Abstract
Introduction
Cognitive behavioural therapy for insomnia (CBT-I) is effective at treating chronic insomnia, yet in-person CBT-I can often be challenging to access. Prior studies have used technology to bridge barriers but have been unable to extensively assess the impact of the digital therapeutic on real-world patient experience and multidimensional outcomes. Among patients with insomnia, our aim is to determine the impact of a prescription digital therapeutic (PDT) (PEAR-003b, FDA-authorised as Somryst; herein called PDT) that provides mobile-delivered CBT-I on patient-reported outcomes (PROs) and healthcare utilisation.
Methods and analysis
We are conducting a pragmatically designed, prospective, multicentre randomised controlled trial that leverages Hugo, a unique patient-centred health data-aggregating platform for data collection and patient follow-up from Hugo Health. A total of 100 participants with insomnia from two health centres will be enrolled onto the Hugo Health platform, provided with a linked Fitbit (Inspire 2) to track activity and then randomised 1:1 to receive (or not) the PDT for mobile-delivered CBT-I (Somryst). The primary outcome is a change in the insomnia severity index score from baseline to 9-week postrandomisation. Secondary outcomes include healthcare utilisation, health utility scores and clinical outcomes; change in sleep outcomes as measured with sleep diaries and a change in individual PROs including depressive symptoms, daytime sleepiness, health status, stress and anxiety. For those allocated to the PDT, we will also assess engagement with the PDT.
Ethics and dissemination
The Institutional Review Boards at Yale University and the Mayo Clinic have approved the trial protocol. This trial will provide important data to patients, clinicians and policymakers about the impact of the PDT device delivering CBT-I on PROs, clinical outcomes and healthcare utilisation. Findings will be disseminated to participants, presented at professional meetings and published in peer-reviewed journals.
Trial registration number
Keywords: SLEEP MEDICINE, Clinical trials, MENTAL HEALTH
Strengths and limitations of this study.
Prospective randomised controlled trial is used to examine the impact of using a novel mobile phone-based, prescription digital therapeutic delivering cognitive behavioural therapy for patients with chronic insomnia.
The strengths of this study include the rigour and reproducibility of the trial design as well as the use of a novel patient-centred health data-aggregating platform (Hugo) for data collection, including healthcare utilisation and patient follow-up.
Limitations include the homogeneous population recruited from two sleep medicine clinics and reliance on participants motivation to engage in and complete both the sleep diaries and behavioural intervention.
Introduction
Insomnia is one of the most prevalent health concerns and imposes a significant physical, psychological and financial burden on patients’ lives.1 Up to 50% of the general adult population experience insomnia symptoms, with 12%–20% meeting criteria for chronic insomnia.2 3 The impact on both the individual and the healthcare system is substantial. Insomnia accounts for over $100 billion of US annual healthcare costs,4–6 and lost productivity related to insomnia costs the US economy approximately $63 billion a year.7 Adults suffering from insomnia also have a higher likelihood of comorbid conditions such as depression, resulting in a reduced quality of life and higher rates of morbidity and mortality.8 The documented high rates and detrimental effects of insomnia and co-occurring disorders, including depression, provide a compelling rationale for identifying effective, accessible, easy-to-use and cost-effective treatments.
There is empirical evidence indicating that cognitive-behavioural therapy for insomnia (CBT-I) can effectively treat chronic insomnia,9–15 including when present with co-occurring disorders like major depression,16 with long-lasting benefits. CBT-I is now recommended as first-line therapy for insomnia15 17 and its primary components include a focus on sleep restriction and consolidation, stimulus control, sleep hygiene and cognitive restructuring.18 However, due to challenges associated with in-person CBT-I, such as lack of trained clinicians, poor access and limited fidelity,19 attention has turned towards use of technology to overcome obstacles and deliver CBT-I interventions (eg, Sleep Healthy Using the Internet: SHUTi).20–23 Despite promising clinical efficacy in randomised controlled trials (RCTs),23 these studies have been unable to rigorously assess impact of the digital therapeutic on patient experience in the real-world.
In light of this gap in knowledge, we designed the PreScription DigitaL ThErapEutic For Patients with Insomnia (SLEEP-I) trial, a pragmatic, multicentre RCT to collect and evaluate real-world data from a mobile CBT-I prescription digital therapeutic (PEAR-003b, FDA-authorised as Somryst, herein called PDT) for patients with insomnia using Hugo,24 a patient-centred data aggregating platform. This approach will allow the concurrent analysis of clinical outcomes data, healthcare utilisation data and data from connected devices. The data generated will be used alongside clinically validated measures of insomnia to yield a multidimensional analysis of patient benefit. The PDT will be delivered via mobile devices to patients with insomnia as six treatment core CBT-I modules over 9 weeks that target three common mechanisms of action: stimulus control, sleep restriction and cognitive restructuring.21 22 25 We will also enrol patients in the Hugo data-aggregating platform to understand patient experience with insomnia by aggregating patients’ electronic health record (EHR) data, survey data on patient-reported outcomes (PROs), healthcare utilisation metrics and patient activity recorded via Fitbit.
