Abstract
We hypothesized that functional imaging with 64Cu-DOTA-trastuzumab PET/CT would predict the response to the antibody–drug conjugate trastuzumab–emtansine (T-DM1). Methods: Ten women with metastatic human epidermal growth factor receptor 2–positive breast cancer underwent 18F-FDG PET/CT and 64Cu-DOTA-trastuzumab PET/CT on days 1 and 2 before treatment with T-DM1. Results: T-DM1–responsive patients had higher uptake than nonresponsive patients. Day 1 minimum SUVmax (5.6 vs. 2.8, P < 0.02), day 2 minimum SUVmax (8.1 vs. 3.2, P < 0.01), and day 2 average SUVmax (8.5 vs. 5.4, P < 0.05) for 64Cu-DOTA-trastuzumab all favored responding patients. Tumor-level response suggested threshold dependence on SUVmax. Patients with a day 2 minimum SUVmax above versus below the threshold had a median time to treatment failure of 28 mo versus 2 mo (P < 0.02). Conclusion: Measurement of trastuzumab uptake in tumors via PET/CT is promising for identifying patients with metastatic breast cancer who will benefit from T-DM1.
Keywords: breast, oncology, PET, breast cancer, breast PET
Overexpression of human epidermal growth factor receptor 2 (HER2) occurs in 15%–20% of breast cancers and determines candidacy for trastuzumab, which improves disease outcome for all stages of HER2-positive breast cancer (1–3).
We have used 64Cu-DOTA-trastuzumab PET/CT to study women with recurrent or metastatic breast cancer (4) and reported a positive correlation between tumor uptake of 64Cu-DOTA-trastuzumab and HER2 status as measured by immunohistochemistry (4,5). Trastuzumab–emtansine (T-DM1) is an antibody–drug conjugate that uses trastuzumab to target HER2-positive breast cancer and deliver its cytotoxic payload, emtansine (6). T-DM1’s mechanism of action and use as a single agent are advantageous for correlating trastuzumab imaging with treatment response. We report the results of a pilot study testing whether pretreatment 64Cu-DOTA-trastuzumab PET/CT can predict benefit from T-DM1 for HER2-positive metastatic breast cancer.
MATERIALS AND METHODS
Patient Selection
Eligibility requirements included metastatic or recurrent HER2-positive breast cancer in patients who were to receive T-DM1, were 18 y old or older, had an Eastern Cooperative Oncology Group performance status of 0–2, had a normal cardiac ejection fraction, and had at least 1 metastasis with a diameter of at least 2.0 cm. Patients could not have received trastuzumab for 4 or more weeks. Eligibility was confirmed by tumor biopsy for HER2 assessment and 18F-FDG PET/CT. The City of Hope Institutional Review Board approved the study, and all patients provided written informed consent (NCT02226276).
Treatment
The patients underwent a clinical examination and toxicity assessment before each cycle of T-DM1. Follow-up 18F-FDG PET/CT was performed after every 2 cycles of T-DM1 for up to 18 mo and at the discretion of the treating oncologist thereafter. Treatment response was evaluated by PERCIST (7).
64Cu-DOTA-Trastuzumab-PET/CT
64Cu was provided by the Mallinckrodt Institute of Radiology, Washington University School of Medicine. Radiolabeled trastuzumab was prepared and administered according to IND 109971 as previously described (4).
Scans were acquired with a Discovery STe 16 PET/CT device (GE Healthcare) operated in 3-dimensional mode. The PET axial field of view and slice thickness were 15.4 cm and 3.3 mm, respectively. PET images were iteratively reconstructed as previously described (5) and had a measured resolution of 9 mm in full width at half maximum. 64Cu-DOTA-trastuzumab PET/CT was performed during the first day (day 1) and second day (day 2) after injection. Quantitative imaging with 64Cu-DOTA-trastuzumab was supported by direct measurement of activity concentrations in peripheral venous blood samples drawn before imaging on days 1 and 2. Measurement of 64Cu-DOTA trastuzumab uptake is described in Supplemental Figure 1 (supplemental materials are available at http://jnm.snmjournals.org).
