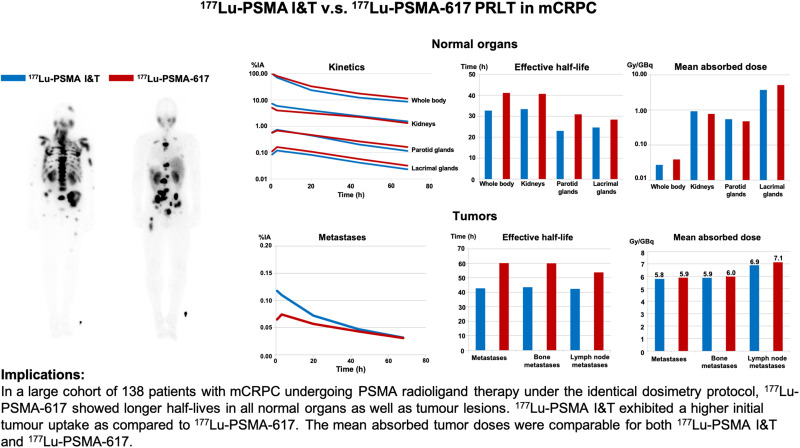
Visual Abstract
Keywords: prostate-specific membrane antigen, dosimetry, 177Lu, PSMA radioligand therapy, 177Lu-PSMA I&T, 177Lu-PSMA-617, theranostics
Abstract
The objective of this study was to determine the safety, kinetics, and dosimetry of the 177Lu-labeled prostate-specific membrane antigen (PSMA) small molecules 177Lu-PSMA I&T and 177Lu-PSMA-617 in a large cohort of patients with metastatic castration-resistant prostate cancer (mCRPC) undergoing PSMA radioligand therapy (PRLT). Methods: In total, 138 patients (mean age, 70 ± 9 y; age range, 46–90 y) with progressive mCRPC and PSMA expression verified by 68Ga-PSMA-11 PET/CT underwent PRLT. Fifty-one patients received 6.1 ± 1.0 GBq (range, 3.4–7.6 GBq) of 177Lu-PSMA I&T, and 87 patients received 6.5 ± 1.1 GBq (range, 3.5–9.0 GBq) of 177Lu-PSMA-617. Dosimetry was performed on all patients using an identical protocol. The mean absorbed doses were estimated with OLINDA software (MIRD Scheme). Treatment-related adverse events were graded according to the Common Terminology Criteria for Adverse Events, version 5.0, of the National Cancer Institute. Results: The whole-body half-lives were shorter for 177Lu-PSMA I&T (35 h) than for 177Lu-PSMA-617 (42 h). The mean whole-body dose of 177Lu-PSMA-617 was higher than that of 177Lu-PSMA I&T (0.04 vs. 0.03 Gy/GBq, P < 0.00001). Despite the longer half-life of 177Lu-PSMA-617, the renal dose was lower for 177Lu-PSMA-617 than for 177Lu-PSMA I&T (0.77 vs. 0.92 Gy/GBq, P = 0.0015). Both PSMA small molecules demonstrated a comparable dose to the parotid glands (0.5 Gy/GBq, P = 0.27). Among all normal organs, the lacrimal glands exhibited the highest mean absorbed doses, 5.1 and 3.7 Gy/GBq, for 177Lu-PSMA-617 and 177Lu-PSMA I&T, respectively. All tumor metastases exhibited a higher initial uptake when using 177Lu-PSMA I&T than when using 177Lu-PSMA-617, as well as a shorter tumor half-life (P < 0.00001). The mean absorbed tumor doses were comparable for both 177Lu-PSMA I&T and 177Lu-PSMA-617 (5.8 vs. 5.9 Gy/GBq, P = 0.96). All patients tolerated the therapy without any acute adverse effects. After 177Lu-PSMA-617 and 177Lu-PSMA I&T, there was a small, statistically significant reduction in hemoglobin, leukocyte counts, and platelet counts that did not need any clinical intervention. No nephrotoxicity was observed after either 177Lu-PSMA I&T or 177Lu-PSMA-617 PRLT. Conclusion: Both 177Lu-PSMA I&T and 177Lu-PSMA-617 PRLT demonstrated favorable safety in mCRPC patients. The highest absorbed doses among healthy organs were in the lacrimal and parotid glands—not, however, resulting in any significant clinical sequel. 177Lu-PSMA-617 demonstrated a higher absorbed dose to the whole-body and lacrimal glands but a lower renal dose than did 177Lu-PSMA I&T. The mean absorbed tumor doses were comparable for both 177Lu-PSMA I&T and 177Lu-PSMA-617. There was a large interpatient variability in the dosimetry parameters. Therefore, individual patient-based dosimetry seems favorable for personalized PRLT.
Prostate cancer is the second most frequent cancer and the fifth leading cause of cancer death in men; in 2020, there were almost 1.4 million new cases and 375,000 deaths worldwide (1). It carries a poor prognosis when it metastasizes aggressively after initial treatment and becomes castration-resistant (2).
A promising treatment modality in the management of metastatic castration-resistant prostate cancer (mCRPC) can be provided when prostate-specific membrane antigen (PSMA) small molecules are radiolabeled; such PSMA-targeted radioligand therapy (PRLT) may use the β-emitting radionuclide 177Lu or the α-emitter 225Ac, as per multiple retrospective studies. 177Lu-PSMA therapy decreased prostate-specific antigen by at least 50% in 32 of 50 men with mCRPC who had progressed after conventional treatment, and the toxicity profile was favorable (3,4). In a randomized, open-label phase 2 trial, TheraP, 177Lu-PSMA therapy produced a higher prostate-specific antigen response and fewer adverse events than did cabazitaxel chemotherapy in mCRPC (5). Furthermore, targeted α-therapy with 225Ac-PSMA has provided durable disease control after failure of 177Lu-PSMA treatment, when all other therapeutic options have been exhausted (6–10).
Currently, the most frequently used PSMA-targeting small-molecule inhibitors are DOTA-PSMA-617 (PSMA-617) and DOTAGA-PSMA I&T (PSMA I&T); “DOTA” and “DOTAGA” denote the cages enclosing the radionuclides, and “I&T” denotes the radionuclide that yields both imaging and therapy. 177Lu (half-life, 6.7 d) is the radionuclide for theranostics, as it emits a cytotoxic β-particle for effective therapy and also has the ability to quantify γ-emissions, enabling diagnostic evaluation and biodistribution using scintigraphy for dosimetry.
