Abstract
Fat injection has been in application for more than two decades, and its usefulness has been well documented. In our article, we want to highlight the various applications and usefulness of this versatile technique. We also want to showcase the methods to ensure good results and maximize the uptake of fat grafts with minimal absorption. Our results in our series of 110 consecutive cases have been very encouraging, with excellent patient satisfaction.
Keywords: Fat grafts, fat injection, lipofilling
INTRODUCTION
Fat grafting has been in vogue since two decades, and it has been used by plastic surgeons for various applications. The present technique was popularized by Sidney Coleman.[1] The fundamental components of this technique are syringe aspiration of fat, proper fat treatment with centrifugation and component separation and finally fat injection into various sites.
Initially, fat was used for correction of facial and body contour defects. As its usefulness become known, it has found various applications, including breast and buttock augmentation, facial rejuvenation, and burns scar treatment, to fill defects of trauma and post-oncological resection defects; to correct deformities of some surgery complications such as post-gynecomastia surgery saucer defects; to correct wasting defects such as in webspace and cheeks, frontal bone defects, and penile augmentation; and to correct congenital defects such as Rhomberg’s disease.
Fat grafts have also been known to contain stem cells and have very good growth potential; they have been used for stem cell research and stem cell therapy such as in spine injuries.
MATERIALS AND METHODS
We report our results on 110 consecutive patients who underwent fat grafting between March 2015 and March 2020. Overall, 10 patients had multiple procedures in the same sitting whereas the rest 100 had fat grafts applied to specific areas. The minimal follow-up period was one year post-procedure. Cases with less follow-up were excluded. Some patients required additional sittings of fat grafting, which we did after one year, and are not included in this study.
Surgical technique
The surgical technique we followed was based on the time-tested Coleman technique, which consists of (1) fat harvest with syringe aspiration, (2) fat processing with syringe inversion or centrifugation with storage in ice packs, and finally (3) fat injection into concerned areas.
The procedure starts with preoperative markings to delineate the specific areas for application of fat grafts. The procedure is done under either general or local anesthesia depending on the amount of fat required; smaller fat grafts can be done under local anesthesia as an office procedure. The wet technique of lipoaspiration with fluid injection at donor site ensures minimal ecchymoses and minimal discomfort postoperatively. We use Luer-Lock syringes of 10 ml and 5 ml; the piston of 5 ml is used as a stop after inserting the cannula into the donor area and aspirating. A to-and-fro motion with gentle negative pressure helps in fat harvest. A combination of slight negative pressure and the curetting action of the cannula through the tissues allows parcels of fat to move through the cannula and Luer-Lock aperture into the barrel of the syringe. The piston is removed after harvesting. The syringes are then kept upside down for gravity separation in a container having ice cubes. This helps in separation of the fat from the fluids and lipid layer. The central area contains the fat needed. The fluid layer is let out by inverting back the syringes, and fat is injected into concerned areas by the fanning technique with fat being injected as the syringe is pulled back. The fat injection at the recipient site should be performed by using small-gauge cannulas in a fanning-out pattern over multiple sessions, rather than a single session.
We use smaller-sized injection cannulas than aspiration cannulas so that fat snugly fits into concerned areas.
Postoperatively, we use ice packs and pressure garments to reduce the swelling.
RESULTS
Our results are shown in Table 1.
Table 1.
Table showing case differentiation
| Male | Female | Total | |
|---|---|---|---|
| Cosmetic: face | 9 | 11 | 20 |
| Cosmetic: breast | 2 | 13 | 15 |
| Cosmetic: buttocks | 2 | 8 | 10 |
| Post-traumatic defect | 12 | 8 | 20 |
| Post-tumor excision defect | 7 | 8 | 15 |
| Rhomberg’s disease | 4 | 6 | 10 |
| Lipodistrophy | 7 | 3 | 10 |
| Penile augmentation | 5 | 5 | |
| Burn scar | 2 | 3 | 5 |
| Total | 50 | 60 | 110 |
Cosmetic uses in face, breast, and buttocks are: 45 cases, post-traumatic defects: 20 cases, post-tumor excision defects: 15, Rhomberg’s disease: 10 cases, lipodystrophy: 10 cases, penile augmentation: 5 cases, and burn scar treatment: 5.
All the cases were followed up for a minimum period of one year.
