Abstract
Background:
Lactate dehydrogenase (LDH), an intra-cellular enzyme present in all cells of the body, catalyses the final step of anaerobic glycolysis. This intra-cellular enzyme is released into the extra-cellular space after tissue disintegration, which is evident in oral squamous cell carcinoma (OSCC). However, investigations comparing Lactate dehydrogenase (LDH) levels in OSCC and healthy controls have shown conflicting findings in both serum and saliva samples. Further, Uric acid's anti-oxidant activity has been demonstrated in several diseases. Several cancers have been linked to increased uric acid levels. However, uric acid levels in oral squamous cell cancer have varied. There exists limitted research comparing serum and salivary uric acid with OSCC. Thus, the present investigation was conducted to evaluate the combined diagnostic abilities of serum and salivary LDH and uric acid in OSCC.
Aim and Objective:
To compare and correlate LDH and uric acid levels in serum and salivary samples of OSCC patients and healthy individuals.
Material and Methods:
LDH levels and uric acid levels were measured using an enzymatic method in serum and salivary samples of OSCC cases (n = 18) and healthy individuals (n = 18).
Results:
This study indicated statistically significant elevated levels of LDH in serum and saliva samples of OSCC patients when compared to healthy individuals. Furthermore, serum and salivary uric acid were higher in OSCC patients than in controls. This increased levels of uric acid was significant only in serum but not in saliva samples. However, salivary uric acid was found to be co-relating with serum uric acid. In addition to this, the receiver operating characteristic (ROC) curve when plotted to assess combined diagnostic abilities of all the investigations to predict oscc, indicating the diagnostic ability to be 77%.
Conclusion:
This study found an increase in uric acid levels in OSCC patients, which contradicts previous existing litratures. Salivary uric acid and LDH levels may be effective indicators for OSCC screening. However, because of the limited sample size, these findings should be viewed with caution.
Keywords: Lactate dehydrogenase, oral squamous cell carcinoma, saliva, serum, uric acid
INTRODUCTION
Oral cancer (OC) is the leading cause of cancer mortality among men in India. High prevalence of OC was also found to be prevalent in Europe, Australia, and New Zealand.[1] The aetiology of OC has been linked to cigarette smoking, human papillomavirus infection, and ultraviolet radiation.[2,3,4,5] OCs have the ability to metastasise to regional lymph nodes before metastasising to distant sites, and this ability to metastasise to regional lymph nodes in patients with oral cancer is recognised as a factor that governs and influences the “disease survival rate” in patients with oral squamous cell carcinoma (OSCC). The delay in diagnosis of OSCC and the tumour's poor response to treatment are two variables that have a continuous influence on the disease survival rate associated with OSCC. As a result, early and accurate diagnosis remains the single most significant factor in determining the prognosis of OSCC. A range of tissue molecular markers have been identified and have been extensively explored in order to predict the early transition from the normal epithelium to pre-malignancy and, subsequently, onto OSCC;[6] however, ideal ones are yet to be established.
In addition to this, advancements in the field of molecular biology have led to the discovery of tumour biomarkers in biological fluids such as blood, saliva, and urine. A more recent study has shown significant elevation of serum levels of the squamous cell carcinoma antigen (SCC-Ag), ferritin, and the carcino embryonic antigen (CEA) in OSCC patients, suggesting for a diagnostic importance of these markers.[7] Further, utilising saliva for early cancer diagnosis in the search for novel clinical indications is a potential technique because of the non-invasive nature of the sample and the simplicity with which it can be collected. In addition to this proteins, peptides, electrolytes, and organic and inorganic salts secreted by the salivary glands, parallel contributions from gingival crevicular fluids and mucosal transudates contribute to the overall composition of the salivary fluid, all of which could serve as markers that could be effectively investigated to understand the disease process. Many salivary biomarkers have been explored for early detection and diagnosis of OSCC, such as protein biomarkers [interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin 1a (IL-1a), interleukin 1b (IL-1b), TNF-a, tissue polypeptide antigen (TPA), Cyfra 21-1, cancer antigen 125 (CA 125), telomerase, Mac-2 binding protein (M2BP), CD44, CD59, profilin, MRP14, glutathione, Mac-2 binding protein (M2BP), squamous cell carcinoma antigen 2, involucrin, calcyclin, cathepsin-G, azurocidin, transaldolase, carbonic anhydrase I, calgizzarin, myeloblastin, vitamin D-binding protein, immunoglobulin heavy chain constant region gamma (IgG), S100 calcium-binding protein, cofilin-1, transferrin, fibrin, α-1-antitrypsin (AAT), secretory leukocyte peptidase inhibitor (SLPI), cystatin A, keratin 36, thioredoxin, haptoglobin (HAP), salivary zinc finger, Protein 510 peptide, a-amylase, and albumin], genomic biomarkers [DNA (promoter hyper-methylation), histone family 3 (HA3), S100 calcium-binding protein P (S100P), spermidine/spermine N1-acetyltransferase EST (SAT), ornithin decarboxylase antizyme 1 (OAZ), P53 gene codon 63, mitochondrial DNAs such as cytochrome cooxidase I and II, tumour suppressor genes (DAPK, DCC, TIMP-31, TIMP-3, MGMT, CCNA1, MINT-31)], transcriptomic biomarkers (IL-1β, IL-8, dual-specificity phosphatase 1 (DUSP1), H3 histone family 3A (H3F3A), long non-coding HOTAIR, miR-125a, miR-200a, and miR-31], metabolomics biomarkers (cadaverine, alanine, serine, glutamine, piperidine, taurine piperidine, choline, pyrroline hydroxycarboxylic acid, beta-alanine, alpha-aminobutyric acid betaine, tyrosine, leucine + isoleucine, histidine, tryptophan, glutamic acid, threonine, carnitine, pipercolic acid, lactic acid, phenylalanine and valine, hypoxanthine, guanine, guanosine, trimethylamine N-oxide, spermidine, pipecolate, methionine), oxidative stress-related molecules [glutathione S-transferase (GST), peroxidase, malondialdehyde (MDA), 8-hydroxy-2- deoxyguanosine (8-OHdG), glutathione S-transferase (GST), reactive nitrogen species (RNS) such as nitric oxide (NO), nitrites (NO2), and nitrates (NO3), superoxide dismutase (SOD)], and glycosylation-related molecules (α-L-fucosidase, sialic acid).[8] However, further study is needed to ensure the reliability and validity of these salivary biomarkers in clinical applications for detection and prevention of OC.