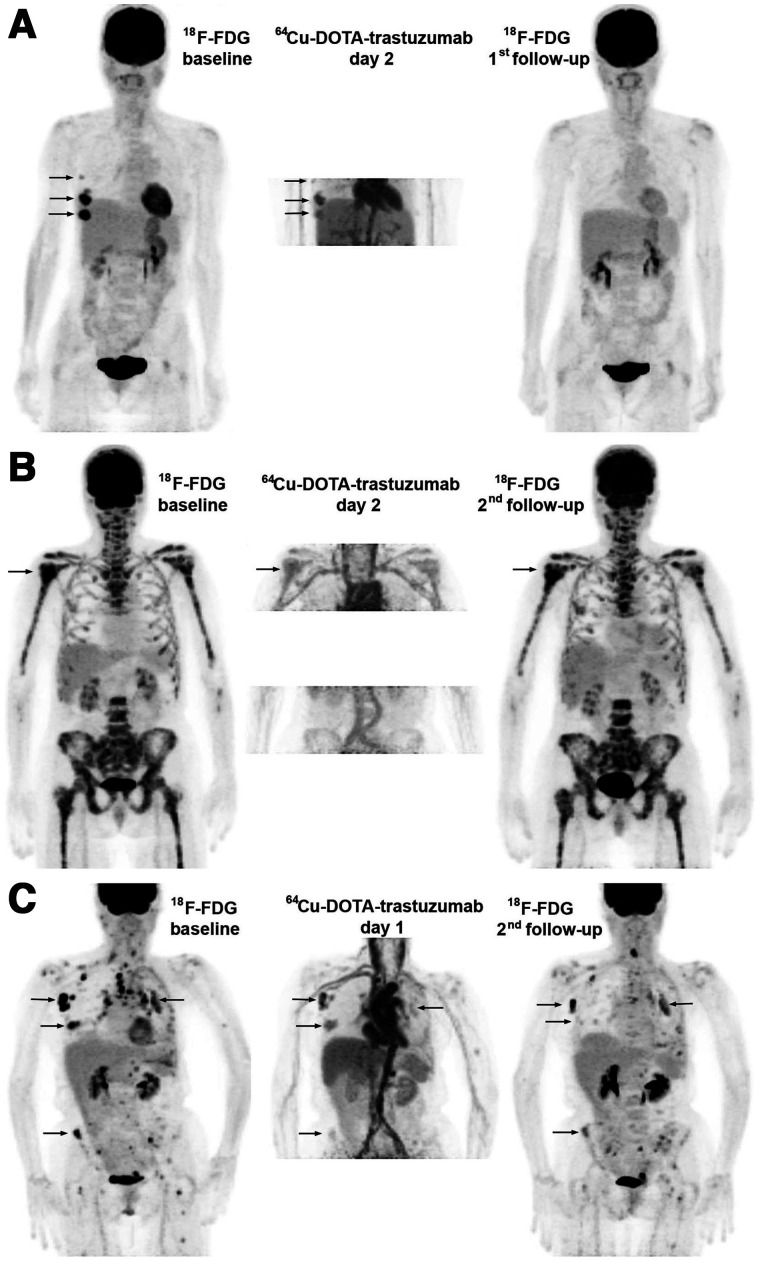
FIGURE 1.
Treatment effects. Images are maximum-intensity projections with upper intensity thresholds (black color) corresponding to SUV of 7 and 10 g/mL for 18F-FDG and 64Cu-DOTA-trastuzumab, respectively. (A) Response with baseline 18F-FDG–positive disease limited to right breast and axilla (arrows). All lesions were well visualized with 64Cu-DOTA-trastuzumab, and follow-up 18F-FDG showed complete response. (B) Nonresponse with extensive 18F-FDG–positive bone metastasis. (Arrow indicates PERCIST target tumor.) Tumor uptake of 64Cu-DOTA-trastuzumab was low (day 2 target tumor SUVmax, 5.5 g/mL). Disease progression occurred after 4 cycles of T-DM1. (C) Nonresponse with widely disseminated 18F-FDG–positive disease. Tumor uptake of 64Cu-DOTA-trastuzumab was variable, and tumor response at second 18F-FDG follow-up (after 4 cycles of T-DM1) was correspondingly mixed.
