Abstract
Preclinical models of cancer have long been paramount to understanding tumor development and advancing the treatment of cancer. Creating preclinical models that mimic the complexity and heterogeneity of human tumors is a key challenge in the advancement of cancer therapy. About ten years ago, we created the mouse oral carcinoma (MOC) cell line models that were derived from 7, 12-dimethylbenz(a) anthracene (DMBA)-induced mouse oral squamous cell cancers. This model has been used in numerous investigations, including studies on tumor biology and therapeutics. We have seen remarkable progress in cancer immunology in recent years, and these cell lines, which are syngeneic to C57BL/6 background, have also been used to study the anti-tumor immune response. Herein, we aim to review the MOC model from its development and characterization to its use in non-immunological and immunological preclinical head and neck squamous cell carcinoma (HNSCC) studies. Integrating and refining these MOC model studies and extending findings to other systems will provide crucial insights for translational approaches aimed at improving head and neck cancer treatment.
Keywords: Oral squamous cell carcinoma, Carcinogen-induced cancer, Immunocompetent mouse models
1. INTRODUCTION
Pre-clinical models of cancers have a long history of use in laboratory investigations and have yielded significant insights into tumor development pathways and therapeutic approaches. In head and neck cancers, preclinical modeling approaches started with the hamster cheek pouch model [1] and have extended to current day carcinogen induced and transgenic mouse models.
The goals of modeling approaches are to allow experimental efforts to understanding mechanistic foundations of head and neck cancer development and therapeutic targeting, ideally coupled to the biology observed in patients. Several groups have reviewed existing head and neck cancer models and their advantages and disadvantages [2] [3]. Considerations in model development include fidelity with human head and neck squamous cell carcinoma (HNSCC) with regard to driver genomic alterations, in vivo biology, and therapeutic responses. In addition, as immunotherapy is now standard of care for recurrent/metastatic HNSCC patients, syngeneic models allowing the study of tumor-specific immunity are highly desired. Ideal syngeneic models also a contain tumor mutation burden as a source for neoantigens for immune targeting. As expected, each specific model system has its advantages and disadvantages that investigators need to consider in their pre-clinical studies.
Here, we review the mouse oral carcinoma (MOC) cell line model, from its development to its use in preclinical HNSCC studies. We first discuss development of the models, their genomic characterization and use in tumor growth and metastasis studies. As of April 2022, nearly 100 manuscripts have utilized MOC models and here, we summarize these studies as they relate to tumor biology and host anti-tumor response, including mechanisms of immune suppression and approaches for cancer immunotherapy.
2. MOC model development
In 2007, we started to focus on developing carcinogen-induced syngeneic oral carcinoma cell line models. At that time, there were few HNSCC syngeneic models and SCC VII, a cell line derived from a spontaneously arising abdominal wall squamous cell carcinoma, was the most commonly used surrogate. We knew that exposing wild-type C67BL/6 (B6) background mice to the carcinogen 7, 12-dimethylbenz(a) anthracene (DMBA) induced squamous cell carcinomas [4] [5] [6]. As this model tended to generate multi-focal lesions, we aimed to generate C57BL/6 derived cell line models from single primary oral cavity tumors. In this fashion, MOC1 was generated from a mucosal lip lesion, MOC22 from a buccal lesion and MOC2 from a floor of mouth mass (Figure 1). These three lines have been the “workhorses” for HNSCC preclinical studies and have a spectrum of phenotypes including indolent (MOC1, 22) and aggressive growth with lymphatic and lung metastasis (MOC2).
Figure 1:
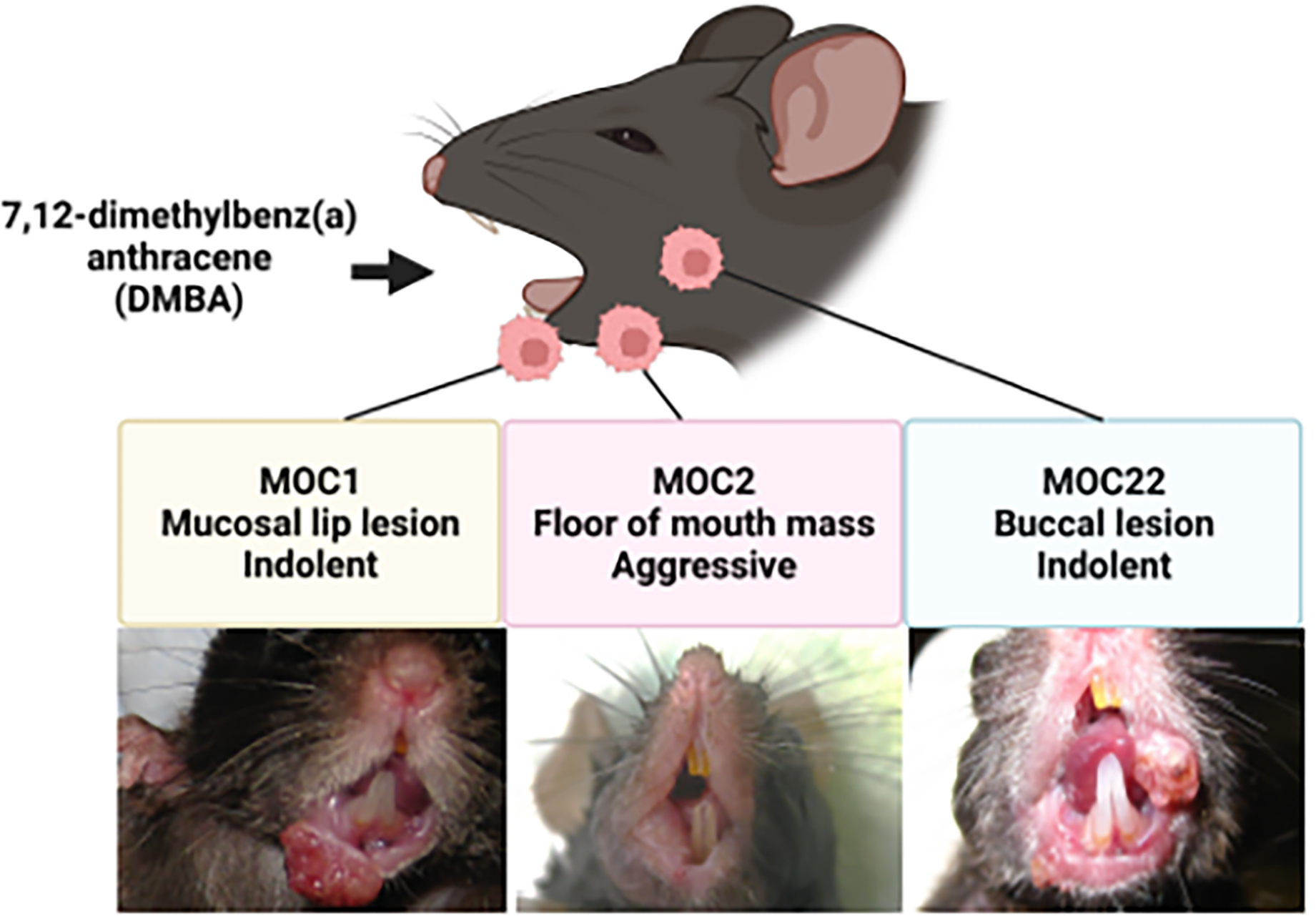
Development of MOC cells. Using 7, 12-dimethylbenz(a) anthracene (DMBA), MOC1 was generated from a mucosal lip lesion (left; original image), MOC2 from a floor of mouth mass (middle; original image), and MOC22 from a buccal lesion (right; representative image).
3. Tumor Growth and Metastasis Studies (non-immune focused)
In early studies, genomic and transcriptional characteristics of MOC lines were characterized using next-generation sequencing (NGS) and expression microarrays [7] [8]. Onken et al. observed conservation of human cancer driver pathway mutations, such as TP53, PI3K, MAPK, NOTCH and JAK/STAT [9]. Moreover, expression analysis revealed a signature of aggressive growth overlapping with human HNSCC, indicating that MOC models mirror some aspects of human disease. Recent genomic analysis of 4-nitroquinoline-1 oxide (4NQO) induced oral cancers has demonstrated the presence of a tobacco related genetic signature not present in DMBA induced cancers. [10]. Although MOC genomics have less overlap with a tobacco related signature, they harbor key driver mutations of human HNSCC (for example Tp53, Notch1, Fat1) and also bear Kras and Hras mutations found in some HNSCC patients.
