Abstract
Introduction
Penicillin allergies are highly prevalent in the healthcare setting and associated with the prescription of second-line inferior antibiotics. More than 85% of all penicillin allergy labels can be removed by skin testing and 96%–99% of low-risk penicillin allergy labels can be removed by direct oral challenge. An internally and externally validated clinical assessment tool for penicillin allergy, PEN-FAST, can identify a low-risk penicillin allergy without the need for skin testing; a score of less than 3 has a negative predictive value of 96.3% (95% CI, 94.1 to 97.8) for the presence of a penicillin allergy. It is hypothesised that PEN-FAST is a safe and effective tool for assessing penicillin allergy in an outpatient clinic setting.
Methods and analysis
This is an international, multicentre randomised control trial using the PEN-FAST tool to risk-stratify penicillin allergy labels in adult outpatients. The study’s primary objective is to evaluate the non-inferiority of using PEN-FAST score-guided management with direct oral challenge compared with standard care (defined as prick and intradermal skin testing followed by oral penicillin challenge). Participants will be randomised 1:1 to the intervention arm (direct oral penicillin challenge) or standard of care arm (skin testing followed by oral penicillin challenge, if skin testing is negative). The sample size of 380 randomised patients (190 per treatment arm) is required to demonstrate non-inferiority.
Ethics and dissemination
The study will be performed according to the guidelines of the Helsinki Declaration and is approved by the Austin Health Human Research Ethics Committee (HREC/62425/Austin-2020) in Melbourne Australia, Vanderbilt University Institutional Review Board (IRB #202174) in Tennessee, USA, Duke University Institutional Review Board (IRB #Pro00108461) in North Carolina, USA and McGill University Health Centre Research Ethics Board in Canada (PALACE/2022-7605). The results of this study will be published and presented in various scientific forums.
Trial registration number
Keywords: IMMUNOLOGY, INFECTIOUS DISEASES, Protocols & guidelines, Public health
Strengths and limitations of this study.
This is an international multicentre, prospective non-inferiority randomised clinical trial.
The investigators will use a validated clinical tool, the PEN-FAST as part of the inclusion criteria in the study.
In terms of the limitations, this study excludes the paediatric population, considering that the clinical tool PEN-FAST was validated in an adult population.
The recruiting institutions are specialised drug allergy centres that offer skin testing as standard of care.
Finally, the conclusions from this trial might not be generalisable beyond the enrolled study population considering that the participants who consent to participate in this trial could be different from those that decline consent.
Introduction
Penicillin allergies are highly prevalent in the healthcare setting and associated with the prescription of second-line inferior antibiotics. Patient-reported penicillin allergies and incorrect antibiotic allergy labels result in poor health outcomes for patients, including increased length of stay and mortality rate during hospitalisation,1 2 and drive inappropriate antibiotic prescribing and antimicrobial resistance, increase side effects from second-line antibiotics and increase healthcare costs.3–7
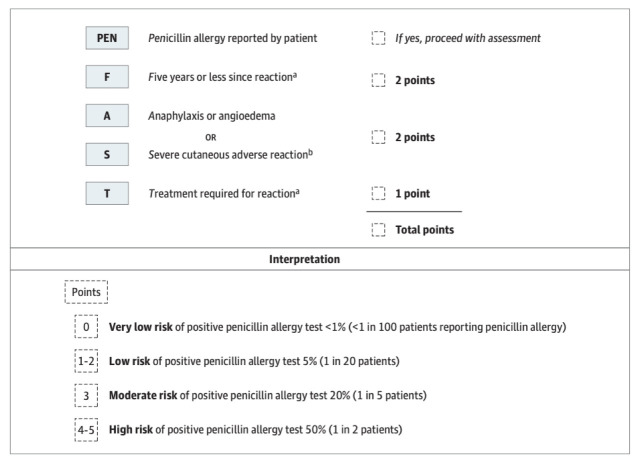
Work from our group has shown that more than 85% of all penicillin allergy labels can be removed by formal skin testing8 and 96%–99% of low-risk penicillin allergies can be removed by point-of-care oral challenge.9–11 We have internally and externally validated a novel penicillin allergy clinician decision rule (PEN-FAST) that can identify low-risk penicillin allergies (figure 1).12 In patients with a reported penicillin allergy (PEN), based on four allergy history criteria (time since reaction ≤5 years (F), anaphylaxis or angioedema (A), severe cutaneous adverse reaction (S) or whether treatment (T) was required for reaction), a PEN-FAST score of <3 is associated with 96.7% negative predictive value.12 A PEN-FAST score of less than 3 classified 460/622 (74%) patients as low-risk.12 Seventeen (3.7%) of these patients had a positive penicillin allergy test. This was subsequently externally validated against a retrospective multicentre cohort of 945 outpatients from Australia and the USA with consistent results.12
Figure 1.
PEN-FAST clinical decision rule. aForms of severe delayed reactions include potential Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms and acute generalised exanthematous pustulosis. Patients with a severe delayed rash with mucosal involvement should be considered to have a severe cutaneous adverse reaction. Acute interstitial nephritis, drug-induced liver injury, serum sickness and isolated drug fever were excluded phenotypes from the derivation and validation cohorts. bIncludes unknown.
