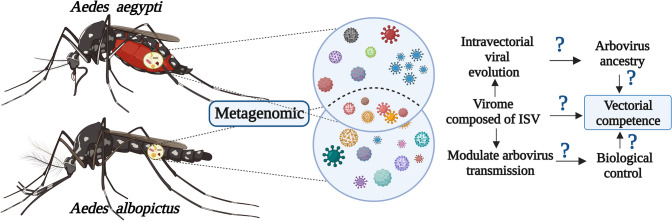
Abstract
Abstract
Aedes aegypti and Aedes albopictus are the main vectors of highly pathogenic viruses for humans, such as dengue (DENV), chikungunya (CHIKV), and Zika (ZIKV), which cause febrile, hemorrhagic, and neurological diseases and remain a major threat to global public health. The high ecological plasticity, opportunistic feeding patterns, and versatility in the use of urban and natural breeding sites of these vectors have favored their dispersal and adaptation in tropical, subtropical, and even temperate zones. Due to the lack of available treatments and vaccines, mosquito population control is the most effective way to prevent arboviral diseases. Resident microorganisms play a crucial role in host fitness by preventing or enhancing its vectorial ability to transmit viral pathogens. High-throughput sequencing and metagenomic analyses have advanced our understanding of the composition and functionality of the microbiota of Aedes spp. Interestingly, shotgun metagenomics studies have established that mosquito vectors harbor a highly conserved virome composed of insect-specific viruses (ISV). Although ISVs are not infectious to vertebrates, they can alter different phases of the arboviral cycle, interfering with transmission to the human host. Therefore, this review focuses on the description of Ae. aegypti and Ae. albopictus as vectors susceptible to infection by viral pathogens, highlighting the role of the microbiota-virome in vectorial competence and its potential in control strategies for new emerging and re-emerging arboviruses.
Graphical Abstract
Keywords: Arbovirus, ISV, Metagenomics, Microbiota, Vectors, Virome
Background
Vector-borne diseases significantly impact public health, affecting approximately 30% of the world's population [1–3]. In particular, viral pathogens transmitted to humans by insects—arboviruses—are one of the main concerns due to the accelerated increase in their incidence and geographical distribution in recent years [4–6], with dengue (DENV), Zika (ZIKV), and chikungunya (CHIKV) the arboviruses of most significant medical importance in the world and requiring active epidemiological surveillance [7–9]. DENV, the most prevalent viral infection in tropical and subtropical countries [10–14], infects between 100 and 400 million people per year, and its incidence has increased 30-fold in recent decades [15, 16]. Since 2000, CHIKV and ZIKV have spread and caused significant outbreaks in Asia and the Americas [17–19].
DENV, CHIKV, and ZIKV are transmitted to humans predominantly through the highly competent mosquitoes Aedes aegypti and Aedes albopictus [20, 21]. Aedes aegypti is a mosquito vector with a wide distribution worldwide, especially in tropical and subtropical environments, and is closely associated with urban areas and areas with environmental disturbances [22, 23]. Contrastingly, Ae. albopictus presents a greater geographical expansion [24], colonizing all five continents [20]. Despite vector control efforts, in recent years an increase in the geographical distribution of Aedes spp. has been detected due to factors associated with climate change [5, 25–27], globalization [28–30], urbanization [29–31], and resistance to different insecticides [32]. As a result, these vector species are considered a serious threat [33], compromising the effectiveness of preventive measures, control programs, and the management of outbreaks of the diseases they are involved in.
Furthermore, the search and discovery of viruses in insect vectors have accelerated in the last decade, thanks to advances in metagenomic sequencing technologies [34–37]. However, studies on vector virome are still scarce [37], and the role of insect-specific viruses—ISVs (viruses that only replicate in arthropod cells)—is virtually unknown, despite the importance in the prevention and control of mosquito-borne diseases. Different investigations indicate that ISVs are closely related to families of pathogenic viruses such as Flaviviridae and Togaviridae [38, 39], where DENV, CHIKV, and ZIKV are found. Therefore, ISVs can become potential pathogenic viruses in invertebrates [40, 41]; on the contrary, they could be highly related to the competition of vectors to transmit arboviruses [42, 43], or serve in the future as biological control agents against known arboviruses [44, 45]. Knowledge about the composition of viral communities in mosquitoes of the genus Aedes will contribute to the understanding of the mosquito–virus–pathogen interaction, as well as to elucidating new control strategies in the face of new arboviral epidemics. This review focuses on the description of the composition and diversity of the microbiota-virome of vectors involved in the transmission of arboviruses such as Ae. aegypti and Ae. albopictus. Also, the role of these microbes in the modulation of vectorial capacity and in the potential strategy of biological control is highlighted.
Aedes aegypti and Aedes albopictus: competent vectors in arboviral transmission
Aedes aegypti (Stegomyia aegypti) and Ae. albopictus (Stegomyia albopicta) are of interest primarily because of their association with emerging and re-emerging infectious diseases [17]. These two mosquito vectors have been described as highly competent in the transmission of arboviral pathogens such as DENV, ZIKV, and CHIKV [5, 22, 23, 29, 33, 46]. The species share several characteristics that give them adaptive advantages over others, making them successful invaders. The rapid spread and adaptation in tropical, subtropical, and temperate zones, and thus expansion of global coverage [5, 21, 26], may be related to large-scale epidemics and recent simultaneous outbreaks [20, 29]. Aedes aegypti is originally from Africa, considered a primary vector of some arboviruses. It has a high potential for pathogen transmission to humans due to its purely anthropophilic habits, reproduction in domestic (urban) and peridomestic environments, use of artificial containers as breeding places [5, 47], and greater availability of natural containers for oviposition [48, 49]. In comparison, Ae. albopictus, known as the tiger mosquito (Asian), is ecologically more flexible, with a more comprehensive geographical range than Ae. aegypti [24, 50]. It is found in suburban, rural, and sylvatic habitats, where it presents a wide range of hosts including humans, livestock, amphibians, reptiles, and birds [24, 29]. Both vectorial species present high ecological plasticity in heterogenous anthropic, climatic, and environmental conditions [25, 31, 50, 51]. Thus, Ae. aegypti and Ae. albopictus also reveal opportunistic feeding patterns in multiple human hosts during a gonotrophic cycle [52], diapause states (metabolism decreased at meager rates of energy expenditure and subsequent inactivity) during the development of eggs in drought conditions [53], resistance to insecticides such as DDT (dichlorodiphenyltrichloroethane) and pyrethroids [54], and versatility in the use of clean or stagnant water hatcheries in urban and natural environments [24].
Aedes aegypti and Ae. albopictus are considered two of the most invasive mosquito species [23, 50]. Competition between the two species in their ranges, whether native or invaded, frequently causes competitive displacement of one of the species [55], which can modify the epidemiology of arboviral diseases [56–58]. However, today, the two still coexist in large regions of the world [21, 59]. Several authors have revealed that the coexistence of Ae. aegypti and Ae. albopictus vector species in the same geographical areas can increase the risk of infection or co-infection for humans, especially during outbreaks or arboviral expansion [22, 26, 46, 60, 61]. Braks et al. [48] demonstrated that habitat is a determining factor in the abundance of the two species. Although Ae. aegypti predominates in urban areas and Ae. albopictus in rural areas, the two species can coexist in peri-urban areas, as demonstrated in several regions of Brazil and in the state of Florida in the United States. Thus, the segregation of different habitats may be a mechanism promoting coexistence between species, which avoids direct competition [55, 62].
Different factors influence the spatio-temporal relationships of virus–vector–human transmission, including vector capacity, vector competence, and host susceptibility [63]. Vector competence is defined as the intrinsic ability of the vector to successfully transmit a virus [63]. It normally comprises the capacity of a vector to acquire, maintain, and transmit a pathogen agent [64]. In contrast, vector capacity involves environmental factors such as temperature, vertebrate host availability, vector feeding behavior, population density, longevity, and predation [63]. Scientific evidence indicates that factors such as climate, vegetation, and building density affect the distribution of both species [5, 51, 57, 65], which determines the probability of arbovirus transmission in each region. In addition, virus–vector dynamics associated with the genetic and immunology background of each population of Aedes and the associated microbiota, including ISVs and arboviral variants, also play essential roles in the spread of the virus by vector species [23, 66, 67].
