Abstract
Although numerous clinical trials have been implemented, an absolutely effective treatment against coronavirus disease 2019 (COVID-19) is still elusive. Interleukin-22 (IL-22) has attracted great interest over recent years, making it one of the best-studied cytokines of the interleukin-10 (IL-10) family. Unlike most interleukins, the major impact of IL-22 is exclusively on fibroblasts and epithelial cells due to the restricted expression of receptor. Numerous studies have suggested that IL-22 plays a crucial role in anti-viral infections through significantly ameliorating the immune cell-mediated inflammatory responses, and reducing tissue injury as well as further promoting epithelial repair and regeneration. Herein, we pay special attention to the role of IL-22 in the lungs. We summarize the latest progress in our understanding of IL-22 in lung health and disease and further discuss maneuvering this cytokine as potential immunotherapeutic strategy for the effective manage of COVID-19.
Keywords: interleukin-22, immunotherapeutic strategy, lung, COVID-19, SARS-CoV-2 infection
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection has affected more than 548 million coronavirus disease 2019 (COVID-19) patients and caused more than 6.3 million deaths globally (https://coronavirus.jhu.edu, Johns Hopkins Coronavirus Resource Center) up to July 2, 2022, with these cases continuously increasing and producing several variants of concern. COVID-19 patients develop a constellation of clinical features, ranging from mild respiratory symptoms to severe acute respiratory syndromes, and even death (1, 2). Multiple evidences have illustrated that cytokine release syndrome (CRS) is the pathological hallmark of critically ill COVID-19 patients, which is characterized by a rapid and sustained systemic increase of more than 20 inflammatory chemokines and cytokines. CRS induced secondary hemophagocytic lymphohistiocytosis and acute respiratory distress syndrome (ARDS) are regarded as main causes of organ injuries that drive the deterioration of COVID-19 (3–7). Therefore, besides direct supplemental oxygen and antiviral therapy for COVID-19 patients, immunotherapeutic strategies potentially alleviate COVID-19 progression and rescue severe or critical illness. Unfortunately, a recent double-blind, randomized Phase III trial (clinicaltrials.gov; NCT04320615) testing the treatment efficacy of an anti-interleukin (IL-6) receptor antibody (tocilizumab) in COVID-19 cases failed to show a significant reduced the mortality (8, 9). Thus, further studies aimed at exploration of novel immunomodulatory therapeutic strategies to treat COVID-19 and development corresponding regimens are urgently warranted.
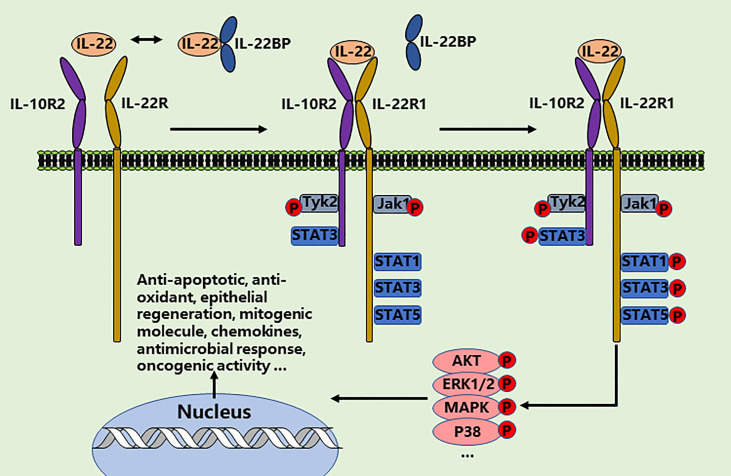
Interleukin-22 (IL-22) has attracted great interest over recent years, making it one of the best-studied cytokines of the interleukin-10 (IL-10) family (10, 11). The function of IL-22 is mediated through directly interacting with its heterodimeric IL-22R1 and IL-10R2 receptor complex (12, 13). Unlike most interleukins, which directly regulate the function of hematopoietic cells, the major impact of IL-22 is exclusively on fibroblasts and epithelial cells due to the restricted expression of receptors in the kidney, lung, liver, pancreas, gastrointestinal tract, skin, and thymus (14). Therefore, IL-22 represents a main communication channel between specialized tissue cell types and the immune system. Signaling occurs via the activation of Jak1/Tyk2 and STAT3 pathway and then activation of anti-apoptotic, mitogenic and antioxidant molecules. Protein kinase B (AKT)/mechanistic target of rapamycin (mTOR), ERK1/2, P38, MAPK, STAT1, and STAT5 pathways are also activated by IL-22 (15–20). Of note, numerous of studies have illustrated that IL-22 plays a pivotal role in anti-viral infections through significantly ameliorating the immune cell-mediated inflammatory responses, as well as reducing lung injury and promoting further airway epithelial repair and regeneration ( Figure 1 ) (21–23). Herein, we summarize recent progress in understanding the biology of IL-22 in lung health, suggesting more immunotherapeutic strategies to maneuver this cytokine for the effective manage of COVID-19 (24–28).
Figure 1.
IL-22/IL-22-R1 signaling events. IL‐22 exerts the therapeutic effects in two steps through a receptor complex composed of IL‐10R2 and IL‐22R1. IL‐22-IL10R2/IL‐22R1 complex leads to the phosphorylation of JAK1 and TYK2 and then phosphorylates the tyrosine residues such as STATs, AKT, ERK1/2, MAPK, and P38 in the cytoplasmic domain of IL‐22R1. The cellular effects of IL-22 are involved in anti-apoptotic and anti-oxidant response, epithelial regeneration, mitogenic molecule expression, chemokines expression, antimicrobial response, and oncogenic activity.
Pathological characteristics of COVID-19
COVID-19 is a complex disorder in which the respiratory performance is accompanied by systemic responses, illustrating viral infection generates widely dysfunctional immune reactions. Generally, the pathological feature of COVID-19 is bilateral diffuse alveolar damage with evidence of neutrophil extracellular traps, fibrin thrombi, and activated platelets in the vessels (25, 26). Infiltrating monocytes, macrophages, and neutrophils are clearly found in both lungs, accompanied by multinucleated syncytial cells infiltration in the alveoli that characterized by amphiphilic granular cytoplasm, large nuclei, and prominent nucleoli indicating viral cytopathic alters (27–31). Additionally, myocardial infarction, kidney damage, and persistent symptoms, such as depression, anxiety, palpitations, chest pain, sleep difficulty, dizziness, and weight loss, also represent the pathological features of critically COVID-19 cases (27–32). The immune profile in COVID-19 patients has been well reviewed, which shows dysfunction in both the innate and adaptive immune (33, 34). Specifically, CD16+ monocytes, γδ T cells, and NK cells are obviously activated, while the percentages of CD4+ T cells, CD8+ T cells, and natural killer (NK) cells are significantly decreased (34). Besides that, T cells show hyperinflammatory responses and enhanced migration abilities, along with evidently increased expression of inhibitory molecules (7, 33, 34). The clonality of B cells and the proportion of plasma B cell compartments are also elevated. The percentages of dendritic cells are obviously decreased; however, the IFN-response cell compartments are increased (34–38). For the mechanisms, multiple evidence has demonstrated that IL-1 axis and IL-6 axis are the most importantly relevant signal transductions in the SARS-CoV-2-induced hyperinflammatory responses (39).
Altogether, the pathological characteristics of COVID-19 are complex, comprising fibrotic processes, hyperinflammation, thromboembolic complications and endothelial cells and lymphocyte dysfunction in the lung. These processes between patients are also highly variable, may be due to the heterogeneity of host immune reaction, which urgently require stratified and novel immunotherapy strategies for COVID-19 management.
