Abstract
Background
Electronic Health Record (EHR) data are increasingly being used to monitor population health on account of their timeliness, granularity, and large sample sizes. While EHR data are often sufficient to estimate disease prevalence and trends for large geographic areas, the same accuracy and precision may not carry over for smaller areas that are sparsely represented by non-random samples.
Methods
We developed small-area estimation models using a combination of EHR data drawn from MDPHnet, an EHR-based public health surveillance network in Massachusetts, the American Community Survey, and state hospitalization data. We estimated municipality-specific prevalence rates of asthma, diabetes, hypertension, obesity, and smoking in each of the 351 municipalities in Massachusetts in 2016. Models were compared against Behavioral Risk Factor Surveillance System (BRFSS) state and small area estimates for 2016.
Results
Integrating progressively more variables into prediction models generally reduced mean absolute error (MAE) relative to municipality-level BRFSS small area estimates: asthma (2.24% MAE crude, 1.02% MAE modeled), diabetes (3.13% MAE crude, 3.48% MAE modeled), hypertension (2.60% MAE crude, 1.48% MAE modeled), obesity (4.92% MAE crude, 4.07% MAE modeled), and smoking (5.33% MAE crude, 2.99% MAE modeled). Correlation between modeled estimates and BRFSS estimates for the 13 municipalities in Massachusetts covered by BRFSS’s 500 Cities ranged from 81.9% (obesity) to 96.7% (diabetes).
Conclusions
Small-area estimation using EHR data is feasible and generates estimates comparable to BRFSS state and small-area estimates. Integrating EHR data with survey data can provide timely and accurate disease monitoring tools for areas with sparse data coverage.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-022-13809-2.
Keywords: Behavioral risk factor surveillance system, Population surveillance, Asthma, Diabetes mellitus, Hypertension, Obesity, Smoking
Background
Electronic Health Record (EHR) data are increasingly being used for public health surveillance by local and national public health jurisdictions. Although data from EHRs are primarily used to manage individuals’ health, they have the potential to facilitate population health surveillance. EHRs cover far more people than traditional public health surveys, and once the infrastructure is in place, EHR data can be refreshed and analyzed in real-time without additional recruitment or interviewing costs [1]. In the past few years [2], several jurisdictions have made enormous progress in accessing and integrating electronic health data from various sources, including Chicago’s Health Atlas [3] and New York’s Macroscrope [4]. This work will focus on chronic disease prevalence estimation using data from Massachusetts’s MDPHnet [5].
Despite the advantages of EHR data over health surveys, estimating disease prevalence using EHR data remains subject to question. The first challenge is the same faced by health surveys in that, despite having numerically higher coverage rates than traditional health surveys in catchment areas of the healthcare system, geographic areas outside the catchment areas are usually underrepresented, therefore direct estimates for these areas are not available and/or not reliable. The second challenge is that the data is not randomly sampled and therefore may not represent the target population. For example, the patient population from MDPHnet overrepresents selected minorities and patients living in high poverty neighborhoods and underrepresents patients living in small rural communities. While MDPHnet has been demonstrated to return statewide estimates consistent with estimates from the Centers for Disease Control and Prevention’s (CDC’s) Behavioral Risk Factor Surveillance System (BRFSS) [6], it remains unclear how MDPHnet performs for local municipalities.
In this paper, we explore the use of small-area estimation (SAE) for disease prevalence surveillance. We integrate individual-level clinical and demographic data from the EHR with community-level socioeconomic data from the American Community Survey and community-level hospitalization data from the state health department. SAE methods “borrow” information from other communities by linking key socio-demographic information to allow estimates to be transferable to off-sampled communities. They have been applied in many contexts with traditional survey data, such as obesity [7], tobacco use [8], COPD [9], and periodontitis [10], and in the more recent CDC project Places: Local Data for Better Health [11]. We sought to extend the techniques of SAE to EHR data for five health conditions — asthma, diabetes, hypertension, obesity, and smoking. We evaluated the performance of increasingly-refined models (i.e. the effect of weighting and inclusion of more predictors in the model) with comparisons to BRFSS 500 Cities estimates for Massachusetts cities and towns. Equipped with this predictive model, we identified the municipalities at greatest risk for the aforementioned health conditions to demonstrate its potential utility for operational public health surveillance.
Methods
SAE methods can be broadly divided into design-based methods (estimates constructed from the sampling design) and model-based methods (estimates relying on a specified model) [12]. However, design-based methods fail for undercovered areas, and for these areas, only a model-based method can be utilized. Multilevel regression and poststratification (MRP) is a model-based SAE approach that was first used to estimate state-level indicators from national polls and has since been extended to track an array of health outcomes from traditional surveys, notably by the CDC’s PLACES project [11]. Additionally, the model-based nature of MRP can adjust the estimation from a nonrepresentative survey as a result of recruiting difficulties [13]. MRPs do not need to be restricted to just survey data — respresentative or not — but can also be applied to EHR data, which can be viewed as a highly nonrepresentative survey. We apply MRP techniques to five health conditions — asthma, diabetes, hypertension, obesity, and smoking — and to evaluate their performance against BRFSS’ 500 Cities estimates for Massachusetts cities and towns. We will first introduce the data sources employed in this study, and then describe the details of our MRP.