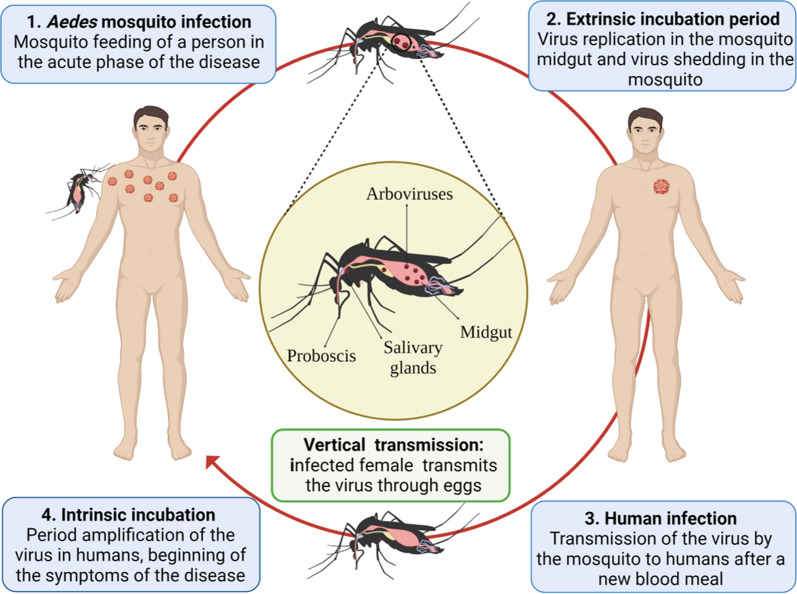
In epidemic arboviruses such as DENV, ZIKV, and CHIKV, the urban (enzootic) cycle is essential for maintaining transmission without requiring other cycles to achieve disease persistence [8, 9, 15]. Transmission of arboviruses by vectors is carried out in various steps (Fig. 1): (1) infection of the vector (female mosquitoes) after feeding with blood from a host during the acute febrile or viremic phase of the disease [15]; (2) extrinsic incubation period, with the replication of the virus in the middle intestine of the mosquito and its spread to distal tissues, until it reaches the salivary glands (reservoir organs for the virus) [68, 69], a process influenced by the ambient temperature, the strain of the virus, immunological response of the mosquito, and the vector capacity [63]; (3) transmission of the virus from the infectious mosquito to a human during a new blood-feeding [9]; and (4) intrinsic incubation period and appearance of disease symptoms. Symptoms develop within an incubation period of 4 to 10 days after being bitten by an infected mosquito and usually last 2 to 7 days. The asymptomatic or symptomatic person can transmit the virus to a new mosquito and keep the epidemic cycle active [15, 33].
Fig. 1.
The urban cycle of arboviruses in humans and mosquitoes. Figure created with BioRender.com
Interestingly, vertical or transovarial transmission, defined as the transfer of pathogens from the infected parent to part of the offspring [70], also occurs when the infected female mosquito transmits the virus through eggs [68] (Fig. 1). This phenomenon can occur especially during interepidemic periods and periods of drought [59, 71, 72]. In endemic areas, during unfavorable periods for horizontal transmission, the ability of some arboviruses to persist in the environment after long periods of few or no documented human cases is not clear [73, 74]. Although vertical transmission occurs at low rates, it can limit effective surveillance for infectious diseases and the achievement of comprehensive arboviral control. In particular, during the extrinsic incubation period, the genetic diversity of the virus population decreases stochastically as the virus crosses different anatomical barriers for transmission [69, 71], such as the midgut, where viral replication occurs in distal organs for dissemination, and salivary glands where transmission is finally ensured. Therefore, virus populations capable of overcoming these tissue barriers are considered to undergo positive or purifying selection where new genotypes may emerge [15, 40, 69], influencing vector competence in mosquitoes.
Microbiota and vector competence in Ae. aegypti and Ae. albopictus
In recent years, studies on the biology of arbovirus-transmitting insects have shown that in addition to disease-causing pathogens, mosquitoes harbor many microorganisms such as bacteria, viruses, fungi, and parasites [23, 75–77]. It has been shown that microbial composition and functionality in mosquitoes are influenced by genetic factors of the host and the environment [78]. Therefore, it is believed that mosquito microbiomes can vary substantially among individuals, life stages, species, and geographical area [36, 79]. In Ae. aegypti and Ae. albopictus, there is evidence that acquisition of the microbiota is attributed primarily to trans-stage transmission from larva to adult and consumption of water, nectar, or other environmental food sources [35, 78, 80]. The vectorial competence, blood consumption, and infection by different pathogens may persistently or transiently change the bacterial composition through alterations in the redox state of metabolism. However, despite these differences, there appears to be a “core microbiota,” a collection of critical bacterial taxa that commonly colonize different mosquito species [35, 81], although the specific roles of these taxa and their relationship to vectorial competence remain unclear.
In the case of adult Ae. aegypti and Ae. albopictus mosquitoes, Proteobacteria, Bacteroides, Firmicutes, and Actinobacteria are the phyla that group more than 99% of the total components of the microbiota community [36]. Some of the bacteria associated with the different body organs of Aedes mosquito species are Acetobacter, Burkholderia, Cupriavidus, Elizabethkingia, Escherichia-Shigella, Ochrobacterium, Pantoea, Serratia, and Sphingomonas at the salivary gland level; Asaia, Bacillus, Chryseobacterium, Chromobacterium, Cupriavidus, Enterobacter, Enterococcus, Klebsiella, Kluyvera, Leucobacter, Pantoea, Pichia, Pseudomonas, Serratia, and Sphingomonas at the midgut level; and Pseudomonas, Acinetobacter, Cupriavidus, Ochrobacterium, Stenotrophomonas, and Wolbachia at the reproductive organ level [35, 36, 75, 82, 83]. Although the bacterial components of the mosquito microbiota are widely investigated [76, 84–86], some studies also include other entities such as fungi and yeasts identified from metagenomic shotgun sequencing [87] and targeted sequencing of the 28S marker (28S rRNA). In laboratory-reared and field-collected Aedes, mainly yeasts such as Candida and Pichia have been identified, as well as a variety of filamentous fungi such as Penicillium [35]. The protists of helminths comprising this microbiota and their influence on the insect physiology remain unknown.
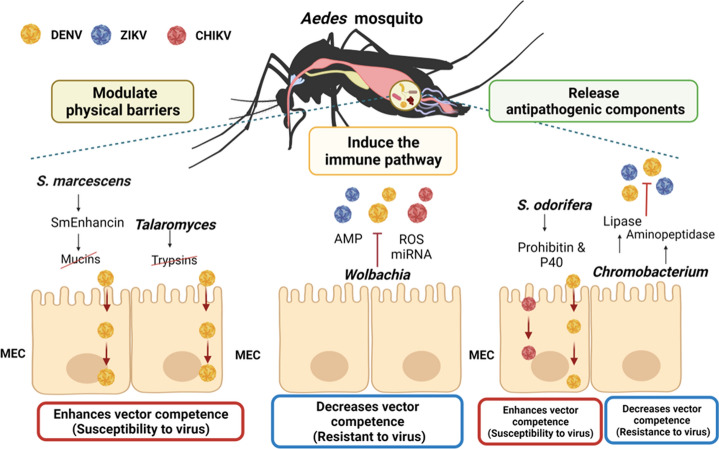
Recent studies have shown that bacterial communities of adult Ae. aegypti and Ae. albopictus can play an essential role in regulating viral invasion by generating resistance or susceptibility to infection against arboviruses and other pathogens [64, 75, 84, 86, 88] (Fig. 2). Thus, some patterns of microbial regulation have been described that ultimately modulate the vectorial competence. Strategies of viral regulation include modulation of physical barriers in midgut epithelial cells (MEC), activation of immune response signaling pathways, and release of antipathogenic components. One of the symbionts that promote susceptibility to arbovirus infection is Serratia marcescens. This can degrade mucins bound to the intestinal membrane of Ae. aegypti by releasing the Sm enhancin protein, which decreases the natural protection of intestinal mucus [89] and thereby promotes the spread of pathogenic viruses such as DENV in the mosquito gut. Similarly, the fungus Talaromyces sp. in this same vector species can suppress the expression of digestive enzymes (trypsin) in the midgut of Aedes mosquitoes and, consequently, increase susceptibility to DENV infection [90] (Fig. 2).
Fig. 2.
Aedes mosquito–microbiota–arbovirus interactions may modulate vectorial competence. Some viral regulatory strategies include the following: Modulation of physical barriers in midgut epithelial cells (MEC). Serratia marcescens by releasing Sm enhancin protein and the fungus Talaromyces by suppressing the expression of digestive enzymes (trypsin) in the midgut of Aedes can promote susceptibility to DENV infection. Activation of immune response signaling pathways and release of antipathogenic components. Wolbachia in the presence of arboviruses can induce antimicrobial peptides (AMP), melanization, and the production of reactive oxygen species (ROS), among others, that restrict arboviral activity. Release of antipathogenic compounds. Chromobacterium sp. Panama (Csp_P), by degrading the arbovirus coat protein, limits the replication of DENV and ZIKV, while Serratia odorifera participates in the interaction between P40 polypeptide and prohibitin, proteins associated with DENV and CHIKV infection in Aedes. Red boxes indicate interactions that increase vector competence (susceptibility to virus infection). Blue boxes indicate interactions that decrease vector competence (resistance to virus infection). CHIKV chikungunya virus, ZIKV Zika virus, DENV dengue virus, ROS reactive oxygen species, AMP antimicrobial peptides, miRNA microRNA, MEC midgut epithelial cells, P40 polyvinentide P40, Pr prohibitin. Figure created with BioRender.com
Wolbachia, an intracellular symbiont prevalent in some insects, has been identified as capable of reducing vectorial competition by manipulating the reproduction of its host insects and generating cytoplasmic incompatibility [75]. Experimental trials show a significant reduction in the transmission of DENV, ZIKV, CHIKV, and yellow fever virus (YFV) in Aedes mosquitoes inoculated with this bacterium [67, 91]. Curiously, negative interactions between Wolbachia and arboviruses are mainly associated with enhanced insect immune response. This occurs by inducing antimicrobial peptides (AMP), melanization, and the production of reactive oxygen species (ROS) [84]. Likewise, it has been demonstrated that this microorganism, by inducing microRNA (miRNA) production, suppresses the expression of essential genes during viral genome methylation [92, 93]. In addition, Wolbachia can compete for resources by sequestering cholesterol and other lipids in insect cells [94], which ultimately limits arboviral infections (Fig. 2).
