Abstract

Mechanochemical and biocatalytic approaches in modern research are two major assets to develop greener processes. In the present study, these modular tools of sustainability are pointed toward the production of versatile and daily employed compounds such as surfactants. Toward this aim, glycolipids, a class of nonionic surfactants composed of ubiquitous and primary metabolites such as sugar and fatty acid moieties, represent a promising alternative to petroleum-derived surface-active agents. Therefore, the combination of biocatalysis with mechanochemistry aiming at glycolipid synthesis seemed a logical step that was taken in this study for the first time. The monoacylated model compound glucose-6-O-decanoate was synthesized with the help of a bead mill apparatus using two different unconventional dissolved reaction systems, namely, menthol-based hydrophobic deep eutectic solvents and 2-methyl-2-butanol, thus reaching up to 12% yield in the latter based on the conversion of vinyl decanoate, after only 90 min of reaction. In addition, a neat reaction system using an excess of vinylated fatty ester as an adjuvant allowed a 27 mM/h space-time yield. The overall significant increase in productivities, up to 6 times, compared to standard heating and shaking methods, shows the tremendous potential of mechanoenzymatic synthesis.
Keywords: biocatalysis, solvent-free, transesterification, glycolipid, lipase, biosurfactants
Short abstract
Mechanoenzymatic glycolipid synthesis is a highly promising strategy that saves time and energy and allows solvent free biocatalytic production.
Introduction
Surfactants are part of our everyday life as they occur in cosmetics, pharmaceuticals, foods, petrochemicals, mining, metallurgy, agrochemicals, fertilizers, and so forth.1,2 Yet most are produced out of crude oil, which means that they rely on a limited resource. In 2017, surfactants had an annual global revenue of USD 43.6 billion.3 The prices of surfactants are suspected to increase because of the high variability in the oil price in recent years as well as due to higher demand.4 An environmentally friendlier alternative to fill the gap of this ending resource could be the use of biosurfactants. In Europe alone, biobased surfactants had an annual growth rate of 3% between 2008 and 2013. Thus, sustainable synthesis routes are needed for the production of glycolipids.5 Moreover, the sales of biosurfactants worldwide are predicted to reach revenue of up to USD 5.52 billion in 2022. As a flagrant example, rhamnolipids alone are foretold to reach an 8% sales growth until 2023.6 Biosurfactants and more particularly glycolipids are sustainable, odorless, nontoxic, and tasteless compounds5,7 characterized by mono-, di-, or oligosaccharides as their hydrophilic head moiety. The hydrophobic tail usually consists of one or more alkyl moieties with varying chain lengths. This versatility leads to a wide variety of glycolipids.8 Moreover, the ester bond between the two building blocks of glycolipids makes them inherently degradable in nature, hence limiting the risk of harmful accumulations in the environment. Glycolipids, like rhamnolipids, sophorolipids, or mannosylerythritol lipids, have been reported to be bioactive: for example, sophorolipids show anti-inflammatory effects in vitro as well as in vivo;9−11 rhamnolipids improve wound healing in rats9,12,13 and inhibit biofilm formation.9,14,15 They can be also effective against certain viruses and fungi and are potential anti-tumor agents.3,16 Moreover, glycolipids are highly surface-active’ they efficiently reduce surface tension, exhibit a low critical micelle concentration, and act as foam stabilizers.9,17 Consequently, these components gained rapid interest for industrialization purposes. Thus, at the industrial scale, the production of glycolipids such as sugar esters is mostly done by chemical synthesis that is cost-effective with reasonably high yields. However, the chemical procedure requires the use of harsh conditions like high temperature, acidic catalysis (e.g., by perfluorinated alkylsulfonic acid), and complicated processing requiring multiple steps of protection, deprotection, and activation (e.g., with allyl, benzyl, and p-methoxybenzyl groups), leading to wasteful side products.18−20 Nonetheless, these compounds are of great interest in the food-, cosmetic-, and pharmaceutical industry. For example, sorbitol-6-O-laurate, a model compound studied by Delavault et al.,21 is structurally related to an emulsifier registered as E493, which is commonly employed for pharmaceutical and food applications and approved by the European Food Safety Authority (EFSA).22 Enzymatic synthesis of glycolipids provides an alternative to chemical synthesis as milder conditions are applicable, for example, relatively low temperature, as well as selective synthesis, which reduces side product formation.23 However, enzymatic glycolipid synthesis faces some challenges as the substrates show a wide range of polarity and simultaneous solubilization of polar sugars and non-polar fatty acids is difficult.24,25
Mechanochemistry is one of IUPACs 10 world-changing technologies and allows accelerated reaction velocities in solvent-free conditions.26 First reported mechanoenzymatic reactions were enzymatic starch liquefaction and saccharification using amylases in a twin screw extruder.27,28 One of the most common biocatalysts used in mechanoenzymology is the extensively studied Novozym 435, also known as the immobilized Candida antarctica lipase B (CALB).29 It was used in the first mechanoenzymatic production of fine chemicals for the kinetic resolution of secondary alcohols by ball milling.30 Kinetic resolution by mechanoenzymology was extensively studied as well, for many active pharmaceutical ingredients due to high enantioselectivity.31 CALB was also applied in further mechanoenzymatic reactions, for example, acylation of primary amines, hydrolysis of amino esters, or ring-opening polymerization of ω-pentadecalactone.32 Moreover, the use of organic solvents, volatile thus problematic in an eco-friendly context,33 and extensive heat to reach the activation energy of certain chemical reactions can be reduced by using mechanochemistry.34
The objective of the present study is to evaluate the potential of mechanoenzymology to improve the performance of the biocatalyzed synthesis of glycolipids using a model reaction producing glucose monodecanoate (Scheme 1). Using a bead mill provided with glass beads, the transesterification of a sugar with a vinylated fatty acid was catalyzed by the commercial lipase formulation Novozym 435.
Scheme 1. Reaction Scheme to Produce Glucose Monodecanoate.
The synthesis was performed under conditions of reduced water content in order to reverse the hydrolysis activity of the lipase. Thus, organic solvents like 2-methyl-2-butanol (2M2B), ethyl acetate, and acetone were used alongside deep eutectic solvents (DES), as well as in solvent-free conditions. DES are non-toxic, non-volatile, and biodegradable and consist of hydrogen bond donors and hydrogen bond acceptors forming supramolecular structures with hydrogen bonding interactions, resulting in a liquid at room temperature.35−37 In this regard, ubiquitous and naturally occurring plant metabolites may be used as hydrogen bond donors and acceptors, for example, sugars, menthol, and choline chloride, among others. DES are reported to have stabilizing effects on enzymes and exhibit high dissolution power.38−40 However, one of the major drawbacks of DES is their high viscosity compared to water and organic solvents.25,41 The authors assumed that use of mechanoenzymology might overcome the drawback intrinsic to DES caused by high viscosity and the challenges of simultaneous substrate solubilization in different solvent systems. Indeed, successful use of mechanoenzymology was reported to be independent of solubility effects.28,42 In the conducted experiments, hydrophobic DES made of menthol and decanoic acid were used.
Materials and Methods
2M2B (purity ≥ 99%) and menthol (purity ≥ 99%), as well as acid washed glass beads (425–600 and 710–1180 μm), were purchased from Sigma Aldrich Chemie GmbH (Taufkirchen, Germany). Glucose (purity ≥ 98%) and the solvents ethyl acetate (purity ≥ 99.5%) and acetone (purity ≥ 99.5%) were acquired from Carl Roth GmbH & Co. KG (Karlsruhe, Germany). Lipase formulation, Novozym 435, was purchased from STREM Chemicals Europe (Bischheim, France). Glucose monodecanoate (purity > 99%) was acquired from Sohena (Tübingen, Germany). VD (purity > 99%) and decanoic acid (purity ≥ 98%) were acquired from Tokyo Chemical Industry Co., Ltd. (TCI Europe, Belgium). All chemicals were used without further purification.