Immunotherapeutic strategies for COVID-19
Despite numerous clinical trials have been implemented, an absolutely effective treatment against COVID-19 is still elusive. Immunomodulatory medications hold huge promise, but their administration should be cautiously considered so that the protective effects are appropriate for the dominant immunopathology and the disease stages to minimize the side-effects ( Table 1 ).
Table 1.
Overview of the candidate immunotherapeutic strategies for the treatment of COVID-19/SARS-CoV-2 infection.
| Drug class | Target | Trial/paper | Overall status and conclusion | Ref |
|---|---|---|---|---|
| Cytokine antagonists | Blocking IL-1 (Canakinumab, Anakinra ) |
NCT04362813 NCT04680949 NCT02735707 NCT04357366 NCT04341584 |
Canakinumab showed no efficacy in clinical trials; Anakinra had promising results and larger clinical trials are pending | (40, 41) |
| Blocking IL-6 (Sarilumab, Tocilizumab) |
NCT02735707 NCT04381936 |
Sarilumab phase 3 trials were stopped; Dexamethasone&Tocilizumab were recommended by NIH for severe cases treatment |
8, 9) | |
| Blocking GM-CSF (mavrilimumab) | NCT04318366 | Phase 2 with primary endpoint was reached | (42) | |
| Blocking TNF-a (Etanercept, Adalimumab), | Case series | Further studies are required | (43) | |
| Blocking CCR5 (Leronlimab) | NCT04678830 | Currently not recommended | (44) | |
| Kinase inhibitors | Blocking JAK1and JAK2 (Baricitinib) |
NCT04401579 NCT04421027 |
Baricitinib has been approved by FDA as EUA, but the risk of adverse consequences remains |
(45) |
| Blocking JAK1and JAK3 (Tofacitinib) | NCT04469114 | The risk of respiratory failure or death was reduced, but the risk of adverse reactions remained | (46) | |
| Inflammasome inhibitors | Blocking Gasdermin D pore (Disulfiram) | Preclinical studies | Lack of efficacy and safety studies | (47) |
| Blocking NLRP3 inflammasome (Melatonin) |
NCT04409522 | As an adjunctive therapy; further larger studies are required | (48) | |
| Immune stimulants | IFNβ-1a, IFNβ-1b, IFN-𝜆 |
NCT04385095 NCT04276688 Case series |
Nebulized IFNs has demonstrated efficacy | (49, 50) |
| Immunomodulatory therapies | Glucocorticoids |
NCT02517489 NCT04244591 |
Only favorable results in critical cases, but accompanied by multiple adverse effects | (51) |
| Neutralizing antibody | Sotrovimab, LY-CoV555 |
NCT04545060 NCT04411628 NCT04427501 NCT04497987 NCT04501978 NCT04518410 NCT04634409 |
Have been approved by FDA as EUA, but VOCs have gradually weaken the efficacies | (52, 53) |
Cytokine immunomodulators
Of note, immunotherapeutic strategies blocking proinflammatory cytokines or their receptors are only meaningful in the hyperinflammatory stages; as in the early stages of mild lung injury or minimal inflammation, cytokines are largely desirable in fighting against virus infection. These include inhibitors of IL-1, IL-6, IL-18, TNF, CCR5 and GM-CSF or their receptors. However, some phase 2 or 3 results suggest no or little benefits in moderate and severe patients, and large-scale clinical data in COVID-19 are needed (8, 40–44, 54). Interferons (IFNs) have been used for many years to treat infectious disorders, cancers and multiple sclerosis, suggesting their potent antiviral effects for COVID-19. Although recent studies have found early nebulized IFN-α/β/𝜆 administration accelerated high-risk patients’ recovery and reduced mortality, the multinational clinical trials on hospitalized severe cases indicated subcutaneous IFNs injection with or without lopinavir treatment had minor effects on length of hospital stay and mortality (49, 50, 55, 56).
Kinase and inflammasome inhibitors
Small molecule inhibitors targeting cytokine-mediated signaling pathway, specifically of Janus kinases (JAKs), have also been well studied for treating COVID-19. For instance, Baricitinib and Tofacitinib were approved for emergency use authorization (EUA) because the clinical symptoms improved when co-treated with remdesivir during SARS-CoV-2 infection. But secondary infection and thromboembolism are the risk of these kinase inhibitors, which is highly dependent on administration time (45, 46). Glucocorticoids, such as dexamethasone, are broad-acting and powerful immunosuppressive and antiphlogistic therapies that can reduce mortality in critical COVID-19 cases (51). However, many clinical studies demonstrated that such efficacies are inevitably accompanied by multiple adverse effects, including viral reactivation, blocking of the host immune responses important for viral clearance, and others (51). In addition, it is indisputable that inflammasomes contribute to the pathogenesis of COVID-19, and trials on the GasderminD-pore inhibitor Disulfiram and the NLRP3 inhibitors Melatonin are ongoing (47, 48).
Neutralizing antibodies
Monoclonal antibody (mAb) is one of most effective immunotherapeutic strategies for the treatment of serious viral infection; thus, lots of mAbs are identified purposing to inhibit SARS-CoV-2 infection through binding to the spike protein. So far, the FDA has approved EUA for LY-CoV555 and Sotrovimab in pediatric and high-risk patients to prophylaxis and treat COVID-19, as these antibodies can reduce mortality and hospitalization (52, 57). Nevertheless, viral mutations present in variants/variants of concern (VOCs) have begun to gradually weaken the efficacies of many mAbs, and it is likely some will become invalid without upgrading to compensate for SARS-CoV-2 evolution (53). Altogether, immune system dysfunction plays a key role in COVID-19 pathogenesis and immunotherapeutic strategies for SARS-CoV-2 infection are promising, future efforts to discover more effective agents are obviously warranted. Also note that COVID-19 remains a field of rapid progress, thus recommendations on drugs and biomarkers will continue to evolve.
Interleukin‐22 biology in the lung
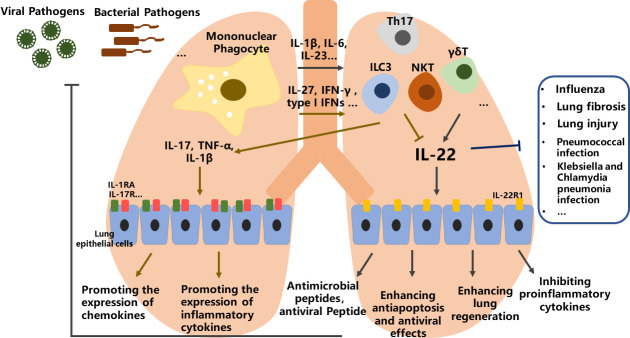
In the lung, stimulation of CD11b+-DCs and alveolar macrophages via their innate pattern recognition receptors leads to the secretion of related proinflammatory cytokines, expression of RORγt, differentiation of ILC3, γδT, Th17 and NKT cells, and production of IL-22 (57). Conversely, CD103+-DCs and plasmacytoid can be stimulated through viral antigens, resulting in the secretion of IL-27, IFN-γ and type I IFNs that block IL-22 expression in lymphocytes (58).
Molecular mechanism
Multiple studies have shown that IL-22 is involved in lung repair, recovery and regeneration during lung infection or injury. From these studies, it is concluded that IL-22 can be protective or proinflammatory, where IL-22/IL-22R axis is important for host protective immunity to both viral infections and bacterial infections. The tissue protective nature of IL-22 via enhancing wound healing and the epithelial barrier through promoting antimicrobial peptides expression, enhancing anti-apoptosis and antiviral effects and alleviating airway inflammation, as well as inhibiting proinflammatory cytokines produced by airway epithelial cells ( Figure 2 ). In contrast, the proinflammatory of IL-22 is evidenced by its ability to promote the expression of chemokines, and inflammatory cytokines such as IL-1β, IL-17, and TNF-α. Whether IL-22 has a protective or proinflammatory effect seems to depend on the related cytokines co-produced by the relevant cells during different stages of the diseases (10, 14).