Pilot dosimetric studies of either 177Lu-PSMA-617 or 177Lu-PSMA I&T were performed to estimate the absorbed doses for normal organs and tumor lesions. An initial study that included 7 patients for whom the pretreatment radiation doses were estimated using a tracer amount of 177Lu-PSMA-617 indicated that the dose-limiting organ seemed to be the parotid glands rather than the kidneys and that the radiation dose to the bone marrow was significantly lower than those to the kidneys and the parotid glands (11). These dosimetric studies were obtained in a small number of patients, however, and were given in tracer amounts or a low therapeutic activity; indeed, few publications have addressed the absorbed doses delivered to tumors after 177Lu-PSMA radionuclide therapy, and dosimetric approaches for calculation of the absorbed doses have varied among studies (11–19).
Therefore, for the first time, to our knowledge, we compared 177Lu-PSMA-617 and 177Lu-PSMA I&T using an identical dosimetry protocol. The Bad Berka dose protocol, used in our daily clinical routine, has been established during more than 15 y in the treatment of more than 1,000 neuroendocrine neoplasm patients undergoing peptide receptor radionuclide therapy (20,21). Dosimetric parameters, such as uptake and estimated mean absorbed dose to organs and tumor lesions, were obtained from these dosimetric calculations to evaluate therapeutic response and possible adverse effects.
For this reason, the aim of this study was to determine the safety, kinetics, and dosimetry of the 177Lu-labeled PSMA small molecules 177Lu-PSMA I&T and 177Lu-PSMA-617 in a large cohort of patients with mCRPC undergoing PRLT under an identical dosimetry protocol.
MATERIALS AND METHODS
Patients
In total, 138 patients (mean age, 70 ± 9 y; age range, 46–90 y) with progressive mCRPC who received 177Lu-PSMA I&T or 177Lu-PSMA-617 PRLT at Zentralklinik Bad Berka were enrolled in this retrospective study. Significant PSMA expression of the metastases was confirmed by 68Ga-PSMA-11 PET/CT (Biograph mCT Flow 64; Siemens). The demographics of the patients and the location of metastases are shown in Table 1.
TABLE 1.
Demographic and Baseline Characteristics of Patients with mCRPC (n = 138)
| Characteristic | All patients | 177Lu-PSMA I&T group | 177Lu-PSMA-617 group |
|---|---|---|---|
| Number of patients | 138 | 51 | 87 |
| Age (y) | 70 ± 9 (46–90) | 71 ± 9 (46–87) | 69 ± 9 (50–90) |
| ISUP grading | |||
| Group 1 | 7 (5.1%) | 2 (3.9%) | 5 (5.7%) |
| Group 2 | 20 (14.5%) | 3 (5.9%) | 17 (19.5%) |
| Group 3 | 21 (15.2%) | 10 (19.6%) | 11 (12.6%) |
| Group 4 | 26 (18.8%) | 6 (11.8%) | 20 (23.0%) |
| Group 5 | 39 (28.3%) | 18 (35.3%) | 21 (24.1%) |
| NA | 25 (18.1%) | 12 (23.5%) | 13 (14.9%) |
| PSA level (ng/mL) | 216.5 ± 538.7 | 90.6 ± 158.7 | 283.3 ± 648.2 |
| Metastases | |||
| Lymph nodes | 109 (79.0%) | 38 (74.5%) | 71 (81.6%) |
| Bone | 108 (78.2%) | 39 (76.5%) | 69 (79.3%) |
| Bone marrow | 11 (8.0%) | 2 (3.9%) | 9 (10.3%) |
| Lung | 15 (10.9%) | 6 (11.8%) | 9 (10.3%) |
| Liver | 12 (8.7%) | 4 (7.8%) | 8 (9.2%) |
| Other | 36 (26.1%) | 10 (19.6%) | 26 (29.9%) |
| Injected activity (GBq) | 6.4 ± 1.0 (3.4–9.0) | 6.1 ± 1.0 (3.4–7.6) | 6.5 ± 1.1 (3.5–9.0) |
ISUP = International Society of Urological Pathology; NA = not available; PSA = prostate-specific antigen.
Qualitative data are number and percentage; continuous data are mean and range.
177Lu-PSMA I&T and 177Lu-PSMA-617 were administered in compliance with the German Medicinal Products Act, AMG §13 2b, and in accordance with the responsible regulatory body (Thüringer Landesamt; that is, the government of Thuringia). All patients underwent PRLT under the compassionate-use clause of the German Medicinal Product Act (22). All procedures performed in studies involving human participants complied with the ethical standards of the institutional or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The decision to perform PRLT was based on exhaustion of all conventional therapy options; took into account age, renal function, and the adverse effects of possible other therapies; and was made by the referring physicians (urologists and oncologists). The study protocol was approved by the local ethics committee (approval 34333/2017/96, Bad Berka). All patients signed a detailed informed consent form before undergoing the treatment, as well as consenting to the use of their anonymized clinical data for scientific purposes. The administered activities are shown in Table 1.
Radiopharmaceuticals and Infusion
177Lu labeling of the DOTAGA-based PSMA ligand PSMA I&T (DOTAGA-(I-y)fk(Sub-KuE)) and the PSMA-617 ligand was performed in our good-manufacturing-practice–certified radiopharmacy using previously published methods (23,24). In brief, the PSMA ligand was incubated with the required radioactivity of 177Lu-Cl3 at 90°C for 30 min in sodium acetate buffer (0.4 M, pH 5.5). To this buffer, 5–10 mg of gentisic acid were added to prevent radiolysis. The reaction solutions were diluted with saline to achieve a suitable volume. After sterile filtration, a sample was taken for quality control (radio–high-performance liquid chromatography, radio–thin-layer chromatography, pH, limulus amebocyte lysate testing, sterility testing, retention sample). Radiochemical purity was more than 95% in all cases (in most labeling procedures, >99%). The radiopharmaceutical was administered intravenously over 10–15 min using a dedicated infusion pump system for radionuclide therapy.