Preparation of DES
The hydrophobic DES were prepared using an equimolar ratio of menthol and decanoic acid according to Hollenbach et al.43
Solvent Screening: Glycolipid Synthesis in the Bead Mill
For synthesis, 0.5 M glucose (50 mg), 0.5 M VD (65 μL), and 20 mg Novozym 435 (33 mg/mL) were mixed in 1.5 mL Superspin microtubes (20170-030; VWR International GmbH, Darmstadt, Germany) with 0.5 g of glass beads (710–1180 μm) and 600 μL of solvent. The tubes containing the reaction mixture were incubated in a MM300 bead mill (Retsch GmbH, Haan, Germany) at 25 Hz (Figure 1). Experiments were performed in triplicates, and three tubes were collected for each time point (5, 10, 15, 30, 45, 60, and 90 min).
Figure 1.

Bead mill apparatus for processing the samples.
Yield, space-time yield, and biocatalyst productivity were calculated as follows
| 1 |
| 2 |
| 3 |
where n is the number of moles in μmol, c the molar concentration in mM, t the operating time in h, and m the mass in g.
Influence of Milling Frequency, the Size of Glass Beads, and Pre-grinding of the Enzyme Formulation in 2M2B: Single Factor Experiments
Different frequencies (5, 10, 17.5, and 25 Hz) were tested in 2M2B at 45 min reaction time without varying any other reaction parameter to evaluate the optimal milling frequency.
To examine the influence of the bead size on the reaction, two different glass bead sizes (425–600 and 720–1180 μm) were tested at a reaction time of 45 min, while all other reaction conditions were kept constant (20 mg Novozym 435, 0.5 g of glass beads, 600 μL of solvent, 25 Hz).
To address the influence of ground enzyme on the reaction, Novozym 435 was pre-ground manually for approximately 10 min with the help of a ceramic mortar until a visibly fine powder was observed (Figure S1). The pre-ground enzyme had a Sauter diameter of 58.6 ± 0.8 μm (Table 1, Figure S2). The pre-ground enzyme was added to the reaction mixtures instead of whole Novozym 435 beads without changing any other parameter.
Table 1. Particle Size of Whole and Ground Enzyme.
| biocatalyst | whole Novozym 435 | ground Novozym 435 |
|---|---|---|
| Sauter diameter d3,2/μm | 598.7 ± 22.2 | 58.6 ± 0.8 |
| d10,3/μm | 467.3 ± 18.2 | 24.7 ± 0.5 |
| median d50,3/μm | 617.3 ± 22.5 | 202.2 ± 5.2 |
| d90,3/μm | 794.1 ± 22.6 | 589.6 ± 17.7 |
| span/μm | 0.53 ± 0.02 | 2.79 ± 0.03 |
Energy consumption of the different methods was calculated as follows
| 4 |
where P is the power of the applied machines in kW and t is the operation time in h.
Influence of the Substrate Ratio
Different substrate ratios of glucose:VD were investigated such as the following: 0.5:0.5 M (1:1), 0.5:0.25 M (2:1), and 0.25:0.5 M (1:2) in 2M2B and in the hydrophobic menthol:decanoic acid DES. All other reaction conditions were kept constant [20 mg Novozym 435, 0.5 g of glass beads (710–1180 μm), 600 μL of solvent, 25 Hz, 90 min].
Influence of Enzyme Concentration
To examine the optimal enzyme concentration, different concentrations of Novozym 435, that is, 20, 33, 50, 100, and 200 mg/mL, were employed in 2M2B and the hydrophobic menthol/decanoic acid DES without varying any other reaction parameter [0.5 M glucose, 0.5 M VD, 0.5 g of glass beads (710–1180 μm), 600 μL of solvent, 25 Hz, 90 min].
Solvent-Free (Neat) Synthesis of Glycolipids Using a Bead Mill Apparatus
To investigate the mechanoenzymatic synthesis in a solvent-free system, 50 mg of glucose and 20 mg Novozym 435 were mixed in superspin tubes with 0.5 g of glass beads (710–1180 μm) alongside different volumes of VD: 33, 65, 130, 260, and 520 μL as both reactants and adjuvants. Samples were incubated for 90 min at 25 Hz in the bead mill.
Glycolipid Synthesis Using Conventional Heating
As a reference, a standard heating method was used. Therefore, the reaction was carried in 2M2B at 50 °C in a rotator with a vortex mixer (program U2) from neoLab (Heidelberg, Germany), which enables thorough mixing of liquid samples in tubes by vertical rotation and vibration, at 90 rpm for 90 min within an oven, with or without glass beads to allow for further comparison between heating methods after data treatment. The same reaction vessels were used as in the mechanoenzymatic synthesis, and the solvent volume of 600 μL was also retained as well as the other reaction conditions [0.5 M glucose, 0.5 M VD, 20 mg Novozym 435, 0.5 g of glass beads (710–1180 μm), 600 μL of solvent, 25 Hz, 90 min].
Quantification of Glycolipids
Samples were centrifuged after the synthesis. Tubes containing organic solvents were treated as follows: 0.2 mL of the supernatant was transferred into a 1.5 mL tube and dry-evaporated using a SpeedVac centrifugal evaporator (H. Saur Laborbedarf, Reutlingen, Germany) and resuspended in 0.2 mL of ethyl acetate, then subsequently analyzed by high-performance liquid chromatography (HPLC).
For tubes containing DES, samples were diluted with ethyl acetate (1:1, v/v) and analyzed by HPLC. Glycolipids were quantified by HPLC-evaporative light scattering detector (ELSD) as described by Hollenbach et al.25 Briefly, reversed-phase chromatography was performed using a Kinetex EVO C18 (2.6 μm, 250 × 4.6 mm) from Phenomenex (Aschaffenburg, Germany) with an accompanying guard column (4 × 3.0 mm ID) of the same phase and an Agilent (Germany) 1260 series liquid chromatograph equipped with a quaternary pump, an autosampler, and a column oven. An ELSD from BÜCHI Labortechnik (Essen, Germany) was operated at 38 °C, a gas flow rate (air) of 1.5 mL/min, and a gain of 1. Mobile phase was a gradient of acetonitrile (A) and water (B) with a total flow rate of 1 mL/min. The gradient started from 40% A-60% B; then from 0 to 10 min, there was a linear gradient up to 35% A-65% B; followed by another linear gradient from 10 to 15 min up to 25% A-75% B. This gradient was held for 5 min, followed by a reconditioning step of the column with 40% A-60% B for 5 min. Limit of detection was 0.0014 mM.25 A commercially available standard of glucose monodecanoate was used for calibration.
Solvent-free samples were diluted with 600 μL of ethyl acetate and thereafter analyzed by HPLC-ELSD.
Structural Elucidation of Glucose Monodecanoate
Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker AVANCE II+ 600 MHz spectrometer (Bruker BioSpin, Rheinstetten, Germany) equipped with a BBI probe head at 300 K. Prior to analysis, 10 mg glucose monodecanoate was dissolved in 0.6 mL CD2Cl2/d6-acetone (4:1, by vol). For structural elucidation, 1D 1H NMR spectroscopy and 2D 1H–1H correlation spectroscopy (COSY), 1H–13C heteronuclear single–quantum correlation (HSQC) spectroscopy, and 1H–13C-heteronuclear multiple-bond correlation (HMBC) spectroscopy were performed. Spectra were processed and analyzed using Topspin 4.1.4 (Bruker BioSpin) and MestReNova 14.2.0 (Mestrelab Research S.L., Santiago de Compostela, Spain). Chemical shifts were reported relative to the 1H and 13C resonance of tetramethylsilane.
One- and two-dimensional NMR experiments were performed to get a detailed structural description of glucose monodecanoate (Figures S3–S6). Two ring systems were identified in 1H–1H COSY, 1H–13C HSQC, and 1H–13C HMBC spectra that can be assigned to the α- and β-anomers of glucose (Table S1). The decanoate chain could be traced in 1H–1H COSY and 1H–13C HMBC spectra. Based on the cross-peaks between glucose protons and the lipid carbonyl in the 1H–13C HMBC spectrum, the position of the acylation could be confirmed. Both glucose anomers were acylated on C6.
The mass spectrometry (MS) for mass identifications was performed with electrospray ionization on a quadrupole Q Exactive Plus (Thermo Fisher Scientific GmbH, Kandel, Germany) and recorded in a positive mode. The raw spectrometric data were treated using MestReNova Suite 2020 (version 14.2.0) (Mestrelab Research S.L., Santiago de Compostela, Spain). m/z 282.279 [MGMD + H]+-3H2O; m/z 299.185 [MGMD + H]+-2H2O; m/z 317.196 [MGMD + H]+-H2O; m/z 335.206 [MGMD + H]+; m/z 352.233 [MGMD + NH4]+; m/z 357.188 [MGMD + Na]+ (Table S2 and Figure S7).