Methods and analysis
We used the SPRINT reporting guidelines to draft this protocol paper outlining the SLEEP-I clinical trial.26 Enrolment for the SLEEP-I study was initiated on 22 December 2021 and is projected to be completed by 31 December 2022.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Overall study design and data collection
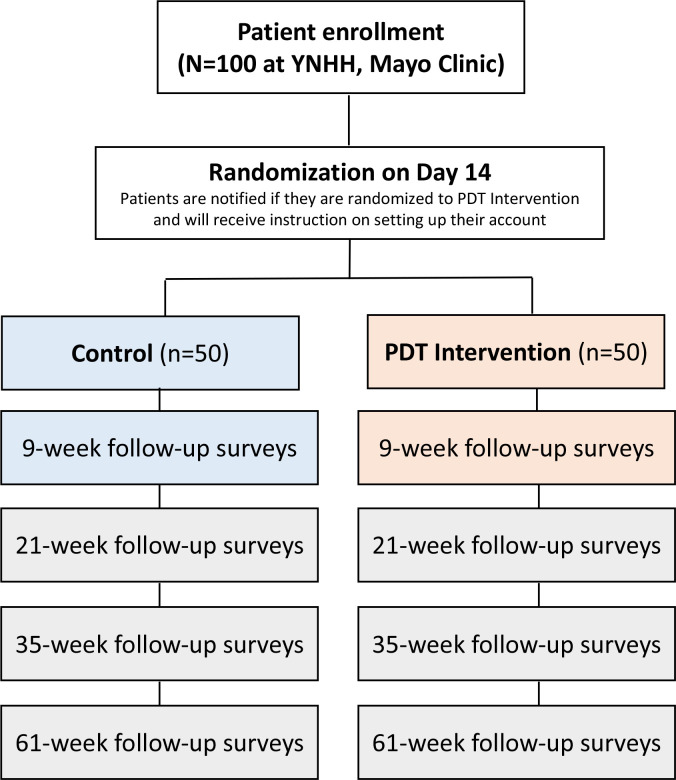
SLEEP-I is a pragmatically designed, prospective, multicentre RCT using a two group parallel design (PDT vs control) by five assessments (baseline, 9 weeks, 21 weeks, 35 weeks and 61 weeks) to evaluate the impact of the use of the PDT on PROs and clinically validated metrics for insomnia (figure 1). This study leverages a patient-centred health data-aggregating platform called Hugo,24 which was initially developed to overcome many of the limitations of traditional clinical trials, such as cost and patient access.27 Using patients’ mobile devices or computers, Hugo aggregates electronic health data for each patient from multiple sources, including electronic health records from hospitals and physicians offices, pharmacies and payors along with data from personal digital devices and wearables, by leveraging Blue Button technology and Application Programming Interfaces. Hugo aggregates electronic health data for patients from multiple sources including EHRs (hospitals, physicians offices, clinics), pharmacies, payors and wearables using hl7 fast healthcare interoperability resources and other application programming interface. Hugo also has the capability of delivering patient surveys through emails or text messages which essentially enables the assessment of PROs and other information without face-to-face interaction with study coordinators after enrolment.24 After the consent process, all participants will be enrolled in the Hugo platform, whereby they will receive near-real time access to their electronic health data from multiple sources, which will be shared with the research team; no data will be directly obtained from healthcare systems.28 This study will also employ use of the Fitbit (Inspire 2) fitness tracker that integrates with Hugo to obtain multiple physiological and sleep measurements including the number of steps per day, sleep (total sleep time in minutes) and self-reported metrics such as weight, height and body mass index (BMI). We chose to use the Fitbit Inspire 2 wearable in this study to measure basic activity and sleep metrics due as their affordability, unobtrusive nature and ease of linkage with mobile devices. In terms of reliability, prior work has demonstrated a high interdevice reliability for steps, distance, energy expenditure and sleep duration for certain Fitbit models. Importantly, for patients wearing the wrist Fitbit overnight, there was almost perfect levels of agreement (96.5%–99.1%) to classify whether the minute-level data were sleep or wake.29 30 That said, sleep diaries and the insomnia severity index (ISI) will form the gold-standard metric for insomnia assessment and the activity tracker will serve as an exploratory variable.
Figure 1.
Study design flow of the SLEEP-I study. SLEEP-I, PDT, prescription digital therapeutic; SLEEP-I, PreScription DigitaL ThErapEutic For Patients with Insomnia; YNHH, Yale-New Haven Health.
Sample selection and screening
Participants will be recruited from two academic health systems: Yale-New Haven Health (YNHH) and the Mayo Clinic. Potential participants with insomnia will be seen initially by a sleep provider at the YNHH and Mayo Sleep Centers to confirm a diagnosis of insomnia based on the International Classification of Sleep Disorders, 3rd edition,31 32 and if eligible, will introduce patients to the Sleep-I study. Participants will then be approached by a study coordinator using both in person and virtual recruitment methods via a referral from a sleep provider. Study flyers will also be used across both sleep centres to advertise the study and allow eligible patients to directly contact the study coordinators. Additional recruitment pathways may be employed to capture patients if enrolment targets are not initially met (eg, recruitment from psychiatry/stress centres, retrospective review of medical charts for recently diagnosed patients, social media outreach). Eligible patients will be consented by the study coordinator, and on enrolment, patients will be asked to sign an electronic consent at YNHH (online supplemental file 1) and the Mayo Clinic (online supplemental file 2) as well as linking their electronic health records and Fitbit to Hugo. All participants will receive paper materials on sleep hygiene and healthy sleep tips, which include behavioural information regarding getting a good night’s sleep (eg, setting a regular bedtime, getting out of bed if remaining awake, exercising regularly, not smoking). Participants randomised to PDT will receive access to the therapeutic for 9 weeks in addition to the sleep hygiene material.
bmjopen-2022-062041supp001.pdf (161KB, pdf)
bmjopen-2022-062041supp002.pdf (305.1KB, pdf)
Inclusion/exclusion criteria
Inclusion criteria will be confirmed prior to the informed consent process. Patients who do not meet all inclusion criteria or meet any of the non-inclusion criteria will not proceed with consent and enrolment. Patients must fulfil the following criteria prior to trial enrolment: (1) aged between 22 and 64 years; (2) English-speaking (both reading and writing in English required) and (3) have a diagnosis of chronic insomnia. Additionally, participants must also be willing and able to give consent to participate in the study, to have an email account (or be willing to create one), to have a smartphone capable of downloading the necessary applications and willing and able to use the PDT, the Hugo data aggregating platform and the syncable device (eg, Fitbit).