Imaging and Dosimetry
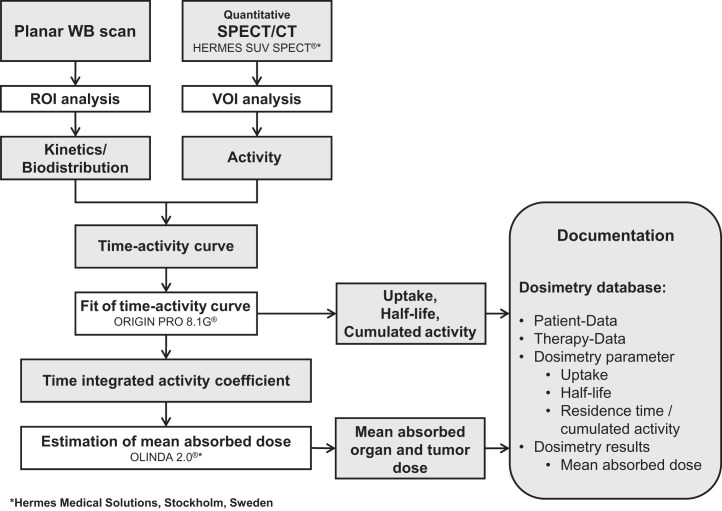
Dose estimation requires an accurate determination of the time-dependent activity of the organs and tumors. Thus, most important is the correct evaluation of the distribution and the kinetics of the administered radiopharmaceutical (25,26). To do so, we adapted the calculation model to our special conditions to establish the Bad Berka dose protocol, which is practicable in daily clinical routine, and to make dosimetry available for each patient. The dosimetric approach is based on the MIRD scheme, and mean absorbed doses are estimated using the software OLINDA, version 2.0 (27–30). The workflow of the Bad Berka dose protocol is shown in Figure 1.
FIGURE 1.
Flowchart of Bad Berka dose protocol. ROI = region of interest; VOI = volume of interest.
At least 5 serial planar whole-body scintigraphy studies and 1 SPECT/CT study were acquired per patient. For planar whole-body imaging, we used the following γ-camera settings: MEDISO spirit DH-V dual-head γ-camera (Medical Imaging Systems), medium-energy general-purpose collimator, 15% energy window, peak at 208 keV, and scan speed of 15 cm/min. Whole-body scintigraphy was performed from 0.5 h after injection (immediately after administration of therapeutic activity and before bladder voiding) to 68 h after injection at a total of at least 5 time points. Additionally, posttherapy SPECT/CT images of the kidneys or tumor-involved regions was done at 24, 48, or 72 h after injection using a Symbia T camera system (Siemens) with the following settings: medium-energy low-penetration collimator, peak at 113 keV and 208 keV (15% energy windows and 20% upper and lower scatter window), 128 × 128 matrix, 32 projections with 30 s per step, and body contouring.
Because the patients were not allowed to empty the bladder before the first scan, the total-body counts acquired immediately after the injection were defined to be 100% of the administered activity. By assessing means of regions of interest, which were drawn manually over the source regions, the scintigraphy studies were analyzed using the Hermes system (Hermes Medical Solutions). Regions of interest were always drawn manually by the same physicist, in collaboration with a nuclear medicine physician, who decided which lesions were suitable for dosimetry; preferably, these target lesions had the highest uptake in each organ. The SPECT/CT scans were reconstructed and quantified using the Hermes SUV SPECT software (Hermes Medical Solutions). Mean absorbed doses to organs and tumors were estimated using OLINDA 2.0. Specifically, mean absorbed doses to tumors and to parotid and lacrimal glands were estimated using the unit density sphere module of OLINDA 2.0. A standard volume was used to assess lacrimal glands, according to the study of Bingham et al. (31).
With this dosimetry protocol, the following parameters were assessed: uptake as a fraction of the administered activity, effective half-life (hours), and mean absorbed organ and tumor doses (Gy/GBq). Organs showing tumor involvement were excluded from dosimetric evaluation.
Toxicity Assessment
All patients were clinically monitored during therapy and for at least 2–4 d afterward as inpatients for possible side effects. Vital parameters were recorded during therapy, and a structured questionnaire documented any delayed complication. Laboratory analysis including hematologic status, renal function, and liver function was performed before each PRLT cycle and in follow-up (restaging was performed regularly until death). Treatment-related adverse events were recorded in accordance with the Common Terminology Criteria for Adverse Events, version 5.0, of the National Cancer Institute.
Statistical Analysis
All dosimetric parameters were determined for the whole body and for normal organs (kidneys, parotid glands, and lacrimal glands), as well as for metastases. Results are given as median values. In comparisons of the 2 177Lu-labeled PSMA ligands, the following parameters were chosen to describe differences between the peptides: uptake at 20 h after injection, effective half-life, and mean absorbed dose. Nonparametric tests for independent samples were used to describe significant differences among ligands. All statistical tests were performed on OriginPro, version 8.1G (OriginLab); P values of less than 0.05 were considered significant.
RESULTS
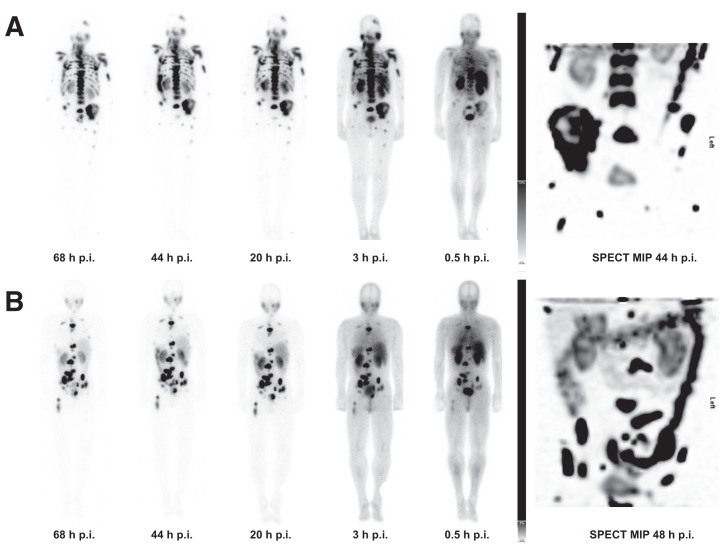
For both 177Lu-PSMA I&T and 177Lu-PSMA-617, strong physiologic uptake was observed in the lacrimal and salivary glands, kidneys, and small intestine on the posttherapy scans, followed by medium to low uptake in the liver and spleen at all time points. The radiopharmaceutical was excreted predominantly through the kidneys, as visualized by an accumulation in the urinary bladder that was dominant on the early scans 0.5 and 3 h after injection. Excellent uptake and retention of both 177Lu-PSMA I&T and 177Lu-PSMA-617 was noted in metastases and in residual or locally recurrent prostate cancer on posttherapy planar and SPECT/CT images (Fig. 2).