Lipofilling represents a simple solution to restore the correct profile of the breast after reconstruction. Lipofilling can be used after reconstruction with implants or muscle flaps with or without tissue expansion. Fat injection is rarely used as the sole treatment, rather it is often performed in combination with other routine techniques of breast surgery.[2] When fat tissue is not perfused, it can die and result in necrotic cysts and even calcification, but this complication can occur in any surgical breast procedure.[3] Fat grafting to the breast could potentially interfere with breast cancer detection; however, no evidence has been found that strongly supports such an interference.[4] Two of our cases for the breast were done for post-gynecomastia saucer defect correction.
Patients with retractile and painful scars compromising the normal daily activity/ mobility of the joint involved can take advantage of lipofilling treatment. Subscar and intrascar fat grafting are relatively recent techniques that improve scar quality.
Facial rejenuvation with fat grafts are commonly used for malar area, lips, and naolabial lines.
Brazilian butt lift with fat augmentation has popularized buttock augmentation.
We did observe some amount of fat absorption mainly in the first three months and this did offer a further fat injection for some patients. However, we have not included any patients who required further fat injections in the present study. The satisfaction rate among patients was also high, as we had explained the chances of absorption of fat preoperatively and the possible need for additional procedures.
We have noted that movements of the parts for which fat augmentation has been done should be restricted for at least one month to ensure good fat uptake. For example for facial rejuvenation, we advise that the patient should be on a soft diet and not indulge in excessive mastication for at least one month postoperatively to ensure good fat uptake. For the breast we advise no aerobics for at least one month postoperatively. For the buttocks we advise patients to sleep in prone position for one month after fat injection. This is to ensure good fat uptake, as we have observed that maximum fat absorption occurs in one month postoperatively.
Preoperative and postoperative photographs of fat injection for breasts are shown in Figures 1 and 2.
Figure 1.
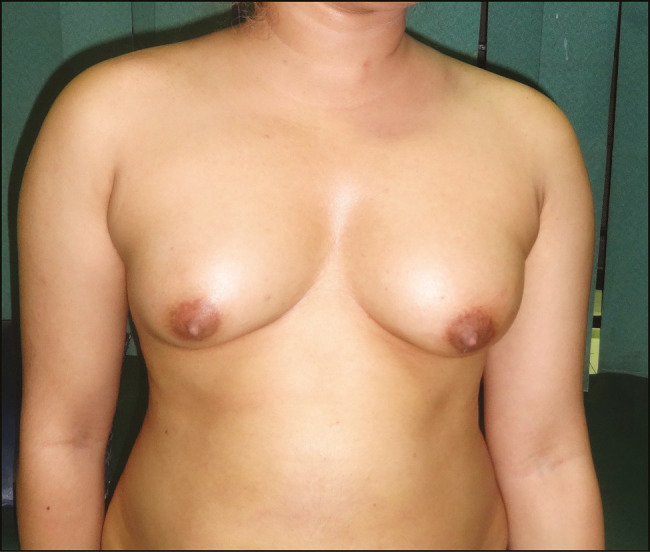
Breast pre-operative photograph
Figure 2.
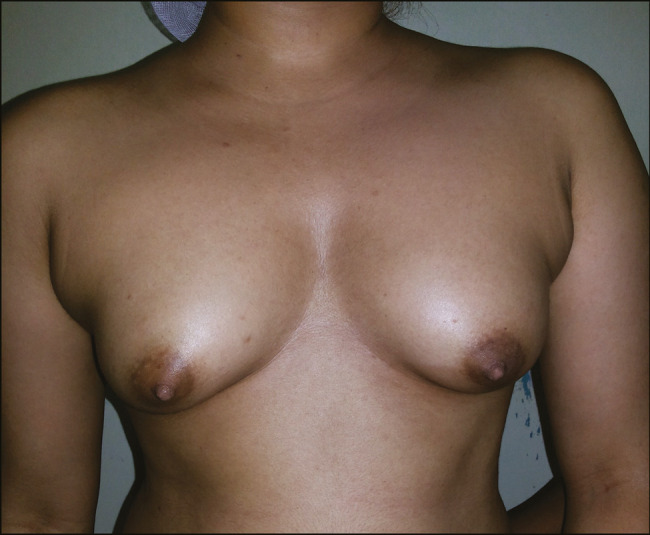
Breast post-operative photograph
Preoperative and postoperative photographs of fat injection for buttocks are shown in Figures 3 and 4.