A prospective randomised controlled trial evaluating the safety of drug challenge in patients with a low-risk penicillin allergy patient group (defined as skin rash, hives, itching or unknown reaction that occurred more than 10 years before the assessment and that did not require emergency medical attention) has been reported.13 Specifically, the authors included 159 allergic patients in the randomisation (1:1) and compared skin testing followed by drug challenge (if skin testing negative) versus a gradual two-step direct challenge. They identified 3/79 patients (3.8%) with a positive challenge (immediate skin manifestations treated with antihistamines, no epinephrine use), showing that the challenge was a safe and effective alternative for delabelling penicillin allergy. Furthermore, a preoperative clinic used three criteria ((1) an allergy history of more than 15 years prior and either an unknown reaction, (2) a non-itchy rash or (3) a type A adverse drug reaction) to identify 119 low-risk patients from a cohort of 219 patients.14 Among these, 55/56 (98%) patients were successfully delabelled (three dose amoxicillin challenge 5/50/500 mg at 20 min interval followed by a 3-day course of amoxicillin) with one patient developed a delayed exanthem.14 Similar, 328 military recruits who reported penicillin or cephalosporin allergy were challenged to a single-dose direct oral challenge with amoxicillin (250 mg).15 Five (1.5%) demonstrated an adverse drug reaction without anaphylaxis and were treated with oral antihistamine and intramuscular epinephrine (to prevent reaction progression).15 The remaining 323 participants also received an intramuscular dose of benzathine penicillin without any reported adverse reactions.15 A prospective 2-year inpatient delabelling study in an intensive care unit setting with 12%–16% prevalence of penicillin allergy labels identified around 60% of penicillin allergy labelled patients as low-risk.11 When challenged with amoxicillin, 203/205 (99%) tolerated their challenge leading to label removal, and only two cases (<1%) had rash that led to return of the allergy label after a challenge or subsequent treatment.10
Direct drug challenges, including penicillin, have been increasingly used in the outpatient setting.16 17 However, no multicentre randomised control trial utilising a validated risk assessment tool has been undertaken to assess the safety of direct oral penicillin challenge in the outpatient setting. In our international multicentre cohort study, we will use the validated clinical tool, PEN-FAST, to determine if a direct oral penicillin challenge, where PEN-FAST score <3, is safe and effective when compared with standard of care penicillin skin testing followed by oral penicillin challenge.
Methods and analysis
Study design
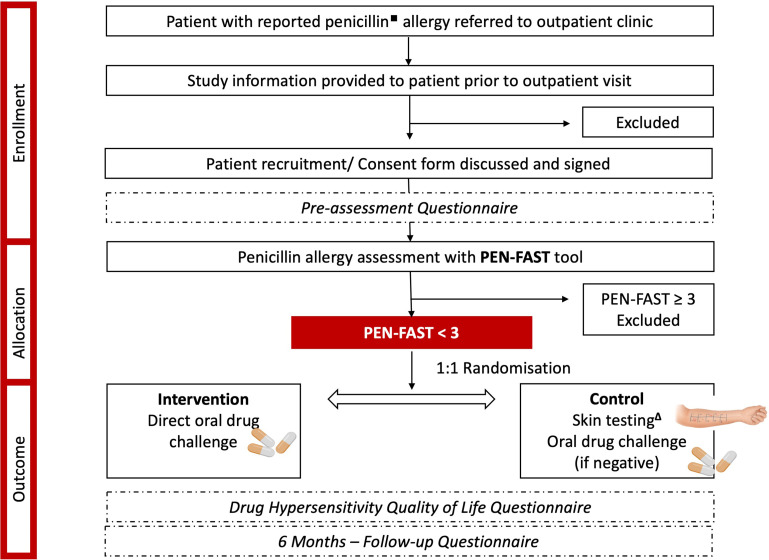
This is an international multicentre, prospective, non-inferiority randomised clinical trial (figure 2) to be conducted in the outpatient drug allergy services at Austin Health (Melbourne, Victoria, Australia), Peter MacCallum Cancer Centre (Melbourne, Victoria, Australia), Royal Melbourne Hospital (Melbourne, Victoria, Australia), Vanderbilt University Medical Centre (Nashville, Tennessee, USA), Duke University Medical Centre (Durham, NC, USA), and McGill University Health Centre (Montreal, Quebec, Canada).
Figure 2.
Overview of the study design. ■Penicillin unspecified, penicillin VK/G, amoxicillin, amoxicillin/clavulanate, ampicillin, semisynthetic antistaphylococcal penicillins. ∆Skin prick testing followed by intradermal testing using standard beta-lactam panel.
Eligibility criteria section
We will include adult patients (≥18 years) reporting a penicillin allergy label where the calculated PEN-FAST score is less than 3. Participants will be excluded if they (1) present any illness that, in the investigator’s judgement, will substantially increase the risk associated with their participation in this study, including neurological or psychological conditions; (2) have a history of type A adverse drug reaction, drug-associated anaphylaxis, idiopathic urticaria/anaphylaxis, mastocytosis, serum sickness, blistering skin eruption or acute interstitial nephritis; (3) report an allergy history that cannot be confirmed or (4) are on concurrent antihistamine therapy or receiving more than stress dose steroids (ie, >50 mg four times a day hydrocortisone or steroid equivalent). Pregnant patients will also be excluded from the study.
The various ethnic backgrounds of the recruited patients will be collected and subcategorised under (1) Caucasian, (2) East Asian, (3) Indo Asian, (4) African, (5) Hispanic or Latino, (6) Aboriginal or Torres Strait Islander and (7) other. All three Australian academic centres evaluate Aboriginal or Torres Strait Islander patients and the McGill University Health Centre covers a large and varied territory, stretching from Montreal to Nunavik in the far north. Both centres in the USA evaluate Hispanic patients, with this population representing 18% of the US total population. All recruiting centres are referred adults from the age of 18 with patient from all stages of life being assessed.
