Summary
Background
Pre-eclampsia and gestational hypertension are pregnancy-related disorders with major maternal cardiovascular implications later in life.
Objectives
The aim of this study was to determine interleukin- 6 levels in women with pre-eclampsia and gestational hypertension and in healthy pregnant controls, and to examine their correlations with characteristics of the women and echocardiographic findings.
Methods
The ELISA method was used to determine serum interleukin-6 in 36 women with gestational hypertension, 37 women with pre-eclampsia and 50 pregnant controls. The echocardiographic examination was performed according to current recommendations by the European Association of Cardiovascular Imaging and the American Society of Echocardiography.
Results
Mean serum interleukin-6 levels were 2.77 pg/ml in the controls, 5.08 pg/ml in the gestational hypertension group and 8.06 pg/ml in the pre-eclampsia group. A significant difference in these levels was present between the controls and both hypertensive groups, but not between the two hypertensive groups. Higher levels correlated with heart chamber enlargement and worse ventricular function.
Keywords: pregnancy, interleukin-6, pre-eclampsia, gestational hypertension, echocardiography, inflammation
Hypertensive disorders of pregnancy (HDP) complicate approximately 5–10% of human pregnancies,1 and are one of the leading causes of maternal mortality in the modern world.2 There is also increasing evidence of elevated cardiovascular risk after pregnancy-induced hypertension – women have a long-term risk of developing arterial hypertension, coronary atherosclerosis, ischaemic heart disease, stroke, type 2 diabetes mellitus, venous thromboembolism and heart failure.3-6
It is hypothesised that the hypertensive disorders of pregnancy, in addition to common risk factors, share some similar mechanisms with heart disease, such as endothelial dysfunction, inflammation, oxidative stress and thrombophilia.7-9 The inflammatory component of pre-eclampsia is characterised by elevated cytokine levels and activated leucocytes as well as stimulation of the angiotensin II type 1 receptor, leading to vasoconstriction. Tumour necrosis factor (TNF)-alpha, interleukin-6 and interleukin-8 are elevated, while antiinflammatory factors such as interleukin-10 are decreased.10-12
Interleukin-6 is a pro-inflammatory cytokine with an established role in the inflammatory response, hypertension and atherosclerosis.13 It has been proven in a rat model that interleukin-6 is involved in elevation of blood pressure in pregnancy due to the reduction of uterine perfusion pressure and it mediates worsening of renal function.14 In another study it was found that it impaired endothelium-dependent relaxation and enhanced constriction of systemic vessels in pregnant rats. This, in turn, suggested its direct role in the vascular resistance in hypertension-complicated pregnancy.15
In humans, higher interleukin-6 levels were measured in the umbilical vein and plasma of 12 women with pre-eclampsia compared to 12 women with normotensive pregnancies.16 Similarly, in another study, higher levels of interleukin-6, interleukin-8 and TNF-alpha were present in maternal and placental blood, adding evidence to the hypothesis of the cytokine’s significant role in the pathogenesis of pre-eclampsia.17 There is also evidence of higher interleukin-6 levels in women with anamnesis of pre-eclampsia, years after the pregnancy, which is interpreted as a sign of long-term endothelial dysfunction for those women.18
Additionally, interleukin-6 levels are known to be elevated in certain cardiovascular diseases. Higher plasma and myocardium levels of interleukin-6 were present in patients with end-stage heart failure compared to recent-onset heart failure.19 Its expression was proved to be induced in ischaemic and reperfused areas during myocardial infarction,20 and it was also able to predict future coronary incidents.21
On the other hand, changes in cardiac structure and function, as assessed echocardiographically, appear to be more pronounced during the course of hypertensive pregnancies compared to normotensive ones.22-24 This suggests a degree of abnormal cardiovascular response of the female organism during pre-eclampsia and gestational hypertension.
In this study we aimed to determine serum levels of interleukin-6 in women with pre-eclampsia and gestational hypertension, and compare them with those of healthy pregnant controls. Additionally, we examined correlations of interleukin-6 levels with some characteristics of the women and echocardiographic findings as a potential link between hypertensive disorders of pregnancy and cardiovascular diseases.
Methods
Between August 2018 and January 2020, a prospective, singlecentre, clinical, epidemiological study was conducted at the Clinic of Cardiology at the University multi-profile hospital Sveti Georgi, Plovdiv, Bulgaria, and 123 pregnant women over 18 years of age were enrolled, 37 with the diagnosis of pre-eclampsia, 36 with gestational hypertension. Fifty healthy pregnant controls were also enrolled. The women were recruited from the Clinic of Obstetrics and Gynecology in the same hospital and some of the controls were referred by local obstetrics and gynecology practices. The study included the analysis of certain biomarkers as well as echocardiographic assessment of the women.
The study was carried out according to the Declaration of Helsinki and approved by the ethics committee of the Medical University – Plovdiv. All of the participants signed a written, informed consent after a detailed explanation about the study and the required procedures.
One hundred and sixteen of the women had singleton pregnancies and nine had bigeminal pregnancies, four in the pre-eclampsia group, two in the gestational hypertension group and three controls. Current weight and height of the women were measured with standardised equipment. Weight before the pregnancy was self-reported.
A diagnosis of pre-eclampsia was established if the women had high blood pressure [office-measured systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg at least twice over the course of a minimum of four hours] registered for the first time after the 20th gestational week, and proteinuria of ≥ 300 mg/l for 24 hours. Gestational hypertension was diagnosed if high blood pressure was registered for the first time after the 20th gestational week, and proteinuria was < 300 mg/l for 24 hours.
Both hypertensive forms were considered early if the hypertension was first discovered before the 34th gestational week.21 Both hypertensive conditions were considered severe if the women had registered SBP ≥ 160 mmHg and/or DBP ≥ 110 mmHg or levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) twice the upper reference limit of the laboratory. No women in the study had thrombocytopaenia, corresponding to the criterion for severe forms of hypertensive disorder of pregnancy (< 100 × 109 cells/l).
In order to ensure that the changes in biomarker levels were not influenced by other conditions, women with recent infections and any serious systemic diseases or organ failure were not asked to participate in the study. Women with chronic arterial hypertension, diabetes mellitus and any known or significant heart diseases discovered in the course of the study were also not included. For ethical reasons women who had pulmonary congestion, encephalopathy, epigastric pain, or HELLP syndrome (all considered forms of severe HDP) or any other medical emergency were not asked to participate in the study as their participation could delay urgent medical interventions. For the control group, women with diagnosed intrauterine retardation of the foetus were not included either.
Venous blood was collected from the women in certified monovettes with a cloth activator. Serum was separated via centrifugation at 3 000 rpm for 10 minutes and then stored at –20°C as recommended by the test kit manufacturer. Serum interleukin-6 levels were determined with solid-phase sandwich ELISA (Diaclone, Besancon, France) with a biotinylated human interleukin-6 antibody.