Figure 2.
The role of IL-22 in lung health and diseases. In the lung, stimulation of mononuclear phagocyte via viral or bacterial pathogens leads to the secretion of related proinflammatory cytokines, then differentiation of ILC3, γδT, Th17 and NKT lymphocytes, and promoting or blocking of IL-22 expression. IL-22 can be protective or proinflammatory through multiple effects, where IL-22/IL-22R axis is important for host protective immunity to both viral infections and bacterial infections.
The regulation IL-22
Transcription factors play crucial roles in controlling the production of IL-22 in lungs (15, 59–61). Interestingly, studies indicate that these transcription factors, such as aryl hydrocarbon receptor (AhR), c-Maf, Batf, and Notch, can sometimes control IL-22 even in the same cells. These transcription factors form complex regulatory networks and regulate the production of IL-22 in context-dependent manners (62–64). As a cytosolic transcription factor, AhR is a harbor that converges several different environmental and cellular signaling to regulate the development of many Th cell lines, including Tr1, Th17 and Treg cells via recognizing multiple natural molecules and small xenobiotics (62–65). AhR directly binds to cMaf and synergistically enhances IL-22 production in ILC3s, Th17 cells, γδ T cells and Th22 cells. Nevertheless, under some certain conditions, AhR may not produce IL-22, and AhR-/- mice can develop severe skin inflammation associated with higher IL-22 and IL-17 expression. The Notch signaling also promotes IL-22 expression via AhR induction in Th17 cells (65). Additionally, prostaglandin E2 (PGE2) enhances IL-22 production from both T cells and ILC3 via binding EP4 and EP2 receptors and blocking IRF-4, as well as activating AhR and cAMP signaling (66, 67) ( Figure 3 ). In the lungs, certain viral or bacterial microorganisms can convert tryptophan (Trp) into derivatives that promote IL-22 secretion. Of importance, IL-22 production from γδ T cells is manipulated through AhR- and Try-dependent mechanism via CD69 (68). And CD69 regulates LAT-1 expression, which determines the intracellular quantity of AhR and the uptake of Trp ( Figure 3 ). Moreover, both CARD-/- and IDO-/- mice can alter downstream Trp metabolites, for instance 3-IAID, with one depriving IL-22-promoting derivatives and the other favoring them, respectively (69). Taken together, these findings illustrate that controlling AhR signaling and Trp metabolites may be a powerful tool for regulating IL-22 functions.
Figure 3.
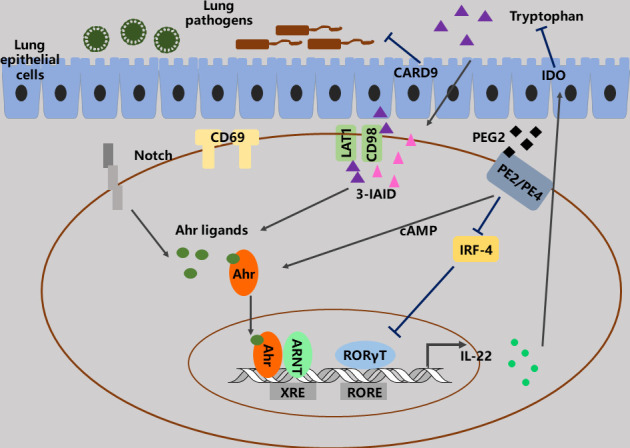
The functions and regulation of IL-22. AhR plays importance roles in the functions and regulation of IL-22 in lungs. A lot of signaling, such as PGE2, LAT1, CD69, CD98 and Notch can converge to AhR signaling to regulate IL-22 production via modulating of AhR ligand.
Protective effects of IL-22
During the early stage of influenza infection, studies has previously illustrated that IL22 is overexpressed by NK and NKT cells in the lung, resulting in the airway and parenchymal epithelium regeneration. Within two days after viral infection, IL-22 gene transcription in Tγδ, Tαβ and innate lymphoid cells is also increased in lung ( Table 2 ). However, IL22BP gene transcription is decreased. During the sublethal stage of viral infection, IL-22 can inhibit lung inflammation, reduce secondary infection and preserve the integrity of lung epithelium (70). Whereas IL-22-/- mice infected with viral shows enhanced collagen accumulation and a defect in epithelial regeneration (70). Moreover, IL-22 can also prevent pulmonary fibrosis in a Bacillus subtilis induced model. Administration of IL-22 alleviates lung fibrosis, while inhibition of IL-22 leads to increased collagen accumulation in the lungs (71). In pneumococcal pneumonia, IL-22 gene is found to increase in the lung together with other cytokines, for example TNFα, IL-6, and IL-1. The absence of IL-22 makes the host vulnerable to pneumococcal infection, which indicates that the presence of IL22 is very important to control pneumococcal infection (72). IL-22 also plays a key role in host resistance to many other lung pathogens. During Klebsiella pneumonia infection, NK cells activation results in IL-22 production to protect lung tissue, and during infection with Streptococcus pneumoniae, TLR5 and DC cells activation leads to a repaid accumulation of ILC3s in the lungs to express IL-22 and defense against bacterial infection (73–75). During Chlamydia muridarum infection, IL-22 levels are rapidly increased in the lungs. Neutralization of IL-22 with anti-IL-22 mAbs can lead to impaired Th17 responses and deterioration of disease. Intranasal injection of IL-22 can significantly enhance Th17 response and have a protective effect following Chlamydia pneumoniae infection (76). After Aspergillus fumigatus pulmonary infection, the fungal burden is increased in IL-22-/- mice, suggesting the critical role of IL-22 in the elimination of this pathogen (77). Additionally, IL-22 can also play a protective role in the barotrauma model, thereby reducing pulmonary edema and disintegration (78).
Table 2.
Immunotherapeutic strategies via targeting IL-22, in lung diseases.
| Diseases | Type | IL-22 treatment | Observed effect | Ref |
|---|---|---|---|---|
| Influenza | Viral | IL-22 deficient mice and recombinant IL-22 injection | Inhibits lung inflammation, reduce secondary infection and preserve the integrity of lung epithelium | (59) |
|
Bacillus
subtilis infection |
Bacterial | Intratracheal treatment with recombinant IL-22 | Protection from lung fibrosis | 60) |
| Pneumococcal pneumonia infection | Bacterial | Systemically administration of recombinant IL-22 | IL22 is important for controlling pneumococcal infection | (61) |
| Klebsiella pneumonia infection | Bacterial | IL-22 neutralizing antibody and recombinant IL-22 injection | Defenses against bacterial infection | (62, 64) |
| Streptococcus pneumoniae infection | Bacterial | Endogenous IL-22 | Defenses against bacterial infection | (63) |
| Chlamydia muridarum infection | Chlamydia | IL-22 neutralizing antibody and intranasal injection | Significantly enhances Th17 response and has protective effects | (65) |
| Aspergillus fumigatus pulmonary infection | Fungal | IL-22 neutralizing antibody and IL-22 deficient mice | Lung defense against Aspergillus fumigatus is mediated by IL-22 production | (66) |
| Ventilator-induced lung injury | Tissue damage | Prophylactic inhalation of IL-22 | Alleviation of ventilator induced lung damage | 67) |
| Bleomycin induced lung injury | Tissue damage | IL-22 neutralizing antibody and recombinant IL-22 injection | Proinflammatory effect of IL-22 depends on the synergistic effect with IL-17 | (68, 69) |
| Asthma models and asthmatic patients | asthmatic | IL-22 neutralizing antibody and recombinant IL-22 injection | IL-22 induces the occurrence of asthma and ameliorates inflammation during asthma exacerbation | 70–74) |
Proinflammatory effect of IL-22
In the animal model of bleomycin induced lung injury, it is demonstrated that IL-22 and IL-17 are predominantly produced by Th17 cells. Inhibiting IL-22 during bleomycin injection can improve airway inflammation, indicating IL-22 has a proinflammatory effect; nevertheless, airway inflammation is also significantly reduced in IL-17-/- mice with still high levels of IL-22 produced by T cells, suggesting IL-22 has a protective function for pulmonary fibrosis in deficiency of IL-17 (79, 80). Therefore, the proinflammatory effect of IL-22 may depend on the synergistic effect with IL-17 ( Table 2 ).