Potentially eligible patients referred to the outpatient clinic reporting a penicillin allergy will be identified and assessed with a standard clinical history and calculation of the PEN-FAST score (figure 1). A penicillin allergy label will be defined as patients reporting an allergy to any of the following drugs: ‘unspecified’, penicillin VK, penicillin G, amoxicillin, amoxicillin/clavulanate, ampicillin, flucloxacillin or dicloxacillin.
Sample size and justification
The null hypothesis is that the difference in the proportion of positive allergy investigations, including drug provocation and skin testing, is not larger than 5% (non-inferiority margin). To achieve 80% power, assuming the event rate in the control group is 4%18 and type 1 error probability of 5% (one-sided), 380 patients need to be randomised (190 per group). If the control group has lower prevalence of the outcome (2.5% or 2.0%), the power of the study will remain at least 80% if up to 4.5% of the intervention group has the outcome. Due to the randomisation, intervention and primary outcome being collected within the same visit, loss to follow-up is expected to be minimal.
Recruitment
Allergy outpatient clinics at the participating centres receive around 800 referrals per year for penicillin allergy patients. Thus, the authors estimate that recruitment will not represent an issue for this study. We estimate that the recruitment period will be 6 months, excluding when the outpatients are closed due to institutional COVID-19 infection prevention-related policies. The start date for this trial is January 2022 with a recruitment period of 8–12 months.
Randomisation
Participants meeting eligibility criteria will be randomised to either the intervention (direct oral penicillin provocation) or standard of care arm (skin testing followed by oral penicillin provocation if negative). Permuted block design randomisation will be used, stratified by the hospital site. Randomisation will be non-blinded and performed using REDCap (Research Electronic Data Capture). The allocation sequence will be developed and uploaded to REDCap by a trial statistician and will remain concealed.
Treatment arms
Prior to the intervention or standard of care procedures, participants will be asked to complete a Drug Hypersensitivity Prequestionnaire (table 1). The goal of this questionnaire is to evaluate the quality of life of patients with drug allergy labels, specifically penicillin. Indeed, drug allergy labels can have a significant impact on healthcare but the patient’s perspective has seldomly been assessed in the past.
Table 1.
Pre-Questionnaire
| Please answer yes, no or non-applicable (N/A) to the following questions. Mark the appropriate box with an x. Veuillez répondre non, oui ou sans objet (N/A) aux questions suivantes. |
Yes | No | N/A |
| 1. Would you like the allergist’s/infectious disease’s physician opinion before taking medications prescribed by other specialists? Souhaitez-vous l'avis de l'allergologue / de l'infectiologue avant de prendre des médicaments prescrits par d'autres spécialistes? | ⬜ | ⬜ | ⬜ |
| 2. Do you talk to others about your allergy problem? Parlez-vous à d'autres personnes de votre problème d'allergie? | ⬜ | ⬜ | ⬜ |
| 3. Is your family aware of your problem? Votre famille est-elle au courant de votre problème? | ⬜ | ⬜ | ⬜ |
| 4. Is your partner conscious of your problem? Votre partenaire est-il au courant de votre problème? | ⬜ | ⬜ | ⬜ |
| 5. Is your family doctor aware of your drug allergy problem? Votre médecin de famille est-il au courant de votre problème d'allergie aux médicaments? | ⬜ | ⬜ | ⬜ |
| 6. Is your community pharmacist aware of your drug allergy problem? Votre pharmacien est-il au courant de votre problème d'allergie aux médicaments? Accepteriez-vous de recevoir à nouveau de la pénicilline dans la communauté après un résultat de test négatif en clinique? | ⬜ | ⬜ | ⬜ |
| 6. Would you be happy to have penicillin again in the community after a negative test result in clinic? | ⬜ | ⬜ | ⬜ |
| The following questions concern the influence your drug allergy has on your quality of life. Answer every question by marking the appropriate box with an x. You may choose from one of the following answers. Les questions suivantes portent sur l'impact de votre allergie médicamenteuse sur votre qualité de vie. Répondez à chaque question en cochant la case appropriée avec un x. Vous pouvez choisir parmi l'une des réponses suivantes. | 0 Not at all Pas du tout |
1 Slightly Légèrement |
2 Moderately Modérément |
3 Very Très |
4 Extremely Extrêmement |
| 0 | 1 | 2 | 3 | 4 | |
| 6. Do you feel different from others? Vous sentez-vous different(e) des autres? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 7. Do you feel unluckier from others? Vous sentez-vous moins chanceux (euse) par rapport aux autres? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 8. Is it that even a little discomfort is a problem for you? Est-ce que même un peu d'inconfort est un problème pour vous? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 9. Is your job efficiency affected by the problem of your allergy to medications? Votre efficacité au travail est-elle affectée par le problème de votre allergie aux médicaments? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 10. Do you feel helpless? Vous sentez-vous impuissant(e)? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 11. Do you sleep badly? Vous dormez mal? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 12. Do you feel embarrassed in relationships with others? Vous sentez-vous gêné(e) dans vos relations avec les autres? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 13. Since you are unable to take medications, does every illness limit you more than other people? Puisque vous êtes incapable de prendre des médicaments, est-ce que chaque maladie vous limite plus que les autres? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 14. Do you have difficulties concentrating? Avez-vous des difficultés à vous concentrer? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 15. Does your allergy problem interfere with your sexual life? Votre problème d'allergie interfère-t-il avec votre vie sexuelle? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 16. Do you feel anguished due to your problem of allergy reaction? Vous sentez-vous angoissé à cause de votre problème de réaction allergique? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 17. Do you feel ill? Vous vous sentez malade? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 18. Are you restricted in your nutrition from fear of substances you might be allergic to? Êtes-vous limité(e) dans votre alimentation par peur de consommer substances auxquelles vous pourriez être allergique? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 19. Are you afraid of being administered a medication during an emergency to which you are allergic? Avez-vous peur de recevoir un médicament auquel vous êtes allergique, en cas d’urgence? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 20. Do you feel you cannot cope with your allergy problem? Pensez-vous que vous ne pouvez pas faire face à votre problème d’allergie? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 21. For each disease, would you be confident that there is a medication that you can safely take? Pour chaque maladie, êtes-vous certain qu'il existe un médicament que vous pouvez prendre en toute sécurité? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 22. Are you afraid you could not deal with the pain? Avez-vous peur de ne pas pouvoir supporter la douleur? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 23. Do you feel anxious due to your problem of allergy reaction? Vous sentez-vous anxieux en raison de votre problème de réaction allergique? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 24. Does your problem influence your relationships with other people? Votre problème influence-t-il vos relations avec les autres? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 25. Are you in a bad mood due to your problem of allergy reaction? Êtes-vous de mauvaise humeur en raison de votre problème de réaction allergique? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 26. Do you feel frightened due to your problem of allergy reaction? Avez-vous peur à cause de votre problème de réaction allergique | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 27. Do you worry every time you take a medication different from ones that cause your allergic reactions? Vous inquiétez-vous à chaque fois que vous prenez un médicament différent de ceux qui provoquent vos réactions allergiques? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 28. Do you feel tired during the day because you sleep badly at night? Vous sentez-vous fatigue(e) pendant la journée parce que vous dormez mal la nuit? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 29. Do you give up leisure activities (sport, vacations and trips) because of your problem? Avez-vous abandonné les activités de loisirs (sport, vacances, voyages) à cause de votre problème? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 30. Does the idea of taking a medicine make you feel anxious? L'idée de prendre un médicament vous rend-il anxieux(euse)? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 31. Are you annoyed by frequent medical controls? Êtes-vous agacé(e) par les contrôles médicaux fréquents? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 33. Does the problem of adverse reaction to medications affect your life? Le problème des réactions indésirables aux médicaments affecte-t-il votre vie? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 34. How likely are you to believe a negative penicillin allergy test result? Quelle est la probabilité que vous croyiez un résultat négatif au test d'allergie à la pénicilline? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
| 35. How likely do you think it is that your penicillin allergy test will be negative? Selon vous, quelle est la probabilité que votre test d'allergie à la pénicilline soit négatif? | ⬜ | ⬜ | ⬜ | ⬜ | ⬜ |
A proposed clinical workflow is described in online supplemental appendix figure 1.
bmjopen-2022-063784supp001.pdf (150.1KB, pdf)
Intervention
Participants will receive a single dose of oral penicillin or penicillin derivatives in the intervention group after baseline vital sign assessment (ie, temperature, heart rate, blood pressure, respiratory rate, oxygen saturation and skin check). Nursing staff will repeat vital signs as needed after oral challenge while observing for signs of an immune-mediated adverse reaction. The vital signs are also performed for all participants 60 min following the oral challenge. If an antibiotic-associated adverse event is noted at any stage, the standard of care treatment will be offered by the attending clinicians (eg, epinephrine for immediate hypersensitivity reaction).
If the patient reports a reaction to either penicillin unspecified, penicillin G, penicillin V, semisynthetic antistaphylococcal penicillins, ampicillin, amoxicillin or amoxicillin/clavulanate, he/she will be administered either the implicated drug or amoxicillin, consistent with site local practice. The dose used for amoxicillin is 250 mg or the lowest available dose according to centre availability. The implicated drug should also be administered at the lowest available dose. For the amoxicillin/clavulanate allergic patients, they will retain an allergy label to clavulanate.
Control
In the control group, routine management will include penicillin skin prick and intradermal beta-lactam inoculation adapted from previously published studies (box 1) followed by oral penicillin challenge if skin testing is negative. Vital signs, observations and management will be the same in both groups, and patients will be able to directly contact a member of the clinical team (phone or email) if any serious or antibiotic-associated adverse events (including non-immune mediated adverse events) occur in the 24–48 hours after oral provocation.
Box 1. Drug allergy testing concentrations.
Skin prick testing (read at 15 min).
Histamine 10 mg/mL.
Sodium chloride 0.9%.
Diater PPL (major determinant).
Diater MDM (minor determinant) (if available).
Ampicillin mg/mL.
Penicillin G 10 000 U/mL.
Intradermal testing (0.02 mL) (read at 15 min)
Sodium chloride 0.9%.
Diater PPL (major determinant).
Diater MDM (minor determinant) (if available).
Ampicillin 25 mg/mL.
Penicillin G 10 000 U/mL.
Follow-up telephone questionnaire
A 6-month postrandomisation telephone questionnaire, based on previous publications,11 will help assess the impact of outpatient delabelling on patient perceptions of their allergy status (box 2).
Box 2. A 6-month follow-up questionnaire—adapted from Wilson et al.21.
English
Telephone survey script.
Verbal consent script for patients who were randomised in the trial.