Figure 3.
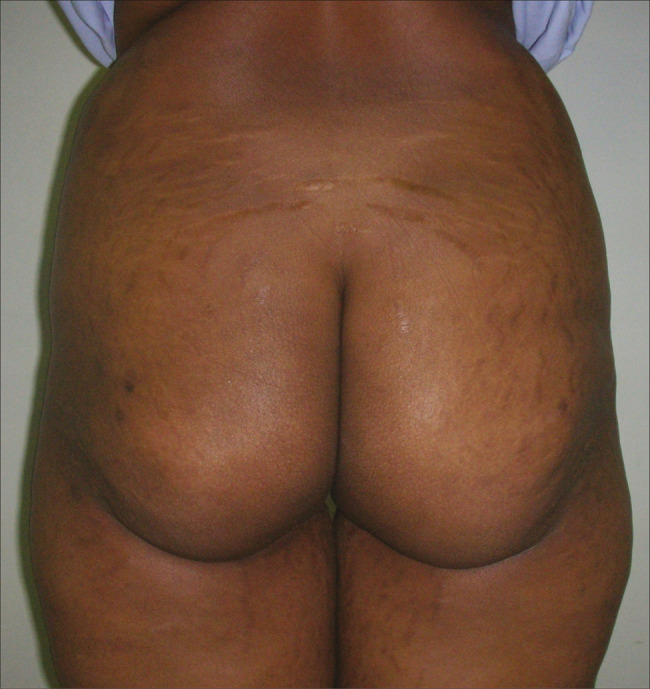
Buttock pre-operative photograph
Figure 4.
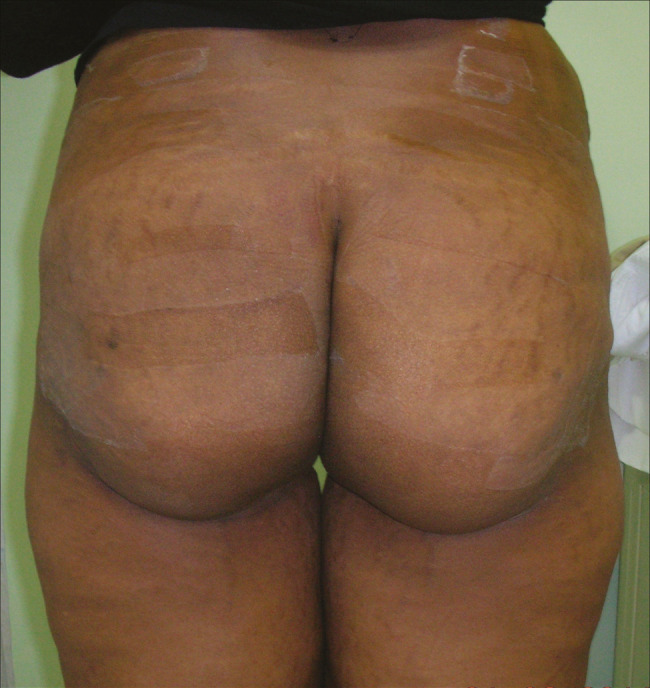
Buttock post-operative photograph
Preoperative and postoperative photographs of fat injection for facial rejuvenation are shown in Figures 5 and 6.
Figure 5.

Facial rejuvenation pre-operative photograph
Figure 6.