Dual effects of IL-22 in asthma
In peripheral blood of asthmatic patients and preclinical asthma models, serum IL-22 levels are increased. The recent studies have indicated IL-22 can induce the occurrence of asthma in preclinical models, but it can ameliorate inflammation during asthma exacerbation (81, 82). In ovalbumin induced asthma animal model, IL-22 neutralization with mAbs during sensitization stage of disease, which is similar to the pulmonary fibrosis research just mentioned above, evidently alleviated lung pathology and airway inflammation. Subcutaneous administration of IL-22 during sensitization leads to worse lung pathology and inflammation (83–85). Conversely, IL-22 neutralization after sensitization can result in increased lung inflammation, whereas IL-22 injection decreases chemokine expression, goblet cell hyperplasia, production of IL-25, eosinophil infiltration and constriction of the airway (82–85). The mechanism behind this dual effect in allergic airway inflammation is unclear, but it may involve inhibiting the production of IL-25 and CCL17 through airway epithelial repair, which may involve IL-10.
In conclusion, IL-22 is involved in a variety of lung diseases, making it a promising target for clinical development.
Potential therapeutic effect of IL-22 for COVID-19
The emergence of SARS-CoV-2, which leads to COVID-19, is one of the most serious public health and epidemics crises in this century. As an emerging virus, there are multiple problems to be clarified in distinguishing the effective immune response of mild and severe diseases.
IL-22 in COVID-19 patients
Although IL-22 does not appear to reduce virus titer during infections, studies have illustrated that IL-22 can reduce the pneumonia severity via the immune regulation and tissue protective or regenerative functions (86, 87). As COVID-19 is a respiratory disorder with similar pathological characteristics and symptoms to other serious pulmonary virus infections, it is reasonable to speculate that IL-22 may also serve to limit the severity of this disease. More importantly, recent studies have shown that IL-22 has potent immune boosting, antiviral, and antibacterial properties to respiratory syncytial virus, which could also extend to manage SARS-CoV-2 infection (88). Accordingly, compared with healthy control individuals, the IL-22+-Tc22 and IL-22+-Th22 numbers in adult-COVID-19 patients increased significantly, whether it is asymptomatic pneumonia, mild pneumonia, or severe pneumonia ( Figure 4 ). These findings further suggest that in the 0-12-year-old age group with asymptomatic disease course and uncomplicated adult cases, IL-22 expressed Tc22 cells are higher, which indicates that IL-22 has a protective effect (89). In contrast, the association between the increase of Tc17 cells and the severity of COVID-19 may reveal the destructive effect of co-expression of IL-17 and IL-22.
Figure 4.
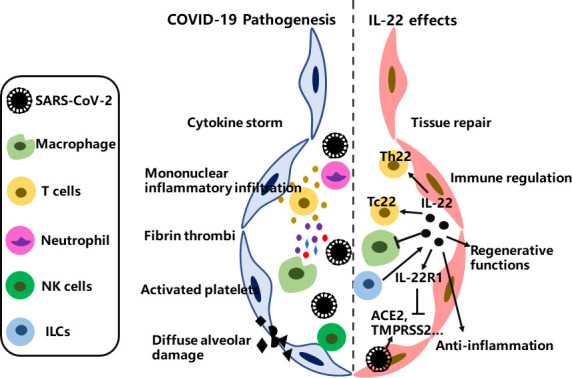
The therapeutic potential of IL-22 for COVID-19. SARS-CoV-2 virus first occupied the airway epithelial barrier; mononuclear inflammatory infiltration increased; and then platelets activation, cytokine storm, fibrin thrombi and diffuse alveolar injury followed. IL-22 can reduce the pneumonia severity through immunomodulation, anti-inflammation and tissue regenerative and repair functions, but the detailed mechanism underlying the therapeutic consequences for COVID-19 remains to be fully clarified.
IL-22/IL-22R1 axis in COVID-19 patients
As the IL-22/IL-22R1 axis is involved in inflammation during virus infection, the expression patterns of IL-22/IL-22R1 on blood hematopoietic cells in SARS-CoV-2 infection have been well studied (90–93). The numbers of IL-22R1+ myeloid DC1, myeloid DC2, and plasmacytoid DC and the proportions of IL-22R1+ intermediate, non-classical, and classical monocytes higher in COVID-19 patients than controls at the presented day. Moreover, high proportions of mDC2 and IL-22R1+ non-classical monocytes show high HLA-DR expression and are therefore activated. Multivariate analysis is performed on all IL-22R1+ myeloid cells to distinguish the disease severity. However, correlation analysis between the concentration of plasma chemokines and IL-22R1+ cell subsets indicates that some subsets have protective effects, while others have pro-inflammatory effects (93, 94). Without stimulation, CD4+-T and NK lymphocytes produced IL-22. CD4+-T cells expressed IL-22R1, and its expression density defines two different functional subsets. The number of IL-22R1+ intermediate monocytes is negatively correlated with IFN-α, CRP and IL-6 in non-serious SARS-CoV-2 infection, whereas pDC and IL-22R1+ classical monocytes are positively correlated with pro-inflammatory chemokines IP-10 and MCP-1 in serious SARS-CoV-2 infections. Besides, researchers further demonstrate that IL-22 can reduce the expression of viral entry receptors, such as ACE2, TMPRSS2, and increase the expression of anti-viral proteins through IL-22/IL-22R1 pathway ( Figure 4 ) (95). Thus, these findings suggest that the IL-22/IL-22R1 signaling is involved in the pathological process of COVID-19, which can be protective during SARS-CoV-2 infections (93–95).
ILCs in COVID-19 patients
Recently, studies have demonstrated the roles of ILCs (innate lymphoid cells) in COVID-19 patients. Blood from severely infected COVID-19 patients were found that had fewer ILC precursor cells and ILCs than those mild cases. Of importance, in these severely infected COVID-19 patients, the expression of CD69 in ILCs was higher, which as a marker for tissue homing and activation. ILCs-CD69+ increased and blood ILCs decreased in severe infections, indicating that severely infected COVID-19 patients have more lung homing and activation (96). Further findings corroborate these results by confirming that higher ILCs abundance in blood was associated with shorter hospitalization. Additionally, the number of ILCs in the blood of hospitalized patients with SARS-CoV-2 infection decreased by 1.8 times (97). Collectively, these studies demonstrate that there are correlations between severe COVID-19 and decreased ILCs in the blood. As IL-22 plays critical functions in epithelial barrier integrity and ILCs are identified as the major source of IL-22 in response to lung pathogens stimulation (98), further studies are urgently needed to assess their exact roles in SARS-CoV-2 infection since current results regarding IL-22 and ILCs in COVID-19 individuals are obtained via analysis of blood periphery ( Figure 4 ).