‘Hello, could I please speak to (patient’s full given name and surname)?’
Hello, I am ________ (name and function in the research team). You have participated in a study on Penicillin allergy, the PALACE Study, about 6 months ago. We are now contacting for the second part of the study to determine what antibiotics you have used after antibiotic allergy testing at our centre (please complete with tercentre name).
Before we proceed further, can I please confirm your full name and date of birth?
Suppose you agree to continue participating in this study. In that case, we will ask you some questions about your allergies and what antibiotics you have taken, and any problems with your antibiotics recently. Usually, the interview takes about 10 min, but if we identify some problems with your allergies and help you solve them, it might take longer. If we identify some issues, we might ask for your permission to contact your local doctor or (please complete with physician name) at the allergy service at our centre that can help you solve these problems. Taking part in this interview is entirely voluntary and will not affect your future care at the (please complete with centre name) or other hospitals.
If the patient is not at home:
‘Is there a time that I could call back to speak with (patient’s name)?’
If the patient is busy:
‘Is there another time that I could call back that would be convenient?’
Patient questions
Do you remember having a test dose of penicillin in the outpatient clinic?
If No, do you agree to schedule a follow-up appointment with (please complete with physician name) to discuss the investigations at the outpatient clinic further?
If Yes, please tell me whether you agree with these statements:
-
‘I felt safe during the test dose’.
Strongly agree.
Agree.
Neutral.
Disagree.
Strongly disagree.
-
‘I recommend the penicillin assessment to other patients with a penicillin allergy’.
Strongly agree.
Agree.
Neutral.
Disagree.
Strongly disagree.
-
What was the result of your penicillin assessment in the clinic?
Penicillin allergy removed.
Penicillin allergy confirmed.
I do not know.
-
Did you have any late reaction to assessment after the observation period?
If Yes, state reaction.
What treatment was required? (eg, General Practitioner visit, antihistamines, topical steroids, admission to hospital).
-
Have you received an antibiotic since the test?
If yes, what was the name of the antibiotic?
If unable to recall, prompt: Was a ‘penicillin’?
If yes (ie, penicillin received), did you have any reaction to the penicillin?
Did you receive a letter about your allergy post-testing? Y/N.
Do you feel you know more about penicillin allergies? Y/N.
Do you feel you know more about your reactions to penicillin? Y/N.
-
Are you still avoiding penicillin(s)?
If Yes, please explain why? Free-text (Investigator to categorise later).
If No, Congratulations. We are happy to hear this. We will further continue with some questions.
-
Do you consider yourself allergic to penicillin? Y/N.
If Yes, the next time you are admitted to the hospital, would you say you are allergic to penicillin?
Do you have any comments about the testing, either good or bad, for us?
If the patient states that they are still avoiding penicillin (Q9) or they consider themselves allergic to penicillin (Q10) and you have assessed them to be able to participate in a qualitative interview, then say:
‘We would like to explore these issues further. This would involve another phone interview. Would you be interested in participating? What would be a good time to call you?’
End—‘That is the end of the questions. Thank you very much for your time’.
French
Script d'enquête téléphonique.
Consentement verbal pour les patients randomisés dans l'étude.
‘Bonjour, pourrais-je parler à (nom et prénom complets du patient)?’
Bonjour, je suis ________ (nom et fonction à l'hôpital). Vous avez participé à une étude sur l'allergie à la pénicilline, l'étude PALACE, il y a environ 6six mois. Nous vous contactons maintenant pour la deuxième partie de l'étude afin de savoir quels antibiotiques vous avez utilisé suite aux tests d'allergie dans notre centre (nommer le centre).
Avant de poursuivre, puis-je confirmer votre nom complet et votre date de naissance?
Si vous acceptez de continuer à participer à cette étude, nous vous poserons quelques questions sur vos allergies et les antibiotiques que vous avez pris ainsi que tout problème que vous auriez rencontré avec la prise d’antibiotiques récemment. Habituellement, l'entretien dure environ 10 minutes min, mais si nous identifions certains problèmes liés à vos allergies et que nous vous aidons à résoudre ces problèmes, cela peut prendre plus de temps. Si nous identifions certains problèmes, nous pouvons vous demander la permission de contacter votre médecin local ou (nommer investigateur) au département d'allergie de notre centre qui peut vous aider à résoudre ces problèmes. La participation à cet entretien est entièrement volontaire et n'affectera pas vos futurs soins dans le (nommer le centre) ou autres hôpitaux.
Si le patient n'est pas à la maison:
‘Y a-t-il un moment où je pourrais rappeler pour parler avec (nom du patient)?’
Si le patient est occupé:
‘Y a-t-il un autre meilleur moment quand je pourrais vous re-contacter?’
Questions pour les patients
Vous souvenez-vous d'avoir eu une dose de pénicilline à la clinique externe?
Si Non, seriez-vous d’accord à organiser une rencontre de suivi avec (nommer l’investigateur)3three pour discuter les investigations que vous avez eu à la clinique d’allergie?
Si Oui, veuillez me dire si vous êtes d'accord avec ces affirmations:
-
‘Je me sentais en sécurité pendant le test’.
Tout à fait d'accord.
D'accord.
Neutre.
Pas d'accord.
Fortement en désaccord.
-
‘Je recommande l'évaluation de la pénicilline à d'autres patients allergiques à la pénicilline’.
Tout à fait d'accord.
D'accord.
Neutre.
Pas d'accord.
Fortement en désaccord.