Antibody scans were interpreted in relation to baseline 18F-FDG scans by a radiologist different from those who evaluated the 18F-FDG PET/CT scans. Quantitative analysis was performed using XD (version 3.6; Mirada Medical). Correction for altered 18F-FDG biodistribution in follow-up examinations is shown in Supplemental Figure 2.
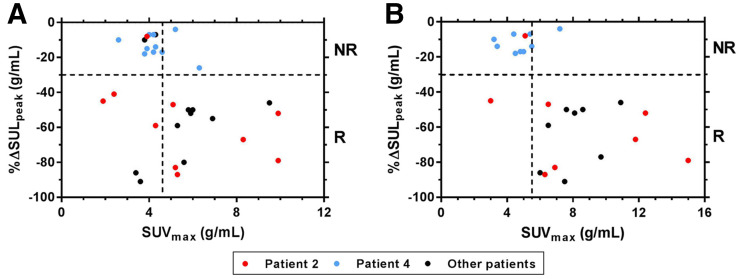
FIGURE 2.
Percentage change in 18F-FDG uptake (%ΔSULpeak) is plotted vs. 64Cu-DOTA-trastuzumab SUVmax measured on days 1 (A) and 2 (B). Horizontal and vertical dashed lines show, respectively, PERCIST threshold for positive response (−30%) and thresholds (day 1, 4.6 g/mL; day 2, 5.5 g/mL) that maximized accuracy of 64Cu-DOTA-trastuzumab uptake in separating responsive from nonresponsive tumors. NR = nonresponsive; R = responsive.
Tumor uptake was measured in terms of voxel SUVmax (4,5). Measurements were limited to tumors measurable for baseline 18F-FDG uptake. Tumor images strongly overlapped by positively imaged adjacent features (e.g., a vessel or organ) were rejected for uptake measurement. Those that were strongly positive and well separated from adjacent features were segmented via a maximum voxel-based thresholding technique (8). The methods used for tumor assessment and numbers of tumors assessed are in Supplemental Tables 1 and 2.
TABLE 1.
64Cu-DOTA-Trastuzumab Uptake and TTF
| Tumor uptake | Patients > threshold | Patients ≤ threshold | |||||
|---|---|---|---|---|---|---|---|
| Metric | Cut point (g/mL)* | n | Median TTF (mo) | n | Median TTF (mo) | Hazard ratio | P † |
| Day 1 average SUVmax | 4.6 | 5 | 18 | 5 | 3 | 0.3 (0.1−1.3) | 0.10 |
| Day 2 average SUVmax | 5.5 | 6 | 23 | 3 | 2 | 0.1 (0.0−0.9) | 0.01 |
| Day 1 minimum SUVmax | 4.6 | 4 | 26 | 6 | 3 | 0.3 (0.1−1.3) | 0.09 |
| Day 2 minimum SUVmax | 5.5 | 5 | 28 | 4 | 2 | 0.1 (0.0−1.0) | 0.02 |
Optimal threshold relating uptake to response for individual tumors.
†Log rank test.
Hazard ratios are >cut point relative to ≤cut point. Data in parentheses are 95% CIs.
Statistical Plan and Analysis
The study was designed to accrue 10 patients to explore the relationship between tumor uptake of 64Cu-DOTA-trastuzumab and tumor response. For individual tumors, a hierarchic (tumor-within-patient) linear mixed-effects model was used to evaluate the association between day 1 or day 2 SUVmax and response. The best SUVmax cut points for individual tumors were used in patient-level response (Fisher exact test), and planned comparisons of average (or minimum) uptake in responsive versus nonresponsive patients used the t test. All P values are 2-sided. Cox regression was used for time to treatment failure (TTF) (the supplemental materials provide additional details).