Interestingly, bacteria with broad-spectrum antipathogenic activity against arboviruses, such as Chromobacterium sp. Panama (Csp_P), can reduce DENV infection in Ae. aegypti by degrading the viral envelope protein through the production of a type of aminopeptidase [95, 96]. This bacterium can also restrict Plasmodium falciparum infection in Ae. gambiae through the antiparasitic protein romidepsin [97]. In contrast, Serratia odorifera can increase DENV or CHIKV infection in Ae. aegypti due to the effect generated by the interaction between the P40 polypeptide, encoded by the bacterium, and prohibitin, a protein related to arboviral infection in mosquito cells [98, 99] (Fig. 2).
These results have opened a window for further research to understand mosquito–-microbiota interactions, their influence on arboviral infection in Ae. aegypti and Ae. albopictus, and the development of novel vector control strategies. Paratransgenesis stands out among the main strategies developed with the use of microbiota to control arboviral and vector-borne diseases [35]. This technique is based on the genetic manipulation of symbiotic bacteria to produce antipathogen effector molecules, followed by the reintroduction of the modified symbiont into the arthropod host to reduce vector competence [75]. Interestingly, genetic engineering research has evaluated molecules generated by “stable” species of the vector microbiota that are commonly present in different mosquito species (“core” microbiota).
Most of these studies have focused on persistent symbionts, capable of secreting antagonistic molecules that are horizontally and/or vertically transmitted, thus allowing self-sustainment of the modified symbionts in the field [36]. In addition, these symbionts must present the potential to survive long enough in the mosquito to ensure the effective and constant production of effectors that limit pathogen replication in the vector [35, 67, 84]. The primary investigations have studied genetic modifications of symbiont microbiota of insects such as Rhodnius prolixus to control the Trypanosoma cruzi parasite, the causative agent of Chagas disease, and Anopheles spp. for the control of the Plasmodium parasite, the causative agent of malaria [84]. In the case of Aedes, the bacterium Asaia is postulated as a promising option for arboviral control due to its ability to colonize both laboratory and field mosquitoes [35, 80, 82, 83, 100]. In addition, this bacterial species has been used in paratransgenesis for malaria control, demonstrating limitations in larval development in Anopheles spp. [35]. Despite the advances achieved, most studies are still in vitro trials due to the possible environmental risk that could be generated by the release of genetically modified organisms and the potential implications of interactions between modified microorganisms and native insect vectors.
Based on advances in next-generation sequencing, especially shotgun metagenomics, much better understanding of the complexity of the Ae. aegypti and Ae. albopictus microbiota in the presence or absence of viral pathogens is expected. Currently, it is important to move towards a deeper understanding of the inherent molecular mechanisms of interaction between the microbiota, the pathogens of Aedes mosquitoes, and their impact on the modulation of vectorial capacity. This new metagenomic next-generation sequencing (mNGS) approach can favor the detection of microorganisms that can be exploited to develop different applications for the efficient management of Aedes vectors and the diseases they transmit. In addition, considering that the population structure of Ae. aegypti and Ae. albopictus is strongly influenced by geography and the type of breeding site, the anthropogenic, environmental, and geographical factors that affect acquisition should also be considered in future studies and abundance of microbial communities. This information could be used to better understand the potential of the microbiome to prevent or increase mosquitoes' ability to transmit medically important arboviral pathogens, depending on the conditions of the habitat where these vector species circulate or coexist.
Metagenomics and the rise of virome studies in mosquitoes
Metagenomic next-generation sequencing (mNGS), also known as shotgun deep-sequencing, is a high-throughput sequencing strategy with high efficiency and a short turnaround time [101]. mNGS is defined as “the application of the modern genomics without the need for isolation and laboratory culture of individual species” [102], and facilitates the understanding of the composition of all genetic material (DNA or RNA) in a clinical or environmental sample [103]. The deployment of mNGS has identified structural and functional diversity of vertebrates and invertebrates microbiomes [35, 78, 104]. In the case of viral communities in insects, even recently there was a “biased” conception of viruses as agents that cause disease; however, today, with the advances in metagenomics studies, the proper structural and functional diversity of insect viromes has begun to be elucidated [104–106]. Thus, metagenomics methods, especially during the last decade, have provided new insights into the complexity of insect-borne viruses [41, 107–109], relevant for active pathogen surveillance and response to emerging and re-emerging infectious diseases [37, 103, 110, 111].
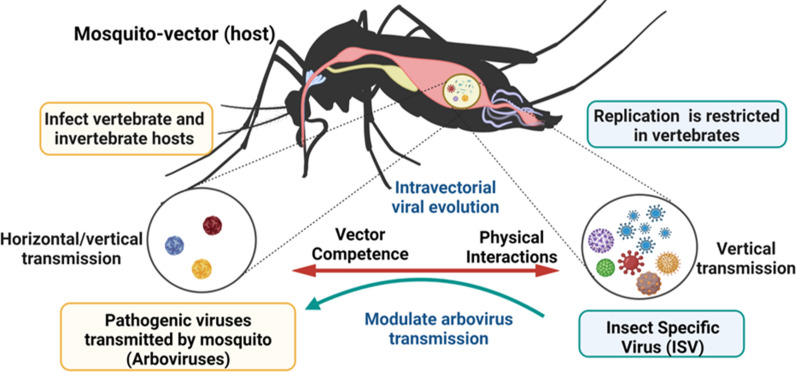
Metagenomic studies in mosquito vectors are relatively recent. This technique is one of the most common sequencing approaches for the characterization of mosquito microbial communities and the description of phylogenetic relationships [35, 100, 112]. With this new approach, it has been possible to elucidate that Ae. aegypti and Ae. albopictus mosquitoes harbor a rich and diverse virome composed mainly of ISVs [39, 107, 113–115]. Unlike arboviruses, which have dual host tropism (vertebrates and arthropod vectors), ISVs replicate exclusively in insect populations and cannot replicate in vertebrate cells or infect humans [41, 116, 117]. Therefore, being naturally associated with arthropods, they can be considered members of viral communities of insects exclusively (viromes), and are not considered pathogens [104, 117]. In adult insects, the highest proportion of ISVs characterized so far correspond to RNA viruses [104] without causing apparent affectation, suggesting their high adaptation and relationship with insects [104].
Metaviromic analyses in mosquitoes carried out recently show a significant increase in the number of specific viral or ISV sequences, both in natural populations and in mosquito-derived cell lines [37, 116, 118]. These findings indicate a higher abundance and prevalence of ISVs in mosquito populations than in arboviruses in approximately 1–2% of individuals [34]. Research on ISVs in mosquito vectors has focused mainly on Culex spp. mosquitoes, Aedes spp., and Anopheles spp. [109, 115, 119–121]. Phylogenetic analyses on the reconstruction of ancestral traits in ISVs indicate that there are variations in the diversity and abundance of viruses between vector species; however, viral families such as Flaviviridae (positive-sense [+] single-stranded RNA [ssRNA]), Bunyaviridae (negative-sense ssRNA [−]), Rhabdoviridae (ssRNA), Reoviridae (double-stranded RNA [dsRNA]), and Togaviridae (+ssRNA) are shared [34, 37–39, 122]. These discoveries have opened a new view on the diversity and evolution of ISVs [38, 39, 116] and their influence on vectorial competence [123] for efficient transmission of human pathogens (arboviruses) [118], in addition to their potential use as biological control agents or new vaccine platforms [44, 45] (Fig. 3).
Fig. 3.
Schematic summary of some characteristics of the Aedes mosquito virome from metagenomic studies. The Aedes virome is formed by arboviruses and in greater proportion by insect-specific viruses (ISV). Arboviruses are pathogenic viruses that are transmitted to humans by mosquito vectors (dual host), while ISVs are viruses that replicate exclusively in insects and are not capable of infecting humans (single host). ISVs possibly evolved and diversified with their insect hosts. Some ISV species could potentially modulate vector competence in Aedes spp. Advances in vector viromics may contribute to the development of strategies to control and prevent arboviral diseases. Figure created with BioRender.com
Phylogenetic analyses and experimental studies have shown that many ISVs isolated from mosquitoes contain ancient and diverse lineages, which possibly evolved and diversified with their host insects [122, 124]. Research on the reconstruction of ancestral traits in ISVs of Bunyavirales has evidenced a basal phylogenetic relationship between the dual-host bunyavirus and insect-specific ancestors [125]. The close relationship found between ISVs and human pathogenic arboviruses [38, 39, 122] has generated the hypothesis about the role they can play in modulating arboviral transmission [44, 45] (Fig. 3). ISVs are found at all stages of life in both male and female mosquitoes. This is associated with their efficient transmission to offspring (transovarial transmission) [104, 118] and coexistence with their insect host over a long period [39, 124]. In addition, there is evidence of the presence of viral RNA in the insect transcriptome and a high incidence of endogenous copies in the insect genome, suggesting a crucial role of ISV in the evolution of RNA viruses [126]. In this way, it could be inferred that, if ISVs are ancestral to arboviruses, they could be studied to understand the evolution from a single host to a dual host, as well as to elucidate the factors that influence the “switch” of viruses from arthropods to viruses with emerging potential.
Virome in Ae. aegypti and Ae. albopictus
Metagenomics studies in Ae. aegypti and Ae. albopictus conducted in different geographical areas of the world (Americas, Asia, Africa, Europe, and Australia) [73, 107, 121, 127–134] have identified that Aedes host a viral community with high diversity. Currently, the available data focus mainly on Ae. aegypti, compared with the virome of Ae. albopictus, possibly because it is considered the primary vector of arboviruses and with a vast geographical distribution [23, 107, 114]. It should be noted that most of the viruses discovered lack a formal taxonomic classification (unclassified viruses), which limits the proper understanding of the diversity of the circulating virome in these vector species. This condition highlights the need to generate more robust databases allowing us to improve the viral characterization of mosquitoes that transmit infectious diseases.