Data sources
MDPHnet: MDPHnet [5] is a distributed health data network in Massachusetts that permits authorized public health officials to submit detailed queries against the EHR data of three large multispecialty group practices that serve a combined patient population of approximately 1.1 million people (about 19% of the state population as of 2020). Each practice group uses open-source software (Electronic Medical Record Support for Public Health) [14] to create, host, and maintain a data repository behind its firewall in accordance with a common data specification. MDPHnet’s current practice partners include Atrius Health, a large multisite, multispecialty ambulatory group serving primarily a well-insured population (approximately 800,000 patients, 29 clinical locations), the Massachusetts League of Community Health Centers (MLCHC), a network of community health centers targeting underserved populations (approximately 500,000 patients, 15 clinical locations), and Cambridge Health Alliance (CHA), a combination safety net–general practice hospital and ambulatory group (approximately 200,000 patients, 15 clinical locations). The overall patient return rate across all 3 practice groups is 82% [15]. Analysis for this study was reviewed and approved by the institutional review board of Harvard Pilgrim Health Care Institute.
We analyzed 5 test conditions — diabetes, asthma, smoking, hypertension, and obesity — using EHR-data derived from MDPHnet (disease definitions are listed in Additional file 1). To facilitate comparisons against BRFSS 500 Cities estimates conducted in 2016, we restricted our analysis to anonymized, individual-level monthly data from MDPHnet from 2016 on individuals aged ≥20 with at least one outpatient visit of any kind in the health care system in the 2 years prior to each index month in 2016. The individual-level characteristics available within MDPHnet are sex, race, and age group. Because MDPHnet has measurements taken each month, we aggregated all 12 months of data for 2016 and included a time trend variable to account for temporal changes. Note that each provider will carry over mostly the same set of patients each month, although some patients may change providers and patient IDs were not provided for this analysis, making it impossible to account for within-person correlations over the months. Therefore, while we utilize all 12 months of data for estimation purposes, we inflated standard errors as if we had 1 month of data to provide conservative confidence intervals.
Community-Level Data: In addition to sex, race, and age compositions, the following seven sociodemographic data were derived from the ACS 2012–2016 5-year estimates for each of the 537 Zip Code Tabulation Areas (ZCTA) of Massachusetts: % never married, % single householder, % with bachelor’s degree, % English spoken at home, % unemployed, % receiving food stamps, and per capita income.
Hospital Discharge Data (“Case Mix”): MA hospital discharge data due to asthma, diabetes (with or without complications), and hypertension (including essential and secondary) for those aged 18 and older were obtained from Massachusetts’ case mix database. Because hospitalizations due to a particular chronic illness are far rarer than prevalent within the population, we developed a calibration model for the discharge data and blended the resulting estimates with our SAE estimators.
Behavioral Risk Factor Surveillance System Data: Our gold standard for validating our model estimates was the CDC’s Behavioral Risk Factor Surveillance System (BRFSS) MA statewide and 500 Cities estimates. For many years, the BRFSS has been a mainstay of public health surveillance for chronic diseases [16]. This telephone survey generates national and state-specific estimates of the prevalence of the major chronic diseases and risk factors that state and local public health departments rely upon to monitor health, plan interventions, and monitor their impact. Recent innovations in BRFSS methods in response to evolving surveillance needs include adding mobile telephone numbers to the sample, imputing measures for the 500 largest cities, incorporating area-level poverty as a predictor, and fielding follow-up surveys to gather clinical care information for conditions such as asthma, diabetes, hypertension, obesity, and smoking. Despite this, BRFSS is disadvantaged by the limited number of questions asked, the self-reported nature of the data, the lack of clinical data, declining response rates [17], and differential nonresponse over sex, age, race/ethnicity, income, and rurality [18].
MRP estimation procedure
We present comparisons of 2016 BRFSS statewide estimates from five candidate models:
M0: Crude estimates with no adjustments
M1: Adjustment for sex, race, age
M2: M1 + American Community Survey
: M1 + calibrated Case Mix data
: M2 + calibrated Case Mix data
To test the influence of separate practice groups’ influence on the estimators, we fit M0, M1, M2 individually for each of the three practice groups and in aggregate; the aggregate model pooled the three separate fits by weighting the estimates by relative coverage by provider per ZCTA. This relative coverage was computed as the coverage of each provider divided by the coverage of all three providers and therefore the three relative coverages sum up to 100% (see Step 3 below for more details). We included this relative coverage to account for the large differences in disease prevalence rates amongst different providers; therefore, provider affiliation was used as a proxy for additional behavioral and sociodemographic characteristics associated with disease risk. We note that for models M1 and M2, we also weighted each race, age, and sex strata by the corresponding census weight in a procedure known as poststratification [9]. We did not fit nor for each provider because the calibration procedure, as described shortly, needs sufficient coverage per provider over the available ZCTAs which was not the situation. The estimation steps are described briefly below, and more mathematical details provided in Additional File 2. All analyses were performed using R version 3.6.1.
