Abstract
Glomus tumors are uncommon, benign solitary tumors derived from the glomus apparatus. We report here a case of a malignant glomus tumor in an 8-year-old child presenting as a multilocular ill-defined radiolucency of the mandible. The lesion microscopically showed sheets of round basophilic cells with high nuclear-cytoplasmic ratio, indistinct cell boundaries, nuclear hyperchromatism and nuclear pleomorphism. Immunohistochemically, the tumor was positive for vimentin and smooth muscle actin.
Keywords: Child, glomus tumor, malignant, mandible, oral, pedodontia, vimentin
INTRODUCTION
The glomus apparatus was identified in 1862 by Sucquet and described in detail by Hoyer in 1877. It is an arteriovenous anastomosis located in the stratum reticularis of the dermis predominantly on the palm and digits of the hand and the ventral surface of the feet.[1] It is involved in thermal regulation. The glomus tumor derived from the glomus apparatus accounts for <2% of soft tissue tumors.[2] It is a rare tumor that usually is seen in distal extremities. Other less common sites of involvement include the nasal cavity, middle ear, stomach, bone, lung and rarely oral cavity (0.6%).[3,4]
We present here a rare case of an intraoral malignant glomus tumor in the mandible and review the literature concerning the glomus tumor of the oral cavity.
CASE REPORT
An 8-year-old girl presented to the Department of Pedodontics, Ragas Dental College and Hospital, with an intraoral growth in the alveolar region of the right body of the mandible of 1-year duration with a history of a gradual increase to the present size of 3 × 3 cm [Figure 1]. The sessile growth was soft in consistency and nontender on palpation. Mucosa over the swelling was smooth and not ulcerated. The first molar and deciduous canine and first molar on the affected side were mobile. The patient gave a history of incisional biopsy done a month earlier, reported as “insufficient tissue for diagnosis.” Orthopantomogram revealed an ill-defined multilocular radiolucency in the right body of the mandible measuring 3.5 × 1.5 cm in size and extending from the apical end of 83 to the mesial aspect of the 47 permanent tooth bud. Forty-six and 84 exhibited root resorption. The permanent tooth buds 44 and 45 were within the radiolucency [Figure 2]. The provisional diagnosis was ameloblastic fibrosarcoma.
Figure 1.
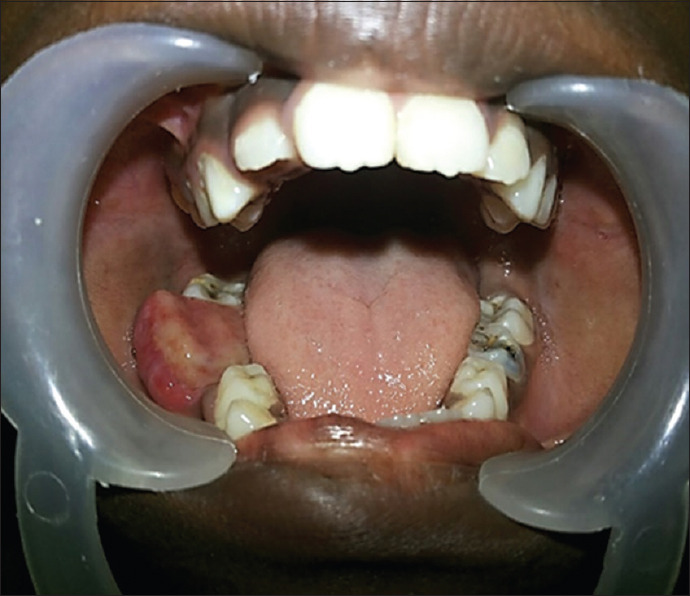
Intraoral growth in the alveolar region body of the mandible
Figure 2.
