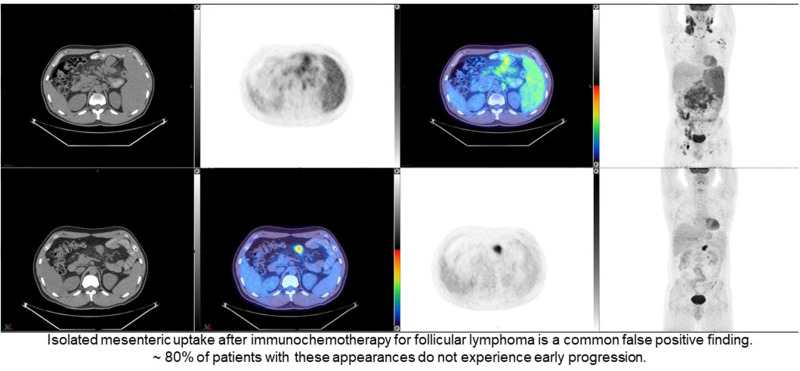
Visual Abstract
Keywords: follicular lymphoma, PET, response assessment
Abstract
Complete metabolic response (CMR) on PET/CT was the sole independent predictor of overall survival in the PET substudy of the phase III GALLIUM trial (NCT01332968) in first-line treatment of high-tumor-burden follicular lymphoma. The aim of this analysis was to further investigate the outcome of patients not achieving CMR. Methods: Two international experts rereviewed PET/CT scans from patients failing to achieve CMR assessed by the Independent Review Committee masked otherwise to committee results. Metabolic response category and Deauville score were assigned. Progression-free survival (PFS) was investigator-assessed with contrast-enhanced CT. Kaplan–Meier methodology was used to estimate landmark PFS and time to next treatment from end of induction by Deauville score. Patients who experienced CT-based progressive disease at the end of induction were excluded. Results: Fifty-four patients were reviewed. Six had CMR, 37 had a partial metabolic response, 2 had no metabolic response, and 9 had progressive metabolic disease. Patients were reassigned to CMR because 18F-FDG uptake was considered inflammatory (n = 2), was considered incidental neoplasia (n = 2), or was visually close to liver uptake but quantitatively lower (n = 2). There was a trend for shorter PFS and time to next treatment for patients with a Deauville score of 5 than a score of 4. High-grade mesenteric uptake at the end of induction was common, occurring in 20 patients with non-CMR, 14 of whom achieved CMR at all other sites. Only 3 of 14 (21%) patients with mesenteric uptake as the only site of disease experienced progression or death within 24 mo, whereas 4 of 6 patients (67%) with mesenteric and additional sites of 18F-FDG–avid disease experienced progression or death within 24 mo. All patients with early progression had measurable disease on contrast-enhanced CT at 18F-FDG–avid sites at the end of induction. Conclusion: After induction immunochemotherapy, CMR was assigned after reassessment in some patients, in whom increased 18F-FDG uptake was considered due to inflammation or incidental neoplasia rather than to lymphoma. Quantitative assessment to confirm the visual impression of residual uptake in lesions is suggested. Isolated mesenteric 18F-FDG uptake is likely a common false-positive finding at the end of induction and does not warrant changes in clinical management or disease surveillance unless there is measurable disease on contrast-enhanced CT or clinical suspicion of active disease.
Patients with follicular lymphoma treated with first-line immunochemotherapy with complete metabolic response (CMR) on PET using the 5-point Deauville score have a better prognosis than patients who do not achieve CMR (1). In a landmark analysis of 508 patients with baseline and end-of-induction PET/CT scans in the prospective phase III GALLIUM study (NCT01332968), the progression-free survival (PFS) 2.5 y from randomization for patients achieving CMR (Deauville score of 1, 2, or 3) was 87.4% (95% CI, 83.7–90.2), compared with 54.9% (95% CI, 40.5–67.3) for patients failing to do so, with a Deauville score of 4 or 5 assigned as non-CMR (2). PET/CT was superior to contrast-enhanced CT for predicting PFS and overall survival. The 2014 Lugano response criteria (3,4) incorporating the Deauville score were superior to the previous standard, the 2007 International Harmonization Project criteria (5). The Lugano criteria also proposed combining the Deauville score with interval changes in 18F-FDG uptake to assign metabolic response categories (3,4), analogous to the anatomic response categories used for CT reporting.