FIGURE 2.
PRLT posttherapy scans and SPECT maximum-intensity-projection (MIP) image. (A) Scans after 177Lu-PSMA I&T. (B) Scans after 177Lu-PSMA-617. p.i. = after injection.
Whole Body
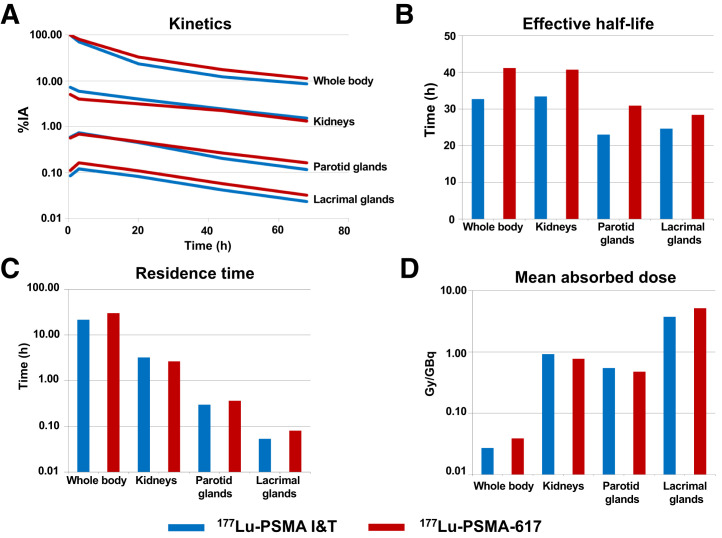
At all time points, retention of 177Lu-PSMA-617 was higher than that of 177Lu-PSMA I&T. The curves demonstrated an initial rapid washout followed by a second slower decline. On that account, the time–activity curves for whole body were fitted to a biexponential function. The half-lives were shorter for 177Lu-PSMA I&T (35 h) than for 177Lu-PSMA-617 (42 h). As a result of calculations from these kinetic parameters, the whole-body mean absorbed dose was higher for 177Lu-PSMA-617 than for 177Lu-PSMA I&T (0.04 vs. 0.03 Gy/GBq, P < 0.00001) (Fig. 3).
FIGURE 3.
Biodistribution and dosimetry results for normal organs in patients treated with different PSMA ligands. (A) Kinetics: median uptakes in percentage administered activity. (B) Median effective half-life in hours. (C) Median residence time in hours. (D) Mean absorbed doses in Gy/GBq.
Kidneys
We analyzed the renal kinetics and kidney dosimetry of the 51 patients treated with 177Lu-PSMA I&T and the 83 patients treated with 177Lu-PSMA-617. Renal uptake was marginally higher for 177Lu-PSMA I&T. For both ligands, uptake declined rapidly between the first scan and 3 h after injection, followed by a slower washout with a longer half-life for 177Lu-PSMA-617. The effective renal half-lives for 177Lu-PSMA-617 and 177Lu-PSMA I&T were 40 and 33 h, respectively (P = 0.00511). As compared with 177Lu-PSMA-617, the residence time was longer for 177Lu-PSMA I&T (P = 0.00138), with initially higher uptake (P < 0.00001), and the resulting renal dose was slightly but statistically significantly higher for 177Lu-PSMA I&T. Calculated absorbed radiation doses of 177Lu-PSMA-617 and 177Lu-PSMA I&T in the kidneys were 0.8 and 0.9 Gy/GBq, respectively (P = 0.0015).
Parotid and Lacrimal Glands
The parotid glands were analyzed in 47 patients treated with 177Lu-PSMA I&T and 80 patients treated with 177Lu-PSMA-617. Both ligands demonstrated a first increase in activity until 3 h after injection before the exponential washout, whereas 177Lu-PSMA-617 showed higher uptake and longer half-lives. The effective half-lives of the parotid glands for 177Lu-PSMA-617 and 177Lu-PSMA I&T were 31 and 23 h, respectively (P < 0.00001); yet, the mean absorbed dose of the different ligands was comparable in the parotid glands (0.5 Gy/GBq) (P = 0.26603) (Fig. 3).
The lacrimal glands were analyzed in 42 patients treated with 177Lu-PSMA I&T and in 69 patients treated with 177Lu-PSMA-617. Uptake was slightly higher and the half-life longer for 177Lu-PSMA-617. The effective half-lives for 177Lu-PSMA-617 and 177Lu-PSMA I&T were 28 and 25 h, respectively (P = 0.00269). The resulting absorbed dose to the lacrimal glands was significantly higher for 177Lu-PSMA-617 than for 177Lu-PSMA I&T (5.1 vs. 3.7 Gy/GBq, P = 0.000617). Notably, among all normal organs, the lacrimal glands exhibited the highest absorbed doses—31 and 22 Gy for 177Lu-PSMA-617 and 177Lu-PSMA I&T, respectively—for an injected activity of 6 GBq.
Tumor Dosimetry
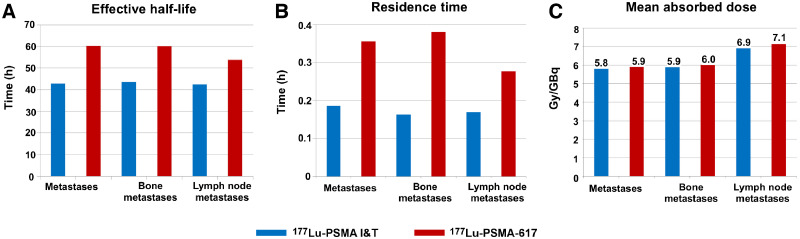
Initial uptake was higher for 177Lu-PSMA I&T than for 177Lu-PSMA-617 (Fig. 4). Fitting of all time–activity curves to monoexponential functions from 20 h after injection led to significantly longer half-lives for 177Lu-PSMA-617. Similar to the results for normal organs, the effective half-life in metastases was longer for 177Lu-PSMA-617 (half-life, 61 h) than for 177Lu-PSMA I&T (half-life, 43 h, P < 0.00001). The mean absorbed tumor doses extended over a wide range, whereas the medians of the mean absorbed tumor doses were comparable (177Lu-PSMA-617 vs. 177Lu-PSMA I&T, 5.9 vs. 5.8 Gy/GBq, P = 0.96257) (Fig. 5).