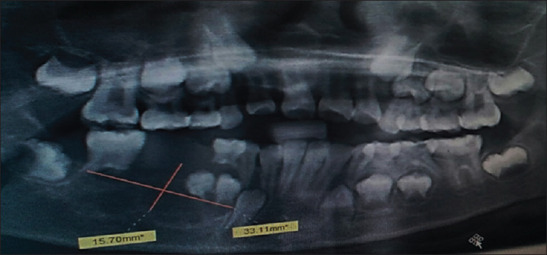
Orthopantomogram revealed ai ill-defined multilocular radiolucency
An excisional biopsy was done. The gross specimen submitted included hard and soft tissue and measured 2 × 4.5 × 2 cm in size [Figure 3].
Figure 3.
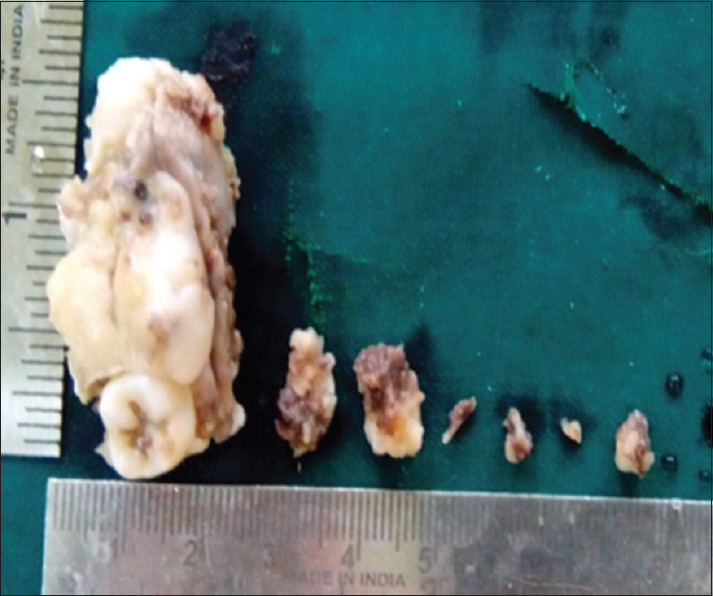
Gross specimen
Histopathological examination showed uniform, monomorphic round blue cells arranged in sheets and cords. The cells were basophilic with a high nuclear-cytoplasmic ratio, indistinct cell boundaries [Figure 4a], foci of nuclear hyperchromatism, nuclear pleomorphism and mitotic figures (9/10HPF) [Figure 4b]. The connective tissue stroma was fibrovascular with numerous dilated capillaries and focal edematous areas. The tumor cells were positive for vimentin and smooth muscle actin (SMA) [Figures 4c and d] and negative for desmin, p63, CD34 and CD45.
Figure 4.
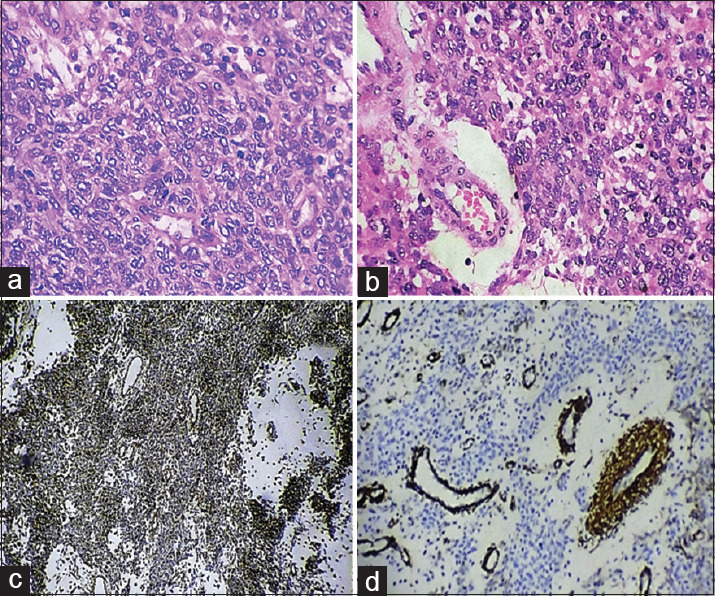
(a) Sheets of round cells with basophilic cytoplasam, high nuclear-cytoplasmic ratio and indistinct cell boundaries (H and E, ×40). (b) The presence of mitotic figures (H and E, ×40). (c) The tumor cells show diffuse positivity for vimentin (immunohistochemistry, ×10). (d) The tumor cells show focal positivity for smooth muscle actin (immunohistochemistry, ×10)
The histopathological features were not consistent with the clinical diagnosis of ameloblastic fibrosarcoma because ameloblastic fibrosarcoma shows scattered odontogenic epithelial cell rests, intertwining fibrils in the connective tissue.