RESULTS
Ten patients were enrolled, and their characteristics are in Supplemental Table 3. Five experienced a response, and 5 were nonresponders; TTF ranged from 1.3 mo (early death) to 46 mo. Two patients continued in long-term follow-up at 62 and 78 mo from the initiation of chemotherapy. Figure 1 compares 64Cu-DOTA-trastuzumab and 18F-FDG PET scans for 3 patients with varying responses to T-DM1.
Fifty-nine 18F-FDG baseline-measurable tumors met the criteria for measurability of 64Cu-DOTA-trastuzumab uptake, and 31 (day 1) and 25 (day 2) were also measured for response. Over half the data for individual tumors came from 2 patients (Figs. 1B and 1C).
Individual tumor response appeared to have a distinct threshold dependence on uptake of 64Cu-DOTA-trastuzumab, especially on day 2 (Fig. 2). Although the optimal uptake threshold settings accurately separated responsive from nonresponsive tumors, those results are not statistically significant in a hierarchical model of tumors within patient for this 10-patient cohort.
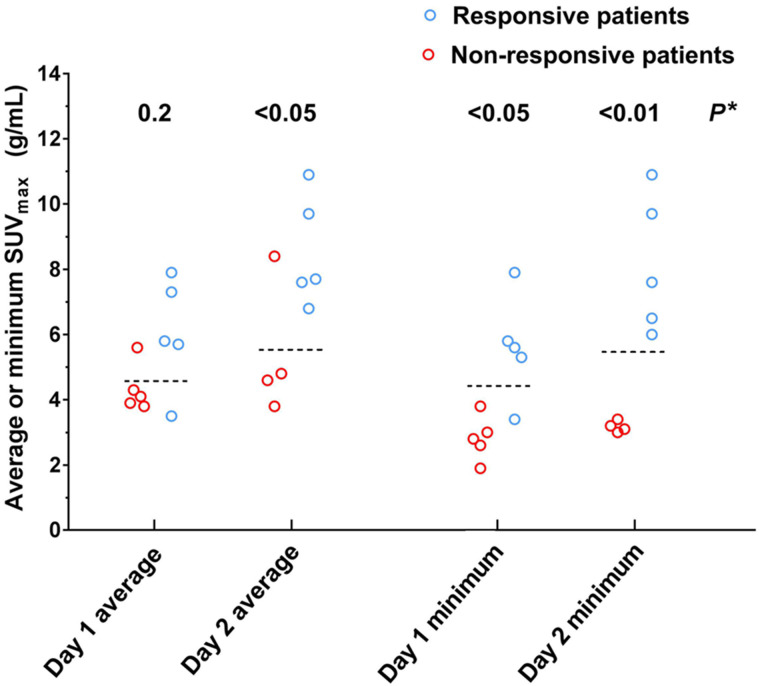
Patient-level response was positively related to tumor uptake of 64Cu-DOTA-trastuzumab, and the thresholds that optimally related uptake to best response for individual tumors also accurately separated patients by response to T-DM1 (Fig. 3). Responsive patients had a significantly higher day 2 average, day 1 minimum, and day 2 minimum SUVmax than nonresponsive patients. In the categoric analysis (intrapatient average or minimum tumor SUVmax > response threshold, yes/no, vs. responsive, yes/no), day 1 results were significant for minimum SUVmax, whereas day 2 results were significant for both metrics. For day 2, all patients with the lowest tumor uptake above the threshold responded, whereas no patients with the lowest tumor uptake below the threshold responded.
FIGURE 3.