The MOC models have been used by multiple investigators to examine factors that drive aggressive growth and metastasis. Several tumor intrinsic or tumor extrinsic (microenvironment) factors appear to drive these phenotypes. Deleted in malignant brain tumors 1 (DMBT1) knockout mice had increased MOC1 tumor growth and incidence of small satellite tumors compared to tumors in DMBT1 wild-type mice, supporting the finding that tumor-mediated suppression of DMBT1 in normal tissues surrounding the tumor leads to tumor invasion and micro-metastasis [11]. Knockout of neutrophil elastase (NE), essential for tumor cell seeding by activating Src/PI3K-dependent Akt signaling, inhibited MOC2 metastasis [12]. Chen et al. demonstrated that overexpression of nerve growth factor receptor (NGFR) drives ESM1-induced migration, invasion, and metastasis using MOC2 cells [13]. MOC cells exhibit either low-CD44 expression, associated with an indolent phenotype, or high-CD44 expression, associated with an aggressive phenotype, similar to human HNSCC [14]. Increased ERK phosphorylation and MAPK signaling downstream of high CD44 in more aggressive tumors led our group to examine trametinib, a MEK inhibitor, in a window of opportunity HNSCC clinical trial [15]. Expression of CD271, which is associated with activation of Slug, an epithelial to mesenchymal transition (EMT)-related transcription factor [16], is restricted to a subset of CD44+ human HNSCC cells. CD44+ CD271+ MOC2 cells were found to be more tumorigenic than CD44− CD271− MOC2 cells [17]. Moreover, early lymph node metastasis was increased following oral inoculation of MOC2 overexpressing CD271. Thus, fundamental tumor growth and metastasis related properties of these molecules and candidate therapeutic targeting strategies were defined in part using MOC models.
Caspase-8, a key factor inducing the extrinsic apoptosis pathway and suppressing necroptotic cell death, is frequently mutated (~10%) in HNSCC [18], and mutations are associated with poor prognosis [19]. Caspase-8 knockdown in wildtype Casp8 MOC1 cells rendered them sensitive to induction of necroptosis with a second mitochondria-derived activator of caspase (SMAC) mimetic, especially in the presence of radiation [19]. These data suggest that HNSCC harboring inactivating caspase-8 mutations may be effectively treated with a combination of radiotherapy and a SMAC mimetic.
Angiogenesis is a key process that is required for tumor growth and metastasis. High expression of ephrin type B receptor 2 (Ephb2), which activates STAT3, is associated with poor prognosis in HNSCC patients [20]. Ephb2 promotes tumor angiogenesis by extracellular vesicles derived from HNSCC cells, and aggressive MOC2 cells had higher blood vessel density than other tumors including MOC1 [20]. The Ephb family is involved in other aspects of tumor growth in addition to angiogenesis. Low expression of Ephrin type B receptor 3 (Ephb3) protein, as is found in MOC1 and MOC2, was associated with tumor growth and migration as well as weakened response to PI3K signaling inhibition in human HNSCC [21].
Cetuximab, which targets the epidermal growth factor receptor (EGFR), is the only approved targeted therapy for head and neck cancer, but response rates remain low. Therefore, elucidating mechanisms of EGFR signaling modulation and anti-EGFR therapy resistance is desired to improve EGFR-targeting response rates in HNSCC. Cetuximab was found to have antibody-dependent cellular cytotoxicity (ADCC) activity in human EGFR expressing MOC1 and MOC2 tumors (MOC1- and MOC2- huEGFR, respectively) but without significant tumor suppression or radiosensitization [22]. However, combined cetuximab and radiation therapy (RT) enhanced ADCC and antitumor activity of cetuximab by increasing natural killer (NK) cell infiltration [22]. Although tumorigenesis of MOC2 is associated with EGFR signaling, MOC2 was resistant to EGFR tyrosine kinase inhibitors [23]. Inhibiting the P2Y2 nucleotide receptor, which induces EGFR transactivation, suppressed MOC2 tumorigenesis by weakening nucleotide-induced intracellular Ca2+ responses and ERK1/2 activation [24].
4. Immunosuppressive Tumor Microenvironment Studies
Immunosuppression in the tumor microenvironment is a critical hurdle to overcome for successful cancer immunotherapy. MOC models have been examined for immunosuppressive cellular mediators leading to immune escape and approaches to circumvent these pathways.
4.1. Myeloid derived suppressor cells
Myeloid derived suppressor cells (MDSCs) are a major cellular immunosuppressive population in HNSCCs. MDSCs consist of two main subsets: granulocytic/polymorphonuclear MDSC (gMDSC or PMN-MDSC) and monocytic MDSC (mMDSC or M-MDSC). PMN-MDSCs increase during tumor progression, resulting in progressive immune cell dysfunction. Depletion of PMN-MDSC recovered antigen-specific T cell responses in tumor infiltrating lymphocytes (TIL) and draining lymph nodes in MOC1. Analysis of TCGA data, showed that 60% of HNSCCs have high expression of CTLA-4 and MDSC-related chemokines and MDSC-rich gene profiles. Depletion of PMN-MDSC augmented the antitumor T cell response of anti-CTLA-4 therapy with formation of immune memory in MOC1 [25].
CXCR2+ PMN-MDSCs suppress the killing ability of tumor-infiltrating lymphocytes and represent the most abundant myeloid cell subset in MOC1 [26]. Although CXCR2 inhibition by itself had no anti-tumor effect, combining with immune checkpoint inhibition or adoptive T cell transfer delayed MOC1 growth [26]. In addition, inhibition of CXCR2+ PMN-MDSCs by CXCR1/2 inhibition augmented the anti-tumor effect of adoptively transferred NK cells in MOC2 by enhancing the infiltration and activation of NK cells [27]. Thus, tumor-infiltrating CXCR2+ PMN-MDSCs can inhibit anti-tumor responses.
Inhibition of Semaphorin4D (Sema4D), a member of a family of transmembrane and soluble proteins that guide axonal sprouting, reduced PMN-MDSC-derived immune suppression and led to activation and infiltration of CD8 T cells with enhanced IFN-γ production MOC1 tumor TIL [28]. This effect resulted in growth delay and prolonged survival of MOC1-bearing mice in combination with immune checkpoint blockade [28].
M-MDSCs upregulate caspase-1 activity and promote proliferation of human HNSCC cells [29]. Adoptive transfer of caspase-1 null bone marrow cells reduced MOC1 growth in T cell depleted mice [29]. Thus, caspase-1 in M-MDSCs leads to direct tumor growth independent of T cells. Taken together, MOC model studies confirmed and extended the crucial role of MDSCs in anti-cancer immune responses.
4.2. Tumor-associated Macrophages
Macrophages exist in a diverse array of phenotypic and functional categories across different pathobiological states with the M1 and M2 states representing extreme ends of this spectrum [30]. In brief, M1 macrophages are antitumor macrophages, while M2-polarized macrophages consist of tumor-associated macrophage (TAM) that contribute to tumor progression and suppression of antitumor immunity. Strategies for polarizing M2 TAMs to M1 are being actively examined, as this would likely improve response to immunotherapy. STAT3 inhibition was found to decrease M2 macrophages and activated CD8+ T cell recruitment, and the combination of STAT3 inhibition with Toll-like receptor (TLR) engagement triggered an RT enhanced antitumor response by activating M1 macrophage and CD8 T cells in MOC2 [31]. Ephb4-ephb2 inhibition with RT increased antitumor effect in MOC2 and the ratio of M1 macrophage to M2 TAMs in human HNSCC [32]. TLR7 agonist treatment of TAMs favored polarization towards M1 macrophages and enhanced tumor-specific CD8 T cell responses in HNSCC cells, and the combination of TLR7 agonism with anti-PD-1 therapy was effective in MOC1 [33].
4.3. Regulatory T cells
Regulatory T cells (Treg), a CD4+ T cell population that suppresses autoimmunity, were discovered in the 1990s [34]. Although Tregs play essential roles in maintaining immune self-tolerance and homeostasis, Tregs obstruct antitumor immunity in tumor bearing hosts and contribute to tumor development and progression. Two reports have suggested that increased infiltration by Tregs is associated with poor prognosis in HNSCC patients [35] [36]. MOC2 is more aggressive, has increased FOXP3+CD4+ Tregs infiltration and reduced MHC class I expression and CD8+ T cell infiltration compared to MOC1 [37]. Depletion of Tregs was found to attenuate MOC2 tumor growth [37]. Although combination therapy with RT, anti-PD-L1 and anti-TIM-3 enhanced anti-tumor effects against MOC2, these effects did not persist [38]. However, Treg depletion induced an anti-tumor immune memory response and tumor rejection, indicating inhibition of Tregs were crucial for memory immune responses [38].
A triple combination of RT, Treg depletion, and anti-CD137 DC agonism induced strong CD8 T cell responses through activation of DC in tumor-draining lymph nodes in RT-resistant MOC2 tumors [39]. In a separate report, this same group showed that anti-CD25 mediated Treg depletion combined with RT reduced MOC2 tumor growth but did not lead to MOC2 tumor rejection [40]. Moreover, targeting STAT3 using an anti-sense oligo decreased Tregs and delayed tumor growth when used in combination with RT, demonstrating that reducing Tregs via STAT3 targeting improved therapeutic response to RT [40].
4.4. Cytokines
Numerous cytokines/chemokines and growth factors contribute to creating an immunosuppressive tumor microenvironment. TNF-related apoptosis-inducing ligand (TRAIL) is a member of the TNF family of proteins that can induce apoptotic cell death. However, nonapoptotic TRAIL signaling via a caspase-8 independent mechanism was associated with tumor metastasis and invasion through nuclear factor-κB (NF-κB)-dependent release of immunosuppressive cytokines[41]. Enforced expression of HNSCC-associated caspase-8 mutants showed that some mutants were capable of mediating TRAIL-induction of immunosuppressive cytokines/chemokines CXCL1, IL-6, or IL-8 [42]. Furthermore, MOC1-caspase-8 knockout cells expressing wild-type but not mutant caspase-8 increased intra-tumoral T cells and NKT cells [42]. Thus, HNSCC-associated caspase-8 mutants contribute to tumorigenesis via loss of function but also alter the tumor microenvironment in HNSCC via TRAIL-induced immunosuppressive cytokines.
