Abstract
Patient: Male, 51-year-old
Final Diagnosis: Adeno virus liver failure
Symptoms: Liver failure
Medication: —
Clinical Procedure: —
Specialty: Transplantology
Objective:
Unusual clinical course
Background:
Human adenovirus is a well-known pathogen that can potentially lead to severe infection in immunocompromised patients. Adenovirus infections in solid-organ transplant recipients can range from asymptomatic to severe, prolonged, disseminated disease, and have a significant impact on morbidity, mortality, and graft survival. The clinical manifestations vary from asymptomatic and flu-like illness to severe life-threatening viremia with multi-organ failure. Post-transplant adenovirus infection is well described in kidney recipients, but in adult liver transplant recipients the impact of the virus is not well described. In this report, a case of disseminated adenovirus infection with subsequent fatal acute liver failure in a post-kidney transplant patient is presented.
Case Report:
A 51-year-old man underwent a deceased kidney transplantation for focal segmental glomerulosclerosis. Shortly after the kidney transplantation, he received multiple plasmapheresis with additional steroid treatments for cellular rejection and reoccurrence of his primary kidney disease. Three weeks after the kidney transplant, he developed a disseminated adenovirus infection with subsequent acute liver failure. Despite the early diagnosis and aggressive treatment, the patient died.
Conclusions:
Patients with organ transplantation with autoimmune background etiology are usually over-immunosuppressed to avoid early rejection. In this population, opportunistic infections are not rare. Fever, general malaise, and transplant organ dysfunction are the first signs of bacterial or viral infection. Early infectious diseases work-up, including tissue biopsy, is fundamental to establish a diagnosis. Broad antibiotic and possible antiviral aggressive treatment are mandatory.
Keywords: Adenovirus Infections, Human; Kidney Transplantation; Liver Failure, Acute
Background
Adenoviruses are DNA viruses that are classified into 7 subgroups (A–G) and these subgroups can be further divided into 52 serotypes [1]. Human adenovirus (HAdV) infections in immuno-competent subjects are common in all age groups of the population, and are usually asymptomatic and self-limiting. In immunocompromised and especially transplant patients, HAdV have been associated with severe, life-threatening infections that have a significant impact on morbidity, mortality, and graft survival [2]. In solid-organ adult transplantation, the incidence seems to be comparable in kidney and liver recipients, at 4.1% and 5.8%, respectively. The incidence is much higher in lung transplant recipients (22.5%) [3–5]. Several risk factors for adenovirus infection in solid-organ adult transplant recipients have already been described. One of the most consistent risk factors is immunosuppressive therapy. Exposure to lytic antibody therapy such as OKT3, thymoglobulin and basiliximab, as well as higher levels of maintenance immunosuppression therapy, have been proven to increase the risk for viral infection [6,7]. Use of adenovirus sero-mismatched donors is also a risk factor for HAdV infection, along with patient age and type of transplanted organ [8].
HAdV infection in kidney transplantation has been well described. In this population, the median time to diagnosis of infection was 1.25 months (range 0.5–75 months), with 76.5% of the infections being diagnosed within 3 months after kidney transplant [6]. Clinical presentation can vary from fever and dysuria to hemorrhagic cystitis, tubular nephritis, and acute graft dysfunction [9,10].
Case Report
Our patient was a 51-year-old man who underwent deceased-donor kidney transplant for focal segmental glomerulosclerosis (FSGS). His pre-transplant serology screening was positive for EBV IgG only. He received standard immunosuppression consisting of thymoglobulin x 3 doses (1.5 mg/kg), mycophenolate mofetil 1000 mg p.o. twice a day, and tacrolimus with a daily goal of 8–10 ng/ml. His postoperative course was complicated by reoccurrence of FSGS requiring apheresis x 6 cycles and rituximab (375 mg/m2). Three weeks after initial transplant, the patient was admitted for open kidney biopsy due to increasing creatinine. The kidney biopsy was suspicious for acute cellular rejection, and the patient received another 2 cycles of apheresis as well as a steroid pulse (Solumedrol 250 mg daily for 3 days with subsequent prednisone taper) and was sent home. After 3 days (25 days after kidney transplantation), he was readmitted for general malaise, fever, and leukopenia. At that point it was found that his liver enzymes were slightly elevated. His condition rapidly deteriorated over the following 24 h with the development of severe acute liver and kidney failure. A liver biopsy was performed, revealing acute adenoviral hepatitis with extensive necrosis (Figures 1, 2). CT abdomen/pelvis with triple i.v. contrast showed moderate hepatic steatosis. There was decreased enhancement along the medial aspect of the right hepatic lobe, including portions of segments 5–8 on the portal venous phase, without an obvious mass or vascular thrombosis.
Figure 1.