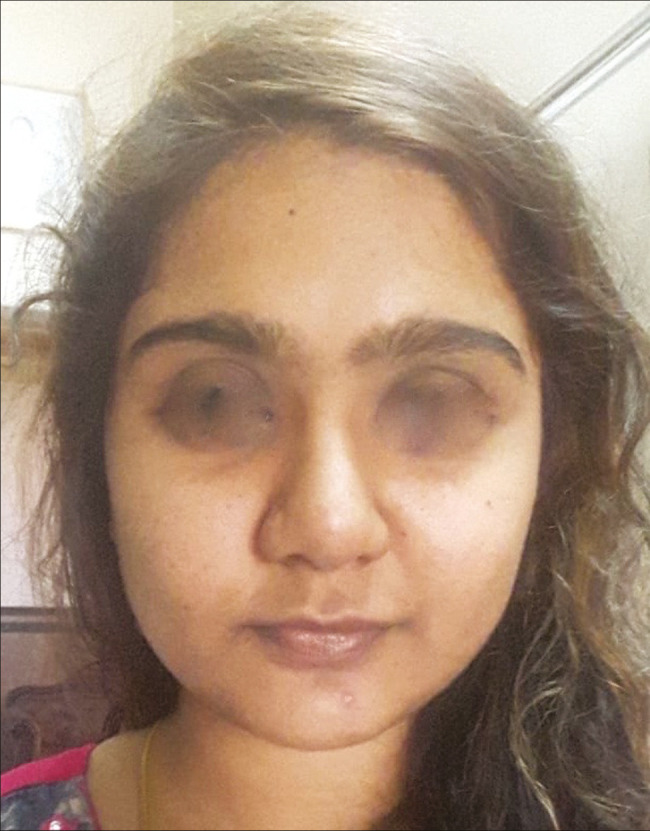
Facial rejuvenation post-operative photograph
Preoperative and postoperative photographs of fat injection for post-traumatic frontal bone defect are shown in Figures 7 and 8.
Figure 7.

Post-traumatic frontal defect pre-operative photograph
Figure 8.

Post-traumatic frontal defect post-operative photograph
Preoperative and postoperative photographs of fat injection for post-tumor excision defect of arm are shown in Figures 9 and 10.
Figure 9.
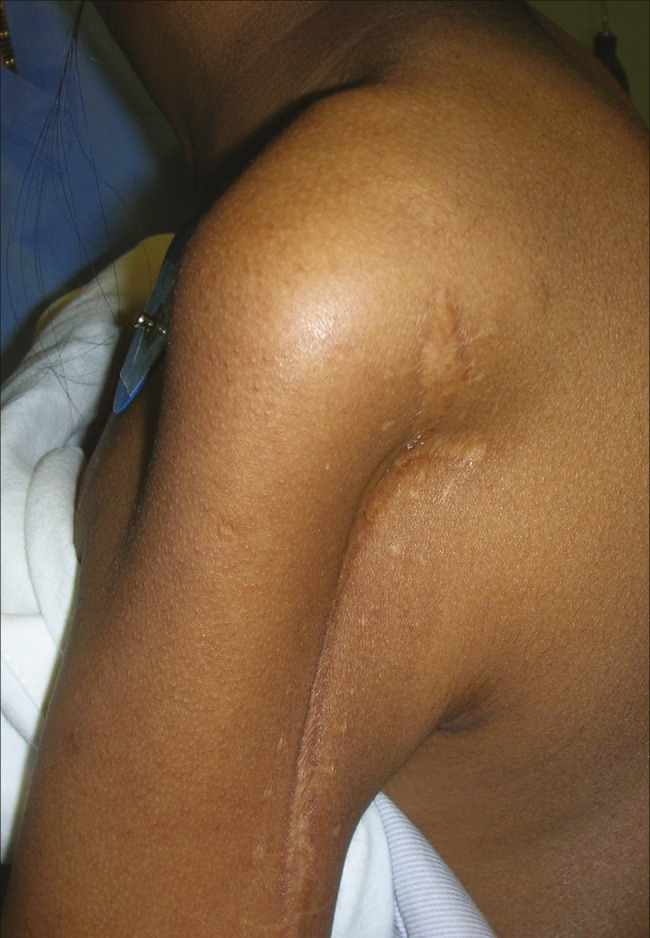
Post-tumor excision defect pre-operative photograph
Figure 10.
