Abstract
Background
Acute exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD) contributes significantly to mortality among patients with COPD in Intensive care unit (ICU). This study aimed to develop a nomogram to predict 30-day mortality among AECOPD patients in ICU.
Methods
In this retrospective cohort study, we extracted AECOPD patients from Medical Information Mart for Intensive Care III (MIMIC-III) database. Multivariate logistic regression based on Akaike information criterion (AIC) was used to establish the nomogram. Internal validation was performed by a bootstrap resampling approach with 1000 replications. The discrimination and calibration of the nomogram were evaluated by Harrell’s concordance index (C-index) and Hosmer–Lemeshow (HL) goodness-of-fit test. Decision curve analysis (DCA) was performed to evaluate its clinical application.
Results
A total of 494 patients were finally included in the study with a mean age of 70.8 years old. 417 (84.4%) patients were in the survivor group and 77 (15.6%) patients were in the non-survivor group. Multivariate logistic regression analysis based on AIC included age, pO2, neutrophil-to-lymphocyte ratio (NLR), prognostic nutritional index (PNI), invasive mechanical ventilation and vasopressor use to construct the nomogram. The adjusted C-index was 0.745 (0.712, 0.778) with good calibration (HL test, P = 0.147). The Kaplan–Meier survival curves revealed a significantly lower survival probability in the high-risk group than that in the low-risk group (P < 0.001). DCA showed that nomogram was clinically useful.
Conclusion
The nomogram developed in this study could help clinicians to stratify AECOPD patients and provide appropriate care in clinical setting.
Keywords: AECOPD, Nomogram, Predictive model, 30-day mortality, Intensive care unit
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a common chronic respiratory disease featured by persistent respiratory symptoms and airflow limitation [1]. COPD is an important public health challenge and associated with high mortality worldwide. According to the WHO, it is estimated that global COPD will rise to the third leading cause of death in 2030, with the corresponding economic burden ranking the fifth [2]. Acute exacerbation of COPD (AECOPD) is defined as an acute worsening of respiratory symptoms in COPD which require additional therapy [1]. AECOPD contributes significantly to mortality among patients with COPD [3], especially for those who require intensive care unit (ICU) admission with a high mortality rate of 16.9% to 48.8% [4, 5]. The severity of exacerbations reflected by clinical results is strongly correlated with patient prognosis. Whereas accurate decision-making and prompt treatment would predict a better prognosis, it is important to identify the factors that is able to predict outcomes in AECOPD patients. Although several studies have investigated independent factors to predict mortality due to AECOPD [6–9], few study especially focused on patients with AECOPD in ICU.
Medical Information Mart for Intensive Care III (MIMIC III) database can provide a wealth of clinical data to be routinely analyzed. The purpose of our study was to determine independent factors affecting mortality for patients with AECOPD in MIMIC III database by nomogram. Nomograms, user-friendly instrument with visualized prediction outcomes, are popular prognostic tools with the ability to predict clinical events by integrating potential risk factors [10]. Nomogram could be easily applied in clinical practice to identify high-risk patients and guide decision-making. Hence, we intend to develop and internally validate a nomogram to predict 30-day mortality after admission to ICU among AECOPD patients.
Material and methods
Data source
We extracted the data of this retrospective study from MIMIC-III version 1.4 (MIMIC-III v1.4) database. MIMIC-III is a large, open, and public database, containing more than 50,000 patients admitted to the ICU at Beth Israel Deaconess Medical Center from 2001 to 2012 [11]. We accessed the MIMIC-III after completion of the Protecting Human Research Participants exam. The establishment and employment of this database were approved by the Institutional Review Boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. No informed consent was required since all the data were de-identified.
Study population, data extraction and outcome
Adult patients (≥ 18 years old) with the diagnosis of AECOPD were selected from the database. The definition of AECOPD was based on the International Classification of Diseases, 9th edition (ICD-9) code 491.21. For patients with multiple hospitalizations, only the first hospitalization was enrolled. Other exclusion criteria included length of ICU stay < 48 h and missing data > 10%.
Data were extracted from MIMIC-III database through Structured Query Language [12]. The data upon admission to ICU were recorded, including age, gender, full blood count (white blood cell (WBC) count, neutrophil count, lymphocyte count, platelet count, hemoglobin), laboratory values (serum albumin, alanine transaminase (ALT), aspartate transaminase (AST), serum creatinine (sCr), serum blood urea nitrogen (BUN), serum sodium, serum potassium, serum calcium), arterial blood gas (pH, partial pressure of oxygen (pO2), partial pressure of carbon dioxide (pCO2), bicarbonate), vital signs (temperature, mean atrial pressure (MAP), heart rate, respiratory rate), comorbidities (hypertension, diabetes mellitus (DM), coronary heart disease (CHD), chronic renal disease (CKD), maligancy), treatment therapy (invasive mechanical ventilation (IMV) and vasopressor) and clinical severity scales (Sequential Organ Failure Assessment (SOFA) score and Simplified Acute Physiology Score II (SAPS II)). The neutrophil-to-lymphocyte ratio (NLR) was defined as the absolute count of neutrophils divided by the absolute count of lymphocytes. The platelet-to-lymphocyte ratio (PLR) was defined as the absolute count of platelets divided by the absolute count of lymphocytes. The prognostic nutritional index (PNI) value was calculated as the following equation: 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count (mm3). For missing variables, predictive mean matching was used to impute numeric features. The primary outcome was 30-day all-cause mortality after admission to ICU.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation (SD) for normal distribution and as the median and interquartile range (IQR) for skewed distribution. Normal distributions were confirmed by Agostino tests. Continuous variables were compared by unpaired Student's test or Mann–Whitney U-test. Categorical variables were compared using the χ2-test or Fisher exact test as appropriate. The median difference (MD) were analyzed by Hodges-Lehmann estimate along with 95% confidence interval (CI).
