AIA | 49.10 ± 4.34 | 40.83 ± 5.41 | 6.37 ± 0.17 | 6.41 ± 0.15 |
Values expressed by mean ± S.E.M of 7 determinations. Comparisons between groups by Two-way ANOVA, followed by the Tuckey Post-Hoc test. CTRL: control; ORX: orchiectomy; AIA: adjuvant-induced arthritis; ORX/AIA: orchiectomy in addition to adjuvant-induced arthritis.
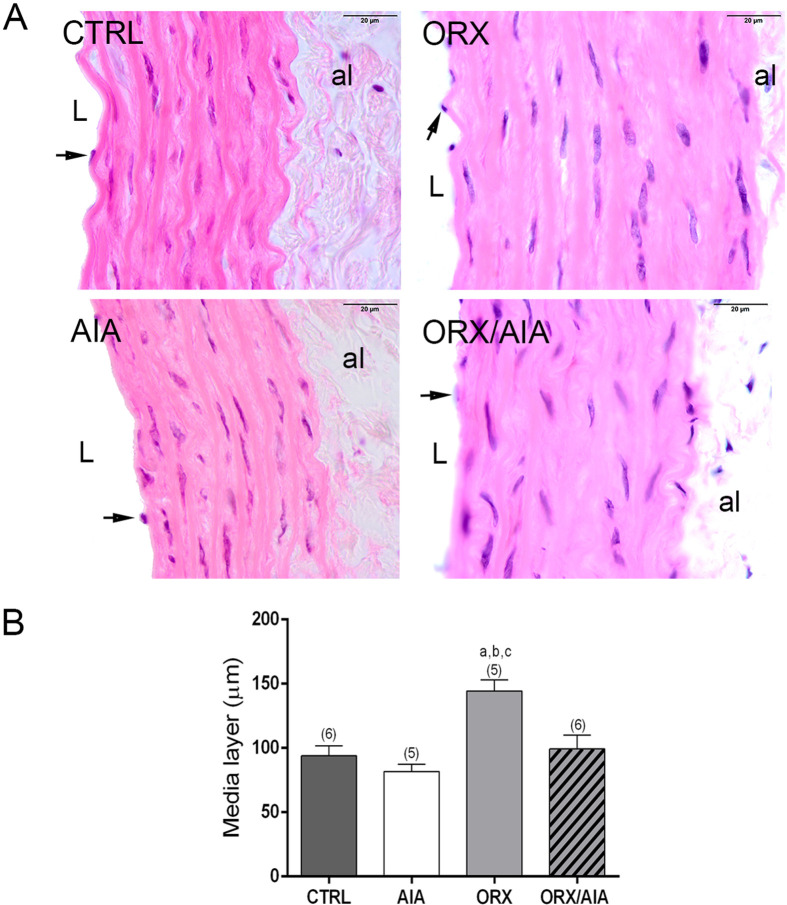
Histomorphometry of the aorta
The ORX, but not AIA, significantly increased the thickness of the media layer of the aorta. This structural modification, however, was not found in animals submitted to both ORX and AIA (Fig. 5).
Fig. 5.
Representative photomicrographs of thoracic aorta sections from CTRL, ORX, AIA and ORX/AIA groups, stained with hematoxylin and eosin, highlighting the media layer (A). al: adventitia layer; L: lumen. Symbols: arrow: endothelial cell. Scale bar=20 µm. Morphometry of the thickness of the media layer obtained in each experimental group (B). Data were compared by a two-way ANOVA test, followed by the Tukey Post-Hoc test, and values expressed by mean ± S.E.M. Superscript letters indicate statistically significant (P≤0.05) differences (a Compared to CTRL group; b Compared to AIA group; c Compared to ORX/AIA group). In parentheses is the number of samples. CTRL: control; ORX: orchiectomy; AIA: adjuvant-induced arthritis; ORX/AIA: orchiectomy in addition to adjuvant-induced arthritis.