Electrocardiograms were performed on all women in order to exclude any significant rhythm or conduction disturbances, which could compromise the results of the study. A thorough transthoracic echocardiographic examination according to a protocol was performed with the cardiovascular ultrasound system General Electric Vivid 9.5 and the echographic recordings were analysed using EchoPAC clinical workstation software version 201 (General Electric Medical System, Milwaukee, WI, USA). Measurements were performed according to the current recommendations of the guidelines of the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE).25,26
Statistical analysis
Statistical analysis was performed using IBM SPSS statistics 25.0 (IBM SPSS Statistics for Windows, SPSS Inc, Chicago, IL, USA) and MedCalc Version 14.8.1 (MedCalc Software, Mariakerke, Belgium). Continuous variables were tested for normality with Kolmogorov–Smirnov and Shapiro–Wilk tests. The Student’s t-test, analysis of variance (ANOVA) test and Bonferroni post hoc test were used to compare the continuous variables that had normal distribution and more than two independent groups with homogeneity of variances. Continuous variables with non-normal distribution were compared with the Kruskal– Wallis test and the Mann–Whitney U-test. The relationship between categorical variables in cross tables was analysed using the χ2 and Fisher’s exact tests.
Correlation analysis was performed using either Pearson’s correlation coefficient or Spearman’s rho according to the normality of the continuous variables. Receiver operating characteristic (ROC) curve analysis was carried out to determine discriminative abilities of interleukin-6. Logistic regression was performed to explain the relationship between variables. Findings with p < 0.05 were considered statistically significant.
Results
The mean maternal age of the study group was 29.93 ± 5.71 years (18–43) and the mean gestational age was 33.72 ± 4.47 weeks (22.0–39.29). The two hypertensive groups and the controls were matched for maternal and gestational age. Between the two hypertensive groups there was no statistical difference for the prevalence of early (72.2 vs 83.8%, p = 0.269) and severe forms (36.1 vs 35.1%, p = 1.000).
There was no statistical difference between the groups for women who defined themselves as smokers (44.4% in gestational hypertension, 45.9% in pre-eclampsia and 54.0% in controls; p > 0.05) and also between the current smokers who reported smoking during the pregnancy (37.5, 47.1 and 51.9%, respectively, p > 0.05). More primigravid women were in the combined hypertensive groups compared to the healthy controls (49.3 vs 24%, p = 0.008), while there was no statistical difference for women with a second (26 vs 44%, p = 0.059) and third or more pregnancies (24.7 vs 32%, p = 0.494).
Women in the hypertensive groups had significantly higher pre-pregnancy body mass index (BMI) compared to the controls (28.58 ± 6.14 kg/m2 in the gestational hypertension group, 27.26 ± 5.68 kg/m2 in the pre-eclampsia group and 22.58 ± 5.11 kg/ m2 in the controls, p < 0.05), current BMI (33.66 ± 5.75, 31.77 ± 5.32 and 27.81 ± 5.49 kg/m2, respectively, p < 0,05) and body surface area (BSA) (1.97 ± 0.20, 1.96 ± 0.18 and 1.83 ± 0.20 m2, respectively, p < 0.05). Current weight gain, calculated as the difference between self-reported pre-pregnancy weight and weight measured at the time of the inclusion in the study did not differ statistically between the three groups (13.69 ± 6.54, 12.94 ± 7.51 and 14.05 ± 6.18 kg, respectively, p > 0.05).
Mean interleukin-6 levels were significantly higher in the gestational hypertension group (5.08 ± 5.16 pg/ml, p = 0.020) and pre-eclampsia group (8.06 ± 12.48 pg/ml, p = 0.002) compared to the controls (2.77 ± 2.43 pg/ml), but the values did not differ significantly between the two hypertensive groups (p = 0.508) despite a tendency for higher levels in the pre-eclampsia group. When analysed according to the severity and onset of the disease, there was no statistical difference between the levels in the newly formed subgroups (Table 1). The difference between the controls and the late forms of both pathologies, as well as the mild form of gestational hypertension was non-significant (Table 2).
Table 1. Comparative analysis of interleukin-6 levels and forms of gestational hypertension and pre-eclampsia.
| Early onset | Late onset | ||||||
| Group | Number | Mean | SD | Number | Mean | SD | p-value |
| GH | 26 | 4.81 | 3.43 | 10 | 5.80 | 8.36 | 0.664 |
| PE | 31 | 6.46 | 7.73 | 6 | 16.34 | 25.78 | 0.888 |
| Mild form | Severe form | ||||||
| Number | Mean | SD | Number | Mean | SD | ||
| GH | 23 | 5.32 | 6.15 | 13 | 4.66 | 2.82 | 0.626 |
| PE | 24 | 8.98 | 15.06 | 13 | 6.37 | 5.26 | 0.604 |
GH: gestational hypertension; PE: pre-eclampsia; SD: standard deviation.
Table 2. Comparative analysis of interleukin-6 levels between different forms of gestational hypertension and pre-eclampsia and the controls.
| GH + PE | Controls | ||||||
| Subgroup | Number | Mean | SD | Number | Mean | SD | p-value |
| Early form of GH | 26 | 4.81 | 3.43 | 50 | 2.77 | 2.43 | 0.019 |
| Late form of GH | 10 | 5.80 | 8.36 | 0.312 | |||
| Mild form of GH | 23 | 5.32 | 6.15 | 0.149 | |||
| Severe form of GH | 13 | 4.66 | 2.82 | 0.014 | |||
| Early form of PE | 31 | 6.46 | 7.73 | 0.002 | |||
| Late form of PE | 6 | 16.34 | 25.78 | 0.256 | |||
| Mild form of PE | 24 | 8.98 | 15.06 | 0.020 | |||
| Severe form of PE | 13 | 6.37 | 5.26 | 0.006 |
GH: gestational hypertension; PE: pre-eclampsia; SD: standard deviation.
Mean levels of interleukin-6 were significantly lower in women who were in their second pregnancy compared to those in the first pregnancy. When analysing the whole study group, women whose pregnancy was the third or more did not differ significantly from those in either first or second pregnancy. When each group was analysed separately, there was a tendency for lower interleukin-6 levels in the second pregnancy for the controls and gestational hypertension groups, while for the pre-eclampsia group, the lowest levels were in women with three or more pregnancies, but the difference was not significant (Table 3).