Furthermore, tissue damage is known to be present in a variety of cancers, including OSCC. As a result, discovering a biomarker that indicates tissue damage might aid in the detection of oral squamous cell carcinoma. Lactate dehydrogenase (LDH), an intra-cellular enzyme found in abundance in all of the body's cells,[9] is regarded as a marker of tissue damage as this intra-cellular enzyme is released into the extra-cellular space whenever there is tissue destruction and cell necrosis.[10] Additionally, it is found that, even a small amount of the LDH enzyme released from the damaged tissue has the ability to elevate the LDH enzyme to a greater levels.[11,12]
LDH has been widely investigated in a variety of cancer types. In a study that compared levels of LDH in various solid tumours, higher levels of LDH were shown to be associated with a poor prognosis.[13] Further studies, such as those conducted by Suh SY et al.[14] and Zhou GQ et al.,[15] have shown that elevated serum LDH levels could be used to predict survival in patients with untreatable cancer and to correlate clinical staging in patients with nasopharyngeal carcinoma, respectively. In addition to this, there have been studies that have indicated higher levels of LDH in patients with oral potential malignant disorders and OC.[16,17,18]
In recent years, salivary LDH levels have been shown to be a diagnostic and prognostic tool for monitoring oral potentially malignant disorders (OPMDs) and OC, culminating in the discovery of novel insights into cancer monitoring. The source of salivary LDH in the oral cavity, according to some studies, may be attributed to the shedding of oral epithelial cells, which distinguish it from the LDH found in the bloodstream.[19] Moreover, alterations in the LDH profile in salivary samples may be associated with pathological diseases such as OSCC. Because of this, salivary LDH may be used to evaluate probable oral mucosal pathologies in a manner similar to other tissue pathologies, such as those in the heart, muscle, or liver, which can be examined using LDH detection in plasma.[19] According to the conclusions of a number of studies on salivary LDH in OSCC, patients had increasing levels of salivary LDH compared to healthy volunteers.[20,21,22] Besides this, another study found a statistically significant difference in the mean salivary LDH levels of oral leucoplakia and OSCC when compared to healthy controls.[23]
Similarly, uric acid, found in human blood plasma, is a potent anti-oxidant. Uric acid is produced as a result of purine metabolism, which is catalysed by the enzyme xanthine oxidoreductase (XOR).[24] This anti-oxidant effect of uric acid is evident in a multitude of disease conditions.[25,26] Hyperuricemia is a condition in which there is an increased level of uric acid in the blood, which is defined as either >7.0 mg/dL or >6.0 mg/dL of serum uric acid concentration.[27] Studies have shown that high levels of serum uric acid are found to be associated with renal illness, cardiovascular disease, and metabolic syndrome, among other conditions.[25,26] Because of its inherent anti-oxidant properties, the levels of uric acid have also been investigated in various malignancies such as gastric cancer,[28] rectal cancer,[29] and head and neck cancer[30] and have been found to be elevated. However, other studies, pertaining to OC, have found the levels of uric acid to be decreasing. As a consequence, studies on the relationship between uric acid and carcinogenesis have produced contradictory findings.[31]
Recently, salivary uric levels have been identified as a novel non-invasive diagnostic and prognostic marker for the monitoring of OPMDs and OSCC patients. In the literature, there are limited studies that show, salivary uric acid levels to be dependent on serum uric acid levels[32], studies that reveal decreased salivary uric acid levels in OSCC[33,34] and increased salivary uric acid levels in head and neck cancer.[35] However, there seems to be a paucity of studies comparing and correlating serum and salivary uric acid with OSCC. As a result, the research was conducted to examine serum and salivary LDH and uric acid levels in OC patients and healthy controls in order to determine if these indicators could be employed successfully as profiles of markers in the identification and monitoring of OSCC patients.
MATERIAL AND METHODS
Study design
A case–control study was performed on serum and saliva samples of OSCC patients and healthy volunteers, to evaluate the utility of serum and salivary LDH and uric acid levels as a profile of markers in OSCC patients.
Study population
A total of 36 subjects who gave consent participated in this study. The 36 participants were divided into two groups, namely, the OSCC group (group I, n = 18), which constituted serum and unstimulated saliva samples of all patients referred for treatment of incisional biopsy-proven primary OSCC with a history of tobacco use at ESIC Dental College and Kidwai Memorial Institute of Oncology, Kalaburgi, from September 2021 to December 2021, and the healthy volunteers group (group II, n = 18), which contained serum and unstimulated saliva samples obtained from healthy volunteers, with no history of tobacco use, any systemic or local illness, or any autoimmune disease. The healthy status of control subjects was determined based on the results of physical examination.
Patients with a history of malignancy other than OSCC; patients undergoing chemotherapy, radiotherapy, or any surgical procedure for OSCC; patients with a history of past systemic or oral malignancy; patients with a prolonged history of antibiotic therapy; patients with a history of aspirin, narcotics, or alcohol consumption and recent anaesthesia; patients with a history of heart failure (myocardial infarction) within the last 2 weeks; and patients taking procainamides and other drugs used to treat arrhythmia, pulmonary infarction, and stroke were excluded. This study was approved by the institutional review board and ethical committee of ESIC Dental College.
Technique proper
All the data pertaining to socio-demographic characteristics, adverse habits, dietary habits, TNM staging, and histopathological diagnosis and grades of OSCC were recorded, and written informed consent was obtained from all participants prior to collection of samples.
Collection of serum samples
A volume of 5 ml of venous blood samples was collected from the antecubital vein from all the the participants, transferred to a sterile test tube, and allowed to clot at room temperature. The serum was then separated out and centrifuged at 2000 rpm for 10 min. The serum thus obtained was transferred into Eppendorf tubes and stored at + 2°C to + 8°C. If assays are not completed within 48 hours or the separated sample is to be stored beyond 48 hours, samples were frozen at -15°C to -20°C.
Collection of saliva samples
The saliva was collected according to the technique described by Kallalli BN et al.[9] To avoid the influence of the circadian rhythm on salivary flow, saliva samples were obtained between 10 a.m. and 12 p.m., and participants were asked to fast for 60–90 minutes before sampling. All the participants were asked to sit in an upright position, with the head inclined forward. Initially, all participants were asked to swallow saliva first and were then asked to spit their saliva into sterile plastic vials to obtain unstimulated saliva samples. After centrifuging the saliva samples, pure saliva without sputum was placed into micro-tubes. Saliva was immediately centrifuged at 2000 rpm for 10 minutes to eliminate squamous cells and cell debris after collection. The supernatants were kept at -20°C until further evaluation.