Particle Size Distribution Measurements
The particle size distribution of the whole and ground enzyme formulation was characterized using a Horiba LA-940 laser diffraction particle analyzer from Retsch Technology GmbH (Haan, Germany).
The pictures were acquired and scaled using a microscope Nikon Eclipse E200 coupled to an NIS Elements D version 4.50 software, both purchased from Nikon Germany (Düsseldorf). For illumination, the spotlight of a KL 1500 LCD goose neck lamp from Schott AG (Mainz, Germany) was placed on the left and right sides of customized glass slides.
Data Treatment and Statistical Analysis
OriginPro software 9.7 (version 2020) (OriginLab Corporation, Northampton, MA, USA) was used for raw data treatment and statistical analysis. Results are presented as mean ± standard deviation (n = 3). Statistical analysis was performed by one-way ANOVA and Tukey test, and the results were considered significant if the p-value was <0.05 if not stated otherwise.
Results
The present work investigates for the first time the synthesis of a glycolipid, namely, a sugar ester, assisted by a mechanoenzymatic method in both dissolved and neat reaction systems. Glucose monodecanoate was produced using a bead mill, and the efficiency was compared to conventional convective thermal heating. For the bead mill-assisted synthesis, different solvent systems were evaluated to mediate the quasi-simultaneous transesterification/esterification reactions.44 Thus, by following the product formation over time, different parameters such as substrate ratio, milling frequency, and enzyme concentration were optimized. Finally, a solvent-free approach was presented and compared to the dissolved reaction systems made of either organic solvent (2M2B) or hydrophobic DES that can both be seen as unconventional media for a biocatalyzed reaction.
Influence of Different Solvents
Figure 2 shows the reaction time course of the mechanoenzymatic glycolipid synthesis in different solvents. Highest yields were obtained in 2M2B (2.2 ± 0.3% corresponding to 11.1 ± 1.4 mM), followed by acetone (1.6 ± 0.4% corresponding to 8.1 ± 2.3 mM). Synthesis in ethyl acetate (1.1 ± 0.2% corresponding to 5.6 ± 0.7 mM) and the hydrophobic (−)-menthol:decanoic acid DES (1.2 ± 0.2% corresponding to 5.9 ± 0.8 mM) resulted in similar yields. Notably, production of glucose monodecanoate in DES without addition of activated fatty acid resulted in considerably lower yields (0.2 ± 0.05% corresponding to 0.8 ± 0.2 mM) in comparison to those achieved with the addition of VD in organic solvents and DES.
Figure 2.

Influence of different solvents on the mechanoenzymatic synthesis of glucose monodecanoate. Highest product titers were achieved in 2M2B. Reaction conditions: substrate ratio glucose: VD is 1:1 (0.5/0.5 M); 33 mg/mL Novozym 435; 0.5 g of glass beads (710–1180 μm); 600 μL of solvent; bead milling frequency: 25 Hz. 2M2B: 2-methyl-2-butanol; DES: hydrophobic (−)-menthol: decanoic acid DES; DES-VD: hydrophobic (−)-menthol: decanoic acid DES without addition of VD. n = 3, p-value < 0.05.
Comparison of Mechanoenzymatic Reactions to Conventional Heating
Samples were incubated in an oven provided with an overhead shaker with vortex mixer to compare the reaction yields of the mechanoenzymatic reaction (Table 2).
Table 2. Comparison of Glucose Monodecanoate by Mechanochemistry Using a Bead Mill vs Conventional Convective Heating after 90 min of Processing.
| solvent | conditiona | glucose monodecanoate/mMb | yield/% |
|---|---|---|---|
| 2M2B | oven w/o glass beads | 8.7 ± 0.6 | 1.7 ± 0.1 |
| oven w/glass beads | 11.5 ± 0.4 | 2.3 ± 0.1 | |
| bead mill (25 Hz) | 11.1 ± 1.4 | 2.2 ± 0.3 | |
| DES | oven w/o glass beads | 4.3 ± 0.2 | 0.9 ± 0.05 |
| oven w/glass beads | 4.4 ± 0.5 | 0.9 ± 0.1 | |
| bead mill (25 Hz) | 5.9 ± 0.8 | 1.2 ± 0.2 |
Oven was used as the standard heating method used as reference at 50 °C provided w/(with) or w/o (without) glass beads in the reaction vessel.
Data are presented as mean values ± standard deviations (n = 3).
Glycolipid titers reached after 90 min without the addition of glass beads were lower in both solvents when using a conventional heating method compared to the bead mill-assisted synthesis. In addition, experiments were performed in which glass beads were added to the reaction vessel but processed by conventional heating instead of the bead mill. These experiments were performed to evaluate whether the lower mechanical forces reached in a shaker provided with glass beads could also reach an intensification of the process similar to the effect observed in the proper bead mill apparatus. Using 2M2B, the initial glycolipid concentration was similar between the mechanoenzymatic approach and the conventional heating method when glass beads were added to the reaction tubes. For the synthesis in DES, however, the addition of glass beads in combination with conventional heating did not achieve the glycolipid titers reached with the bead mill. Furthermore, the synthesis in the bead mill resulted not only in higher product concentration but also in lower energy consumption. The operation of the ball mill consumed 0.27 kW h within the reaction time of 90 min, whereas the energy consumption of the shaker and heating oven was 0.99 kW h.
Influence of Frequency, Pre-grinding of Enzyme Formulation, and Glass Bead Size
Herein, 2M2B was used as a solvent and the reaction time was set to 45 min. The influence of the milling frequency as well as the effect of pre-grinding on the enzyme activity was evaluated. For whole enzyme formulation, the product concentration increased with an increase in shaking frequency up to the highest one allowed by the milling apparatus (Figure 3). Using pre-ground enzyme formulation, a frequency of 17.5 Hz resulted in similar yields as 25 Hz. Consequently, the milling frequency could be reduced when a pre-ground enzyme formulation was used accordingly. However, the maximal product titers reached could not be increased by the pre-grinding.
Figure 3.
Influence of the milling frequency and a pre-grinding step on mechanoenzymatic glycolipid production yields. Reaction conditions: substrate ratio glucose/VD is 1:1 (0.5/0.5 M) at a reaction time of 45 min; 33 mg/mL Novozym 435; 0.5 g glass beads (710–1180 μm); 600 μL of solvent. (a–c) Statistically significant differences (p < 0.05).
Two different sizes of glass beads were investigated in this study, that is, 425–600 and 720–1180 μm of diameter. The bigger beads resulted in a slightly higher product titer (10.2 ± 1.1 mM) compared to the smaller beads (9.2 ± 0.8 mM). However, this difference was not statistically significant.
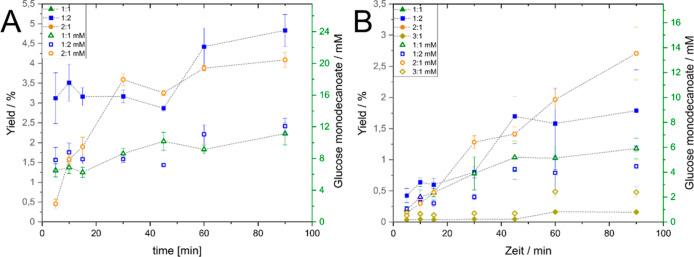
Influence of Substrate Ratio
The substrate ratio was accessed as a reaction parameter for optimization in 2M2B as well as in DES. For both solvent systems, a substrate ratio of 2:1 (glucose:VD) resulted in significantly higher glycolipid concentrations than the other substrate ratios investigated (Figure 4). However, in terms of yields, this observation was non-significant in DES, and in 2M2B, the yield was the highest at a substrate ratio of 1:2. In DES, the product concentration was slightly higher for an equimolar ratio than for a 1:2 ratio. However, there was no statistically significant differences between an equimolar ratio and a substrate ratio of 1:2. In 2M2B, the yields achieved for a substrate ratio of 1:2 were significantly higher compared to the equimolar ratio.