Exclusion criteria will include: (1) pregnancy; (2) shift work or family/other commitments that interfere with the establishment of regular night-time sleep patterns; (3) if wake/sleep time is outside the ranges of 4:00–10:00 hours (wake time) and 20:00–02:00 hours (bed-time), respectively; (4) absence of reliable internet access and smartphone; (5) a reported diagnosis of psychosis, schizophrenia or bipolar disorder or any medical disorders contraindicated with sleep restriction (eg, individuals with unstable or untreated medical or psychiatric conditions, specifically bipolar disorder and seizure disorder); (6) current involvement in a non-medication treatment programme for insomnia (participants are still eligible if they are taking traditional sleep medications) and (7) those with untreated coexisting sleep conditions (eg, sleep apnea) and those who have failed CBT-I in the past.
Intervention and method of assignment/randomisation
After signing informed consent documentation, participants will complete their baseline questionnaires and, over the following 2 weeks, will complete their baseline sleep diaries. Patients will be randomised 1:1 to the PDT or the control arm using a randomisation algorithm via Hugo. A total of 100 participants with chronic insomnia from two health centres (50 at each site) will be enrolled in Hugo, provided with a linked Fitbit (Inspire 2) to track activity and then randomised 1:1 to receive (or not) the PDT for mobile-delivered CBT-I. Patients will be notified if they are randomised to the treatment arm on day 14 by the study coordinators and will be provided with instructions on how to set up and create their PDT account if randomised to the PDT arm. The study will not employ blinding as patients will need to know if they are completing the PDT-delivered treatment.
The treatment duration will be 9 weeks, and there will be a 21-week, 35-week and 61-week follow-up. All patients will be evaluated at baseline, as well as prompted to complete additional assessments at weeks 9-week, 21-week, 35-week and 61-week postrandomisation (figure 1). The PDT intervention will deliver CBT-I via mobile devices as six treatment core modules over 9 weeks. Using the Hugo platform, we will also collect patient-generated engagement data, healthcare utilisation and patient activity/clinical outcomes for patients with insomnia.
Patients will use their own mobile devices but will be given the necessary syncable devices to keep (ie, Fitbit Inspire 2) and will receive a stipend for their time contributed as part of this study. This stipend will cover the consent process, initial setup and baseline questionnaire (3 hours), questionnaires provided at 9-week, 21-week, 35-week and 61-week postrandomisation, and the time it takes to sync and use the provided devices (3 hours per timepoint).
CBT-I intervention description
The intervention is the FDA-market-cleared PDT (Somryst) that delivers digital CBT-I via a mobile device that addresses maladaptive behaviours, dysfunctional thoughts and routines that can perpetuate sleep problems. Digital CBT-I is modelled on face-to-face CBT-I, which is usually delivered in weekly sessions over a period of 6–8 weeks. The intervention in this study delivers six treatment Cores (learning modules) that cover various specific CBT-I therapy content which has been previously described in detail.33 34 Cores of the PDT are completed sequentially and take approximately 30–45 min to complete. Each new Core is made available 1 week after the completion of the previous Core. Between Cores 1 and 2, at least five daily sleep diaries (integrated into the programme) within a 7-day period must also be entered to unlock the next Core. Furthermore, the participant must complete five out of seven sleep diaries between each Core in order to receive an updated Sleep Window. The digital therapeutic uses the Consensus Sleep Diary as recommended by the expert consensus panel which has been previously described.35 Participants will have access to the programme for 9 weeks, after which time their access will be expired. Although all Cores can be completed in as little as 6 weeks, the intervention will be available for 9 weeks prior to postassessment to allow users sufficient time to access all Core materials, as well as implement new behaviours, strategies and techniques.33 34
Sociodemographic and clinical characteristics
Baseline characteristics including sociodemographic, socioeconomic status, risk factors, comorbidities and sleep characteristics will be collected via self-report through Hugo at enrolment (table 1). Patients will also self-report the use of over-the-counter medications, including medications to assist with sleep and/or insomnia.
Table 1.
Sociodemographic and clinical variables obtained at baseline from self-report
| Sociodemographics/socioeconomic status | Risk factors | Comorbidities | Sleep history |
| Age | Hypertension | Alcohol use | Sleep difficulties |
| Sex | Diabetes | Coronary heart disease | Insomnia treatments |
| Race/ethnicity | High cholesterol | A heart attack (also called myocardial infarction) | Length of sleep problems |
| Marital status | Smoking history | Cancer | |
| Employment/working status | Depression | ||
| Education status | PTSD | ||
| Annual income level | General anxiety disorder | ||
| Stroke/TIA | |||
| Chronic pain | |||
| Asthma or lung problems |
PTSD, post-traumatic stress disorder; TIA, transient ischaemic attack.
Outcome measures
The primary, secondary and exploratory study outcomes, including the timing of data collection/administration of measures collected through Hugo and Fitbit, are presented in table 2. PROs collected in this study include the ISI score,36 the Epworth Sleepiness Scale (ESS),37 the Patient Health Questionnaire-8 (PHQ-8),38 the Generalized Anxiety Disorder-7 (GAD-7),39 the Perceived Stress Scale (PSS-10)40 and the Short-Form-12 (SF-12).41
Table 2.