Step 1 (Crude estimation of ZCTA-level disease prevalence per practice group): Direct prevalence estimates of the disease outcomes were computed based only off the available patients within each practice group in each ZCTA; many ZCTAs were inestimable due to lack of coverage from any of the three providers. This step forms M0 for Atrius, CHA, and MLCHC separately.
Step 2 (Adjusted estimation of ZCTA-level disease prevalence per practice group): A generalized linear mixed model (GLMM) was used to estimate ZCTA-level prevalence for each outcome within each practice group as a function of predictors. Model M1 included sex, race, and age while M2 included the full set of predictors listed under Community Level Data.
Step 3 (Pooling of crude and adjusted estimates from practice groups): Models M0, M1, M2 were then averaged over providers according to a relative coverage weighting, the weights were computed in each ZCTA as the number of patients subscribed to each provider divided by the total number subscribed to all three providers in that particular ZCTA. Stratum-specific weights were computed in a similar fashion. If data were not available for a stratum within a ZCTA, we rolled-up and replaced with the relative coverage for the overall ZCTA. If no data existed for the ZCTA, we rolled-up once again and used the overall relative coverage of the three data sources. This step forms M0, M1, M2 over all three providers.
Step 4 (Incorporation of case mix data with pooled): Hospitalization data was available for three of the five disease outcomes (asthma, diabetes, hypertension). These data contain rates of hospitalization due to a specified complication as measured by number of hospitalizations with that specified complication divided by the population of each ZCTA over the years 2011–2015. Hospitalizations do not equate to general prevalences; we therefore calibrated these crude hospitalization proportions such that the overall proportions for MA matched the overall predictions from M1 and M2 and the variation of proportions over ZCTAs matched the variation from M1 and M2 predictions. This formed and , our calibrated case mix estimates. For the other two outcomes without available case mix (obesity and smoking), their model values are deferred to M1 and M2.
Step 5 (Roll-up from ZCTAs to municipalities and statewide estimates): The 537 ZCTAs of MA were aggregated to the 351 municipalities of MA and to the entirety of MA.
Ranking municipalities
Ranking was determined by the following two-step procedure. First, the interquartile range (IQR) outlier detection test retains municipalities if their prevalence is greater than Q3 + 1.5(Q3 − Q1), where Q1 is the first quartile (25 percentile) and Q3 is the third quartile (75 percentile). Next, this shortlist is ordered based off the lower bound in a 95% confidence interval for the true proportion in that municipality; this system is known as Wilson score ranking and accounts for high prevalences which may be due to small sample sizes.
Results
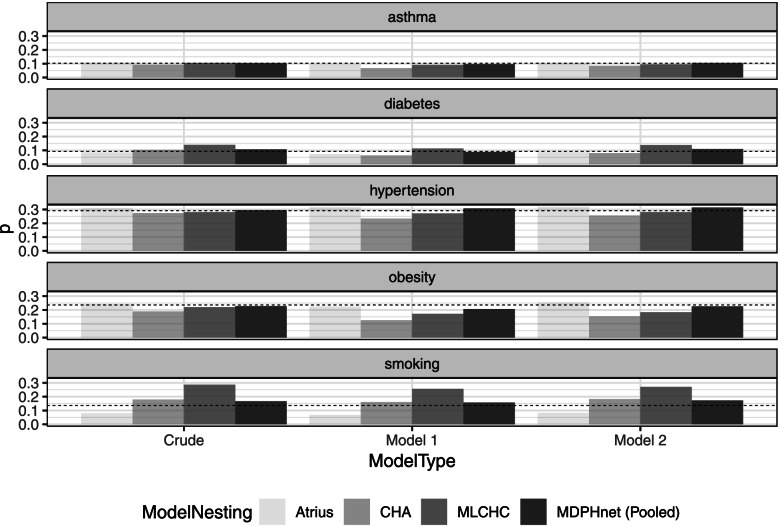
Statewide estimates of the prevalence of asthma, diabetes, hypertension, obesity, and smoking from models M0, M1, M2 vs. BRFSS are presented in Fig. 1. and are not included in Fig. 1 because they were calibrated to have matching statewide estimates as M1 and M2. The pooled estimates from M0, M1, M2 closely matched BRFSS statewide estimates. However, estimates differed widely between practices, indicating substantial between-practice differences that did not resolve even after accounting for sociodemographic characteristics. Indeed, for each model M0, M1, and M2, the ranges (largest provider prediction minus smallest) were as follows: asthma (1.61, 2.78, 1.99%), diabetes (5.40, 5.00, 5.84%), hypertension (3.49, 8.39, 6.25%), obesity (5.27, 9.86, 10.1%), and smoking (20.6, 18.8, 18.8%), indicating that obesity and smoking estimates are very provider-dependent. In addition to aggregate results, we benchmarked MDPHnet predictions against the 2016 BRFSS / CDC 500 Cities estimates for cities found within MA, namely Boston, Brockton, Cambridge, Fall River, Lawrence, Lowell, Lynn, New Bedford, Newton, Quincy, Somerville, Springfield, and Worcester.