Estimation of serum and salivary LDH
Determination of LDH in serum and saliva was performed using a semi-automatic analyser machine (ErbaCHEM 5X, s.r.no: S2002125) and an LDH-P (S.L) (Manufacture name: Agappe, mfgdt: 11/2020, lot no: 30110184) reagent kit. The kit contained two reagents, LDH-P (S.L) as reagent 1 (R1) containing Tris buffer (pH 7.4)–80 mmol/L pyruvate–1.6 mmol/L sodium chloride–200 mmol/L and LDH-P (S.L) as reagent 2 (R2) containing 240 mmol/L of NADH. The working reagent was prepared by mixing 4 volumes of reagent 1 (R1) with 1 volume of reagent 2 (R2). A 1000 μL of the working reagent was then mixed with 10 μL of the serum/saliva sample and incubated at 37°C for 1 minute. Then, the change in absorbance per minute (▴OD/min) during 3 minutes at 340 nm of light was obtained. LDH activities were measured using the following formula and were tabulated accordingly.
LDH-P activity (U/L) = (▴OD/min) × 16030 (Provided factor)
Estimation of serum and salivary uric acid
The serum and salivary uric acid levels were determined using the uricase–Trinder end point method using an ERBA uric acid DES kit (Uric Acid Des-Dynamic Extended Stability with Lipid Clearing Agent Modified Trinder Method, End Point) (manufacturer name: Erba, mfgdt: 06/2021, lot no: B062124). The kit contained the uric acid reagent and uric acid standard. The uric acid working reagent (1000 μL) was pipetted onto each of three test tubes containing 20 μL of the serum/saliva sample (test), 20 μL of distilled water (blank), and 20 μL of the uric acid standard (standard) and mixed well. All the test tubes were then incubated at 37°C for 5 minutes. Then, the absorbance of the standard and each test at 505 nm against the reagent blank was measured, and the uric acid levels were calculated using the formula below and were tabulated accordingly.
Uric acid (mg/dl) = (Absorbance of test/Absorbance of standard) x Concentration of the standard (mg/dl)
Absorbance of standard
Statistical analysis
Each group's mean score of serum uric acid, salivary uric acid, serum LDH, and salivary LDH was computed, and the results were compared and correlated within and between groups. For serum uric acid, salivary uric acid, serum LDH, and salivary LDH, the Kolmogorov–Smirnov test was used to analyse the distribution of samples within the two groups. In the two groups, serum uric acid, salivary uric acid, and serum LDH scores followed a normal distribution. To compare and correlate within and between the groups, the parametric test, the independent t test, was used. Salivary LDH levels in controls, on the other hand, did not follow a normal distribution. To compare and correlate within and between the groups, the non-parametric Mann–Whitney U test was used. To correlate serum uric acid, salivary uric acid, serum LDH, and salivary LDH, Karl Pearson's correlation was used. Using a receiver operating characteristic (ROC) curve and quantifying the area under the curve (AUC), the combined diagnostic abilities of all screening tests were evaluated.
RESULTS
A total of 36 individuals were evaluated for serum and salivary LDH and uric acid levels in OSCC patients and healthy volunteers. There were 16 (88.89%) males and two (11.11%) females in the OSCC group and 13 (72.22%) males and five (27.78%) females in the control group. The mean age in the OSCC group was 44.67 years, and the mean age in the control group was 34.56 years. Out of 18 participants included in the OSCC group, 16 were in stage IV, one was in stage I, and one was in stage II. There were no patients in stage III OSCC. The mean age of the OSCC group was higher than that of the control group.
The mean serum LDH in group I (OSCC) was 341.61 ± 111.75 U/L (mean ± SD), and in group II (controls), it was 244.83 ± 37.98 U/L (mean ± SD). When an independent t test was applied to compare mean serum LDH levels in the OSCC group and control group, the mean serum LDH levels statistically significantly (p = 0.0014) differentiated the OSCC group from control group, as indicated in Table 1 and Graph 1. Further, the mean salivary LDH in group I (OSCC) was 660.44 ± 748.29 U/L (mean ± SD), and in group II (controls), it was 220.78 ± 40.85 U/L (mean ± SD). Because the data did not show normal distribution in the Kolmogorov–Smirnov test, the Mann–Whitney U test was applied to compare the mean salivary LDH levels between the OSCC group and the control group, which showed a statistically significant (p = 0.0082) difference between the two groups, as indicated in Table 2 and Graph 2.
Table 1.
Comparison of the OSCC group and control group with the mean of serum LDH scores by the independent t test
| Investigations | OSCC group | Controls | Mean Difference | t | p | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Mean | Sth.Dev. | Mean | Sth.Dev. | ||||
| Serum LDH | 341.61 | 111.75 | 244.83 | 37.98 | 96.78 | 3.4788 | 0.0014* |
*P<0.05
Graph 1.
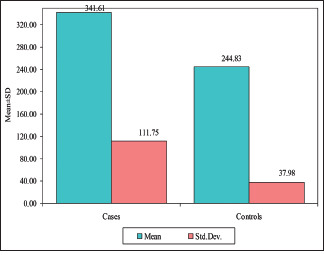
Comparison of the OSCC group and control group with the mean of serum LDH scores
Table 2.
Comparison of the OSCC group and control group with salivary LDH by Mann–Whitney U test
| Investigations | OSCC group | Controls | Z | p | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Mean | Sth.Dev. | Mean rank | Mean | Sth.Dev. | Mean rank | |||
| Salivary LDH | 660.44 | 748.29 | 23.17 | 220.78 | 40.85 | 13.83 | 2.6418 | 0.0082* |
*P<0.05
Graph 2.
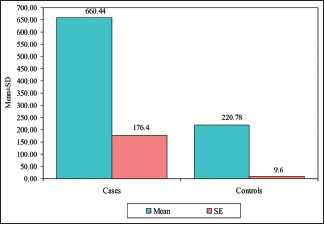
Comparison of the OSCC group and control group with salivary LDH
The mean serum uric acid in group I (OSCC) was 6.92 ± 3.02 mg/dl (mean ± SD), and in group II (controls), was 5.06 ± 1.33 mg/dl (mean ± SD). The mean salivary uric acid in group I (OSCC) was 5.88 ± 2.75 mg/dl (mean ± SD), and in group II (controls), was 4.73 ± 1.48 mg/dl (mean ± SD). An independent t test when used to compare mean serum uric acid levels between the OSCC group and control group showed a statistically significant (p = 0.0223) difference between the two groups, as indicated in Table 3 and Graph 3. However, the independent t test did not demonstrate any significant (p = 1.5554) difference between the OSCC group and the control group for mean salivary uric acid.
Table 3.