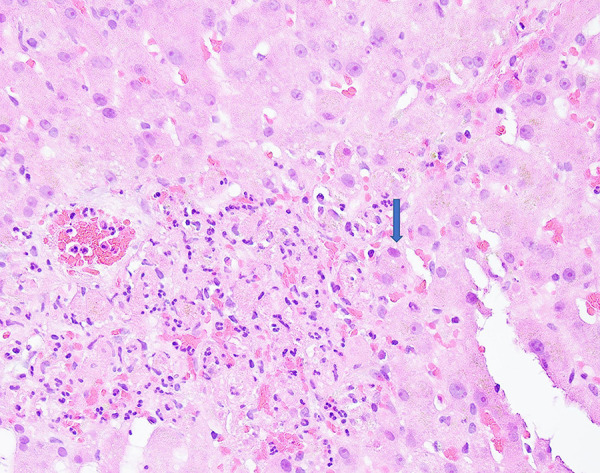
Adenovirus hepatitis. High-power view of the liver biopsy shows a focus of neutrophilic inflammatory infiltrate with necrotic hepatocytes. The adjacent viable hepatocytes demonstrate large and basophilic smudgy nuclei-adenovirus inclusion (arrow, ×600).
Figure 2.
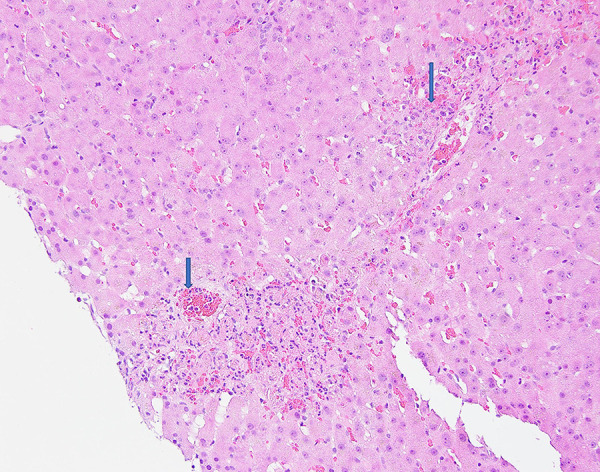
Adenovirus hepatitis: Low-power view of the liver biopsy shows extensive hepatic necrosis.
The patient became hemodynamically unstable, requiring several vasopressors. His liver enzymes markedly increased (ALT 10 000 IU/l) and synthetic function tests worsened (INR 7) (Table 1). He also developed severe hyperkalemia despite hemodialysis. Viral blood serology revealed acute adenoviral infection (DNA levels 7×108 copies/ml). Liver transplant work-up was started for potential liver transplantation on day 2, but unfortunately the patient died 72 h after hospital admission.
Table 1.
Index laboratory tests at presentation and the day of death.
| Lab | Index | ||||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 – Death | ||
| WBC | 1.4 | 1.0 | 3.5 | 1.5 | |
| AST | 546 | 1992 | 6340 | 6420 | |
| ALT | 556 | 1323 | 11800 | 12460 | |
| Alkaline phosphatase | 87 | 101 | 274 | 199 | |
| Total bilirubin | 0.4 | 1.2 | 5.7 | 3.6 | |
| INR | 1.8 | 2.1 | 3.78 | 6.88 | |
| Na | 136 | 135 | 143 | 164 | |
| K | 4.0 | 4.0 | 5.2 | 7.0 | |
| Creatinine | 1.7 | 1.72 | 3.05 | 1.7 | |
| Lactate | – | – | 13.9 | >18 | |
Discussion
FSGS recurrence after kidney transplantation is a major risk factor for graft loss. Idiopathic FSGS recurs post-transplant in one-third of cases and is associated with a five5-fold higher risk of graft loss [11]. Plasmapheresis and rituximab are the most frequent treatments. Response to treatment is associated with significantly better outcomes but is achieved in only half of cases. In this case scenario, the patient also received thymoglobulin and pulse steroids as a treatment of acute cellular rejection of the kidney. The adenovirus infection with subsequent acute liver failure was likely precipitated by his increased immunosuppression regimen.
Acute liver failure (ALF) is a consequence of a quick and vast insult to the liver, resulting in a multisystem illness characterized by severe coagulopathy, encephalopathy, and subsequent multi-organ failure. The most common cause of acute liver failure in transplant patients is viral infections and drug-induced liver injury [12]. In immunocompromised recipients, typical viral infections that could lead to ALF are herpes virus, Epstein-Barr virus, and cytomegalovirus, and, rarely, adenovirus and parvoviruses. In fact, there is only 1 case described similar to this [13].
Currently, transplant protocols for solid-organ transplant do not include the evaluation of anti-adenovirus serostatus. It is considered to be of little benefit due to the existence of many different serotypes. Thus, patients cannot be stratified into high-/low-risk groups (as is in CMV or Herpes screening).