Primary, secondary and exploratory outcomes
| Weeks | |||||
| Enrolment (baseline) | 9W | 21W | 35W | 61W | |
| Primary outcome | |||||
| ISI score (change in ISI from baseline to 9-week postrandomisation) | x | ||||
| Secondary outcomes | |||||
| Patient-reported outcomes (Hugo) | |||||
| ISI score | x | x | x | x | |
| ESS | x | x | x | x | x |
| PHQ-8 | x | x | x | x | x |
| GAD-7 | x | x | x | x | x |
| PSS-10 | x | x | x | x | x |
| SF-12 | x | x | x | x | x |
| Healthcare utilisation outcomes (Hugo) | |||||
| No. outpatient visits | x | x | x | x | |
| No specialty care visits | x | x | x | x | |
| No. medication refills for sleep | x | x | x | x | |
| No. of medication refills for psychotropic medications | x | x | x | x | |
| Health Utility Scores (Hugo) | |||||
| Health utility scores (SF-12) | x | x | x | x | |
| Sleep diaries* | |||||
| SE | x | x | x | x | x |
| SOL (min) | x | x | x | x | x |
| WASO (min) | x | x | x | x | x |
| Number of awakenings | x | x | x | x | x |
| Sleep quality (scale score) | x | x | x | x | x |
| Time in bed | x | x | x | x | x |
| Total sleep time | x | x | x | x | x |
| Exploratory outcomes (FitBit feature comparisons) | |||||
| Steps per day | x | x | x | x | x |
| Sleep (total sleep time in minutes) | x | x | x | x | x |
| Weight | x | x | x | x | x |
| Height | x | x | x | x | x |
| Body mass index | x | x | x | x | x |
*Definition of key sleep outcomes:
SE: The ratio of TST to TIB.
SOL: The length of time that it takes between ‘lights out’ or intention to sleep and first onset of sleep.
Sleep quality: One's satisfaction of the sleep experience, integrating aspects of sleep initiation, sleep maintenance, sleep quantity, and refreshment on awakening.
WASO: Total time awake between initial sleep onset and final morning awakening.
ESS, Epworth Sleepiness Scale; GAD-7, Generalized Anxiety Disorder-7; ISI, insomnia severity index; PHQ-8, Patient Health Questionnaire-8; PSS, Perceived Stress Scale; SE, sleep efficiency; SF, Short-Form; SOL, sleep onset latency; TIB, time in bed; TST, total sleep time; WASO, waking after sleep onset.
The primary outcome is a change in the ISI score36 from baseline to 9-week postrandomisation. The ISI questionnaire is a 7-item global index of self-reported insomnia symptom severity that has been shown to be valid, reliable and sensitive to changes in insomnia treatment36 42 and validated for online use.43
The secondary outcomes will be ascertained at baseline, 9-week, 21-week, 35-week and 61-week postrandomisation: (1) healthcare utilisation outcomes data available in patients’ electronic medical records (EMRs) through Hugo, which may include the number of outpatient visits and specialty care visits, number of medication refills for sleep and psychotropic medications, comparing PDT to control at all follow-up time points (9 weeks, 21 weeks, 35 weeks and 61 weeks); (2) change from baseline to 21, 35 and 61 weeks in the ISI, comparing PDT to control and (3) change from baseline to 9-week, 21-week, 35-week and 61-week postrandomisation in individual PROs including daytime sleepiness (ESS),37 depressive symptoms (PHQ-8),38 anxiety (GAD-7),39 stress (PSS-10)40 and health status (SF-12),41 comparing PDT to control. The ESS is the most widely used tool for measuring daytime sleepiness for clinical and research purposes.44 45 It is a simple, self-administered, eight-item questionnaire that measures the risk of falling asleep in eight specific situations that are commonly met. A score of less than 10 is considered normal. The higher the score (from 10 to 24), the greater the reported subjective daytime sleepiness.37
The PHQ-8 is a measure of depressive symptoms in the general population.46 Participants indicate the frequency with which they have been bothered by eight depressive symptoms (eg, ‘little interest or pleasure in doing things’) in the prior 2 weeks. Response options range from 0 (not at all) to 3 (nearly every day) and are summed to create the total symptom severity score. The GAD-7 is a validated screening tool and measure of severity of generalised anxiety disorder39 and contains seven items, with each response ranked from 0 (not at all sure) to 3 (nearly every day). A GAD-7 of 0–4 indicates minimal anxiety, of 5–9 indicates mild anxiety, 10–14 indicates moderate anxiety and of 15+ indicates severe anxiety.47
The PSS-10 is a global perceived stress scale where respondents are evaluated on the degree to which they perceived their life situations over the past month to be unpredictable, uncontrollable or overloaded, with higher scores indicating greater stress. Last, the SF-12 instrument measures overall physical and mental health status through 12 items.41 Both the Physical Component Summary and Mental Component Summary scores were used for this study and range from 0 to 100, with higher scores indicating a greater level of physical or mental functioning.
Asides from PROs administered in this study, additional secondary outcomes include: (1) Change in sleep outcomes collected through sleep diaries (sleep efficiency, sleep onset latency (SOL) (minutes), waking after sleep onset (WASO) (minutes), number of awakenings, sleep quality (scale score), time in bed and total sleep time, from baseline to 9-week, 21-week, 35-week and 61-week postrandomisation comparing PDT to control. Following the baseline assessment that will include questionnaires as described above, patients will complete 10 days of sleep diaries within a 14-day window as well as at all follow-up time periods. The sleep diaries are part of recent guidelines from the American Academy of Sleep Medicine17 48 as outcomes to be considered in evaluation of efficacy and clinical significance. Other secondary outcomes will include; (2) Change in (and total) health utility scores using the SF-12 among patients randomised to receive PDT versus the control only at 9-week, 21-week, 35-week and 61-week postrandomisation. Last, for patients randomised to the PDT, we will examine the relationship between engagement with the PDT and clinical outcomes, particularly the sleep-specific outcomes (ISI and diary-derived sleep metrics). More specifically, engagement will be operationalised by evaluating engagement and adherence rates with the PDT findings from the in-therapeutic software application data including: (1) core completion rates and (2) intervention sleep diary completion rates. Other variables will also be explored, including number of times the PDT is logged into/opened.
