Abstract
Introduction
A multicountry monkeypox disease (MPX) outbreak began in May 2022 in Europe, leading to the assessment as a potential Public Health Emergency of International Concern (PHEIC) on June 23, 2022. Some observational studies have partially characterised clinical features, hospitalisations, and deaths. However, no systematic reviews of this MPX outbreak have been published.
Methods
We performed a systematic review with meta-analysis, using five databases to assess clinical features, hospitalisations, complications and deaths of MPX confirmed or probable cases. Observational studies, case reports and case series, were included. We performed a random-effects model meta-analysis to calculate the pooled prevalence and 95% confidence interval (95% CI). In addition, we carried out a subgroup analysis according to the continents and a sensitivity analysis excluding studies classified as having a high risk of bias.
Results
A total of 19 articles were included, using only 12 articles in the quantitative synthesis (meta-analysis). For 1958 patients, rash (93%, 95% CI 80–100%), fever (72%, 95% CI 30–99%), pruritus (65%, 95% CI 47–81%), and lymphadenopathy (62%, 47–76%), were the most prevalent manifestations. Among the patients, 35% (95% CI 14–59%) were hospitalised. Some 4% (95% CI 1–9%) of hospitalised patients had fatal outcomes (case fatality rate, CFR).
Conclusion
MPX is spreading rapidly, with a third of hospitalised patients, but less than 5% with fatal outcomes. As this zoonotic virus spreads globally, countries must urgently prepare human resources, infrastructure and facilities to treat patients according to the emerging guidelines and the most reliable clinical information.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12941-022-00527-1.
Keywords: Monkeypox, Orthopoxvirus, Poxviridae, Zoonotic, Clinical features, Laboratory, Outcomes, Epidemic
Introduction
Rationale
Monkeypox virus (MPXV), an Orthopoxvirus, is a genus that includes the smallpox virus [1, 2]. This emerging zoonotic agent was discovered in 1958 in Denmark among laboratory monkeys but had been causing human disease since 1970 in endemic countries in Africa [3–6]. This pathogen causes a two-clinical stage illness, including a prodrome during its invasive period and a cutaneous phase defined as the skin eruption [7]. Although described over decades, the current ongoing outbreak outside Africa seems to present atypical clinical manifestations compared to cases reported before 2022 in endemic countries [8–10]. The MPXV, taxonomically, is currently part of the genus Orthopoxvirus, which belongs to the subfamily Chordopoxvirinae in the family Poxviridae. That family is part of the order Chitovirales included in the class Pokkesviricetes. This class belongs to the phylum Nucleocytoviricota, in the kingdom Bamfordvirae, in the realm Varidnaviria [11–14].
Members of the genus Orthopoxvirus cause disease in humans and animals, as well as other members of the family Poxviridae affecting birds, goats, cervids, crocodiles, rabbits, and insects [6]. In the case of smallpox, this epidemic disease was eradicated by the 1980s after a successful global vaccination campaign. Since there, no vaccination against smallpox has continued [15]. As expected, several similarities and differences in the epidemiology, clinical features, and management of smallpox and monkeypox have been identified. These are enveloped double‐stranded DNA viruses with a genome ranging from 130 to 300 kbp (around 190 kbp for monkeypox) [16]. Therefore, a complete clinical characterisation of MPX disease, as well as cutaneous lesions, hospitalisation and outcome, is required.
Although only two months have elapsed since the global spreading of MPX in 2022 [17], some studies and case reports have been already published in major international scientific and medical journals from European and other countries with travel- and non-travel-related cases [8, 9, 18, 19]. Many of these reports have started to answer clinical questions, including evolution and outcomes, potential risk factors, and clinical, especially dermatological findings [20]; however, a systematic review consolidates what has been learned from each study or reported case is to date missing. Although systematic reviews and meta-analyses usually include randomised clinical trials (RCTs) and aim to provide a more precise estimate of the effect of a treatment or risk factor for disease, they also have been extensively used, especially during the last decades, to synthesised observational studies [21–23]. However, RCTs are not feasible or available in many situations, and only data from observational studies are accessible [23]. That is the case for the clinical, hospitalisation, and outcome features of MPX.
Objectives
To summarise the clinical features of MPX reported in currently available observational studies.
To assess the clinical spectrum of the cutaneous manifestations and their frequency.
To examine the outcome of MPX cases, including the proportion of patients requiring hospitalisation and those with fatal outcomes.
Methods
Protocol and registration
This protocol follows the recommendations established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [24], and it has been reported in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42022336855).
Databases and search strategy
A comprehensive search about the prevalence of clinical manifestations, characteristics of the lesions and complications of patients diagnosed with monkeypox was conducted on June 7, 2022, through the following databases: PubMed, Scopus, Embase, Ovid-Medline and Web of Science. No restrictions regarding language or publication date were applied. The search strategy was built using the Peer Review of Electronic Search Strategies (PRESS) Checklist, and we carried out a hand search of the reference lists of the included studies and preprints databases (The Lancet Preprints, medRxiv and ResearchSquare). The complete search strategy is available in Additional file 1: Table S1.
Study selection and data extraction
This systematic review had a comprehensive scope, including the subsequent observational studies: cross-sectional, cohort, case–control, case reports and case series. In addition, studies assessing the prevalence of the various clinical manifestations, characteristics of the lesions and complications of patients with a probable or confirmed monkeypox diagnosis, regardless of age, were included. The definitions of possible and confirmed cases for each study are described in Additional file 1: Table S2. Case series with more than 10 cases were included in the qualitative and quantitative synthesis; however, studies with less than ten were included only in the qualitative synthesis. Scoping reviews, narrative reviews, systematic reviews and conference abstracts were excluded.
All the articles resulting from the electronic search were exported to the data management software “Rayyan QCRI”, and duplicate records were removed. Titles and abstracts were independently screened by four reviewers ( JRU-B, EAA-B, MDM-R and EAH-B). After identifying the potential references to be included, the reviewers independently assessed the full text of each article. Conflicts or discrepancies in decisions were resolved through debate among the total of the authors, then a consensus was reached. The data from the included articles were extracted through a data extraction sheet built in Microsoft Excel. The following information was extracted: author, year of publication, and the number of probable or confirmed cases affected by the clinical characteristics or complications. We used the Web-based tool “WebPlotDigitizer” to extract data from graphs in case it was not available in numerical format [25].
Risk of bias assessment
We used the original Newcastle–Ottawa Scale (NOS) to assess the risk of bias in case–control and cohort studies, and the NOS was adapted for cross-sectional studies (NOS-CS). In both scales, a score of 7 or more stars was considered a low risk of bias, while a score of 6 or fewer stars was considered a high risk of bias. The quality assessment of case reports and case series were assessed with the Joanna Briggs Institute’s Checklist for Case Reports and Checklist for Case Series, respectively. As with the NOS and the NOS-C, the cut-off points for both checklists were seven stars. We assigned a star to every item answered as “Yes”; otherwise, it did not receive a star. In case multiple items were “Not applicable” for an article, the quality of the study was finally decided by consensus on a case-by-case basis. This examination was done independently by two reviewers (JRU-B and EAA-B).
Assessment of publication bias
The publication bias assessment in proportional meta-analysis is an evaluation that is not recommended in the current literature. That is because conventional funnel plots and Egger’s test are inaccurate for these analyses. The reason behind this is that funnel plots were created assuming that studies with positive results were published more frequently when compared to studies with negative results; however, in a meta-analysis of proportions, there is no consensus on what a positive result is. Moreover, there is no evidence that proportions adjust correctly to funnel plots or Egger’s tests [26, 27].
Statistical approach
The information collected from the included articles was combined using STATA 16.0. We conducted a pooled analysis of the various clinical manifestations, characteristics of the lesions and complications of patients with probable/confirmed monkeypox. A random-effects model (Dersimonian and Laird) was used for the quantitative analysis. The 95% Confidence Intervals for the proportions reported in each study were calculated using the Clopper-Pearson Method. The Freeman-Tukey Double Arcsine Transformation was used as the variance stabiliser. The Cochran’s Q test and the I2 statistic were used to assess the between-study-heterogeneity; values equal to or greater than 60% were classified as high heterogeneity for the I2 statistic, and a P-value < 0.05 was a sign of heterogeneity in the Cochran's Q test. In addition, we carried out a subgroup analysis according to the continent where the studies were conducted (when there were at least two studies to meta-analyse) and a sensitivity analysis excluding studies classified as having a high risk of bias.