The most important component of therapy remains supportive care and decreasing the amount of immunosuppression. The use of antiviral agents has not been FDA approved for the treatment of adenoviral infection or disease and is not supported by prospective randomized clinical trials [2]. In most transplant centers, however, intravenous cidofovir is considered the standard practice for treatment of severe adenovirus disease [14,15]. Unfortunately, in this clinical case the aggressiveness of the HAdV infection was so prominent that there was not enough time for more than supportive treatment.
Conclusions
Screening for rare viruses should be considered higher priority for all transplant patients with abnormal liver function. Liver biopsy and broad-spectrum antibiotics and, in some cases, viral agents need to be started empirically early in the clinical course. The prognosis for transplant patients with ALF is grim and these patients are probably poor candidates for liver transplantation. Over-immunosuppression should be avoided to prevent rare opportunistic viral infections in transplanted patients.
Abbreviations
- HAdV
human adenovirus;
- FSGS
focal segmental glomerulosclerosis;
- ALF
acute liver failure
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Echavarria M. Adenoviruses in immunocompromised hosts. ClinMicrobiol Rev. 2008;21:704–15. doi: 10.1128/CMR.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Florescu DF, Hoffman JA, AST Infectious Diseases Community of Practice Adenovirus in solid organ transplantation. Am J Transplant. 2013;13(Suppl. 4):206–11. doi: 10.1111/ajt.12112. [DOI] [PubMed] [Google Scholar]
- 3.Watcharananan SP, Avery R, Ingsathit A. Adenovirus disease after kidney transplantation: course of infection and outcome in relation to blood viral load and immune recovery. Am J Transplant. 2011;11:1308–14. doi: 10.1111/j.1600-6143.2011.03479.x. [DOI] [PubMed] [Google Scholar]
- 4.Haq A, Gregston A, Elwir S, Spak CW. Treatment of viral hepatitis due to adenovirus in a liver transplantation recipient: The clinical use of cidofovir and intravenous immunoglobulin. Liver Transpl. 2022;28(3):505–7. doi: 10.1002/lt.26266. [DOI] [PubMed] [Google Scholar]
- 5.Humar A. Reactivation of viruses in solid organ transplant patients receiving cytomegalovirus prophylaxis. Transplantation. 2006;82:S9–S14. doi: 10.1097/01.tp.0000230432.39447.8b. [DOI] [PubMed] [Google Scholar]
- 6.Felipe C, Ferreira AN, de Paula M, Viana L. Incidence and risk factors associated with cytomegalovirus infection after the treatment of acute rejection during the first year in kidney transplant recipients receiving preemptive therapy. Transpl Infect Dis. 2019;21(6):e13106. doi: 10.1111/tid.13106. [DOI] [PubMed] [Google Scholar]
- 7.Cintra-Cabrera M, Suárez-Benjumea A, Bernal-Blanco G. Resistant cytomegalovirus infection after renal transplantation: Literature review. Transplant Proc. 2018;50(2):575–77. doi: 10.1016/j.transproceed.2017.09.058. [DOI] [PubMed] [Google Scholar]
- 8.Al-Heeti OM, Cathro HP, Ison MG. Adenovirus infection and transplantation. Transplantation. 2022;106(5):920–27. doi: 10.1097/TP.0000000000003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofland CA, Eron LJ, Washecka RM. Hemorrhagic adenovirus cystitis after renal transplantation. Transplant Proc. 2004;36:3025–27. doi: 10.1016/j.transproceed.2004.10.090. [DOI] [PubMed] [Google Scholar]
- 10.Hatlen T, Mroch H, Tuttle K. Disseminated adenovirus nephritis after kidney transplantation. Kidney Int Rep. 2017;3(1):19–23. doi: 10.1016/j.ekir.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uffing A, Pérez-Sáez MJ, Mazzali M, et al. Recurrence of FSGS after kidney transplantation in adults. Clin J Am Soc Nephrol. 2020;15(2):247–56. doi: 10.2215/CJN.08970719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Grady JG. Acute liver failure. Postgrad Med J. 2005;81:148–54. doi: 10.1136/pgmj.2004.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norris SH, Butler TC, Glass N, Tran R. Fatal hepatic necrosis caused by disseminated type 5 adenovirus infection in a renal transplant recipient. Am J Nephrol. 1989;9(2):101–5. doi: 10.1159/000167945. [DOI] [PubMed] [Google Scholar]
- 14.Florescu DF, Schaenman JM. AST Infectious Diseases Community of Practice. Adenovirus in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13527. doi: 10.1111/ctr.13527. [DOI] [PubMed] [Google Scholar]
- 15.Servais AM, Keck M, Leick M. Viral enteritis in intestinal transplant recipients. Transpl Infect Dis. 2020;22(2):e13248. doi: 10.1111/tid.13248. [DOI] [PubMed] [Google Scholar]