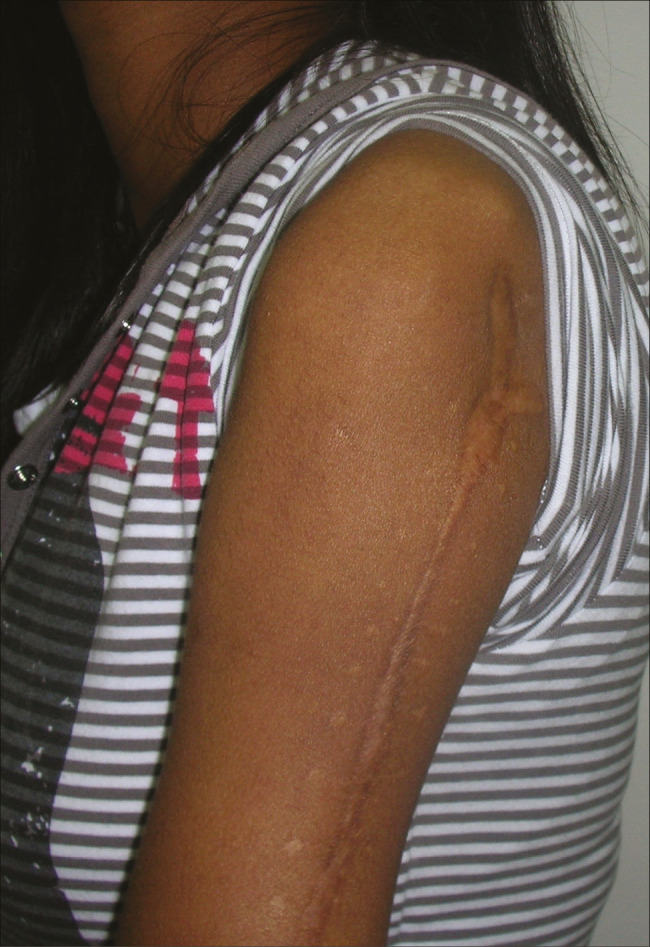
Post-tumor excision defect post-operative photograph
Preoperative and postoperative photographs of fat injection for penile augmentation are shown in Figures 11 and 12.
Figure 11.

Penile augmentation pre-operative photograph
Figure 12.

Penile augmentation post-operative photograph
Preoperative and postoperative photographs of fat injection for Rhomberg’s disease are shown in Figures 13 and 14.
Figure 13.

Rhomberg’s disease pre-operative photograph
Figure 14.

Rhomberg’s disease post-operative photograph
DISCUSSION
Fat, being a readily available donor area, needs to be utilized well and excellent results can be obtained. Fat is filled with ideal properties: It naturally integrates into tissues, is autologous, and is 100% biocompatible. Fat, being an autograft, has very little chances of rejection unlike an allograft. Also, it gives an added benefit of reducing the fat bulges of the donor area and helps in body contouring. Further, fat cells have a high stem cell potential and the retained fat has a growth potential that can be enhanced with good nutrition and diet.
Since fat is universal, multiple donor areas can be chosen although our preferred areas have been the abdomen and thighs. Campbell et al. found an inverse relationship between cellular damage and the diameter of the instrument used to extract fat.[5] Erdim et al.[6] reported higher graft viability with lipoaspirates that were obtained by using a 6-mm cannula rather than a 4-mm or 2-mm cannula. We use 3- or 4-mm cannulas for aspiration and 1- or 2-mm cannulas for injection. The size of cannulas varies with areas for injection, with smaller cannulas used for the face and bigger ones for the breast and buttocks.
Micro- and nanofat grafts, typically harvested with cannulas as small as 0.7 mm in diameter, can be used to treat delicate areas of the face such as the eyelids and lips.[7]
Small-gauge cannulas are believed to reduce trauma to the recipient site, thus reducing the risks of bleeding, hematoma formation, and poor graft oxygen diffusion.[8] Because revascularization starts at the periphery, ischemic time is longer in the center of the graft.[9] Therefore, fat reinjection in multiple small-volume sessions is preferred over one single injection.[9]
Tonnard et al. highlighted the clinical application of micro- and nanofat grafts compared with macrofats.[10]
The nanofat grafts were devoid of adipocytes, and the native architecture was disrupted.[10,11] However, the nanografts retained a rich supply of ASCs, which were similar to the ASCs in the macro- and microfat samples in terms of proliferation and differentiation.[10,11] In several clinical cases, the use of nanofat grafts has resulted in improved skin quality by six months postoperatively.[10,11] Therefore, the author suggests that although nanografts do not contain viable adipocytes, the high content of stem cells in these grafts may be clinically useful for skin rejuvenation.[10,11]
During aspiration, the yellow fat is definitely a better harvest than fat that is blood tinged. We advise change of donor area for aspiration when aspirate becomes blood tinged.