Results
Study selection and characteristics
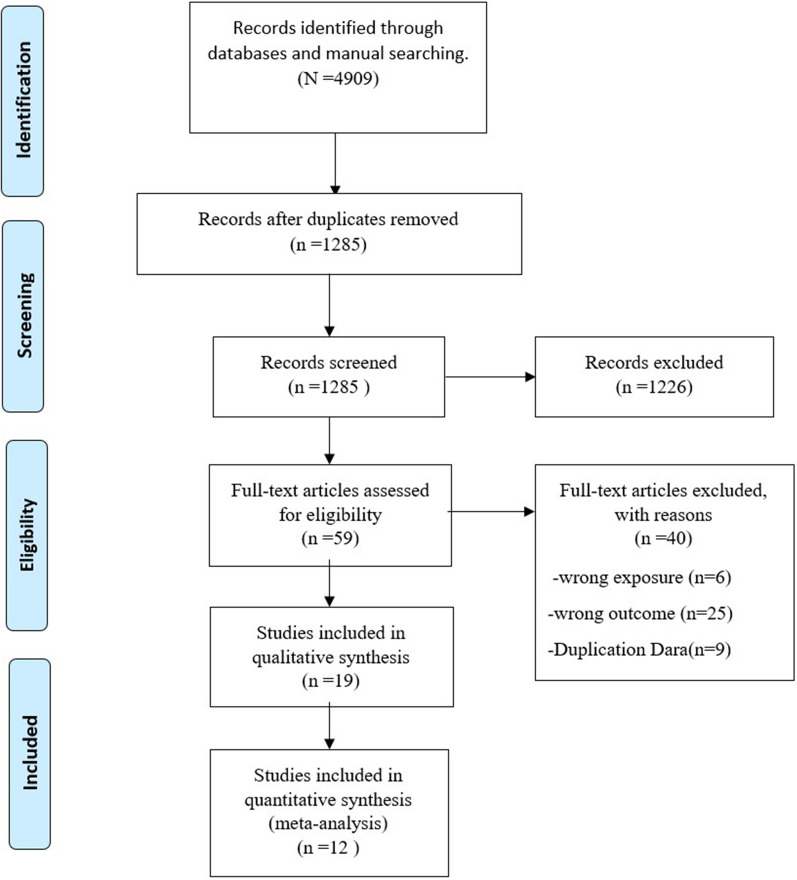
The systematic search retrieved 4909 references across all databases, and duplicates were removed. Titles and abstracts screened the remaining 1285 references, and 59 articles remained for selection by full-text. After screening, 12 were included for quantitative synthesis (meta-analyses), and 19 studies were included in the qualitative synthesis [28–46]. The main characteristics of all included studies are summarised in Tables 1 and 2, and the study selection process is briefly described in the PRISMA flow diagram (Fig. 1).
Table 1.
Characteristics and main clinical manifestations of the included studies
| Author | Country | Year | Participants(male/female) | Median/mean /range age (IQR/SD) | Classification of the cases presented | Smallpox vaccination history (includes visible smallpox scar) |
|---|---|---|---|---|---|---|
| Formenty et al. | Sudan | 2010 | 19 (9/10) | 8 months–32 years | Confirmed cases: 10 Probable cases: 9 | NR |
| Huhn et al. | USA | 2005 | 34 (18/16) | 26 (6–47) | All patients are confirmed cases | 7/34 |
| Yinka-Ogunleye et al. | Nigeria | 2019 | 122 (84/38) | 29 (2 days old-50 y), | Confirmed cases: 118 Probable cases: 4 | NR |
| Whitehouse et al. | Democratic Republic of Congo | 2021 | 1057 (568/469) | 14 (6–23.9) | All patients are confirmed cases | 97/1057 |
| Ježek et al. | Democratic Republic of Congo | 1988 | 338 (182/156) | 3 months–69 years | All patients are confirmed cases | 43/338 |
| Kalthan et al. | Central African Republic | 2016 | 12 (6/6) | 31.6 (12.37) | Confirmed cases:4 Probable cases:8 | NR |
| Pittman et al. | Democratic Republic of Congo | 2022 | 216 (138/78) | 14 (9.9) | All patients are confirmed cases | 4/216 |
| Breman et al. | Central and West Africa (5 countries) | 1980 | 47 (21/26) | 8 years (7 months-35 years) | Confirmed cases:35, Probable cases:12 | 4/47 |
| Perez-Duque et al. | Portugal | 2022 | 27 (27/0) | 33 (22–59) | All patients are confirmed cases | 1/27 |
| Learned et al. | Democratic Republic of Congo | 2003 | 11 (8/3) | 9.6 (7.3) | Confirmed cases:3, Probable cases:8 | 0/11 |
| Mande et al. | Democratic Republic of Congo | 2022 | 21 (14/7) | 16 (4–30) | All patients are confirmed cases | NR |
| Girometti et al. | United Kingdom | 2022 | 54 (54/0) | 41 (34- 45) | All patients are confirmed cases | NR |
| Vaughan et.al | United Kingdom | 2018 | 2 (2/0) | NR | All patients are confirmed cases | NR |
| Antinori et al. | Italy | 2022 | 4 (4/0) | NR | All patients are confirmed cases | 1/4 |
| Hammerschlag et al. | Australia | 2022 | 1 (1/0) | NR | Confirmed Case | NR |
| De Nicolas-Ruanes et al. | Spain | 2022 | 1 (1/0) | 30 | Confirmed Case | NR |
| Reynolds et al. | Sierra Leone | 2019 | 1 (1/0) | 35 | Confirmed Case | NR |
| Erez et al. | Israel | 2019 | 1 (1/0) | NR | Confirmed Case | NR |
| Adler et al. | United Kingdom | 2022 | 7 (4/3) | NR | All patients are confirmed cases | NR |
| Clinical manifestations (affected patients/total patients) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymphadenopathy | Photophobia | Myalgias | Fever | Arthralgia | Pruritus | Conjunctivitis | Rash | Diarrhoea | Cough | Headache | Fatigue | Sore throat | Difficulty breathing | Nausea or vomiting |
| 15/19 | NR | 15/19 | 16/19 | 15/19 | NR | 11/19 | 19/19 | 2/9 | 10/19 | 11/19 | 12/19 | NR | 12/19 | 2/19 |
| 19/34 | NR | 19/34 | 28/34 | 2/34 | NR | 3/34 | 32/34 | 2/34 | 16/34 | 22/34 | NR | 21/34 | 6/34 | 11/34 |
| 45/65 | NR | 42/67 | 81/92 | NR | 57/78 | NR | 122/122 | NR | NR | 61/77 | NR | 45/77 | NR | NR |
| 876/1034 | 332/999 | 754/1003 | 1023/1057 | NR | 542/1012 | 210/1016 | 1057/1057 | NR | 561/1024 | 793/1011 | 888/1029 | 246/325 | NR | 250/1010 |
| 163/295 | NR | NR | NR | NR | NR | NR | 295/295 | NR | 100/280 | NR | NR | NR | NR | NR |
| 6/12 | NR | NR | 11/12 | NR | NR | NR | 12/12 | NR | NR | 2/12 | NR | NR | NR | NR |
| 213/216 | 5/216 | 15/216 | 1/216 | 21/216 | NR | 20/216 | 215/216 | 10/216 | 104/216 | 51/216 | NR | 169/216 | 15/216 | 23/216 |
| 18/45 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 14/27 | NR | 5/27 | 13/27 | NR | NR | NR | 14/27 | NR | NR | 7/27 | NR | NR | NR | NR |
| 2/11 | 7/8 | NR | NR | NR | 6/8 | NR | 8/11 | NR | NR | NR | 1/11 | NR | NR | NR |
| 10/21 | NR | NR | 20/21 | NR | NR | NR | 21/21 | NR | NR | NR | NR | NR | NR | NR |
| 30/54 | NR | 16/54 | 31/54 | NR | NR | NR | 6/54 | NR | NR | N | 36/54 | 6/54 | NR | NR |
| 2/2 | NR | NR | 2/2 | NR | 1/2 | NR | 2/2 | NR | NR | NR | NR | NR | NR | NR |
| 2/4 | NR | 1/4 | 2/4 | NR | 2/4 | NR | 4/4 | NR | NR | NR | NR | NR | NR | NR |
| NR | NR | NR | 1/1 | NR | NR | NR | 1/1 | NR | NR | NR | NR | NR | NR | NR |
| 1/1 | NR | 1/1 | 1/1 | 1/1 | NR | NR | 1/1 | NR | NR | NR | NR | NR | NR | NR |
| 1/1 | NR | 1/1 | 1/1 | NR | NR | NR | 1/1 | NR | NR | NR | NR | NR | NR | NR |
| 1/1 | NR | 1/1 | NR | NR | NR | 1/1 | NR | NR | NR | NR | NR | NR | NR | |
| 5/7 | NR | NR | NR | NR | 1/7 | 1/7 | 7/7 | NR | NR | NR | NR | 2/7 | NR | NR |
IQR, interquartile range; SD, standard deviation; NR, not reported
Table 2.