-
Quel a été le résultat de votre évaluation concernant l’allergie à la pénicilline à la clinique?
Allergie à la pénicilline supprimée.
Allergie à la pénicilline confirmée.
Je ne sais pas.
-
Avez-vous présenté une réaction retardée à l'évaluation après la période d'observation?
Si oui, indiquez la réaction:
Quels traitements ont été nécessaire ? (eg, visite chez le médecin généraliste, antihistaminiques, stéroïdes topiques, admission à l'hôpital).
-
Avez-vous reçu un antibiotique depuis le test?
Si oui, quel était le nom de l'antibiotique?
Si vous ne pouvez pas vous en souvenir, demandez : est-ce que c’était une ‘pénicilline’?
Si oui (c.-à-d. pénicilline reçue), avez-vous présenté une réaction à la pénicilline?
Avez-vous reçu une lettre concernant votre allergie suite à l’évaluation? O/N.
Avez-vous l'impression d'en savoir plus sur les allergies à la pénicilline? O/N.
Avez-vous l'impression d'en savoir plus sur vos réactions à la pénicilline? O/N.
-
Évitez-vous toujours la (les) pénicilline(s)?
Si oui, veuillez expliquer pourquoi? Texte libre (le chercheur classera plus tard).
-
Vous considérez-vous allergique à la pénicilline? O/N.
Si oui, la prochaine fois que vous serez hospitalisé, diriez-vous que vous êtes allergique à la pénicilline?
Si non, félicitations. Nous sommes heureux d'entendre cela. Nous allons continuer avec quelques questions.
-
Avez-vous des commentaires sur les tests, qu'ils soient bons ou mauvais, que vous aimeriez nous transmettre? [(texte libre]).
Si le patient déclare qu'il évite toujours la pénicilline (9) ou qu'il seSE considère allergique à la pénicilline (10) et que vous l'avez évalué pour pouvoir participer à un entretien qualitatif, alors dire:
‘Nous aimerions approfondir ceci. Cela impliquerait un autre entretien téléphonique. Seriez-vous intéressé à participer? Quel serait le bon moment pour vous appeler?’
Fin—‘C'est la fin des questions. Merci beaucoup pour votre temps’.
Adverse events
A serious adverse event will be defined as any adverse drug event/experience occurring at any dose that, in the opinion of the investigators, is causal for any of these outcomes: (1) death; (2) life-threatening reaction; (3) inpatient hospitalisation; (4) results in persistent or significant disability/incapacity; (5) congenital anomaly or birth defect or (6) requires intervention to prevent permanent impairment or damage.
An antibiotic-associated immune-mediated adverse event will include any immune-mediated (immediate (IgE) or non-immediate (T-cell)) reaction within 48 hours of oral provocation judged by two independent reviewers. An antibiotic-associated adverse event will include any non-immune mediated reaction (eg, nausea, vomiting and diarrhoea) within 48 hours of oral provocation judged by two independent reviewers.
Withdrawals and stopping criteria
Participants may withdraw from the study at any point. In these circumstances, the participant’s data collected before the withdrawal might be included in the analysis. However, participants may request their data be destroyed if not already used in analysis. No withdrawals following randomisation will be replaced.
Data management
Participants’ clinical details and demographics will be recorded on electronic uniform data collection forms directly on REDCap. The collected data from every institution will then be stored on an electronic database on password-protected computers. The data from all recruiting sites will be hosted by a single REDCap database at the Austin Health. The participation sites will only have access to their locally entered patient data. A study monitor will be assigned at the Austin Health that will be responsible for data entry completion, error and consistency. According to the local institutional review board regulations, all data for this study will be retained. All electronic and paper data will be destroyed by hospital policy at the time.
Outcomes
The primary outcome is the difference in the proportion of positive oral challenges (ie, immune-mediated reaction).
Secondary outcomes are listed in box 3 and include (1) feasibility outcomes evaluating eligibility to screened ratio, the recruitment to eligibility ratio and the intervention to recruitment ratio; (2) safety outcomes including protocol compliance and adverse reactions (antibiotic-associated immune-mediated adverse event or severe adverse drug reaction) and (3) exploratory efficacy outcomes. The exploratory efficacy outcomes are determined by the number of patients with non-immune reactions, the number of immediate and delayed positive skin testing. Other important elements are the time from randomisation to delabelling, the number of appointments required for delabelling, participant’s quality of life box 3 as well as measuring the impact on delabelling 6 months after the procedure (box 3). In this context, delabelling is defined as removing a patient’s reported allergy if no adverse immune-mediated reaction is noted following a direct oral provocation with the implicated drugs.
Box 3. Secondary outcomes.
Feasibility outcome measures:
Proportion of patients referred to the outpatient allergy clinic that are eligible for intervention (i.e randomisation) as per protocol (eligibility to screened ratio).
Feasibility of recruitment defined as the proportion of patients consenting to participate in the study as per protocol from eligible patients (recruitment to eligibility ratio).
Feasibility of intervention delivery defined as the proportion of patients randomised to the intervention arm who had the intervention delivered as per protocol (intervention to recruitment ratio).
Safety outcome measures:
The proportion of patients with a penicillin allergy who experience an antibiotic-associated immune-mediated adverse event OR severe adverse drug reaction as per protocol definitions.
The proportion of patients with a penicillin allergy who experience an antibiotic associated non-immune-mediated adverse event.
The proportion of patients who will respect the protocol (protocol compliance).
Exploratory efficacy outcomes:
Proportion of patients with positive penicillin skin test.