FIGURE 4.
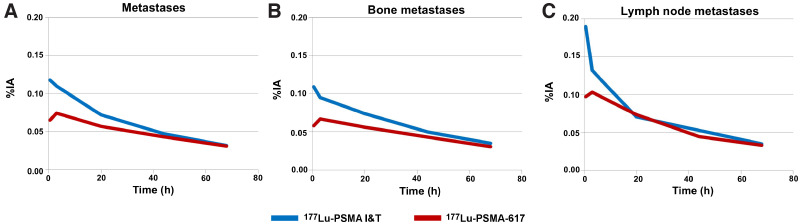
Kinetics of metastases and comparative results from 96 metastases (bone, lymph node, liver, lung, and other) of patients treated with 177Lu-PSMA I&T and from 179 tumor lesions (bone, lymph node, liver, lung, and other) of patients treated with 177Lu-PSMA-617. (A) Median kinetics of all types of metastases. (B) Median kinetics of bone metastases. (C) Median kinetics of lymph node metastases. After administration of therapeutic activity, higher initial uptakes and faster washout for 177Lu-PSMA I&T were observed in bone and lymph node lesions. In contrast, curves of 177Lu-PSMA-617 showed initial increase until 3 h after injection. %IA = percentage injected activity.
FIGURE 5.
Comparative dosimetry results of metastases. (A) Median effective half-life. (B) Median residence time. (C) Mean absorbed dose.
Bone and lymph node lesions were considered separately because most of the investigated lesions were bone or lymph node metastases. After administration of the therapeutic activity, the early elimination phase differed between the 2 ligands, demonstrating higher initial uptake and faster washout for 177Lu-PSMA I&T in bone and lymph node lesions (Fig. 4). 177Lu-PSMA-617 had a longer effective half-life than did 177Lu-PSMA I&T in bone metastases (60 vs. 43 h, P < 0.00001) and lymph node metastases (55 vs. 42 h, P = 0.0275). However, the mean doses absorbed by bone metastases were comparable (177Lu-PSMA-617 vs. 177Lu-PSMA I&T, 6.0 vs. 5.9 Gy/GBq, P = 0.82564), as were the doses to lymph node metastases (177Lu-PSMA-617 vs. 177Lu-PSMA I&T, 7.1 vs. 6.9 Gy/GBq, P = 0.94015). For both ligands, the mean absorbed tumor dose was higher for lymph node lesions than for bone lesions (Fig. 5). The mean tumor-to-kidney absorbed dose ratio was slightly higher for 177Lu-PSMA-617 (7.6) than for 177Lu-PSMA I&T (6.3).
Treatment Toxicity
In no patients receiving either 177Lu-PSMA I&T or 177Lu-PSMA-617 were any serious acute side effects seen, nor were any seen during short-term follow-up (after 2 cycles of PRLT) or long-term follow-up (after 2–6 cycles of PRLT, with follow-up according to the last restaging; observation period, 3.2–48.5 mo; mean ± SD, 17.4 ± 11.9 mo; median, 13.2 mo). No change in blood pressure, heart rate, or body temperature was observed during therapy. The most common adverse effect was mild fatigue, which was observed in 20% of the patients and lasted a few days after therapy, more frequently after the first cycle. Five patients (3.6%) reported mild, reversible xerostomia—2 patients (3.9%) in the 177Lu-PSMA I&T group and 3 (3.4%) in 177Lu-PSMA-617 group—after 2–6 cycles of treatment and in follow-up. Xerophthalmia was not reported by any patients. No other adverse symptoms were noticed during the entire follow-up period.
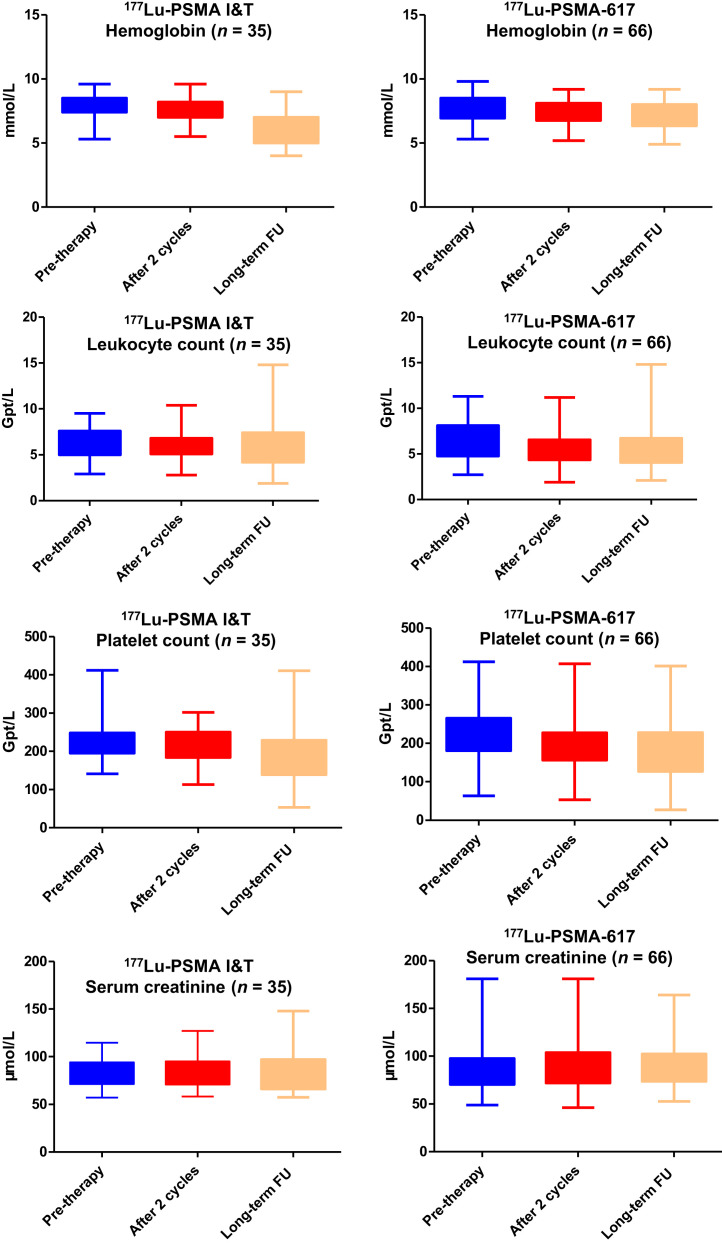
Hematotoxicity and nephrotoxicity after 177Lu-PSMA I&T and 177Lu-PSMA-617 PRLT are detailed in Tables 2 and 3 and in Figure 6. There was no evidence of renal toxicity after either 177Lu-PSMA I&T or 177Lu-PSMA-617 PRLT, as determined by serum creatinine, creatinine clearance using the Cockcroft–Gault formula, or tubular extraction rate as determined by 99mTc-mercaptoacetyltriglycine renal scintigraphy, which was performed before therapy and then every 3 mo during follow-up. No grade 3 or 4 nephrotoxicity, according to the Common Terminology Criteria for Adverse Events, was observed during any treatment cycle or during the longer follow-up. There was a small, statistically significant reduction in hemoglobin, leukocyte counts, and platelet counts after 177Lu-PSMA-617 and 177Lu-PSMA I&T (Fig. 6), although the absolute differences were minimal and clinically insignificant. Remarkably, patients with low blood cell counts before therapy did not exhibit a decrease in blood cell counts after either 177Lu-PSMA I&T or 177Lu-PSMA-617 therapy.