Characteristics of the rash and complications associated with MPX
| Author | Rash (affected patients/total patients) | Lesions on mucous membranes (affected patients/total patients) | Number of lesions (affected patients/total patients) | Complications (affected patients/total patients) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monomorphic | Pleomorphic | Rash site | Body Distribution | Ocular lesions | Secondary bacterial skin infection | Hemorrhagic pustules | Ulcerated or necrotic lesions | Deaths | Hospitalised patients | |||
| Formenty et.al. | NR | NR | Entire Body: 8/11 Head and/or neck: 3/8 Arms and/or hands: 3/8 Legs and/or feets: 3/8 Chest and/or Abdomen: | NR | Oral cavity: 6/8 | < 100: 3/6 > 100:3/6 | NR | NR | NR | NR | 0/19 | 7/19 |
| Huhn et.al. | 21/31 | 9/31 |
Entire Body: 7/31 Head or neck: 20/32 Arms or hands: 26/32 Pelvic area and groin: 3/32 Legs or feet: 21/32 Chest or Abdomen:18/32 |
Centrifugal: 15/31 Centripetal:1/31 |
Oral cavity: 7/34 | < 100: 24/30 > 100: 6/30 | NR | 19/40 | 2/34 | 8/34 | 0/34 | 9/34 |
| Yinka-Ogunleye et al. | 25/40 | 15/40 | Head and/or neck: 68/71 Arms and/or hands: 55/70 pelvic area and groin: 44/65 Legs and/or feets: 63/69 Chest and/or Abdomen:56/70 Palms: 48/70 Sole of the foot: 42/66 | NR | Genitals: 25/40 | < 100: 16/40 > 100: 24/40 | NR | 19/40 | NR | NR | 7/122 | NR |
| Whitehouse et al. | 286/319 | NR |
Head and/or neck:1036/1057 Arms and/or hands: 1026/1057 Pelvic area and groin: 300/1057 Legs and/or feets: 786/1057 Chest and/or Abdomen:1028/1057 Palms: 1009/1057 v: 885/1057 |
Centrifugal: 989/1025 Centripetal: 33/1025 |
Oral cavity 570/1018 Genitals: 300/1057 |
< 100: 510/1043 > 100: 533/1043 |
NR | NR | NR | NR | NR | NR |
| Ježek et al. | 233/295 | 62/295 | Head and/or neck: 256/295 Palms: 206/295 Sole of the foot: 196/295 |
Centrifugal: 245/295 Centripetal: 13/295 |
Oral cavity: 207/295 Genitals: 88/295 Conjunctiva: 57/295 |
< 100: 77 /295 > 100: 218/295 |
11/295 | 48/295 | 0/295 | NR | 33/338 | NR |
| Kalthan et.al | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 3/12 | 10/12 |
| Pittman et al. | NR | NR | NR | NR | Oral cavity: 53/216 | NR | NR | 18/216 | 6/216 | NR | 3/216 | NR |
| Breman et.al. | NR | NR | NR | NR | NR | < 100: 24/47 > 100: 23/47 | 1/47 | NR | NR | NR | 8/47 | NR |
| Perez-Duque | NR | NR | Pelvic area and groin: 6/27 | NR | Genitals: 6/27 | NR | NR | NR | NR | 11/27 | 0/27 | 3/27 |
| Learned et.al. | NR | NR | NR | NR | NR | < 100: 6/8 > 100:2/8 | 1/11 | NR | NR | NR | 0/11 | 8/11 |
| Mande et al. | 18/21 | 3/21 |
Arms and/or hands: 18/21 Sole of the foot:15/21 |
NR | NR | NR | 8/21 | NR | NR | NR | NR | NR |
| Girometti et.al. | NR | 48/54 |
Head or neck: 11/54 Arms or hands: 11/54 Pelvic area and groin: 51/54 Legs or feet: 11/54 Chest or Abdomen: 14/54 |
NR | Oral cavity:4/54 | NR | NR | 6/54 | NR | NR | NR | 5/54 |
| Vaughan et al. | NR | NR | Pelvic area and groin: 1/2 | NR | Oral cavity: 1/2 | NR | NR | NR | NR | NR | NR | NR |
| Antinori et al. | NR | NR |
Head or neck: 1/4 Pelvic area and groin: 3/4 Legs or feet: 3/4 Chest or Abdomen: 2/4 Sole of the foot:1/4 |
NR | Anal or rectal area: 2/4 | NR | NR | NR | NR | NR | NR | NR |
| Hammerschlag et al. | NR | NR |
Pelvic area and groin: 1/1 Head or neck: 1/1 Chest or Abdomen: 1/1 |
NR | NR | NR | NR | NR | NR | NR | NR | NR |
| De Nicolas-Ruanes et al. | NR | NR | Pelvic area and groin: 1/1 | NR | Anal or rectal area: 1/1 | NR | NR | NR | NR | 1/1 | NR | NR |
| Erez et al. | NR | NR | Entire Body: 1/1 | NR | NR | NR | NR | NR | NR | 1/1 | NR | NR |
| Adler et al. | NR | 7/7 |
Head or neck:7/7 Arms or hands: 3/7 pelvic area and groin:4/7 Legs or feet: 1/7 Chest or Abdomen:7/7 Palm:4/7 Sole of the foot:2/7 |
NR | NR | NR | NR | 0/7 | NR | 2/7 | 0/7 | 7/7 |
NR not reported
Fig. 1.
PRISMA Flow Diagram
Our review included nine cross-sectional studies, three cohort studies, four case reports, and three case series. Most of them were conducted in African countries (9 studies); the most extensive study was from Whitehouse et al. [31], and the smallest was from Learned et al. [37]. Case reports and case series were included only for qualitative synthesis and were the studies with the smallest populations. A total of 1958 patients from the remaining study designs were pooled in the quantitative synthesis. Males accounted for 57.6% (1129), and the age ranged from 2 days old to 69 years old. Most cross-sectional studies were at low risk of bias, and just two were at high risk. Likewise, all cohort studies, case series and case reports were at low risk of bias (Additional file 1: Table S3).
Monkeypox clinical findings
Regarding the clinical manifestations, rash (93%, 95% CI: 80–100%), fever (72%, 95% CI: 30–99%), pruritus (65%, 95% CI: 47–81%), and lymphadenopathy (62%, 95% CI:47–76%), were among the most prevalent clinical manifestations (Fig. 2). Other manifestations included fatigue (60%, 95% CI: 32–85%), sore throat (57%, 95% CI: 36–77%), headache (50%, 95% CI: 25–75%), cough (47%, 95% CI: 38–57%), myalgias (45%, 95% CI: 16–76%), photophobia (32%, 95% CI: 3–71%), arthralgia (26%, 95% CI: 1–65%), difficult breathing (25%, 95% CI: 3–58%), conjunctivitis (19%, 95% CI: 9–32%), nausea/vomiting (19%, 95% CI: 9–30%), diarrhea (4%, 95% CI: 2–7%) (Fig. 2).
Fig. 2.