Galectin-1 (Gal1) is part of a carbohydrate-binding protein family, is highly overexpressed in HNSCC and is associated with poor prognosis [43]. Tumor-secreted Gal1 mediated immune evasion by interrupting T cell migration into tumors and upregulating PD-L1 expression [44]. Gal1 knockout enhanced the antitumor effect of anti-PD-1 blockade with increased T cell infiltration into MOC2 tumors [44].
On the other hand, the chemokine CXCL14 was found to act as a tumor infiltrating lymphocyte modulator and tumor suppressor in HNSCC. Parikh et al. used MOC1 and MOC2 to investigate the tumor suppressive mechanism of CXCL14 and found that CXCL14 expression was higher in non-metastatic MOC1 compared to metastatic MOC2 [45].
5. Cancer Immunotherapy Studies
A fundamental approach of cancer immunotherapy is elicitation and expansion of tumor-reactive effector immune cells. Furthermore, immunomodulators that convert “cold” tumors into “hot” immunotherapy sensitive targets are desired. Many strategies for effective cancer immunotherapy have been examined in the MOC model.
5.1. Immune Checkpoint Blockade
Immune checkpoint inhibitors (ICIs), that inhibit immune evasion and enhance tumor antigen specific T cell activity, have revolutionized clinical management of various types of tumors including HNSCC. However, many patients don’t response including due to tumor cell heterogeneity derived resistance. Zhou et al. developed an anti-PD1 resistant MOC1 model (MOC1-esc1) [46]. Compared to the parental anti-PD1 sensitive MOC1, scRNASeq and scTCRSeq revealed distinct dynamics of CD8 TILs in ICI responsive and unresponsive tumors. Understanding T cell dynamics in resistant tumors and how tumor cell heterogeneity can be overcome are key hurdles for immunotherapy success.
Total tumor burden is another factor involved in the resistance to ICI. Pai et al., using mouse tumor models including MOC1, demonstrated that the combination therapy of anti-CTLA-4 and anti-PD-1 had lower antitumor immunity and higher IFN-γ production and sensitivity to T cell apoptosis in the low tumor burden (LTB) state than in the high tumor burden (HTB) state [47]. Upregulated IFN-γ production in the LTB state led to immune resistance through apoptosis of the dominant tumor-specific T cells via activation-induced cell death (AICD), showing the paradoxical role of IFN-γ in cancer immunotherapy.
To enhance the effects of ICIs, various approaches have been considered. Giardi et al. developed soluble microneedles (MN) as a novel local ICI delivery strategy using MOC1 and other mouse HNSCC cell lines [48]. Anti-CTLA-4 therapy using MN had a high response rate that was dependent on CD8 T cells and cDC1s with the beneficial effect of reduced immune related adverse events (irAEs) in MOC1-bearing mice [48].
Recently, neoadjuvant ICIs prior to surgery have been examined in several HNSCC clinical trials with the goal to prevent recurrent and metastasis [49]. Neoadjuvant immunotherapy has been proposed to generate systemic immune response against multiple tumor antigens, including those in micrometastases. In pre-clinical surgical approaches using MOC1 and MOC22 models, neoadjuvant anti-PD-1 therapy prolonged survival with formation of effective immunologic memory against re-challenge of tumor cells lacking dominant antigens compared to surgery alone or adjuvant anti-PD-1 therapy [50]. These data provide strong rationale and mechanistic insights on neoadjuvant immunotherapy for HNSCC. On the other hand, Sharon et al. showed combinational effects of adjuvant therapies in a MOC1-ova surgical model [51]. Although adjuvant anti-PD-1 therapy after surgical resection was ineffective, local delivery of antigen-specific T cells into the resection cavity was effective. Collectively, strategies for cancer immunotherapy have been developed not only to treat patients after recurrence or metastasis but also to prevent these deadly occurrences.
5.2. Cancer Vaccines
Cancer antigen vaccination targeting is directed against two major groups: tumor-associated antigens (TAA) and neoantigens which are tumor specific antigens (TSA). Development of next generation genomic technologies have made cancer mutation specific (neoantigen) targeted therapy without autoimmune side effects that may arise with targeting self-antigens. In MOC lines, high immunogenicity MOC22 had a higher mutation burden than in low immunogenicity MOC2 [52]. Among the mutations in MOC22, a mutated ICAM1 protein (mICAM1) was identified as a bona fide Class I-Kb restricted neoantigen. In addition, the ICAM1 mutation also contributed to a Class II epitope that was critical for successful vaccination by inducing both a CD4+ and CD8+ T cell response [53]. An alternative approach with tumor membrane vesicle (TMV) vaccine plus anti-PD-1 therapy was effective against MOC1 and MOC2 [54]. A biomaterials-based mesoporous silica rod (MSR) cancer vaccine targeting HPV-16 E7 had efficacy against MOC2 with enforced expression of E6/E7 [55] and an adenovirus vaccine targeting HPV-6 E6 induced antitumor T cell response against MOC1 engineered for E6 expression [56]. Together, these studies illustrate utility of the MOC models in both TSA and TAA vaccine approaches.
5.3. Adoptive Cell Therapy
Adoptive Cell Therapy (ACT)-based cancer immunotherapy is also an optimal strategy to efficiently introduce expanded tumor-reactive immune cells. Knochelmann et al. evaluated the characteristics of TILs from MOC2 and MOC22 compared to HNSCC patient TILs for the translational research of TIL ACT [57]. These TILs could be expanded ex vivo and were similar to human TILs in the high expression of the exhausted markers and their functional dynamics. ACT of NK cells expressing CARs that target specific tumor antigens represents a promising approach and has recently been established in MOC models. High-affinity NK cells (haNKs) engineered to express a CAR specific against PD-L1 suppressed tumor growth of MOC1 [58]. Moreover, the potent antitumor ACT effect of haNKs, engineered to express high-affinity CD16, endoplasmic reticulum (ER) -retained IL-2 and a CAR targeting PD-L1, was also demonstrated using MOC1 [59].
5.4. Immune System Modulators
Innate immune stimulators such as toll like receptor (TLR) agonists and stimulator of interferon genes (STING) agonists have been investigated as treatments that break tumor immune tolerance and activate anti-cancer immunity. Intra-tumoral injection of TLR7 and TLR9 agonists suppressed tumor growth and enhanced the effect of anti-PD-1 blockade by decreasing TAMs and promoting the infiltration of antigen-specific CD8 T cells in MOC1 [33].
Activating STING by noncanonical cyclic dinucleotide (CDN) decreased tumor progression in MOC1 but not MOC2 [60]. MOC2 had resistance for STING ligand therapy compared to MOC1, which was associated with IL-10 production from MOC2 [61]. Tan et al. showed using several HNSCC cell lines including MOC2-E6/E7 that SOX2, an oncoprotein of HNSCC, inhibited Type I IFN activation by enhancing autophagy-dependent degradation of STING [62]. HPV16-E7 immune evasion was shown to be effected by NLRX-1 mediated STING degradation in MOC2-E6/E7 [63], while intratumor injection of STING-loaded biomaterials suppressed MOC2-E6/E7 growth [64].
Therapeutic cytokine therapy also modulates tumor microenvironments and may enhance antitumor immunity. IL-2 has been used clinically to activate T and NK cells in several settings. IL-2 and IL-2 receptor complexes with longer half-lives have shown effects on immune activity and in combination with ICI and RT in MOC2 [65]. While dexamethasone was known as complement inhibitor and inhibition of complement C3a and C5a signaling suppressed antitumor immunity in MOC2 [66], IL-2 and IL-2 receptor complexes bypassed dexamethasone-derived immunosuppression in MOC1 [67]. Therapeutic IL-12 therapy is known to improve suppressive tumor microenvironments in preclinical models, but has failed due to severe adverse events in clinical trials [68]. Administration of a tumor-targeted IL-12 antibody fusion protein (NHS-rmIL-12), designed to eliminate adverse systemic effects activated antitumor immunity against MOC22 [69]. As determine by single-cell transcriptomics, the expression pattern of IL-12R in MOC22 was similar to that of human HNSCC, consistent with human pathway parallels in this preclinical model [69].
Tumor and immune metabolic states are well recognized factors that influence antitumor immunity [70]. The diabetes drug metformin has been found to be valuable in protecting against cancer and demonstrated enhanced effects with cancer immunotherapy [71]. Munoz et al. showed that a cancer vaccine and metformin combination enhanced the antitumor effect and decreased lung metastasis compared with cancer vaccine alone in MOC2 [72].