Figure 4.
Influence of substrate ratio on product titers and yields. (A) In 2M2B; (B) in (−)-menthol:decanoic acid DES. Tested ratios: 1:1 (0.5 M glucose: 0.5 M VD), 1:2 (0.25 M glucose: 0.5 M VD), and 2:1 (0.5 M glucose: 0.25 M VD). For equimolar ratio and 2:1, the curves of yield and concentration are overlapping. Reaction conditions: 33 mg/mL of Novozym 435; 0.5 glass beads (710–1180 μm); 600 μL of solvent; 25 Hz milling frequency. Data are presented as mean values ± standard deviations (n = 3).
Influence of Enzyme Loading
In order to increase the productivity of the mechanoenzymatic process, different enzyme concentrations were tested (Figure 5). Glucose monodecanoate concentration increased with increasing enzyme concentration up to 100 mg/mL in 2M2B and 50 mg/mL in DES. At higher enzyme concentrations, the product titers decreased. The influence of the enzyme concentration was more pronounced in 2M2B than in DES. For the mechanoenzymatic synthesis in 2M2B, the glycolipid concentration was quadrupled (from 11.9 ± 1.9 mM at 20 mg/mL to 39.5 ± 3.5 mM at 100 mg/mL), while the product concentration in DES was only doubled with optimized enzyme concentration (from 4.6 ± 1.8 mM at 20 mg/mL to 9.0 ± 0.4 mM at 50 mg/mL).
Figure 5.
Influence of enzyme loading on mechanoenzymatic production of glucose monodecanoate. (A) In 2M2B; (B) in DES. Reaction conditions: substrate ratio glucose: VD is 1:1 (0.5/0.5 M); 0.5 g glass beads (710–1180 μm); 600 μL solvent; 25 Hz milling frequency; 90 min. (a–c) Statistically significant differences (p < 0.05).
Solvent-Free Synthesis
Neat reaction systems, also known as solvent-free systems, have drawn considerable attention over the past decades as they allow almost complete riddance of solvents and thus simplify waste management strategies.45,46 Their use in mechanoenzymology is highly relevant as liquid substrates; for example, fatty esters in our case, can play the role of an adjuvant to enhance mass transfer. With those considerations, neat systems are therefore a tool of high interest for green chemistry and more particularly biocatalysis. It therefore appeared logical to investigate a solvent-free method in the frame of the mechanoenzymatic glycolipid synthesis.
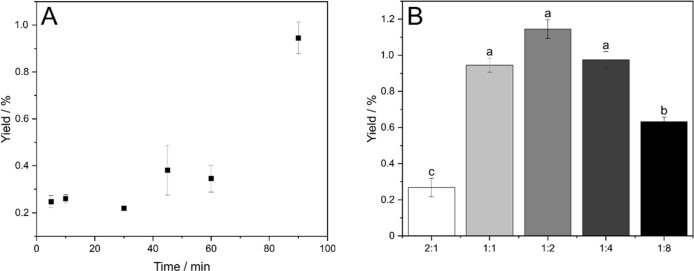
Figure 6A shows the reaction time course. The data indicate that after the reaction time of 90 min, a steady state is not yet reached. However, longer reaction times could not be addressed due to limitations in the maximal operation time of the milling apparatus.
Figure 6.
Solvent-free glycolipid synthesis. (A) Reaction time course of glucose monodecanoate production in a solvent-free mechanoenzymatic synthesis (substrate ratio glucose:VD is 1:1). (B) Influence of VD ratio on glucose monodecanoate yields in solvent-free mechanoenzymatic synthesis (substrate ratio glucose:VD is 2:1; 1:1; 1:2; 1:4; 1:8). Reaction conditions: 20 mg Novozym 435; 50 mg glucose; 0.5 g glass beads (710–1180 μm); 90 min, 25 Hz milling frequency. (a–c) Statistically significant differences (p < 0.05).
Different volumes of VD were evaluated in this study. The yield of glucose monodecanoate was the best at a slight excess of C10 vinylated fatty ester or equimolar ratio of the substrates (Figure 6B). Upon 520 μL of VD, an inhibition of the reaction was observed. The maximum achieved yield in the solvent-free system was 1.1 ± 0.07%, while in 2M2B, yields up to 11.4 ± 2.4% and in DES up to 2.0 ± 0.2% were reached.
Discussion
In this study, a mechanoenzymatic approach for glycolipid synthesis was evaluated. Mechanochemistry has been widely applied in various fields of chemistry;47−50 however, modern mechanochemistry has emerged only recently in biocatalysis, as first reported in 2016.30,48 We demonstrated the suitability of mechanoenzymatic glycolipid synthesis in two different unconventional dissolved reaction solvents such as organic solvents and hydrophobic DES. Most generally and until now, glycolipids have been produced in various organic solvents using conventional heating.51−56 By means of mechanoenzymatic synthesis, yields were the highest in 2M2B, also known as tert-amyl alcohol, which is a solvent reported to be compatible with food and pharmaceutical applications.57 With a log P of 1.1, 2M2B is less polar than the other investigated organic solvents, ethyl acetate, and acetone, which turns out to be valuable for biocatalysis as solvents of medium polarity are most suitable for glycolipid synthesis by allowing sugar dissolution without hindering enzyme activity.43,52,53,58 Indeed, highly polar solvents can strip off water from the essential hydration shell of the enzyme, ending up in partial or complete denaturation of the latter.59−61
The mechanoenzymatic synthesis was compared to a conventional heating and mixing method. Higher product titers and yields were reached in the bead mill than in the conventional method. Additionally, the synthesis using conventional heating was performed with adjunction of glass beads in the reaction vessel and reached similar glycolipid concentrations as in the bead mill. This observation was made significant using 2M2B, while only minor effects were observed in DES. This indicates that in DES, the influence of shear mechanical forces was not as prevalent as in 2M2B when adding glass beads to the reaction mixtures to enhance the conversion. This conjecture is valid whether the synthesis was assisted by the heated overhead shaker or the bead mill. The difference in behavior of the reaction in 2M2B and DES is most likely due to the higher viscosity of the latter, which is known to affect mass transfer directly. The improved performances of the mechanoenzymatic reaction might be due to an increase in interface energy and decrease in activation energy, which is well-known for mechanochemistry.50 These effects allow for high space-time yields and thus save a considerable amount of time. The comparison of our study with glycolipid syntheses described in the literature confirms higher space-time yields by mechanoenzymatic synthesis, which is 2–6 times higher than the reported productivity for sugar ester synthesis in the literature (Table 3). However, the comparison also shows that the yields in the study presented here are lower despite much greater space-time yields because no steady state was reached prior to attaining 90 min of reaction time. Hence, longer reaction times and temperature control will most likely increase the yields of the mechanoenzymatic reaction further. Unfortunately, this could not be investigated in this study due to a limited maximum running time of the ball mill used to prevent overheating of the unit.
Table 3. Yield and Space-Time Yield of Enzymatic Glycolipid Production by Different Methods in Different Solvent Systems.
| method | solvent (n/na; enzyme quantity; biocatalyst) | reaction time/h | yield/%c | space-time yield/mM/hc | biocatalyst productivity/μmolproduct/h/mgbiocatalyst | refs |
|---|---|---|---|---|---|---|
| BM | 2M2B (1:1; 33 mg/mL, Novozym 435) | 0.75 | 2.0 ± 0.2 | 13.5 ± 1.5 | 0.27 ± 0.03 | this work |
| 2M2B (1:2; 100 mg/mL; Novozym 435) | 1.5 | 11.4 ± 2.4 | 19.0 ± 3.9 | 0.13 ± 0.03 | this work | |
| 2M2B (1:1; 100 mg/mL Novozym 435) | 1.5 | 7.9 ± 0.7 | 26.3 ± 2.3 | 0.17 ± 0.01 | this work | |
| 2M2B (2:1; 33 mg/mL; Novozym 435) | 1.5 | 4.1 ± 0.2 | 13.6 ± 0.6 | 0.27 ± 0.01 | this work | |
| DES (2:1; 33 mg/mL; Novozym 435) | 1.0 | 2.0 ± 0.2 | 6.6 ± 0.5 | 0.13 ± 0.01 | this work | |
| Solvent-free(1:2; 20 mg; Novozym 435) | 1.5 | 1.1 ± 0.07 | 27.0 ± 1.9 | 0.09 ± 0.006 | this work | |
| conv | 2M2B | 1.5 | 1.9 ± 0.2 | 6.3 ± 0.8 | 0.12 ± 0.02 | this work |
| DES | 1.5 | 0.9 ± 0.05 | 2.9 ± 0.15 | 0.06 ± 0.003 | this work | |
| conv. | DES (3:1; 20 mg/mL; Novozym 435) | 24 | 11 | 6.8 | 0.34 | (43) |
| conv. | 2M2B (1:1; 10% SP 435) | 72 | 55 | 6.4 | 0.06 | (52)b |
| conv. | IL/t-butanol (1:2; 5% Candida antarctica lipase B) | 72 | 60 | 4.2 | 0.08 | (62)b |
Unless indicated, the molar ratio n/n corresponds to the glucose and VD.