Univariate Cox proportional hazard analysis was performed to explore the potential confounders associated with 30-day mortality. Subsequently, variables with P values < 0.1 in univariate were used to establish multivariate Cox regression by the backward step-down process based on the Akaike information criterion (AIC) to estimate the hazard ratio (HR) and 95% CI. The final model minimized the score of AIC in order to have fewest variables. The variance inflation factor (VIF) was calculated to detect the potential collinearity between continuous variables. When VIF > 10, collinearity was considered to exist and it will be solved by regularization. Nomogram was constructed based on the multivariate Cox regression results to visualize the model [13]. The Harrell’s concordance index (C-index) and receiver operating characteristic (ROC) curve were used to evaluate the discrimination ability of the nomogram. The nomogram was then calibrated graphically by visual examination of the calibration plot with the Hosmer–Lemeshow (HL) goodness-of-fit test. Internal validation of the final multivariate model was performed using a bootstrap resampling approach with 1000 replications. Decision curve analysis (DCA) was performed to assess the clinical usefulness of the nomogram by quantifying the standardized net benefits at different threshold probabilities [14]. Finally, according to the median of risk score, all patients were divided into the high-risk and low-risk groups, and the survival curve with a log-rank test was used to verify the prognostic value of nomogram.
All analyses were conducted using R software (version 3.6.3) and two-sided p values less than 0.05 were considered statistically significant in each statistical analysis.
Results
Baseline characteristics of the included patients
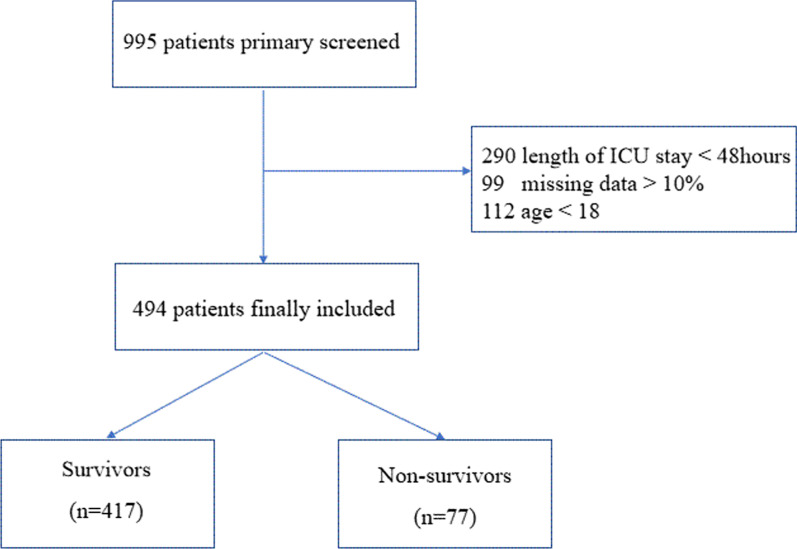
995 patients with AECOPD in the MIMIC-III database were primary screened. 501 patients were excluded due to length of ICU stay < 48 h, missing data > 10% or age < 18 (Fig. 1). Finally, a total of 494 patients with AECOPD were included in our study, with a mean age of 70.8 years old and 50.2% male. The baseline characteristics of the study population were shown in Table 1. 417 (84.4%) patients were in the survivor group and 77 (15.6%) patients were in the non-survivor group. Non-survivors tended to be elder compared with the survivors (75.3 ± 8.6 years old vs. 69.9 ± 10.5 years old, P < 0.001). Deceased patients had significantly higher levels of neutrophil count (MD = 1600/μL, 95%CI, 300 to 2900/μL), BUN (MD = 4.0 mmol/L, 95%CI, 1.0 to 8.0 mmol/L), NLR (MD = 3.8, 95%CI, 1.5 to 7.1) and PLR (MD = 63.4, 95%CI, 9.3 to 119.9). While, the levels of lymphocyte count (MD = − 2600/μL, 95%CI, − 4000 to − 1000/μL), serum albumin (MD = − 0.3 g/dL, 95%CI, − 0.5 to − 0.1 g/dL), partial pressure of oxygen (MD = -16 mmHg, 95%CI, − 30 to − 2 mmHg) and PNI (MD = − 3.2, 95%CI, − 4.9 to − 1.6) were significantly lower in non-survivors. Clinical severities, such as SAPS II (MD = 6, 95%CI, 3 to 9) and SOFA (MD = 1, 95%CI, 0 to 2), tended to be more severe in the non-survivor group when compared with the survivor group. Non-survivors were more likely to be treated with IMV (61% vs. 46.3%, P = 0.002) and vasopressor (54.5% vs. 32.4%, P < 0.001). In terms of vital signs, there were no significant differences between the two groups.
Fig. 1.
The flow chart of the included population
Table 1.