The Lugano criteria have been suggested as a suitable platform for response-adapted therapy in follicular lymphoma (2), with different management strategies already being tested for patients with advanced follicular lymphoma with CMR versus non-CMR after first-line immunochemotherapy using the Deauville score as a binary measure of response (6,7). This suggested suitability may be partly because there are no published data to our knowledge about ordinal Deauville scores or metabolic response categories, nor is there information about patterns of 18F-FDG uptake that might be associated with early progression or death in patients with follicular lymphoma and non-CMR at the end of induction. Patients with lymphoma usually have high response rates, which make analysis of the small numbers of patients with non-CMR challenging. It is becoming clear, however, that a positive PET finding may not carry uniform prognostic weight, with clinical trials on aggressive lymphomas reporting that patients with a PET score of 5 have inferior outcomes to patients with a PET score of 4 and treating this group differently (8–11). The aims of this ancillary analysis from the GALLIUM PET study were, therefore, to report PFS according to Deauville score and metabolic response category and to evaluate any PET/CT findings, such as disease distribution, on end-of-induction scans that might be associated with early progression or death in patients with non-CMR.
MATERIALS AND METHODS
The details of the GALLIUM trial (12) and the PET analysis (2) have been reported previously. In brief, previously untreated patients with advanced-stage grades 1–3a follicular lymphoma requiring immunochemotherapy were eligible. Patients were randomized 1:1 to receive either obinutuzumab or rituximab followed by the same maintenance antibody for up to 2 y. The chemotherapy was cyclophosphamide, vincristine, and prednisone; cyclophosphamide, doxorubicin, vincristine, and prednisone; or bendamustine, decided by the treating site. PFS was assessed by investigators using contrast-enhanced CT scans. PFS24 was defined as progression or death within 24 mo from the end of induction.
PET/CT response was a secondary endpoint, with scans mandated for the first 170 patients and optional thereafter. All PET/CT scans were performed according to a standardized study protocol with prespecified time windows. The GALLIUM study was approved by local Institutional Review Boards and conducted in accordance with the Declaration of Helsinki and good clinical practice. All patients gave written informed consent.
Baseline and end-of-induction PET/CT and contrast-enhanced CT scans were read prospectively by an independent review committee using the International Harmonization Project criteria, which were the international standard at the time. The PET/CT scans were retrospectively reviewed by the independent review committee using the 2014 Lugano criteria (3,4). Patients with progressive metabolic disease in GALLIUM had an unexpectedly longer PFS than patients with a partial metabolic response on independent review committee evaluation, though this difference did not reach statistical significance. Two international experts were invited to rereview baseline and end-of-induction PET/CT scans that had been assessed as non-CMR by the independent review committee. Deauville score and metabolic response category were assigned without knowledge of the independent review committee results or any clinical information at the time of scan review, with differences between the 2 reviewers resolved by consensus. A Deauville score of 5 was assigned when uptake in lesions that were present at baseline was at least 3 times higher than the SUVmax in the liver at response or in the presence of new lesions considered to be lymphoma-related. Lymphomatous lesions were considered measurable if 2 dimensions were recorded on contrast-enhanced CT, with the longest diameter being at least 15 mm for nodal sites and at least 10 mm for extranodal sites (4).
Kaplan–Meier methodology was used to estimate landmark PFS distributions and time to next antilymphoma treatment from the end of induction therapy for each response category. Patients who experienced progressive disease (CT-based assessment) at the end of induction were excluded from the landmark analyses.
RESULTS
By independent review committee assessment, there were 55 patients with non-CMR without CT-defined progressive disease at the end of induction. One patient was excluded from further analysis, as not all 18F-FDG–avid sites present on the baseline scan were included on the end-of-induction PET/CT scan. Median follow-up for these 54 patients was 75.1 mo (range, 5.9–92.3 mo) from the end of induction.
Six of the remaining 54 patients in the current analysis were reassigned to CMR. Thirty-seven patients had a partial metabolic response, 2 patients had no metabolic response, and 9 patients had progressive metabolic disease.