Discussion
RA is an autoimmune disease that involves complex and not yet fully understood pathophysiological mechanisms that can extend beyond the joints. Deepening the understanding of this disease often depends on more invasive experimental approaches. Thus, animal models are essential in the search for information regarding arthritis that can be extrapolated to the treatment of RA in human beings.
In the present study, the first step was to ensure the effectiveness of the experimental model. The AIA-induced reduction in body weight, observed in animals submitted or not to ORX, indicates sarcopenia, a condition commonly described in this arthritis model. Moreover, a previous study revealed that sarcopenia is more severe at the moment when the animals of the present study were assessed (20).
On the other hand, ORX also induced a slight body weight reduction which may indicate a lack of the testosterone anabolic effect (21). The lack of testosterone, which is a direct result of ORX, was also observed by the significant reduction in the wet weight of both the prostate and seminal vesicle. Actually, reductions in the wet weight of these testosterone-dependent organs have been used as indicators of ORX effectiveness (22). In addition, the testosterone in serum was below the detection limit in animals submitted to ORX, thereby confirming the effectiveness of this procedure.
Interestingly, AIA slightly reduced the prostate and seminal vesicle wet weights, but this reduction reached statistical significance only in the seminal vesicle. This suggests that, at some point in its development, AIA may have promoted a reduction in circulating testosterone levels. Supporting this hypothesis, we observed recently a drastic reduction in circulating levels in rats, 15 days after AIA induction. This reduction in testosterone was reversed and was no longer significant at about 40 days after AIA induction (10).
Once observed the effectiveness of both ORX and AIA, we proceed to the analysis of these animals’ aortas. The immunohistochemistry revealed that Nox1, Nox2 and Nox4 were immunostained in the intima, media and adventitia layers of aortas from all tested rats with or without treatments with ORX and/or AIA, although the staining intensity was not uniform among layers and treatments. The wide distribution of these Nox isoforms over the different layers of the aortas is in agreement with previous studies (23). These same authors also argue that pro-inflammatory cytokines produced in joints affected by AIA, such as TNF, can induce Nox1, Nox2 and Nox4 in these tissues. In fact, studies are showing AIA-induced elevation of different Nox isoforms as well as other proteins that are components of the NADPH oxidase complex (4, 24). In rat aortas, there are evidences of AIA-induced increase of gp91phox (also named Nox2) (5, 25), p22phox (5, 24,25,26) and p47phox (5, 24). However, further studies are still needed to identify the participation of other NADPH oxidase isoforms in vascular disorders induced by AIA, as well as to know their histological location in rat aortas.
In the present study, the expression pattern of Nox1, Nox2 and Nox4 in the aorta may also have been influenced by AIA. AIA apparently increased the immunostaining of these proteins mainly in the intima and sub-intima, as well as in the transition of the media and adventitia layers. This characteristic distribution pattern of Nox1, Nox2 and Nox4 may be due to the distribution route of pro-inflammatory cytokines in these tissues. These cytokines access the aortic tissues through the bloodstream and thus the intima is more exposed to these mediators. The distribution of cytokines in the aorta can also occur by the adventitial layer, through the vasa vasorum (27). Thus, it is possible to infer that these regions may be expressing more Nox because they are more exposed to inflammatory cytokines. Notably, this apparent AIA-induced increment of immunostaining also was observed in the group that had previously undergone ORX.
In addition, ORX may also have influenced the expression of Nox1, Nox2 and Nox4 in the studied animals. A reduction in Nox1 immunostaining was clearly observed in ORX animals, which was less evident in relation to Nox2 and Nox4. This apparent reduction in the presence of Nox1 was followed by a decrease in its quantification by Western blotting. It is noteworthy that this reduction of Nox1 detection was not statistically significant. However, the correspondence that exists between the weaker staining in the immunohistochemistry and the reduction, although not statistically significant, of its detection by Western blotting reinforces the hypothesis of Nox1 reduction in ORX animals. These data suggest that testosterone participates in the induction of Nox1 expression in the aorta. In this sense, it was demonstrated that testosterone increased the expression of Nox1 in cultured rat mesenteric arteries’ smooth muscle cells (14).