The exploratory outcomes will be ascertained at 9-week, 21-week, 35-week and 61-week postrandomisation. Physical and sleep activity measured using Fitbit (steps per day, sleep (total sleep time in minutes) and self-reported metrics such as weight, height and BMI from baseline to 9-week, 21-week, 35-week and 61-week postrandomisation comparing PDT to control.
Data analysis plan
All analyses of results from this RCT will be conducted as intent-to-treat to avoid the effects of crossover and dropout.49 We will report baseline descriptive statistics for the overall study, by site, and for both the control and treatment arms of the study. Baseline data will be compared using Pearson χ2 tests or Fisher exact tests for dichotomous and/or categorical variables and student’s t-tests for continuous variables. If variables are deemed as non-parametric, we will use a median test such as a Mann-Whitney U-test, where appropriate.
For the primary outcome, we will use a t-test to compare the ISI scores36 for the intervention (PDT+Fitbit) and control group (Fitbit only) at baseline. We will then use a 2 (group)×2 (time) repeated measures analysis of variance (RM ANOVAs) to compare prechanges to postchanges from baseline to 9 weeks across groups.21 25 Paired sample t tests by group will be used to examine time effects within each condition (if the overall interaction effect is significant). At weeks 21, 35 and 61 postrandomisation, we will also perform the same analysis, but this will be as an exploratory secondary endpoint. If a patient drops out, we will carry forward the most recent PRO response. As this is an RCT, we expect that confounding will be minimal. If patients are missing outcome data, we will use the last observation carried forward for the patient-reported outcome. Missing covariates will be set to missing.
For the secondary outcomes using the PROs, we will calculate the change in the ESS,37 PHQ-8,38 GAD-7,7 PSS-1040 and SF-12,41 and the scores at baseline and at 9-week, 21-week, 35-week and 61-week postrandomisation and perform a comparison between patients randomised to the intervention (PDT+Fitbit) and patients randomised to the control (Fitbit only). Based on our prior work using SHUTi,50 we will examine the change in scores between groups using a mixed model repeated measures ANOVA51 with an unstructured matrix and estimated df with Satterthwaite’s correction. We will present df alongside F-test statistics and t statistics. For the secondary clinical/healthcare utilisation outcomes, we will compare the PDT to the control at all follow-up time points. These comparisons will be compared using t-tests at each time point.
For the sleep diary outcomes,21 we will calculate the change in each sleep outcome from baseline to 9-week, 21-week, 35-week and 61-week postrandomisation comparing PDT to the control based on sleep diaries. We will examine the change in scores between groups using a mixed model repeated measures ANOVA.51 Paired-sample t-tests will be used to examine time effects within each condition if the overall interaction effect is significant.
For the secondary health utilities outcome, we will calculate the change in health utility scores from baseline to 9-week, 21-week, 35-week and 61- week postrandomisation and perform a comparison between patients randomised to the intervention (PDT+Fitbit) and patients randomised to the control (Fitbit only). Health utilities scores are derived from the SF-6D algorithm as applied to the SF-12 data.52
For the secondary engagement outcome, we will examine the relationship between engagement with PDT and clinical outcomes in the PDT arm at all follow-up time points. Correlations between engagement and clinical outcomes will be evaluated using both Pearson’s correlation coefficient and Spearman’s rank correlation as follows. Change from baseline (follow-up–baseline) will be calculated for ISI, sleep diary-derived metrics of SOL and WASO and PHQ-8, at both the end of treatment and the end of all follow-ups. These will be correlated with core completion rates, sleep diary completion rate and the number of times the PDT is opened. In addition, clinical outcomes among those who complete all six cores of treatment will be examined.
Last, the exploratory physical and sleep activity outcomes (measured using Fitbit) will again be compared from baseline to 9 weeks, 21 weeks, 35 weeks and 61 weeks comparing PDT to control. We will examine the change in scores between groups using a mixed model repeated measures ANOVA.51 Paired-sample t-tests will be used to examine time effects within each condition if the overall interaction effect is significant. We will use a Bonferroni correction to adjust for an increased likelihood of a type I error due to multiple comparisons. A value of p<0.05 will be considered statistically significant. All analyses will be conducted in SAS (V.9.4) and performed at the Mayo Clinic.
Sample size calculation
Our sample size was determined assuming 90% power to detect an effect size of d=0.52 for the main outcome (change in ISI from baseline to 9-week postrandomisation),50 with alpha 0.05 using the PASS software (PASS 15) to detect a clinically meaningful change.53 This effect size is 1/2 to 1/3 of what we have seen previously. Because this effect size is smaller than the levels demonstrated in RCTs, we are adequately powered to detect changes of interest in the main ISI outcome. We further note that this calculation is conservative because the analysis may optionally draw from outcome values recorded at baseline and each follow-up time. Because the models assume that each outcome is normally distributed, the outcome effects represent the average amount each outcome is expected to change with the incremental shift in any explanatory variable. We will recruit a total of 100 participants and will randomly assign them to treatment and control arms based on a 50% probability of assignment. We also assume a 25% rate of dropout between baseline and the end of follow-up, resulting in an effective sample size of N=80. The dropout attrition rate is based on prior research on the SHUTi intervention where study dropout attrition at 1 year has been as high as 50%50 although another study was as low as 4%21 at the end of treatment evaluation. This may be due to the fact that differential dropout is frequently greater in active than control conditions in clinical trials due to the added psychological effort required in the active group and/or to attainment of treatment goals.