Fig. 1.
Comparison of increasingly refined model predictions from MDPHnet vs BRFSS estimates: Massachusetts (2016) statewide aggregate. Model 1 is the sex-race-age poststratification with coverage weighting. Model 2 is the fully loaded model with coverage weighting
Tables 1 and 2 provide mean absolute error (MAE) and correlation coefficient (R) metrics to assess concordance between MDPHnet-based estimates and BRFSS. MAE and R represent two different qualities: MAE computes the averaged positive errors and therefore larger detractions from zero indicate greater differences in sampling methodology, target population definitions, model assumptions, etc. between MDPHnet estimates and BRFSS estimates; R characterizes relative agreement between MDPHnet estimates and BRFSS estimates, and therefore a higher R indicates MDPHnet would provide a more similar list of “hotspot” municipalities to that provided by BRFSS based off rankings despite differences in actual disease prevalences.
Table 1.
Mean absolute error (percentage points) for various model fits vs. 2016 BRFSS 500 Cities estimates
| M0 | M1 | M2 | ||||
|---|---|---|---|---|---|---|
| Asthma | Atrius | 1.97 | 2.05 | 0.82 | – | – |
| CHA | 2.70 | 4.82 | 2.29 | – | – | |
| MLCHC | 3.30 | 2.51 | 1.91 | – | – | |
| MDPHnet | 2.24 | 1.83 | 1.02 | 1.35 | 0.61 | |
| Diabetes | Atrius | 2.45 | 3.46 | 1.38 | – | – |
| CHA | 2.86 | 4.81 | 2.88 | – | – | |
| MLCHC | 4.35 | 2.33 | 5.31 | – | – | |
| MDPHnet | 3.13 | 1.84 | 3.48 | 1.30 | 3.68 | |
| Hypertension | Atrius | 4.82 | 2.18 | 2.46 | – | – |
| CHA | 5.62 | 7.94 | 4.02 | – | – | |
| MLCHC | 3.53 | 3.25 | 2.22 | – | – | |
| MDPHnet | 2.60 | 2.33 | 1.48 | 1.33 | 2.23 | |
| Obesity | Atrius | 5.29 | 5.13 | 2.99 | – | – |
| CHA | 8.68 | 15.2 | 9.06 | – | – | |
| MLCHC | 6.55 | 9.64 | 6.95 | – | – | |
| MDPHnet | 4.92 | 6.92 | 4.07 | 6.92 | 4.07 | |
| Smoking | Atrius | 9.15 | 12.47 | 8.94 | – | – |
| CHA | 3.08 | 5.80 | 3.54 | – | – | |
| MLCHC | 8.92 | 6.60 | 8.53 | – | – | |
| MDPHnet | 5.33 | 2.84 | 2.99 | 2.84 | 2.99 |
Table 2.
Correlation coefficients (out of 100) for various model fits vs. 2016 BRFSS 500 Cities estimates
| M0 | M1 | M2 | ||||
|---|---|---|---|---|---|---|
| Asthma | Atrius | 24.1 | −7.4 | 68.8 | – | – |
| CHA | 23.9 | − 36.5 | 45 | – | – | |
| MLCHC | 57.3 | 77.7 | 90.9 | – | – | |
| MDPHnet | 40.8 | 55.3 | 88.9 | 92.6 | 96.0 | |
| Diabetes | Atrius | 51.8 | −0.6 | 82.6 | – | – |
| CHA | 0.4 | −68.6 | 48.5 | – | – | |
| MLCHC | 45.1 | 7.5 | 90.8 | – | – | |
| MDPHnet | 67.1 | 77.1 | 96.7 | 83.3 | 91.5 | |
| Hypertension | Atrius | 54.6 | 83.7 | 85.5 | – | – |
| CHA | −0.4 | −20.6 | 59.9 | – | – | |
| MLCHC | 86.6 | 94.2 | 96.7 | – | – | |
| MDPHnet | 76.5 | 85.9 | 92.9 | 96.7 | 93.7 | |
| Obesity | Atrius | 77.6 | 70.6 | 88.3 | – | – |
| CHA | 51 | −37.0 | 52.5 | – | – | |