Table 3. Comparative analysis of interleukin-6 levels according to gravidity, smoking status of the women and smoking during pregnancy by group.
| Gravidity | ||||||||||
| 1 | 2 | 3+ | ||||||||
| Groups | Number | Mean | SD | Number | Mean | SD | Number | Mean | SD | |
| Whole sample | 48 | 6.25a | 10.02 | 41 | 4.61be | 7.51 | 34 | 3.84a | 3.14 | |
| Controls | 12 | 3.64a | 2.89 | 22 | 1.97a | 1.72 | 16 | 3.20 | 2.71 | |
| GH | 20 | 6.25a | 6.12 | 7 | 3.50 | 2.47 | 9 | 3.73a | 3.90 | |
| PE | 16 | 8.21a | 15.85 | 12 | 10.09a | 12.19 | 9 | 5.09a | 3.01 | |
| GH + PE | 36 | 7.12 | 11.36 | 19 | 7.66a | 10.17 | 18 | 4.41a | 3.45 | |
| Smoking status | ||||||||||
| Never | Former | Current | ||||||||
| Number | Mean | SD | Number | Mean | SD | Number | Mean | SD | ||
| Whole sample | 43 | 5.07a | 10.68 | 15 | 2.60 | 2.68 | 60 | 5.70b | 6.36 | |
| Controls | 13 | 1.64a | 1.68 | 10 | 2.69 | 2.17 | 27 | 3.34be | 2.69 | |
| GH | 15 | 2.88 | 2.48 | 2 | 4.57 | 6.41 | 16 | 7.41b | 6.43 | |
| PE | 15 | 10.22 | 17.00 | 3 | 1.00 | 1.03 | 17 | 7.84a | 9.03 | |
| GH + PE | 30 | 6.55a | 12.51 | 5 | 2.43 | 3.82 | 33 | 7.63b | 7.76 | |
| Smoking during pregnancy | ||||||||||
| No | Yes | |||||||||
| Number | Mean | SD | Number | Mean | SD | p-value | ||||
| Whole sample | 32 | 6.13 | 7.90 | 28 | 5.21 | 4.05 | 0.646 | |||
| Controls | 13 | 3.48 | 3.29 | 14 | 3.20 | 2.11 | 0.794 | |||
| GH | 10 | 7.22 | 8.21 | 6 | 7.73 | 1.50 | - | |||
| PE | 9 | 8.72 | 11.36 | 8 | 6.85 | 6.06 | 0.743 | |||
| GH + PE | 19 | 7.93 | 9.57 | 14 | 7.23 | 4.57 | 0.358 | |||
GH: gestational hypertension; PE: pre-eclampsia; SD: standard deviation.
Same letters in the rows signify the lack of a statistical difference, while different letters signify the presence of a significant difference (p < 0.05).
Subgroups with n < 8 were not analysed due to lack of statistical representability.
Women who stated that they were smokers had significantly higher levels of serum interleukin-6 than non-smokers in the whole study group, as well as when separately analysing the controls, the gestational hypertension group and the combined hypertensive groups. For those who were smokers, smoking during pregnancy did not lead to significantly different levels of serum interleukin-6 in any of the groups analysed (Table 3). ROC curve analysis was used to determine the ability to differentiate between the hypertensive and normotensive pregnancies using interleukin-6 levels. The area under the curve (AUC) for differentiating between women with gestational hypertension and the controls was 0.65 at a cut-off point of 4 pg/ml (p = 0.020) (Fig. 1). The AUC for differentiating between women with pre-eclampsia and the controls was 0.70 at a cut-off point of 2.82 pg/ml (p = 0.002) (Fig. 2). The AUC for differentiating between the combined hypertensive group and the controls was 0.67 at a cut-off point of 2.5 pg/ml (p = 0.001) (Fig. 3).
Fig. 1.
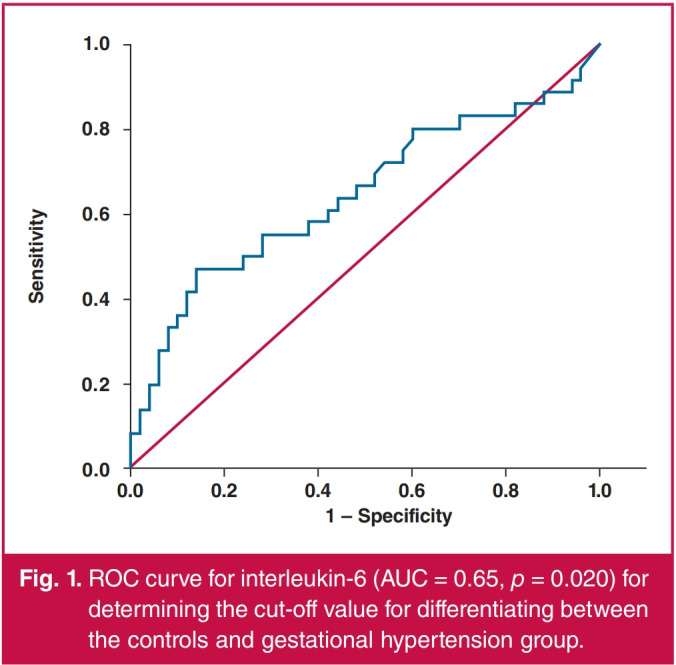
ROC curve for interleukin-6 (AUC = 0.65, р = 0.020) for determining the cut-off value for differentiating between the controls and gestational hypertension group.
Fig. 2.
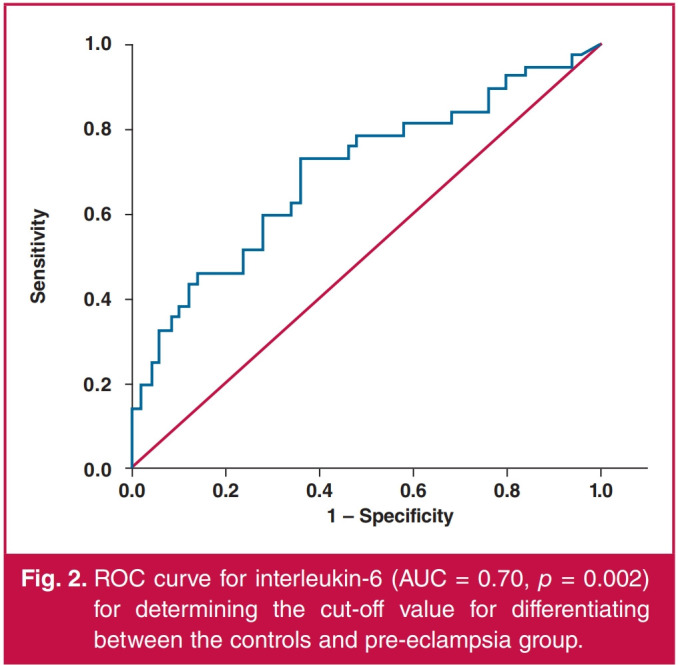
ROC curve for interleukin-6 (AUC = 0.70, р = 0.002) for determining the cut-off value for differentiating between the controls and pre-eclampsia group.
Fig. 3.