Two of the 6 patients reassigned to CMR by both reviewers, compared with the independent review committee, had new uptake at sites not involved at baseline. These areas of new uptake were considered to be more in keeping with a sarcoidlike reaction and inflammatory uptake in degenerative disease at a facet joint than with new sites of follicular lymphoma, with CMR at all other sites. Two patients had abnormal uptake determined by the reviewers to be due to a neoplasm different from follicular lymphoma, also in the context of CMR elsewhere. One of these patients had unchanged uptake in an incidental thyroid nodule, in keeping with a probable thyroid neoplasm. Follow-up information was not available. The second patient had increasing 18F-FDG uptake in a focus in the cecum and in a probable liver metastasis. The cecal uptake was later confirmed to be colonic adenocarcinoma on biopsy (Fig. 1). Two patients had low-grade residual uptake in the mesentery and the subpectoral region, respectively, which were close to the intensity of maximum uptake in the liver visually but less than the liver on quantitative measurement and hence reassigned as CMR. Five of the 6 patients reassigned to CMR did not experience PFS24. The remaining patient, with inflammatory arthropathy, experienced progression, presenting with a new parotid node 11 mo after the end of induction, a site of initial disease on the baseline scan that had responded at the end of induction on both PET/CT and contrast-enhanced CT.
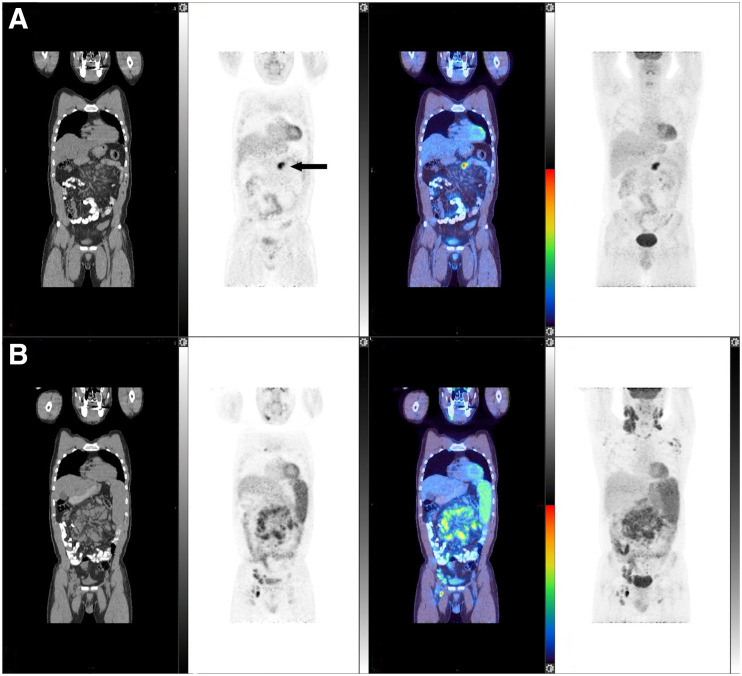
FIGURE 1.
Patient scan with CT, PET, fused, and maximum-intensity-projection coronal images, from left to right. Scans at end of induction and at baseline are shown in A and B, respectively. 18F-FDG uptake is seen in focus in cecum (arrowed in A on PET image) and in liver (arrowed in A on maximum-intensity-projection image) at end of induction, which was increased compared with baseline scan (arrowed in B on PET and maximum-intensity-projection images). There was CMR at other sites, including left supraclavicular and upper abdominal nodes. Patient did not experience progression from follicular lymphoma, but biopsy of cecum confirmed adenocarcinoma. Images are scaled to a maximum SUV of 7 for display.