Fat processing is necessary, because the lipoaspirate contains not only adipocytes but also collagen fibers, blood, and debris. These elements can cause inflammation at the recipient site, which can be detrimental for the fat graft.[9] Blood must be extracted, because blood accelerates the degradation of the transplanted fat.[12] Moreover, the injection of debris gives an erroneous impression of the volume of correction because the debris will be absorbed after a few hours.[9]
Coleman suggested a processing method that has gained popularity and has been since integrated in many fat-transfer clinical protocols. Aspirated fat in syringes is spun at 3000 rpm for 3 min to isolate the fat.[13] After the centrifugation, three layers are observed: The first layer includes lipids, which can be poured off by using absorbent material; the second layer consists of fatty tissue; and the third layer contains blood, tissue fluid, and local anesthetic and is ejected from the base of the syringe. The middle layer is routinely used for adipose tissue grafting.[14,15,16,17]
The benefits of centrifugation have been controversial and we have found good results with our technique of inversion of aspirated syringes, ensuring adequate time of at least 15 min for proper fat sedimentation and discarding the fluid component. Also, the fat needs proper cold storage with ice packs during the time that it is outside the body.
Also, the ease of fat injection ensures it to be suitable as an office procedure and does not require any sophisticated equipment. In cases of lipodystrophy, the causative factors need to be treated and at least one year of stabilization is required before attempting fat injection. The same applies to Rhomberg’s disease, where we wait for at least 1 year of stabilization before attempting fat grafting.
The major complications of facial rejuvenation by lipofilling are possibly attributable to the injection of fat grafts in “dangerous” areas such as the glabella and nasolabial folds.[18,19] In fact, fat grafts may cause cerebral or ocular artery thrombosis, with an increase in local pressure, resulting in a reflux of the fat into the ophthalmic artery and the internal carotid artery.[18,19] To limit this risk and the risks of fat embolism and serious consequences, verification of an absence of blood reflux into the syringe prior to the injection, slow injection at low pressure, and the use of a blunt-tip cannula are recommended.[18,19]
The complication rates with this technique are minimal, and some amount of fat nodularity and hardness encountered can be easily tackled with appropriate massaging techniques. We advise a short course of oral antibiotics to ensure no infection rates. Ice packs and enzyme preparations such as serratiopeptidase are useful in reducing the swelling postoperatively. Pressure garments are advised for at least three months postoperatively.
CONCLUSION
Fat injection is an art and the plastic surgeon being an artist needs to ensure good care in achieving good results for his efforts. Rohrich et al. have classed it as number three in the list of top innovations in plastic surgery.[20] As with all surgeries, fat injection has a learning curve and skills can be sharpened with experience.
Very satisfying results can be achieved, and fat can be a very powerful arsenal in achieving good results in body contouring. The minimal risks involved in this procedure make it a very popular surgery in the coming times. In this article, we have showcased the various applications of fat grafting and this can be broadened with time.
Due to their stem cell potential, fat cells have also been used in spine surgeries; they have been used by neurosurgeons for CSF leak closures, by ENT surgeons for laryngeal palsy, and by cleft surgeons for augmenting the posterior pharyngeal wall for velopharyngeal incompetence. These are only a few of various other applications and fat may be used in many other ways.
In conclusion, we have tried to highlight the various applications and usefulness of this versatile technique. We have showcased the methods to ensure good results and maximize uptake of fat grafts with minimal absorption. We have achieved good results and excellent patient satisfaction in our series.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/ have given his/ her/ their consent for his/ her/ their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
None.