Concerns of Interleukin‐22 application
Although IL-22 possesses definite immunomodulatory properties and tissue-protective effects, mainly via inhibiting apoptosis and promoting proliferation of epithelial cells, these same effects have also been involved in pathological states such as psoriasis, rheumatoid arthritis, and malignant tumors (99, 100). Additionally, functions of IL-22 have been implicated in host defense within barrier-tissues such as the skin, oral mucosa, and intestine (100–102). The functional outcomes of IL-22 in immunomodulation may be either protective or pathologic, suggesting that it is an extremely controversial interleukin (96–98). Systemic administration of IL-22 can upregulate the expression of proinflammatory cytokines including G-CSF and IL-6, and chemokines like chemokine (C-X-C motif) ligands CXCL1, CXCL5, CXCL9, which is sufficient to cause an inflammatory response (99). Studies indicate that IL-22 serum levels in psoriasis and rheumatoid arthritis patients are much higher than that the health individuals, and these also correlate with the disease severity (103, 104). Of note, IL-22-/- mice significantly alleviate collagen induced arthritis (105). In rheumatoid arthritis tissues, synovial fibroblasts are considered to be the key cellular target of IL-22, which may drive the proliferation of this cell type through STAT3 (106). Besides, synovial-fibroblasts activation via IL-22 can upregulate the expression of NF-KB ligand and CCL2, which promote inflammation and joint destruction (106, 107). Inhibition of IL-22 biological activity can also reduce the severity of psoriasis, which is also consistent with the psoriasis like symptoms in IL-22 transgenic mice (108–110). Keratinocytes are targets of IL-22 in psoriasis, and IL-22 regulates the expression of key inflammatory parameters by keratinocytes, including matrix metalloproteinases-1, IL-20 and CXCL5 (108–111). It is worth mentioning that studies have also suggested that IL-22 may also promote lung pathology during chronic exposure to Aspergillus fumigatus (112). As overactivation of STAT3 in a variety of human tumors, IL-22 is considered to be associated with many tumorigeneses (113, 114). Similarly, elevated levels of IL-22 can also be detected in human cancers, including hepatocellular carcinoma and gastric cancer as well as non-small cell lung cancer (15). The related consequences of high levels of IL-22 have been fully illustrated in the diethylnitrosamin induced hepatocellular carcinoma model, where IL-22 transgenic mice show increased and IL–22-/- mice decreased tumor formation (115). These complicated biological functions result in the difficulties of IL-22 clinical application.
Conclusions and future perspectives
In summary, it can be concluded that IL-22 is a crucial modulator of epithelial homeostasis and a regulator of host defense in the lung. It provides communication channels that allow hematopoietic cells, especially lymphocytes, to trigger pleiotropic responses in the epithelium to maintain barrier homeostasis against pulmonary pathogens while protecting the lungs from invasion or damage. IL-22 participates in various lung diseases through epithelial protection or regeneration, making it an extremely attractive cytokine for the treatment of COVID-19. Of note, the FDA has approved several research groups to study the efficacy of IL-22 during SARS-CoV-2 infection (116–118). These clinical trials suggest that IL-22 treatment can shorten the duration of Intensive Care Unit (ICU) stay. Therefore, it is necessary to further comprehensively understand the specific mechanisms and functions of IL-22 in regulating the lung microenvironment, which could enable to identify novel immunotherapeutic strategy for COVID-19. However, IL-22 has been demonstrated to promote tumor growth and accelerate inflammation in a few animal models. The therapeutic consequences of IL-22 in either direction for COVID-19 treatment should be evaluated, which will provide useful insights into its role in lung health.
Author contributions
WC and SF wrote the manuscript. WC, YL and DJ designed the structures and supervised the work. The final version of this paper has been approved by all authors.
Funding
Our work is partially supported by Shanghai Municipal Science and Technology Major Project [No.2018SHZDZX01] and National Natural Science Foundation of China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan. China Lancet (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of COVID-19: current state of the science. Immunity (2020) 52:910–41. doi: 10.1016/j.immuni.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong LR, Perlman S. Immune dysregulation and immunopathology induced by SARS-CoV-2 and related coronaviruses - are we our own worst enemy? Nat Rev Immunol Nov (2021) 22:47–56. doi: 10.1038/s41577-021-00656-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li J, Lai S, Gao GF, Shi. The emergence W. Genomic diversity and global spread of SARS-CoV-2. Nature (2021) 600:408–18. doi: 10.1038/s41586-021-04188-6 [DOI] [PubMed] [Google Scholar]
- 5. Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science (2020) 369:eabc8511. doi: 10.1126/science.abc8511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuri-Cervantes L, Pampena MB, Meng W, Rosenfeld AM, Ittner CAG, Weisman AR, et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol (2020) 5:eabd7114. doi: 10.1126/sciimmunol.abd7114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song J, Zhang C, Fan X, Meng F, Xu Z, Xia P, et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat Commun (2020) 11:3410. doi: 10.1038/s41467-020-17240-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhartet JD, et al. Tocilizumab in patients hospitalized with covid-19 pneumonia. N Engl J Med (2021) 384:20–30. doi: 10.1056/NEJMoa2030340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown MJ, Alazawi W, Kanoni S. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med (2021) 384:1491–502. doi: 10.1056/NEJMc2108482 [DOI] [PubMed] [Google Scholar]
- 10. Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discovery (2014) 13:21–38. doi: 10.1038/nrd4176 [DOI] [PubMed] [Google Scholar]
- 11. Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL‐10‐related T cell‐derived inducible factor (IL‐TIF), a novel cytokine structurally related to IL‐10 and inducible by IL‐9. J Immunol (2000) 164:1814–9. doi: 10.4049/jimmunol.164.4.1814 [DOI] [PubMed] [Google Scholar]
- 12. Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptorrelated proteins CRF2-4 and IL-22R. J Biol Chem (2000) 275:31335–9. doi: 10.1074/jbc.M005304200 [DOI] [PubMed] [Google Scholar]
- 13. Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, et al. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rβ) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem (2001) 276:2725–32. doi: 10.1074/jbc.M007837200 [DOI] [PubMed] [Google Scholar]
- 14. Rutz S, Wang X, Ouyang W. The IL-20 subfamily of cytokines–from host defence to tissue homeostasis. Nat Rev Immunol (2014) 14:783–95. doi: 10.1038/nri3766 [DOI] [PubMed] [Google Scholar]
- 15. Ouyang W, O'Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity (2019) 50:871–91. doi: 10.1016/j.immuni.2019.03.020 [DOI] [PubMed] [Google Scholar]
- 16. Wang S, Yao Y, Yao M, Fu P, Wang W. Interleukin-22 promotes triple negative breast cancer cells migration and paclitaxel resistance through JAK-STAT3/MAPKs/AKT signaling pathways. Biochem Biophys Res Commun (2018) 503:1605–9. doi: 10.1016/j.bbrc.2018.07.088 [DOI] [PubMed] [Google Scholar]
- 17. Calautti E, Avalle L, Poli V. Psoriasis: A STAT3-centric view. Int J Mol Sci (2018) 19:171. doi: 10.3390/ijms19010171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bai L, Fang H, Xia S, Zhang R, Li L, Ochando J, et al. STAT1 activation represses IL-22 gene expression and psoriasis pathogenesis. Biochem Biophys Res Commun (2018) 501:563–9. doi: 10.1016/j.bbrc.2018.05.042 [DOI] [PubMed] [Google Scholar]