Baseline and clinical characteristics of the study population
| Characteristics | All (n = 494) | Survivors (n = 417) | Non-survivors (n = 77) | P value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 70.8 ± 10.4 | 69.9 ± 10.5 | 75.3 ± 8.6 | < 0.001a |
| Gender, n (%) | ||||
| Male | 248 (50.2) | 210 (50.4) | 38 (49.4) | 0.871b |
| Female | 246 (49.8) | 207 (49.6) | 39 (50.6) | 0.871b |
| Laboratory test, median (IQR) | ||||
| WBC count (103/μL) | 11.1 (8.0, 15.6) | 10.9 (8.0, 15.1) | 12.0 (9.1, 17.7) | 0.084c |
| Neutrophil count (103/μL) | 9.2 (6.2, 13.2) | 8.8 (6.1, 13.0) | 10.4 (7.2, 15.1) | 0.019c |
| Lymphocyte count (103/μL) | 0.9 (0.5, 1.6) | 0.9 (0.5, 1.6) | 0.8 (0.4, 1.2) | 0.015c |
| Platelet count (103/μL) | 236 (175, 308) | 239 (175, 307) | 224 (162, 309) | 0.434c |
| Hemoglobin (g/dL) | 10.8 (9.6, 12.3) | 10.9 (9.6, 12.4) | 10.6 (9.5, 11.5) | 0.145c |
| Serum albumin (g/dL) | 3.2 (2.7, 3.6) | 3.3 (2.8, 3.7) | 2.9 (2.6, 3.4) | < 0.001c |
| Serum sodium (mmol/L) | 138 (134, 141) | 137 (134, 141) | 137 (134, 140) | 0.592c |
| Serum potassium (mmol/L) | 4.2 (3.7, 4.7) | 4.2 (3.7, 4.7) | 4.1 (3.5, 4.6) | 0.080c |
| Serum calcium (mmol/L) | 1.13 (1.07, 1.19) | 1.13 (1.10, 1.19) | 1.13 (1.10, 1.16) | 0.982c |
| pH | 7.34 (7.25, 7.40) | 7.34 (7.26, 7.40) | 7.32 (7.25, 7.40) | 0.503c |
| pO2 (mmHg) | 103 (74, 187) | 107 (76, 192) | 88 (61, 167) | 0.018c |
| pCO2 (mmHg) | 67 (53, 72) | 53 (44, 72) | 52 (46, 71) | 0.810c |
| Bicarbonate (mmol/L) | 28 (25, 33) | 28 (25, 33) | 30 (25,34) | 0.574c |
| ALT (IU/L) | 23 (15, 40) | 23 (15, 40) | 21 (15, 40) | 0.975c |
| AST (IU/L) | 27 (18, 42) | 26 (18, 42) | 27 (17, 46) | 0.968c |
| Serum creatinine (mg/dL) | 1.0 (0.7, 1.4) | 1.0 (0.7, 1.4) | 1.0 (0.7, 1.3) | 0.787c |
| BUN (mmol/L) | 25 (18, 38) | 24 (18, 36) | 28 (21, 44) | 0.021c |
| Mean vital signs, median (IQR) | ||||
| Temperature (℃) | 36.7 (36.4, 37.1) | 36.7 (36.3, 37.1) | 36.7 (36.4, 37.1) | 0.738c |
| MAP (mmHg) | 77 (71, 85) | 77 (71, 84) | 75 (70, 83) | 0.176c |
| Heart rate (min−1) | 90 (80, 100) | 89 (80, 99) | 90 (81, 103) | 0.142c |
| Respiratory rate (min−1) | 20 (17, 23) | 20 (17, 23) | 20 (17, 23) | 0.587c |
| Comorbidities, n (%) | ||||
| Hypertension | 246 (49.8) | 216 (51.8) | 30 (39.0) | 0.038b |
| DM | 137 (27.7) | 124 (29.7) | 13 (16.9) | 0.021b |
| CHD | 112 (22.7) | 94 (22.5) | 18 (23.4) | 0.352b |
| CKD | 83 (16.8) | 75 (18.0) | 8 (10.4) | 0.101b |
| Maligancy | 131 (26.5) | 108 (25.9) | 23 (29.9) | 0.468b |
| Inflammatory indicators, median (IQR) | ||||
| NLR | 8.8 (5.0, 16.6) | 8.8 (5.0, 16.6) | 14.6 (6.6, 31.5) | < 0.001c |
| PLR | 246.3 (133.9, 467.7) | 237.5 (129.5, 436.9) | 295.9 (175.6,593.2) | 0.022c |
| PNI | 33.3 (28.6, 37.6) | 33.8 (29.2, 38.0) | 30.1 (26.2, 35.4) | < 0.001c |
| Scoring system, median (IQR) | ||||
| SAPSII | 39 (32, 47) | 38 (31, 45) | 44 (38, 51) | < 0.001c |
| SOFA | 4 (2, 6) | 4 (2, 6) | 5 (4, 7) | 0.007c |
| Treatment, n (%) | ||||
| IMV | 240 (48.6) | 193 (46.3) | 47 (61.0) | 0.002b |
| Vasopressor | 177 (35.8) | 135 (32.4) | 42 (54.5) | < 0.001b |
Normally distributed data are presented as the mean ± SD, non-normally distributed data are presented as median (IQR) and categorical variables are presented as n (%)
aThe analysis was performed by using independent samples Student's T test
bThe analysis was performed by using χ2-test
cThe analysis was performed by using Mann–Whitney U-test
ALT alanine transaminase, AST aspartate transaminase, BUN blood urea nitrogen, CHD coronary heart disease, CKD chronic kidney disease, DM diabetes mellitus, IMV invasive mechanical ventilation, MAP mean atrial pressure, NLR neutrophil-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, PNI prognostic nutritional index, SAPSII simplified acute physiology score II, SOFA sequential organ failure assessment, WBC white blood cell
Univariate and multivariate prognostic analyses
Univariate Cox regression analysis showed that age (HR = 1.057, 95% CI, 1.030 to 1.085; P < 0.001), neutrophil count (HR = 1.034, 95% CI, 1.004 to 1.065; P = 0.026), serum albumin (HR = 0.547, 95% CI, 0.379 to 0.787; P = 0.001), NLR (HR = 1.009, 95% CI, 1.003 to 1.016; P = 0.006), PNI (HR = 0.941, 95% CI, 0.908 to 0.975; P < 0.001), SAPSII (HR = 1.037, 95% CI, 1.004 to 1.065; P = 0.026), SOFA (HR = 1.099, 95% CI, 1.020 to 1.184; P = 0.013), IMV (HR = 2.602, 95% CI, 1.339 to 5.057; P = 0.005) and vasopressor use (HR = 2.206, 95% CI, 1.408 to 3.455; P < 0.001) were significantly associated with 30-day mortality. After considering collinearity, multivariate Cox regression analysis based on AIC identified that age (HR = 1.066, 95% CI, 1.037 to 1.095; P < 0.001), pO2 (HR = 0.997, 95% CI, 0.995 to 0.999; P = 0.009), NLR (HR = 1.006, 95% CI, 1.001 to 1.013; P = 0.048), PNI (HR = 0.958, 95% CI, 0.923 to 0.994; P = 0.024), IMV (HR = 2.516, 95% CI, 1.265 to 5.005; P = 0.008) and vasopressor use (HR = 2.042, 95% CI, 1.267 to 3.292; P = 0.003) were independent risk factors for 30-day mortality (Table 2).