Discussion
Knowledge gained from the SLEEP-I RCT will assist in improving the PDT which could then improve outcomes for individuals with chronic insomnia, which is one of the most common health concerns and imposes a significant burden on patients’ lives.1 Although CBT-I is the main treatment for insomnia, there are many challenges associated with in-person CBT-I such as poor access and lack of trained clinicians.19 This will be the first controlled study to address these important gaps in clinical care by examining the impact of a mobile-delivered PDT device delivering CBT for insomnia using Hugo, a novel data science aggregating platform, to inform the field on the impact of a PDT for chronic insomnia on clinical domains (change in insomnia severity) and important related domains of patient satisfaction and healthcare utilisation.
Results from this study will advance our understanding of: (1) how novel ways of collecting and aggregating clinical and PROs data can support informed clinical decision-making; (2) digital therapeutic engagement and its relationship to clinical outcomes and (3) evaluation of data from linked devices by providing novel information on a prescription digital therapeutic for insomnia, connected with the Hugo platform. The outcome of this research will provide crucial data to inform the latest thinking about how data from both digital therapeutics and EHR systems can be used to evaluate real-world clinical and utilisation outcomes. These data will be used to demonstrate the value of implementing technology within healthcare systems, supporting the broad uptake of similar technology platforms. In addition, they will inform reimbursement discussions with payers to support coverage of and broad access to effective digital therapeutics.
This RCT has several potential study limitations. First, this study sample is quite small and will be relatively homogeneous given that participants will be recruited from sleep medicine clinics and may not represent those who only present at primary care or any medical disorders contraindicated with sleep restriction, and participants will be drawn from two urban sleep centres. Future studies should aim to enrol larger and more heterogeneous samples to improve the generalisability of the findings, such as those comparisons by sex and race/ethnicity to determine which patients most benefit from treatment, based on specific risk factors.54–56 Second, this study relies on participants motivation and/or willingness to complete sleep diaries/intervention cores and PROs. Third, our findings will be based on self-report measures or PROs (eg, depression, stress, anxiety) versus a clinician-administered interview at all assessment points,
Ethics and dissemination
Ethics approval
The SLEEP-I RCT is sponsored by the National Evaluation System for health Technology Coordinating Center. Ethics approval was obtained independently at each of the two health systems, including at Yale University on 30 August 2021 (#2000029050) and Mayo Clinic on 14 February 2022 (#20–0 06 319). Any amendments to the protocol are first reviewed by each of the two local institutional review boards prior to implementation and also receive approval from the study sponsor. This RCT is also registered at ClinicalTrials.gov (NCT04909229) and was first posted on 1 June 2021.
Serious adverse events are not expected in this study where participants will be using their own digital devices. However, if there are device-related adverse events, they will be reported immediately, followed by a written report within five calendar days of the PIs becoming aware of the event to the IRB (using the appropriate forms from the website) and any appropriate funding and regulatory agencies. The investigators will apprise fellow investigators and study personnel of all UPIRSOs and adverse events that occur during the conduct of this research project via email as they are reviewed by the PIs. The investigator team will make clear that any sync-able data, including PROs, will not be reviewed in real-time by researchers and will not be provided to the clinical care team and, therefore, any adverse or severe symptoms should be reported directly to their physician(s) or emergency room physicians as they would have in the normal course of care.
Dissemination plan
The results from this RCT will be presented at both scientific meetings and submitted for publication to peer-reviewed journals. Additionally, study results will be shared with stakeholders and enrolled study participants.
Supplementary Material
Footnotes
Twitter: @DrRachelDreyer, @mollyjeffery
Collaborators: N/A.
Contributors: RPD: Conception and design, data collection, analysis and interpretation, writing the article, critical revision of the article, final approval. AB: Data collection, critical revision of the article, writing the article, final approval. HKY: Critical revision of the article, writing the article, final approval. LS: Critical revision of the article, writing the article, final approval. NDS: Conception and design, analysis and interpretation, writing the article, critical revision of the article, final approval. LE: Data collection, critical revision of the article, writing the article, final approval. BK: Critical revision of the article, writing the article, final approval. MMJ: Critical revision of the article, writing the article, final approval. MD: Conception and design, data collection, analysis and interpretation, writing the article, critical revision of the article, final approval. KE: Critical revision of the article, writing the article, final approval. FT: Conception and design, data collection, analysis and interpretation, writing the article, critical revision of the article, final approval. JSR: Conception and design, analysis and interpretation, writing the article, critical revision of the article, final approval, overall study responsibility.