TABLE 2.
Hematotoxicity and Nephrotoxicity after 177Lu-PSMA I&T PRLT According to Common Terminology Criteria for Adverse Events, version 5.0 (n = 35)
| Numbers of patients with… | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anemia | Leukocytopenia | Thrombocytopenia | Nephrotoxicity | |||||||||
| Grade | Pretherapy | After 2 cycles | Long- term FU | Pretherapy | After 2 cycles | Long- term FU | Pretherapy | After 2 cycles | Long- term FU | Pretherapy | After 2 cycles | Long- term FU |
| CTC-1 | 21 | 30 | 24 | 5 | 3 | 6 | 1 | 2 | 8 | 5 | 5 | 7 |
| CTC-2 | 1 | 2 | 8 | 1 | 1 | 2 | 0 | 1 | 1 | 0 | 0 | 0 |
| CTC-3 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| CTC-4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CTC-5 | NA | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 |
FU = follow-up; CTC = Common Terminology Criteria grade; NA = not applicable before therapy (grade 5 represents death).
TABLE 3.
Hematotoxicity and Nephrotoxicity After 177Lu-PSMA-617 PRLT According to Common Terminology Criteria for Adverse Events, version 5.0 (n = 66)
| Numbers of patients with… | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anemia | Leukocytopenia | Thrombocytopenia | Nephrotoxicity | |||||||||
| Grade | Pretherapy | After 2 cycles | Long- term FU | Pretherapy | After 2 cycles | Long- term FU | Pretherapy | After 2 cycles | Long- term FU | Pretherapy | After 2 cycles | Long- term FU |
| CTC-1 | 44 | 45 | 46 | 8 | 12 | 12 | 7 | 12 | 15 | 11 | 12 | 12 |
| CTC-2 | 7 | 14 | 14 | 1 | 3 | 5 | 1 | 2 | 4 | 2 | 1 | 2 |
| CTC-3 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 4 | 0 | 0 | 0 |
| CTC-4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CTC-5 | NA | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 |
FU = follow-up; CTC = Common Terminology Criteria grade; NA = not applicable before therapy (grade 5 represents death).
FIGURE 6.
Comparison of laboratory parameters (hemoglobin, leukocyte, platelet, and serum creatinine) before therapy, after 2 cycles, and after 2–6 cycles with long-term follow up (FU) (observation period, 3.2–48.5 mo; mean ± SD, 17.4 ± 11.9 mo; median, 13.2 mo) for 177Lu-PSMA I&T and 177Lu-PSMA-617 PRLT.
DISCUSSION
In this study of a large cohort of mCRPC patients treated at a single center, we used an identical dosimetry protocol when depicting the biodistribution and dosimetric analysis results after either 177Lu-PSMA I&T or 177Lu-PSMA-617 PRLT therapy.
177Lu-PSMA-617 exhibited a higher mean absorbed dose for whole body and lacrimal glands and showed longer half-lives in all normal organs and in tumor lesions, with the highest tumor doses being estimated for lymph node lesions. The initial tumor uptake was higher for 177Lu-PSMA I&T than for 177Lu-PSMA-617. The mean absorbed tumor doses extended over a wide range, whereas the medians of the mean absorbed tumor doses were comparable for both 177Lu-PSMA I&T and 177Lu-PSMA-617.
177Lu-PSMA-617 had an estimated mean absorbed dose of 0.8 Gy/GBq for kidneys, 0.4 Gy/GBq for parotid glands, and 5.1 Gy/GBq for lacrimal glands (median values). Comparable results were reported by Zechmann et al. after they performed dosimetric estimations with 131I-labeled PSMA ligands (32). Kabasakal et al. reported comparable organ doses in 7 patients who had received a pretherapeutic dose of 177Lu-PSMA-617. The dosimetric approach used was based on planar imaging, to which attenuation correction was applied using PET/CT images (11). Delker et al. also evaluated the dosimetry of 177Lu-PSMA-617 in 5 patients using whole-body scans and quantitative SPECT/CT. They estimated a slightly lower renal dose of 0.6 Gy/GBq than the 0.8 Gy/GBq found in the current study. Similarly, 1.4 Gy/GBq was reported for parotid glands, compared with 1.6 Gy/GBq in our study (mean values). Delker et al. reported mean absorbed tumor doses in the range of 1.2–47.5 Gy (14). In the current study, with a much larger patient population, we found a wider range of mean absorbed tumor doses (between 1.0 and 670 Gy per cycle for individual patients). The highest absorbed tumor dose, 670 Gy, was achieved in a lymph node metastasis in an mCRPC patient with lymph node and liver metastases during his second cycle of PRLT with 6.0 GBq of 177Lu-PSMA-617.