Pool prevalences forest plots of main clinical findings
Cutaneous lesions characteristics of monkeypox
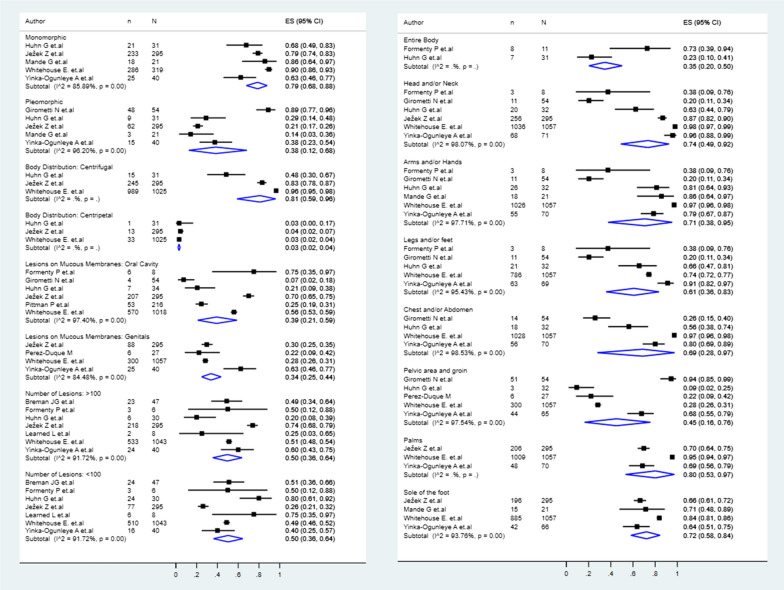
The cutaneous lesions were mostly monomorphic (79%, 95% CI: 68–88%), instead of pleomorphic (38%, 95% CI: 12–68%), with a centrifugal distribution (81%, 95% CI: 59–96%), rather than centripetal (3%, 95% CI: 2–4%) (Fig. 3). About the number of lesions, in 50% (95% CI: 36–64%), there were < 100 lesions, and 50% (95% CI: 36–64%) had ≥ 100 lesions. Regarding the lesion distribution, these were located at head/neck (74%, 95% CI: 49–92%), hand palms (80%, 95% CI: 53–97%), foot soles (72%, 95% CI: 58–84%), arms/hands (71%, 95% CI: 38–95%), chest/abdomen (69%, 95% CI: 28–97%), legs/feet (61%, 95% CI: 36–83%), pelvic area and groins (45%, 95% CI: 16–76%), oral cavity (39%, 95% CI: 21–59%), mucosae of genitals (34%, 95% CI: 25–44%), and at the entire body (35%, 95% CI: 20–50%) (Fig. 3).
Fig. 3.
Pool prevalences forest plots of the cutaneous lesion characteristics and sites
Complications, hospitalizations and deaths associated with monkeypox
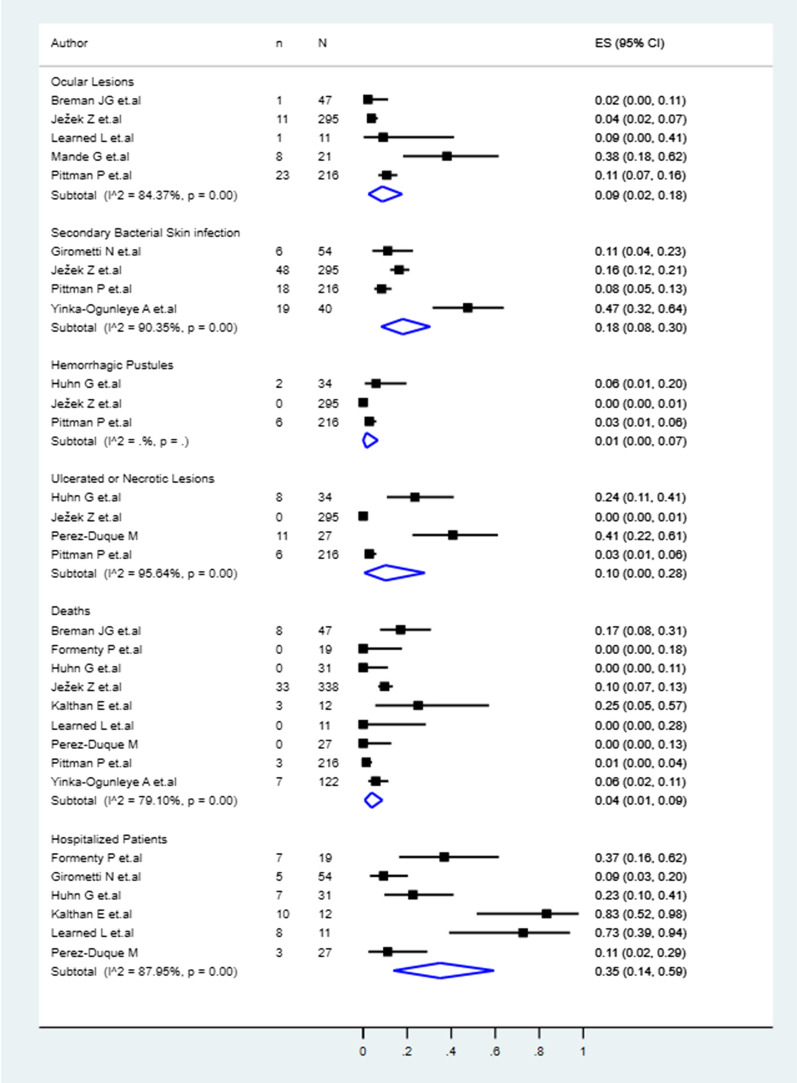
Among the patients, 9% (95% CI 2–18%) presented ocular lesions, 18% (8–30%) secondary bacterial skin infections, 1% (0–7%) haemorrhagic pustules, and 10% (0–28%) ulcerated or necrotic lesions (Fig. 4). From the patients, 35% (14–59%) were hospitalized, and 4% (1–9%) had fatal outcomes (Fig. 4).
Fig. 4.
Pool prevalences forest plots of the MPX complications, hospitalisations and associated deaths
Subgroup analysis
In the subgroup analysis, fever, myalgias and lymphadenopathy occurred similarly between African and European patients (Additional file 1: Table S4). When comparing the frequency of rash, this was significantly higher in African studies (100%, 95% CI: 100–100%) than in European (22%, 95% CI: 14–32%) (Additional file 1: Table S1). At the same time, the distribution of the rash in the pelvic area and groins was significantly higher in European studies (75%, 95% CI: 65–84%) compared to the African (30%, 95% CI: 28–33%) (Additional file 1: Table S3). In addition, lymphadenopathy was slightly more frequent in Africa (65%, 95% CI: 47%-81%) than in Europe (54%, 95% CI: 43–65%). African patients were significantly more hospitalised (64%, 33–90%) than European (10%, 4–17%). Although no deaths have been reported in Europeans so far, all the deaths corresponded to African patients.
Sensitivity analysis
Regarding to the sensitivity analysis (Additional file 1: Table S5), the most common clinical findings were rash (93%, 95% CI: 80–100%), cough (47%, 95% CI: 38–57%), headache (55%, 95% CI: 29–81%), fatigue (75%, 95% CI: 55–90%), throat sore (57%, 95% CI:36–77%), lymphadenopathy (67%, 95% CI: 51–81%), and pruritus (55%, 95% CI: 52–58%).Monkeypox lesions were mainly monomorphic (79%, 95% CI: 68–88%) with a centrifugal body distribution (81%, 95% CI: 59–96%), and with more than 100 lesions (52%, 95% CI: 37–66%). The most common sites of rash were head and/or neck (74%, 95% CI: 49–72%), arms and/or hands (71%, 95% CI: 38–95%), and palms (80%, 95% CI: 53–97%). The most prevalent complications were secondary bacterial skin infection (18%, 95% CI: 8–30%), hospitalization (18%, 95% CI: 8–30%), and ulcerated or necrotic lesions (10%, 95% CI: 0–28%).
Discussion
Over the last months, more than 18,000 cases of monkeypox have been reported outside Africa, in more than 70 countries (up to July 27, 2022), for the first time in more than five decades since the virus was first detected in humans [47–50]. Monkeypox is an emerging condition outside endemic countries, rapidly spreading to multiple countries and continents due to different factors, including its potential sexual transmission [51, 52]. Preparedness at different levels, facing a new clinical disease, demands efforts in epidemiological, diagnostic, therapeutic, and preventive fields during a potential pandemic [53] threatening to spread to new territories and areas with the risk of epidemics [41, 54–59].