In those studies, esters of sugars with slight structural variations were synthesized.
Averaged values.
Furthermore, the effect of enzyme formulation pre-grinding was accessed. Increased activity of ground immobilized C. antarctica lipase B was previously reported in conventional heating as well as in mechanoenzymology.63,64 We observed that for the ground enzyme formulation, a lower milling frequency during the mechanoenzymatic reaction was necessary to reach the same product concentrations. The described effect is likely attributed to diffusional effects, because a higher surface of the biocatalyst carrier and a better distribution in the reaction mixture is achieved by grinding as the particles get smaller. The Sauter diameter of the biocatalyst particles was 10 times smaller for the ground enzyme formulation than for the whole one, thus adding evidence to our conjecture.
For conventional convective heating, a high amount of dissolved sugar proved to be beneficial for enzymatic glycolipid synthesis in different solvent systems.24,43,65−67 Likewise, this study revealed a higher product concentration in the presence of glucose excess. The best substrate ratio was 2:1 (glucose:VD, n/n) for 2M2B as well as for DES.
To enhance product concentration, the biocatalyst loading was evaluated, resulting in an optimal enzyme concentration of 100 mg/mL in 2M2B and 50 mg/mL in DES. Higher enzyme loading resulted in a significant decrease in glycolipid concentration, which is also reported for other transesterification reactions.43,68−70 On the one hand, this observation might be due to product hydrolysis by the excess enzyme and the water formed as a by-product.43,44 On the other hand, a high enzyme excess could affect mass transfer, as a higher enzyme concentration correlates to a higher observed viscosity of the reaction mixture. In this study, samples with 200 g/L enzyme formulation load displayed a paste-like behavior after ball mill application and could no longer be associated to a proper liquid like the other samples. Optimal enzyme formulation concentrations of 50 mg/mL and higher are not uncommon with Novozym 435. For example, Delavault et al. revealed 50 mg/mL as ideal for the synthesis of sorbitol laurate21 and enzyme concentrations of, for example, 150 mg/mL solvent or 190 mg per mmol substrate, have also been reported in mechanoenzymatic reactions.68,71 Hereby, it is to consider that in the case of enzymes immobilized on beads, the non-catalytic material makes up a large part of the total mass (90 to >99 wt %).72,73 For Novozym 435, a protein content of 30–55 mg/g is reported in the literature.74 Therefore, 100 mg/mL Novozym 435 corresponds to only 3–5 mg/mL of protein. Moreover, Novozym 435 is a highly stable lipase formulation, and the reusability of this biocatalyst has been proven also in mechanoenzymatic reactions, reducing the enzyme costs in each consecutive cycle.30,64,75 However, a valid recycling strategy has to be developed and evaluated to ensure economic applicability of such a process.
Solvent-free strategies are gaining interest as they enable more sustainable production by preventing the formation of wastes, increasing volumetric productivity, and accordingly minimizing downstream processing.45,46,50,76−78 However, semisolid reaction mixtures thereby pose a huge challenge. In previous studies involving solvent-free syntheses of sugar esters, the practical difficulties in processing semisolid mixtures were addressed by high-pressure homogenization and addition of crude sugar esters or conditions under reduced pressures.79,80 We did overcome the problem of immiscibility of the substrates, glucose and VD, owing to a mechanoenzymatic strategy.
In the solvent-free synthesis, we observed highest conversion employing a slight excess of fatty acid ester in contrast to the dissolved reaction systems, using either 2M2B or DES, in which a slight excess of sugar performed best. This observation can be explained by the fact that the acyl acceptor, namely, glucose, is solid, while the vinyl fatty ester is liquid (at room temperature) and provides properties similar to those of an adjuvant. Thus, a higher amount of fatty acid leads to a higher proportion of liquid in the reaction mixture and hence to a better mixing. Still, an inhibitory effect was observed above a certain ratio of the fatty acid in solvent-free synthesis. It is known for different solvent systems that fatty acids inhibit glycolipid synthesis as well as different transesterification reactions.55,81−83 Indeed, transesterification reactions follow a ping–pong bi bi mechanism and therefore the formation of non-productive complexes with either fatty acids or sugar is possible.81,82,84
Conclusions
The study provides first evidence for the applicability of mechanoenzymatic reactions in glycolipid production. We have shown that lipase-mediated glycolipid synthesis is possible in organic solvents as well as in DES using the mechanical shear forces provided by a bead mill apparatus. We achieved considerably higher space-time yields by mechanoenzymatic synthesis compared to a variety of conventional heating and mixing systems. Therefore, the mechanoenzymatic approach is most likely a promising strategy for glycolipid synthesis and biocatalysis as a whole, to save time and energy.
Moreover, we presented a solvent-free enzymatic synthesis for a glucose fatty acid ester for the first time, showing that the challenges of a semisolid system could be successfully overcome by a mechanoenzymatic approach.
Acknowledgments
We gratefully thank the Open Access Publishing Fund of Karlsruhe Institute of Technology. Furthermore, the authors gratefully acknowledge Sebastian Höhne and Dr. Ulrike S. van der Schaaf (Institute of Process Engineering in Life Sciences I: Food Process Engineering) for the support with the laser diffraction particle analyzer, Jens Rudat (Institute of Process Engineering in Life Sciences II: Technical Biology) for the support with the microscope, as well as Karolin Kohnle and Lara Hirsch (Institute of Organic Chemistry) for the support with the MS.
Glossary
Abbreviations
- DES
deep eutectic solvents
- VD
vinyl decanoate
- 2M2B
2-methyl-2-butanol
- ELSD
evaporative light scattering detector
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acssuschemeng.2c01727.
Microscopical images of ground and whole Novozym 435; cumulative particle size distribution of ground and whole Novozym 435; NMR spectra of glucose monodecanoate; mass spectrum of glucose monodecanoate; chemical shifts obtained by NMR of glucose monodecanoate; and results of MS of glucose monodecanoate (PDF)
Author Present Address
§ Biotechnological Conversion, Technikum Laubholz, Blaubeuren, Germany
Author Contributions
R.H. and A.D. contributed equally. This study was conceptualized by R.H. and A.D. Investigations were performed by R.H., A.D., and L.G. NMR analysis was performed by H.S. and C.M.-G. The work was supervised by C.S. and K.O. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
R.H. was supported by the European Regional Development Fund and the Ministry of Science, Research and the Arts of the State of Baden-Württemberg within the research center ZAFH InSeL (Grant #32-7545.24-20/6/3). C.M.-G. acknowledges funding by the Helmholtz-Society.
The authors declare no competing financial interest.
Supplementary Material
References
- Shete A. M.; Wadhawa G.; Banat I. M.; Chopade B. A. Mapping of patents on bioemulsifier and biosurfactant: A review. J. Sci. Ind. Res. 2006, 65, 91–115. [Google Scholar]
- Perinelli D. R.; Lucarini S.; Fagioli L.; Campana R.; Vllasaliu D.; Duranti A.; et al. Lactose oleate as new biocompatible surfactant for pharmaceutical applications. Eur. J. Pharm. Biopharm. 2018, 124, 55–62. 10.1016/j.ejpb.2017.12.008. [DOI] [PubMed] [Google Scholar]
- Banat I. M.; Carboué Q.; Saucedo-Castañeda G.; de Jesús Cázares-Marinero J. Biosurfactants: The green generation of speciality chemicals and potential production using Solid-State fermentation (SSF) technology. Bioresour. Technol. 2021, 320, 124222. 10.1016/j.biortech.2020.124222. [DOI] [PubMed] [Google Scholar]
- Surfactants Market Research Report: Market Size, Industry Outlook, Market Forecast, Demand Analysis,Market Share, Market Report 2021-2026 [Internet]. Available from: https://www.industryarc.com/Report/15201/surfactants-market.html.