- 19. Chen W, Zhang X, Fan J, Zai W, Luan J, Li Y, et al. Tethering interleukin-22 to apolipoprotein a-I ameliorates mice from acetaminophen-induced liver injury. Theranostics (2017) 7:4135–48. doi: 10.7150/thno.20955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen W, Luan J, Wei G, Zhang X, Fan J, Zai W, et al. In vivo hepatocellular expression of interleukin-22 using penetratin-based hybrid nanoparticles as potential anti-hepatitis therapeutics. Biomaterials (2018) 187:66–80. doi: 10.1016/j.biomaterials.2018.09.046 [DOI] [PubMed] [Google Scholar]
- 21. Zhang Z, Zou J, Shi Z, Zhang B, Etienne-Mesmin L, Wang Y, et al. IL-22-induced cell extrusion and IL-18-induced cell death prevent and cure rotavirus infection. Sci Immunol (2020) 5:eabd2876. doi: 10.1126/sciimmunol.abd2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neil JA, Matsuzawa-Ishimoto Y, Kernbauer-Hölzl E, Schuster SL, Sota S, Venzon M, et al. IFN-I and IL-22 mediate protective effects of intestinal viral infection. Nat Microbiol (2019) 4:1737–49. doi: 10.1038/s41564-019-0470-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shabgah AG, Navashenaq JG, Shabgah OG, Mohammadi H, Sahebkar A. Interleukin-22 in human inflammatory diseases and viral infections. Autoimmun Rev (2017) 16:1209–18. doi: 10.1016/j.autrev.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 24. Shi Z, Zou J, Zhang X, Zhao X, Noriega J, Zhang B, et al. Segmented filamentous bacteria prevent and cure rotavirus infection. Cell (2019) 179:644–58. doi: 10.1016/j.cell.2019.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Heide RSV. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from new Orleans. Lancet Respir Med (2020) 8:681–6. doi: 10.1016/S2213-2600(20)30243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keam S, Megawati D, Patel SK, Tiwari R, Dhama K, Harapan H. Immunopathology and immunotherapeutic strategies in severe acute respiratory syndrome coronavirus 2 infection. Rev Med Virol (2020) 30:e2123. doi: 10.1002/rmv.2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med (2020) 8:420–2. doi: 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ (2020) 371:m3862. doi: 10.1136/bmj.m3862 [DOI] [PubMed] [Google Scholar]
- 29. Behnood SA, Shafran R, Bennett SD, Zhang AXD, Mahoney LL, Stephenson TJ, et al. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: A meta-analysis of controlled and uncontrolled studies. J Infect (2022) 84:158–70. doi: 10.1016/j.jinf.2021.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fahriani M, Ilmawan M, Fajar JK, Maliga HA, Frediansyah A, Masyeni S, et al. Persistence of long COVID symptoms in COVID-19 survivors worldwide and its potential pathogenesis - a systematic review and meta-analysis. Narra J (2021) 1:e36. doi: 10.52225/narraj.v1i2.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fajar JK, Ilmawan M, Mamada S, Mutiawati E, Husnah M, Yusuf H, et al. Global prevalence of persistent neuromuscular symptoms and the possible pathomechanisms in COVID-19 recovered individuals: A systematic review and meta-analysis. Narra J (2021) 1:e48. doi: 10.52225/narra.v1i3.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med (2020) 383:590–2. doi: 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Z, Shen Q. H, Chang, vaccines for COVID-19: A systematic review of immunogenicity, current development, and future prospects. Front Immunol (2022) 13:843928. doi: 10.3389/fimmu.2022.843928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Antia R, Halloran ME. Transition to endemicity: Understanding COVID-19. Immunity (2021) 54:2172–6. doi: 10.1016/j.immuni.2021.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ogega CO, Skinner NE, Blair PW, Park H, Littlefield K, Ganesan A, et al. Durable SARS-CoV-2 b cell immunity after mild or severe disease. J Clin Invest (2021) 131:e145516. doi: 10.1172/JCI145516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Siracusano G, Pastori C, Lopalco L. Humoral immune responses in COVID-19 patients: a window on the state of the art. Front Immunol (2020) 11:1049. doi: 10.3389/fimmu.2020.01049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boyton RJ, Altmann DM. The immunology of asymptomatic SARS-CoV-2 infection: what are the key questions? Nat Rev Immunol (2021) 21:762–8. doi: 10.1038/s41577-021-00631-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shi L, Wang L, Xu R, Zhang C, Xie Y, Liu K, et al. Mesenchymal stem cell therapy for severe COVID-19. Signal Transduct Target Ther (2021) 6:339. doi: 10.1038/s41392-021-00754-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe (2020) 27:992–1000. doi: 10.1016/j.chom.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Caricchio R, Abbate A, Gordeev I, Meng J, Hsue PY, Neogi T, et al. Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19. JAMA (2021) 326:230–9. doi: 10.1001/jama.2021.9508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou T, Damsky W, Weizman OE, McGeary MK, Hartmann KP, Rosen CE, et al. IL-18BP is a secreted immune checkpoint and barrier to IL-18 immunotherapy. Nature (2020) 583:609–14. doi: 10.1038/s41586-020-2422-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Luca GD, Cavalli G, Campochiaro C, Della-Torre E, Angelillo P, Tomelleri A, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: A single-centre, prospective cohort study. Lancet Rheumatol (2020) 2:e465–73. doi: 10.1016/S2665-9913(20)30170-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duret PM, Sebbag E, Mallick A, Gravier S, Spielmann L, Messer L. Recovery from COVID-19 in a patient with spondyloarthritis treated with TNF-alpha inhibitor etanercept. Ann Rheum Dis (2020) 79:1251–2. doi: 10.1136/annrheumdis-2020-217362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patterson BK, Seethamraju H, Dhody K, Corley MJ, Kazempour K, Lalezari JP, et al. Disruption of the CCL5/RANTES-CCR5 pathway restores immune homeostasis and reduces plasma viral load in critical COVID-19. medRxiv (2020) 2020:20084673. doi: 10.1101/2020.05.02.20084673 [DOI] [Google Scholar]
- 45. Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. ACTT-2 study group members, baricitinib plus remdesivir for hospitalized adults with covid-19. N Engl J Med (2020) 384:795–807. doi: 10.1056/NEJMoa2031994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, Derde L, Leavis H, Crevel R, et al. A guide to immunotherapy for COVID-19. Nat Med 28(2022) 28:39–50. doi: 10.1038/s41591-021-01643-9 [DOI] [PubMed] [Google Scholar]
- 47. Adrover JM, Carrau L, Daßler-Plenker J, Bram Y, Chandar V, Houghton S, et al. Disulfiram inhibits neutrophil extracellular trap formation and protects rodents from acute lung injury and SARS-CoV-2 infection. JCI Insight (2022) 7:e157342. doi: 10.1172/jci.insight.157342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Farnoosh G, Akbariqomi M, Badri T, Bagheri M, Izadi M, Saeedi-Boroujeni A, et al. Efficacy of a low dose of melatonin as an adjunctive therapy in hospitalized patients with COVID-19: A randomized, double-blind clinical trial. Arch Med Res (2022) 53:79–85. doi: 10.1016/j.arcmed.2021.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Monk PD, Marsden RJ, Tear VJ, Brookes J, Batten TN, Mankowski M, et al. Inhaled interferon beta COVID-19 study group, safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: A randomised, double-blind, placebo-controlled, phase 2 trial, lancet respir. Med (2020) 9:196–206. doi: 10.1016/S2213-2600(20)30511-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang N, Zhan Y, Zhu L, Hou Z, Liu F, Song P, et al. Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients. Cell Host Microbe (2020) 28:455–64. doi: 10.1016/j.chom.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA (2020) 324:1330–41. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hatzl S, Krause R, Schilcher G. Early treatment with sotrovimab for covid-19. N Engl J Med (2022) 386:1480. doi: 10.1056/NEJMc2201606 [DOI] [PubMed] [Google Scholar]