An alleged AIA-induced increase in NOX expression, however, was not confirmed by the Western blotting experiments. This weakens the hypothesis that AIA has modified the expression of these proteins in the aorta, as Western blotting is the most adequate method for this quantification. Nevertheless, we cannot rule out that an AIA-induced increase in Nox1, Nox2 and Nox4 expression may have occurred in these aortas. As Western blotting is done in the homogenate of the vessel, it may not have been possible to detect small differences in the presence of these enzymes, mainly if they occurred only in parts of the aorta.
As we know, Nox1 and Nox2 are enzymes that produce superoxide anions, while Nox4 has been identified as a producer of hydrogen peroxide (23). Thus, an increase in the expression of these enzymes, mainly Nox1 and Nox2, would lead to an increase in oxidative stress and consequent endothelial dysfunction. However, the ACh-induced aorta relaxation was not changed by both AIA and/or ORX. Notably, the ACh-induced relaxation in rat aorta is a phenomenon mainly mediated by endothelium-derived nitric oxide (28) and, thus, local oxidative stress could attenuate such response to the extent that it may reduce NO bioavailability (29). These aortas were further challenged with ACh in the presence of apocynin, which can reduce the degree of oxidative stress in these preparations (30). In this condition of lower oxidative stress, although a slightly increased ACh-induced aorta relaxation occurred in all groups, there was no statistical difference in Rmax or pEC50 between groups. This suggests that neither AIA nor ORX changed the local production of NO or the redox balance in the studied animals. Still, concerning these ACh-induced relaxing responses, it was observed that they were slightly lower in ORX animals, compared to the others. This slight reduction of ACh-induced relaxation observed in ORX animals does not appear to be due to an increase in local oxidative stress, since it was observed even in the presence of apocynin. Although not significant, this difference may indicate that ORX may change mechanisms that modulate ACh responses in the aorta. There is evidence of reduction of NO and increment of thromboxane A2 synthesis in the aorta of ORX rats (31, 32). These changes may alter the balance between vasoconstrictor/vasodilator local mechanisms mobilized by ACh, thereby justifying at least partially the observed response modification.
The results of both Western blotting and vascular responsiveness experiments conflict with previous studies, in which an increase in NADPH oxidases subunits and the consequent oxidative stress were increased by AIA (4, 5, 25). Like in the present study, those authors harvest aortas on the 21st day after the onset of AIA in the paw contralateral to the induction. We chose to harvest the aortas at this time, as we had already observed that AIA-related joint volume increment is higher at this moment (20). It is noteworthy, however, that AIA is a reversible model of arthritis, in which the apex of increased joint volume is not necessarily reached at the moment when inflammatory activity is at its highest. Perhaps, vascular changes may develop at different times depending on the rat strain. The changes in the expression of NADPH oxidase complex proteins previously reported were observed in Lewis rats (5, 24,25,26), whereas in the present study we used Wistar rats. Thus, in these Wistar rats, vascular changes may not be at the highest point at this moment in the AIA evolution.
Assuming that structural changes caused by AIA could be present in these aortas even if the local inflammatory process was in regression, we also performed the morphometry of these arteries. The obtained data showed that the media layer thickness was not changed by AIA in these aortas, but it was significantly increased by ORX. These data are in agreement with previously reported findings (15), that reinforce the protective role of testosterone in the cardiovascular system (33). According to Pawlowska-Olszewska (15), this media layer thickening may be associated with significant changes in the volume of extracellular matrix components in these vessels. Possibly, this structural change can be related to the non-significant reduction of ACh-induced vasodilation response, that was observed in ORX animals. This increase in the thickness of the media layer implies an increase in the diffusion distance of NO through the aorta, increasing the chances that it will be degraded before promoting its pharmacological actions. Curiously, this structural change caused by ORX was not observed in ORX/AIA animals. Nevertheless, other experimental approaches are required to explore the mechanism of this ORX-AIA interaction in the morphology of these vessels.