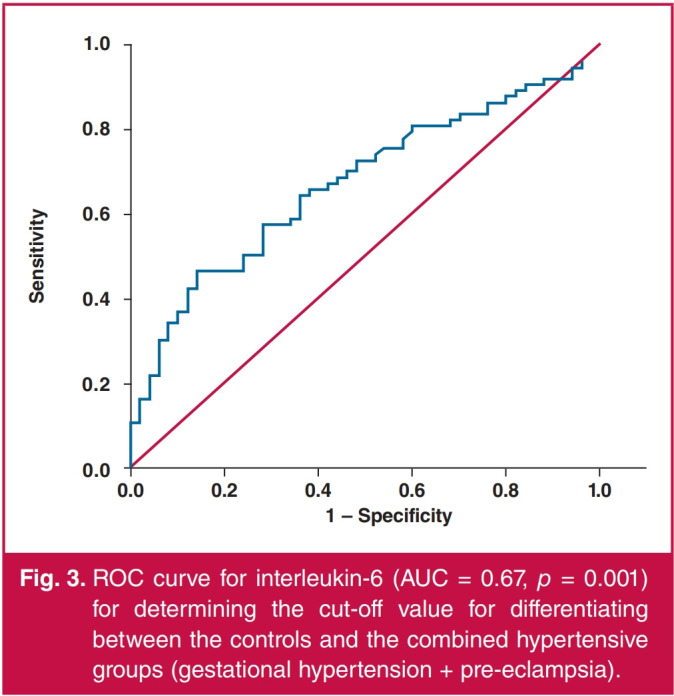
ROC curve for interleukin-6 (AUC = 0.67, р = 0.001) for determining the cut-off value for differentiating between the controls and the combined hypertensive groups (gestational hypertension + pre-eclampsia).
The respective sensitivity, specificity, positive and negative predictive values and accuracy of the cut-off values are given in Table 4. Binary logistic regression gave an odds ratio of 4.80 (95% CI: 1.90–12.13) for women with interleukin-6 levels greater than or equal to the provided cut-off points to have pre-eclampsia, and an odds ratio of 3.21 (95% CI: 1.30–7.92) for having gestational hypertension. The odds ratio for the presence of either gestational hypertension or pre-eclampsia was 3.13 (95% CI: 1.48–6.62).
Table 4. Cut-off values of interleukin-6 and values of the validation criteria for differentiation between the controls and women with pre-eclampsia and gestational hypertension.
| Group | Cut off (pglml) | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Accuracy (%) |
| GH | >4 | 56 | 72 | 59 | 69 | 65 |
| PE | 2.82 | 73 | 64 | 60 | 76 | 68 |
GH: gestational hypertension; PE: pre-eclampsia.
Correlation analysis gave positive correlations of interleukin-6 levels in the whole study group with BMI before pregnancy (r = 0.266), current BMI (r = 0.284) and BSA (r = 0.223). In the control group, all of those correlations were significant and stronger (r = 0.305; r = 0.466; r = 0.468, respectively), and also significant positive correlations with gestational age (r = 0.488) and current weight gain (r = 0.382) were present. No significant correlations existed in the gestational hypertension or pre-eclampsia groups when analysed separately. The levels did not correlate in any of the groups with maternal age, and in the hypertensive groups they did not correlate with the maximum detected systolic or diastolic blood pressure (Table 5).
Table 5. Correlation coefficients between interleukin-6 levels and certain characteristics of the women.
| Characteristics | All women | Controls | GH | PE |
| Maternal age | 0.002 | -0.105 | 0.171 | 0.061 |
| Gestational age | 0.101 | 0.488* | -0.039 | -0.068 |
| BMI before pregnancy | 0.266** | 0.305* | 0.065 | -0.007 |
| Current BMI | 0.284** | 0.466** | 0.103 | -0.040 |
| BSA | 0.223* | 0.468* | -0.101 | -0.085 |
| Current weight gain | 0.169 | 0.382° | 0.069 | 0.009 |
| Maximum SBP | - | - | 0.275 | 0.045 |
| Maximum DBP | - | - | -0.001 | 0.255 |
*p < 0.05, **p < 0.01, **p < 0.001
GH: gestational hypertension; PE: pre-eclampsia; BMI: body mass index; BSA:
body surface area; SBP: systolic blood pressure; DBP: diastolic blood pressure.
Using the data from the echocardiographic examination of the women, we found several correlations between interleukin-6 levels and certain parameters. Of the left-sided parameters (Table 6) in the whole study group, interleukin-6 levels correlated positively with the anterior–posterior diameter of the left atrium (LA), end-diastolic diameter (EDD) of the left ventricle (LV), end-systolic diameter (ESD) of the LV, left ventricular mass index (LVMI), septal thickness in diastole, stroke volume and biplane end-diastolic volume of the LV. The mean E/e′ ratio and the global longitudinal strain of the LV had a negative correlation with the S wave of the lateral mitral annulus.
Table 6. Correlation coefficients between interleukin-6 levels and left-sided echocardiographic parameters.
| Parameters | Whole study group | Controls | GH | PE | GH + PE |
| AP diameter of LA | 0.239° | 0.318* | 0.081 | 0.139 | 0.089 |
| Ind AP diameter of LA | 0.032 | -0.122 | 0.171 | 0.104 | 0.132 |
| Ind LA volume | 0.094 | -0.108 | 0.033 | 0.212 | 0.107 |
| Septum (diastolic) | 0.184° | 0.285° | -0.105 | -0.158 | -0.133 |
| Posterior wall (diastolic) | 0.118 | 0.210 | -0.118 | -0.109 | -0.102 |
| EDD | 0.293** | 0.356* | 0.117 | 0.358* | 0.238* |
| ESD | 0.317 | 0.401* | 0.219 | 0.333* | 0.268* |
| Ind EDD | 0.085 | -0.065 | 0.187 | 0.316 | 0.257 |
| Ind ESD | 0.128 | 0.021 | 0.252 | 0.353* | 0.278* |
| EDV biplane | 0.186* | 0.179 | -0.171 | 0.327* | 0.078 |
| Ind EDV biplane | 0.061 | -0.070 | -0.207 | 0.407* | 0.112 |
| ESV biplane | 0.146 | 0.225 | -0.193 | 0.227 | 0.000 |
| Ind ESV biplane | 0.047 | 0.022 | -0.192 | 0.220 | 0.011 |
| LVMI | 0.234** | 0.441" | 0.221 | -0.025 | 0.081 |
| EF (S) | -0.023 | -0.130 | 0.152 | 0.040 | 0.099 |
| SV (VTI) | 0.215* | 0.322* | 0.052 | 0.150 | 0.097 |
| Cardiac index | 0.162 | 0.135 | 0.163 | 0.237 | 0.219 |
| E/A | 0.069 | 0.007 | 0.295 | -0.129 | 0.089 |
| E-DT | -0.128 | 0.032 | -0.019 | -0.140 | -0.066 |
| e' medial | -0.097 | -0.271 | 0.357* | 0.079 | 0.189 |
| e' lateral | -0.133 | -0.232 | 0.110 | 0.047 | 0.061 |
| e' mean | -0.112 | -0.258 | 0.244 | 0.074 | 0.137 |
| E/e' mean | 0.206* | 0.176 | 0.123 | -0.209 | -0.003 |
| S medial | -0.174 | -0.173 | 0.123 | -0.178 | -0.046 |
| S lateral | -0.255** | -0.225 | 0.076 | -0.300 | -0.143 |
| LV GLS | 0.211 | 0.220 | -0.146 | -0.140 | -0.093 |
*p < 0.05, **p < 0.01, ***p < 0.001
GH: gestational hypertension; PE: pre-eclampsia; AP: anterior–posterior;
LA: left atrium; ind: indexed to BSA; EDD: end-diastolic dimension; ESD: end-systolic dimension; EDV: end-diastolic volume of the left ventricle; ESV: end-systolic volume of the left ventricle; LVMI: left ventricular mass index; EF (S): ejection fraction of the left ventricle, Simpson’s method; SV: stroke volume, calculated using VTI; E/A: ratio of the E wave and A wave of the mitral flow; E–DT: deceleration time of the E wave; e′: peak early diastolic velocity of the mitral annulus; E/e′: ratio of the E wave of the mitral flow and e′ of the mitral annulus; S: peak systolic velocity of the mitral annulus; LV GLS: global longitudinal strain of the left ventricle.