- Grüninger J.; Delavault A.; Ochsenreither K. Enzymatic glycolipid surfactant synthesis from renewables. Process Biochem. 2019, 87, 45–54. 10.1016/j.procbio.2019.09.023. [DOI] [Google Scholar]
- Farias C. B. B.; Almeida F. C. G.; Silva I. A.; Souza T. C.; Meira H. M.; Soares da Silva R. de C. F.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. 10.1016/j.ejbt.2021.02.002. [DOI] [Google Scholar]
- Lima T. M. S.; Procópio L. C.; Brandão F. D.; Carvalho A. M. X.; Tótola M. R.; Borges A. C. Biodegradability of bacterial surfactants. Biodegradation 2011, 22, 585–592. 10.1007/s10532-010-9431-3. [DOI] [PubMed] [Google Scholar]
- Pöhnlein M.; Hausmann R.; Lang S.; Syldatk C. Enzymatic synthesis and modification of surface-active glycolipids. Eur. J. Lipid Sci. Technol. 2015, 117, 145–155. 10.1002/ejlt.201400418. [DOI] [Google Scholar]
- Marchant R.; Banat I. M. Microbial biosurfactants: Challenges and opportunities for future exploitation. Trends Biotechnol. 2012, 30, 558–565. 10.1016/j.tibtech.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Bluth M. H.; Kandil E.; Mueller C. M.; Shah V.; Lin Y. Y.; Zhang H.; et al. Sophorolipids block lethal effects of septic shock in rats in a cecal ligation and puncture model of experimental sepsis. Crit. Care Med. 2006, 34, E188 10.1097/01.ccm.0000196212.56885.50. [DOI] [PubMed] [Google Scholar]
- Hardin R.; Pierre J.; Schulze R.; Mueller C. M.; Fu S. L.; Wallner S. R.; et al. Sophorolipids Improve Sepsis Survival: Effects of Dosing and Derivatives. J. Surg. Res. 2007, 142, 314–319. 10.1016/j.jss.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Stipcevic T.; Piljac A.; Piljac G. Enhanced healing of full-thickness burn wounds using di-rhamnolipid. Burns 2006, 32, 24–34. 10.1016/j.burns.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sana S.; Datta S.; Biswas D.; Auddy B.; Gupta M.; Chattopadhyay H. Excision wound healing activity of a common biosurfactant produced by Pseudomonas sp. Wound Med. 2018, 23, 47–52. 10.1016/j.wndm.2018.09.006. [DOI] [Google Scholar]
- Rodrigues L. R.; Banat I. M.; Mei H. C.; Teixeira J. A.; Oliveira R. Interference in adhesion of bacteria and yeasts isolated from explanted voice prostheses to silicone rubber by rhamnolipid biosurfactants. J. Appl. Microbiol. 2006, 100, 470–480. 10.1111/j.1365-2672.2005.02826.x. [DOI] [PubMed] [Google Scholar]
- Aleksic I.; Petkovic M.; Jovanovic M.; Milivojevic D.; Vasiljevic B.; Nikodinovic-Runic J.; et al. Anti-biofilm properties of bacterial di-rhamnolipids and their semi-synthetic amide derivatives. Front. Microbiol. 2017, 8, 2454. 10.3389/fmicb.2017.02454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu S. A.; Twigg M. S.; Naughton P. J.; Marchant R.; Banat I. M.. Biosurfactants as Anticancer Agents: Glycolipids Affect Skin Cells in a Differential Manner Dependent on Chemical Structure. Pharmaceutics 2022, 14(). https://doi.org/10.3390/pharmaceutics14020360. 10.3390/pharmaceutics14020360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbach R.; Oeppling S.; Delavault A.; Völp A. R.; Willenbacher N.; Rudat J.; et al. Comparative study on interfacial and foaming properties of glycolipids in relation to the gas applied for foam generation. RSC Adv. 2021, 11, 34235–34244. 10.1039/d1ra06190a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornaghi L. F.; Poulsen S. A. Microwave-accelerated Fischer glycosylation. Tetrahedron Lett. 2005, 46, 3485–3488. 10.1016/j.tetlet.2005.03.126. [DOI] [Google Scholar]
- Oikawa M.; Tanaka T.; Fukuda N.; Kusumoto S. One-pot preparation and activation of glycosyl trichloroacetimidates: Operationally simple glycosylation induced by combined use of solid-supported, reactivity-opposing reagents. Tetrahedron Lett. 2004, 45, 4039–4042. 10.1016/j.tetlet.2004.03.170. [DOI] [Google Scholar]
- Roy B.; Mukhopadhyay B. Sulfuric acid immobilized on silica: an excellent catalyst for Fischer type glycosylation. Tetrahedron Lett. 2007, 48, 3783–3787. 10.1016/j.tetlet.2007.03.165. [DOI] [Google Scholar]
- Delavault A.; Opochenska O.; Laneque L.; Soergel H.; Muhle-Goll C.; Ochsenreither K.; et al. Lipase-catalyzed production of sorbitol laurate in a “2-in-1” deep eutectic system: Factors affecting the synthesis and scalability. Molecules 2021, 26, 2759. 10.3390/molecules26092759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulation (E. G.) No. 1333/2008 of the European Parliaments and of the council on food additives; Official Journal of the Europe, 2014; pp 1–330. http://data.europa.eu/eli/reg/2008/1333/oj.