Finally, it should be noted that the absence of functional or structural changes in the aorta at this time of AIA evolution does not exclude that such changes may occur in other vascular beds. Arthritis’ repercussions may not happen in the same way throughout the entire cardiovascular system. Studies in humans and animals suggest that the effects of arthritis on microcirculation may be different than those observed in the large vessels (18, 34, 35).
Conclusion
The present study showed weak clues of increased expression of Nox1, Nox2 and Nox4 as a result of AIA, as well as of Nox1 reduction caused by ORX. In addition, the endothelial function was not modified in the aortas of these animals by both AIA and/or ORX. On the other hand, ORX increased significantly the aorta media layer thickness in the studied animals, which was apparently mitigated by AIA. The present study, however, does not exclude that further AIA-induced changes may occur in other vascular beds since the effects of arthritis cardiovascular system are territory-dependent.
Ethics Standards
All experiments and procedures were performed in accordance with international guidelines for the care and use of laboratory animals and approved by the Ethics Committee on Animal Use of Marília Medical School (protocol nº 1026/14).
Conflicts of Interest
The authors declare that they have no competing interests.
Acknowledgements
The authors thank Alisson Douglas Ventura Neves, Priscilla Bianca de Oliveira and Rosa Maria dos Santos Sabatini for technical assistance. Financial support for this study was provided by the São Paulo Research Foundation (FAPESP), through a Regular Research Grant (Process nº. 2016/08450-3, Principal Investigator AB Chies and Collaborator MA Spadella).
References
- 1.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016; 388(10055): 2023–38. doi: 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 2.Meyer PW, Anderson R, Ker JA, Ally MT. Rheumatoid arthritis and risk of cardiovascular disease. Cardiovasc J S Afr. 2018; 29(5): 317–21. doi: 10.5830/CVJA-2018-018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.England BR, Thiele GM, Anderson DR, Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ. 2018; 361: k1036. doi: 10.1136/bmj.k1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haruna Y, Morita Y, Komai N, Yada T, Sakuta T, Tomita N, et al. Endothelial dysfunction in rat adjuvant-induced arthritis: vascular superoxide production by NAD(P)H oxidase and uncoupled endothelial nitric oxide synthase. Arthritis Rheum. 2006; 54(6): 1847–55. doi: 10.1002/art.21891 [DOI] [PubMed] [Google Scholar]
- 5.Haruna Y, Morita Y, Yada T, Satoh M, Fox DA, Kashihara N. Fluvastatin reverses endothelial dysfunction and increased vascular oxidative stress in rat adjuvant-induced arthritis. Arthritis Rheum. 2007; 56(6): 1827–35. doi: 10.1002/art.22632 [DOI] [PubMed] [Google Scholar]
- 6.Mäki-Petäjä KM, Cheriyan J, Booth AD, Hall FC, Brown J, Wallace SM, et al. Inducible nitric oxide synthase activity is increased in patients with rheumatoid arthritis and contributes to endothelial dysfunction. Int J Cardiol. 2008; 129(3): 399–405. doi: 10.1016/j.ijcard.2008.02.011 [DOI] [PubMed] [Google Scholar]