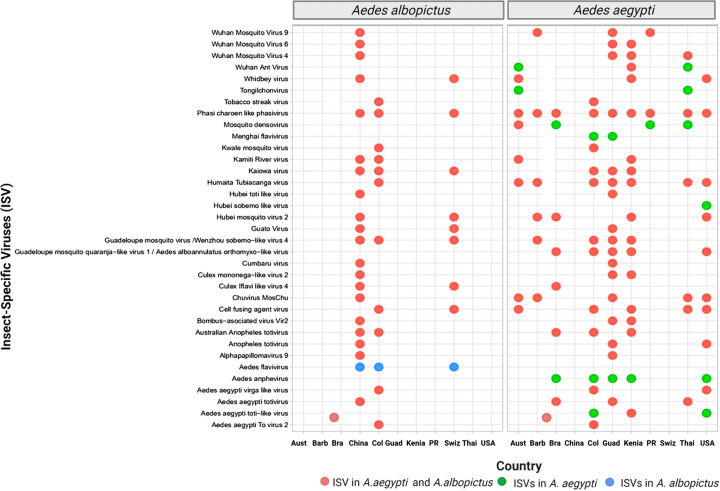
Curiously, in these investigations, similarity in the composition of the virome has been evidenced in Aedes species, made up mainly of the families Flaviviridae, Totiviridae, Phenuiviridae, Orthomyxoviridae, Virgaviridae, and Secoviridae. In addition, this research highlights the conservation of viral species in the Aedes virome, such as Phasi Charoen-like virus (PCLV) (Phenuiviridae), Humaita-Tubiacanga (HTV) (unclassified viruses), Guadeloupe mosquito virus (GMV)/Wenzhou sobemo-like virus 4 (unclassified viruses), cell fusing agent virus (CFAV; Flaviviridae), Guadeloupe mosquito quaranja-like virus 1/Aedes alboannulatus orthomyxo-like virus (Orthomyxoviridae), and Australian Anopheles totivirus (Totiviridae) (Fig. 4), demonstrating the presence of a “core virome,” perhaps associated with its ecology, similar food sources, selective host pressures, and microbial interactions [67, 112]. Therefore, it is considered that the “core virome” in Ae. aegypti and Ae. albopictus likely plays a role in mosquito homeostasis and may also have implications in vector competition for arbovirus transmission [39, 118, 132]. Future studies are needed to fully understand these complex interactions.
Fig. 4.
Conservation of insect-specific viruses (ISV) (virome) in Ae. aegypti and Ae. albopictus in countries of different geographical areas of the world. Pink circles indicate the presence of shared ISVs in Ae. aegypti and Ae. albopictus, yellow circles indicate unique ISVs in Ae. aegypti, and blue circles indicate unique ISVs in Ae. albopictus. Aust Australia, Barb Barbados, Bra Brazil, Nig Nigeria, Guad Guadeloupe, Ken Kenya, PR Puerto Rico, Suiz Switzerland, Tha Thailand, USA United States of America
In most metavirome studies carried out so far, the viral families Flaviviridae, Orthomyxoviridae, Totiviridae, and Phenuiviridae have been the most prevalent (Fig. 4). This suggests a possible origin from the ancestral Aedes mosquito and evolution with the vector in different parts of the planet. This is consistent with several authors that propose processes of co-evolution and diversification between ISVs and the insect [113, 116, 121, 135], as previously suggested for the families Bunyaviridae, Flaviviridae, and Rhabdoviridae [38, 123]. In particular, the family Totiviridae has been mainly associated with fungi and plants [136]. However, its prevalence has increased among arthropods, possibly due to horizontal virus transfer mechanisms [132]. According to Shi et al. [137], interspecies virus transmission is a joint event across the ISV landscape, and it has likely been a significant factor in the evolutionary history of such viruses. Considering this new vision on the virome of Ae. aegypti and Ae. albopictus mosquitoes, subsequent studies could focus their attention on investigating how these viruses could influence mosquito–virus–pathogen interactions in the dynamics of arbovirus–insect transmission.
Some investigations of viral communities in Ae. aegypti and Ae. albopictus also show a stable virome profile at different life stages (larva, pupa, and adult), grown in the laboratory and collected in the field [107, 127]. In this way, several studies suggest that the “core virome” can be acquired vertically (from parent to offspring) [127] or from the environment [127]. Accordingly, studies by Coatsworth et al. [132] confirmed the vertical transfer of ISVs in Ae. aegypti by metagenomic studies conducted over several generations. Similarly, Thannesberger et al. [128] showed that the local ecosystem could play a preponderant role in the composition of viral communities in mosquito vectors. Interestingly, most metaviromic analyses in Aedes mosquitoes collected from the field show the presence of a “core virome” with more extraordinary diversity than the virome of laboratory mosquitoes. This is probably associated with the variable geographical and environmental conditions found in nature versus the standardized conditions of water and resources available in the laboratory [37, 129, 130]. The evident contrast between the scenarios in which field and laboratory mosquitoes are exposed could reflect the viral diversity of their respective environments [132]. Thus, future investigations could identify the impact of different biological and environmental variables on ISVs.
Comparative analyses between the virome of Ae. aegypti and Ae. albopictus show differences in viral composition and diversity. Metagenomic studies show a higher ISV richness in Ae. aegypti [73, 107, 128, 131, 132], related to its high susceptibility to arbovirus infection [23, 29, 63]. In contrast, the virome of Ae. albopictus presents a wide diversity of viruses associated with vertebrates, insects, plants, bacteria, and fungi [24, 127, 129, 130], which can probably be explained by the different cycle, ecotope, and environmental factors, including breeding and feeding sites [29, 80]. In addition, it must be highlighted that specific viral species have been identified for each vector and geographical region. However, unique ISVs have been detected in Aedes anphevirus (AeAV; Xinmoviridae) and Aedes aegypti totivirus (Totiviridae), while in Ae. albopictus only the ISV Aedes flavivirus (AeFV; Flaviviridae) has been found. These differences in the virome composition of these vectors could reflect essential differences in evolutionary history and host immune responses [78, 81] and differences in virus–mosquito interactions, potentially related to vector competence [35, 45, 112].
ISVs seems to modulate arbovirus infections in Ae. aegypti and Ae. albopictus
Considering the role of some symbiont members of the microbiota of insect vectors, it is considered that some ISVs may also have a similar effect on vector competition by suppressing or enhancing arbovirus replication in insect vectors [45, 104, 111, 117]. Some authors have proposed that mosquito-associated ISVs could have potential applications as (i) biological control agents against vector-borne diseases, (ii) diagnostic therapies, and (iii) new vaccine platforms [34, 35, 44, 45, 104, 117, 118].
The first characterized ISVs belong to the genus Flavivirus, as the CFAV, isolated from a culture in a cell line of Ae. aegypti [138]. This virus can also replicate in Ae. albopictus cell lines; however, it does not show a cytopathic effect on invertebrate cell lines. Recent studies by Zhang et al. [139] found that CFAV infection significantly improved DENV replication, possibly due to an increase in the expression of ribonuclease kappa (RNASEK), known to promote infection of endocytosis-dependent viruses and pH-dependent entry. The authors indicate that increased CFAV-induced RNASEK expression will likely contribute to improved DENV replication in CFAV-infected cells [139].
Research conducted by Schultz et al. [42] examined cell lines of Aedes species. The suppression of the arbovirus in the presence of ISV CFAV and PCLV demonstrates that dual ISV infection managed to decrease the growth of ZIKV, DENV, and La Crosse encephalitis virus (LACV) by up to 90% in immunocompetent cells of Ae. albopictus and Ae. aegypti. Another study characterized Aedes anphevirus (AeAV), a negative-sense RNA virus of the order Mononegavirales, capable of infecting laboratory colonies, wild mosquitoes, and cell lines of Ae. aegypti and Ae. albopictus worldwide [140]. It was identified that Ae. aegypti cells, co-infected with AeAV and Wolbachia, improved AeAV replication and slightly reduced DENV replication in vitro [140]. These data suggest that there are mechanisms of viral competition [141] as exclusion by superinfection. They may involve competition or modification of cellular resources that reduce receptor binding, viral entry, RNA replication, and translation of the secondary virus [42, 45, 142] or mechanisms of both positive and negative regulation of the antiviral immune response of the vector [39].
A similar study evaluated Nhumirim virus (NHUV) (Flaviviridae) pre-inoculated or inoculated simultaneously with ZIKV and dengue-2 virus (DENV-2) in Ae. albopictus cells, showing a significant reduction of these viruses [43]. Additionally, trials with individuals of Ae. aegypti also demonstrated decreased ZIKV infection rates in mosquitoes inoculated with NHUV compared with those not exposed [43]. Likewise, in Culex quinquefasciatus, a decrease in the transmission rates of West Nile virus (WNV) was observed when the vector was previously exposed to NHUV [143]. These results indicate that some ISV species could modulate vector competition in Aedes spp. and Culex.
On the other hand, Nazar et al. [144] investigated the ability of Eilat virus (EILV), an ISV of the Togaviridae family, to interfere with viruses of the same family, such as Sindbis virus (SINV), CHIKV, western equine encephalitis viruses (WEEV), eastern equine encephalitis virus (EEEV), and Venezuelan equine encephalitis virus (VEEV). In vitro results in Ae. aegypti C7/10 cells showed that EILV infection reduced replication of pathogenic viruses regardless of virus or multiplicity of infection. In addition, in vivo trials in Aedes mosquitoes pre-inoculated with EILV and fed blood containing CHIKV also showed a reduction in the rate of infection and the spread of CHIKV [144]. These results suggest different interactions between ISVs and arboviruses, such as competitive inhibition and superinfection exclusion [123]. However, it should be noted that studies on the relationship between ISV–arbovirus–microbiota of insects are only just emerging. Therefore, new investigations are needed to better understand this type of interaction, which may result in the development of new approaches for the control and prevention of arboviral transmission.