In the controls, the first six of the correlation remained significant and were stronger, while the latter four were not present. In the gestational hypertension group, there was a weak positive correlation only with the e′ wave of the medial mitral annulus, but no correlation with the mean value of the medial and lateral e′ waves. In the pre-eclampsia group, there were positive correlations with the EDD and ESD of the LV as well as the indexed ESD and the indexed and non-indexed end-diastolic volume. In the combined hypertensive group, there were correlations with the indexed and non-indexed ESD and ESD of the LV.
Of the analysed right-sided parameters (Table 7), fewer correlations were observed. In the controls the interleukin-6 levels correlated negatively with the right ventricle (RV) fractional area change (FAC) (r = –0.389, p < 0.01) and positively with the end-systolic area (ESA) of the RV (r = 0.286, p < 0.05). In the pre-eclampsia group, the levels correlated negatively with the E/e′ ratio of the tricuspid inflow (r = –0.367, p < 0.05) and positively with the FAC (r = 0.325, p < 0.05). There were no correlations in the whole study group, the gestational hypertension and the combined hypertension groups.
Table 7. Correlation coefficients between interleukin-6 levels and right-sided echocardiographic parameters.
| Parameters | Whole study group | Controls | GH | PE | GH + PE |
| Ind RA volume | 0.133 | 0.024 | 0.253 | 0.178 | 0.199 |
| EDA | 0.071 | 0.097 | -0.121 | 0.110 | -0.001 |
| ESA | 0.120 | 0.286* | -0.058 | -0.046 | -0.066 |
| Ind EDA | -0.058 | -0.249 | -0.106 | 0.203 | 0.067 |
| Ind ESA | -0.004 | 0.022 | -0.010 | -0.036 | -0.029 |
| FAC | -0.139 | -0.389* | -0.146 | 0.325* | 0.072 |
| TAPSE | 0.021 | -0.046 | 0.008 | 0.255 | 0.114 |
| TV E | -0.020 | 0.010 | 0.136 | -0.248 | -0.050 |
| TV E-DT | -0.046 | 0.031 | -0.313 | 0.002 | -0.152 |
| TV E/A | -0.034 | 0.001 | 0.134 | -0.159 | 0.007 |
| TV e' | -0.033 | -0.052 | 0.157 | 0.125 | 0.112 |
| TV E/e' | -0.021 | -0.010 | -0.007 | -0.367* | -0.168 |
| TV e'la' | -0.018 | 0.049 | 0.023 | -0.020 | -0.024 |
| TV S wave | -0.052 | 0.057 | 0.049 | -0.122 | -0.026 |
| RV GLS | 0.147 | 0.139 | 0.078 | -0.094 | -0.019 |
| AT of PV | -0.016 | -0.074 | 0.106 | -0.052 | 0.040 |
*p < 0.05, **p < 0.01, ***p < 0.001,
GH: gestational hypertension; PE: pre-eclampsia; Ind: indexed to BSA; RA: right atrial; EDA: end-diastolic area of the right ventricle; ESA: end-systolic area of the right ventricle; FAC: fractional area change of the right ventricle; TAPSE: tricuspid annular plane systolic excursion; TV E: peak velocity of early tricuspid inflow wave; TV E.DT: deceleration time of the E wave; TV E/A: the ratio between the peak velocities of the early and late tricuspid inflow waves; TV E–DT: peak early diastolic velocity of the tricuspid annulus; TV E/A: the ratio of the E wave of the tricuspid flow and the e′of the tricuspid annulus; TV e′/a′: the ratio between the peak velocities of the early and late velocities of the tricuspid annulus; TV S wave: peak systolic velocity of the tricuspid annulus; RV GLS: global longitudinal strain of the right ventricle (free wall); AT of PV: acceleration time of the pulmonary valve.
Discussion
The presence of higher levels of interleukin-6 in women with gestational hypertension and pre-eclampsia in our study confirms the inflammatory component and endothelial dysfunction in these conditions.27,28 Similar to our study, a number of other studies also found elevated interleukin-6 levels in women with pre-eclampsia,29-32 while a study by Borekci et al. did not find such an association.33
In a study by Xiao et al.,34 104 women with pre-eclampsia were analysed and compared to 75 controls, and significantly elevated levels were found in both early- and late-onset severe forms, but not in the mild cases. In the pre-eclampsia group, there was a significant difference in levels between the mild and severe forms, but no difference between early and late onset of the disease.
Similarly, in our study there was no statistical difference when comparing between early and late onset of both hypertensive disorders, however, only the early-onset disorder had statistically higher interleukin-6 levels compared to the controls. This finding could be in line with the hypothesis of several authors of the presence of different risk factors and underlying pathogenic mechanisms for the development of early- versus late-onset pre-eclampsia.35,36 The less pronounced inflammatory response in late-onset pre-eclampsia and gestational hypertension could also explain the known lower frequency of maternal and foetal complications compared to the early-onset forms.37,38
Conversely to Xiao et al.,34 we did not find a significant difference between the mild and severe forms in both the gestational hypertension and the pre-eclampsia groups, which could be due to the fact that most of the women, by the time of inclusion in the study, had only blood pressure values as an indicator of severity. The authors of an extensive review39 dealing with differences and similarities between early- and late-onset pre-eclampsia commented on the more pronounced immunological response in early-onset pre-eclampsia most likely explaining the higher probability of multi-organ damage. They consider the studies comparing early- and late-onset pre-eclampsia to be limited, especially when it comes to interleukin-6 levels.