5.5. Targeted Therapy
Resistance to cancer immunotherapy may involve a variety of immunosuppressive signaling pathways and disabling them may lead to effective immunotherapy. The combination of anti-PD-L1 blockade and regorafenib, an oral multi kinase inhibitor that targets VEGFR1-3, TIE2, PDGFR-β, FGFR, KIT, RET, and RAF, was effective in activating CD8 T cells, polarization of M1-like macrophages, and decreasing MDSC and Treg in TILs and tumor-draining lymph node of MOC1 [73]. Enhancer of zeste homolog 2 (EZH2) -targeting therapy enhanced antigen presenting ability by upregulating MHC class I expression and synergized with anti-PD-1 therapy in MOC1-esc1 [74]. Aryl hydrocarbon receptor (AhR)-deficient MOC1 cells reduced expression of multiple immune checkpoint molecules compared to control of MOC1 cells and were rejected in C57BL/6 mice [75]. Inhibition of cIAP1/2 and XIAP, which are essential components of TNF receptor signaling pathway, augmented antitumor efficacy of anti-PD-1 therapy plus radiation therapy or chemotherapy and killing ability of T cells in MOC1 [76] [77]. Knockdown of FAT1 circular RNA (circRNA), a continuous loop single stranded RNA connecting 5’ and 3’ ends, in MOC1 enhanced CD8+ T cell infiltration into tumor and the effect of anti-PD-1 therapy [78].
Mammalian target of rapamycin (mTOR) has been implicated in multiple important intracellular pathways including the MAPK, phosphoinositide-3-kinase (PI3K) and NF-κB circuits. Activation of these signaling pathways contributes not only to tumor development and treatment resistance but also the immunosuppressive tumor microenvironment. mTOR inhibition and MEK1/2 inhibition had distinct immune related effects against immunogenic MOC1 and poorly immunogenic MOC2 [79, 80] [81]. The PI3K/mTOR inhibitor rapamycin improved survival of both MOC1 and MOC2 and MEK inhibitor PD901 improved survival of MOC2 but not MOC1[79]. Rapamycin resulted in activation of MAPK pathway and upregulation of CD44 expression but PD901 reduced CD44 expression and suppressed tumor growth in MOC2 [80]. Although PD901 suppressed IFN-γ production and PD-L1 expression on MOC1 [81], rapamycin enhanced IFN-γ production and activated CD8 T cells in TILs, which lead to additional anti-tumor effects with anti-PD-L1 therapy in MOC1 [79] [80]. Meanwhile, inhibition of fibroblast growth factor receptor (FGFR) which is upstream of MAPK signaling upregulated MHC class I and class II in MOC1 and enhanced the antitumor activity of T cell-based immunotherapy [82].
A significant percentage of HNSCCs have mutations of the PI3K signaling pathway and PI3K inhibition is a desirable goal for most HNSCC patients. Although pik3cg knockout did not affect tumor growth of MOC2, it increased expression of PD-1 and release of IFN-γ and IL-17 in TILs [83]. PI3Kδ/γ inhibitor changed the tumor microenvironment and augmented the antitumor effect of anti-PD-L1 therapy in MOC1 but not MOC2 [84]. However, high doses of a PI3Kδ/γ inhibitor canceled the effect of anti-PD-L1 blockade due to suppression of antigen-specific T cell function in MOC1[84]. HNSCC with intact pik3ca have aberrant PI3K/AKT/mTOR signaling due in part to phosphorylation of human epidermal growth factor receptor 3 (HER3) and PI3K recruitment [85]. Although MOC1 was resistant to HER3 inhibitors due to Hras mutation, the combination with anti-PD-1 blockade improved survival in MOC1[85].
Many cancer cells have lost G1 checkpoint function leading to uncontrolled proliferation with a dependence on the G2/M checkpoint. Thus, targeting this cell cycle checkpoint is a promising therapeutic approach. WEE1 is a tyrosine kinase that controls the G2/M cell cycle, and inhibition of WEE1 can drive tumor cell death. In MOC1-SIINFEKL engineered cell line, Wee1 inhibition enhanced antitumor immunity and had combinatorial impact with anti-PD-1 therapy [86]. While cancer cells stimulate G2/M cell cycle checkpoint in response to granzyme B and RT, inhibition of WEE1 combined with RT enhanced responses of ICI and antitumor immunity in MOC1 by augmenting CD8 T cell response [87]. Furthermore, inhibition of WEE1 had a synergistic effect with NK cellular therapies. The combination of WEE1 kinase inhibition and adoptive transfer of NK cells enhanced tumor growth control and prolonged survival in MOC2 by increasing DNA damage and sensitivity to granzyme B [88]. Targeting of p53, which has a panoply of effects on cancer cells, with scL-53, a cationic liposome nanocomplex enveloped anti-transferrin receptor single-chain antibody fragment (scL), had an anti-tumor effect in combination with anti-PD-1 in MOC1 tumor bearing mice [89].
In HPV-associated HNSCC, HPV E5 was associated with poor response of ICI in MOC2 engineered to express HPV-E5 and E5 reduced expression of HLA in HNSCC patients [90]. The antiviral rimantadine which can inhibit E5 demonstrated significant antitumor effects against an HPV-E5-expressing tumor cells and upregulated MHC on multiple tumor cells suggesting that E5 may be a novel target in HPV-associated HNSCC [90].
Near-infrared photoimmunotherapy (NIR-PIT) is a novel cancer treatment that involves conjugation of a tumor targeted monoclonal antibody with the silica-phthalocyanine dye photoabsorber, IRDye700DX (IR700) that upon photoactivation, induces tumor cell death while sparing normal tissues. Notably, NIR-PIT elicits an antitumor immune response in the host by inducing immunogenic cell death [91]. NIR-PIT targeting CD44 showed potent antitumor effects on both immunogenic MOC1 and poorly immunogenic MOC2 [92]. CD44-targeted NIR-PIT combined with anti-CTLA-4 therapy showed stronger tumor growth inhibition than either alone in MOC1 [93]. Although NIR-PIT targeting CTLA-4 against MOC2-luc delayed tumor growth [94], CD44 and CTLA-4 dual-targeted NIR-PIT did not show synergy in MOC2-luc [95]. On the other hand, NIR-PIT targeting CD44 enhanced the antitumor effect of anti-PD-1 blockade by increasing the number of activated CD8 T cells in TILs, indicating that it could improve low immunogenicity of MOC2-luc [96]. CD44-targeted NIR-PIT in combination with CD25-targeted NIR-PIT or IL-15 treatment enhanced the therapeutic effects compared to CD44-targeted NIR-PIT monotherapy in MOC1 [97] [98]. As Okada et al. demonstrated the efficacy of endoscopic CD44-targeted NIR-PIT therapy for MOC2-luc [99], further pre-clinical studies may lead this novel technology to broader clinical application.
5.6. Chemotherapy and Radiotherapy
Chemotherapy, which induces immunogenic cell death, has been widely explored as a promising strategy for effective immunotherapy [100]. Cisplatin plus anti-PD-1 therapy induced immunogenic cell death was evident with enhanced calreticulin, MHC-class I, and PD-L1 in MOC1 [101]. Concurrent therapy of moderate-dose cisplatin and anti-PD-1/PD-L1 blockade augmented antitumor impact without reducing the number of intra-tumoral immune stimulatory or suppressor cells in MOC1 [102].
RT plays a crucial role in HNSCC treatment and has been investigated in MOC models. Cunningham et al. demonstrated that ultra-high dose rate radiotherapy (FLASH) approach reduced toxicity without compromising efficacy against MOC1 and MOC2 [103]. Moreover, RT has the potential to enhance immune responses in both innate and adaptive immune arms and could be a reasonable immunoadjuvant because of its already widespread use in HNSCC. High-dose RT enhanced priming of antigen-specific T cells in MOC1-ova [104]. Moreover, high-dose hypo-fractioned RT increased CD8+ T cell activation, IFN-γ production, and MHC class I expression and reduced gMDSC accumulation in MOC1, resulting in better synergy with anti-PD-1 therapy compared to low-dose daily fractionated RT [105]. RT activated intratumoral cDC1s and CD8+ T cells in MOC1 but not MOC2 [106]. RT activated a Type I IFN response with STING involvement in MOC2 [107], but the combination of RT and STING agonist showed no synergistic effect in MOC1 which expresses minimal STING, indicating that STING is important to augment effect of RT [108]. Knitz et al. demonstrated using HNSCC cells including MOC2 that RT resulted in activation and proliferation of effector T cells through STAT1 phosphorylation and CXCL9/10 release [109]. Furthermore, the FMS-like tyrosine kinase 3 ligand (FLT3) secreted by NK cells was crucial for maintaining responsiveness to radiotherapy in MOC2 in the presence of anti-NK cell antibody [110]. NK cells were essential for the antitumor immune response to RT combined with ataxia telangiectasia and Rad3-related inhibitor (ATRi) in MOC2 [111]. Thus, treatment using antitumor NK cell activity may be beneficial even if an effective anti-tumor T-cell response is not achieved.
Definitive CRT is the treatment of choice for many HNSCC patients. Although CRT is most effective when anti-tumor immunity is enhanced, CRT may suppress systemic immune responses. Hanoteau et al. examined the immunomodulatory effect of cyclophosphamide (CTX) and the small molecule inducible nitric oxide synthase (iNOS) inhibitor L-n6-(1-iminoethyl)-lysine (L-NIL) to enhance CRT outcome in MOC2 [112]. CTX/L-NIL therapy increased the number of tumor antigen-specific T cells and M1 macrophages in TIL and decreased gMDSCs, resulting in improving CRT efficacy [112] [113]. CTX/L-NIL combined with ICI and RT treated mice rejected both MOC2 and MOC2-E6E7 [113]. Understanding the combination of immunomodulatory chemotherapy or CRT with immunotherapy will enhance approaches to achieve complementary immune activation.