Clinical findings and the evolution of the disease and outcomes constitute critical knowledge that should be carefully studied when a new infectious disease emerges or reemerge. Recently, in this context of the monkeypox outbreak, several questions have been raised, including what the full spectrum of illness is, which proportion of patients present complications, need hospitalisation, and may evolve to fatal forms [60–62]. In this systematic review and meta-analysis, we tried to initially summarise clinical data on monkeypox cases published during the outbreak's first weeks. As a result, we analysed more than 1900 patients for major clinical manifestations (most of them men). Our findings are consistent with the expected.
Initial observations from imported cases [46, 63–66], confirmed by this systematic review, suggest that monkeypox's clinical presentation and evolution would differ from the findings of African studies before 2022 and the publications in non-endemic European countries. This review confirms that rash with pruritus, fever, and lymphadenopathy are critical clinical findings, but now, in connection with the potential sexual transmission or transmission due to close contact during sex, is associated more in the 2022 outbreak with rash in the pelvic area and groins (75%) compared to its frequency in African patients (30%). The rash also seems to be different between the clinical presentation in Africa and Europe, with higher frequency in endemic countries (100%), whilst relatively low in European patients (22%). In some cases, yet to be confirmed in case series and more extensive studies, patients may present with solitary or few lesions [43]. Nevertheless, it is to note that 50% of the patients show 100 or more cutaneous lesions, which corresponds to the severe skin lesion severity score of the World Health Organization [67].
Even more, and fortunately, the disease is milder in the current outbreak and outside Africa, with a rate of hospitalisation approximately of 1:6 between Europeans versus African patients, with no deaths reported so far in the ongoing outbreak outside endemic countries. This pattern is probably associated with the circulation of the milder West African clade. A recent systematic review exclusively assessing the case fatality rates (CFR%) by clades found that the CFR% for the West African clade (3.6%, 95% CI 1.7–6.8%) was significantly lower than the Central African clade (10.6%, 8.4–13.3%) [68].
Given the higher frequency of rash (93%) but also of fever (72%), the differential diagnoses of monkeypox will be broad and also dependent on the local epidemiology, including multiple vaccine-preventable diseases such measles, varicella, or even arboviral diseases in the tropics (e.g. dengue, chikungunya, Zika) [69], as well as other established sexually transmitted infections, such as syphilis and AIDS dermatitis, but also the increasing report of this zoonotic infection among people living with HIV/AIDS [18, 36, 41, 42, 70–74]. Moreover, cutaneous lesions or rash appear, as confirmed in this systematic review, head/neck, hand palms and foot soles (> 70%) [17]. Nevertheless, as discussed, genitals and even the oral cavity (in more than a third of patients) should also be explored, looking for lesions. Recently, a call was made not only for physicians but for dental surgeons to assess monkeypox as a differential and possible diagnosis during the current outbreak [75].
A critical differentiating clinical finding is lymphadenopathy (62%), which is not present in other diseases such as smallpox or varicella [76]. However, even lymphadenopathy does not differ significantly in frequency between Africans and Europeans, and probably across the years of study, confirming their importance as a clinical finding in monkeypox [77, 78].
Although most patients did not require hospitalisation (65%), multiple complications may occur, ranging from haemorrhagic pustules (1%) to secondary bacterial skin infections (18%), that may lead to fatal outcomes that have occurred among African patients, but even 10% of European patients were hospitalised.
Case–control studies and cohort studies derived from the 2022 outbreak are necessary to better define the disease's clinical evolution. Clinical characterisation by disease phases and correlation with viremia and viral DNA detection in other body fluids is currently key to understanding disease and transmission. More studies are needed to elucidate the risk factors for severe illness and death. That will allow for identifying groups most likely to have poor outcomes so that we can focus on prevention and treatment efforts. It is supposed that very young children, pregnant women [79, 80], elderly and immunocompromised persons are at higher risk of complicated disease, but this needs further assessment [81].
The laboratory abnormalities were not included in this systematic review. There is a lack of studies, more reports are needed, and only a few data are reported in recent case reports [45] and some studies before 2022 [29]. Leukopenia, thrombocytopenia, elevated blood urea nitrogen, increased hepatic transaminases (ALT/AST), and hypoalbuminemia has been reported in the past and is associated with monkeypox disease [29]. In the recent case series in the United Kingdom of imported illness from 2018 to 2021, transaminitis was reported in association with antiviral treatment in three patients using brincidofovir. Antiviral therapy is another gap in available information regarding its use and apparent efficacy and safety as a treatment for monkeypox [46, 82]. Multiple aspects of monkeypox were not addressed before the 2003 United States outbreak [83].
Our results showed that there is still a need for more comprehensive clinical studies, including short- and long-term follow-up cohort assessments. More studies from outside Africa, now that 37 non-endemic countries have reported cases are necessary to contribute to the growing volume of data, in addition to the increasing number of studies appearing in 2022 from African countries [84–86]. Even more, assessing the impact of spreading events, including the pride parties and festivals on Grand Canary island in Spain or Belgium, needs better evaluations [18, 36, 41, 42, 71–73]. More studies are also required from non-European and non-African countries, including North and Latin America, the Middle East and Oceania, regions already affected by monkeypox [8, 42, 49]. Further clinical data is crucial to elucidate the clinical spectrum of the disease. Up to now, the clinical findings are consistent regardless of report type (cross-sectional studies or case reports). However, more data are needed to define the risk factors for hospitalisation and possible fatal outcomes outside Africa. In other resource-constrained settings, different from Africa, supply chains, including those for drugs, masks and personal protection equipment, will be rechallenged, although at a lower level than COVID-19 [58].
The results of this systematic review highlight the findings that may assist clinicians anywhere in the globe in suspecting the possibility of monkeypox infection in those with recent travel to areas with the ongoing transmission or among contacts of confirmed cases, according to global and national definitions. Early recognition of cases will allow clinicians to ensure adequate clinical monitoring, supportive interventions, and preventing further transmission by implementing infection control measures [1, 2, 31, 67, 73, 87]. There is a need for prospective studies to evaluate the epidemiology, pathogenesis, duration of viral shedding, and the clinical spectrum of disease associated with this zoonotic emerging viral infection [88].
To effectively protect populations and healthcare workers in the face of the arrival and spreading of this emerging viral pathogen, constant evaluation of available evidence is essential to guide clinical suspicion, diagnosis, management and mitigation of transmission of monkeypox.
Limitations
This review has several limitations. First, few studies are available for inclusion. Most are from Africa and Europe. It would be better to include as many studies with a broad geographic scope to understand monkeypox comprehensively. More detailed patient information, particularly regarding clinical outcomes, was unavailable in many studies at the time of analyses; however, the data in this review allow a first synthesis of the clinical characteristics of monkeypox. Finally, possible heterogeneity of the studies included should be considered for different variables. Additionally, we could not identify detailed information from the patients for many variables. In addition, the variability of the populations, including their nationality, origin or belonging to some specific ethnicity and other epidemiological aspects that they could possess, as well as the differences in the designs of the studies reviewed.
Conclusions
Infection with the monkeypox virus is associated with significant cutaneous compromise. Therefore, most patients will not require hospitalisation. Similar to other viral pathogens, monkeypox presents a progressive course of fever in most cases. A significant distinguishing factor includes lymphadenopathy. Eliciting a history of recent travel to areas with ongoing outbreaks of this emerging pathogen or contact with a confirmed case of monkeypox should prompt clinicians to initiate isolation precautions and obtain laboratory confirmation by PCR. Additional research is needed to elucidate viral and host factors in the pathogenesis of severe and fatal infections.
Supplementary Information
Additional file 1: Table S1. Search strategies. Table S2. Cases Definitions. Table S3. Quality assessment of included studies. Table S4. Analysis of subgroups according to continents. Table S5. Sensitivity analysis according to the risk of bias.
Acknowledgements
None.
Author contributions
VBZ, and AJRM, formulated the research questions, designed the study, developed the preliminary search strategy and drafted the manuscript. DKBA, JRUB, EAAB, EAHB, and MDMR refined the search strategy by conducting iterative database queries and incorporating new search terms. JRUB, EAAB, EAHB, MDMR, DBKA, VBZ, and AJRM searched and collected the articles. JRUB, EAAB, EAHB, MDMR, and VBZ conducted the quality assessment. All authors critically reviewed the manuscript for relevant intellectual content. All authors have read and approved the final version of the manuscript. All authors read and approved the final manuscript.