Table 2.
Univariate and multivariate analysis of Cox-proportional hazards model for the risk of 30-day mortality
| Univariate (HR, 95% CI) | P value | Multivariate (HR, 95% CI) | VIF | P value | |
|---|---|---|---|---|---|
| Age | 1.057 (1.030, 1.085) | < 0.001 | 1.066 (1.037, 1.095) | 1.3 | < 0.001 |
| Male | 0.973 (0.622, 1.521) | 0.904 | / | – | – |
| Laboratory tests | |||||
| WBC count | 1.025 (0.999, 1.052) | 0.063 | / | – | – |
| Neutrophil count | 1.034 (1.004, 1.065) | 0.026 | / | – | / |
| Lymphocyte count | 0.886 (0.709, 1.107) | 0.287 | / | – | – |
| Platelet count | 1.000 (0.998, 1.002) | 0.779 | – | – | – |
| Hemoglobin | 0.930 (0.829, 1.043) | 0.213 | – | – | – |
| Serum albumin | 0.547 (0.379, 0.787) | 0.001 | – | – | – |
| Serum sodium | 0.995 (0.954, 1.037) | 0.799 | – | – | – |
| Serum potassium | 0.772 (0.580, 1.027) | 0.076 | – | – | – |
| Serum calcium | 1.257 (0.056, 28.164) | 0.885 | – | – | – |
| pH | 0.474 (0.059, 3.820) | 0.483 | – | – | – |
| pO2 | 0.998 (0.996, 1.000) | 0.079 | 0.997 (0.995, 0.999) | 1.1 | 0.009 |
| pCO2 | 1.000 (0.990, 1.011) | 0.957 | – | – | – |
| Bicarbonate | 1.010 (0.975, 1.045) | 0.588 | – | – | – |
| ALT | 0.999 (0.997, 1.001) | 0.409 | – | – | – |
| AST | 0.999 (0.997, 1.001) | 0.484 | – | – | – |
| Serum creatinine | 1.058 (0.881, 1.270) | 0.547 | – | – | – |
| BUN | 1.006 (0.997, 1.015) | 0.192 | – | – | – |
| Mean vital signs | |||||
| Temperature | 0.934 (0.641, 1.361) | 0.723 | – | – | – |
| MAP | 0.984 (0.962, 1.007) | 0.175 | – | – | – |
| Heart rate | 1.012 (0.997, 1.028) | 0.122 | – | – | – |
| Respiratory rate | 1.018 (0.964, 1.075) | 0.524 | – | – | – |
| Comorbidities | |||||
| Hypertension | 0.635 (0.402, 1.004) | 0.052 | – | – | – |
| DM | 0.710 (0.581, 1.025) | 0.056 | – | – | – |
| CHD | 1.383 (0.697, 1.745) | 0.225 | – | – | – |
| CKD | 0.564 (0.271, 1.173) | 0.125 | – | – | – |
| Malignancy | 1.190 (0.731, 1.939) | 0.484 | – | – | – |
| Inflammatory indicators | |||||
| NLR | 1.009 (1.003, 1.016) | 0.006 | 1.006 (1.001, 1.013) | 1.4 | 0.048 |
| PLR | 1.000 (1.000, 1.001) | 0.108 | – | – | – |