Studies focusing on interleukin-6 levels, specifically in women with gestational hypertension, are rare and usually those women are part of a larger hypertension-in-pregnancy group.40,41 Nonetheless evidence of an inflammatory response in such women does exist. Zhang et al.42 found significantly higher levels of interleukin-6 in women with gestational hypertension compared to the controls, but unlike in our study, in their study the levels of interleukin-6 in gestational hypertension were significantly lower than in the pre-eclampsia group. Most studies deal with early- and late-onset forms of pre-eclampsia, however, our study proved a more pronounced inflammation in the earlyonset forms of gestational hypertension as well, which we believe to be an important finding.
It is interesting to note that in our study, in the control group, the levels of interleukin-6 were positively correlated with a number of parameters: pre-pregnacy and current BMI, weight gain and gestational age, while in the hypertensive groups, such correlations did not exist. This result could lead to the conclusion that during a hypertensive pregnancy, higher levels of interleukin-6 are mostly determined by the presence of the hypertensive disorder of pregnancy itself, and the influence of other factors is negligible. Similar to our study, Friis et al. found higher levels of interleukin-6 and other inflammatory markers in pregnant women with a higher BMI.43 Another group of authors found a positive correlation between foeto-maternal adiposity and inflammatory markers, including interleukin-6.44
The levels of interleukin-6 were higher in smokers for our whole study group, the controls and the gestational hypertension group, but not for the pre-eclampsia group. This result corresponds to a 2017 study45 in which the authors found higher interleukin-6 levels in non-pregnant smokers, as well as a moderate positive correlation between its levels and serum amyloid A:low-density lipoprotein levels, a marker for oxidative stress. Sunyer et al.46 also proved that interleukin-6 levels were significantly higher in the group of ever-smokers from a total of 1 003 people who survived myocardial infarction.
With regard to the echocardiographic examination in our study, in the whole study group, higher levels of interleukin-6 correlated with more pronounced structural changes of the LV and also with some parameters indicating worse diastolic function as well as worse longitudinal strain of the LV. Indexing to BSA led to the disappearance of the correlations between interleukin-6 and the EDD and ESD of the LV and the ESA of the RV. This is likely due to the moderate positive correlation between interleukin-6 and BSA. In the pre-eclampsia group, however, where higher interleukin-6 levels did not correspond to higher BSA, the indexing to BSA rendered the correlation with the EDD of the LV non-significant, but enhanced the correlation with the ESD and the EDD of the LV.
In the combined hypertension group, the correlation with EDD remained weaker but significant after indexing. However, it is worth noting that interleukin-6 had a positive correlation with RV FAC and a negative correlation with the E/e′ ratio of the tricuspid valve for the pre-eclampsia group alone. Those correlations were not present for the gestational hypertension group and the correlation with FAC in the controls was negative. Although these particular results might seem paradoxical, they could be explained by the presence of an increased contractility, which is believed to be the initial RV response to a higher afterload,47,48 likely happening as a result of the generalised vasoconstriction in pre-eclampsia, which is not as pronounced in gestational hypertension.49 Therefore higher levels of interleukin-6 could indicate the initial compensatory stages of RV involvement in the systemic syndrome of pre-eclampsia, despite corresponding to a seemingly better function.
Interleukin-6 had very few correlations in the gestational hypertension group, which could lead to the conclusion that despite significantly higher interleukin-6 levels in this population, the biomarker does not seem to directly correspond to cardiac changes as assessed echocardiographically. We are not aware of another study examining correlations between interleukin-6 levels and echocardiographic changes in human pregnancy, normotensive or otherwise.
A study by Ding et al.50 analysed the association between interleukin-6 and heart function in a pre-eclampsia model of pregnant rats. The administration of interleukin-6 further worsened the tissue Doppler mitral systolic peak velocity (Sm) and early diastolic (Ea) peak velocities in the rats with reduced uterine perfusion pressure, and increased the medial E/Ea ratio. It also increased serum concentrations of cardiac troponin I, the MB fraction of creatinine phosphokinase (CK), myoglobin and brain natriuretic peptide (BNP). The authors concluded that interleukin-6 is complicit in the myocardial damage occurring in such pregnancies. They further proved that inhibiting interleukin-6 with tocilizumab improved the E/Ea ratio and lowered the expression of BNP and CK-MB.
We identified several studies assessing the relationship between interleukin-6 levels and echocardiographic parameters in non-pregnant populations. In a study by Pauli et al.51 enrolling early-onset coronary artery disease patients and healthy controls, interleukin-6 had a negative correlation with echographic measurements of the diameter of the ascending aorta, shortening fraction of the LV and EDD of the RV.
In a different study, increased interleukin-6 levels were related to the presence of LV diastolic dysfunction, defined as mitral E/e′ mean ratio ≥ 15 in patients indicated for coronary angiography.52 In patients with systemic atherosclerosis, it had negative correlations with medial, lateral and mean e′ wave of the mitral annulus and a positive correlation with the mitral inflow E/e′ ratio.53 In a cardiac magnetic resonance study its levels correlated inversely and strongly with the regional LV function.54
Our study proves that the exaggerated inflammatory activation during hypertensive disorders of pregnancy, as expressed by interleukin-6 levels, is additionally associated with more pronounced changes in cardiac structure and function in pre-eclampsia. On one hand, we believe this link could be interpreted as proof of the role of hypertensive disorders of pregnancy, and especially pre-eclampsia, as a risk factor for future adverse cardiac outcomes. Additionally, if implemented in clinical practice, measuring higher interleukin-6 levels could be interpreted as a need for stricter follow up and control of the individual risk factors of those women in order to improve their cardiovascular profile. It is not without merit to identify biomarkers that could be used as surrogates of cardiac changes, as this could greatly influence post-pregnancy care for women.
Conclusions
Serum interleukin-6 levels were significantly elevated in women with both gestational hypertension and pre-eclampsia compared to healthy pregnant women. When analysed in subgroups the difference remained significant for severe and early forms of both conditions, as well as the mild form of pre-eclampsia. In pre-eclampsia, but not in gestational hypertension, higher levels significantly correllated with echocardiographic changes, indicative of chamber remodelling and dysfunction. Interleukin-6 levels were higher in smokers, but were not statistically different between those who smoked during pregnancy and those who did not.
Positive correlations with maternal anthropometric characteristics and gestational age were present in the controls, but not in the hypertensive groups. Women with interleukin-6 levels higher than the provided cut-off points had an OR of approximately 4.80 for the presence of pre-eclampsia and 3.21 for gestational hypertension. Interleukin-6 could therefore be used in clinical practice to improve complex care of women with hypertensive pregnancies.
Acknowledgments
The study was financially supported in the purchase of the biomarker kits by a scientific project of the Medical University – Plovdiv, DPDP N19/2019 via the Doctoral and Postdoctoral Projects programme.
Contributor Information
Dolina Gencheva, Email: Dolina.Gencheva@mu-plovdiv.bg.