- 53. Hoffmann M, Arora P, Gross R, Seidel A, Hörnich BF, Hahn AS, et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell (2021) 184:2384–93. doi: 10.1016/j.cell.2021.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kooistra EJ, Waalders NJB, Grondman I, Janssen NAF, de Nooijer AH, Netea MG, et al. RCI-COVID-19 study group, anakinra treatment in critically ill COVID-19 patients: A prospective cohort study. Crit Care (2020) 24:688. doi: 10.1186/s13054-020-03364-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. WHO Solidarity Trial Consortium. Pan H, Peto R, Henao-Restrepo A, Preziosi M, Sathiyamoorthy V, et al. Repurposed antiviral drugs for Covid-19–interim WHO solidarity trial results. N Engl J Med (2020) 384:497–511. doi: 10.1056/NEJMoa2023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Andreakos E, Tsiodras S. COVID-19: Lambda interferon against viral load and hyperinflammation. EMBO Mol Med (2020) 12:e12465. doi: 10.15252/emmm.202012465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jones BE, Brown-Augsburger PL, Corbett KS, Westendorf K, Davies J, Cujec TP, et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci Transl Med (2021) 13:eabf1906. doi: 10.1126/scitranslmed.abf1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dudakov JA, Hanash AM, van den Brink MR. Interleukin 22: immunobiology and pathology. Annu Rev Immunol (2015) 33:747–85. doi: 10.1146/annurev-immunol-032414-112123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, et al. The aryl hydrocarbon receptor interacts with c-maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol (2010) 11:854–61. doi: 10.1038/ni.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol (2010) 11:846–53. doi: 10.1038/ni.1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of t(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature (2008) 453:65–71. doi: 10.1038/nature06880 [DOI] [PubMed] [Google Scholar]
- 62. Qiu J, Guo X, Chen ZM, He J, Sonnenberg GF, Artis D, et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity (2013) 39:386–99. doi: 10.1016/j.immuni.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stockinger B, Meglio PD, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol (2014) 32:403–32. doi: 10.1146/annurev-immunol-032713-120245 [DOI] [PubMed] [Google Scholar]
- 64. Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of notch. Nat Immunol (2011) 13:144–51. doi: 10.1038/ni.2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Alam MS, Maekawa Y, Kitamura A, Tanigaki K, Yoshimoto T, Kishihara K, et al. Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc Natl Acad Sci USA (2010) 107:5943–8. doi: 10.1073/pnas.0911755107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Robb CT, McSorley HJ, Lee J, Aoki T, Yu C, Crittenden S, et al. Prostaglandin E2 stimulates adaptive IL-22 production and promotes allergic contact dermatitis. J Allergy Clin Immunol (2018) 141:152–62. doi: 10.1016/j.jaci.2017.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Duffin R, O’Connor RA, Crittenden S, Forster T, Yu C, Zheng X, et al. Prostaglandin E2 constrains systemic inflammation through an innate lymphoid cell-IL-22 axis. Science (2016) 351:1333–8. doi: 10.1126/science.aad9903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Araújo EF, Preite NW, Veldhoen M, Loures FV, Calich VLG. Pulmonary paracoccidioidomycosis in AhR deficient hosts is severe and associated with defective treg and Th22 responses. Sci Rep (2020) 10:11312. doi: 10.1038/s41598-020-68322-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Michaudel C, Bataille F, Maillet I, Fauconnier L, Colas C, Sokol H, et al. Ozone-induced aryl hydrocarbon receptor activation controls lung inflammation via interleukin-22 modulation. Front Immunol (2020) 11:144. doi: 10.3389/fimmu.2020.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ivanov S, Renneson J, Fontaine J, Barthelemy A, Paget C, Fernandez EM, et al. Interleukin-22 reduces lung inflammation during influenza a virus infection and protects against secondary bacterial infection. J Virol (2013) 87:6911–24. doi: 10.1128/JVI.02943-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Simonian PL, Wehrmann F, Roark CL, Born WK, O'Brien RL, Fontenot AP. γδ T cells protect against lung fibrosis via IL-22. J Exp Med (2010) 207:2239–53. doi: 10.1084/jem.20100061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Trevejo-Nunez G, Elsegeiny W, Conboy P, Chen K, Kolls JK. Critical role of IL-22/IL22-RA1 signaling in pneumococcal pneumonia. J Immunol (2016) 197:1877–83. doi: 10.4049/jimmunol.1600528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against gram-negative bacterial pneumonia. Nat Med (2008) 14:275–81. doi: 10.1038/nm1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Maele LV, Carnoy C, Cayet D, Ivanov S, Porte R, Deruy E, et al. Activation of type 3 innate lymphoid cells and interleukin 22 secretion in the lungs during streptococcus pneumoniae infection. J Infect Dis (2014) 210:493–503. doi: 10.1093/infdis/jiu106 [DOI] [PubMed] [Google Scholar]
- 75. Xu X, Weiss ID, Zhang HH, Singh SP, Wynn TA, Wilson MS, et al. Conventional NK cells can produce IL-22 and promote host defense in klebsiella pneumoniae pneumonia. J Immunol (2014) 192:1778–86. doi: 10.4049/jimmunol.1300039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Peng Y, Gao X, Yang J, Shekhar S, Wang S S, Zhao W, et al. IL-22 promotes Th1/Th17 immunity in chlamydial lung infection. Mol Med (2014) 20:109–19. doi: 10.2119/molmed.2013.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gessner MA, Werner JL, Lilly LM, Nelson MP, Metz AE, Dunaway CW, et al. Dectin-1-dependent interleukin-22 contributes to early innate lung defense against aspergillus fumigatus. Infect Immun (2012) 80:410–17. doi: 10.1128/IAI.05939-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hoegl S, Bachmann M, Scheiermann P, Goren I, Hofstetter C, Pfeilschifter J, et al. Protective properties of inhaled IL-22 in a model of ventilator-induced lung injury. Am J Respir Cell Mol Biol (2011) 44:369–76. doi: 10.1165/rcmb.2009-0440OC [DOI] [PubMed] [Google Scholar]
- 79. Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med (2010) 207:1293–305. doi: 10.1084/jem.20092054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gu P, Wang D, Zhang J, Wang X, Chen Z, Gu L, et al. Protective function of interleukin-22 in pulmonary fibrosis. Clin Transl Med (2021) 11:e509. doi: 10.1002/ctm2.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Farfariello V, Amantini C, Nabissi M, Morelli MB, Aperio C, Caprodossi S, et al. IL-22 mRNA in peripheral blood mononuclear cells from allergic rhinitic and asthmatic pediatric patients. Pediatr Allergy Immunol (2011) 22:419–23. doi: 10.1111/j.1399-3038.2010.01116.x [DOI] [PubMed] [Google Scholar]
- 82. Besnard AG, Sabat R, Dumoutier L, Renauld J, Willart M, Lambrecht B, et al. Dual role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A, am. J Respir Crit Care Med (2011) 183:1153–63. doi: 10.1164/rccm.201008-1383OC [DOI] [PubMed] [Google Scholar]
- 83. Nakagome K, Imamura M, Kawahata K, Harada H, Okunishi K, Matsumoto T, et al. High expression of IL-22 suppresses antigen-induced immune responses and eosinophilic airway inflammation via an IL-10-associated mechanism. J Immunol (2011) 187:5077–89. doi: 10.4049/jimmunol.1001560 [DOI] [PubMed] [Google Scholar]
- 84. Takahashi K, Hirose K, Kawashima S, Niwa Y, Wakashin H, Iwata A, et al. IL-22 attenuates IL-25 production by lung epithelial cells and inhibits antigeninduced eosinophilic airway inflammation. J Allergy Clin Immunol (2011) 128:1067–76. doi: 10.1016/j.jaci.2011.06.018 [DOI] [PubMed] [Google Scholar]