- Burek B. O.; Dawood A. W. H.; Hollmann F.; Liese A.; Holtmann D. Process Intensification as Game Changer in Enzyme Catalysis. Front Catal. 2022, 2, 858706. 10.3389/fctls.2022.858706. [DOI] [Google Scholar]
- Flores M. V.; Naraghi K.; Engasser J.; Halling P. J.. Influence of Glucose Solubility and Dissolution Rate on the Kinetics of Lipase Catalyzed Synthesis of Glucose Laurate in 2-Methyl 2-Butanol, 2002, 78, 815. https://doi.org/ 10.1002/bit.10263. [DOI] [PubMed] [Google Scholar]
- Hollenbach R.; Bindereif B.; van der Schaaf U. S.; Ochsenreither K.; Syldatk C. Optimization of Glycolipid Synthesis in Hydrophilic Deep Eutectic Solvents. Front. Bioeng. Biotechnol. 2020, 8, 382. 10.3389/fbioe.2020.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomollón-Bel F. Ten Chemical Innovations That Will Change Our World. Chem. Int. 2020, 42, 3–9. 10.1515/ci-2020-0402. [DOI] [Google Scholar]
- Hakulin S.; Linko Y.-Y.; Linko P.; Seiler K.; Seibel W. Enzymatic Conversion of Starch in Twin-Screw HTST-Extruder. Starke 1983, 35, 411–414. 10.1002/star.19830351203. [DOI] [Google Scholar]
- Gatt E.; Rigal L.; Vandenbossche V. Biomass pretreatment with reactive extrusion using enzymes: A review. Ind. Crops Prod. 2018, 122, 329–339. 10.1016/j.indcrop.2018.05.069. [DOI] [Google Scholar]
- Ortiz C.; Ferreira M. L.; Barbosa O.; dos Santos J. C. S.; Rodrigues R. C.; Berenguer-Murcia Á.; et al. Novozym 435: The “perfect” lipase immobilized biocatalyst?. Catal. Sci. Technol. 2019, 9, 2380–2420. 10.1039/c9cy00415g. [DOI] [Google Scholar]
- Hernández J. G.; Frings M.; Bolm C. Mechanochemical Enzymatic Kinetic Resolution of Secondary Alcohols under Ball-Milling Conditions. ChemCatChem 2016, 8, 1769–1772. 10.1002/cctc.201600455. [DOI] [Google Scholar]
- Pérez-Venegas M.; Mechanoenzymology J. E. State of the Art and Challenges towards Highly Sustainable Biocatalysis. ChemSusChem 2021, 14, 2682–2688. 10.1002/cssc.202100624. [DOI] [PubMed] [Google Scholar]
- Kaabel S.; Friščić T.; Auclair K. Mechanoenzymatic Transformations in the Absence of Bulk Water: A More Natural Way of Using Enzymes. ChemBioChem 2020, 21, 742–758. 10.1002/cbic.201900567. [DOI] [PubMed] [Google Scholar]
- Sheldon R. A. Green solvents for sustainable organic synthesis: State of the art. Green Chem. 2005, 7, 267–278. 10.1039/b418069k. [DOI] [Google Scholar]
- Fischer F.; Wenzel K. J.; Rademann K.; Emmerling F. Quantitative determination of activation energies in mechanochemical reactions. Phys. Chem. Chem. Phys. 2016, 18, 23320–23325. 10.1039/c6cp04280e. [DOI] [PubMed] [Google Scholar]
- Dai Y.; Witkamp G. J.; Verpoorte R.; Choi Y. H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. 10.1016/j.foodchem.2015.03.123. [DOI] [PubMed] [Google Scholar]
- Hammond O. S.; Bowron D. T.; Edler K. J. Liquid structure of the choline chloride-urea deep eutectic solvent (reline) from neutron diffraction and atomistic modelling. Green Chem. 2016, 18, 2736. 10.1039/c5gc02914g. [DOI] [Google Scholar]
- Gutiérrez M. C.; Ferrer M. L.; Mateo C. R.; del Monte F. D. Freeze-drying of aqueous solutions of deep eutectic solvents: A suitable approach to deep eutectic suspensions of self-assembled structures. Langmuir 2009, 25, 5509–5515. 10.1021/la900552b. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Jacoby W. A.; Wan C. Ternary deep eutectic solvents for effective biomass deconstruction at high solids and low enzyme loadings. Bioresour. Technol. 2019, 279, 281–286. 10.1016/j.biortech.2019.01.126. [DOI] [PubMed] [Google Scholar]
- Procentese A.; Johnson E.; Orr V.; Garruto Campanile A.; Wood J. A.; Marzocchella A.; et al. Deep eutectic solvent pretreatment and subsequent saccharification of corncob. Bioresour. Technol. 2015, 192, 31–36. 10.1016/j.biortech.2015.05.053. [DOI] [PubMed] [Google Scholar]
- Monhemi H.; Housaindokht M. R.; Moosavi-Movahedi A. A.; Bozorgmehr M. R. How a protein can remain stable in a solvent with high content of urea: Insights from molecular dynamics simulation of Candida antarctica lipase B in urea: Choline chloride deep eutectic solvent. Phys. Chem. Chem. Phys. 2014, 16, 14882–14893. 10.1039/c4cp00503a. [DOI] [PubMed] [Google Scholar]
- Dai Y.; van Spronsen J.; Witkamp G. J.; Verpoorte R.; Choi Y. H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. 10.1016/j.aca.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Arciszewski J.; Auclair K. Mechanoenzymatic Reactions Involving Polymeric Substrates or Products. ChemSusChem 2022, 15, e202102084 10.1002/cssc.202102084. [DOI] [PubMed] [Google Scholar]
- Hollenbach R.; Ochsenreither K.; Syldatk C. Enzymatic synthesis of glucose monodecanoate in a hydrophobic deep eutectic solvent. Int. J. Mol. Sci. 2020, 21, 4342. 10.3390/ijms21124342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcens D.; Grau E.; Grelier S.; Cramail H.; Peruch F. Impact of Fatty Acid Structure on CALB-Catalyzed Esterification of Glucose. Eur. J. Lipid Sci. Technol. 2020, 122, 1900294. 10.1002/ejlt.201900294. [DOI] [Google Scholar]
- de Sousa R. R.; da Silva A. S.; Fernandez-Lafuente R.; Ferreira-Leitão V. S. Simplified method to optimize enzymatic esters syntheses in solvent-free systems: Validation using literature and experimental data. Catalysts 2021, 11, 1357. 10.3390/catal11111357. [DOI] [Google Scholar]
- Tufvesson P.; Annerling A.; Hatti-Kaul R.; Adlercreutz D. Solvent-free enzymatic synthesis of fatty alkanolamides. Biotechnol. Bioeng. 2007, 97, 447–453. 10.1002/bit.21258. [DOI] [PubMed] [Google Scholar]
- Bolm C.; Hernández J. G. From Synthesis of Amino Acids and Peptides to Enzymatic Catalysis: A Bottom-Up Approach in Mechanochemistry. ChemSusChem 2018, 11, 1410–1420. 10.1002/cssc.201800113. [DOI] [PubMed] [Google Scholar]
- Friščić T.; Mottillo C.; Titi H. M. Mechanochemistry for Synthesis. Angew. Chem., Int. Ed. 2020, 59, 1018–1029. 10.1002/ange.201906755. [DOI] [PubMed] [Google Scholar]
- Pérez-Venegas M.; Juaristi E.; Juaristi E. Mechanochemical and Mechanoenzymatic Synthesis of Pharmacologically Active Compounds: A Green Perspective. ACS Sustain. Chem. Eng. 2020, 8, 8881–8893. 10.1021/acssuschemeng.0c01645. [DOI] [Google Scholar]
- Galant O.; Cerfeda G.; McCalmont A. S.; James S. L.; Porcheddu A.; Delogu F.; et al. Mechanochemistry Can Reduce Life Cycle Environmental Impacts of Manufacturing Active Pharmaceutical Ingredients. ACS Sustain. Chem. Eng. 2022, 10, 1430–1439. 10.1021/acssuschemeng.1c06434. [DOI] [Google Scholar]
- Walsh M. K.; Bombyk R. A.; Wagh A.; Bingham A.; Berreau L. M. Synthesis of lactose monolaurate as influenced by various lipases and solvents. J. Mol. Catal. B: Enzym. 2009, 60, 171–177. 10.1016/j.molcatb.2009.05.003. [DOI] [Google Scholar]
- Šabeder S.; Habulin M. Lipase-catalyzed synthesis of fatty acid fructose esters. J. Food Eng. 2006, 77, 880–886. 10.1016/j.jfoodeng.2005.08.016. [DOI] [Google Scholar]
- Bouzaouit N.; Bidjou-haiour C. Response Surface Methodological Study of Glucose Laurate Synthesis Catalyzed by Immobilized Lipase from Candida cylindracea. Biol. Forum. 2016, 8, 420. [Google Scholar]; Available from: https://researchtrend.net/bfij/pdf/60%20CHAHRA%20BIDJOU-HAIOUR.pdf
- Zhao Y.; Liu J.; Deng L.; Wang F.; Tan T. Optimization of Candida sp. 99-125 lipase catalyzed esterification for synthesis of monoglyceride and diglyceride in solvent-free system. J. Mol. Catal. B: Enzym. 2011, 72, 157. 10.1016/j.molcatb.2011.05.014. [DOI] [Google Scholar]