| PNI | 0.941 (0.908, 0.975) | < 0.001 | 0.958 (0.923, 0.994) | 1.0 | 0.024 |
| Scoring system | |||||
| SAPSII | 1.037 (1.020, 1.054) | < 0.001 | – | – | – |
| SOFA | 1.099 (1.020, 1.184) | 0.013 | – | – | – |
| Treatment | |||||
| MV | 2.602 (1.339, 5.057) | 0.005 | 2.516 (1.265, 5.005) | 1.2 | 0.008 |
| Vasopressor | 2.206 (1.408, 3.455) | < 0.001 | 2.042 (1.267, 3.292) | 1.4 | 0.003 |
ALT alanine transaminase, AST aspartate transaminase, BUN blood urea nitrogen, CHD coronary heart disease, CKD, chronic kidney disease, DM diabetes mellitus, IMV invasive mechanical ventilation, MAP mean atrial pressure, NLR neutrophil-to-lymphocyte ratio PLR platelet-to-lymphocyte ratio, PNI prognostic nutritional index, SAPSII simplified acute physiology score II, SOFA sequential organ failure assessment, VIF variance inflation factor, WBC white blood cell
Construction and internal validation of the prognostic nomogram
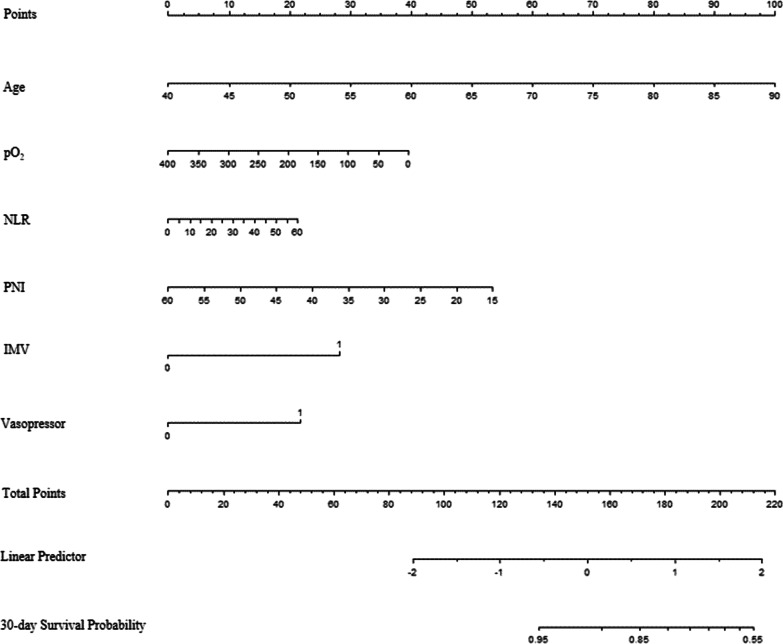
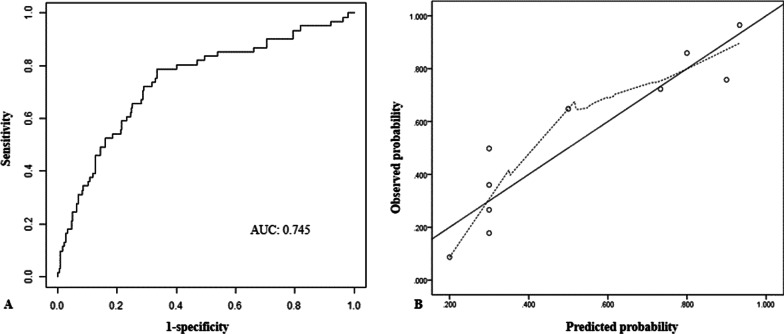
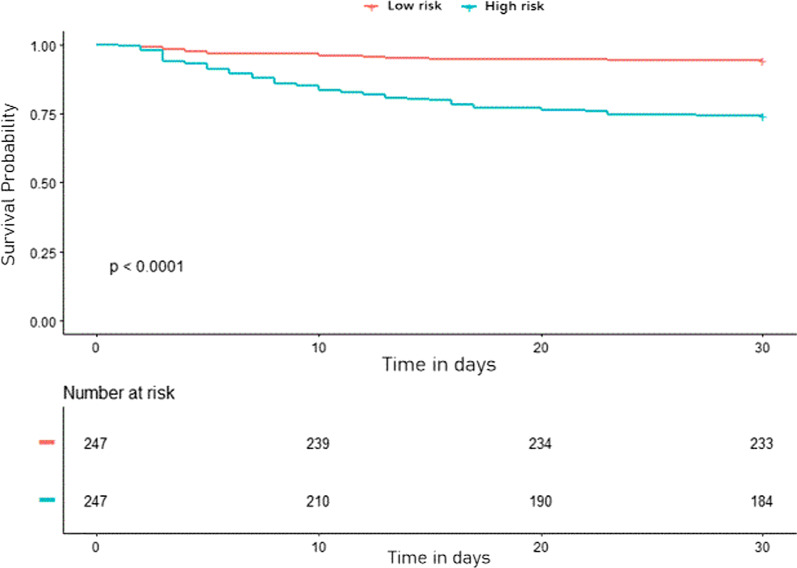
The nomogram for predicting the probability of 30-day survival among AECOPD patients was constructed based on the multivariate Cox regression model (Fig. 2). Every specific value of these factors was allocated a score on the points scale. By adding up these scores, the total score was calculated. The discrimination power of the nomogram was evaluated by the C-index values and ROC curves. As the relatively same sample size of our study, we adopted 1000 bootstrap for internal validation. After 1000 samples, the adjusted C-index was 0.745 (95% CI, 0.712 to 0.778) with sensitivity 78.7% and specificity 67.4% (Fig. 3A). The calibration curve showed that the prediction results of the nomogram model were in good agreement with the actual observations (HL test, P = 0.147) (Fig. 3B). In addition, the Kaplan–Meier survival curves revealed a significantly lower survival probability in the high-risk group than that in the low-risk group (P < 0.001), which indicated the substantial discriminatory power of the nomogram to stratify the risk (Fig. 4).
Fig. 2.