Relationship between patient best response to T-DM1 and measured tumor uptake of 64Cu-DOTA-trastuzumab. Dashed lines show optimal thresholds relating response to uptake for individual tumors (day 1, 4.6 g/mL; day 2, 5.5 g/mL). Group mean SUVmax (responsive vs. nonresponsive patients) was significantly different (t test) for day 2 average (8.5 vs. 5.4, P < 0.05), day 1 minimum (5.6 vs. 2.8, P < 0.02), and day 2 minimum (8.1 vs. 3.2, P < 0.01). (P = 0.08 for day 1 average). *Fisher exact test for response vs. threshold.
TTF was positively related to measured tumor uptake of 64Cu-DOTA-trastuzumab (Table 1). For day 2, the relationship was statistically significant for both patient-level uptake metrics. Depending on the metric, the day 2 tumor response threshold discriminated patients with a median TTF of 2 versus 23 or 28 mo.
The supplemental materials include additional details about patients, treatment, response assessment, and 64Cu-DOTA-trastuzumab image analysis.
DISCUSSION
We demonstrated a significant association between tumor uptake of 64Cu-DOTA-trastuzumab and patient benefit (response and TTF) from treatment with T-DM1. The ZEPHIR trial found pretreatment tumor imaging with 89Zr-trastuzumab PET/CT to be predictive of patient response and TTF in T-DM1 therapy for HER2-positive metastatic breast cancer (9,10). The pretreatment work-up included 18F-FDG PET/CT. 89Zr-trastuzumab PET/CT scans acquired 4 d after injection were assessed by radiologists’ qualitative inspection.
Our quantitative study corroborates the ZEPHIR trial’s finding that tumor uptake of trastuzumab on PET/CT correlates with patient response and outcome with T-DM1. Using SUVmax measurement, moreover, we found an apparent threshold relationship between tumor response and tumor uptake 1 and 2 d after injection of 64Cu-DOTA-trastuzumab. The response thresholds for individual tumors also accurately separated patients by response and TTF. Based on published data for 89Zr-trastuzumab (11,12), we estimate that the 64Cu-DOTA-trastuzumab SUVmax T-DM1 response threshold is modestly greater than blood pool SUV at 4 d postinjection (Supplemental Fig. 3 and associated text). This is consistent with the criterion for “relevant” tumor uptake of 89Zr-trastuzumab vis-à-vis response to T-DM1 in the ZEPHIR trial.
The current study demonstrated some disadvantages of 64Cu (half-life, 12.8 h) relative to 89Zr (half-life, 3.3 d). 64Cu does not provide whole-body coverage with an acceptable scan duration and adequate count density for accurate and precise measurement of tumor uptake. In the current study, the low count rate limited coverage of disease burden on day 2. Overlap with images of adjacent blood vessels reduced the number of tumor images measurable for 64Cu-DOTA-trastuzumab SUVmax. Variations in time between injection and scan (tscan − tinj) imposed by difficulty in scheduling research scans amid clinical operations added noise to measurements of tumor SUVmax. The resulting error is inversely related to tscan − tinj and thus inherently much worse for scans on days 1 and 2 than scans on day 4 or later. The problem is well illustrated by the patient (Fig. 1A) with the shortest day 1 time to scan (16 h). Although the patient had a complete response to T-DM1, SUVmax was below the empiric response threshold for both of her measurable tumors on day 1, whereas on day 2, SUVmax was above the threshold.
Despite limitations imposed by the relatively short half-life of 64Cu, we have demonstrated that measurement of tumor uptake of trastuzumab at 1–2 d after injection can be effective in identifying patients unlikely to benefit from T-DM1 therapy. Further work is required to develop measurement of trastuzumab uptake as a predictor of clinical benefit from T-DM1. Trastuzumab imaging may identify patients who could benefit from T-DM1 and thus avoid the toxicity of chemotherapy. Trastuzumab imaging may also identify women who might not otherwise be considered for HER2-directed treatment (13).
We previously reported on the patient depicted in Figure 1C, whose disease demonstrated heterogeneous uptake of 64Cu-DOTA-trastuzumab and a correspondingly mixed response to T-DM1 (14). This case suggests that trastuzumab imaging may identify patients who could benefit from combining T-DM1 with chemotherapy or other treatments.