Conclusions
Aedes aegypti and Ae. albopictus mosquitoes that transmit viruses of medical and economic importance, such as DENV, CHIKV, and ZIKV, among others, continue to be a major threat to global public health. Currently, the geographical spread of these mosquito vectors, and thus of arboviruses, is increasingly extending to new regions under the influence of multiple social, environmental, and ecological factors. In recent years, shotgun metagenomic sequencing has improved our understanding of the composition and, in part, the functionality of the Aedes microbiota/virome. These advances have revealed the critical role that some of the resident microorganisms play in host fitness, especially in the presence and absence of viral pathogens. It is known that Ae. aegypti and Ae. albopictus share a core set of microorganisms, especially bacterial and viral. However, many aspects of this area of knowledge remain unclear. Therefore, understanding the true diversity of the mosquito microbiome and its interactions with the host, as well as its dynamics in the face of arbovirus infection events, is critical, given the evidence as potential biological control agents. Recent metavirological analyses indicate that the “core virome” of Ae. aegypti and Ae. albopictus, composed mainly of ISVs, has the potential to alter the susceptibility of mosquitoes to certain arboviruses, as well as to show evolutionary relationships with these pathogens. Further studies, especially of ISVs, including isolation, whole-genome sequencing, and even functional assays, are thus warranted to better understand their origins, pathogenic potential, molecular mechanisms affecting vector competence, and potential biotechnological applications.
It is therefore emphasized that microbiota–ISV–arbovirus interactions in the mosquito (host) form an ecologically complex system, influenced by the geographical and environmental conditions to which the vector is exposed. For this reason, future analyses should also consider the anthropogenic, environmental, and geographical factors that influence the acquisition and abundance of the microbial communities of Ae. aegypti and Ae. albopictus, and identify the change in the composition and diversity of the microbiota/virome in the different life stages of Aedes (pupa, larva and adult), at both the field and laboratory levels. This information could be used to better understand the potential of the microbiota to prevent or enhance the mosquito's ability to transmit arboviral pathogens of medical importance, depending on the habitat conditions where these vector species circulate or coexist. Finally, it is concluded that the path is open for further strengthening of omics and bioinformatics sciences, towards a better understanding of Aedes insect biology and arbovirus epidemiology, and the development of potential “novel” arboviral intervention strategies.
Acknowledgements
We thank the Dirección de Investigación e Innovación from Universidad del Rosario for covering the publication fees of this manuscript.
Abbreviations
- ISV
Insect-specific viruses
- AMP
Antimicrobial peptides
- CHIKV
Chikungunya virus
- DENV
Dengue virus
- DDT
Dichlorodiphenyltrichloroethane
- miRNA
MicroRNA
- MEC
Midgut epithelial cells
- PCR
Polymerase chain reaction
- P40
Polyvinentide P40
- Pr
Prohibitin.
- ROS
Reactive oxygen species
- ZIKV
Zika virus
Author contributions
JDR conceived the article. MG drafted the manuscript, with contributions from DM, MM and JDR. All authors read and approved the final manuscript.
Funding
Open Access funding provided by Colombia Consortium. This work was supported by Dirección de Investigación e Innovación from Universidad del Rosario.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marcela Gómez, Email: alida.gomez@urosario.edu.co.
David Martinez, Email: davidf.martinez@urosario.edu.co.
Marina Muñoz, Email: claudia.munoz@urosario.edu.co.
Juan David Ramírez, Email: juand.ramirez@urosario.edu.co, Email: juan.ramirezgonzalez@mountsinai.org.
References
- 1.Golding N, Wilson AL, Moyes CL, Cano J, Pigott DM, Velayudhan R, Brooker SJ, Smith DL, Hay SI, Lindsay SW. Integrating vector control across diseases. BMC Med. 2015;13:249. doi: 10.1186/s12916-015-0491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Global vector control response 2017–2030. Geneva: World Health Organization; 2017. [Google Scholar]
- 3.Mboera LE, Mweya CN, Rumisha SF, Tungu PK, Stanley G, Makange MR, Misinzo G, De Nardo P, Vairo F, Oriyo NM. The risk of dengue virus transmission in Dar es Salaam, Tanzania during an epidemic period of 2014. PLoS Negl Trop Dis. 2016;10:e0004313. doi: 10.1371/journal.pntd.0004313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzman MG, Harris E. Dengue. Lancet. 2015;385:453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- 5.Kraemer MUG, Reiner RC, Jr, Brady OJ, Messina JP, Gilbert M, Pigott DM, et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019;4:854–863. doi: 10.1038/s41564-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paixao ES, Teixeira MG, Rodrigues LC. Zika, chikungunya and dengue: the causes and threats of new and re-emerging arboviral diseases. BMJ Glob Health. 2018;3:e000530. doi: 10.1136/bmjgh-2017-000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young PR. Arboviruses: a family on the move. Adv Exp Med Biol. 2018;1062:1–10. doi: 10.1007/978-981-10-8727-1_1. [DOI] [PubMed] [Google Scholar]
- 9.Gould E, Pettersson J, Higgs S, Charrel R, de Lamballerie X. Emerging arboviruses: Why today? One Health. 2017;4:1–13. doi: 10.1016/j.onehlt.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299–309. doi: 10.2147/CLEP.S34440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilder-Smith A, Ooi EE, Vasudevan SG, Gubler DJ. Update on dengue: epidemiology, virus evolution, antiviral drugs, and vaccine development. Curr Infect Dis Rep. 2010;12:157–164. doi: 10.1007/s11908-010-0102-7. [DOI] [PubMed] [Google Scholar]
- 13.Patterson J, Sammon M, Garg M. Dengue, Zika and Chikungunya: emerging arboviruses in the new world. West J Emerg Med. 2016;17:671–679. doi: 10.5811/westjem.2016.9.30904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sukhralia S, Verma M, Gopirajan S, Dhanaraj PS, Lal R, Mehla N, et al. From dengue to Zika: the wide spread of mosquito-borne arboviruses. Eur J Clin Microbiol Infect Dis. 2019;38:3–14. doi: 10.1007/s10096-018-3375-7. [DOI] [PubMed] [Google Scholar]
- 15.Guzman MG, Gubler DJ, Izquierdo A, Martinez E, Halstead SB. Dengue infection. Nat Rev Dis Primers. 2016;2:16055. doi: 10.1038/nrdp.2016.55. [DOI] [PubMed] [Google Scholar]
- 16.Pollett S, Melendrez MC, Maljkovic Berry I, Duchene S, Salje H, Cummings DAT, et al. Understanding dengue virus evolution to support epidemic surveillance and counter-measure development. Infect Genet Evol. 2018;62:279–295. doi: 10.1016/j.meegid.2018.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver SC, Charlier C, Vasilakis N, Lecuit M. Zika, Chikungunya, and other emerging vector-borne viral diseases. Annu Rev Med. 2018;69:395–408. doi: 10.1146/annurev-med-050715-105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ketkar H, Herman D, Wang P. Genetic determinants of the re-emergence of arboviral diseases. Viruses. 2019;11:150. doi: 10.3390/v11020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones R, Kulkarni MA, Davidson TMV. Arbovirus vectors of epidemiological concern in the Americas: a scoping review of entomological studies on Zika, dengue and chikungunya virus vectors. PLoS ONE. 2020;15:e0220753. doi: 10.1371/journal.pone.0220753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leta S, Beyene TJ, De Clercq EM, Amenu K, Kraemer MUG, Revie CW. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int J Infect Dis. 2018;67:25–35. doi: 10.1016/j.ijid.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espinal MA, Andrus JK, Jauregui B, Waterman SH, Morens DM, Santos JI, et al. Emerging and reemerging Aedes-transmitted arbovirus infections in the region of the Americas: implications for health policy. Am J Public Health. 2019;109:387–392. doi: 10.2105/AJPH.2018.304849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Souza-Neto JA, Powell JR, Bonizzoni M. Aedes aegypti vector competence studies: a review. Infect Genet Evol. 2019;67:191–209. doi: 10.1016/j.meegid.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 2009;11:1177–1185. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Brady OJ, Golding N, Pigott DM, Kraemer MU, Messina JP, Reiner RC, Jr, et al. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasit Vectors. 2014;7:338. doi: 10.1186/1756-3305-7-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl Trop Dis. 2019;13:e0007213. doi: 10.1371/journal.pntd.0007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goindin D, Delannay C, Ramdini C, Gustave J, Fouque F. Parity and longevity of Aedes aegypti according to temperatures in controlled conditions and consequences on dengue transmission risks. PLoS ONE. 2015;10:e0135489. doi: 10.1371/journal.pone.0135489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraemer MU, Sinka ME, Duda KA, Mylne A, Shearer FM, Brady OJ, et al. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci Data. 2015;2:150035. doi: 10.1038/sdata.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer SV, Tesh RB, Vasilakis N. The emergence of arthropod-borne viral diseases: a global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017;166:155–163. doi: 10.1016/j.actatropica.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu-Helmersson J, Quam M, Wilder-Smith A, Stenlund H, Ebi K, Massad E, Rocklov J. Climate change and Aedes vectors: 21st century projections for dengue transmission in Europe. EBioMedicine. 2016;7:267–277. doi: 10.1016/j.ebiom.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morales D, Ponce P, Cevallos V, Espinosa P, Vaca D, Quezada W. Resistance status of Aedes aegypti to deltamethrin, malathion, and temephos in Ecuador. J Am Mosq Control Assoc. 2019;35:113–122. doi: 10.2987/19-6831.1. [DOI] [PubMed] [Google Scholar]