Comparison of the OSCC group and control group with the mean of serum uric acid and salivary uric acid by independent t test
| Investigations | OSCC group | Controls | Mean Difference | t | p | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Mean | Sth.Dev. | Mean | Sth.Dev. | ||||
| Serum uric acid | 6.92 | 3.02 | 5.06 | 1.33 | 1.86 | 2.3946 | 0.0223* |
| Salivary uric acid | 5.88 | 2.75 | 4.73 | 1.48 | 1.14 | 1.5554 | 0.1291 |
*P<0.05
Graph 3.
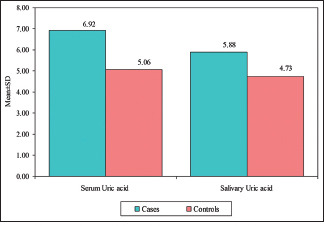
Comparison of the OSCC group and control group with the mean of serum uric acid and salivary uric acid
Furthermore, Karl Pearson's correlation showed a statistically significant (p = 0.0001) strong positive correlation (r = 0.8745) between serum uric acid and salivary uric acid and a weak positive correlation (r = 0.4973) between serum uric acid and serum LDH with a P value of 0.0360, as indicated in [Table 4], whereas in control groups, a strong positive correlation (r = 0.8048) was seen between serum uric acid and serum LDH and a weak positive correlation was seen between serum uric acid and salivary LDH (r = 0.5451, P = 0.0190) and between serum LDH and salivary LDH (r = 0.6282, P = 0.0050), as indicated in [Table 5]. All other correlations were statistically insignificant.
Table 4.
Correlations among serum uric acid, salivary uric acid, serum LDH, and salivary LDH in the OSCC group by Karl Pearson’s correlation coefficient
| Investigations | Summary | Serum Uric acid | Salivary Uric acid | Serum LDH | Salivary LDH |
|---|---|---|---|---|---|
| Serum uric acid | r | --- | |||
| P | --- | ||||
| Salivary uric acid | r | 0.8745 | --- | ||
| P | 0.0001* | --- | |||
| Serum LDH | r | 0.4973 | 0.3203 | --- | |
| P | 0.0360* | 0.1950 | --- | ||
| Salivary LDH | r | 0.4460 | 0.1675 | 0.4328 | --- |
| P | 0.0640 | 0.5070 | 0.0730 | --- |
*P<0.05
Table 5.
Correlations among serum uric acid, salivary uric acid, serum LDH, and salivary LDH in controls by Karl Pearson’s correlation coefficient
| Investigations | Summary | Serum Uric acid | Salivary Uric acid | Serum LDH | Salivary LDH |
|---|---|---|---|---|---|
| Serum uric acid | r | --- | |||
| P | --- | ||||
| Salivary uric acid | r | 0.3555 | --- | ||
| P | 0.1480 | --- | |||
| Serum LDH | r | 0.8048 | 0.3816 | --- | |
| P | 0.0001* | 0.1180 | --- | ||
| Salivary LDH | r | 0.5451 | -0.0083 | 0.6282 | --- |
| P | 0.0190* | 0.9740 | 0.0050* | --- |
*P<0.05
Moreover, In the OSCC group, there were 72.22 percent (n = 13) cases of moderately differentiated carcinoma and 27.78 percent (n = 05) cases of well-differentiated carcinoma. The mean serum LDH concentration in cases of moderately differentiated carcinoma was 319.92 U/L, whereas the mean serum LDH level in cases of well-differentiated squamous cell carcinoma was 398 U/L. The mean salivary LDH of patients with moderately differentiated carcinoma was 762.53 U/L, whereas the mean salivary LDH of patients with well-differentiated squamous cell carcinoma was 395 U/L. It was shown that the mean blood uric acid level in cases of moderately differentiated carcinoma was 7.25 mg/dl, whereas the level in cases of well-differentiated squamous cell carcinoma was 6.04 mg/dl. The mean salivary uric acid level in cases of moderately differentiated carcinoma was 5.90 mg/dl, whereas the mean salivary uric acid level in cases of well-differentiated squamous cell carcinoma was 5.8 mg/dl. In all of the cases included in the research, there was no poorly differentiated carcinoma. When all of these findings were looked at statistically, they did not show any significant differences between them as the P value was more than 0.05.
In addition to this, the ROC curve, when plotted to assess the combined diagnostic abilities of all the investigations, found that the ROC curve was far away from the diagonal line and the AUC measured was 0.7778, indicating the diagnostic abilities by 77%, as indicated in Graph 4.
Graph 4.
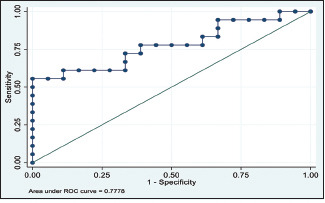
ROC curve for combined diagnostic abilities of all the investigations
The findings of the study implicated that both serum and salivary LDH and uric acid were higher in OSCC patients than in controls. However, findings pertaining to salivary uric acid should be watched cautiously as it was not significant. However, by the findings of correlation analysis, it appears that any changes in salivary uric acid levels are simply a reflection of serum uric acid.
DISCUSSION
LDH, which has the enzyme commission number EC 1.1.1.27 and belongs to the class of oxidoreductases, is known to catalyse the reversible conversion of lactate to pyruvate with the concomitant oxidation/reduction of NADH to NAD + and vice versa.[36] LDH plays a vital role in the Warburg effect, which is a phenomenon typically used by cancer cells in which tumour cells convert from an aerobic to a predominantly anaerobic metabolism, in which glucose is converted to lactate. Elevated lactate concentrations have been shown to predict tumour malignancy, recurrence, survival, and metastasis in cancer patients.[37,38,39] The Warburg effect appears to be a frequent feature of malignant cells, which is critical for their tumourigenic potential[40] and is one of the most significant features that arise during the development of OSCC.[41,42,43]
Aside from this, clinical evidence suggests that increased levels of LDH are a prognostic marker in several types of cancer, including colorectal cancer,[44] nasopharyngeal carcinoma,[45,46] lung cancer,[47,48,49] breast cancer,[50,51] prostate cancer,[52] germ cell cancer,[53,54] melanoma,[55,56] and OC.[57] In contrast to this, a few studies have demonstrated that LDH is a non-specific diagnostic marker for cancer.[58,59,60] In addition, a non-invasive technique for estimating LDH levels in the saliva of patients with OSCC has yielded inconsistent and equivocal results.[20,21,22,23] Because of the findings of the previous study, the current study sought to determine whether serum and salivary LDH levels could be used to effectively identify and monitor OC patients.