Nomogram to calculate risk score and predict 30-day survival probability in AECOPD patients. Scores were assigned for age, PO2, NLR level, PNI level, treatment of IMV and vasopressor by drawing a line upward from the corresponding values to the ‘score’ line. The sum of all these scores, plotted on the ‘Total score’ line, corresponds to predictions of 30-day survival probability. IMV invasive mechanical ventilation, NLR, neutrophil-to-lymphocyte ratio; PNI prognostic nutritional index; PO2 partial pressure of oxygen
Fig. 3.
The ROC curve (A) and calibration curve (B) of the nomogram in predicting 30-day mortality among AECOPD patients in ICU after 1000 bootstrap
Fig. 4.
The Kaplan–Meier survival curves classified by low-risk group and high-risk group
Clinical usefulness of the prognostic nomogram
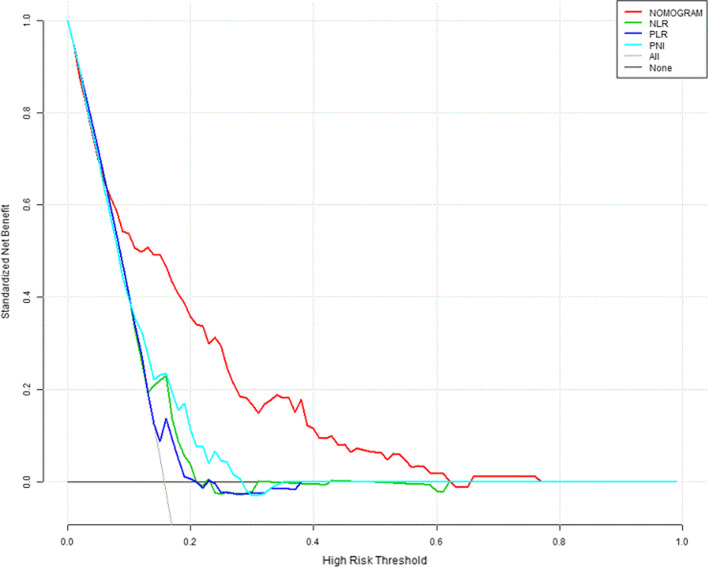
The DCA curve was plotted to perform a clinical application of this nomogram. Using data from the whole cohort, the DCA showed that if the threshold probability of a patient or doctor is 10%-60%, the prognostic model had a better positive net gain by risk stratification than NLR, PLR and PNI, indicating that it has good potential as a clinical application (Fig. 5).
Fig. 5.
Decision curve analysis for 30-day survival. The x-axis showed the threshold probability. The y-axis represented net benefit. Black line meant that all patients were dead and gray line represented that none patients were dead. The red line displayed the benefit of the nomogram. The green line displayed the benefit of NLR. The blue line displayed the benefit of PLR. The lake blue line displayed the benefit of PNI. NLR neutrophil-to-lymphocyte ratio; PLR platelet-to-lymphocyte ratio; PNI prognostic nutritional index
Discussion
The prognosis of patients with COPD exacerbations requiring ICU admission is generally poor. In this study, the 30-day mortality was 15.6%, which is consistent with findings of previous studies [4, 5]. Our main purpose was to use clinical data obtained from MIMIC III database to evaluate risk factors associated with 30-day mortality of patients with COPD exacerbation admitted to ICU. Then, we constructed a prognostic nomogram for AECOPD patients using individual patients’ status on admission to ICU. Six risk factors, namely, age, pO2, NLR, PNI, IMV and vasopressors use were included to establish a prognostic nomogram. This nomogram demonstrated good discrimination assessed by the C-index and calibration evaluated by HL goodness of fit test. Thus, this nomogram could be efficiently and effectively applied in clinical practice.
The impact of age as a prognostic factor for AECOPD patients is well known. Previous studies have demonstrated the role of age as a determinant of prognosis [4, 15, 16]. Issues related to increasing age, such as frailty, sarcopenia and co-morbidity, might affect prognosis [17]. Besides, elder patients usually present with atypical symptoms, such as muscle weakness, vertigo, confusion and leg edema during severe exacerbations [18]. What’s more, their respiratory system and immune function are impaired and more susceptible to pulmonary infection [6]. Hypoxemia is another frequently recognized prognostic factor. Several studies have reported its impact on poor prognosis in AECOPD patients [19–21]. Additionally, 48.6% of patients treated with IMV in our study and hypoxemia treated with IMV further increase the risk of mortality according to the nomogram. In a study by Brown et al. [22], 38.7% of patients required IMV and multivariate analysis demonstrated that the requirement for IMV was significantly correlated with in-hospital mortality. Cao et al. also found that requirement for IMV was a significant predictor of in-hospital mortality of AECOPD [23]. Vasopressor is the first-line to elevate blood pressure and hypotension occurs more frequently in those non-survivors [24].
The measurement of NLR and PNI are cost-effective and easily performed in clinical laboratories. The neutrophil–lymphocyte ratio, calculated by both neutrophil and lymphocyte counts, is a biomarker to predict systemic inflammation [25]. Several studies have utilized NLR as a marker of inflammation and severity for AECOPD patients. LEE et al. prospectively evaluated the value of NLR in patients with AECOPD, stable disease, and healthy controls. Compared to stable disease and healthy controls, NLR was significantly correlated with AECOPD [26]. Other studies also confirmed that NLR was elevated in AECOPD patients when compared with COPD and healthy controls [27–29]. NLR is also a prognostic biomarker in COPD. Yao et al. enrolled 303 patients with AECOPD. NLR values were significantly higher in non-survivors than those who survived in hospital [28]. Prognostic nutritional index is reflected by serum albumin concentration and peripheral total lymphocyte count. Serum albumin is a hallmark of nutritional status. Hypoalbuminemia could also reflect poor clinical status and persistent inflammation. Several studies have suggested that hypoalbuminemia is associated with increased mortality among AECOPD patients [17, 30, 31]. Lymphocyte count, another biomarker of immune status and inflammation, could predict risk of mortality in AECOPD patients. Lymphocytopenia has been proved to be correlated with increased mortality in COPD patients with acute exacerbation [23, 32]. Then, Peng et al. investigated the role of PNI in predicting mortality among AECOPD patients and they observed that the risk of 30-day mortality significantly increased with the downgraded of PNI [33].