- 33.Conway MJ, Colpitts TM, Fikrig E. Role of the vector in arbovirus transmission. Annu Rev Virol. 2014;1:71–88. doi: 10.1146/annurev-virology-031413-085513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Almeida JP, Aguiar ER, Armache JN, Olmo RP, Marques JT. The virome of vector mosquitoes. Curr Opin Virol. 2021;49:7–12. doi: 10.1016/j.coviro.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Scolari F, Casiraghi M, Bonizzoni M. Aedes spp. and their microbiota: a review. Front Microbiol. 2019;10:2036. doi: 10.3389/fmicb.2019.02036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mancini MV, Damiani C, Accoti A, Tallarita M, Nunzi E, Cappelli A, et al. Estimating bacteria diversity in different organs of nine species of mosquito by next generation sequencing. BMC Microbiol. 2018;18:126. doi: 10.1186/s12866-018-1266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atoni E, Zhao L, Karungu S, Obanda V, Agwanda B, Xia H, et al. The discovery and global distribution of novel mosquito-associated viruses in the last decade (2007–2017) Rev Med Virol. 2019;29:e2079. doi: 10.1002/rmv.2079. [DOI] [PubMed] [Google Scholar]
- 38.Roundy CM, Azar SR, Rossi SL, Weaver SC, Vasilakis N. Insect-specific viruses: a historical overview and recent developments. Adv Virus Res. 2017;98:119–146. doi: 10.1016/bs.aivir.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Ohlund P, Lunden H, Blomstrom AL. Insect-specific virus evolution and potential effects on vector competence. Virus Genes. 2019;55:127–137. doi: 10.1007/s11262-018-01629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halbach R, Junglen S, van Rij RP. Mosquito-specific and mosquito-borne viruses: evolution, infection, and host defense. Curr Opin Insect Sci. 2017;22:16–27. doi: 10.1016/j.cois.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Bolling BG, Vasilakis N, Guzman H, Widen SG, Wood TG, Popov VL, et al. Insect-specific viruses detected in laboratory mosquito colonies and their potential implications for experiments evaluating arbovirus vector competence. Am J Trop Med Hyg. 2015;92:422–428. doi: 10.4269/ajtmh.14-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schultz MJ, Frydman HM, Connor JH. Dual Insect specific virus infection limits Arbovirus replication in Aedes mosquito cells. Virology. 2018;518:406–413. doi: 10.1016/j.virol.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romo H, Kenney JL, Blitvich BJ, Brault AC. Restriction of Zika virus infection and transmission in Aedes aegypti mediated by an insect-specific flavivirus. Emerg Microbes Infect. 2018;7:181. doi: 10.1038/s41426-018-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nouri S, Matsumura EE, Kuo YW, Falk BW. Insect-specific viruses: from discovery to potential translational applications. Curr Opin Virol. 2018;33:33–41. doi: 10.1016/j.coviro.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Patterson EI, Villinger J, Muthoni JN, Dobel-Ober L, Hughes GL. Exploiting insect-specific viruses as a novel strategy to control vector-borne disease. Curr Opin Insect Sci. 2020;39:50–56. doi: 10.1016/j.cois.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lozano-Fuentes S, Kenney JL, Varnado W, Byrd BD, Burkhalter KL, Savage HM. Susceptibility and vectorial capacity of American Aedes albopictus and Aedes aegypti (Diptera: Culicidae) to American Zika virus strains. J Med Entomol. 2019;56:233–240. doi: 10.1093/jme/tjy114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shragai T, Tesla B, Murdock C, Harrington LC. Zika and chikungunya: mosquito-borne viruses in a changing world. Ann N Y Acad Sci. 2017;1399:61–77. doi: 10.1111/nyas.13306. [DOI] [PubMed] [Google Scholar]
- 48.Braks MA, Honorio NA, Lourencqo-De-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida. J Med Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- 49.Dos Santos AP, Urbinatti PR, da Rocha CR, de Sa Almeida RMM, Ribeiro SS, de Lima-Camara TN. Parity and gonotrophic discordance of females of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in the city of Sao Paulo, SP, Brazil. J Vector Ecol. 2019;44:233–240. doi: 10.1111/jvec.12354. [DOI] [PubMed] [Google Scholar]
- 50.Bonizzoni M, Gasperi G, Chen X, James AA. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol. 2013;29:460–468. doi: 10.1016/j.pt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ciota AT, Chin PA, Ehrbar DJ, Micieli MV, Fonseca DM, Kramer LD. Differential effects of temperature and mosquito genetics determine transmissibility of arboviruses by Aedes aegypti in Argentina. Am J Trop Med Hyg. 2018;99:417–424. doi: 10.4269/ajtmh.18-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delatte H, Desvars A, Bouetard A, Bord S, Gimonneau G, Vourc'h G, et al. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Reunion. Vector Borne Zoonotic Dis. 2010;10:249–258. doi: 10.1089/vbz.2009.0026. [DOI] [PubMed] [Google Scholar]
- 53.Delatte H, Gimonneau G, Triboire A, Fontenille D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J Med Entomol. 2009;46:33–41. doi: 10.1603/033.046.0105. [DOI] [PubMed] [Google Scholar]
- 54.Coleman M, Hemingway J, Gleave KA, Wiebe A, Gething PW, Moyes CL. Developing global maps of insecticide resistance risk to improve vector control. Malar J. 2017;16:86. doi: 10.1186/s12936-017-1733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Juliano SA, Lounibos LP, O'Meara GF. A field test for competitive effects of Aedes albopictus on A. aegypti in South Florida: differences between sites of coexistence and exclusion? Oecologia. 2004;139:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamgang B, Wilson-Bahun TA, Irving H, Kusimo MO, Lenga A, Wondji CS. Geographical distribution of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and genetic diversity of invading population of Ae. albopictus in the Republic of the Congo. Wellcome Open Res. 2018;3:79. doi: 10.12688/wellcomeopenres.14659.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tedjou AN, Kamgang B, Yougang AP, Njiokou F, Wondji CS. Update on the geographical distribution and prevalence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae), two major arbovirus vectors in Cameroon. PLoS Negl Trop Dis. 2019;13:e0007137. doi: 10.1371/journal.pntd.0007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lwande OW, Obanda V, Lindstrom A, Ahlm C, Evander M, Naslund J, et al. Globe-trotting Aedes aegypti and Aedes albopictus: risk factors for arbovirus pandemics. Vector Borne Zoonotic Dis. 2020;20:71–81. doi: 10.1089/vbz.2019.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferreira-de-Lima VH, Camara DCP, Honorio NA, Lima-Camara TN. The Asian tiger mosquito in Brazil: Observations on biology and ecological interactions since its first detection in 1986. Acta Trop. 2020;205:105386. doi: 10.1016/j.actatropica.2020.105386. [DOI] [PubMed] [Google Scholar]
- 60.Ruckert C, Weger-Lucarelli J, Garcia-Luna SM, Young MC, Byas AD, Murrieta RA, et al. Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat Commun. 2017;8:15412. doi: 10.1038/ncomms15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson-Bahun TA, Kamgang B, Lenga A, Wondji CS. Larval ecology and infestation indices of two major arbovirus vectors, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazzaville, the capital city of the Republic of the Congo. Parasit Vectors. 2020;13:492. doi: 10.1186/s13071-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Camara DC, Codeco CT, Juliano SA, Lounibos LP, Riback TI, Pereira GR, et al. Seasonal differences in density but similar competitive impact of Aedes albopictus (Skuse) on Aedes aegypti (L.) in Rio de Janeiro, Brazil. PLoS ONE. 2016;11:e0157120. doi: 10.1371/journal.pone.0157120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alonso-Palomares LA, Moreno-Garcia M, Lanz-Mendoza H, Salazar MI. Molecular basis for arbovirus transmission by Aedes aegypti mosquitoes. Intervirology. 2018;61:255–264. doi: 10.1159/000499128. [DOI] [PubMed] [Google Scholar]