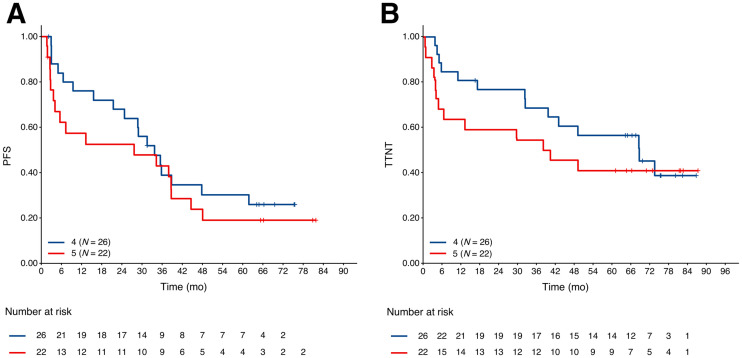
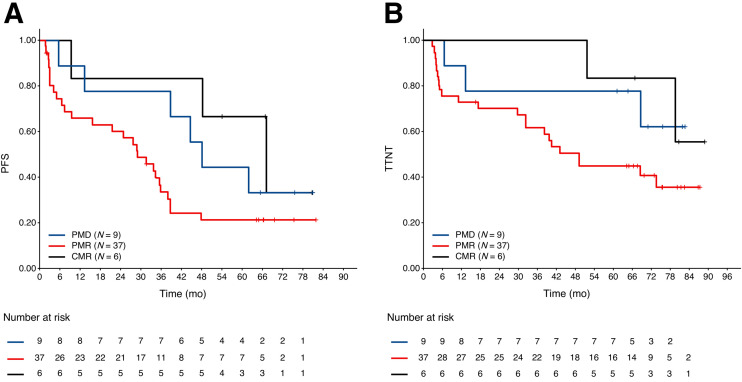
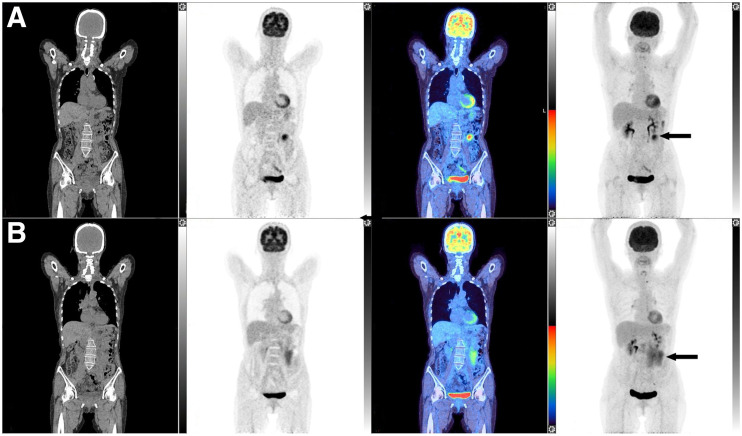
Forty-eight patients were assigned as non-CMR by the current reviewers, 26 with a Deauville score of 4 and 22 with a Deauville score of 5. The PFS and time to next antilymphoma treatment for patients with a Deauville score of 5 was shorter than for patients with a Deauville score of 4, but the differences did not reach statistical significance. The hazard ratios were 1.33 (95% CI, 0.69–2.59; P = 0.39) and 1.37 (95% CI, 0.64–2.92; P = 0.41) for PFS and time to next antilymphoma treatment, respectively (Fig. 2). Fourteen of 37 (37.8%) patients with a partial metabolic response, 2 of 2 patients with no metabolic response, and 2 of 9 (22.2%) patients with progressive metabolic disease experienced PFS24 (Fig. 3), prompting a careful review of appearances that might not represent lymphoma and could represent false-positive uptake. Six of the 9 patients with progressive metabolic disease had increased or new uptake in the mesentery, 2 with measurable disease on CT. In 5 of these patients, the mesentery was the only site of disease, and only 1 of the 9 patients with the largest residual mesenteric node (42 × 24 mm) experienced progression from follicular lymphoma within the residual mesenteric site 5.5 mo after end-of-induction PET scans, at which time the patient commenced antilymphoma treatment (Fig. 4). The remaining 5 patients did not experience early progression (Fig. 5). One patient died without progression. Median follow-up for nonprogressing patients was 74.5 mo (range, 16.5–0.9 mo).
FIGURE 2.
Landmark Kaplan–Meier plots of PFS (A) and time to next antilymphoma treatment (B) by Deauville scores 4 and 5 at end of induction. TTNT = time to next antilymphoma treatment.
FIGURE 3.
Landmark Kaplan–Meier plots of PFS (A) and time to next antilymphoma treatment (B) at end of induction by metabolic response category in patients classified as non-CMR by independent review committee. PMD = progressive metabolic disease; PMR = partial metabolic response; TTNT = time to next antilymphoma treatment.
FIGURE 4.
Patient scan with CT, PET, fused, and maximum-intensity-projection coronal images, from left to right. Increased 18F-FDG uptake is seen in mesentery on end-of-induction PET/CT scan (A) (SUVmax, 11.4; arrowed in A and B), with CMR at all other sites demonstrated on baseline scan (B). Lesion measured 42 × 24 mm on contrast-enhanced CT, and patient experienced progression of mesenteric node at 5.5 mo on contrast-enhanced CT. Images are scaled to a maximum SUV of 7 for display.
FIGURE 5.
Patient scan with CT, PET, fused, and maximum-intensity-projection coronal images, from left to right. Residual 18F-FDG uptake is seen in mesentery on end-of-induction PET/CT scan (A) (SUVmax, 11.6; arrowed in A), with CMR at all other sites demonstrated on baseline scan (B). Patient did not experience progression. Images are scaled to a maximum SUV of 7 for display.