This study still had some limitations. First, the database has large missing data on height and weight, so we could not evaluate the impact of body mass index on mortality. Second, the data for the nomogram were obtained from a single center and selection bias could not be avoided. Third, the generalization of our nomogram should be interpreted with caution due to the absence of external validation. So, we would require multicenter prospective studies to further investigate the clinical practice of our nomogram.
Conclusion
The nomogram developed in our study could help clinicians to predict risk of 30-day mortality in ICU and assist them to stratify patients and provide appropriate care in clinical setting.
Acknowledgements
Not applicable.
Abbreviations
- AECOPD
Acute exacerbation of chronic obstructive pulmonary disease
- AIC
Akaike information criterion
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- BUN
Blood urea nitrogen
- CHD
Coronary heart disease
- CKD
Chronic renal disease
- DCA
Decision curve analysis
- DM
Diabetes mellitus
- HR
Hazard ratio
- ICU
Intensive care unit
- IMV
Invasive mechanical ventilation
- IQR
Interquartile range
- MAP
Mean atrial pressure
- MIMIC
Medical information mart for intensive care
- NLR
Neutrophil-to-lymphocyte ratio
- pO2
Partial pressure of oxygen
- pCO2
Partial pressure of carbon dioxide
- PLR
Platelet-to-lymphocyte ratio
- PNI
Prognostic nutritional index
- ROC
Receiver operating characteristic
- sCr
Serum creatinine
- SAPS II
Simplified acute physiology score II
- SOFA
Sequential organ failure assessment
- VIF
Variance inflation factor
- WBC
White blood cell
Author contributions
P.J.C. wrote the manuscript. G.W.W. did the statistical work. W.Y extracted the data. Y.T.Y. designed the study. J.X.Y. reviewed and revised the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The datasets generated and analyzed during the current study are available in the MIMIC III repository (https://physionet.org/works/MIMICIIIClinicalDatabase/files/).
Declarations
Ethics approval and consent to participate
The establishment and employment of this database were approved by the Institutional Review Boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. All methods were performed in accordance with the relevant guidelines and regulations. No informed consent was required since all the data were de-identified.
Consent for publication
Not applicable.
Competing interests
None of the authors has any potential conflicts of interest related to this article to declare and the results of this report have been produced, analyzed, and interpreted without any outside participation.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiang-Chen Peng and Wen-Wen Gong contributed equally to the manuscript.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 2.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 3.Lima FV, Yen TY, Patel JK. Trends in In-hospital outcomes among adults hospitalized with exacerbation of chronic obstructive pulmonary disease. COPD. 2015;12(6):636–642. doi: 10.3109/15412555.2015.1020151. [DOI] [PubMed] [Google Scholar]
- 4.Singanayagam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(2):81–89. doi: 10.1513/AnnalsATS.201208-043OC. [DOI] [PubMed] [Google Scholar]
- 5.Ongel EA, Karakurt Z, Salturk C, Takir HB, Burunsuzoglu B, Kargin F, et al. How do COPD comorbidities affect ICU outcomes? Int J Chron Obstruct Pulmon Dis. 2014;9:1187–1196. doi: 10.2147/copd.s70257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pei Z, Sun Y, Wang S, Chen Y, Yang T, Huang K, et al. Estimating mortality among inpatients with acute exacerbation of chronic obstructive pulmonary disease using registry data. NPJ Primary Care Respir Med. 2020;30(1):28. doi: 10.1038/s41533-020-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slenter RH, Sprooten RT, Kotz D, Wesseling G, Wouters EF, Rohde GG. Predictors of 1-year mortality at hospital admission for acute exacerbations of chronic obstructive pulmonary disease. Respir Int Rev Thorac Dis. 2013;85(1):15–26. doi: 10.1159/000342036. [DOI] [PubMed] [Google Scholar]
- 8.Yohannes AM, Baldwin RC, Connolly MJ. Predictors of 1-year mortality in patients discharged from hospital following acute exacerbation of chronic obstructive pulmonary disease. Age Ageing. 2005;34(5):491–496. doi: 10.1093/ageing/afi163. [DOI] [PubMed] [Google Scholar]
- 9.Steriade AT, Davidoiu A, Afrasinei A, Tudose C, Radu D, Necula D, et al. Predictors of long-term mortality after hospitalization for severe COPD exacerbation. Maedica. 2019;14(2):86–92. doi: 10.26574/maedica.2019.14.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(8):1364–1370. doi: 10.1200/jco.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 11.Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamison DC. Structured Query Language (SQL) fundamentals. Current protocols in bioinformatics. 2003;Chapter 9:Unit9 2. 10.1002/0471250953.bi0902s00. [DOI] [PubMed]
- 13.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56(2):337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 14.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Mak Int J Soc Med Decis Mak. 2006;26(6):565–574. doi: 10.1177/0272989x06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spannella F, Giulietti F, Cocci G, Landi L, Lombardi FE, Borioni E, et al. Acute exacerbation of chronic obstructive pulmonary disease in oldest adults: predictors of in-hospital mortality and need for post-acute care. J Am Med Dir Assoc. 2019;20(7):893–898. doi: 10.1016/j.jamda.2019.01.125. [DOI] [PubMed] [Google Scholar]