- 85. Badi YE, Pavel AB, Pavlidis S, Riley JH, Bates S, Kermani NZ, et al. Mapping atopic dermatitis and anti-IL-22 response signatures to type 2-low severe neutrophilic asthma. J Allergy Clin Immunol (2022) 149:89–101. doi: 10.1016/j.jaci.2021.04.010 [DOI] [PubMed] [Google Scholar]
- 86. Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, Fattman CL, et al. Il-22 is essential for lung epithelial repair following influenza infection. Am J Pathol (2013) 182:1286–96. doi: 10.1016/j.ajpath.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kumar P, Thakar MS, Ouyang W, Malarkannan S. IL-22 from conventional nk cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol (2013) 6:69–82. doi: 10.1038/mi.2012.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Das S, Croix CS, Good M, Chen J, Zhao J, Hu S, et al. Interleukin-22 inhibits respiratory syncytial virus production by blocking virus-mediated subversion of cellular autophagy. iScience (2020) 23:101256. doi: 10.1016/j.isci.2020.101256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cagan E, Tezcan G, Simsek A, Kizmaz MA, Dombaz F, Asan A, et al. The age-dependent role of th22, tc22, and tc17 cells in the severity of pneumonia in covid-19 immunopathogenesis. Viral Immunol (2022) (35):318–27. doi: 10.1089/vim.2021.0132 [DOI] [PubMed] [Google Scholar]
- 90. Hebert KD, Mclaughlin N, Zhang Z, Cipriani A, Alcorn JF, Pociask DA. Il-22ra1 is induced during influenza infection by direct and indirect tlr3 induction of STAT1. Respir Res (2019) 20:184. doi: 10.1186/s12931-019-1153-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brias SG, Stack G, Stacey MA, Redwood AJ, Humphreys IR. The role of il-22 in viral infections: paradigms and paradoxes. Front Immunol (2016) 7:211. doi: 10.3389/fimmu.2016.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zenewicz LA. Il-22: there is a gap in our knowledge. ImmunoHorizons (2018) 2:198–207. doi: 10.4049/immunohorizons.1800006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Albayrak N, Cano CO, Karimi S, Dogahe D, Praet AV, Godefroid A, et al. Distinct expression patterns of interleukin-22 receptor 1 on blood hematopoietic cells in SARS-CoV-2 infection. Front Immunol (2022) 13:769839. doi: 10.3389/fimmu.2022.769839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Savan R, McFarland AP, Reynolds DA, Feigenbaum L, Ramakrishnan K, Karwan M, et al. A novel role for il-22r1 as a driver of inflammation. Blood (2011) 117:575–84. doi: 10.1182/blood-2010-05-285908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Klooster JPT, Bol-Schoenmakers M, van Summeren K, van Vliet ALW, de Haan CAM, van Kuppeveld FJM, et al. Enterocytes, fibroblasts and myeloid cells synergize in anti-bacterial and anti-viral pathways with IL22 as the central cytokine. Commun Biol (2021) (4):631. doi: 10.1038/s42003-021-02176-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Garcıa M, Kokkinou E, Garća AC, Parrot T, Palma Medina LM, Maleki KT, et al. Innate lymphoid cell composition associates with covid-19 disease severity. Clin Transl Immunol (2020) 9:e1224. doi: 10.1002/cti2.1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hoffmann JP, Kolls JK, McCombs JE. Regulation and function of ILC3s in pulmonary infections. Front Immunol (2021) 12:672523. doi: 10.3389/fimmu.2021.672523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Alcorn JF. Il-22 plays a critical role in maintaining epithelial integrity during pulmonary infection. Front Immunol (2020) 11:1160. doi: 10.3389/fimmu.2020.01160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by il-22. Nat Imm (2011) 12:383–90. doi: 10.1038/ni.2025 [DOI] [PubMed] [Google Scholar]
- 100. Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol (2010) 107:1–29. doi: 10.1016/B978-0-12-381300-8.00001-0 [DOI] [PubMed] [Google Scholar]
- 101. Fabre T, Molina MF, Soucy G, Goulet JP, Willems B, Villeneuve JP, et al. Type 3 cytokines IL-17A and IL-22 drive TGF-beta-dependent liver fibrosis. Sci Immunol (2018) 3:eaar7754. doi: 10.1126/sciimmunol.aar7754 [DOI] [PubMed] [Google Scholar]
- 102. Powell N, Pantazi E, Pavlidis P, Tsakmaki A, Li K, Yang F, et al. Interleukin-22 orchestrates a pathological endoplasmic reticulum stress response transcriptional programme in colonic epithelial cells. Gut (2020) 69:578–90. doi: 10.1136/gutjnl-2019-318483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Nakajima H, Nakajima K, Tarutani M, Morishige R, Sano S. Kinetics of circulating Th17 cytokines and adipokines in psoriasis patients. Arch Dermatol Res (2011) 303:451–5. doi: 10.1007/s00403-011-1159-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Leipe J, Schramm MA, Grunke M, Baeuerle M, Dechant C, Nigg AP, et al. Interleukin 22 serum levels are associated with radiographic progression in rheumatoid arthritis. Ann Rheum Dis (2011) 70:1453–7. doi: 10.1136/ard.2011.152074 [DOI] [PubMed] [Google Scholar]
- 105. Geboes L, Dumoutier L, Kelchtermans H, Schurgers E, Mitera T, Renauld JC, et al. Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheumatol (2009) 60:390–5. doi: 10.1002/art.24220 [DOI] [PubMed] [Google Scholar]
- 106. Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, et al. Expression of interleukin-22 in rheumatoid arthritis:potential role as a proinflammatory cytokine. Arthritis Rheumatol (2005) 52:1037–46. doi: 10.1002/art.20965 [DOI] [PubMed] [Google Scholar]
- 107. Kim KW, Kim HR, Park JY, Park JS, Oh HJ, Woo YJ, et al. Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts. Arthritis Rheumatol (2012) 64:1015–23. doi: 10.1002/art.33446 [DOI] [PubMed] [Google Scholar]
- 108. Van Belle AB, de Heusch M, Lemaire MM, Hendrickx E, Warnier G, Dunussi Joannopoulos K, et al. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol (2012) 188:462–9. doi: 10.4049/jimmunol.1102224 [DOI] [PubMed] [Google Scholar]
- 109. Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: implications for human liver disease progression. Hepatology (2011) 54:252–61. doi: 10.1002/hep.24339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Li C, Xu M, Coyne J J, Wang W, Davila ML, Wang Y, et al. Psoriasis-associated impairment of CCL27/CCR10-derived regulation leads to IL-17A/IL-22-producing skin T-cell overactivation. J Allergy Clin Immunol (2021) 147:759–63. doi: 10.1016/j.jaci.2020.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bellone M, Brevi A, Huber S. Microbiota-propelled t helper 17 cells in inflammatory diseases and cancer. Microbiol Mol Biol Rev (2020) (84):e00064-19. doi: 10.1128/MMBR.00064-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lilly LM, Gessner MA, Dunaway CW, Metz AE, Schwiebert L, Weaver CT, et al. The β-glucan receptor dectin-1 promotes lung immunopathology during fungal allergy via IL-22. J Immunol (2012) 189:3653–60. doi: 10.4049/jimmunol.1201797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Johnston PA, Grandis JR. STAT3 signaling: anticancer strategies and challenges. Mol Interv (2011) 11:18–26. doi: 10.1124/mi.11.1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Thilakasiri PS, Dmello RS, Nero TL, Parker MW, Ernst M, Chand AL. Repurposing of drugs as STAT3 inhibitors for cancer therapy. Semin Cancer Biol (2021) 68:31–46. doi: 10.1016/j.semcancer.2019.09.022 [DOI] [PubMed] [Google Scholar]
- 115. Jiang R, Tan Z, Deng L, Chen Y, Xia Y, Gao Y, et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology (2011) 54:900–9. doi: 10.1002/hep.24486 [DOI] [PubMed] [Google Scholar]
- 116. ClinicalTrials.gov . A study to evaluate the safety and efficacy of mstt1041a (astegolimab) or uttr1147a in patients with severe covid-19 pneumonia (covastil) (2020). Available at: https://clinicaltrials.gov/ct2/show/NCT04386616.
- 117. Clinicaltrials.gov . Study of f-652 (il-22: igg2 fusion protein) in patients with moderate to severe COVID-19 (2020). Available at: https://clinicaltrials.gov/ct2/show/NCT04498377.
- 118. McAleer JP, Kolls JK. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol Rev (2014) 260:129–44. doi: 10.1111/imr.12183 [DOI] [PMC free article] [PubMed] [Google Scholar]