- 7.Palma Zochio Tozzato G, Taipeiro EF, Spadella MA, Marabini Filho P, de Assis MR, Carlos CP, et al. Collagen-induced arthritis increases inducible nitric oxide synthase not only in aorta but also in the cardiac and renal microcirculation of mice. Clin Exp Immunol. 2016; 183(3): 341–9. doi: 10.1111/cei.12728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Sijl AM, Peters MJ, Knol DK, de Vet HC, Gonzalez-Gay MA, Smulders YM, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum. 2011; 40(5): 389–97. doi: 10.1016/j.semarthrit.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 9.Perez-Garcia LF, Te Winkel B, Carrizales JP, Bramer W, Vorstenbosch S, van Puijenbroek E, et al. Sexual function and reproduction can be impaired in men with rheumatic diseases: a systematic review. Semin Arthritis Rheum. 2020; 50(3): 557–73. doi: 10.1016/j.semarthrit.2020.02.002 [DOI] [PubMed] [Google Scholar]
- 10.Santos CR, Benjamin ACA, Chies AB, Domeniconi RF, Zochio GP, Spadella MA. Adjuvant-induced arthritis affects testes and ventral prostate of Wistar rats. Andrology. 2020; 8(2): 473–85. doi: 10.1111/andr.12693 [DOI] [PubMed] [Google Scholar]
- 11.Brubaker WD, Li S, Baker LC, Eisenberg ML. Increased risk of autoimmune disorders in infertile men: analysis of US claims data. Andrology. 2018; 6(1): 94–8. doi: 10.1111/andr.12436 [DOI] [PubMed] [Google Scholar]
- 12.Gubbels Bupp MR, Jorgensen TN. Androgen-induced immunosuppression. Front Immunol. 2018; 9: 794. doi: 10.3389/fimmu.2018.00794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kataoka T, Hotta Y, Maeda Y, Kimura K. Testosterone deficiency causes endothelial dysfunction via elevation of asymmetric dimethylarginine and oxidative stress in castrated rats. J Sex Med. 2017; 14(12): 1540–8. doi: 10.1016/j.jsxm.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 14.Chignalia AZ, Schuldt EZ, Camargo LL, Montezano AC, Callera GE, Laurindo FR, et al. Testosterone induces vascular smooth muscle cell migration by NADPH oxidase and c-Src-dependent pathways. Hypertension. 2012; 59(6): 1263–71. doi: 10.1161/HYPERTENSIONAHA.111.180620 [DOI] [PubMed] [Google Scholar]
- 15.Pawlowska-Olszewska M, Puzio I, Harrison AP, Borkowski L, Tymicki G, Grabos D. Supplementation with camelina oil prevents negative changes in the artery in orchidectomized rats. J Physiol Pharmacol. 2018; 69(1): 109–16. [DOI] [PubMed] [Google Scholar]
- 16.Costa TJ, Ceravolo GS, dos Santos RA, de Oliveira MA, Araújo PX, Giaquinto LR, et al. Association of testosterone with estrogen abolishes the beneficial effects of estrogen treatment by increasing ROS generation in aorta endothelial cells. Am J Physiol Heart Circ Physiol. 2015; 308(7): H723–32. doi: 10.1152/ajpheart.00681.2014 [DOI] [PubMed] [Google Scholar]
- 17.Bordy R, Totoson P, Prati C, Marie C, Wendling D, Demougeot C. Microvascular endothelial dysfunction in rheumatoid arthritis. Nat Rev Rheumatol. 2018; 14(7): 404–20. doi: 10.1038/s41584-018-0022-8 [DOI] [PubMed] [Google Scholar]
- 18.Totoson P, Maguin-Gaté K, Nappey M, Prati C, Wendling D, Demougeot C. Microvascular abnormalities in adjuvant-induced arthritis: relationship to macrovascular endothelial function and markers of endothelial activation. Arthritis Rheumatol. 2015; 67(5): 1203–13. doi: 10.1002/art.39065 [DOI] [PubMed] [Google Scholar]
- 19.White RM, Rivera CO, Davison CA. Nitric oxide-dependent and -independent mechanisms account for gender differences in vasodilation to acetylcholine. J Pharmacol Exp Ther. 2000; 292(1): 375–80. [PubMed] [Google Scholar]
- 20.Pita LM, Spadella MA, Montenote MC, Oliveira PB, Chies AB. Repercussions of adjuvant-induced arthritis on body composition, soleus muscle, and heart muscle of rats. Braz J Med Biol Res. 2020; 53(3): e8969. doi: 10.1590/1414-431x20198969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, et al. Testosterone Gel Study Group Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000; 85(8): 2839–53. [DOI] [PubMed] [Google Scholar]
- 22.de Souza Rossignoli P, Pereira OC, Chies AB. Orchidectomy enhances the effects of phenylephrine in rat isolated portal vein. Clin Exp Pharmacol Physiol. 2010; 37(3): 368–74. doi: 10.1111/j.1440-1681.2009.05313.x [DOI] [PubMed] [Google Scholar]