Funding
The research was financed by the Universidad Peruana de Ciencias Aplicadas through grant A-024-2021.
Declarations
Ethics approval and consent to participate
Approval was not required.
Competing interests
All authors report no potential conflicts, except AJRM. AJRM is Deputy Editor-in-Chief of Annals of Clinical Microbiology and Antimicrobials. All authors have submitted the ICMJE Form for Disclosure of Potential.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Monkeypox—United Kingdom of Great Britain and Northern Ireland. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON383. Accessed 23 June 2022.
- 2.Monkeypox outbreak toolbox. https://www.who.int/emergencies/outbreak-toolkit/disease-outbreak-toolboxes/monkeypox-outbreak-toolbox. Accessed 23 June 2022.
- 3.Bleyer JG. Ueber auftreten von variola unter affen der genera mycetes und cebus bei vordringen einer pockenepidemie im urwaldgebiete an den nebenflüssen des alto uruguay in südbrasilien. Muench Med Wochenschr. 1922;69:1009–1010. [Google Scholar]
- 4.Magnus PV, Andersen EK, Petersen KB, Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand. 1959;46(2):156–176. doi: 10.1111/j.1699-0463.1959.tb00328.x. [DOI] [Google Scholar]
- 5.Heberling RL, Kalter SS. Induction, course, and transmissibility of monkeypox in the baboon (Papio cynocephalus) J Infect Dis. 1971;124(1):33–38. doi: 10.1093/infdis/124.1.33. [DOI] [PubMed] [Google Scholar]
- 6.Bonilla-Aldana DK, Rodriguez-Morales AJ. Is monkeypox another reemerging viral zoonosis with many animal hosts yet to be defined? Vet Q. 2022;42(1):148–150. doi: 10.1080/01652176.2022.2088881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farahat RA, Abdelaal A, Shah J, Ghozy S, Sah R, Bonilla-Aldana DK, Rodriguez-Morales AJ, McHugh TD, Leblebicioglu H. Monkeypox outbreaks during COVID-19 pandemic: are we looking at an independent phenomenon or an overlapping pandemic? Ann Clin Microbiol Antimicrob. 2022;21(1):26. doi: 10.1186/s12941-022-00518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basgoz N, Brown CM, Smole SC, Madoff LC, Biddinger PD, Baugh JJ, Shenoy ES. Case 24–2022: a 31-year-old man with perianal and penile ulcers, rectal pain, and rash. N Engl J Med. 2022 doi: 10.1056/NEJMcpc2201244. [DOI] [PubMed] [Google Scholar]
- 9.Patrocinio-Jesus R, Peruzzu F. Monkeypox Genital Lesions. N Engl J Med. 2022 doi: 10.1056/NEJMicm2206893. [DOI] [PubMed] [Google Scholar]
- 10.Ortiz-Martínez Y, Rodríguez-Morales AJ, Franco-Paredes C, Chastain DB, Gharamti AA, Vargas Barahona L, Henao-Martínez AF. Monkeypox – a description of the clinical progression of skin lesions: a case report from Colorado, USA. Ther Adv Infect Dis. 2022;9:20499361221117726. doi: 10.1177/20499361221117726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020 doi: 10.3390/v12111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doty JB, Malekani JM, Kalemba LN, Stanley WT, Monroe BP, Nakazawa YU, Mauldin MR, Bakambana TL, LiyandjaDjaLiyandja T, Braden ZH, et al. Assessing monkeypox virus prevalence in small mammals at the human-animal interface in the democratic Republic of the Congo. Viruses. 2017 doi: 10.3390/v9100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakazawa Y, Mauldin MR, Emerson GL, Reynolds MG, Lash RR, Gao J, Zhao H, Li Y, Muyembe JJ, Kingebeni PM, et al. A phylogeographic investigation of African monkeypox. Viruses. 2015;7(4):2168–2184. doi: 10.3390/v7042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sood A, Sui Y, McDonough E, Santamaria-Pang A, Al-Kofahi Y, Pang Z, Jahrling PB, Kuhn JH, Ginty F. Comparison of multiplexed immunofluorescence imaging to chromogenic immunohistochemistry of skin biomarkers in response to monkeypox virus infection. Viruses. 2020;12(8):78. doi: 10.3390/v12080787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.León-Figueroa DA, Bonilla-Aldana DK, Pachar M, Romaní L, Saldaña-Cumpa HM, Anchay-Zuloeta C, Diaz-Torres M, Franco-Paredes C, Suárez JA, Ramirez JD, et al. The never ending global emergence of viral zoonoses after COVID-19? The rising concern of monkeypox in Europe, North America and beyond. Travel Med Infect Dis. 2022;49:102362. doi: 10.1016/j.tmaid.2022.102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen-Gihon I, Israeli O, Shifman O, Erez N, Melamed S, Paran N, Beth-Din A, Zvi A. Identification and Whole-Genome Sequencing of a Monkeypox Virus Strain Isolated in Israel. Microbiol Resour Announc. 2020 doi: 10.1128/MRA.01524-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Morales AJ. Monkeypox and the importance of cutaneous manifestations for disease suspicion. Microbes, Infection and Chemotherapy. 2022;2:e1450. doi: 10.54034/mic.e1450. [DOI] [Google Scholar]
- 18.Bížová B, Veselý D, Trojánek M, Rob F. Coinfection of syphilis and monkeypox in HIV positive man in Prague. Czech Republic. Travel Med Infect Dis. 2022 doi: 10.1016/j.tmaid.2022.102368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mileto D, Riva A, Cutrera M, Moschese D, Mancon A, Meroni L, Giacomelli A, Bestetti G, Rizzardini G, Gismondo MR, et al. New challenges in human monkeypox outside Africa: a review and case report from Italy. Travel Med Infect Dis. 2022 doi: 10.1016/j.tmaid.2022.102386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bragazzi NL, Kong JD, Mahroum N, Tsigalou C, Khamisy-Farah R, Converti M, Wu J. Epidemiological trends and clinical features of the ongoing monkeypox epidemic: a preliminary pooled data analysis and literature review. J Med Virol. 2022 doi: 10.1002/jmv.27931. [DOI] [PubMed] [Google Scholar]
- 21.Cardona-Ospina JA, Henao-SanMartin V, Acevedo-Mendoza WF, Nasner-Posso KM, Martinez-Pulgarin DF, Restrepo-Lopez A, Valencia-Gallego V, Collins MH, Rodriguez-Morales AJ. Fatal Zika virus infection in the Americas: a systematic review. Int J Infect Dis. 2019;88:49–59. doi: 10.1016/j.ijid.2019.08.033. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Morales AJ, Cardona-Ospina JA, Fernanda Urbano-Garzon S, Sebastian Hurtado-Zapata J. Prevalence of post-chikungunya infection chronic inflammatory arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2016;68(12):1849–1858. doi: 10.1002/acr.22900. [DOI] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drevon D, Fursa SR, Malcolm AL. Intercoder Reliability and Validity of WebPlotDigitizer in Extracting Graphed Data. Behav Modif. 2017;41(2):323–339. doi: 10.1177/0145445516673998. [DOI] [PubMed] [Google Scholar]
- 26.Barker TH, Migliavaca CB, Stein C, Colpani V, Falavigna M, Aromataris E, Munn Z. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol. 2021;21(1):189. doi: 10.1186/s12874-021-01381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67(8):897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Formenty P, Muntasir MO, Damon I, Chowdhary V, Opoka ML, Monimart C, Mutasim EM, Manuguerra JC, Davidson WB, Karem KL, et al. Human monkeypox outbreak caused by novel virus belonging to Congo Basin clade, Sudan, 2005. Emerg Infect Dis. 2010;16(10):1539–1545. doi: 10.3201/eid1610.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huhn GD, Bauer AM, Yorita K, Graham MB, Sejvar J, Likos A, Damon IK, Reynolds MG, Kuehnert MJ. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41(12):1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 30.Yinka-Ogunleye A, Aruna O, Dalhat M, Ogoina D, McCollum A, Disu Y, Mamadu I, Akinpelu A, Ahmad A, Burga J, et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19(8):872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitehouse ER, Bonwitt J, Hughes CM, Lushima RS, Likafi T, Nguete B, Kabamba J, Monroe B, Doty JB, Nakazawa Y, et al. Clinical and Epidemiological Findings from Enhanced Monkeypox Surveillance in Tshuapa Province, Democratic Republic of the Congo During 2011–2015. J Infect Dis. 2021;223(11):1870–1878. doi: 10.1093/infdis/jiab133. [DOI] [PubMed] [Google Scholar]
- 32.Jezek Z, Grab B, Szczeniowski M, Paluku KM, Mutombo M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull World Health Organ. 1988;66(4):459–464. [PMC free article] [PubMed] [Google Scholar]
- 33.Kalthan E, Dondo-Fongbia JP, Yambele S, Dieu-Creer LR, Zepio R, Pamatika CM. Twelve cases of monkeypox virus outbreak in Bangassou District (Central African Republic) in December 2015. Bull Soc Pathol Exot. 2016;109(5):358–363. doi: 10.1007/s13149-016-0516-z. [DOI] [PubMed] [Google Scholar]
- 34.Pittman PR, Martin JW, Kingebeni PM, Tamfum JJM, Wan Q, Reynolds MG, Quinn X, Norris S, Townsend MB, Satheshkumar PS, et al. Clinical characterization of human monkeypox infections in the Democratic Republic of the Congo. MedRxiv. 2022;46:593. [Google Scholar]