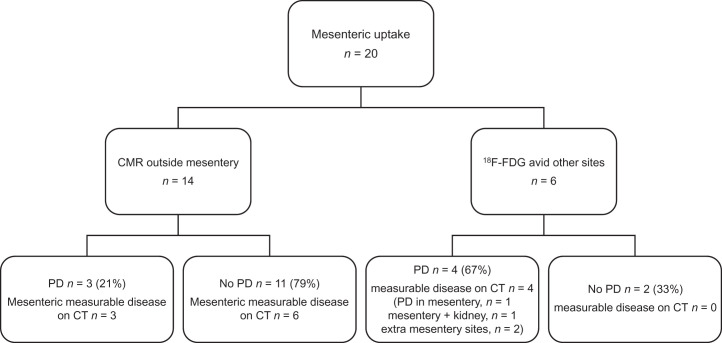
In the current analysis of a subgroup of patients assigned as non-CMR, high residual or new mesenteric uptake associated with lymph nodes or mesenteric stranding was a common finding. Twenty of 48 (41.7%) patients had abnormal 18F-FDG uptake in the mesentery at the end of induction (Fig. 6). All 20 patients had mesenteric involvement at baseline. The location of mesenteric uptake was not always identical at baseline and response but, allowing for bowel or mesenteric movement and reduction in other nodal responding masses, was concerning for the presence of residual lymphoma. Fourteen of 20 (70.0%) patients had mesenteric uptake in the context of CMR at all other baseline 18F-FDG–avid sites, with a median mesenteric SUVmax of 10.6 (range, 3.1–18.7), only 3 of whom (21.0%) progressed; all had measurable mesenteric disease on CT and commenced antilymphoma treatment within 3 mo of progression. Six of 20 patients had mesenteric uptake and additional sites of abnormal 18F-FDG uptake, 4 of whom progressed within 24 mo (66.7%); the progression in 2 of these 4 patients was at mesenteric sites, and one of these 2 patients also had progression of renal disease. All had measurable mesenteric disease on CT. Three of the patients with PFS24 commenced antilymphoma treatment within 3 mo of progressive disease, and 1 commenced treatment 26 mo after progression on contrast-enhanced CT. The presence of measurable disease in the mesentery was difficult to assess on the low-dose CT scan acquired as part of the PET/CT examination, and the size of lesions measured during this post hoc review was not reliable when compared with the measurements obtained on dedicated contrast-enhanced CT scans of the abdomen and pelvis.
FIGURE 6.
PFS24 events on follow-up CT in patients with abnormal 18F-FDG uptake in mesentery at end of induction. PD = progressive disease.
The change in SUVmax with treatment has been suggested as a marker of inadequate response in aggressive non-Hodgkin lymphoma (13,14). The mean reduction in SUVmax was 35.5% among patients who had a PFS24 event (range, −91.4 to +12.1%) and was 34.3% (range, −91.1 to +121.3%) among patients who remained alive without progression.
DISCUSSION
Few patients treated with immunochemotherapy for high-tumor-burden follicular lymphoma fail to achieve a CMR at the end of induction. In this ancillary analysis of the 12% (55) of patients with non-CMR from the GALLIUM PET study, as assessed by the independent review committee, the current reviewers reassigned 6 patients to CMR. Four were reassigned by virtue of new or persistent sites of 18F-FDG uptake not considered to be follicular lymphoma (new uptake was considered to be due to a sarcoidlike reaction and an inflammatory arthropathy in 2 patients, and persistent uptake was considered to be due to an incidental thyroid nodule and colonic cancer in another 2 patients). New areas of uptake unlikely to be related to lymphoma, especially in the context of response elsewhere, are not considered to represent progressive metabolic disease in the Lugano criteria and are designated by the suffix X after the PET score given to residual lymphomatous lesions (e.g., score 3X or 4X) (3) and by implication to sites of persistent or increased uptake attributable to other etiologies. Two patients with residual uptake close in intensity to the liver visually but measured as quantitatively lower than maximum liver uptake were also reassigned to CMR. It is recommended that visual assessment be confirmed using quantitative assessment to avoid misinterpretation, which can be influenced by the adjacent background (15,16). Only the patient with inflammatory arthropathy experienced progression, at a different nodal site.