- 64.Cansado-Utrilla C, Zhao SY, McCall PJ, Coon KL, Hughes GL. The microbiome and mosquito vectorial capacity: rich potential for discovery and translation. Microbiome. 2021;9:111. doi: 10.1186/s40168-021-01073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carrington LB, Seifert SN, Armijos MV, Lambrechts L, Scott TW. Reduction of Aedes aegypti vector competence for dengue virus under large temperature fluctuations. Am J Trop Med Hyg. 2013;88:689–697. doi: 10.4269/ajtmh.12-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Viglietta M, Bellone R, Blisnick AA, Failloux AB. Vector specificity of arbovirus transmission. Front Microbiol. 2021;12:773211. doi: 10.3389/fmicb.2021.773211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gabrieli P, Caccia S, Varotto-Boccazzi I, Arnoldi I, Barbieri G, Comandatore F, et al. Mosquito trilogy: microbiota, immunity and pathogens, and their implications for the control of disease transmission. Front Microbiol. 2021;12:630438. doi: 10.3389/fmicb.2021.630438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gubler DJ. Human arbovirus infections worldwide. Ann N Y Acad Sci. 2001;951:13–24. doi: 10.1111/j.1749-6632.2001.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 69.Ruckert C, Ebel GD. How do virus-mosquito interactions lead to viral emergence? Trends Parasitol. 2018;34:310–321. doi: 10.1016/j.pt.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lequime S, Paul RE, Lambrechts L. Determinants of arbovirus vertical transmission in mosquitoes. PLoS Pathog. 2016;12:e1005548. doi: 10.1371/journal.ppat.1005548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harapan H, Michie A, Sasmono RT. Dengue: a minireview. Viruses. 2020;12:829. doi: 10.3390/v12080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.da Costa CF, Dos Passos RA, Lima JBP, Roque RA, de Souza SV, Campolina TB, et al. Transovarial transmission of DENV in Aedes aegypti in the Amazon basin: a local model of xenomonitoring. Parasit Vectors. 2017;10:249. doi: 10.1186/s13071-017-2194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boyles SM, Mavian CN, Finol E, Ukhanova M, Stephenson CJ, Hamerlinck G, et al. Under-the-radar dengue virus infections in natural populations of Aedes aegypti mosquitoes. MSphere. 2020 doi: 10.1128/mSphere.00316-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferreira-de-Lima VH, Lima-Camara TN. Natural vertical transmission of dengue virus in Aedes aegypti and Aedes albopictus: a systematic review. Parasit Vectors. 2018;11:77. doi: 10.1186/s13071-018-2643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao H, Cui C, Wang L, Jacobs-Lorena M, Wang S. Mosquito microbiota and implications for disease control. Trends Parasitol. 2020;36:98–111. doi: 10.1016/j.pt.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang W, Wang S, Jacobs-Lorena M. Use of microbiota to fight mosquito-borne disease. Front Genet. 2020;11:196. doi: 10.3389/fgene.2020.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Villegas LEM, Campolina TB, Barnabe NR, Orfano AS, Chaves BA, Norris DE, et al. Zika virus infection modulates the bacterial diversity associated with Aedes aegypti as revealed by metagenomic analysis. PLoS ONE. 2018;13:e0190352. doi: 10.1371/journal.pone.0190352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strand MR. Composition and functional roles of the gut microbiota in mosquitoes. Curr Opin Insect Sci. 2018;28:59–65. doi: 10.1016/j.cois.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muturi EJ, Ramirez JL, Rooney AP, Kim CH. Comparative analysis of gut microbiota of mosquito communities in central Illinois. PLoS Negl Trop Dis. 2017;11:e0005377. doi: 10.1371/journal.pntd.0005377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scolari F, Sandionigi A, Carlassara M, Bruno A, Casiraghi M, et al. Exploring changes in the microbiota of Aedes albopictus: comparison among breeding site water, larvae, and adults. Front Microbiol. 2021;12:624170. doi: 10.3389/fmicb.2021.624170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caragata EP, Tikhe CV, Dimopoulos G. Curious entanglements: interactions between mosquitoes, their microbiota, and arboviruses. Curr Opin Virol. 2019;37:26–36. doi: 10.1016/j.coviro.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Diaz S, Camargo C, Avila FW. Characterization of the reproductive tract bacterial microbiota of virgin, mated, and blood-fed Aedes aegypti and Aedes albopictus females. Parasit Vectors. 2021;14:592. doi: 10.1186/s13071-021-05093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohlmann TWR, Vogels CBF, Goertz GP, Pijlman GP, Ter Braak CJF, Beest DE, et al. Impact of gut bacteria on the infection and transmission of pathogenic arboviruses by biting midges and mosquitoes. Microb Ecol. 2020;80:703–717. doi: 10.1007/s00248-020-01517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hegde S, Rasgon JL, Hughes GL. The microbiome modulates arbovirus transmission in mosquitoes. Curr Opin Virol. 2015;15:97–102. doi: 10.1016/j.coviro.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coon KL, Vogel KJ, Brown MR, Strand MR. Mosquitoes rely on their gut microbiota for development. Mol Ecol. 2014;23:2727–2739. doi: 10.1111/mec.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guegan M, Zouache K, Demichel C, Minard G, Van Tran V, Potier P, et al. The mosquito holobiont: fresh insight into mosquito-microbiota interactions. Microbiome. 2018;6:49. doi: 10.1186/s40168-018-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chandler JA, Liu RM, Bennett SN. RNA shotgun metagenomic sequencing of northern California (USA) mosquitoes uncovers viruses, bacteria, and fungi. Front Microbiol. 2015;6:185. doi: 10.3389/fmicb.2015.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma E, Zhu Y, Liu Z, Wei T, Wang P, Cheng G. Interaction of viruses with the insect intestine. Annu Rev Virol. 2021;8:115–131. doi: 10.1146/annurev-virology-091919-100543. [DOI] [PubMed] [Google Scholar]
- 89.Wu P, Sun P, Nie K, Zhu Y, Shi M, Xiao C, et al. A gut commensal bacterium promotes mosquito permissiveness to arboviruses. Cell Host Microbe. 2019;25:101–112.e105. doi: 10.1016/j.chom.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 90.Anglero-Rodriguez YI, Talyuli OA, Blumberg BJ, Kang S, Demby C, Shields A, et al. An Aedes aegypti-associated fungus increases susceptibility to dengue virus by modulating gut trypsin activity. Elife. 2017 doi: 10.7554/eLife.28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dutra HL, Rocha MN, Dias FB, Mansur SB, Caragata EP, Moreira LA. Wolbachia blocks currently circulating zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe. 2016;19:771–774. doi: 10.1016/j.chom.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang G, Hussain M, O'Neill SL, Asgari S. Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti. Proc Natl Acad Sci U S A. 2013;110:10276–10281. doi: 10.1073/pnas.1303603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Terradas G, Joubert DA, McGraw EA. The RNAi pathway plays a small part in Wolbachia-mediated blocking of dengue virus in mosquito cells. Sci Rep. 2017;7:43847. doi: 10.1038/srep43847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Terradas G, McGraw EA. Wolbachia-mediated virus blocking in the mosquito vector Aedes aegypti. Curr Opin Insect Sci. 2017;22:37–44. doi: 10.1016/j.cois.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 95.Ramirez JL, Short SM, Bahia AC, Saraiva RG, Dong Y, Kang S, et al. Chromobacterium Csp_P reduces malaria and dengue infection in vector mosquitoes and has entomopathogenic and in vitro anti-pathogen activities. PLoS Pathog. 2014;10:e1004398. doi: 10.1371/journal.ppat.1004398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saraiva RG, Fang J, Kang S, Anglero-Rodriguez YI, Dong Y, Dimopoulos G. Aminopeptidase secreted by Chromobacterium sp. Panama inhibits dengue virus infection by degrading the E protein. PLoS Negl Trop Dis. 2018;12:e0006443. doi: 10.1371/journal.pntd.0006443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saraiva RG, Huitt-Roehl CR, Tripathi A, Cheng YQ, Bosch J, Townsend CA, et al. Chromobacterium spp. mediate their anti-Plasmodium activity through secretion of the histone deacetylase inhibitor romidepsin. Sci Rep. 2018;8:6176. doi: 10.1038/s41598-018-24296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Apte-Deshpande A, Paingankar M, Gokhale MD, Deobagkar DN. Serratia odorifera a midgut inhabitant of Aedes aegypti mosquito enhances its susceptibility to dengue-2 virus. PLoS ONE. 2012;7:e40401. doi: 10.1371/journal.pone.0040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Apte-Deshpande AD, Paingankar MS, Gokhale MD, Deobagkar DN. Serratia odorifera mediated enhancement in susceptibility of Aedes aegypti for chikungunya virus. Indian J Med Res. 2014;139:762–768. [PMC free article] [PubMed] [Google Scholar]
- 100.Minard G, Mavingui P, Moro CV. Diversity and function of bacterial microbiota in the mosquito holobiont. Parasit Vectors. 2013;6:146. doi: 10.1186/1756-3305-6-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang J, Jiang E, Yang D, Wei J, Zhao M, Feng J, et al. Metagenomic next-generation sequencing versus traditional pathogen detection in the diagnosis of peripheral pulmonary infectious lesions. Infect Drug Resist. 2020;13:567–576. doi: 10.2147/IDR.S235182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen K, Pachter L. Bioinformatics for whole-genome shotgun sequencing of microbial communities. PLoS Comput Biol. 2005;1:106–112. doi: 10.1371/journal.pcbi.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kiselev D, Matsvay A, Abramov I, Dedkov V, Shipulin G, Khafizov K. Current trends in diagnostics of viral infections of unknown etiology. Viruses. 2020;12:211. doi: 10.3390/v12020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Altinli M, Schnettler E. Symbiotic interactions between mosquitoes and mosquito viruses. Front Cell Infect Microbiol. 2021 doi: 10.3389/fcimb.2021.694020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xiao P, Li C, Zhang Y, Han J, Guo X, Xie L, et al. Metagenomic sequencing from mosquitoes in China reveals a variety of insect and human viruses. Front Cell Infect Microbiol. 2018;8:364. doi: 10.3389/fcimb.2018.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mokili JL, Rohwer F, Dutilh BE. Metagenomics and future perspectives in virus discovery. Curr Opin Virol. 2012;2:63–77. doi: 10.1016/j.coviro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi C, Beller L, Deboutte W, Yinda KC, Delang L, Vega-Rua A. Stable distinct core eukaryotic viromes in different mosquito species from Guadeloupe, using single mosquito viral metagenomics. Microbiome. 