DISCUSSION
The glomus tumor is a distinct neoplasm of perivascular cells that resemble modified smooth muscle cells seen in the glomus body. The glomus body is most frequently encountered in the subungual region, lateral areas of the digits and the palm where it is involved in thermal regulation. Glomus tumors are usually solitary, painful and well-circumscribed and treated by simple excision. Rarely, they can be multiple.[1] Table 1 lists the cases of oral glomus tumors reported in the literature from 1949 to 2015.
Table 1.
Cases of glomus tumor affecting the oral cavity
| Author | Year | Age/sex | Anatomic location | IHC profile |
|---|---|---|---|---|
| Von Langer[5] | 1949 | 52 male | Hard palate | Not available |
| King[6] | 1954 | 32 male | Gingiva | Not available |
| Kirschner and Strassburg[7] | 1962 | 56 male | Gingiva/alveolar mucosa | Not available |
| Grande and D’Angelo[8] | 1962 | 42 male | Hard palate | Not available |
| Frankel[9] | 1965 | 13 male | Buccal mucosa | Not available |
| Harris and Griffin[10] | 1965 | 35 female | Periodontium/gingiva | Not available |
| Sidhu and Subherwal[11] | 1967 | 10 female | Hard palate | Not available |
| Charles (multiple lesions)[12] | 1976 | 17 female | Hard palate | Not available |
| Sato et al.[13] | 1979 | 29 male | Tongue | Not available |
| Tajima et al.[14] | 1981 | 63 female | Tongue | Not available |
| Saku et al.[15] | 1985 | 45 male | Buccal mucosa | Actin +, smooth muscle myosin + |
| Ficarra et al.[16] | 1986 | 51 female | Upper lip | Not available |
| Moody et al.[17] | 1986 | 65 female | Upper lip | Vimentin +, Factor VIII -, CD45 -, A-BGA -, cytokeratin - |
| Stajcić and Bojić[18] | 1987 | 55 male | Tongue | Not available |
| Geraghty et al.[19] | 1992 | 71 male | Hard palate | Alpha actin -, neuron-specific enolase -, Chromogranin -, desmin - |
| Kusama et al.[20] | 1995 | 57 male | Upper lip | S-100 +, actin +, desmin +, vimentin +, Factor VIII - |
| Sakashita et al.[21] | 1997 | 54 male | Upper lip | Vimentin +, SMA +, Factor VIII - |
| Yu et al. (multiple lesions)[22] | 2000 | 54 female | Left mandibular area, lip, anterior buccal mucosa | SMA +, S-100 - |
| Kessaris et al.[23] | 2001 | 46 female | Hard palate | Vimentin +, S-100 +, actin -, desmin -, chromogranin -, neuron-specific enolase -, epithelial membrane antigen -, cytokeratin -, Factor VIII - |
| Rallis et al.[24] | 2004 | 85 female | Upper lip | SMA +, MSA+Vimentin +, desmin -, AE1/3 -, S-100 - Epithelial membrane antigen -Neuron-specific enolase - CD3, CD31, CD34, CD45, CD20 - Cytokeratin -, Leu 7 - |
| Lanza et al.[25] | 2005 | 65 male | Lower lip | Not available |
| Boros et al.[3] | 2010 | 34 male | Lower lip | SMA +, MSA+S-100 +, keratin - Epithelial membrane antigen - CD34 -, CD31 -, chromogranin - |
| Per Durand III et al.[26] | 2010 | 11 female | Lower lip | Epithelial membrane antigen, S-100 SMA+, vimentin +, pan cytokeratin |
| Biswas et al.[4] | 2014 | 38 male | The floor of the mouth | Not available |
| Mohan et al.[27] | 2015 | 15 female | Upper lip | Not available |