Proportion of patients with non-immune-mediated positive oral provocation.
Proportion of patients with severe adverse reaction—anaphylaxis/death.
Time from randomisation to delabelling.
Number of appointments required for penicillin allergy delabelling.
Assessment with the prequestionnaire and the 6-month follow-up questionnaire (table 1 and box 2)
Statistical analysis
Results will be presented according to Consolidated Standards of Reporting Trials guidelines. The analysis will be according to intention-to-treat, with additional per-protocol analysis. Descriptive statistics for participant characteristics, penicillin allergy history and why participants did not undergo an assessment will be presented. To ensure consistency, all continuous variables will be presented as median (IQR) and categorical variables as frequency (percentage). The immediate result will be presented as the absolute difference of the primary outcome between groups with a 90% CI (due to one-sided 5% significance used in sample calculation). If the upper level of this interval is greater than the non-inferiority margin (5%), the null hypothesis cannot be rejected. Feasibility outcomes will be presented as a percentage with 95% CI. All other secondary outcomes will be presented as the difference in proportion with 95% CI. Continuous outcomes (time from randomisation to delabelling, number of appointments and quality of life) will be compared using negative binomial or linear regression (depending on the distribution). Missing data are expected to be minimal. If present, it will be imputed using multiple imputations separately for each treatment arm.19 Sensitivity analysis will include complete case. No interim analysis will be performed. Subgroup analysis will be performed by including interaction term in binomial regression, results will be expressed as risk differences. Statistical analysis plan will be made available online prior to the completion of recruitment. All analysis will be conducted using StataCorp. 2019 (Stata Statistical Software: Release V.16. College Station, TX: StataCorp LLC).
Governance
An independent data safety management board (DSMB) will review the study’s progress and monitor adherence to the protocol, participant recruitment, outcomes, complications and other issues related to participant safety. The DSMB will be comprised of a minimum of two clinicians (infectious disease or allergy immunology specialist and one statistician). They will also monitor the assumptions underlying sample size calculations for the study and alert the investigators if an increased recruitment effort is required. The DSMB will recommend whether the study should continue or be terminated and consider participant safety or other circumstances as grounds for early termination, including compelling internal or external evidence of treatment differences or feasibility of addressing the study hypotheses (eg, poor participant enrolment).
The DSMB will be advisory to the Trial Management Group (TMG). The TMG will be comprised by Sponsor-Investigator, the trial statistician, the trial coordinator and other important members from the coordinating site. The TMG will oversee the day-to-day conduct of a clinical trial, including safety oversight activities and/or acting on advice from other individual(s) or group(s) providing safety oversight. The TMG will also be responsible for communicating important protocol modifications (eg, changes to eligibility criteria, outcomes and analyses) to relevant parties, such as the investigators. Each investigator will be responsible to inform their respective Institutional Review Board.
Patient and public involvement
No patient involved.
Ethics and dissemination
The study will be performed according to the guidelines of the Helsinki Declaration20 and is approved by the Austin Health Human Research Ethics Committee (HREC/62425/Austin-2020) in Melbourne Australia, Vanderbilt University Institutional Review Board (IRB #202174) in Tennessee, USA, Duke University Institutional Review Board (IRB #Pro00108461) in North Carolina, USA and McGill University Health Centre Research Ethics Board in Canada (PALACE/2022-7605).
All eligible participants will be provided with a verbal explanation of the project and written information included in the consent form. One of the study investigators will thoroughly assess the participant’s competence and capacity to make a good informed decision before the participants are recruited. All participants will be deemed competent if they (1) can comprehend and retain information relevant to making the decision, (2) understand the information and implications of the decision and (3) are able to evaluate the information and decide. For competent non-English/French speaking participants an interpreter can be used as needed.
Combining these routinely collected data and information derived from this study will provide helpful clinical insights into the management of penicillin allergy, and we plan to publish our findings. The final data set will be the propriety of the Austin Health and contractual agreements were signed between all participating institutions and the Austin Health. The Investigational team will determine authorship concerning the International Committee of Medical Journal Editors guidelines. The results of this research project will be published and presented in various scientific forums without any identifying information about participants. The data collected from all the recruited centres mentioned above will be analysed together and might serve for local practice change in the implicated hospitals but might also be considered part of new drug allergy guidelines. The entire protocol, the participant-level data set and the statistical code will be available on request after the study is completed and findings published.
The ability to deliver point-of-care penicillin allergy testing for a large cohort of patients with diverse ethnic backgrounds, without skin testing, will improve patient access to testing and utilisation of preferred penicillin antibiotics.
Supplementary Material
Footnotes
Twitter: @AnaCopaescu
Contributors: JT and A-MC planned the study design and wrote the protocol. FJ contributed to the protocol manuscript as well as the ethics submission process. SV provided valuable information concerning the suitable statistical analysis and the study design. A-MC, FJ, MR, KC, NEH, NAT, CS, EP and JT will be responsible for patient recruitment and data collection. A-MC, FJ, SV, EP and JT will analyse and interpret the clinical data as well as structure the initial draft report. A-MC, FJ, SV, MR, KC, NEH, NAT, CS, EP and JT reviewed the protocol and this manuscript.