The major route of excretion for both 177Lu-labeled PSMA ligands is the kidneys, as noted by the predominant urinary excretion in the bladder. The high uptake in the kidneys may, however, be due to PSMA expression in renal tissue, because substantial uptake of the radiopharmaceutical was noticed, especially on the early 177Lu-PSMA posttherapy images. Blocking of specific PSMA binding in the kidney tissue by 2-(phosphonomethyl)pentanedioic acid has been validated in preclinical studies, but this compound currently has limited availability for clinical use and also blocks tumor uptake (33). There was no evidence of renal toxicity after either 177Lu-PSMA-617 or 177Lu-PSMA I&T PRLT, inasmuch as there was no significant change in serum creatinine, in creatinine clearance as obtained by the Cockcroft–Gault formula, or in tubular extraction rate as determined by 99mTc-mercaptoacetyltriglycine renal scintigraphy.
According to the presented dosimetry results, we summarized the maximum activity and the number of possible therapy cycles to reach dose limits for both PSMA ligands (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org). Regarding the parotid glands, the maximum number of therapy cycles to reach the dose limit was 16 or 18, assuming an injected activity of 6 GBq of 177Lu-PSMA I&T or 177Lu-PSMA-617, respectively, per cycle. The renal dose, on the other hand, would limit the number of cycles to 4 in the case of 177Lu-PSMA I&T and 5 in the case of 177Lu-PSMA-617, if the 23-Gy rule, as known from external-beam radiotherapy, were used (34). However, the high number of cycles according to the current dose limit derived from external-beam radiotherapy may not reflect the true clinical status of the patients after radionuclide therapy. The absorbed dose to parotid glands in those patients who reported mild, reversible xerostomia in the present study was still under the dose limit. More therapy cycles with an accumulative dose over the absorbed dose limit of 23 Gy were feasible without any relevant side effects to the kidneys. Therefore, the limit for renal dose from external-beam radiotherapy may not apply to PRLT.
In metastases, despite the increased effective half-life and residence time of 177Lu-PSMA-617, as compared with 177Lu-PSMA I&T, the resulting mean absorbed tumor doses were not significantly different for these 2 ligands. This result is most probably due to the higher initial uptake of 177Lu-PSMA I&T than of 177Lu-PSMA-617. In addition, the nonhomogeneously distributed sample of metastases in both patient cohorts could have had an influence, as the median volume of metastases was lower for patients treated with PSMA I&T (median volume, 3 cm3) than for those treated with PSMA-617 (median volume, 6 cm3). When lesions with similar residence times are compared, smaller lesions will get the higher mean absorbed dose.
For the dosimetry results, high interpatient variability was found, especially concerning the mean absorbed doses; this finding was not unexpected since the group of patients was very heterogeneous. In addition, earlier results from a study on peptide receptor radionuclide therapy also demonstrated a high intrapatient variability in patients undergoing therapy with different peptides; even in a large cohort of patients, we found a broad range of results (20,21). This finding implies that the median or mean value of a dosimetric parameter varies among patients. Although the variability may be attributed to differences in biologic behavior among the different ligands, it may be also ascribable to widely differing prior therapies.
This study had a few limitations, such as its retrospective design. No strict pretest criteria for selection of patients were applied, and the baseline characteristics of the 2 groups were heterogeneous. Additionally, the wide interpatient variability should be addressed in further studies: inasmuch as significant variations were found even in the large cohort of patients, median values of absorbed doses among patients should not be the only criterion for planning PRLT. Besides the described methods for individual dosimetry, interindividual differences should be considered.
CONCLUSION
Both 177Lu-PSMA I&T and 177Lu-PSMA-617 PRLT demonstrated favorable safety in mCRPC patients. The highest absorbed doses among healthy organs were observed for the lacrimal and parotid glands, not, however, resulting in any significant clinical side effects. 177Lu-PSMA-617 showed longer half-lives in all normal organs and in tumor lesions than did 177Lu-PSMA I&T. 177Lu-PSMA I&T exhibited a higher initial tumor uptake than did 177Lu-PSMA-617. The mean absorbed tumor doses were comparable for both 177Lu-PSMA I&T and 177Lu-PSMA-617. The results of this study further demonstrated that estimation of mean absorbed doses to critical organs and tumor lesions is necessary when evaluating the risks of PRLT and, therefore, when describing the clinical benefit to the patient. Individual patient-based dosimetry seems favorable for personalized PRLT.
DISCLOSURE
This study was supported by the International Centers for Precision Oncology (ICPO) Foundation, the National University of Singapore Start-up Grant (NUHSRO/2021/097/Startup/13; NUHSRO/2020/133/ Startup/08), and NUS School of Medicine Nanomedicine Translational Research Programme (NUHSRO/2021/034/TRP/09/Nanomedicine). No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENT
We thank the patients for participating in this study; the research support staff for their support; and the physician colleagues, nursing staff, and nuclear medicine technologists of Radioisotope Therapy Ward D3 at Zentralklinik Bad Berka for patient care, past and present. We are grateful to Dr. Robert Sklaroff for critically reading the manuscript.
KEY POINTS
QUESTION: Do 177Lu-PSMA I&T and 177Lu-PSMA-617 differ in safety, biodistribution, and dosimetry for PRLT in patients with mCRPC?
PERTINENT FINDINGS: In a large cohort of 138 patients with mCRPC undergoing PRLT under an identical dosimetry protocol, 177Lu-PSMA-617 showed longer half-lives in all normal organs and in tumor lesions; 177Lu-PSMA I&T exhibited a higher initial tumor uptake than did 177Lu-PSMA-617. The mean absorbed tumor doses were comparable for both 177Lu-PSMA I&T and 177Lu-PSMA-617.