Funding: This work was supported by the Medical Device Innovation Consortium (MDIC) on behalf of the National Evaluation System for health Technology (NEST) Coordinating Center (6292-2019-R2TC-B18), initiative funded by the U.S. Food and Drug Administration (FDA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views nor the endorsements of the Department of Health and Human Services or the FDA. While MDIC provided feedback on project conception and design, the organisation will play no role in collection, management, analysis and interpretation of the data, nor preparation, review and approval of the manuscript. The research team, not the funder, made the decision to submit the manuscript for publication
Competing interests: RPD reports research funding from the American Heart Association, the Canadian Institutes of Health Research and from the Medical Device Innovation Consortium (MDIC) as part of the National Evaluation System for health Technology Coordinating Center (NESTcc), Food and Drug Administration (FDA). JSR received research support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing, from the FDA to establish the Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) program (U01FD005938), MDIC as part of the NESTcc, AHRQ (R01HS022882), NHLBI of the NIH (R01HS025164, R01HL144644), and from the Laura and John Arnold Foundation to establish the Good Pharma Scorecard at Bioethics International and to establish the Collaboration for Research Integrity and Transparency (CRIT) at Yale. NDS is currently employed by Delta Airlines; when he was employed by the Mayo Clinic, he reported receiving research support through Mayo Clinic from the Food and Drug Administration, the Centers of Medicare & Medicaid Innovation under the Transforming Clinical Practice Initiative, the Agency for Healthcare Research and Quality (grants R01HS025164, R01HS025402, R03HS025517 and K12HS026379), the National Heart, Lung and Blood Institute of the National Institutes of Health (grants R56HL130496, R01HL131535 and R01HL151662), the National Science Foundation, the Medical Device Innovation Consortium as part of the National Evaluation System for Health Technology, and the Patient-Centered Outcomes Research Institute to develop a Clinical Data Research Network. FT is an employee of Pear Therapeutics, that develops and distributes prescription digital therapeutics for health concerns and disease. Other authors report no other conflicts of interest.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Upon completion of the clinical trial, data will be made available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Institutional Review Board approval was obtained at Yale University (IRB number#2000029050) and the Mayo Clinic (IRB number#20-006319). Participants gave informed consent to participate in the study before taking part.
References
- 1.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev 2002;6:97–111. 10.1053/smrv.2002.0186 [DOI] [PubMed] [Google Scholar]
- 2.Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America insomnia survey (AIS). Sleep 2011;34:997–1011. 10.5665/SLEEP.1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; international statistical classification of diseases and related health problems, tenth revision; and research diagnostic Criteria/International classification of sleep disorders, second edition criteria: results from the America insomnia survey. Biol Psychiatry 2011;69:592–600. 10.1016/j.biopsych.2010.10.023 [DOI] [PubMed] [Google Scholar]
- 4.Fullerton DSP. The economic impact of insomnia in managed care: a clearer picture emerges. Am J Manag Care 2006;12:S246–52. [PubMed] [Google Scholar]
- 5.Stoller MK. Economic effects of insomnia. Clin Ther 1994;16:873–97. [PubMed] [Google Scholar]
- 6.Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep 2007;30:263–73. 10.1093/sleep/30.3.263 [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC, Berglund PA, Coulouvrat C, et al. Insomnia and the performance of US workers: results from the America insomnia survey. Sleep 2011;34:1161–71. 10.5665/SLEEP.1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med 2007;3:S7–10. 10.5664/jcsm.26929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morin CM, Hauri PJ, Espie CA, et al. Nonpharmacologic treatment of chronic insomnia. An American Academy of sleep medicine review. Sleep 1999;22:1134–56. 10.1093/sleep/22.8.1134 [DOI] [PubMed] [Google Scholar]
- 10.Morin CM. Insomnia: psychological assessment and management. New York: Guilford, 1993. [Google Scholar]
- 11.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry 1994;151:1172–80. 10.1176/ajp.151.8.1172 [DOI] [PubMed] [Google Scholar]
- 12.Murtagh DR, Greenwood KM. Identifying effective psychological treatments for insomnia: a meta-analysis. J Consult Clin Psychol 1995;63:79–89. 10.1037/0022-006X.63.1.79 [DOI] [PubMed] [Google Scholar]
- 13.Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia:update of the recent evidence (1998-2004). Sleep 2006;29:1398–414. 10.1093/sleep/29.11.1398 [DOI] [PubMed] [Google Scholar]
- 14.NIH State-of-the-Science conference statement on manifestations and management of chronic insomnia in adults. NIH Consens State Sci Statements 2005;22:1–30. [PubMed] [Google Scholar]
- 15.Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of physicians. Ann Intern Med 2016;165:125–33. 10.7326/M15-2175 [DOI] [PubMed] [Google Scholar]
- 16.Jansson-Fröjmark M, Norell-Clarke A. The cognitive treatment components and therapies of cognitive behavioral therapy for insomnia: a systematic review. Sleep Med Rev 2018;42:19–36. 10.1016/j.smrv.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 17.Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med 2021;17:255–62. 10.5664/jcsm.8986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell MD, Gehrman P, Perlis M, et al. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract 2012;13:40. 10.1186/1471-2296-13-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas A, Grandner M, Nowakowski S, et al. Where are the behavioral sleep medicine providers and where are they needed? A geographic assessment. Behav Sleep Med 2016;14:687–98. 10.1080/15402002.2016.1173551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritterband LM, Thorndike FP, Ingersoll KS, et al. Effect of a web-based cognitive behavior therapy for insomnia intervention with 1-year follow-up: a randomized clinical trial. JAMA Psychiatry 2017;74:68–75. 10.1001/jamapsychiatry.2016.3249 [DOI] [PubMed] [Google Scholar]
- 21.Ritterband LM, Thorndike FP, Gonder-Frederick LA, et al. Efficacy of an Internet-based behavioral intervention for adults with insomnia. Arch Gen Psychiatry 2009;66:692–8. 10.1001/archgenpsychiatry.2009.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorndike FP, Ritterband LM, Gonder-Frederick LA, et al. A randomized controlled trial of an Internet intervention for adults with insomnia: effects on comorbid psychological and fatigue symptoms. J Clin Psychol 2013;69:1078–93. 10.1002/jclp.22032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zachariae R, Lyby MS, Ritterband LM, et al. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia - A systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev 2016;30:1–10. 10.1016/j.smrv.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 24.Health H, 2021. Available: https://hugo.health/ [Accessed 01 Jul 2021].