- 35.Breman JG, Kalisa R, Steniowski MV, Zanotto E, Gromyko AI, Arita I. Human monkeypox, 1970–79. Bull World Health Organ. 1980;58(2):165–182. [PMC free article] [PubMed] [Google Scholar]
- 36.Perez Duque M, Ribeiro S, Martins JV, Casaca P, Leite PP, Tavares M, Mansinho K, Duque LM, Fernandes C, Cordeiro R, et al. Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Euro Surveill. 2022 doi: 10.2807/1560-7917.ES.2022.27.22.2200424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Learned LA, Reynolds MG, Wassa DW, Li Y, Olson VA, Karem K, Stempora LL, Braden ZH, Kline R, Likos A, et al. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am J Trop Med Hyg. 2005;73(2):428–434. doi: 10.4269/ajtmh.2005.73.428. [DOI] [PubMed] [Google Scholar]
- 38.Mande G, Akonda I, De Weggheleire A, Brosius I, Liesenborghs L, Bottieau E, Ross N, Gembu G-C, Colebunders R, Verheyen E, et al. Enhanced surveillance of monkeypox in Bas-Uélé, Democratic Republic of Congo: the limitations of symptom-based case definitions. MedRxiv. 2022;32:238. doi: 10.1016/j.ijid.2022.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Girometti N, Byrne R, Bracchi M, Heskin J, McOwan A, ... Epidemiological characteristics and clinical features of confirmed human monkeypox virus cases in individuals attending a sexual health Centre in London …. papersssrncom. [DOI] [PMC free article] [PubMed]
- 40.Vaughan A, Aarons E, Astbury J, Balasegaram S, Beadsworth M, Beck CR, Chand M, O'Connor C, Dunning J, Ghebrehewet S, et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018 doi: 10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antinori A, Mazzotta V, Vita S, Carletti F, Tacconi D, Lapini LE, D'Abramo A, Cicalini S, Lapa D, Pittalis S, et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 2022 doi: 10.2807/1560-7917.ES.2022.27.22.2200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hammerschlag Y, MacLeod G, Papadakis G, Adan Sanchez A, Druce J, Taiaroa G, Savic I, Mumford J, Roberts J, Caly L, et al. Monkeypox infection presenting as genital rash, Australia, May 2022. Euro Surveill. 2022 doi: 10.2807/1560-7917.ES.2022.27.22.2200411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Nicolas-Ruanes B, Vivancos MJ, Azcarraga-Llobet C, Moreno AM, Rodriguez-Dominguez M, Berna-Rico ED, Garcia-Mouronte E, Carron-Herrero A, McGee A, Galan JC, et al. Monkeypox virus case with maculopapular exanthem and proctitis during the Spanish outbreak in 2022. JEADV. 2022 doi: 10.1111/jdv.18300. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds MG, Wauquier N, Li Y, Satheshkumar PS, Kanneh LD, Monroe B, Maikere J, Saffa G, Gonzalez JP, Fair J, et al. Human monkeypox in sierra leone after 44-year absence of reported cases. Emerg Infect Dis. 2019;25(5):1023–1025. doi: 10.3201/eid2505.180832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erez N, Achdout H, Milrot E, Schwartz Y, Wiener-Well Y, Paran N, Politi B, Tamir H, Israely T, Weiss S, et al. Diagnosis of Imported Monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25(5):980–983. doi: 10.3201/eid2505.190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, Osborne JC, Rampling T, Beadsworth MB, Duncan CJ, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22(8):1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hutin YJ, Williams RJ, Malfait P, Pebody R, Loparev VN, Ropp SL, Rodriguez M, Knight JC, Tshioko FK, Khan AS, et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7(3):434–438. doi: 10.3201/eid0703.017311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reed KD, Melski JW, Graham MB, Regnery RL, Sotir MJ, Wegner MV, Kazmierczak JJ, Stratman EJ, Li Y, Fairley JA, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350(4):342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 49.Cimerman S, Chebabo A, Cunha CAD, Barbosa AN, Rodriguez-Morales AJ. Human monkeypox preparedness in Latin America - Are we ready for the next viral zoonotic disease outbreak after COVID-19? Br J Infect Dis. 2022;26(3):102372. doi: 10.1016/j.bjid.2022.102372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Morales AJ, Lopardo G, Verbanaz S, Orduna T, Lloveras S, Azeñas-Burgoa JM, Escalera-Antezana JP, Alvarado-Arnez LE, Barbosa AN, Diaz-Quijano F, et al. Latin America: Situation and preparedness facing the multi-country human monkeypox outbreak. Lancet Regional Health Am. 2022;13:100318. doi: 10.1016/j.lana.2022.100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez-Morales AJ, Lopardo G. Monkeypox: another sexually transmitted infection? Pathogens. 2022;11(7):713. doi: 10.3390/pathogens11070713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zambrano PG, Acosta-España JD, Mosquera Moyano F, Altamirano Jara MB. Sexually or intimately transmitted infections: A look at the current outbreak of monkeypox in 2022. Travel Med Infect Dis. 2022 doi: 10.1016/j.tmaid.2022.102383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paules CI, Marston HD, Fauci AS. Coronavirus Infections-More Than Just the Common Cold. JAMA. 2020 doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 54.Cimerman S, Chebabo A, Cunha CAD, Rodríguez-Morales AJ. Deep impact of COVID-19 in the healthcare of Latin America: the case of Brazil. Br J Infect Dis. 2020;24(2):93–95. doi: 10.1016/j.bjid.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cimerman S, Chebabo A, Cunha CAD, Rodríguez-Morales AJ. One year after the arrival of COVID-19 in Latin America: what have we learned in Brazil and other countries? Br J Infect Dis. 2021;25(2):101571. doi: 10.1016/j.bjid.2021.101571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ihekweazu C, Yinka-Ogunleye A, Lule S, Ibrahim A. Importance of epidemiological research of monkeypox: is incidence increasing? Expert Rev Anti Infect Ther. 2020;18(5):389–392. doi: 10.1080/14787210.2020.1735361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez-Morales AJ, Cardona-Ospina JA, Collins MH. Emerging and re-emerging vector-borne and zoonotic diseases. Front Med. 2021;8:714630. doi: 10.3389/fmed.2021.714630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez-Morales AJ, Paniz-Mondolfi AE, Faccini-Martínez ÁA, Henao-Martínez AF, Ruiz-Saenz J, Martinez-Gutierrez M, Alvarado-Arnez LE, Gomez-Marin JE, Bueno-Marí R, Carrero Y, et al. The constant threat of zoonotic and vector-borne emerging tropical diseases: living on the edge. Front Trop Dis. 2021;2:676905. doi: 10.3389/fitd.2021.676905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguez-Morales AJ, Gallego V, Escalera-Antezana JP, Méndez CA, Zambrano LI, Franco-Paredes C, Suárez JA, Rodriguez-Enciso HD, Balbin-Ramon GJ, Savio-Larriera E, et al. COVID-19 in Latin America: the implications of the first confirmed case in Brazil. Travel Med Infect Dis. 2020;35:101613. doi: 10.1016/j.tmaid.2020.101613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rudan I. The COVID-19 pandemic: SARS-CoV-2, childhood hepatitis and monkeypox raise five new questions for the global health research community. J Glob Health. 2022;22:01002. doi: 10.7189/jogh.12.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kozlov M. Monkeypox outbreaks: 4 key questions researchers have. Nature. 2022;606(7913):238–239. doi: 10.1038/d41586-022-01493-6. [DOI] [PubMed] [Google Scholar]
- 62.Kupferschmidt K. Monkeypox vaccination plans take shape amid questions. Science (New York, NY) 2022;376(6598):1142–1143. doi: 10.1126/science.add3743. [DOI] [PubMed] [Google Scholar]
- 63.Costello V, Sowash M, Gaur A, Cardis M, Pasieka H, Wortmann G, Ramdeen S. Imported Monkeypox from International Traveler, Maryland, USA, 2021. Emerg Infect Dis. 2022;28(5):1002–1005. doi: 10.3201/eid2805.220292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hobson G, Adamson J, Adler H, Firth R, Gould S, Houlihan C, Johnson C, Porter D, Rampling T, Ratcliffe L, et al. Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Euro Surveill. 2021 doi: 10.2807/1560-7917.ES.2021.26.32.2100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kyaw WM, Vasoo S, Ho HJA, Chan M, Yeo TW, Manauis CM, Ang H, De Pratim P, Ang BSP, Chow ALP. Monitoring healthcare professionals after monkeypox exposure: experience from the first case imported to Asia. Infect Control Hosp Epidemiol. 2020;41(3):373–375. doi: 10.1017/ice.2019.362. [DOI] [PubMed] [Google Scholar]
- 66.Yong SEF, Ng OT, Ho ZJM, Mak TM, Marimuthu K, Vasoo S, Yeo TW, Ng YK, Cui L, Ferdous Z, et al. Imported Monkeypox, Singapore. Emerg Infect Dis. 2020;26(8):1826–1830. doi: 10.3201/eid2608.191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clinical management and infection prevention and control for monkeypox: Interim rapid response guidance, 2022. https://www.who.int/publications/i/item/WHO-MPX-Clinical-and-IPC-2022.1. Accessed 10 Jun 2022.