IHC: Immunohistochemical, SMA: Smooth muscle actin, MSA: Muscle-specific actin
The term glomangioma for the benign tumor was coined by Bailey in 1935. Masson described the occurrence of three histologic patterns –i) angiomatous-most common, ii) solid comprising cellular areas of smooth muscle cells and epithelioid cells and iii) degenerative with hyalinization, edema and mucoid changes in a myxoid stroma. However, these patterns may be mixed in varying proportions in any glomus tumor.[4,25]
The World Health Organization classifies glomus tumors as glomangioma (prominent vascular component), glomangiomyoma (prominent smooth muscle component) and solid glomus tumor (prominent cellular component).[3,28]
Variants of glomus tumors include (1) Glomangiomatosis, a benign, diffuse-growing glomus tumor; (2) Symplastic glomus tumor, characterized by marked nuclear atypia (representing a degenerative phenomenon) and no other features of malignancy and (3) Malignant glomus tumor or glomangiosarcoma, which accounts for approximately 1% of all glomus tumors.[1]
Our present case showed sheets of round cells with basophilic cytoplasm, high nuclear-cytoplasmic ratio, nuclear hyperchromatism, nuclear pleomorphism and mitotic figures (9/10HPF), all suggestive of a malignant glomus tumor
Histological differential diagnosis included hemangiopericytoma, myopericytoma, leiomyosarcoma and gastrointestinal stromal tumor (GIST) [Table 2].
Table 2.
Differential diagnosis
| Differential diagnosis | Clinical features | Histopathology | IHC profile |
|---|---|---|---|
| Hemangiopericytoma | Benign tumor | Ovoid to spindle cells | CD34 +, SMA - |
| Myopericytoma | Benign neoplasm of pericytes | Spindle-shaped cells | SMA +, CD34 -, desmin - |
| Leiomyosarcoma | Malignant Smooth muscle tumor | Spindle to round cells | Desmin +, SMA +, MSA +, CD34 - |
| GIST | Neoplasm in the gastrointestinal tract | Spindle cells arranged in fasciitis | c-KIT +, CD34 +, SMA +, desmin - |
IHC: Immunohistochemical, SMA: Smooth muscle actin, MSA: Muscle-specific actin, GIST: Gastrointestinal stromal tumor
Hemangiopericytoma is a benign tumor, clinically usually large and situated deep in the connective tissue. Histopathologically, the neoplastic cells of hemangiopericytoma have ovoid to spindle morphology and immunohistochemically show positivity for CD34 and negativity for SMA. Immunohistochemically, our present case was negative for CD34 and positive for SMA.
Myopericytoma is a tumor of neoplastic pericytes with smooth muscle differentiation around vascular channels. The neoplastic cells of myopericytoma are spindle, while in the present case, the neoplastic cells were round to oval. Myopericytoma is positive for SMA and CD34 as was our present case.
Leiomyosarcoma is a malignant tumor of smooth muscles. The neoplastic cells of a leiomyosarcoma have a spindle to round morphology, whereas neoplastic cells of the glomus tumor are round to oval in morphology. Leiomyosarcoma is positive for desmin which was negative in the present case.