Nine patients in this analysis using Lugano criteria were assigned as having progressive metabolic disease by expert reviewers, and the observation that 7 of these patients did not experience early progression was surprising and prompted scrutiny of all scans of patients with non-CMR. Persistent or new focal uptake in the mesentery was relatively common (20/48; 42%) in this non-CMR population; only 1 of these patients did not have baseline abnormal mesenteric uptake. An important finding was that just 3 of the 14 patients (21%) with mesenteric uptake who had CMR at all other sites developed early progression, and among patients who progressed in mesenteric sites, all had measurable disease on CT at the time of the first non-CMR assignment. Mesenteric uptake with CMR elsewhere was observed in 3 patients who received obinutuzumab and 11 with rituximab, suggesting it was not related to mesenteric reactivity to the administration of a more potent antibody. This observation suggests that mesenteric uptake has a high likelihood of being false-positive, perhaps inflammatory or representing a delayed metabolic response, especially in the context of CMR elsewhere. In our series, disease progression never occurred in mesenteric sites in the absence of measurable disease on CT at the end of induction. Regression of mesenteric lesions, however, also occurred in 4 patients who had measurable mesenteric disease on the contrast-enhanced CT at the end of induction with CMR at other sites, only 1 of whom progressed at another site 4 mo after the end of induction. By comparison, 15 of 34 patients with nonmesenteric 18F-FDG uptake had a PFS24 event (44%), which was similar to the rate of progression or death of 45% previously reported in the non-CMR population at 2.5 y.
Mesenteric panniculitis on CT has been reported in around 2% of patients with non-Hodgkin lymphoma (17), most commonly follicular lymphoma (18), and is also associated with solid cancers, autoimmune disease, infection, and abdominal trauma. It is more commonly found in male patients (18–20). On biopsy, these appearances are associated with inflammation and fat necrosis (19). Radiologic features that help to distinguish lymphomatous involvement from inflammatory uptake at diagnosis are reported to be nodules at least 1 cm in size, increased attenuation giving the appearance of a misty mesentery, sometimes calcification and the absence of a fat ring or halo around the mesenteric vessels, or a pseudocapsule (17,19). 18F-FDG uptake was reported in only 2 of 44 patients who underwent PET scanning in a retrospective review of all patients with the appearance of mesenteric panniculitis on abdominal CT scans over a 5-y period in a single U.S. radiology network and has been used as a feature to differentiate lymphoma from other causes of panniculitis (18,20). After treatment for lymphoma, distinguishing CT features may disappear, and calcification may occur within nodal masses. Case reports have suggested that follow-up CT imaging may be helpful to determine whether CT changes suggestive of panniculitis resolve, remain stable, or progress along with the underlying lymphoma (17,18,20). Stable changes were reported in 80%—and improvement in appearances in 9%—of cases using CT in 1 series (18). Ishiyama et al. recently reported 3 patients with lymphoma, where new mesenteric uptake on PET/CT with CMR elsewhere, similar to our findings, showed reduced 18F-FDG uptake over time, with an excisional biopsy in 1 patient with follicular lymphoma demonstrating fat necrosis and histiocyte infiltration (21). Four patients in our series with abnormal 18F-FDG uptake in the mesentery in isolation and measurable disease on CT who did not experience disease progression showed regression of lesions on contrast-enhanced CT suggesting an inflammatory component that may be treatment-related. Delayed response in the bone marrow and large nodal masses, also likely inflammatory, are recognized in aggressive lymphomas with focal 18F-FDG uptake at diagnosis (16,22). The Lugano classification considered that “in the case of persistent focal changes in the bone marrow in the context of nodal response consideration should be given to further evaluation with MRI, biopsy or an interval scan” (4). Our findings suggest that similar caution should be exercised in the case of persistent or new sites of mesenteric uptake in patients with follicular lymphoma, especially in the context of response at other disease sites. The use of change in SUV did not appear to be helpful for response discrimination or determining whether mesenteric uptake was a false-positive.
This review was limited by the small number of patients who fail to achieve CMR with effective treatment of follicular lymphoma. The reviewers in the current analysis were masked to independent review committee results but were aware that patients had been categorized as non-CMR. The review was not powered to determine the association of Deauville score or metabolic response category with patient outcomes, although there was a trend for shorter PFS and time to next antilymphoma treatment in patients with a Deauville score of 5 as previously reported in aggressive lymphomas (8–11).