- 68.Bunge EM, Hoet B, Chen L, Lienert F, Weidenthaler H, Baer LR, Steffen R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2):e0010141. doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paniz-Mondolfi AE, Rodriguez-Morales AJ, Blohm G, Marquez M, Villamil-Gomez WE. ChikDenMaZika Syndrome: the challenge of diagnosing arboviral infections in the midst of concurrent epidemics. Ann Clin Microbiol Antimicrob. 2016;15(1):42. doi: 10.1186/s12941-016-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhunu CP, Mushayabasa S, Hyman JM. Modelling HIV/AIDS and monkeypox co-infection. Appl Math Comput. 2012;218(18):9504–9518. doi: 10.1016/j.amc.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kluge H, Ammon A. Monkeypox in Europe and beyond—tackling a neglected disease together. Euro Surveill. 2022 doi: 10.2807/1560-7917.ES.2022.27.24.2200482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miura F, van Ewijk CE, Backer JA, Xiridou M, Franz E, Op de Coul E, Brandwagt D, van Cleef B, van Rijckevorsel G, Swaan C et al: Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill 2022. 10.2807/1560-7917.ES.2022.27.24.2200448 [DOI] [PMC free article] [PubMed]
- 73.Vivancos R, Anderson C, Blomquist P, Balasegaram S, Bell A, Bishop L, Brown CS, Chow Y, Edeghere O, Florence I, et al. Community transmission of monkeypox in the United Kingdom, April to May 2022. Euro Surveill. 2022 doi: 10.2807/1560-7917.ES.2022.27.22.2200422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ortiz-Martínez Y, Zambrano-Sanchez G, Rodríguez-Morales AJ. Monkeypox and HIV/AIDS: When the outbreak faces the epidemic. Int J STD AIDS. 2022 doi: 10.1177/09564624221114191. [DOI] [PubMed] [Google Scholar]
- 75.Murphy S. Monkeypox. Br Dent J. 2022;232(11):760. doi: 10.1038/s41415-022-4358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014;58(2):260–267. doi: 10.1093/cid/cit703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saxena SK, Ansari S, Maurya VK, Kumar S, Jain A, Paweska JT, Tripathi AK, Abdel-Moneim AS. Re-emerging human monkeypox: a major public-health debacle. J Med Virol. 2022 doi: 10.1002/jmv.27902. [DOI] [PubMed] [Google Scholar]
- 78.Quarleri J, Delpino MV, Galvan V. Monkeypox: considerations for the understanding and containment of the current outbreak in non-endemic countries. GeroScience. 2022 doi: 10.1007/s11357-022-00611-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dashraath P, Nielsen-Saines K, Mattar C, Musso D, Tambyah P, Baud D. Guidelines for pregnant individuals with monkeypox virus exposure. Lancet. 2022 doi: 10.1016/S0140-6736(22)01063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mbala PK, Huggins JW, Riu-Rovira T, Ahuka SM, Mulembakani P, Rimoin AW, Martin JW, Muyembe JT. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the democratic Republic of Congo. J Infect Dis. 2017;216(7):824–828. doi: 10.1093/infdis/jix260. [DOI] [PubMed] [Google Scholar]
- 81.European Centers for Disease Control and Prevention (ECDC): Monkeypox multi-country outbreak—apid risk assessment; 2022. https://www.ecdc.europa.eu/en/publications-data/risk-assessment-monkeypox-multi-country-outbreak. Accessed 23 June 2022.
- 82.Hutson CL, Kondas AV, Mauldin MR, Doty JB, Grossi IM, Morgan CN, Ostergaard SD, Hughes CM, Nakazawa Y, Kling C, et al. Pharmacokinetics and efficacy of a potential smallpox therapeutic, brincidofovir, in a lethal monkeypox virus animal model. MSphere. 2021 doi: 10.1128/mSphere.00126-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rodríguez-Morales AJ, Ortiz-Martínez Y, Bonilla-Aldana DK. What has been researched about Monkeypox? A bibliometric analysis of an old zoonotic virus causing global concern. New Microbes New Infect. 2022;47:100993. doi: 10.1016/j.nmni.2022.100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amao LK, Olatunji DI, Igbodo G, Okoli SC, Amaechi I, Goni MI, Ehiakhamen O, Aderinola O, Ogunleye A, Ogunbode O, et al. Trend and enhanced surveillance of Monkeypox during COVID-19 pandemic in Nigeria. J Public Health Afr. 2022;13(1):2184. doi: 10.4081/jphia.2022.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eseigbe EE, Akude C, Osagie IA, Eseigbe P. Human monkey pox virus infection in plateau state, north central nigeria: a report of two cases. West Afr J Med. 2021;38(12):1242–1246. doi: 10.55891/wajm.v38i12.55. [DOI] [PubMed] [Google Scholar]
- 86.Guagliardo SAJ, Monroe B, Moundjoa C, Athanase A, Okpu G, Burgado J, Townsend MB, Satheshkumar PS, Epperson S, Doty JB, et al. Asymptomatic orthopoxvirus circulation in humans in the wake of a monkeypox outbreak among chimpanzees in cameroon. Am J Trop Med Hyg. 2020;102(1):206–212. doi: 10.4269/ajtmh.19-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ortiz-Martínez Y, Galvis-Cataño LM, Arias-Rodríguez D, Romero-Dager C, Bonilla-Aldana DK, Rodriguez-Morales AJ. YouTube and 2022 Monkeypox outbreak: opportunities for awareness and infection control. J Hosp Infect. 2022 doi: 10.1016/j.jhin.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, Palich R, Nori A, Reeves I, Habibi MS, et al. Monkeypox Virus Infection in Humans across 16 Countries—April-June 2022. N Engl J Med. 2022 doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Search strategies. Table S2. Cases Definitions. Table S3. Quality assessment of included studies. Table S4. Analysis of subgroups according to continents. Table S5. Sensitivity analysis according to the risk of bias.