GIST is a mesenchymal neoplasm that arises in the gastrointestinal tract. In pediatric patients, GIST occurs as a component of Carney's triad (gastric GIST, extraadrenal paraganglioma and pulmonary chondroma). GIST has spindle cells and shows immunohistochemistry (IHC) positivity for c-KIT and CD34. The present case was in a pediatric patient, however, the cells were round and were negative for CD34.
The clinical, histopathological features (round cells, cellular and nuclear pleomorphism and mitotic figures) and IHC features (vimentin and SMA positivity) led to the diagnosis of malignant glomus tumor.
CONCLUSION
Malignant glomus tumor is one of the rare sarcomas in the oral cavity
It is a high-grade sarcoma and should be treated immediately to avoid metastasis
It is necessary to consider malignant glomus tumor as one of the differential diagnoses in round cell sarcoma histopathologically
To our knowledge, this is the first case of malignant glomus tumor reported in the oral cavity.
Declaration of patient consent
The authors certify that they have obtained allappropriate patient consent forms. In the form the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.De Chiara A, Apice G, Mori S, Silvestro G, Losito SN, Botti G, et al. Malignant glomus tumour: A case report and review of the literature. Sarcoma. 2003;7:87–91. doi: 10.1080/1357714031000081207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gopal MG, Manjunath NC, Kumar S, Ramesh M, Nandini AS. Glomangioma: a rare case report. Journal of Evolution of Medical and Dental Sciences. 2014;3(1):127–133. [Google Scholar]
- 3.Boros AL, Davis JP, Sedghizadeh PP, Yamashita DD. Glomus tumor: Report of a rare case affecting the oral cavity and review of the literature. J Oral Maxillofac Surg. 2010;68:2329–34. doi: 10.1016/j.joms.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Biswas KD, Sen A, Guha G, Misra S, Sinha R. Glomus tumor in floor of mouth: a case report. Journal of Evolution of Medical and Dental Sciences. 2014;3((22)):6036–6043. [Google Scholar]
- 5.Von Langer R. Glomustumor am harten Gaumen. Wein Med Wochenschr. 1949;99:67. [PubMed] [Google Scholar]
- 6.King ES. Glomus tumor. Aust N Z J Surg. 1954;23:280. doi: 10.1111/j.1445-2197.1954.tb05057.x. [DOI] [PubMed] [Google Scholar]
- 7.Kirschner H, Strassburg M. A glomus tumor localized on the alveolar process of the lower jaw. Dtsch Zahnarzl Z. 1962;17:912. [Google Scholar]
- 8.Grande R, D’Angelo E. A glomic tumor of the hard palate. Arch Ital Laringol. 1962;70:89. [Google Scholar]
- 9.Frankel VG. Occurrence of a leiomyofibromangioma (Glomangiomas) Dtsch Zahnartzl Z. 1965;20:168. [Google Scholar]
- 10.Harris R, Griffin CJ. Glomus tumour of the periodontal tissues. Aust Dent J. 1965;10:33–7. doi: 10.1111/j.1834-7819.1965.tb01597.x. [DOI] [PubMed] [Google Scholar]
- 11.Sidhu SS, Subherwal GL. Glomus tumor of the palate. Indian Dent. 1967;39:167. [PubMed] [Google Scholar]
- 12.Charles NC. Multiple glomus tumors of the face and eyelid. Arch Ophthalmol. 1976;94:1283–5. doi: 10.1001/archopht.1976.03910040155005. [DOI] [PubMed] [Google Scholar]