| MLCHC | 56.4 | 83.3 | 86.6 | – | – | |
| MDPHnet | 57.5 | 86.7 | 81.9 | 83.3 | 86.6 | |
| Smoking | Atrius | 81 | 30 | 73.6 | – | – |
| CHA | 73.9 | 14.4 | 70.2 | – | – | |
| MLCHC | 52.5 | 68.6 | 86.7 | – | – | |
| MDPHnet | 82.5 | 85.8 | 93.9 | 85.8 | 93.9 |
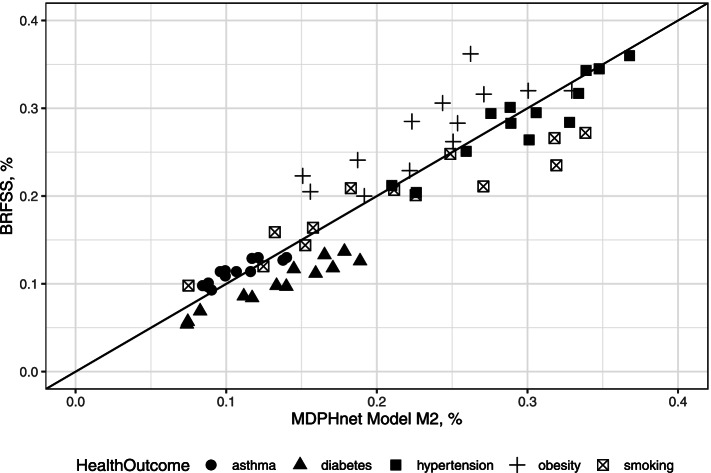
For the vast majority of target cities, M2 provided the closest concordance with BRFSS estimates using both MAE and R; case mix adjustment did not add much. For M2, we see that these two sets of estimates were especially close for asthma, diabetes, hypertension, and smoking but less so for obesity. The MAEs between M2 and BRFSS were asthma (1.02%), diabetes (3.48%), hypertension (1.48%), obesity (4.07%), and smoking (2.99%). The R2 between model (3) and BRFSS were asthma (88.9%), diabetes (96.7%), hypertension (92.9%), obesity (81.9%), smoking (93.9%), which all represent strong agreement in the ranking of municipality outcome prevalences. Furthermore, Table 2 provides additional evidence of strong associations between choice of providers and health outcomes, with varying correlations for each provider-specific estimates vs BRFSS estimates. Figure 2 provides a graphical representation of the information provided in Tables 1 and 2. Within each disease category, MAE is the average of the distances from the diagonal line and R2 characterizes how colinear (and therefore, how concordant) the estimates are between M2 and BRFSS. We note that MDPHnet coverage of an area also heavily influences predictions; Fig. 3 indicates a downward difference in MDPHnet estimates vs BRFSS estimates as coverage increases.
Fig. 2.
Comparison of MDPHnet M2 vs BRFSS: Massachusetts (2016) small-area estimates within the 13 overlapping municipalities from the 2016 BRFSS 500 Cities. Each marker indicates a single location and condition. The diagonal line marks where perfect agreement between MDPHnet and BRFSS would lie
Fig. 3.
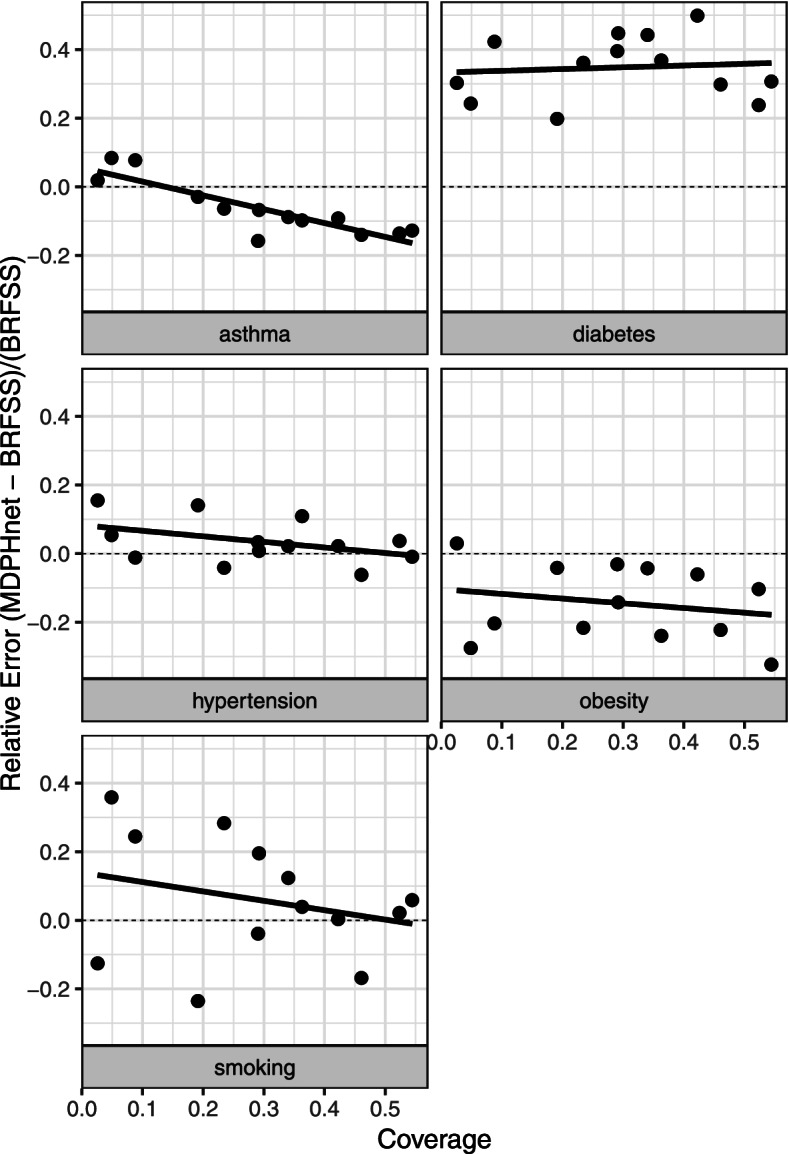
Relative error of MDPHnet M2 from BRFSS estimates vs MDPHnet coverage within the 13 overlapping municipalities from the 2016 BRFSS 500 cities
In addition to small-area estimates for larger municipalities found within MA, we use M2 to estimate the prevalences of each of the 5 target conditions for the remaining 338 municipalities in Massachusetts and to pinpoint those with the highest prevalence rates (Additional file 3 for this list of predictions). Table 3 contains five subtables corresponding to each health outcome and lists the top five at-risk municipalities under the methods described in Ranking municipalities Section and based off predictions from M2. From this procedure, we identified 17 municipalities for asthma, 11 for diabetes, 1 for hypertension, 4 for obesity, and none for smoking.
Table 3.
Rankings of highest at-risk municipalities for each disease outcome