CONCLUSION
Tumor uptake of 64Cu-DOTA-trastuzumab, measured in terms of SUVmax at 1–2 d after injection, was positively associated with patient response and TTF in T-DM1 therapy of HER2-positive metastatic breast cancer. The relationship between SUVmax and tumor response appeared to have a sharp threshold, and the threshold for individual tumor response was also effective in separating patients who did and did not benefit from T-DM1. Thus, measurement of trastuzumab uptake in tumors via PET/CT is highly promising for patient selection in treatment of metastatic breast cancer with T-DM1.
DISCLOSURE
Production of 64Cu at Washington University School of Medicine is supported by the Department of Energy. The clinical trial was funded by the Baum family and the Yvonne Craig Aldrich Foundation. The Biostatistics and Mathematical Modeling Cores provided support under P30CA033572. Joanne E. Mortimer is a consultant for Puma, Astra-Zeneca, Novartis, Pfizer, and Karyopharm Pharmaceuticals. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is tumor uptake of 64Cu-DOTA-trastuzumab as measured by PET/CT predictive of treatment benefit from T-DM1 in metastatic HER2-positive breast cancer?
PERTINENT FINDINGS: Response to T-DM1 was positively associated with tumor uptake of 64Cu-DOTA-trastuzumab, measured as SUVmax. Tumor response appeared to have a distinct threshold dependence on SUVmax, and the response threshold for individual tumors accurately separated patients with respect to response and TTF.
IMPLICATIONS FOR PATIENT CARE: Pretreatment functional imaging of trastuzumab may help in selecting patients likely to benefit from T-DM1.
REFERENCES
- 1. Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med. 2015;66:111–128. [DOI] [PubMed] [Google Scholar]
- 2. Giordano SH, Temin S, Chandarlapaty S, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2–positive breast cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36:2736–2740. [DOI] [PubMed] [Google Scholar]
- 3. Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–2122. [DOI] [PubMed] [Google Scholar]
- 4. Mortimer JE, Bading JR, Colcher DM, et al. Functional imaging of human epidermal growth factor receptor 2–positive metastatic breast cancer using 64Cu-DOTA-trastuzumab PET. J Nucl Med. 2014;55:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mortimer JE, Bading JR, Park JM, et al. Tumor uptake of 64Cu-DOTA-trastuzumab in patients with metastatic breast cancer. J Nucl Med. 2018;59:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burris HA, III, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)–positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29:398–405. [DOI] [PubMed] [Google Scholar]
- 7. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med, 2009;50(suppl 1):122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med. 2004;45:1519–1527. [PubMed] [Google Scholar]
- 9. Clark AS, DeMichele A, Mankoff D. HER2 imaging in the ZEPHIR study. Ann Oncol. 2016;27:555–557. [DOI] [PubMed] [Google Scholar]
- 10. Gebhart G, Lamberts LE, Wimana Z, et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol. 2016;27:619–624. [DOI] [PubMed] [Google Scholar]
- 11. Laforest R, Lapi SE, Oyama R, et al. [89Zr]trastuzumab: evaluation of radiation dosimetry, safety, and optimal imaging parameters in women with HER2-positive breast cancer. Mol Imaging Biol. 2016;18:952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Donoghue JA, Lewis JS, Pandit-Taskar N, et al. Pharmacokinetics, biodistribution, and radiation dosimetry for 89Zr-trastuzumab in patients with esophagogastric cancer. J Nucl Med. 2018;59:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ulaner GA, Hyman DM, Ross DS, et al. Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer using 89Zr-trastuzumab PET/CT. J Nucl Med. 2016;57:1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mortimer JE, Shively JE. Functional imaging of human epidermal growth factor receptor 2–positive breast cancers and a note about NOTA. J Nucl Med. 2019;60:23–25. [DOI] [PubMed] [Google Scholar]