IMPLICATIONS FOR PATIENT CARE: The results of this study demonstrate that estimation of mean absorbed doses to critical organs and tumor lesions is necessary when evaluating the risks of PRLT and, therefore, when describing the clinical benefit to the patient.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. Halabi S, Vogelzang NJ, Kornblith AB, et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol. 2008;26:2544–2549. [DOI] [PubMed] [Google Scholar]
- 3. Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–833. [DOI] [PubMed] [Google Scholar]
- 4. Violet J, Sandhu S, Iravani A, et al. Long-term follow-up and outcomes of retreatment in an expanded 50-patient single-center phase II prospective trial of 177Lu-PSMA-617 theranostics in metastatic castration-resistant prostate cancer. J Nucl Med. 2020;61:857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. [DOI] [PubMed] [Google Scholar]
- 6. Kratochwil C, Bruchertseifer F, Giesel FL, et al. 225Ac-PSMA-617 for PSMA-targeted alpha-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med. 2016;57:1941–1944. [DOI] [PubMed] [Google Scholar]
- 7. Kratochwil C, Haberkorn U, Giesel FL. 225Ac-PSMA-617 for therapy of prostate cancer. Semin Nucl Med. 2020;50:133–140. [DOI] [PubMed] [Google Scholar]
- 8. Zacherl MJ, Gildehaus FJ, Mittlmeier L, et al. First clinical results for PSMA-targeted α-therapy using 225Ac-PSMA-I&T in advanced-mCRPC patients. J Nucl Med. 2021;62:669–674 . [DOI] [PubMed] [Google Scholar]
- 9. Rosar F, Hau F, Bartholoma M, et al. Molecular imaging and biochemical response assessment after a single cycle of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy in mCRPC patients who have progressed on [177Lu]Lu-PSMA-617 monotherapy. Theranostics. 2021;11:4050–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Doelen MJ, Mehra N, van Oort IM, et al. Clinical outcomes and molecular profiling of advanced metastatic castration-resistant prostate cancer patients treated with 225Ac-PSMA-617 targeted alpha-radiation therapy. Urol Oncol. 2021;39:729.e7–729.e16. [DOI] [PubMed] [Google Scholar]
- 11. Kabasakal L, AbuQbeitah M, Aygun A, et al. Pre-therapeutic dosimetry of normal organs and tissues of 177Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:1976–1983. [DOI] [PubMed] [Google Scholar]
- 12. Violet J, Jackson P, Ferdinandus J, et al. Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. J Nucl Med. 2019;60:517–523. [DOI] [PubMed] [Google Scholar]
- 13. Okamoto S, Thieme A, Allmann J, et al. Radiation dosimetry for 177Lu-PSMA I&T in metastatic castration-resistant prostate cancer: absorbed dose in normal organs and tumor lesions. J Nucl Med. 2017;58:445–450. [DOI] [PubMed] [Google Scholar]
- 14. Delker A, Fendler WP, Kratochwil C, et al. Dosimetry for 177Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:42–51. [DOI] [PubMed] [Google Scholar]
- 15. Barna S, Haug AR, Hartenbach M, et al. Dose calculations and dose-effect relationships in 177Lu-PSMA I&T radionuclide therapy for metastatic castration-resistant prostate cancer. Clin Nucl Med. 2020;45:661–667. [DOI] [PubMed] [Google Scholar]
- 16. Hou X, Brosch J, Uribe C, et al. Feasibility of single-time-point dosimetry for radiopharmaceutical therapies. J Nucl Med. 2021;62:1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jackson PA, Hofman MS, Hicks RJ, Scalzo M, Violet J. Radiation dosimetry in 177Lu-PSMA-617 therapy using a single posttreatment SPECT/CT scan: a novel methodology to generate time- and tissue-specific dose factors. J Nucl Med. 2020;61:1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rinscheid A, Kletting P, Eiber M, Beer AJ, Glatting G. Influence of sampling schedules on [177Lu]Lu-PSMA dosimetry. EJNMMI Phys. 2020;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peters SMB, Prive BM, de Bakker M, et al. Intra-therapeutic dosimetry of [177Lu]Lu-PSMA-617 in low-volume hormone-sensitive metastatic prostate cancer patients and correlation with treatment outcome. Eur J Nucl Med Mol Imaging. 2022;49:460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wehrmann C, Senftleben S, Zachert C, Muller D, Baum RP. Results of individual patient dosimetry in peptide receptor radionuclide therapy with 177Lu DOTA-TATE and 177Lu DOTA-NOC. Cancer Biother Radiopharm. 2007;22:406–416. [DOI] [PubMed] [Google Scholar]
- 21. Schuchardt C, Kulkarni HR, Prasad V, Zachert C, Muller D, Baum RP. The Bad Berka dose protocol: comparative results of dosimetry in peptide receptor radionuclide therapy using 177Lu-DOTATATE, 177Lu-DOTANOC, and 177Lu-DOTATOC. Recent Results Cancer Res. 2013;194:519–536. [DOI] [PubMed] [Google Scholar]
- 22.“Compassionate use” programmes. Federal Institute for Drugs and Medical Devices website. https://www.bfarm.de/EN/Medicinal-products/Clinical-trials/Compassionate-Use/_node.html. Published 2013. Accessed May 25, 2022.
- 23. Baum RP, Kulkarni HR, Schuchardt C, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57:1006–1013. [DOI] [PubMed] [Google Scholar]
- 24. Weineisen M, Schottelius M, Simecek J, et al. 68Ga- and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med. 2015;56:1169–1176. [DOI] [PubMed] [Google Scholar]
- 25. Stabin MG, Siegel JA. Physical models and dose factors for use in internal dose assessment. Health Phys. 2003;85:294–310. [DOI] [PubMed] [Google Scholar]
- 26. Sgouros G. Dosimetry of internal emitters. J Nucl Med. 2005;46(suppl 1):18S–27S. [PubMed] [Google Scholar]
- 27. Siegel JA, Thomas SR, Stubbs JB, et al. MIRD pamphlet no. 16: techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med. 1999;40(suppl):37S–61S. [PubMed] [Google Scholar]
- 28. Bolch WE, Eckerman KF, Sgouros G, Thomas SR. MIRD pamphlet no. 21: a generalized schema for radiopharmaceutical dosimetry—standardization of nomenclature. J Nucl Med. 2009;50:477–484. [DOI] [PubMed] [Google Scholar]
- 29. Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–1027. [PubMed] [Google Scholar]
- 30. Stabin MG, Siegel JA. RADAR dose estimate report: a compendium of radiopharmaceutical dose estimates based on OLINDA/EXM version 2.0. J Nucl Med. 2018;59:154–160. [DOI] [PubMed] [Google Scholar]
- 31. Bingham CM, Castro A, Realini T, Nguyen J, Hogg JP, Sivak-Callcott JA. Calculated CT volumes of lacrimal glands in normal Caucasian orbits. Ophthal Plast Reconstr Surg. 2013;29:157–159. [DOI] [PubMed] [Google Scholar]
- 32. Zechmann CM, Afshar-Oromieh A, Armor T, et al. Radiation dosimetry and first therapy results with a 124I/131I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41:1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kratochwil C, Giesel FL, Leotta K, et al. PMPA for nephroprotection in PSMA-targeted radionuclide therapy of prostate cancer. J Nucl Med. 2015;56:293–298. [DOI] [PubMed] [Google Scholar]
- 34. Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. [DOI] [PubMed] [Google Scholar]