CONCLUSION
In some patients initially considered as non-CMR, CMR was assigned on rereview because alternative pathology, rather than lymphoma, was considered to be the cause of new or increased 18F-FDG uptake, and use of the suffix X in this circumstance is recommended as per international guidelines (3). Quantitative assessment to confirm the visual impression of residual uptake in lesions is also suggested to avoid erroneous classification of non-CMR (14,16). Mesenteric 18F-FDG uptake is a common false-positive finding at the end of induction in patients with follicular lymphoma who fail to achieve CMR, most likely because of inflammatory uptake rather than residual lymphoma, especially in the context of CMR at all other disease sites. On the basis of our results, contrast-enhanced CT is recommended to determine whether mesenteric uptake is accompanied by measurable disease on CT, as early progression did not occur in patients without corresponding CT abnormalities. Furthermore, no changes in clinical management or disease surveillance strategy are recommended when isolated mesenteric 18F-FDG uptake is present at the end of induction without measurable disease on CT or a clinical suspicion of active disease. These patients and their families can be reassured and managed as other patients with CMR.
DISCLOSURE
Sally Barrington reports funding from the National Institute for Health Research and Social Care (NIHR) [RP-2-16-07-001] and an honorarium from F. Hoffmann-La Roche Ltd for educational material. Third-party editorial assistance, under the direction of Sally Barrington, was provided by Louise Profit, PhD, and Zoe Toland of Ashfield MedComms, an Ashfield Health company, and was funded by F. Hoffmann-La Roche Ltd. Farheen Mir is a former employee of F. Hoffmann-La Roche Ltd. Tarec Christoffer El-Galaly is a former employee of F. Hoffmann-La Roche Ltd and has received a speaker fee from Abbvie. Andrea Knapp, Tina Nielsen, and Denis Sahin are employees of and own equity in F. Hoffmann-La Roche Ltd. Michael Wenger is currently employed by Novartis and is a former employee of Genentech Inc. Lale Kostakoglu reports consultancy fees from F. Hoffmann-La Roche Ltd. and Genentech, Inc., and travel expenses from F. Hoffmann-La Roche Ltd. Judith Trotman reports research funding from F. Hoffmann-La Roche Ltd., Celgene, Janssen, PCYC, and Beigene. Michel Meignan reports funding from Novartis, F. Hoffmann-La Roche Ltd, Celgene, and Gilead Sciences to support organization of PILM 2021 and research support from F. Hoffmann-La Roche Ltd. The GALLIUM study is sponsored by F. Hoffmann-La Roche Ltd. King’s College London and UCL Comprehensive Cancer Imaging Centre are funded by the CRUK and EPSRC in association with the MRC and Department of Health and Social Care (England). This work was also supported by the Wellcome/EPSRC Centre for Medical Engineering at King’s College London (WT 203148/Z/16/Z). No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
We thank Dr. Bhupinder Sharma for his suggestions on preparing the manuscript and Anastasiia Kinkolykh and Dirk Klingbiel for their contributions to data analyses.
KEY POINTS
QUESTION: What is the outcome of patients from the GALLIUM trial with follicular lymphoma treated with first-line immunochemotherapy who did not achieve CMR?
PERTINENT FINDINGS: In the PET substudy of the GALLIUM trial, CMR on PET/CT was the sole independent predictor of overall survival in patients with follicular lymphoma; here, we investigated the outcome of patients who did not achieve a CMR. We found that isolated mesenteric 18F-FDG uptake is likely a false-positive finding at the end of induction and does not warrant changes in clinical management or disease surveillance unless there is measurable disease on contrast-enhanced CT or clinical suspicion of active disease.
IMPLICATIONS FOR PATIENT CARE: Our results highlight that isolated mesenteric 18F-FDG uptake is a false-positive finding at the end of induction in follicular lymphoma. We suggest contrast-enhanced CT for assessment, if appropriate, as early progression never occurred in the absence of measurable disease; this is important for clinical practice as these patients and their families can be reassured and managed as other patients with CMR.