Conflict of interest
The author has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
REFERENCES
- 1.Coleman SR. Structural fat grafting. Aesthet Surg J. 1998;18:386, 388. doi: 10.1016/S1090-820X(98)70098-6. [DOI] [PubMed] [Google Scholar]
- 2.Raposio E, Caregnato P, Barabino P, Gualdi A, Orefice A, Spagnolo A, et al. Computer-based preoperative planning for breast reconstruction in the woman with unilateral breast hypoplasia. Miner Chir. 2002;57:711–4. [PubMed] [Google Scholar]
- 3.Pinsolle V, Chichery A, Grolleau JL, Chavoin JP. Autologous fat injection in poland’s syndrome. J Plast Reconstr Aesthet Surg. 2008;61:784–91. doi: 10.1016/j.bjps.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 4.Coleman SR, Saboeiro AP. Fat grafting to the breast revisited: Safety and efficacy. Plast Reconstr Surg. 2007;119:775–85; discussion 786-7. doi: 10.1097/01.prs.0000252001.59162.c9. [DOI] [PubMed] [Google Scholar]
- 5.Campbell G-L, Laudenslager N, Newman J. The role of cannula diameter in improved adipocyte viability: A quantitative analysis. Aesthet Surg J. 2006;26:287–9. doi: 10.1016/j.asj.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Erdim M, Tezel E, Numanoglu A, Sav A. The effect of mechanical stress on adipocyte morphology and metabolism. Am J Cosmet Surg. 1987;4:89–94. [Google Scholar]
- 7.Mazzola RF. Fat injections for facial rejuvenation: 17 years experience in 1720 patients. J Cosmet Dermatol. 2003;2:119–25. doi: 10.1111/j.1473-2130.2004.00060.x. [DOI] [PubMed] [Google Scholar]
- 8.Pu LL, Coleman SR, Cui X, Ferguson RE, Jr, Vasconez HC. Autologous fat grafting: In search of the optimal technique. Surg Innov. 2014;21:327–36. doi: 10.1177/1553350613518846. [DOI] [PubMed] [Google Scholar]
- 9.Mojallal A, Foyatier JL. Particle size in fat graft retention: A review on the impact of harvesting technique in lipofilling surgical outcomes. Adipocyte. 2014;3:273–9. doi: 10.4161/21623945.2014.957987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H. Fat Injection: From Filling to Regeneration. St. Louis, MO: Quality Medical Publishing; 2009. pp. 373–422. [Google Scholar]
- 11.Gause TM, Kling RE, Sivak WN, Marra KG, Rubin JP, Kokai LE. Nanofat grafting: Basic research and clinical applications. Plast Reconstr Surg. 2013;132:1017–26. doi: 10.1097/PRS.0b013e31829fe1b0. [DOI] [PubMed] [Google Scholar]
- 12.Sommer B, Sattler G. The effect of different factors on the survival of transplanted adipocytes. Ann Chir Plast Esthet. 2004;49:426–36. doi: 10.1016/j.anplas.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Coleman SR. Effect of centrifugation and washing on adipose graft viability: A new method to improve graft efficiency. J Plast Reconstr Aesthet Surg. 2013;66:712–9. doi: 10.1016/j.bjps.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Gir P, Brown SA, Oni G, Kashefi N, Mojallal A, Rohrich RJ. Cryopreservation of fat tissue and application in autologous fat graft: In vitro and in vivo study. Aesthet Plast Surg. 2012;36: 714–22. doi: 10.1007/s00266-011-9848-z. [DOI] [PubMed] [Google Scholar]
- 15.Wilson A, Butler PE, Seifalian AM. Facial augmentation with structural fat grafting. Clin Plast Surg. 2006;33:567–77. doi: 10.1016/j.cps.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Tuin AJ, Domerchie PN, Schepers RH, Willemsen JC, Dijkstra PU, Spijkervet FK, et al. Adipose-derived stem cells for clinical applications: A review. Cell Prolif. 2011;44:86–98. doi: 10.1111/j.1365-2184.2010.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conde-Green A, de Amorim NF, Pitanguy I. What is the current optimal fat grafting processing technique? A systematic review. J Craniomaxillofac Surg. 2016;44:45–55. doi: 10.1016/j.jcms.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Boureaux E, Chaput B, Bannani S, Herlin C, De Runz A, Carloni R, et al. Treatment of infraorbital dark circles by autologous fat transplantation: A pilot study. Br J Dermatol. 2009;160:1022–5. doi: 10.1111/j.1365-2133.2009.09066.x. [DOI] [PubMed] [Google Scholar]
- 19.Park SH, Sun HJ, Choi KS. Sudden unilateral visual loss after autologous fat injection into the nasolabial fold. Clin Ophthalmol. 2008;2: 679–83. [PMC free article] [PubMed] [Google Scholar]
- 20.Rohrich RJ, Rosen J, Longaker MT. So you want to be an innovator? Plast Reconstr Surg. 2010;126:1107–9. doi: 10.1097/PRS.0b013e3181e3b854. [DOI] [PubMed] [Google Scholar]