Rosen Mihaylov, RAMUS Independent Medical Diagnostic Laboratory and Jordanka Filaretova Medical College, Sofia, Bulgaria.
Blagovesta Pencheva, RAMUS Independent Medical Diagnostic Laboratory, Sofia, Bulgaria.
References
- 1.Vest AR, Cho LS. Hypertension in pregnancy. Curr Atheroscler Rep. 2014;16(3):395. doi: 10.1007/s11883-013-0395-8. [DOI] [PubMed] [Google Scholar]
- 2.Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J. et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323–333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Br Med J. 2007;335(7627):974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53(6):944–951. doi: 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 5.Stuart JJ, Tanz LJ, Missmer SA, Rimm EB, Spiegelman D, James-Todd TM. et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Ann Intern Med. 2018;169(4):224–232. doi: 10.7326/M17-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riise HK, Sulo G, Tell GS, Igland J, Nygard O, Vollset SE. et al. Incident coronary heart disease after preeclampsia: role of reduced fetal growth, preterm delivery, and parity. J Am Heart Assoc. 2017;6(3):e004158. doi: 10.1161/JAHA.116.004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansson SR, Naav A, Erlandsson L. Oxidative stress in preeclampsia and the role of free fetal hemoglobin. Front Physiol. 2015;5:516. doi: 10.3389/fphys.2014.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelius DC. Preeclampsia: from inflammation to immunoregulation. Clin Med Insights Blood Disord. 2018;11:1179545X17752325. doi: 10.1177/1179545X17752325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simcox LE, Ormesher L, Tower C, Greer IA. Thrombophilia and pregnancy complications. Int J Mol Sci. 2015;16(12):28418–28428. doi: 10.3390/ijms161226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamarca B, Brewer J, Wallace K. IL-6-induced pathophysiology during pre-eclampsia: potential therapeutic role for magnesium sulfate? Int J Interferon Cytokine Mediat Res. 2011;2011(3):59–64. doi: 10.2147/IJICMR.S16320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A, Satyam A, Sharma JB. Leptin, IL-10, and inflammatory markers (TNF-alpha, IL-6 and IL-8) in preeclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol. 2007;58(1):21–30. doi: 10.1111/j.1600-0897.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 12.Bowen RS, Gu Y, Zhang Y, Lewis DF, Wang Y. Hypoxia promotes interleukin-6 and -8 but reduces interleukin-10 production by placental trophoblast cells from preeclamptic pregnancies. J Soc Gynecol Investig. 2005;12:428–432. doi: 10.1016/j.jsgi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Young JL, Libby P, Schonbeck U. Cytokines in the pathogenesis of atherosclerosis. Thromb Haemostasis. 2002;88:554–567. [PubMed] [Google Scholar]
- 14.Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin-6. Hypertension. 2006;48(4):711–716. doi: 10.1161/01.HYP.0000238442.33463.94. [DOI] [PubMed] [Google Scholar]
- 15.Orshal JM, Khalil RA. Interleukin-6 impairs endothelium-dependent NO-cGMP-mediated relaxation and enhances contraction in systemic vessels of pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2004;286(6):R1013–R1023. doi: 10.1152/ajpregu.00729.2003. [DOI] [PubMed] [Google Scholar]
- 16.Takacs P, Green KL, Nikaeo A, Kauma SW. Increased vascular endothelial cell production of interleukin-6 in severe preeclampsia. Am J Obstet Gynecol. 2003;188(3):740–744. doi: 10.1067/mob.2003.134. [DOI] [PubMed] [Google Scholar]
- 17.Tosun M, Celik H, Avci B, Yavuz E, Alper T, Malatyalioğlu E. Maternal and umbilical serum levels of interleukin-6, interleukin-8, and tumor necrosis factor-alpha in normal pregnancies and in pregnancies complicated by preeclampsia. J Matern Fetal Neonatal Med. 2010;23(8):880–886. doi: 10.3109/14767051003774942. [DOI] [PubMed] [Google Scholar]
- 18.Bokslag A, Franssen C, Alma LJ, Kovacevic I, Kesteren FV, Teunissen PW. et al. Early-onset preeclampsia predisposes to preclinical diastolic left ventricular dysfunction in the fifth decade of life: An observational study. PLoS One. 2018;13(6):e0198908. doi: 10.1371/journal.pone.0198908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubota T, Miyagishima M, Alvarez RJ, Kormos R, Rosenblum WD, Demetris AJ. et al. Expression of proinflammatory cytokines in the failing human heart: comparison of recent-onset and end-stage congestive heart failure. J Heart Lung Transplant. 2000;19(9):819–824. doi: 10.1016/s1053-2498(00)00173-x. [DOI] [PubMed] [Google Scholar]
- 20.Gwechenberger M, Mendoza LH, Youker KA, Frangogiannis NG, Smith CW, Michael LH. et al. Cardiac myocytes produce interleukin- 6 in culture and in viable border zone of reperfused infarctions. Circulation. 1999;99(4):546–551. doi: 10.1161/01.cir.99.4.546. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentrationof IL-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 22.Melchiorre K, Sutherland GR, Baltabaeva A, Liberati M, Thilaganathan B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension. 2011;57(1):85–93. doi: 10.1161/HYPERTENSIONAHA.110.162321. [DOI] [PubMed] [Google Scholar]
- 23.Melchiorre K, Sutherland GR, Watt-Coote I, Liberati M, Thilaganathan B. Severe myocardial impairment and chamber dysfunction in preterm preeclampsia. Hypertens Pregnancy. 2012;31(4):454–471. doi: 10.3109/10641955.2012.697951. [DOI] [PubMed] [Google Scholar]
- 24.Fayers S, Moodley J, Naidoo DP. Cardiovascular haemodynamics in pre-eclampsia using brain naturetic peptide and tissue Doppler studies . Cardiovasc J Afr. 2013;24(4):130–136. doi: 10.5830/CVJA-2013-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. Eur Heart J Cardiovasc Imaging. Eur Heart J Cardiovasc Imaging. 2015;2016;2016;161717(3)(4)(9):233–270. 412, 969. doi: 10.1093/ehjci/jev014. Erratum in: Erratum in: [DOI] [PubMed] [Google Scholar]
- 26.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 27.Lamarca B. Endothelial dysfunction. An important mediator in the pathophysiology of hypertension during pre-eclampsia. Minerva Ginecol. 2012;64(4):309–320. [PMC free article] [PubMed] [Google Scholar]
- 28.Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Bohm M. et al. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res. 2004;94(4):534–541. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- 29.Teran E, Escudero C, Moya W, Flores M, Vallance P, Lopez-Jaramillo P. Elevated C-reactive protein and pro-inflammatory cytokines in Andean women with pre-eclampsia. Int J Gynaecol Obstet. 2001;75:243–249. doi: 10.1016/s0020-7292(01)00499-4. [DOI] [PubMed] [Google Scholar]