REFERENCES
- 1. Trotman J, Luminari S, Boussetta S, et al. Prognostic value of PET-CT after first-line therapy in patients with follicular lymphoma: a pooled analysis of central scan review in three multicentre studies. Lancet Haematol. 2014;1:e17–e27. [DOI] [PubMed] [Google Scholar]
- 2. Trotman J, Barrington SF, Belada D, et al. Prognostic value of end-of-induction PET response after first-line immunochemotherapy for follicular lymphoma (GALLIUM): secondary analysis of a randomised, phase 3 trial. Lancet Oncol. 2018;19:1530–1542. [DOI] [PubMed] [Google Scholar]
- 3. Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol. 2014;32:3048–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. [DOI] [PubMed] [Google Scholar]
- 6. Federico M, Mannina D, Versari A, et al. Response oriented maintenance therapy in advanced follicular lymphoma: results of the interim analysis of the FOLL 12 trial conducted by the Fondazione Italiana Linfomi. Hematol Oncol. 2019;37:153–154. [Google Scholar]
- 7. Pettitt AR, Barrington S, Kalakonda N, et al. NCRI PETReA trial: a phase 3 evaluation of PET-guided, response-adapted therapy in patients with previously untreated, advanced-stage, high-tumour-burden follicular lymphoma. Hematol Oncol. 2019;37:67–68. [Google Scholar]
- 8. Barrington SF, Phillips EH, Counsell N, et al. Positron emission tomography score has greater prognostic significance than pretreatment risk stratification in early-stage Hodgkin lymphoma in the UK RAPID study. J Clin Oncol. 2019;37:1732–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ceriani L, Martelli M, Gospodarowicz MK, et al. Positron emission tomography/computed tomography assessment after immunochemotherapy and irradiation using the Lugano classification criteria in the IELSG-26 study of primary mediastinal B-cell lymphoma. Int J Radiat Oncol Biol Phys. 2017;97:42–49. [DOI] [PubMed] [Google Scholar]
- 10. Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374:2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hertzberg M, Gandhi M, Butcher B, et al. Early treatment intensification with R-ICE chemotherapy followed by autologous stem cell transplantation (ASCT) using Zevalin-BEAM for patients with poor risk diffuse large B-cell lymphoma (DLBCL) as identified by interim PET/CT scan performed after four cycles of R-CHOP-14: a multicenter phase II study of the Australasian Leukaemia Lymphoma Study Group (ALLG) [abstract]. Blood. 2015;126:815. [Google Scholar]
- 12. Marcus R, Davies A, Ando K, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med. 2017;377:1331–1344. [DOI] [PubMed] [Google Scholar]
- 13. Lin C, Itti E, Haioun C, et al. Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med. 2007;48:1626–1632. [DOI] [PubMed] [Google Scholar]
- 14. Itti E, Lin C, Dupuis J, et al. Prognostic value of interim 18F-FDG PET in patients with diffuse large B-cell lymphoma: SUV-based assessment at 4 cycles of chemotherapy. J Nucl Med. 2009;50:527–533. [DOI] [PubMed] [Google Scholar]
- 15. Itti E, Juweid ME, Haioun C, et al. Improvement of early 18F-FDG PET interpretation in diffuse large B-cell lymphoma: importance of the reference background. J Nucl Med. 2010;51:1857–1862. [DOI] [PubMed] [Google Scholar]
- 16. Barrington SF, Kluge R. FDG PET for therapy monitoring in Hodgkin and non-Hodgkin lymphomas. Eur J Nucl Med Mol Imaging. 2017;44:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khasminsky V, Ram E, Atar E, Steinminz A, Issa N, Bachar GN. Is there an association between mesenteric panniculitis and lymphoma? A case control analysis. Clin Radiol. 2017;72:844–849. [DOI] [PubMed] [Google Scholar]
- 18. Ehrenpreis ED, Roginsky G, Gore RM. Clinical significance of mesenteric panniculitis-like abnormalities on abdominal computerized tomography in patients with malignant neoplasms. World J Gastroenterol. 2016;22:10601–10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horton KM, Lawler LP, Fishman EK. CT findings in sclerosing mesenteritis (panniculitis): spectrum of disease. Radiographics. 2003;23:1561–1567. [DOI] [PubMed] [Google Scholar]
- 20. Zissin R, Metser U, Hain D, Even-Sapir E. Mesenteric panniculitis in oncologic patients: PET-CT findings. Br J Radiol. 2006;79:37–43. [DOI] [PubMed] [Google Scholar]
- 21. Ishiyama M, Matesan M. Mesenteric panniculitis mimicking early recurrence at end-of-treatment evaluation in malignant lymphoma: differentiation by active surveillance with F-18 FDG PET/CT imaging. Radiol Case Rep. 2020;15:1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schöder H, Polley MC, Knopp MV, et al. Prognostic value of interim FDG-PET in diffuse large cell lymphoma: results from the CALGB 50303 clinical trial. Blood. 2020;135:2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]