Pembrolizumab alone or combined with cisplatin and 5-fluorouracil chemotherapy is the current standard of care in treatment of first-line recurrent/metastatic HNSCC [114]. These encouraging advances have been tempered by several failed trials where ICI has been combined with chemoradiotherapy or radiotherapy alone. For example, the Javelin Head and Neck 100 Phase III clinical trial showed that adding avelumab to cisplatin-radiotherapy did not improve outcomes compared to chemoradiotherapy (CRT) [115]. In another negative trial, combining EGFR inhibition with anti-PD-L1 therapy concomitant with radiotherapy in locoregionally advanced HNSCC was found to be inferior to platinum-based chemoradiotherapy in the phase III GORTEC-REACH trial [116]. Thus, how preclinical findings above relate to the clinical trial data to date is an important question to clarify. These clinical trial failures suggest that we need a deeper understanding and consideration of novel therapeutics and approaches including by testing in appropriate preclinical model systems. For example, recent findings from Saddawi-Konefka et al. show that ablation of lymph node tissue directly impacted ICI therapeutic response [117]. These findings may represent the explanation for the disappointing clinical trial results combining ICI with radiation or chemoradiation. However, they also illustrate that preclinical systems can highlight relevant immunobiology to consider in clinical trial.
6. Conclusion and Future perspectives
In recent years, remarkable progress in cancer biology and therapeutics have happened with the development of genomic technologies and the successes of cancer immunotherapy. As a result, numerous new clinical trials are ongoing in search of better treatments for patients. However, a high rate of human clinical trials have failed despite promising results in mouse models, or some clinical trials are being conducted without strong pre-clinical evidence. How to balance the use of faithful pre-clinical models and clinical translation is a key question in the field. Understanding the similarities and differences with each model and human tumors will enable the most optimal use of HNSCC preclinical models. We need to complement each model with objective prediction of effects in clinical practice, leading to translation of HNSCC treatment from bench to bedside.
Figure 2:
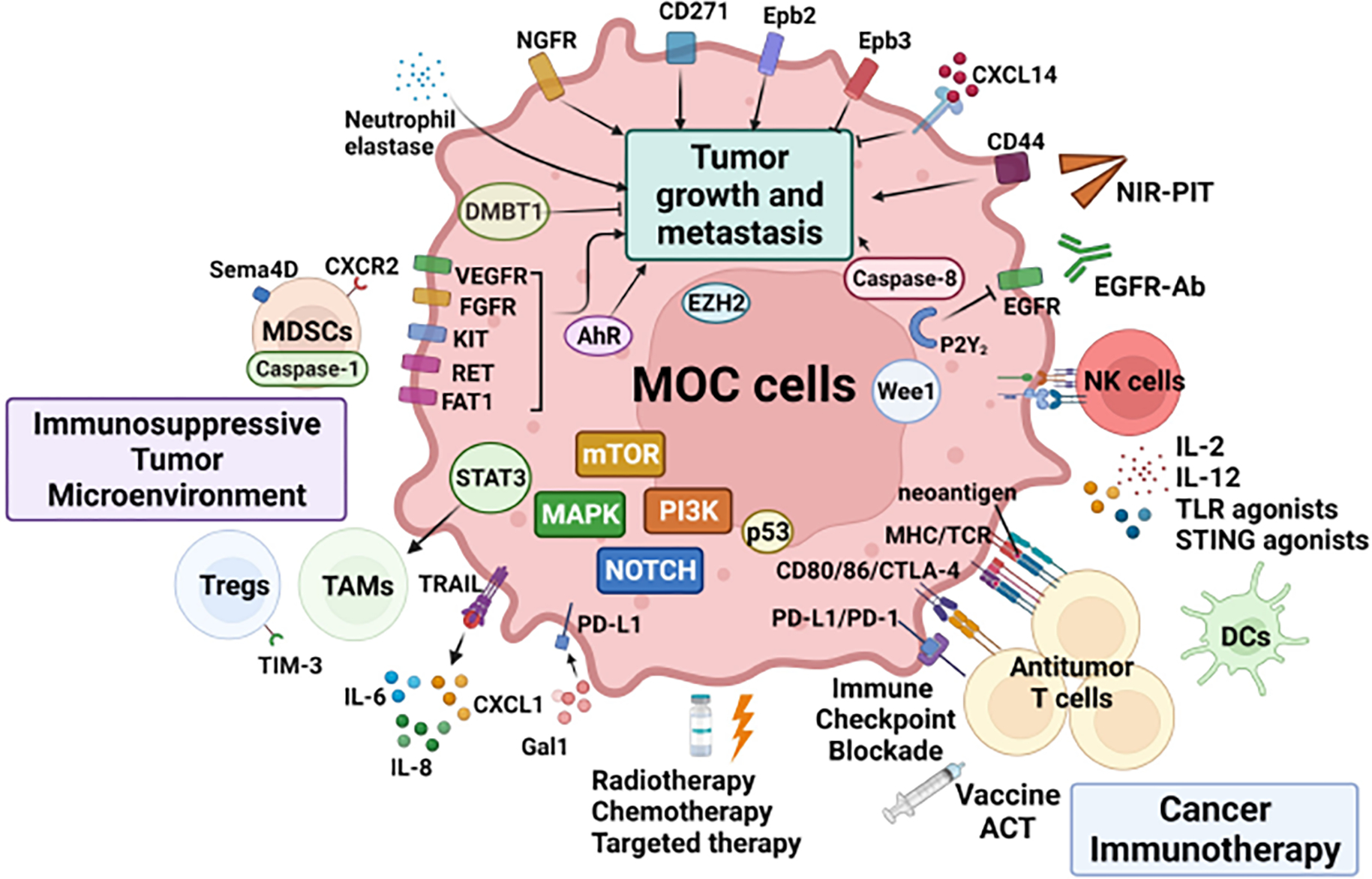
Graphical Abstract of MOC model studies.
Highlights.
The mouse oral carcinoma (MOC) cell line models were developed from 7, 12-dimethylbenz(a) anthracene (DMBA)-induced primary mouse oral squamous cell cancers over 10 years ago.
MOC models have been used in numerous head and neck squamous cell cancer (HNSCC) investigations, including studies on oral cancer tumor biology, host anti-tumor immune responses and therapeutic approach.
Three cell lines (MOC1, 2, and 22) have key driver mutations of human HNSCC and different tumor biological characteristics, therapeutic sensitivity, and immune response.
Understanding and integrating MOC model with human tumor biology may facilitate optimization of various therapeutic approaches and clinical translation for HNSCCs.
Acknowledgements:
We thank all members of the Uppaluri lab (past and present) for development and discussions on the MOC model. We aimed to be comprehensive in this review and apologize in advance for oversight of any studies.
Role of Funding Source:
RU is funded by NIH/NCI/NIDCR U01DE029188 and NIH/NIDCR R01DE027736. The funding source had no role in the preparation of this review.