- 30.Sharma A, Satyam A, Sharma JB. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol. 2007;58:21–30. doi: 10.1111/j.1600-0897.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 31.Jonsson Y, Ruber M, Matthiesen L, Berg G, Nieminen K, Sharma S. et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006;70(1–2):83–91. doi: 10.1016/j.jri.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Singh A, Sharma D, Raghunandan C, Bhattacharjee J. Role of inflammatory cytokines and eNOS gene polymorphism in pathophysiology of pre-eclampsia. Am J Reprod Immunol. 2010;63(3):244–251. doi: 10.1111/j.1600-0897.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- 33.Borekci B, Aksoy H, Al RA, Demircan B, Kadanali S. Maternal serum interleukin-10, interleukin-2 and interleukin-6 in pre-eclampsia and eclampsia. Am J Reprod Immunol. 2007;58(1):56–64. doi: 10.1111/j.1600-0897.2007.00491.x. [DOI] [PubMed] [Google Scholar]
- 34.Xiao JP, Yin YX, Gao YF, Lau S, Shen F, Zhao M. et al. The increased maternal serum levels of IL-6 are associated with the severity and onset of preeclampsia. Cytokine. 2012;60(3):856–860. doi: 10.1016/j.cyto.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 35.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obst Gynecol. 2013;209(6):544.e1–544.e12. doi: 10.1016/j.ajog.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Kucukgoz Gulec U, Ozgunen FT, Buyukkurt S, Guzel AB, Urunsak IF, Demir SC. et al. Comparison of clinical and laboratory findings in early- and late-onset preeclampsia. J Maternal-Fetal Neonatal Med. 2013;26(12):1228–1233. doi: 10.3109/14767058.2013.776533. [DOI] [PubMed] [Google Scholar]
- 37.Madazli R, Yuksel MA, Imamoglu M, Tuten A, Oncul M, Aydin B. et al. Comparison of clinical and perinatal outcomes in early- and lateonset preeclampsia. Arch Gynecol Obstet. 2014;290(1):53–57. doi: 10.1007/s00404-014-3176-x. [DOI] [PubMed] [Google Scholar]
- 38.Stubert J, Ullmann S, Dieterich M, Diedrich D, Reimer T. Clinical differences between early- and late-onset severe preeclampsia and analysis of predictors for perinatal outcome. J Perinat Med. 2014;42(5):617–627. doi: 10.1515/jpm-2013-0285. [DOI] [PubMed] [Google Scholar]
- 39.Raymond D, Peterson E. A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv. 2011;66(8):497–506. doi: 10.1097/OGX.0b013e3182331028. [DOI] [PubMed] [Google Scholar]
- 40.Mtali YS, Lyimo MA, Luzzatto L, Massawe SN. Hypertensive disorders of pregnancy are associated with an inflammatory state: evidence from hematological findings and cytokine levels. BMC Preg Childbirth. 2019;19(1):237. doi: 10.1186/s12884-019-2383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parikh NI, Laria B, Nah G, Singhal M, Vittinghoff E, Vieten C. et al. Cardiovascular disease-related pregnancy complications are associated with increased maternal levels and trajectories of cardiovascular disease biomarkers during and after pregnancy. J Womens Health. (Larchmt);2020;29(10):1283–1291. doi: 10.1089/jwh.2018.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang JY, Cao XX, Wen HX, Zhang HY. Correlation analysis of levels of inflammatory cytokines and nitric oxide in peripheral blood with urine proteins and renal function in patients with gestational hypertension . Exp Ther Med. 2019;17(1):657–662. doi: 10.3892/etm.2018.7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friis CM, Paasche Roland MC, Godang K, Ueland T, Tanbo T, Bollerslev J. et al. Adiposity-related inflammation: effects of pregnancy. Obesity. (Silver Spring);2013;21(1):E124–130. doi: 10.1002/oby.20120. [DOI] [PubMed] [Google Scholar]
- 44.Farah N, Hogan AE, O’Connor N, Kennelly MM, O’Shea D, Turner MJ. Correlation between maternal inflammatory markers and fetomaternal adiposity. Cytokine. 2012;60(1):96–99. doi: 10.1016/j.cyto.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 45.Jamil A, Rashid A, Naveed A, Asim M. Effect of smoking on interleukin- 6 and correlation between IL-6 and serum amyloid a-low density lipoprotein in smokers. J Postgrad Med Inst. 2017;31:336–338. [Google Scholar]
- 46.Sunyer J, Forastiere F, Pekkanen J, Plana E, Kolz M, Pistelli R. et al. AIRGENE Study Group. Interaction between smoking and the interleukin- 6 gene affects systemic levels of inflammatory biomarkers. Nicotine Tob Res. 2009;11(11):1347–1353. doi: 10.1093/ntr/ntp144. [DOI] [PubMed] [Google Scholar]
- 47.Dewachter L, Dewachter C. Inflammation in right ventricular failure: does it matter? Front Physiol. 2018;9:1056. doi: 10.3389/fphys.2018.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension . J Am Coll Cardiol. 2017;69:236–243. doi: 10.1016/j.jacc.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 49.Cunnigham FG, Leveno KJ, Bloom SL, Dashe JS, Hoffman BL, Casey BM, Williams Obstetrics. 24th edn. New York: McGraw-Hill Education: 2014. [Google Scholar]
- 50.Ding L, Bai C, Liu Y. Interleukin-6 contributes to myocardial damage in pregnant rats with reduced uterine perfusion pressure. Braz J Med Biol Res. 2018;51(8):e6921. doi: 10.1590/1414-431X20186921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pauli N, Puchałowicz K, Kuligowska A, Krzystolik A, Dziedziejko V, Safranow. Associations between IL-6 and echo-parameters in patients with early onset coronary artery disease. et al. Diagnostics (Basel) 2019;9(4):189. doi: 10.3390/diagnostics9040189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dinh W, Futh R, Nickl W, Krahn T, Ellinghaus P, Scheffold T. et al. Elevated plasma levels of TNF-alpha and interleukin-6 in patients with diastolic dysfunction and glucose metabolism disorders. Cardiovasc Diabetol. 2009;8:58. doi: 10.1186/1475-2840-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jurisic Z, Martinovic-Kaliterna D, Marasovic-Krstulovic D, Perkovic D, Tandara L, Salamunic I. et al. Relationship between interleukin-6 and cardiac involvement in systemic sclerosis. Rheumatology (Oxford) 2013;52(7):1298–1302. doi: 10.1093/rheumatology/ket131. [DOI] [PubMed] [Google Scholar]
- 54.Yan AT, Yan RT, Cushman M, Redheuil A, Tracy RP, Arnett DK. et al. Relationship of interleukin-6 with regional and global left-ventricular function in asymptomatic individuals without clinical cardiovascular disease: insights from the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2010;31(7):875–882. doi: 10.1093/eurheartj/ehp454. [DOI] [PMC free article] [PubMed] [Google Scholar]