- Lin X.-S.; Wen Q.; Huang Z. L.; Cai Y. Z.; Halling P. J.; Yang Z. Impacts of ionic liquids on enzymatic synthesis of glucose laurate and optimization with superior productivity by response surface methodology. Process Biochem. 2015, 50, 1852. 10.1016/j.procbio.2015.07.019. [DOI] [Google Scholar]
- Lee S. H.; Ha S. H.; Hiep N. M.; Chang W. J.; Koo Y. M. Lipase-catalyzed synthesis of glucose fatty acid ester using ionic liquids mixtures. J. Biotechnol. 2008, 133, 486–489. 10.1016/j.jbiotec.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Khaled N.; Montet D.; Pina M.; Graille J. Fructose oleate synthesis in a fixed catalyst bed reactor. Biotechnol. Lett. 1991, 13, 167–172. 10.1007/bf01025812. [DOI] [Google Scholar]
- Hollenbach R.; Ochsenreither K.; Syldatk C.. Parameters Influencing Lipase-Catalyzed Glycolipid Synthesis by (Trans-)Esterification Reaction. Advances in Biochemical Engineering/Biotechnology; Springer: Berlin, Heidelberg, 2021. [DOI] [PubMed] [Google Scholar]
- Idris A.; Bukhari A. Immobilized Candida antarctica lipase B: Hydration, stripping off and application in ring opening polyester synthesis. Biotechnol. Adv. 2012, 30, 550–563. 10.1016/j.biotechadv.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Nakashima K.; Okada J.; Maruyama T.; Kamiya N.; Goto M. Activation of lipase in ionic liquids by modification with comb-shaped poly(ethylene glycol). Sci. Technol. Adv. Mater. 2006, 7, 692–698. 10.1016/j.stam.2006.06.008. [DOI] [Google Scholar]
- Yang L.; Dordick J. S.; Garde S. Hydration of enzyme in nonaqueous media is consistent with solvent dependence of its activity. Biophys. J. 2004, 87, 812–821. 10.1529/biophysj.104.041269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganske F.; Bornscheuer U. T. Optimization of lipase-catalyzed glucose fatty acid ester synthesis in a two-phase system containing ionic liquids and t-BuOH. J. Mol. Catal. B: Enzym. 2005, 36, 40–42. 10.1016/j.molcatb.2005.08.004. [DOI] [Google Scholar]
- Durand E.; Lecomte J.; Baréa B.; Piombo G.; Dubreucq E.; Villeneuve P. Evaluation of deep eutectic solvents as new media for Candida antarctica B lipase catalyzed reactions. Process Biochem. 2012, 47, 2081–2089. 10.1016/j.procbio.2012.07.027. [DOI] [Google Scholar]
- Pérez-Venegas M.; Tellez-Cruz M. M.; Solorza-Feria O.; López-Munguía A.; Castillo E.; Juaristi E. Thermal and Mechanical Stability of Immobilized Candida antarctica Lipase B: an Approximation to Mechanochemical Energetics in Enzyme Catalysis. ChemCatChem 2020, 12, 803–811. 10.1002/cctc.201901714. [DOI] [Google Scholar]
- Woudenberg-van Oosterom M.; van Rantwijk F.; Sheldon R. A. Regioselective Acylation of Disaccharides in tert-Butyl Alcohol Catalyzed by Candida antarctica Lipase. Biotechnol. Bioeng. 1996, 49, 328–33. [DOI] [PubMed] [Google Scholar]
- Lee S. H.; Koo Y. M.; Ha S. H. Influence of ionic liquids under controlled water activity and low halide content on lipase activity. Korean J. Chem. Eng. 2008, 25, 1456–1462. 10.1007/s11814-008-0239-3. [DOI] [Google Scholar]
- Shin D. W.; Mai N. L.; Bae S. W.; Koo Y. M. Enhanced lipase-catalyzed synthesis of sugar fatty acid esters using supersaturated sugar solution in ionic liquids. Enzyme Microb. Technol. 2019, 126, 18–23. 10.1016/j.enzmictec.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Jiang L.; Ye L. dan; Gu J.; Su W. ke; Ye Ye W. Mechanochemical enzymatic synthesis of 1,4-dihydropyridine calcium antagonists and derivatives. J. Chem. Technol. Biotechnol. 2019, 94, 2555–2560. 10.1002/jctb.6051. [DOI] [Google Scholar]
- Arcos J. A.; Hill C. G.; Otero C. Kinetics of the lipase-catalyzed synthesis of glucose esters in acetone. Biotechnol. Bioeng. 2001, 73, 104–110. 10.1002/bit.1042. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Liu Y. H.; Shang Z. R.; Hu H. C.; Zhang Z. H. Supported molybdenum on graphene oxide/Fe3O4: An efficient, magnetically separable catalyst for one-pot construction of spiro-oxindole dihydropyridines in deep eutectic solvent under microwave irradiation. Catal. Commun. 2017, 88, 39–44. 10.1016/j.catcom.2016.09.028. [DOI] [Google Scholar]
- Pérez-Venegas M.; Rodríguez-Treviño A. M.; Juaristi E. Dual Mechanoenzymatic Kinetic Resolution of (±)-Ketorolac. ChemCatChem 2020, 12, 1782–1788. 10.1002/cctc.201902292. [DOI] [Google Scholar]
- de Souza S. P.; de Almeida R. A. D.; Garcia G. G.; Leão R. A. C.; Bassut J.; de Souza R. O. M. A.; et al. Immobilization of lipase B from Candida antarctica on epoxy-functionalized silica: characterization and improving biocatalytic parameters. J. Chem. Technol. Biotechnol. 2018, 93, 105–111. 10.1002/jctb.5327. [DOI] [Google Scholar]
- Sheldon R. A.; Woodley J. M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801. 10.1021/acs.chemrev.7b00203. [DOI] [PubMed] [Google Scholar]
- José C.; Bonetto R. D.; Gambaro L. A.; Guauque Torres M. D. P.; Foresti M. L.; Ferreira M. L.; et al. Investigation of the causes of deactivation-degradation of the commercial biocatalyst Novozym 435 in ethanol and ethanol-aqueous media. J. Mol. Catal. B: Enzym. 2011, 71, 95–107. 10.1016/j.molcatb.2011.04.004. [DOI] [Google Scholar]
- Singh R. K.; Tiwari M. K.; Singh R.; Lee J. K. From protein engineering to immobilization: Promising strategies for the upgrade of industrial enzymes. Int. J. Mol. Sci. 2013, 14, 1232–1277. 10.3390/ijms14011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güvenç A.; Kapucu N.; Mehmetoğlu Ü. The production of isoamyl acetate using immobilized lipases in a solvent-free system. Process Biochem. 2002, 38, 379–386. 10.1016/s0032-9592(02)00099-7. [DOI] [Google Scholar]
- Dossat V.; Combes D.; Marty A. Efficient lipase catalysed production of a lubricant and surfactant formulation using a continuous solvent-free process. J. Biotechnol. 2002, 97, 117–124. 10.1016/s0168-1656(02)00021-4. [DOI] [PubMed] [Google Scholar]
- Adnani A.; Basri M.; Chaibakhsh N.; Ahangar H. A.; Salleh A. B.; Rahman R. N. Z. R. A.; et al. Chemometric analysis of lipase-catalyzed synthesis of xylitol esters in a solvent-free system. Carbohydr. Res. 2011, 346, 472–479. 10.1016/j.carres.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Ye R.; Hayes D. G.; Burton R.; Liu A.; Harte F. M.; Wang Y. Solvent-free lipase-catalyzed synthesis of technical-grade sugar esters and evaluation of their physicochemical and bioactive properties. Catalysts 2016, 6, 78. 10.3390/catal6060078. [DOI] [Google Scholar]
- Sarney D. B.; Vulfson E. N. Enzymatic Synthesis of Sugar Fatty Acid Esters in Solvent-Free Media. Enzym. Nonaqueous Solv. 2003, 15, 531–543. 10.1385/1-59259-112-4:531. [DOI] [Google Scholar]
- Lopresto C. G.; Calabrò V.; Woodley J. M.; Tufvesson P. Kinetic study on the enzymatic esterification of octanoic acid and hexanol by immobilized Candida antarctica lipase B. J. Mol. Catal. B: Enzym. 2014, 110, 64. 10.1016/j.molcatb.2014.09.011. [DOI] [Google Scholar]
- Serri N. A.; Kamaruddin A. H.; Long W. S. Studies of reaction parameters on synthesis of Citronellyl laurate ester via immobilized Candida rugosa lipase in organic media. Bioprocess Biosyst. Eng. 2006, 29, 253–260. 10.1007/s00449-006-0074-z. [DOI] [PubMed] [Google Scholar]
- Ha S. H.; Hiep N. M.; Lee S. H.; Koo Y. M. Optimization of lipase-catalyzed glucose ester synthesis in ionic liquids. Bioprocess Biosyst. Eng. 2010, 33, 63–70. 10.1007/s00449-009-0363-4. [DOI] [PubMed] [Google Scholar]
- Zaidi A.; Gainer J. L.; Carta G.; Mrani A.; Kadiri T.; Belarbi Y.; et al. Esterification of fatty acids using nylon-immobilized lipase in n-hexane: Kinetic parameters and chain-length effects. J. Biotechnol. 2002, 93, 209–216. 10.1016/s0168-1656(01)00401-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.