In the present study, the mean serum LDH in the OSCC group was higher than the mean serum LDH in controls, which was consistent with the previous studies conducted by Gholizadeh N et al.,[57] Pereira T et al.,[22] Joshi et al.,[16] Masahiro et al.,[61] and Rathore A et al.,[62] which also showed serum LDH levels higher in OSCC groups. This increase in serum LDH may be attributed primarily to hypoxia-inducible factor 1-a (HIF1-a), an important transcription factor that upregulates a set of genes involved in angiogenesis, cell survival, erythropoiesis,[61,63] and glycolytic enzymes such as LDH,[64] and second to malignant tumour tissues or contiguous tissues damaged by the tumour-liberating LDH enzyme into circulation.[65,66] This is again supported by a study conducted by Uehara M et al.[67] and Cai H et al.,[68] which demonstrated the role of HIF-1 alpha in lymph node metastasis and prognosis in patients with OSCC and the role of lactate dehydrogenase A (LDHA) in promoting OSCC cell proliferation, migration, and invasion by facilitating glycolysis and epithelial mesenchymal transition (EMT) progression, respectively.
Oral epithelial shedding is considered to be the origin of salivary LDH, which differs from the LDH seen in plasma.[19] Oral squamous cell cancer may also change the LDH profile in saliva. This study also examined salivary LDH levels in OSCC patients and healthy volunteers and found that salivary LDH rates were increased in OSCC patients compared to healthy volunteers. The findings of the study were in par with the studies conducted by Achalli S et al.,[20] Metgud R et al.,[21] and Pereira T et al.[22] This increase in salivary LDH may be related to the 'Warburg effect' and increased lactate production by tumour cells as they receive less oxygen by virtue of its different organisation, and therefore, their energy production will be dependent on anaerobic glycolysis;[69] as a result, subsequent necrosis and progressive degenerative changes in OSCC proportionally increase the salivary LDH levels in OSCC.[70]
Uric acid plays an important role in the body's anti-oxidant defences. Because of its anti-oxidant properties, uric acid may have cancer-preventive benefits. Uric acid has the ability to scavenge free radicals and chelate transitional metal ions by inhibiting peroxynitrite-induced protein nitrosylation, lipid and protein peroxidation, and the inactivation of tetrahydrobiopterin.[71] Uric acid, on the other hand, has been shown to have pro-oxidant properties in several studies.[71] It is considered that every time, there is an increase in oxidative stress within a cell, and anti-oxidants operate against oxidants and eliminate oxidative stress[72]; as an antioxidant, uric acid is consumed and therefore declines, which may be suggestive of the disease processes.[73] Uric acid also promotes anti-oxidant protection against oxidant- and radical-induced ageing and cancer.[74] Other studies[75,76] have found uric acid to be an independent risk factor for cancer incidence and mortality. However, other studies have indicated increased levels of serum uric acid in cancer.[77,78,79] Studies comparing serum uric acid levels in OC patients and healthy controls have revealed a significant fall in serum uric levels in OC patients.[80,81] Studies that measured salivary uric acid levels in patients with OSCC and healthy volunteers found that OSCC patients had lower levels of salivary uric acid when compared to healthy participants.[33,34] In contrast to these findings, studies have also shown no association between salivary uric acid levels and OSCC individuals.[82] As a consequence, the specific association between uric acid levels in serum or saliva in OSCC patients remains obscure. As a result, the study looked into the relationship between serum and salivary uric acid levels in patients with OSCC and healthy volunteers.
In this study, there was an increase in mean serum uric acid levels and mean salivary uric acid levels in the OSCC group compared to healthy volunteers. These increased mean serum uric acid levels in the OSCC group statistically significantly differentiated the OSCC group from healthy volunteers, whereas mean salivary uric acid failed to differentiate the OSCC group from healthy volunteers. These findings of the study contradict all earlier studies and are in line with the study conducted by Dhankhar R et al.,[35] who also showed significantly higher levels of uric acid in patients with head and neck cancers. These findings of the study may be attributed to the small environmental exposures, genetic background[83] diet, habits, time of collection,[31] and sample size considered.
Further, the study also correlated the results of all the investigations in the OSCC group and found a statistical significant (p = 0.0001) strong positive correlation (r = 0.8745) between serum uric acid and salivary uric acid and a weak positive correlation (r = 0.4973) between serum uric acid and serum LDH with a P value of 0.0360, whereas in control groups, a strong positive correlation (r = 0.8048) was seen between serum uric acid and serum LDH and a weak positive correlation was seen between serum uric acid and salivary LDH (r = 0.5451, P = 0.0190) and between serum LDH and salivary LDH (r = 0.6282, P = 0.0050). These findings implied that there exists a linear correlation between serum and salivary uric acid, and salivary uric acid reflects changes in the serum[32] and the source of salivary LDH is from shedding of oral epithelial cells.[19]
Additionally, the study explored the diagnostic abilities of all the screening investigation tests (serum uric acid, salivary uric acid, serum LDH, and salivary LDH) using ROC curves as this includes all the possible decision thresholds from diagnostic test results. The ROC curve was away from the chance diagonal line, and the AUC was 0.7778, indicating 77% of times that these combined diagnostic tests were able to distinguish OSCC from healthy volunteers.
However, the present study has its own limitations in that it does not evaluate the association between uric acid levels, LDH levels, and various degrees of epithelial dysplasia, the degree of differentiation of OSCC, and the development of distant metastases in individuals with OSCC. As the main goal of the study was to look at serum and salivary LDH and uric acid levels in people with OC and people who were healthy, the study did not compare and correlate serum and salivary LDH and uric acid levels with different grades of epithelial dysplasia. The distant metastasis was not assessed as no distant metastasis was found in any of the cases of OSCC that were consecutively added into the study group.