Footnotes
Conflict of interest statement
RU serves on a Merck head and neck cancer advisory board. The MOC models developed by RU have been filed with the Washington University Office of Technology Management and are licensed for distribution by Kerafast. All other authors have no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference:
- [1].Salley JJ. Experimental carcinogenesis in the cheek pouch of the Syrian hamster. J Dent Res. 1954;33:253–62. [DOI] [PubMed] [Google Scholar]
- [2].Mery B, Rancoule C, Guy JB, Espenel S, Wozny AS, Battiston-Montagne P, et al. Preclinical models in HNSCC: A comprehensive review. Oral Oncol. 2017;65:51–6. [DOI] [PubMed] [Google Scholar]
- [3].Li Q, Dong H, Yang G, Song Y, Mou Y, Ni Y. Mouse Tumor-Bearing Models as Preclinical Study Platforms for Oral Squamous Cell Carcinoma. Front Oncol. 2020;10:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ku TK, Nguyen DC, Karaman M, Gill P, Hacia JG, Crowe DL. Loss of p53 expression correlates with metastatic phenotype and transcriptional profile in a new mouse model of head and neck cancer. Mol Cancer Res. 2007;5:351–62. [DOI] [PubMed] [Google Scholar]
- [5].Lin LM, Chen YK, Lai DL, Huang YL. Minimal arecaidine concentrations showing a promotion effect during DMBA-induced hamster cheek pouch carcinogenesis. J Oral Pathol Med. 1996;25:65–8. [DOI] [PubMed] [Google Scholar]
- [6].Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 2009;4:1350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chalivendra V, Kanchi KL, Onken MD, Winkler AE, Mardis E, Uppaluri R. Genomic analysis to define molecular basis of aggressiveness in a mouse model of oral cancer. Genom Data. 2015;3:61–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li H, Ngan HL, Liu Y, Chan HHY, Poon PHY, Yeung CK, et al. Comprehensive Exome Analysis of Immunocompetent Metastatic Head and Neck Cancer Models Reveals Patient Relevant Landscapes. Cancers (Basel). 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Onken MD, Winkler AE, Kanchi KL, Chalivendra V, Law JH, Rickert CG, et al. A surprising cross-species conservation in the genomic landscape of mouse and human oral cancer identifies a transcriptional signature predicting metastatic disease. Clin Cancer Res. 2014;20:2873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang Z, Wu VH, Allevato MM, Gilardi M, He Y, Luis Callejas-Valera J, et al. Syngeneic animal models of tobacco-associated oral cancer reveal the activity of in situ anti-CTLA-4. Nat Commun. 2019;10:5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Singh P, Banerjee R, Piao S, Costa de Medeiros M, Bellile E, Liu M, et al. Squamous cell carcinoma subverts adjacent histologically normal epithelium to promote lateral invasion. J Exp Med. 2021;218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Deryugina E, Carre A, Ardi V, Muramatsu T, Schmidt J, Pham C, et al. Neutrophil Elastase Facilitates Tumor Cell Intravasation and Early Metastatic Events. iScience. 2020;23:101799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen C, Shin JH, Eggold JT, Chung MK, Zhang LH, Lee J, et al. ESM1 mediates NGFR-induced invasion and metastasis in murine oral squamous cell carcinoma. Oncotarget. 2016;7:70738–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Judd NP, Winkler AE, Murillo-Sauca O, Brotman JJ, Law JH, Lewis JS, Jr., et al. ERK1/2 regulation of CD44 modulates oral cancer aggressiveness. Cancer Res. 2012;72:365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Uppaluri R, Winkler AE, Lin T, Law JH, Haughey BH, Nussenbaum B, et al. Biomarker and Tumor Responses of Oral Cavity Squamous Cell Carcinoma to Trametinib: A Phase II Neoadjuvant Window-of-Opportunity Clinical Trial. Clin Cancer Res. 2017;23:2186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chung MK, Jung YH, Lee JK, Cho SY, Murillo-Sauca O, Uppaluri R, et al. CD271 Confers an Invasive and Metastatic Phenotype of Head and Neck Squamous Cell Carcinoma through the Upregulation of Slug. Clin Cancer Res. 2018;24:674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Murillo-Sauca O, Chung MK, Shin JH, Karamboulas C, Kwok S, Jung YH, et al. CD271 is a functional and targetable marker of tumor-initiating cells in head and neck squamous cell carcinoma. Oncotarget. 2014;5:6854–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Uzunparmak B, Gao M, Lindemann A, Erikson K, Wang L, Lin E, et al. Caspase-8 loss radiosensitizes head and neck squamous cell carcinoma to SMAC mimetic-induced necroptosis. JCI Insight. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sato S, Vasaikar S, Eskaros A, Kim Y, Lewis JS, Zhang B, et al. EPHB2 carried on small extracellular vesicles induces tumor angiogenesis via activation of ephrin reverse signaling. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bhatia S, Griego A, Lennon S, Oweida A, Sharma J, Rohmer C, et al. Role of EphB3 Receptor in Mediating Head and Neck Tumor Growth, Cell Migration, and Response to PI3K Inhibitor. Mol Cancer Ther. 2018;17:2049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jin WJ, Erbe AK, Schwarz CN, Jaquish AA, Anderson BR, Sriramaneni RN, et al. Tumor-Specific Antibody, Cetuximab, Enhances the In Situ Vaccine Effect of Radiation in Immunologically Cold Head and Neck Squamous Cell Carcinoma. Front Immunol. 2020;11:591139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Korpela SP, Hinz TK, Oweida A, Kim J, Calhoun J, Ferris R, et al. Role of epidermal growth factor receptor inhibitor-induced interferon pathway signaling in the head and neck squamous cell carcinoma therapeutic response. J Transl Med. 2021;19:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Woods LT, Jasmer KJ, Munoz Forti K, Shanbhag VC, Camden JM, Erb L, et al. P2Y2 receptors mediate nucleotide-induced EGFR phosphorylation and stimulate proliferation and tumorigenesis of head and neck squamous cell carcinoma cell lines. Oral Oncol. 2020;109:104808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clavijo PE, Moore EC, Chen J, Davis RJ, Friedman J, Kim Y, et al. Resistance to CTLA-4 checkpoint inhibition reversed through selective elimination of granulocytic myeloid cells. Oncotarget. 2017;8:55804–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sun L, Clavijo PE, Robbins Y, Patel P, Friedman J, Greene S, et al. Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Greene S, Robbins Y, Mydlarz WK, Huynh AP, Schmitt NC, Friedman J, et al. Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer Models. Clin Cancer Res. 2020;26:1420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Clavijo PE, Friedman J, Robbins Y, Moore EC, Smith E, Zauderer M, et al. Semaphorin4D Inhibition Improves Response to Immune-Checkpoint Blockade via Attenuation of MDSC Recruitment and Function. Cancer Immunol Res. 2019;7:282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zeng Q, Fu J, Korrer M, Gorbounov M, Murray PJ, Pardoll D, et al. Caspase-1 from Human Myeloid-Derived Suppressor Cells Can Promote T Cell-Independent Tumor Proliferation. Cancer Immunol Res. 2018;6:566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cassetta L, Pollard JW. Tumor-associated macrophages. Curr Biol. 2020;30:R246–R8. [DOI] [PubMed] [Google Scholar]
- [31].Moreira D, Sampath S, Won H, White SV, Su YL, Alcantara M, et al. Myeloid cell-targeted STAT3 inhibition sensitizes head and neck cancers to radiotherapy and T cell-mediated immunity. J Clin Invest. 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bhatia S, Oweida A, Lennon S, Darragh LB, Milner D, Phan AV, et al. Inhibition of EphB4-Ephrin-B2 Signaling Reprograms the Tumor Immune Microenvironment in Head and Neck Cancers. Cancer Res. 2019;79:2722–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sato-Kaneko F, Yao S, Ahmadi A, Zhang SS, Hosoya T, Kaneda MM, et al. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T Cells and Human Disease. Annu Rev Immunol. 2020;38:541–66. [DOI] [PubMed] [Google Scholar]
- [35].Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6301–11. [DOI] [PubMed] [Google Scholar]
- [36].Chuckran CA, Cillo AR, Moskovitz J, Overacre-Delgoffe A, Somasundaram AS, Shan F, et al. Prevalence of intratumoral regulatory T cells expressing neuropilin-1 is associated with poorer outcomes in patients with cancer. Sci Transl Med. 2021;13:eabf8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Judd NP, Allen CT, Winkler AE, Uppaluri R. Comparative analysis of tumor-infiltrating lymphocytes in a syngeneic mouse model of oral cancer. Otolaryngol Head Neck Surg. 2012;147:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Oweida A, Hararah MK, Phan A, Binder D, Bhatia S, Lennon S, et al. Resistance to Radiotherapy and PD-L1 Blockade Is Mediated by TIM-3 Upregulation and Regulatory T-Cell Infiltration. Clin Cancer Res. 2018;24:5368–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Knitz MW, Bickett TE, Darragh LB, Oweida AJ, Bhatia S, Van Court B, et al. Targeting resistance to radiation-immunotherapy in cold HNSCCs by modulating the Treg-dendritic cell axis. J Immunother Cancer. 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Oweida AJ, Darragh L, Phan A, Binder D, Bhatia S, Mueller A, et al. STAT3 Modulation of Regulatory T Cells in Response to Radiation Therapy in Head and Neck Cancer. J Natl Cancer Inst. 2019;111:1339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Henry CM, Martin SJ. Caspase-8 Acts in a Non-enzymatic Role as a Scaffold for Assembly of a Pro-inflammatory “FADDosome” Complex upon TRAIL Stimulation. Mol Cell. 2017;65:715–29 e5. [DOI] [PubMed] [Google Scholar]