- 16.Edwards L, Perrin K, Wijesinghe M, Weatherall M, Beasley R, Travers J. The value of the CRB65 score to predict mortality in exacerbations of COPD requiring hospital admission. Respirology (Carlton, Vic) 2011;16(4):625–629. doi: 10.1111/j.1440-1843.2011.01926.x. [DOI] [PubMed] [Google Scholar]
- 17.Yu X, Zhu GP, Cai TF, Zheng JY. Establishment of risk prediction model and risk score for in-hospital mortality in patients with AECOPD. Clin Respir J. 2020;14(11):1090–1098. doi: 10.1111/crj.13246. [DOI] [PubMed] [Google Scholar]
- 18.Hogea SP, Tudorache E, Fildan AP, Fira-Mladinescu O, Marc M, Oancea C. Risk factors of chronic obstructive pulmonary disease exacerbations. Clin Respir J. 2020;14(3):183–197. doi: 10.1111/crj.13129. [DOI] [PubMed] [Google Scholar]
- 19.Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest. 2003;124(2):459–467. doi: 10.1378/chest.124.2.459. [DOI] [PubMed] [Google Scholar]
- 20.Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163(10):1180–1186. doi: 10.1001/archinte.163.10.1180. [DOI] [PubMed] [Google Scholar]
- 21.Matkovic Z, Huerta A, Soler N, Domingo R, Gabarrús A, Torres A, et al. Predictors of adverse outcome in patients hospitalised for exacerbation of chronic obstructive pulmonary disease. Respir Int Rev Thorac Dis. 2012;84(1):17–26. doi: 10.1159/000335467. [DOI] [PubMed] [Google Scholar]
- 22.Brown H, Dodic S, Goh SS, Green C, Wang WC, Kaul S, et al. Factors associated with hospital mortality in critically ill patients with exacerbation of COPD. Int J Chron Obstr Pulmon Dis. 2018;13:2361–2366. doi: 10.2147/copd.s168983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Y, Xing Z, Long H, Huang Y, Zeng P, Janssens JP, et al. Predictors of mortality in COPD exacerbation cases presenting to the respiratory intensive care unit. Respir Res. 2021;22(1):77. doi: 10.1186/s12931-021-01657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morasert T, Jantarapootirat M, Phinyo P, Patumanond J. Prognostic indicators for in-hospital mortality in COPD with acute exacerbation in Thailand: a retrospective cohort study. BMJ Open Respir Res. 2020 doi: 10.1136/bmjresp-2019-000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faria SS, Fernandes PC, Jr, Silva MJ, Lima VC, Fontes W, Freitas-Junior R, et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience. 2016;10:702. doi: 10.3332/ecancer.2016.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SJ, Lee HR, Lee TW, Ju S, Lim S, Go SI, et al. Usefulness of neutrophil to lymphocyte ratio in patients with chronic obstructive pulmonary disease: a prospective observational study. Korean J Intern Med. 2016;31(5):891–898. doi: 10.3904/kjim.2015.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylan M, Demir M, Kaya H, Selimoglu Sen H, Abakay O, Carkanat A, et al. Alterations of the neutrophil-lymphocyte ratio during the period of stable and acute exacerbation of chronic obstructive pulmonary disease patients. Clin Respir J. 2017;11(3):311–317. doi: 10.1111/crj.12336. [DOI] [PubMed] [Google Scholar]
- 28.Yao C, Liu X, Tang Z. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chron Obstr Pulmon Dis. 2017;12:2285–2290. doi: 10.2147/copd.s141760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurtipek E, Bekci TT, Kesli R, Sami SS, Terzi Y. The role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in exacerbation of chronic obstructive pulmonary disease. JPMA J Pak Med Assoc. 2015;65(12):1283–1287. [PubMed] [Google Scholar]
- 30.Hasegawa W, Yamauchi Y, Yasunaga H, Sunohara M, Jo T, Matsui H, et al. Factors affecting mortality following emergency admission for chronic obstructive pulmonary disease. BMC Pulm Med. 2014;14:151. doi: 10.1186/1471-2466-14-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haja Mydin H, Murphy S, Clague H, Sridharan K, Taylor IK. Anemia and performance status as prognostic markers in acute hypercapnic respiratory failure due to chronic obstructive pulmonary disease. Int J Chron Obstr Pulm Dis. 2013;8:151–157. doi: 10.2147/copd.s39403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaya M, Usami O, Nakayama S, Tode N, Yamada A, Ito S, et al. Malnutrition, airflow limitation and severe emphysema are risks for exacerbation of chronic obstructive pulmonary disease in japanese subjects: a retrospective single-center study. Int J Chron Obstr Pulm Dis. 2020;15:857–868. doi: 10.2147/copd.s238457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng JC, Nie F, Li YJ, Xu QY, Xing SP, Gao Y. prognostic nutritional index as a predictor of 30-day mortality among patients admitted to intensive care unit with acute exacerbation of chronic obstructive pulmonary disease: a single-center retrospective cohort study. Med Sci Monit Int Med J Exp Clin Res. 2022;28:e934687. doi: 10.12659/msm.934687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The datasets generated and analyzed during the current study are available in the MIMIC III repository (https://physionet.org/works/MIMICIIIClinicalDatabase/files/).