CONCLUSION
The present study is in the opinion that the combined profile of serum uric acid levels, salivary uric acid levels, serum LDH levels, and salivary LDH levels may serve as a useful marker for screening in OSCC. Future studies should look more into the relationship between LDH and uric acid levels in people with different grades of epithelial dysplasia and different grades of OSCC and healthy people, and using the study designs to overcome the limitations of this study becomes necessary before interpreting the findings of this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Gupta R, Sinha DN, Mehrotra R. Relationship between type of smokeless tobacco and risk of cancer: A systematic review. Indian J Med Res. 2018;148:56–76. doi: 10.4103/ijmr.IJMR_2023_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br J Cancer. 2015;112:580–93. doi: 10.1038/bjc.2014.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han AY, Kuan EC, Mallen-St Clair J, Alonso JE, Arshi A, St John MA. Epidemiology of squamous cell carcinoma of the lip in the United States: A population-based cohort analysis. JAMA Otolaryngol Head Neck Surg. 2016;142:1216–23. doi: 10.1001/jamaoto.2016.3455. [DOI] [PubMed] [Google Scholar]
- 5.Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58((5 suppl 2)):S129–32. doi: 10.1016/j.jaad.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 6.Kumar KV, Hema KN. Extracellular matrix in invasion and metastasis of oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2019;23:10–6. doi: 10.4103/jomfp.JOMFP_97_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu YH, Lin PY, Yang JH, Kuo YS, Wu YC. Serum levels and positive rates of tumor biomarkers in oral precancer patients. J Formosan Med Assoc. 2021;120:1324–31. doi: 10.1016/j.jfma.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Khurshid Z, Zafar MS, Khan RS, Najeeb S, Slowey PD, Rehman IU. Role of salivary biomarkers in oral cancer detection. Adv Clin Chem. 2018;86:23–70. doi: 10.1016/bs.acc.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Kallalli BN, Rawson K, Muzammil, Singh A, Awati MA, Shivhare P. Lactate dehydrogenase as a biomarker in oral cancer and oral submucous fibrosis. J Oral Pathol Med. 2016;45:687–90. doi: 10.1111/jop.12451. [DOI] [PubMed] [Google Scholar]
- 10.Victor AD, Dios PD, Sierra RT. Relationship between lactate dehydrogenase activity in saliva and oral health status. Arch Oral Biol. 2007;52:911–5. doi: 10.1016/j.archoralbio.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Garba IH, Ubom GH. Total serum lactate dehydrogenase activity in acute Plasmodium falciparum malaria infections. Singapore Med J. 2005;46:632–4. [PubMed] [Google Scholar]
- 12.George J, Chandrasekar G. Lactated dehydrogenase isoenzymes in Dimethylnitrosamine induced hepatic fibrosis in rats. J Clin Biochem Nutr. 1997;22:51–6. [Google Scholar]
- 13.Zhang J, Yao YH, Li BG, Yang Q, Zhang PY, Wang HT. Prognostic value of pretreatment serum lactate dehydrogenase level in patients with solid tumors: A systematic review and meta-analysis? Sci Rep. 2015;5:9800. doi: 10.1038/srep09800. doi: 10.1038/srep09800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suh SY, Ahn HY. Lactate dehydrogenase as a prognostic factor for survival time of terminally ill cancer patients: A preliminary study. Eur J Cancer. 2007;43:1051–9. doi: 10.1016/j.ejca.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 15.Zhou GQ, Ren XY, Mao YP, Chen L, Sun Y, Liu LZ, et al. Prognostic implications of dynamic serum lactate dehydrogenase assessments in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Sci Rep. 2016;6:22326. doi: 10.1038/srep22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi PS, Chougule M, Dudanakar M, Golgire S. Comparison between salivary and serum lactate dehydrogenase levels in patients with oral leukoplakia and oral squamous cell carcinoma — A pilot study. Int J Oral Maxillofac Pathol. 2012;3:7–13. [Google Scholar]
- 17.Anuradha CD, Devi CS. Studies on enzymes of clinical significance in oral sub mucous fi brosis. J Clin Biochem Nutr. 1998;24:45–52. [Google Scholar]
- 18.Kamath VV, Satelur K, Komali Y. Biochemical markers in oral sub mucous fibrosis: A review and update. Dent Res J. 2013;10:576–84. [PMC free article] [PubMed] [Google Scholar]
- 19.Nagler RM, Lischinsky S, Diamond E, Klein I, Reznick AZ. New insightsinto salivary lactate dehydrogenase of human subjects. J Lab Clin Med. 2001;137:363–9. doi: 10.1067/mlc.2001.114710. [DOI] [PubMed] [Google Scholar]
- 20.Achalli S, Babu S, Bhat S, Chadha R, Kumari S, Shetty SR. Salivary lactate dehydrogenase levels in oral leucoplakia and oral squamous cell carcinoma: A biochemical andclinicopathological study. J Cancer Res Ther. 2012;8:123–5. doi: 10.4103/0973-1482.92226. [DOI] [PubMed] [Google Scholar]
- 21.Metgud R, Patel S. Estimation of salivary lactate dehydrogenase in oral leukoplakia and oral squamous cell carcinoma: A biochemical study. J Cancer Res Ther. 2015;11:119–23. doi: 10.4103/0973-1482.138193. [DOI] [PubMed] [Google Scholar]
- 22.Pereira T, Pereira S, Shetty S. Estimation of serum lactate dehydrogenase level in patients with oral premalignant lesions/conditions and oral squamous cell carcinoma: A clinicopathological study. J Cancer Res Ther. 2015;11:78–82. doi: 10.4103/0973-1482.150352. [DOI] [PubMed] [Google Scholar]
- 23.Shetty SR, Chadha R, Babu S, Kumari S, Bhat S, Achalli S. Salivary lactate dehydrogenase levels in oral leukoplakia and oral squamous cell carcinoma: A biochemical and clinicopathological study. J Can Res Ther. 2012;8:123–5. doi: 10.4103/0973-1482.92226. [DOI] [PubMed] [Google Scholar]
- 24.Sautin YY, Johnson RJ. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27:608–19. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richette P, Perez-Ruiz F, Doherty M, Jansen TL, Nuki G, Pascual E, et al. Improving cardiovascular and renal outcomes in gout: What should we target? Nat Rev Rheumatol. 2014;10:654–61. doi: 10.1038/nrrheum.2014.124. [DOI] [PubMed] [Google Scholar]
- 26.Battelli MG, Bortolotti M, Polito L, Bolognesi A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim Biophys Acta Mol Basis Dis. 2018;8:2557–65. doi: 10.1016/j.bbadis.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11:4145–51. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 28.Subandrate, Lea KY, Safyudin The correlation between uric acid and stages of malignancy among gastric cancer patient in Palembang, Indonesia. Journal of Physics Conference Series; 2019;(1246):012062. [Google Scholar]