Funding: This research received no specific grant from any funding agency in public, commercial or not-for-profit sectors. A-MC receives support from the Montreal General Hospital Foundation and Research Institute of the McGill University Health Centre (RI-MUHC) and was awarded the University of Melbourne Research Scholarship, the Anna Maria Solinas Laroche Career Award in Immunology and the Anita Garbarino Girard/Anna Maria Solinas/Dr. Phil Gold Award of Distinction.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Charneski L, Deshpande G, Smith SW. Impact of an antimicrobial allergy label in the medical record on clinical outcomes in hospitalized patients. Pharmacotherapy 2011;31:742–7. 10.1592/phco.31.8.742 [DOI] [PubMed] [Google Scholar]
- 2.Huang K-HG, Cluzet V, Hamilton K, et al. The impact of reported beta-lactam allergy in hospitalized patients with hematologic malignancies requiring antibiotics. Clin Infect Dis 2018;67): :27–33. 10.1093/cid/ciy037 [DOI] [PubMed] [Google Scholar]
- 3.Trubiano JA, Chen C, Cheng AC, et al. Antimicrobial allergy 'labels' drive inappropriate antimicrobial prescribing: lessons for stewardship. J Antimicrob Chemother 2016;71:1715–22. 10.1093/jac/dkw008 [DOI] [PubMed] [Google Scholar]
- 4.MacFadden DR, LaDelfa A, Leen J, et al. Impact of reported beta-lactam allergy on inpatient outcomes: a multicenter prospective cohort study. Clin Infect Dis 2016;63:904–10. 10.1093/cid/ciw462 [DOI] [PubMed] [Google Scholar]
- 5.Trubiano JA, Pai Mangalore R, Baey Y-W, et al. Old but not forgotten: antibiotic allergies in general medicine (the AGM study). Med J Aust 2016;204:273. 10.5694/mja15.01329 [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal KG, Lu N, Zhang Y, et al. Risk of meticillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: population based matched cohort study. BMJ 2018;361:k2400. 10.1136/bmj.k2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moran R, Devchand M, Smibert O, et al. Antibiotic allergy labels in hospitalized and critically ill adults: a review of current impacts of inaccurate labelling. Br J Clin Pharmacol 2019;85:492–500. 10.1111/bcp.13830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trubiano JA, Thursky KA, Stewardson AJ, et al. Impact of an integrated antibiotic allergy testing program on antimicrobial stewardship: a multicenter evaluation. Clin Infect Dis 2017;65:166–74. 10.1093/cid/cix244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trubiano JA, Smibert O, Douglas A, et al. The safety and efficacy of an oral penicillin challenge program in cancer patients: a multicenter pilot study. Open Forum Infect Dis 2018;5:ofy306. 10.1093/ofid/ofy306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koo G, Stollings JL, Lindsell C, et al. Low-risk penicillin allergy delabeling through a direct oral challenge in immunocompromised and/or multiple drug allergy labeled patients in a critical care setting. J Allergy Clin Immunol Pract 2022;10:1660–3. 10.1016/j.jaip.2022.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone CA, Stollings JL, Lindsell CJ, et al. Risk-stratified management to remove low-risk penicillin allergy labels in the ICU. Am J Respir Crit Care Med 2020;201:1572–5. 10.1164/rccm.202001-0089LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trubiano JA, Vogrin S, Chua KYL, et al. Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med 2020;180:745. 10.1001/jamainternmed.2020.0403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mustafa SS, Conn K, Ramsey A. Comparing direct challenge to penicillin skin testing for the outpatient evaluation of penicillin allergy: a randomized controlled trial. J Allergy Clin Immunol Pract 2019;7:2163–70. 10.1016/j.jaip.2019.05.037 [DOI] [PubMed] [Google Scholar]
- 14.Savic L, Gurr L, Kaura V, et al. Penicillin allergy de-labelling ahead of elective surgery: feasibility and barriers. Br J Anaesth 2019;123:e110–6. 10.1016/j.bja.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 15.Tucker MH, Lomas CM, Ramchandar N, et al. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of marine recruits. J Allergy Clin Immunol Pract 2017;5:813–5. 10.1016/j.jaip.2017.01.023 [DOI] [PubMed] [Google Scholar]
- 16.Kuruvilla M, Shih J, Patel K, et al. Direct oral amoxicillin challenge without preliminary skin testing in adult patients with allergy and at low risk with reported penicillin allergy. Allergy Asthma Proc 2019;40:57–61. 10.2500/aap.2019.40.4184 [DOI] [PubMed] [Google Scholar]
- 17.Stevenson B, Trevenen M, Klinken E, et al. Multicenter Australian study to determine criteria for low- and high-risk penicillin testing in outpatients. J Allergy Clin Immunol Pract 2020;8:681–9. 10.1016/j.jaip.2019.09.025 [DOI] [PubMed] [Google Scholar]
- 18.Sousa-Pinto B, Tarrio I, Blumenthal KG, et al. Accuracy of penicillin allergy diagnostic tests: A systematic review and meta-analysis. J Allergy Clin Immunol 2021;147:296–308. 10.1016/j.jaci.2020.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan TR, White IR, Salter AB, et al. Should multiple imputation be the method of choice for handling missing data in randomized trials? Stat Methods Med Res 2018;27:2610–26. 10.1177/0962280216683570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Medical Association . WMA Declaration of Helsinki - Ethical Principles for medical research involving human subjects, 2013. Available: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ [Accessed 26 Jul 2020]. [DOI] [PubMed]
- 21.Wilson A, Trubiano JA, Chua KYL. Patient perspectives on antibiotic allergy delabeling: Enablers and barriers. J Allergy Clin Immunol Pract 2020;8:3637–9. 10.1016/j.jaip.2020.07.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-063784supp001.pdf (150.1KB, pdf)