- 13.Sato M, Shirasuna K, Sakuda M, Yanagawa T, Yoshida H, Imai J, et al. Fine structure of a glomus tumor of the tongue and expression of C type virus in its tumor cells. Int J Oral Surg. 1979;8:199. doi: 10.1016/s0300-9785(79)80019-8. [DOI] [PubMed] [Google Scholar]
- 14.Tajima Y, Weathers DR, Neville BW, Benoit PW, Pedley DM. Glomus tumor (glomangioma) of the tongue: A light and electron microscopic study. Oral Surg Oral Med Oral Pathol. 1981;52:288. doi: 10.1016/0030-4220(81)90268-1. [DOI] [PubMed] [Google Scholar]
- 15.Saku T, Okabe H, Matsutani K, Sasaki M. Glomus tumor of the cheek: An immunohistochemical demonstration of actin and myosin. Oral Surg Oral Med Oral Pathol. 1985;60:65–71. doi: 10.1016/0030-4220(85)90218-x. [DOI] [PubMed] [Google Scholar]
- 16.Ficarra G, Merrell PW, Johnston WH, Hansen LS. Intraoral solitary glomus tumor (glomangioma): Case report and literature review. Oral Surg Oral Med Oral Pathol. 1986;62:306–11. doi: 10.1016/0030-4220(86)90013-7. [DOI] [PubMed] [Google Scholar]
- 17.Moody GH, Myskow M, Musgrove C. Glomus tumor of the lip.A case report and immunohistochemical study. Oral Surg Oral Med Oral Pathol. 1986;62:312–8. doi: 10.1016/0030-4220(86)90014-9. [DOI] [PubMed] [Google Scholar]
- 18.Stajcić Z, Bojić P. Intraoral glomus tumour.A case report. J Craniomaxillofac Surg. 1987;15:376–8. doi: 10.1016/s1010-5182(87)80087-2. [DOI] [PubMed] [Google Scholar]
- 19.Geraghty JM, Thomas RW, Robertson JM, Blundell JW. Glomus tumour of the palate: Case report and review of the literature. Br J Oral Maxillofac Surg. 1992;30:398–400. doi: 10.1016/0266-4356(92)90210-a. [DOI] [PubMed] [Google Scholar]
- 20.Kusama K, Chu L, Kidokoro Y, Kouzu M, Uehara T, Honda M, et al. Glomus tumor of the upper lip. J Nihon Univ Sch Dent. 1995;37:97–101. doi: 10.2334/josnusd1959.37.97. [DOI] [PubMed] [Google Scholar]
- 21.Sakashita H, Miyata M, Nagao K. Glomus tumor in the upper lip.A case report. Int J Oral Maxillofac Surg. 1997;26:301–2. doi: 10.1016/s0901-5027(97)80876-4. [DOI] [PubMed] [Google Scholar]
- 22.Yu HJ, Kwon SJ, Bahn JY, Park JM, Park YW. Localized multiple glomus tumors of the face and oral mucosa. J Dermatol. 2000;27:211–3. doi: 10.1111/j.1346-8138.2000.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 23.Kessaris P, Klimis T, Zanakis S. Glomus tumour of the hard palate: Case report and review. Br J Oral Maxillofac Surg. 2001;39:478–9. doi: 10.1054/bjom.2001.0721. [DOI] [PubMed] [Google Scholar]
- 24.Rallis G, Komis C, Mahera H. Glomus tumor: A rare location in the upper lip. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:327–36. doi: 10.1016/S1079210404001027. [DOI] [PubMed] [Google Scholar]
- 25.Lanza A, Moscariello A, Villani R, Colella G. Glomus tumor of the lower lip.A case report. Minerva Stomatol. 2005;54:687–90. [PubMed] [Google Scholar]
- 26.Dérand P, 3rd, Warfvinge G, Thor A. Glomangioma: A case presentation. J Oral Maxillofac Surg. 2010;68:204–7. doi: 10.1016/j.joms.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Mohan M, Pandya K, Pradeep S. Glomangioma on the upper lip is rare. Indian Dent Res Rev. 2015;10:46–7. [Google Scholar]
- 28.Fletcher DC, Unni KK, Mertens F, editors. World Health Organization Classification of Tumors. Glomus tumors. Pathology and Genetics of Tumors of Soft Tissue and Bone. Lyon: IARC; 2002. p. 137. [Google Scholar]