- 23.Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ. NADPH oxidases in vascular pathology. Antioxid Redox Signal. 2014; 20(17): 2794–814. doi: 10.1089/ars.2013.5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verhoeven F, Totoson P, Maguin-Gaté K, Prigent-Tessier A, Marie C, Wendling D, et al. Glucocorticoids improve endothelial function in rheumatoid arthritis: a study in rats with adjuvant-induced arthritis. Clin Exp Immunol. 2017; 188(2): 208–18. doi: 10.1111/cei.12938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakuta T, Morita Y, Satoh M, Fox DA, Kashihara N. Involvement of the renin-angiotensin system in the development of vascular damage in a rat model of arthritis: effect of angiotensin receptor blockers. Arthritis Rheum. 2010; 62(5): 1319–28. doi: 10.1002/art.27384 [DOI] [PubMed] [Google Scholar]
- 26.Totoson P, Maguin-Gaté K, Prigent-Tessier A, Monnier A, Verhoeven F, Marie C, et al. Etanercept improves endothelial function via pleiotropic effects in rat adjuvant-induced arthritis. Rheumatology (Oxford). 2016; 55(7): 1308–17. doi: 10.1093/rheumatology/kew062 [DOI] [PubMed] [Google Scholar]
- 27.Tesfamariam B. Periadventitial local drug delivery to target restenosis. Vascul Pharmacol. 2017; S1537-1891(17)30235-5. [DOI] [PubMed] [Google Scholar]
- 28.Vizioli EO, Spadin MD, Corrêa FM, Viaro F, Evora PR, Chies AB. Acetylcholine-induced aortic relaxation studied in salbutamol treated rats. J Smooth Muscle Res. 2005; 41(5): 271–81. doi: 10.1540/jsmr.41.271 [DOI] [PubMed] [Google Scholar]
- 29.Santilli F, D’Ardes D, Davì G. Oxidative stress in chronic vascular disease: from prediction to prevention. Vascul Pharmacol. 2015; 74: 23–37. doi: 10.1016/j.vph.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 30.Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, et al. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008; 51(2): 211–7. doi: 10.1161/HYPERTENSIONAHA.107.100214 [DOI] [PubMed] [Google Scholar]
- 31.Martorell A, Blanco-Rivero J, Aras-López R, Sagredo A, Balfagón G, Ferrer M. Orchidectomy increases the formation of prostanoids and modulates their role in the acetylcholine-induced relaxation in the rat aorta. Cardiovasc Res. 2008; 77(3): 590–9. doi: 10.1093/cvr/cvm059 [DOI] [PubMed] [Google Scholar]
- 32.del Campo M, Sagredo A, del Campo L, Villalobo A, Ferrer M. Time-dependent effect of orchidectomy on vascular nitric oxide and thromboxane A2 release. Functional implications to control cell proliferation through activation of the epidermal growth factor receptor. PLoS One. 2014; 9(7): e102523. doi: 10.1371/journal.pone.0102523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mäkinen J, Järvisalo MJ, Pöllänen P, Perheentupa A, Irjala K, Koskenvuo M, et al. Increased carotid atherosclerosis in andropausal middle-aged men. J Am Coll Cardiol. 2005; 45(10): 1603–8. doi: 10.1016/j.jacc.2005.01.052 [DOI] [PubMed] [Google Scholar]
- 34.Sandoo A, Carroll D, Metsios GS, Kitas GD, Veldhuijzen van Zanten JJ. The association between microvascular and macrovascular endothelial function in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 2011; 13(3): R99. doi: 10.1186/ar3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandoo A, Kitas GD, Carroll D, Veldhuijzen van Zanten JJ. The role of inflammation and cardiovascular disease risk on microvascular and macrovascular endothelial function in patients with rheumatoid arthritis: a cross-sectional and longitudinal study. Arthritis Res Ther. 2012; 14(3): R117. doi: 10.1186/ar3847 [DOI] [PMC free article] [PubMed] [Google Scholar]