| Rank | Municipality | 2012–2016 ACS Population | Prevalence | Confidence Interval |
|---|---|---|---|---|
| (a) | Top 5 MA asthma-risk municipalities by Wilson lower bound | |||
| 1 | Revere | 42,125 | 0.170 | (0.167, 0.174) |
| 2 | Orange | 6061 | 0.167 | (0.157, 0.176) |
| 3 | Malden | 48,521 | 0.155 | (0.151, 0.158) |
| 4 | Chelsea | 27,849 | 0.149 | (0.145, 0.154) |
| 5 | North Adams | 12,390 | 0.145 | (0.139, 0.151) |
| (b) | Top 5 MA diabetes-risk municipalities by Wilson lower bound | |||
| 1 | Brockton | 67,992 | 0.189 | (0.186, 0.192) |
| 2 | Everett | 33,062 | 0.188 | (0.183, 0.192) |
| 3 | Lawrence | 55,244 | 0.178 | (0.175, 0.182) |
| 4 | Chelsea | 27,849 | 0.174 | (0.170, 0.178) |
| 5 | New Bedford | 71,200 | 0.171 | (0.168, 0.174) |
| (c) | Top 5 MA hypertension-risk municipalities by Wilson lower bound | |||
| 1 | Lenox | 4094 | 0.435 | (0.420, 0.450) |
| (d) | Top 5 MA obesity-risk municipalities by Wilson lower bound | |||
| 1 | Lawrence | 55,244 | 0.329 | (0.326, 0.333) |
| 2 | Chelsea | 27,849 | 0.324 | (0.318, 0.329) |
| 3 | Randolph | 26,684 | 0.304 | (0.298, 0.309) |
| 4 | Brockton | 67,992 | 0.301 | (0.297, 0.304) |
| (e) | Top 5 MA smoking-risk municipalities by Wilson lower bound | |||
| NA | NA | NA | NA | NA |
Discussion
This paper demonstrates small-area estimation techniques are useful to hone EHR-based disease prevalence estimates for areas that are underrepresented in EHR data and can pinpoint communities with statistically higher disease rates to help public health agencies target interventions. In general, the inclusion of socioeconomic and community data fine-tunes estimates because it constitutes ecological factors which are associated with health outcomes. We found that including sociodemographic factors improved agreement with BRFSS estimates above and beyond adjusting for sex, race, age, and relative coverage and was far superior to a crude model. Intuitively, a model fit with more data from a target ZCTA should be given more weight in its prediction, which our relative coverage weighting exactly stipulates.
Compared to traditional health surveys, EHRs offer larger and more pertinent data thus potentially offering useful insights for real-time chronic disease monitoring and expected trends. The use of EHRs for public health surveillance has been limited due to concerns about the non-random coverage of patients. We demonstrate that EHR-based estimates drawn from multiple practice groups and adjusted using individual demographics, the relative contribution by practice, and community-level socioeconomic data can provide disease prevalence estimates that are very similar to small area estimates derived from traditional health surveys. This may allow for broader utilization of EHR data to facilitate timely and detailed public health surveillance for both large and small areas.