- [42].Cui Z, Dabas H, Leonard BC, Shiah JV, Grandis JR, Johnson DE. Caspase-8 mutations associated with head and neck cancer differentially retain functional properties related to TRAIL-induced apoptosis and cytokine induction. Cell Death Dis. 2021;12:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Saussez S, Camby I, Toubeau G, Kiss R. Galectins as modulators of tumor progression in head and neck squamous cell carcinomas. Head Neck. 2007;29:874–84. [DOI] [PubMed] [Google Scholar]
- [44].Nambiar DK, Aguilera T, Cao H, Kwok S, Kong C, Bloomstein J, et al. Galectin-1-driven T cell exclusion in the tumor endothelium promotes immunotherapy resistance. J Clin Invest. 2019;129:5553–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Parikh A, Shin J, Faquin W, Lin DT, Tirosh I, Sunwoo JB, et al. Malignant cell-specific CXCL14 promotes tumor lymphocyte infiltration in oral cavity squamous cell carcinoma. J Immunother Cancer. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhou L, Zeng Z, Egloff AM, Zhang F, Guo F, Campbell KM, et al. Checkpoint blockade-induced CD8+ T cell differentiation in head and neck cancer responders. J Immunother Cancer. 2022;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pai CS, Huang JT, Lu X, Simons DM, Park C, Chang A, et al. Clonal Deletion of Tumor-Specific T Cells by Interferon-gamma Confers Therapeutic Resistance to Combination Immune Checkpoint Blockade. Immunity. 2019;50:477–92 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gilardi M, Saddawi-Konefka R, Wu VH, Lopez-Ramirez MA, Wang Z, Soto F, et al. Microneedle-mediated intratumoral delivery of anti-CTLA-4 promotes cDC1-dependent eradication of oral squamous cell carcinoma with limited irAEs. Mol Cancer Ther. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Egloff AM, Uppaluri R. Preoperative immunotherapy for head and neck cancers: state of art. Curr Opin Oncol. 2022. [DOI] [PubMed] [Google Scholar]
- [50].Friedman J, Moore EC, Zolkind P, Robbins Y, Clavijo PE, Sun L, et al. Neoadjuvant PD-1 Immune Checkpoint Blockade Reverses Functional Immunodominance among Tumor Antigen-Specific T Cells. Clin Cancer Res. 2020;26:679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sharon S, Baird JR, Bambina S, Kramer G, Blair TC, Jensen SM, et al. A platform for locoregional T-cell immunotherapy to control HNSCC recurrence following tumor resection. Oncotarget. 2021;12:1201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zolkind P, Przybylski D, Marjanovic N, Nguyen L, Lin T, Johanns T, et al. Cancer immunogenomic approach to neoantigen discovery in a checkpoint blockade responsive murine model of oral cavity squamous cell carcinoma. Oncotarget. 2018;9:4109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Shibata H, Xu N, Saito S, Zhou L, Ozgenc I, Webb J, et al. Integrating CD4(+) T cell help for therapeutic cancer vaccination in a preclinical head and neck cancer model. Oncoimmunology. 2021;10:1958589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bommireddy R, Munoz LE, Kumari A, Huang L, Fan Y, Monterroza L, et al. Tumor Membrane Vesicle Vaccine Augments the Efficacy of Anti-PD1 Antibody in Immune Checkpoint Inhibitor-Resistant Squamous Cell Carcinoma Models of Head and Neck Cancer. Vaccines (Basel). 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dharmaraj N, Piotrowski SL, Huang C, Newton JM, Golfman LS, Hanoteau A, et al. Anti-tumor immunity induced by ectopic expression of viral antigens is transient and limited by immune escape. Oncoimmunology. 2019;8:e1568809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lee MY, Metenou S, Brough DE, Sabzevari H, Bai K, Jochems C, et al. Preclinical study of a novel therapeutic vaccine for recurrent respiratory papillomatosis. NPJ Vaccines. 2021;6:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Knochelmann HM, Rivera-Reyes AM, Wyatt MM, Smith AS, Chamness R, Dwyer CJ, et al. Modeling ex vivo tumor-infiltrating lymphocyte expansion from established solid malignancies. Oncoimmunology. 2021;10:1959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Robbins Y, Greene S, Friedman J, Clavijo PE, Van Waes C, Fabian KP, et al. Tumor control via targeting PD-L1 with chimeric antigen receptor modified NK cells. Elife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fabian KP, Padget MR, Donahue RN, Solocinski K, Robbins Y, Allen CT, et al. PD-L1 targeting high-affinity NK (t-haNK) cells induce direct antitumor effects and target suppressive MDSC populations. J Immunother Cancer. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Moore E, Clavijo PE, Davis R, Cash H, Van Waes C, Kim Y, et al. Established T Cell-Inflamed Tumors Rejected after Adaptive Resistance Was Reversed by Combination STING Activation and PD-1 Pathway Blockade. Cancer Immunol Res. 2016;4:1061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Baird JR, Bell RB, Troesch V, Friedman D, Bambina S, Kramer G, et al. Evaluation of Explant Responses to STING Ligands: Personalized Immunosurgical Therapy for Head and Neck Squamous Cell Carcinoma. Cancer Res. 2018;78:6308–19. [DOI] [PubMed] [Google Scholar]
- [62].Tan YS, Sansanaphongpricha K, Xie Y, Donnelly CR, Luo X, Heath BR, et al. Mitigating SOX2-potentiated Immune Escape of Head and Neck Squamous Cell Carcinoma with a STING-inducing Nanosatellite Vaccine. Clin Cancer Res. 2018;24:4242–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Luo X, Donnelly CR, Gong W, Heath BR, Hao Y, Donnelly LA, et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J Clin Invest. 2020;130:1635–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Leach DG, Dharmaraj N, Piotrowski SL, Lopez-Silva TL, Lei YL, Sikora AG, et al. STINGel: Controlled release of a cyclic dinucleotide for enhanced cancer immunotherapy. Biomaterials. 2018;163:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Pieper AA, Rakhmilevich AL, Spiegelman DV, Patel RB, Birstler J, Jin WJ, et al. Combination of radiation therapy, bempegaldesleukin, and checkpoint blockade eradicates advanced solid tumors and metastases in mice. J Immunother Cancer. 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gadwa J, Bickett TE, Darragh LB, Knitz MW, Bhatia S, Piper M, et al. Complement C3a and C5a receptor blockade modulates regulatory T cell conversion in head and neck cancer. J Immunother Cancer. 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kono M, Yamaki H, Komatsuda H, Kumai T, Hayashi R, Wakisaka R, et al. IL-2 complex recovers steroid-induced inhibition in immunochemotherapy for head and neck cancer. Transl Oncol. 2022;18:101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lacy MQ, Jacobus S, Blood EA, Kay NE, Rajkumar SV, Greipp PR. Phase II study of interleukin-12 for treatment of plateau phase multiple myeloma (E1A96): a trial of the Eastern Cooperative Oncology Group. Leuk Res. 2009;33:1485–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hong Y, Robbins Y, Yang X, Mydlarz WK, Sowers A, Mitchell JB, et al. Cure of syngeneic carcinomas with targeted IL-12 through obligate reprogramming of lymphoid and myeloid immunity. JCI Insight. 2022;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhao S, Peralta RM, Avina-Ochoa N, Delgoffe GM, Kaech SM. Metabolic regulation of T cells in the tumor microenvironment by nutrient availability and diet. Semin Immunol. 2021;52:101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Cha JH, Yang WH, Xia W, Wei Y, Chan LC, Lim SO, et al. Metformin Promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated Degradation of PD-L1. Mol Cell. 2018;71:606–20 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Munoz LE, Huang L, Bommireddy R, Sharma R, Monterroza L, Guin RN, et al. Metformin reduces PD-L1 on tumor cells and enhances the anti-tumor immune response generated by vaccine immunotherapy. J Immunother Cancer. 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chiang IT, Lee YH, Tan ZL, Hsu FT, Tu HF. Regorafenib enhances antitumor immune efficacy of anti-PD-L1 immunotherapy on oral squamous cell carcinoma. Biomed Pharmacother. 2022;147:112661. [DOI] [PubMed] [Google Scholar]
- [74].Zhou L, Mudianto T, Ma X, Riley R, Uppaluri R. Targeting EZH2 Enhances Antigen Presentation, Antitumor Immunity, and Circumvents Anti-PD-1 Resistance in Head and Neck Cancer. Clin Cancer Res. 2020;26:290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kenison JE, Wang Z, Yang K, Snyder M, Quintana FJ, Sherr DH. The aryl hydrocarbon receptor suppresses immunity to oral squamous cell carcinoma through immune checkpoint regulation. Proc Natl Acad Sci U S A. 2021;118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Xiao R, Allen CT, Tran L, Patel P, Park SJ, Chen Z, et al. Antagonist of cIAP1/2 and XIAP enhances anti-tumor immunity when combined with radiation and PD-1 blockade in a syngeneic model of head and neck cancer. Oncoimmunology. 2018;7:e1471440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ye W, Gunti S, Allen CT, Hong Y, Clavijo PE, Van Waes C, et al. ASTX660, an antagonist of cIAP1/2 and XIAP, increases antigen processing machinery and can enhance radiation-induced immunogenic cell death in preclinical models of head and neck cancer. Oncoimmunology. 2020;9:1710398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Jia L, Wang Y, Wang CY. circFAT1 Promotes Cancer Stemness and Immune Evasion by Promoting STAT3 Activation. Adv Sci (Weinh). 2021;8:2003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Moore EC, Cash HA, Caruso AM, Uppaluri R, Hodge JW, Van Waes C, et al. Enhanced Tumor Control with Combination mTOR and PD-L1 Inhibition in Syngeneic Oral Cavity Cancers. Cancer Immunol Res. 2016;4:611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Cash H, Shah S, Moore E, Caruso A, Uppaluri R, Van Waes C, et al. mTOR and MEK1/2 inhibition differentially modulate tumor growth and the immune microenvironment in syngeneic models of oral cavity cancer. Oncotarget. 