2019;7:121. doi: 10.1186/s40168-019-0734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xiao P, Han J, Zhang Y, Li C, Guo X, Wen S, et al. Metagenomic analysis of Flaviviridae in mosquito viromes isolated from Yunnan Province in China reveals genes from Dengue and Zika viruses. Front Cell Infect Microbiol. 2018;8:359. doi: 10.3389/fcimb.2018.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He X, Yin Q, Zhou L, Meng L, Hu W, Li F, et al. Metagenomic sequencing reveals viral abundance and diversity in mosquitoes from the Shaanxi-Gansu-Ningxia region, China. PLoS Negl Trop Dis. 2021;15:e0009381. doi: 10.1371/journal.pntd.0009381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cholleti H, Hayer J, Fafetine J, Berg M, Blomstrom AL. Genetic characterization of a novel picorna-like virus in Culex spp. mosquitoes from Mozambique. Virol J. 2018;15:71. doi: 10.1186/s12985-018-0981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Petit MJ, Shah PS. Mapping arbovirus-vector interactions using systems biology techniques. Front Cell Infect Microbiol. 2018;8:440. doi: 10.3389/fcimb.2018.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dennison NJ, Jupatanakul N, Dimopoulos G. The mosquito microbiota influences vector competence for human pathogens. Curr Opin Insect Sci. 2014;3:6–13. doi: 10.1016/j.cois.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramos-Nino ME, Fitzpatrick DM, Tighe S, Eckstrom KM, Hattaway LM, Hsueh AN, et al. High prevalence of Phasi Charoen-like virus from wild-caught Aedes aegypti in Grenada, W.I. as revealed by metagenomic analysis. PLoS ONE. 2020;15:e0227998. doi: 10.1371/journal.pone.0227998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramos-Nino ME, Fitzpatrick DM, Eckstrom KM, Tighe S, Hattaway LM, Hsueh AN, et al. Metagenomic analysis of Aedes aegypti and Culex quinquefasciatus mosquitoes from Grenada, West Indies. PLoS ONE. 2020;15:e0231047. doi: 10.1371/journal.pone.0231047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shi C, Liu Y, Hu X, Xiong J, Zhang B, Yuan Z. A metagenomic survey of viral abundance and diversity in mosquitoes from Hubei province. PLoS ONE. 2015;10:e0129845. doi: 10.1371/journal.pone.0129845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Calisher CH, Higgs S. The discovery of arthropod-specific viruses in hematophagous arthropods: an open door to understanding the mechanisms of arbovirus and arthropod evolution? Annu Rev Entomol. 2018;63:87–103. doi: 10.1146/annurev-ento-020117-043033. [DOI] [PubMed] [Google Scholar]
- 117.Elrefaey AM, Abdelnabi R, Rosales Rosas AL, Wang L, Basu S, Delang L. Understanding the mechanisms underlying host restriction of insect-specific viruses. Viruses. 2020;12:964. doi: 10.3390/v12090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Agboli E, Leggewie M, Altinli M, Schnettler E. Mosquito-specific viruses-transmission and interaction. Viruses. 2019;11:873. doi: 10.3390/v11090873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sadeghi M, Popov V, Guzman H, Phan TG, Vasilakis N, Tesh R, et al. Genomes of viral isolates derived from different mosquitos species. Virus Res. 2017;242:49–57. doi: 10.1016/j.virusres.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shi M, Neville P, Nicholson J, Eden JS, Imrie A, Holmes EC. High-resolution metatranscriptomics reveals the ecological dynamics of mosquito-associated RNA viruses in Western Australia. J Virol. 2017 doi: 10.1128/JVI.00680-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zakrzewski M, Rasic G, Darbro J, Krause L, Poo YS, Filipovic I, et al. Mapping the virome in wild-caught Aedes aegypti from Cairns and Bangkok. Sci Rep. 2018;8:4690. doi: 10.1038/s41598-018-22945-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bolling BG, Weaver SC, Tesh RB, Vasilakis N. Insect-specific virus discovery: significance for the arbovirus community. Viruses. 2015;7:4911–4928. doi: 10.3390/v7092851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vasilakis N, Tesh RB. Insect-specific viruses and their potential impact on arbovirus transmission. Curr Opin Virol. 2015;15:69–74. doi: 10.1016/j.coviro.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dudas G, Obbard DJ. Are arthropods at the heart of virus evolution? Elife. 2015 doi: 10.7554/eLife.06837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marklewitz M, Zirkel F, Kurth A, Drosten C, Junglen S. Evolutionary and phenotypic analysis of live virus isolates suggests arthropod origin of a pathogenic RNA virus family. Proc Natl Acad Sci U S A. 2015;112:7536–7541. doi: 10.1073/pnas.1502036112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li CX, Shi M, Tian JH, Lin XD, Kang YJ, Chen LJ, et al. Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. Elife. 2015 doi: 10.7554/eLife.05378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shi C, Zhao L, Atoni E, Zeng W, Hu X, Matthijnssens J, et al. Stability of the virome in lab- and field-collected Aedes albopictus mosquitoes across different developmental stages and possible core viruses in the publicly available virome data of Aedes mosquitoes. MSystems. 2020 doi: 10.1128/mSystems.00640-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thannesberger J, Rascovan N, Eisenmann A, Klymiuk I, Zittra C, Fuehrer HP, et al. Highly sensitive virome characterization of Aedes aegypti and Culex pipiens complex from central Europe and the Caribbean reveals potential for interspecies viral transmission. Pathogens. 2020 doi: 10.3390/pathogens9090686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kubacki J, Flacio E, Qi W, Guidi V, Tonolla M, Fraefel C. Viral metagenomic analysis of Aedes albopictus mosquitos from Southern Switzerland. Viruses. 2020;12:929. doi: 10.3390/v12090929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.He W, Chen Y, Zhang X, Peng M, Xu D, He H, et al. Virome in adult Aedes albopictus captured during different seasons in Guangzhou City, China. Parasit Vectors. 2021;14:415. doi: 10.1186/s13071-021-04922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.da Silva Ferreira R, de Toni Aquino da Cruz LC, de Souza VJ, da Silva Neves NA, de Souza VC, Filho LCF, et al: Insect-specific viruses and arboviruses in adult male culicids from Midwestern Brazil. Infect Genet Evol 2020, 85:104561. [DOI] [PubMed]
- 132.Coatsworth H, Bozic J, Carrillo J, Buckner EA, Rivers AR, Dinglasan RR, et al. Intrinsic variation in the vertically transmitted core virome of the mosquito Aedes aegypti. Mol Ecol. 2022;29:19–577. doi: 10.1111/mec.16412. [DOI] [PubMed] [Google Scholar]
- 133.Frey KG, Biser T, Hamilton T, Santos CJ, Pimentel G, Mokashi VP, et al. Bioinformatic characterization of mosquito viromes within the Eastern United States and Puerto Rico: discovery of novel viruses. Evol Bioinform Online. 2016;12:1–12. doi: 10.4137/EBO.S38518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oguzie JU, Nwangwu UC, Oluniyi PE, Olumade TJ, George UE, Kazeem A, et al. Metagenomic sequencing characterizes a wide diversity of viruses in field mosquito samples in Nigeria. Scientifc Reports. 2022;12:7616. doi: 10.1038/s41598-022-11797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang L, Rosales Rosas AL, De Coninck L, Shi C, Bouckaert J, Matthijnssens J, et al. Establishment of Culex modestus in Belgium and a Glance into the Virome of Belgian mosquito species. MSphere. 2021 doi: 10.1128/mSphere.01229-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang Y, Guo X, Zhang S, Zhao Q, Sun Q, Zhou H, et al. Discovery of two novel totiviruses from Culex tritaeniorhynchus classifiable in a distinct clade with arthropod-infecting viruses within the family Totiviridae. Arch Virol. 2018;163:2899–2902. doi: 10.1007/s00705-018-3871-1. [DOI] [PubMed] [Google Scholar]
- 137.Shi M, Zhang YZ, Holmes EC. Meta-transcriptomics and the evolutionary biology of RNA viruses. Virus Res. 2018;243:83–90. doi: 10.1016/j.virusres.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stollar V, Thomas VL. An agent in the Aedes aegypti cell line (Peleg) which causes fusion of Aedes albopictus cells. Virology. 1975;64:367–377. doi: 10.1016/0042-6822(75)90113-0. [DOI] [PubMed] [Google Scholar]
- 139.Zhang G, Asad S, Khromykh AA, Asgari S. Cell fusing agent virus and dengue virus mutually interact in Aedes aegypti cell lines. Sci Rep. 2017;7:6935. doi: 10.1038/s41598-017-07279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Parry R, Asgari S. Aedes anphevirus: an insect-specific virus distributed worldwide in Aedes aegypti mosquitoes that has complex interplays with Wolbachia and Dengue virus infection in cells. J Virol. 2018 doi: 10.1128/JVI.00224-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vogels CBF, Ruckert C, Cavany SM, Perkins TA, Ebel GD, Grubaugh ND. Arbovirus coinfection and co-transmission: a neglected public health concern? PLoS Biol. 2019;17:e3000130. doi: 10.1371/journal.pbio.3000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Karpf AR, Lenches E, Strauss EG, Strauss JH, Brown DT. Superinfection exclusion of alphaviruses in three mosquito cell lines persistently infected with Sindbis virus. J Virol. 1997;71:7119–7123. doi: 10.1128/jvi.71.9.7119-7123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Goenaga S, Kenney JL, Duggal NK, Delorey M, Ebel GD, Zhang B, et al. Potential for co-infection of a mosquito-specific Flavivirus, Nhumirim virus, to block West Nile virus transmission in mosquitoes. Viruses. 2015;7:5801–5812. doi: 10.3390/v7112911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nasar F, Erasmus JH, Haddow AD, Tesh RB, Weaver SC. Eilat virus induces both homologous and heterologous interference. Virology. 2015;484:51–58. doi: 10.1016/j.virol.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.