Strengths and limitations
MDPHnet demonstrates the great potential for EHR-based chronic disease surveillance. EHR-based surveillance can monitor large populations with clinical information such as diagnosis and pharmacy data. The electronic nature of the data facilitates real-time statistical analysis. However, EHRs contain information only on those people receiving health care, who may have characteristics different from those not receiving health care. Because the proportion of a population receiving care may depend in part on health insurance coverage, EHR-based surveillance may perform worse in jurisdictions with lower insurance coverage. In addition, MDPHnet data suggest that the validity of small-area estimates may vary depending on the proportion of the local population covered by the EHR and the number of participating providers. Small-area estimates may be imprecise if the participating EHRs cover only a small portion of the population. Ultimately, this concern can be mitigated by adding more practice groups’ EHR data into existing surveillance systems and therefore increasing the proportion of the population under surveillance. Improved EHR coverage would be the first step in improving record keeping of healthcare-seeking behaviors, access, afforability, and insurance coverage. To the extent one can measure these metrics, incorporating them into SAE models would better inform predictions. Further work is necessary to determine the impact of these factors and to validate EHR-based estimates for racial/ethnic subgroups, as modeling based on just demographic characteristics alone is not enough.
Finally, EHR-based surveillance cannot replace traditional population-based surveillance surveys like BRFSS. Surveillance surveys collect data on health behaviors and self-perceived health status in the general population which are not typically available in EHRs (such as exercise, diet, and well-being) as well as data on people not in care. Nevertheless, while we treated BRFSS as the gold standard in this exercise, surveys continue to encounter selection bias and the concordance between BRFSS and MDPHnet SAE estimates does not necessarily exhibit the true chronic disease prevelances.
Conclusions
Development of SAEs can be critical during an emerging public health event, especially one distributed over a wide geographic area. In MA and other jurisdictions, survey response rates are declining, and results from traditional surveillance systems are unreliable for smaller municipalities or ZCTAs because these areas do not have sufficient samples to produce robust estimates. While EHR-based surveillance is still in its infancy, replicating and extending the models developed in this paper offer prospects of detailed and timely public health surveillance for small and large jurisdictions across the country. Standardizing methods and algorithms and sharing technological solutions will help facilitate this work.
The methods in this work were adapted from statistical techniques that have been used for identifying individual- and community-level risk factors. Our approach is straightforward and cost-effective, can be easily translated into routine practice and implemented with existing data via readily available statistical software packages. Proper adaptation of these methods may expand the scope of existing national health surveillance and data collection systems and provide health information and specific recommendations that are directly relevant to local communities and governments.
Supplementary Information
Additional file 1. MDPHnet Disease Identification Criteria. Conditions disease definitions for asthma, diabetes, hypertension, obesity, smoking.
Additional file 2. Details of the Estimation Procedure. Contains more statistical details regarding the estimation procedure discussed in MRP estimation procedure Section.
Additional file 3. Predictions from each model (M0, M1, M2) for each test condition (asthma, diabetes, hypertension, obesity, smoking) for each practice (Atrius, CHA, MLCHC, aggregate) in each of the 351 municipalities.
Acknowledgments
Not applicable.
Abbreviations
- ACS
American Community Survey
- BRFSS
Behavioral Risk Factor Surveillance System
- CDC
Centers for Disease Control and Prevention
- CHA
Cambridge Health Alliance
- EHR
Electronic health records
- IQR
Interquartile range
- MA
Massachusetts
- MAE
Mean absolute error
- MLCHC
Massachusetts League of Community Health Centers
- SAE
Small-area estimation
- ZCTA
ZIP code tabulation area
Authors’ contributions
TC performed all data wrangling and analysis, production of tables and figures, and write-up of the manuscript. MK conceptualized the idea which brought forth this paper and WL provided statistical input; both MK and WL reviewed and approved the final manuscript. BZ provided data preparation. The author(s) read and approved the final manuscript.
Funding
Funding for this work was provided by the Massachusetts Department of Public Health.
Availability of data and materials
MDPHnet and hospital discharge data are not publicly available but are available from the corresponding author upon reasonable request. BRFSS 2016 estimates are available from the Centers for Disease Control and Prevention [https://chronicdata.cdc.gov/500-Cities-Places/500-Cities-Local-Data-for-Better-Health-2018-relea/rja3-32tc]. 2012–2016 ACS data are available from the US Census Bureau [https://www.census.gov/acs/www/data/data-tables-and-tools/data-profiles/2016/].