2015;6:36400–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Shah S, Caruso A, Cash H, Waes CV, Allen CT. Pools of programmed death-ligand within the oral cavity tumor microenvironment: Variable alteration by targeted therapies. Head Neck. 2016;38:1176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kono M, Komatsuda H, Yamaki H, Kumai T, Hayashi R, Wakisaka R, et al. Immunomodulation via FGFR inhibition augments FGFR1 targeting T-cell based antitumor immunotherapy for head and neck squamous cell carcinoma. Oncoimmunology. 2022;11:2021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Anderson K, Ryan N, Alkhimovitch A, Siddiqui A, Oghumu S. Inhibition of PI3K Isoform p110gamma Increases Both Anti-Tumor and Immunosuppressive Responses to Aggressive Murine Head and Neck Squamous Cell Carcinoma with Low Immunogenicity. Cancers (Basel). 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Davis RJ, Moore EC, Clavijo PE, Friedman J, Cash H, Chen Z, et al. Anti-PD-L1 Efficacy Can Be Enhanced by Inhibition of Myeloid-Derived Suppressor Cells with a Selective Inhibitor of PI3Kdelta/gamma. Cancer Res. 2017;77:2607–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Wang Z, Goto Y, Allevato MM, Wu VH, Saddawi-Konefka R, Gilardi M, et al. Disruption of the HER3-PI3K-mTOR oncogenic signaling axis and PD-1 blockade as a multimodal precision immunotherapy in head and neck cancer. Nat Commun. 2021;12:2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Sun L, Moore E, Berman R, Clavijo PE, Saleh A, Chen Z, et al. WEE1 kinase inhibition reverses G2/M cell cycle checkpoint activation to sensitize cancer cells to immunotherapy. Oncoimmunology. 2018;7:e1488359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Patel P, Sun L, Robbins Y, Clavijo PE, Friedman J, Silvin C, et al. Enhancing direct cytotoxicity and response to immune checkpoint blockade following ionizing radiation with Wee1 kinase inhibition. Oncoimmunology. 2019;8:e1638207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Friedman J, Morisada M, Sun L, Moore EC, Padget M, Hodge JW, et al. Inhibition of WEE1 kinase and cell cycle checkpoint activation sensitizes head and neck cancers to natural killer cell therapies. J Immunother Cancer. 2018;6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Moore EC, Sun L, Clavijo PE, Friedman J, Harford JB, Saleh AD, et al. Nanocomplex-based TP53 gene therapy promotes anti-tumor immunity through TP53- and STING-dependent mechanisms. Oncoimmunology. 2018;7:e1404216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Miyauchi S, Sanders PD, Guram K, Kim SS, Paolini F, Venuti A, et al. HPV16 E5 Mediates Resistance to PD-L1 Blockade and Can Be Targeted with Rimantadine in Head and Neck Cancer. Cancer Res. 2020;80:732–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ogawa M, Tomita Y, Nakamura Y, Lee MJ, Lee S, Tomita S, et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget. 2017;8:10425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Nagaya T, Nakamura Y, Okuyama S, Ogata F, Maruoka Y, Choyke PL, et al. Syngeneic Mouse Models of Oral Cancer Are Effectively Targeted by Anti-CD44-Based NIR-PIT. Mol Cancer Res. 2017;15:1667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Maruoka Y, Furusawa A, Okada R, Inagaki F, Fujimura D, Wakiyama H, et al. Near-Infrared Photoimmunotherapy Combined with CTLA4 Checkpoint Blockade in Syngeneic Mouse Cancer Models. Vaccines (Basel). 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Okada R, Kato T, Furusawa A, Inagaki F, Wakiyama H, Choyke PL, et al. Local Depletion of Immune Checkpoint Ligand CTLA4 Expressing Cells in Tumor Beds Enhances Antitumor Host Immunity. Adv Ther (Weinh). 2021;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kato T, Okada R, Furusawa A, Inagaki F, Wakiyama H, Furumoto H, et al. Simultaneously Combined Cancer Cell- and CTLA4-Targeted NIR-PIT Causes a Synergistic Treatment Effect in Syngeneic Mouse Models. Mol Cancer Ther. 2021;20:2262–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wakiyama H, Furusawa A, Okada R, Inagaki F, Kato T, Maruoka Y, et al. Increased Immunogenicity of a Minimally Immunogenic Tumor after Cancer-Targeting Near Infrared Photoimmunotherapy. Cancers (Basel). 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Maruoka Y, Furusawa A, Okada R, Inagaki F, Fujimura D, Wakiyama H, et al. Combined CD44- and CD25-Targeted Near-Infrared Photoimmunotherapy Selectively Kills Cancer and Regulatory T Cells in Syngeneic Mouse Cancer Models. Cancer Immunol Res. 2020;8:345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Maruoka Y, Furusawa A, Okada R, Inagaki F, Wakiyama H, Kato T, et al. Interleukin-15 after Near-Infrared Photoimmunotherapy (NIR-PIT) Enhances T Cell Response against Syngeneic Mouse Tumors. Cancers (Basel). 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Okada R, Furusawa A, Inagaki F, Wakiyama H, Kato T, Okuyama S, et al. Endoscopic near-infrared photoimmunotherapy in an orthotopic head and neck cancer model. Cancer Sci. 2021;112:3041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. [DOI] [PubMed] [Google Scholar]
- [101].Park SJ, Ye W, Xiao R, Silvin C, Padget M, Hodge JW, et al. Cisplatin and oxaliplatin induce similar immunogenic changes in preclinical models of head and neck cancer. Oral Oncol. 2019;95:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Tran L, Allen CT, Xiao R, Moore E, Davis R, Park SJ, et al. Cisplatin Alters Antitumor Immunity and Synergizes with PD-1/PD-L1 Inhibition in Head and Neck Squamous Cell Carcinoma. Cancer Immunol Res. 2017;5:1141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Cunningham S, McCauley S, Vairamani K, Speth J, Girdhani S, Abel E, et al. FLASH Proton Pencil Beam Scanning Irradiation Minimizes Radiation-Induced Leg Contracture and Skin Toxicity in Mice. Cancers (Basel). 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Morisada M, Moore EC, Hodge R, Friedman J, Cash HA, Hodge JW, et al. Dose-dependent enhancement of T-lymphocyte priming and CTL lysis following ionizing radiation in an engineered model of oral cancer. Oral Oncol. 2017;71:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Morisada M, Clavijo PE, Moore E, Sun L, Chamberlin M, Van Waes C, et al. PD-1 blockade reverses adaptive immune resistance induced by high-dose hypofractionated but not low-dose daily fractionated radiation. Oncoimmunology. 2018;7:e1395996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Blair TC, Bambina S, Alice AF, Kramer GF, Medler TR, Baird JR, et al. Dendritic Cell Maturation Defines Immunological Responsiveness of Tumors to Radiation Therapy. J Immunol. 2020;204:3416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Jagodinsky JC, Jin WJ, Bates AM, Hernandez R, Grudzinski JJ, Marsh IR, et al. Temporal analysis of type 1 interferon activation in tumor cells following external beam radiotherapy or targeted radionuclide therapy. Theranostics. 2021;11:6120–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hayman TJ, Baro M, MacNeil T, Phoomak C, Aung TN, Cui W, et al. STING enhances cell death through regulation of reactive oxygen species and DNA damage. Nat Commun. 2021;12:2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Knitz MW, Darragh LB, Bickett TE, Bhatia S, Bukkapatnam S, Gadwa J, et al. Loss of cancer cell STAT1 improves response to radiation therapy and promotes T cell activation in head and neck squamous cell carcinoma. Cancer Immunol Immunother. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Bickett TE, Knitz M, Darragh LB, Bhatia S, Van Court B, Gadwa J, et al. FLT3L Release by Natural Killer Cells Enhances Response to Radioimmunotherapy in Preclinical Models of HNSCC. Clin Cancer Res. 2021;27:6235–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Patin EC, Dillon MT, Nenclares P, Grove L, Soliman H, Leslie I, et al. Harnessing radiotherapy-induced NK-cell activity by combining DNA damage-response inhibition and immune checkpoint blockade. J Immunother Cancer. 2022;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Hanoteau A, Newton JM, Krupar R, Huang C, Liu HC, Gaspero A, et al. Tumor microenvironment modulation enhances immunologic benefit of chemoradiotherapy. J Immunother Cancer. 2019;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Newton JM, Hanoteau A, Liu HC, Gaspero A, Parikh F, Gartrell-Corrado RD, et al. Immune microenvironment modulation unmasks therapeutic benefit of radiotherapy and checkpoint inhibition. J Immunother Cancer. 2019;7:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a andomized, open-label, phase 3 study. Lancet. 2019;394:1915–28. [DOI] [PubMed] [Google Scholar]
- [115].Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a andomized, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22:450–62. [DOI] [PubMed] [Google Scholar]
- [116].Hecht M, von der Grun J, Semrau S, Muller S, Weissmann T, Gaipl US, et al. [Highlights from the 2021 ASCO and ESMO annual meetings on radiotherapy of head and neck cancer]. HNO. 2022;70:258–64. [DOI] [PubMed] [Google Scholar]
- [117].Robert Saddawi-Konefk AOF, Faraji Farhoud, Clubb Lauren, Allevato Michael M., Anang Nana-Ama A. S., Jensen Shawn M., Wang Zhiyong, Wu Victoria H., Yung Bryan S., Riyam Al Msari Ida Franiak Pietryga, Molinolo Alfredo A., Mesirov Jill P., Simon Aaron B., Fox Bernard A., Bui Jack D., Sharabi Andrew, Cohen Ezra E. W., Califano Joseph A., Silvio Gutkind J. Lymphatic-Preserving Treatment Sequencing with Immune Checkpoint Inhibition Unleashes cDC1-Dependent Antitumor Immunity in HNSCC. bioRxiv. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]