- 29.Yuan C, Xu X-H, Wang X-L, Xu L, Chen Z, Li Y-Q. Relationship between serum uric acid and metastatic and nonmetastatic rectal cancer patients with undergoing no chemotherapy? Medicine. 2016;95:e5463. doi: 10.1097/MD.0000000000005463. doi: 10.1097/MD.0000000000005463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dequanter D, Dok R, Nuyts S. Basal oxidative stress ratio of head and neck squamous cell carcinomas correlates with nodal metastatic spread in patients under therapy. Onco Targets Ther. 2017;10:259–63. doi: 10.2147/OTT.S118980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaiswal A, Madaan S, Acharya N, Kumar S, Talwar D, Dewani D. Salivary uric acid: A noninvasive wonder for clinicians? Cureus. 2021;13:e19649. doi: 10.7759/cureus.19649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh U, Solanki V, Mehrotra S, Sharma R. An evaluation of applicability of salivary uric acid measurement in preeclampsia and normal pregnancy and its correlation with serum uric acid. J ObstetGynaecol India. 2019;69:62–8. doi: 10.1007/s13224-018-1124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh H, Shetty P, V SS, Patidar M. Analysis of salivary antioxidant levels in different clinical staging and histological grading of oral squamous cell carcinoma: Noninvasive technique in dentistry. J Clin Diagn Res. 2014;8:ZC08–11. doi: 10.7860/JCDR/2014/9119.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salian V, Demeri F, Kumari S. Estimation of salivary nitric oxide and uric acid levels in oral squamous cell carcinoma and healthy controls. Clin Cancer Investig J. 2015;4:516–9. [Google Scholar]
- 35.Dhankhar R, Dahiya K, Sharma TK, Ghalaut VS, Atri R, Kaushal V. Diagnostic significance of adenosine deaminase, uric acid and C-reactive protein levels in patients of head and neck carcinoma. Clin Lab. 2011;57:795–8. [PubMed] [Google Scholar]
- 36.Schumann G, Bonora R, Ceriotti F, Clerc-Renaud P, Ferrero CA, Férard G, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. Part 3. Reference procedure for the measurement of catalytic concentration of lactate dehydrogenase. Clin Chem Lab Med. 2002;40:643–8. doi: 10.1515/CCLM.2002.111. [DOI] [PubMed] [Google Scholar]
- 37.Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:349–53. doi: 10.1016/s0360-3016(01)01630-3. [DOI] [PubMed] [Google Scholar]
- 38.Walenta S, Mueller-Klieser WF. Lactate: Mirror and motor of tumor malignancy. Semin Radiat Oncol. 2004;14:267–74. doi: 10.1016/j.semradonc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfør K, Rofstad EK, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60:916–21. [PubMed] [Google Scholar]
- 40.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 42.Chen G, Zhang Y, Liang J, Li W, Zhu Y, Zhang M, et al. Deregulation of hexokinase II is associated with glycolysis, autophagy, and the epithelial-mesenchymal transition in tongue squamous cell carcinoma under hypoxia? Biomed Res Int. 2018;2018:8480762. doi: 10.1155/2018/8480762. doi: 10.1155/2018/8480762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen G, Liu H, Zhang Y, Liang J, Zhu Y, Zhang M, et al. Silencing PFKP inhibits starvation-induced autophagy, glycolysis, and epithelial mesenchymal transition in oral squamous cell carcinoma. Exp Cell Res. 2018;370:46–57. doi: 10.1016/j.yexcr.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Trarbach T, Folprecht G, et al. Prognostic and predictive role of lactate dehydrogenase 5 expression in colorectal cancer patients treated with PTK787/ZK 222584 (vatalanib) antiangiogenic therapy. Clin Cancer Res. 2011;17:4892–900. doi: 10.1158/1078-0432.CCR-10-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turen S, Ozyar E, Altundag K, Gullu I, Atahan IL. Serum lactate dehydrogenase level is a prognostic factor in patients with locoregionally advanced nasopharyngeal carcinoma treated with chemoradiotherapy. Cancer Investig. 2007;25:315–21. doi: 10.1080/07357900701209103. [DOI] [PubMed] [Google Scholar]
- 46.Jin Y, Ye X, Shao L, Lin BC, He CX, Zhang BB, et al. Serum lactic dehydrogenase strongly predicts survival in metastatic nasopharyngeal carcinoma treated with palliative chemotherapy. Eur J Cancer. 2013;49:1619–26. doi: 10.1016/j.ejca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 47.Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung cancer: The Southwest Oncology Group experience. J ClinOncol Off J Am Soc Clin Oncol. 1991;9:1618–26. doi: 10.1200/JCO.1991.9.9.1618. [DOI] [PubMed] [Google Scholar]
- 48.Hermes A, Gatzemeier U, Waschki B, Reck M. Lactate dehydrogenase as prognostic factor in limited and extensive disease stage small cell lung cancer—a retrospective single institution analysis. Respir Med. 2010;104:1937–42. doi: 10.1016/j.rmed.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 49.Tas F, Aydiner A, Demir C, Topuz E. Serum lactate dehydrogenase levels at presentation predict outcome of patients with limited-stage small-cell lung cancer. Am J ClinOncol. 2001;24:376–8. doi: 10.1097/00000421-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 50.Brown JE, Cook RJ, Lipton A, Coleman RE. Serum lactate dehydrogenase is prognostic for survival in patients with bone metastases from breast cancer: A retrospective analysis in bisphosphonatetreated patients. Clin Cancer Res. 2012;18:6348–55. doi: 10.1158/1078-0432.CCR-12-1397. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto N, Watanabe T, Katsumata N, Omuro Y, Ando M, Fukuda H, et al. Construction and validation of a practical prognostic index for patients with metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 1998;16:2401–8. doi: 10.1200/JCO.1998.16.7.2401. [DOI] [PubMed] [Google Scholar]