Declarations
Ethics approval and constent to participate
This study was approved by the Harvard Pilgrim Health Care Institutional Review Board under IRB Reference #1352491 and was considered as non-human subjects research because data were either publicly available or de-identified. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable to this study.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim RS, Shankar V. Prevalence estimation by joint use of big data and health survey: a demonstration study using electronic health records in New York city. BMC Med Res Methodol. 2020;20:1–10. doi: 10.1186/s12874-020-00956-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perlman SE, McVeigh KH, Thorpe LE, Jacobson L, Greene CM, Gwynn RC. Innovations in population health surveillance: using electronic health Records for Chronic Disease Surveillance. Am J Public Health. 2017;107(6):853–857. doi: 10.2105/AJPH.2017.303813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chicago Health Atlas. http://www.chicagohealthatlas.org/. Accessed 10 Jan 2021.
- 4.Newton-Dame R, McVeigh KH, Schreibstein L, et al. Design of the New York City Macroscope: innovations in population health surveillance using electronic health records. EGEMS (Wash DC) 2016;4(1):1265. doi: 10.13063/2327-9214.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel J, Brown JS, Land T, Platt R, Klompas M. MDPHnet: secure, distributed sharing of electronic health record data for public health surveillance, evaluation, and planning. Am J Public Health. 2014;104(12):2265–2270. doi: 10.2105/AJPH.2014.302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klompas M, Cocoros NM, Menchaca JT, et al. State and local chronic disease surveillance using electronic health record systems. Am J Public Health. 2017;107(9):1406–1412. doi: 10.2105/AJPH.2017.303874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W, Kelsey JL, Zhang Z, et al. Small-area estimation and prioritizing communities for obesity control in Massachusetts. Am J Public Health. 2009;99(3):511–519. doi: 10.2105/AJPH.2008.137364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Land T, Zhang Z, Keithly L, Kelsey JL. Small-area estimation and prioritizing communities for tobacco control efforts in Massachusetts. Am J Public Health. 2009;99(3):470–479. doi: 10.2105/AJPH.2007.130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Holt JB, Lu H, et al. Multilevel regression and poststratification for small-area estimation of population health outcomes: a case study of chronic obstructive pulmonary disease prevalence using the behavioral risk factor surveillance system. Am J Epidemiol. 2014;179(8):1025–1033. doi: 10.1093/aje/kwu018. [DOI] [PubMed] [Google Scholar]
- 10.Eke P, Zhang X, Lu H, et al. Predicting periodontitis at state and local levels in the United States. J Dent Res. 2016;95(5):515–522. doi: 10.1177/0022034516629112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.PLACES: Local Data for Better Health. https://www.cdc.gov/places/index.html. Accessed 31 Mar 2021.
- 12.Rahman A. A review of small area estimation problems and methodological developments. https://researchoutput.csu.edu.au/ws/portalfiles/portal/25229570/A_Review_of_Small_Area_Estimation.pdf. Accessed 28 May 2022.
- 13.Wang WRD, Goel S, Gelman A. Forecasting elections with non-representative polls. Int J Forecast. 2015;31(3):980–991. doi: 10.1016/j.ijforecast.2014.06.001. [DOI] [Google Scholar]
- 14.Klompas M, Lazarus R, Daniel J, Haney G, Campion F, Kruskal B. Electronic medical record support for public health (ESP): automated detection and reporting of statutory notifiable diseases to public health authorities. Adv Dis Surv. 2007;3(3):1–5. [Google Scholar]
- 15.Cocoros NM, Ochoa A, Eberhardt K, Zambarano B, Klompas M. Denominators matter: understanding medical encounter frequency and its impact on surveillance estimates using EHR data. EGEMS (Wash DC) 2019;7(1):31. doi: 10.5334/egems.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.500 Cities: Local Data for Better Health. https://www.cdc.gov/500cities. Accessed 31 Mar 2021.
- 17.Czajka JL, Beyler A. Background paper declining response rates in federal surveys: trends and implications. Mathematica Policy Res. 2016;1:1–86. [Google Scholar]
- 18.Crouch E, Radcliff E, Strompolis M, Hartley SN. Behavioral risk factor surveillance system state survey on exposure to adverse childhood experiences (ACEs): who declines to respond? Child Youth Serv Rev. 2018;91:259–262. doi: 10.1016/j.childyouth.2018.06.024. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. MDPHnet Disease Identification Criteria. Conditions disease definitions for asthma, diabetes, hypertension, obesity, smoking.
Additional file 2. Details of the Estimation Procedure. Contains more statistical details regarding the estimation procedure discussed in MRP estimation procedure Section.
Additional file 3. Predictions from each model (M0, M1, M2) for each test condition (asthma, diabetes, hypertension, obesity, smoking) for each practice (Atrius, CHA, MLCHC, aggregate) in each of the 351 municipalities.
Data Availability Statement
MDPHnet and hospital discharge data are not publicly available but are available from the corresponding author upon reasonable request. BRFSS 2016 estimates are available from the Centers for Disease Control and Prevention [https://chronicdata.cdc.gov/500-Cities-Places/500-Cities-Local-Data-for-Better-Health-2018-relea/rja3-32tc]. 2012–2016 ACS data are available from the US Census Bureau [https://www.census.gov/acs/www/data/data-tables-and-tools/data-profiles/2016/].