- 25.Thorndike FP, Saylor DK, Bailey ET, et al. Development and perceived utility and impact of an Internet intervention for insomnia. E J Appl Psychol 2008;4:32–42. 10.7790/ejap.v4i2.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan A-W, Tetzlaff JM, Altman DG, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhruva SRJ, Shah N, et al. New federal rules pave the way for Patient-Driven health information exchange and real-world evidence on COVID-19 surveillance and treatment. Health Affairs Blog 2020. 10.1377/hblog20200506.368396 [DOI] [Google Scholar]
- 28.Dhruva SS, Ross JS, Akar JG. Aggregating multiple real-world data sources using a patient-centered health data sharing platform: an 8-week cohort study among patients undergoing bariatric surgery or catheter ablation of atrial fibrillation. NPJ Digital Medicine 2020;3:60. 10.1038/s41746-020-0265-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evenson KR, Goto MM, Furberg RD. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act 2015;12:159. 10.1186/s12966-015-0314-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haghayegh S, Khoshnevis S, Smolensky MH, et al. Accuracy of Wristband Fitbit models in assessing sleep: systematic review and meta-analysis. J Med Internet Res 2019;21:e16273. 10.2196/16273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest 2014;146:1387–94. 10.1378/chest.14-0970 [DOI] [PubMed] [Google Scholar]
- 32.American Academy of Sleep Medicine . International classification of sleep disorders. 3rd ED. American Academy of Sleep Medicine, 2014. [Google Scholar]
- 33.Thorndike FP, Berry RB, Gerwien R, et al. Protocol for digital real-world evidence trial for adults with insomnia treated via mobile (DREAM): an open-label trial of a prescription digital therapeutic for treating patients with chronic insomnia. J Comp Eff Res 2021;10:569–81. 10.2217/cer-2021-0004 [DOI] [PubMed] [Google Scholar]
- 34.Morin CM. Profile of Somryst prescription digital therapeutic for chronic insomnia: overview of safety and efficacy. Expert Rev Med Devices 2020;17:1239–48. 10.1080/17434440.2020.1852929 [DOI] [PubMed] [Google Scholar]
- 35.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep 2012;35:287–302. 10.5665/sleep.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med 2001;2:297–307. 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 37.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness scale. Sleep 1991;14:540–5. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 38.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 40.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 41.Ware J, Kosinski M, Keller SD. A 12-Item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 42.Morin CM, Belleville G, Bélanger L, et al. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34:601–8. 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorndike FP, Ritterband LM, Saylor DK, et al. Validation of the insomnia severity index as a web-based measure. Behav Sleep Med 2011;9:216–23. 10.1080/15402002.2011.606766 [DOI] [PubMed] [Google Scholar]
- 44.Sargento P, Perea V, Ladera V, et al. The Epworth Sleepiness scale in Portuguese adults: from classical measurement theory to Rasch model analysis. Sleep Breath 2015;19:693–701. 10.1007/s11325-014-1078-6 [DOI] [PubMed] [Google Scholar]
- 45.Cho YW, Lee JH, Son HK, et al. The reliability and validity of the Korean version of the Epworth Sleepiness scale. Sleep Breath 2011;15:377–84. 10.1007/s11325-010-0343-6 [DOI] [PubMed] [Google Scholar]
- 46.Kroenke K, Strine TW, Spitzer RL, et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–73. 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 47.Kroenke K, Spitzer RL, Williams JBW, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med 2007;146:317–25. 10.7326/0003-4819-146-5-200703060-00004 [DOI] [PubMed] [Google Scholar]
- 48.Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of sleep medicine systematic review, meta-analysis, and grade assessment. J Clin Sleep Med 2021;17:263–98. 10.5664/jcsm.8988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fisher LD, Dixon DO, Herson J. Intention to treat in clinical trials. In: Peace Ke. New York: Statistical issues in drug research and developmentMarcel Dekker, 1990: 331–50. [Google Scholar]
- 50.Christensen H, Batterham PJ, Gosling JA, et al. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight study): a randomised controlled trial. Lancet Psychiatry 2016;3:333–41. 10.1016/S2215-0366(15)00536-2 [DOI] [PubMed] [Google Scholar]
- 51.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York, NY, NY: Springer, 2011. [Google Scholar]
- 52.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851–9. 10.1097/01.mlr.0000135827.18610.0d [DOI] [PubMed] [Google Scholar]
- 53.PASS 15 Power Analysis and Sample Size Software . ncsscom/software/pass. Kaysville, Utah, USA: NCSS, LLC, 2017. [Google Scholar]
- 54.Kingsbury JH, Buxton OM, Emmons KM. Sleep and its relationship to racial and ethnic disparities in cardiovascular disease. Curr Cardiovasc Risk Rep 2013;7:387–94. 10.1007/s12170-013-0330-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang B, Wing Y-K. Sex differences in insomnia: a meta-analysis. Sleep 2006;29:85–93. 10.1093/sleep/29.1.85 [DOI] [PubMed] [Google Scholar]
- 56.Fan J, Song F, Bachmann MO. Justification and reporting of subgroup analyses were lacking or inadequate in randomized controlled trials. J Clin Epidemiol 2019;108:17–25. 10.1016/j.jclinepi.2018.12.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-062041supp001.pdf (161KB, pdf)
bmjopen-2022-062041supp002.pdf (305.1KB, pdf)
Data Availability Statement
Upon completion of the clinical trial, data will be made available upon reasonable request.