- 52.Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 53.Von Eyben FE, Madsen EL, Liu F, Amato R, Fritsche H. Serum lactate dehydrogenase isoenzyme 1 as a prognostic predictor for metastatic testicular germ cell tumours. Br J Cancer. 2000;83:1256–9. doi: 10.1054/bjoc.2000.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerlinger M, Wilson P, Powles T, Shamash J. Elevated LDH predicts poor outcome of recurrent germ cell tumours treated with dose dense chemotherapy. Eur J Cancer. 2010;46:2913–8. doi: 10.1016/j.ejca.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Egberts F, Kotthoff EM, Gerdes S, Egberts JH, Weichenthal M, Hauschild A. Comparative study of YKL-40, S-100B and LDH as monitoring tools for stage IV melanoma. Eur J Cancer. 2012;48:695–702. doi: 10.1016/j.ejca.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 56.Agarwala SS, Keilholz U, Gilles E, Bedikian AY, Wu J, Kay R, et al. LDH correlation with survival in advanced melanoma from two large, randomised trials (Oblimersen GM301 and EORTC 18951) Eur J Cancer. 2009;45:1807–14. doi: 10.1016/j.ejca.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 57.Gholizadeh N, AlipanahiRamandi M, Motiee-Langroudi M, Jafari M, Sharouny H, Sheykhbahaei N. Serum and salivary levels of lactate dehydrogenase in oral squamous cell carcinoma, oral lichen planus and oral lichenoid reaction. BMC Oral Health. 2020;20:314. doi: 10.1186/s12903-020-01306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kopperschläger G, Kirchberger J. Methods for the separation of lactate dehydrogenases and clinical significance of the enzyme. J Chromatogr B Biomed Appl. 1996;684:25–49. doi: 10.1016/0378-4347(96)00133-8. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz MK. Lactic dehydrogenase. An old enzyme reborn as a cancer marker? Am J ClinPathol. 1991;96:441–3. doi: 10.1093/ajcp/96.4.441. [DOI] [PubMed] [Google Scholar]
- 60.Augoff K, Hryniewicz-Jankowska A, Tabola R. Lactate dehydrogenase 5: An old friend and a new hope in the war on cancer. Cancer Lett. 2015;358:1–7. doi: 10.1016/j.canlet.2014.12.035. doi: 10.1016/j.canlet. 2014.12.035. [DOI] [PubMed] [Google Scholar]
- 61.Masahiro D. Clinical and experimental studies of serum LDH activity and LDH Isoenzymes of patients with oral cancer. J Kyunshu Dent Soc. 1971;24:821–46. [Google Scholar]
- 62.Rathore A, Nagarajappa AK, Sreedevi Evaluation of serum lactate dehydrogenase in oral squamous cell carcinoma, oral leukoplakia and oral submucous fibrosis. J Indian Acad Oral Med Radiol. 2015;27:29–34. [Google Scholar]
- 63.Scartozzi M, Giampieri R, Maccaroni E, Del Prete M, Faloppi L, Bianconi M, et al. Pre-treatment lactate dehydrogenase levels as predictor of efficacy of first-line bevacizumab-based therapy in metastatic colorectal cancer patients. Br J Cancer. 2012;106:799–804. doi: 10.1038/bjc.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maxwell PH, Pugh CW, Ratcliffe PJ. Activation of the HIF pathway in cancer. CurrOpin Genet Dev. 2001;11:293–9. doi: 10.1016/s0959-437x(00)00193-3. [DOI] [PubMed] [Google Scholar]
- 65.Joshi PS, Golgire S. A study of salivary lactate dehydrogenase isoenzyme levels in patients with oral leukoplakia and squamous cell carcinoma by gel electrophoresis method. J Oral Maxillofacial Pathol. 2014;18:39–44. doi: 10.4103/0973-029X.141342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.PriyaShirish J, Madhuri C, Mahesh D, Someshwar G. Comparison between salivary and serum lactate dehydrogenase levels in patients with oral leukoplakia and oral squamous cell carcinoma-A pilot study. Int J Oral Maxillofacial Pathol. 2012;3:7–12. [Google Scholar]
- 67.Uehara M, Sano K, Ikeda H, Nonaka M, Asahina I. Hypoxia-inducible factor 1 alpha in oral squamous cell carcinoma and its relation to prognosis. Oral Oncol. 2009;45:241–6. doi: 10.1016/j.oraloncology.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 68.Cai H, Li J, Zhang Y, Liao Y, Zhu Y, Wang C, et al. LDHA promotes oral squamous cell carcinoma progression through facilitating glycolysis and epithelial–mesenchymal transition? Front Oncol. 2019;9:1446. doi: 10.3389/fonc.2019.01446. doi: 10.3389/fonc. 2019.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakazato K, Mogushi K, Kayamori K, Tsuchiya M, Takahashi KI, Sumino J, et al. Glucose metabolism changes during the development and progression of oral tongue squamous cell carcinomas. Oncol Lett. 2019;18:1372–80. doi: 10.3892/ol.2019.10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iglesias-Velazquez O, Lopez-Pintor RM, González-Serrano J, Casanas E, Torres J, Hernandez G. Salivary LDH in oral cancer and potentially malignant disorders: A systematic review and meta-analysis. Oral Dis. 2022;28:44–56. doi: 10.1111/odi.13630. [DOI] [PubMed] [Google Scholar]
- 71.Kang DH, Ha SK. Uric acid puzzle: Dual role as antioxidantand pro-oxidant? Electrolyte Blood Pressure. 2014;12:1–6. doi: 10.5049/EBP.2014.12.1.1. doi: 10.5049/EBP. 2014.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Skak-Nielsen H, Torp-Pedersen C, Finer N, Caterson ID, Van Gaal L, James WP, et al. Uric acid as a risk factor for cardiovascular disease and mortality in overweight/obese individuals. PLoS One. 2013;8:e59121. doi: 10.1371/journal.pone.0059121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proceedings of the national academy of sciences of the United States of America. 1981;78:6858–62. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strasak AM, Rapp K, Hilbe W, Oberaigner W, Ruttmann E, Concin H, et al. Serum uric acid and risk of cancer mortality in a large prospective male cohort. Cancer Causes Control. 2007;18:1021–9. doi: 10.1007/s10552-007-9043-3. [DOI] [PubMed] [Google Scholar]
- 76.Fini MA, Elias A, Johnson RJ, Wright RM. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin Transl Med. 2012;1:16. doi: 10.1186/2001-1326-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuo CF, See LC, Yu KH, Chou IH, Chiou MJ, Luo SF. Significance of serum uric acid levels on the risk of allcause and cardiovascular mortality. Rheumatology. 2013;52:127–34. doi: 10.1093/rheumatology/kes223. [DOI] [PubMed] [Google Scholar]
- 78.Baeksgaard L, Sørensen JB. Acute tumor lysis syndrome in solid tumors—a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187–92. doi: 10.1007/s00280-002-0556-x. [DOI] [PubMed] [Google Scholar]
- 79.Sevanian A, Davies KJA, Hochstein P. Serum urate as an antioxidant for ascorbic acid. Am J Clin Nutr. 1991;54((Suppl 6)):1129S–34S. doi: 10.1093/ajcn/54.6.1129s. [DOI] [PubMed] [Google Scholar]
- 80.Ara SA, Ashraf S, Patil BM. Evaluation of serum uric acid levels in patients with oral squamous cell carcinoma. Indian J Dent Res. 2016;27:178–83. doi: 10.4103/0970-9290.183128. [DOI] [PubMed] [Google Scholar]
- 81.Lawal AO, Kolude B, Adeyemi BF. Serum uric Acid levels in oral cancer patients seen at tertiary institution in Nigeria. Ann Ib Postgrad Med. 2012;10:9–12. [PMC free article] [PubMed] [Google Scholar]
- 82.Almadori G, Bussu F, Galli J, Limongelli A, Persichilli S, Zappacosta B, et al. Salivary glutathione and uric acid levels in patients with head and neck squamous cell carcinoma. Head Neck. 2007;29:648–54. doi: 10.1002/hed.20579. [DOI] [PubMed] [Google Scholar]
- 83.Yan S, Zhang P, Xu W, Liu Y, Wang B, Jiang T, et al. Serum uric acid increases risk of cancer incidence and mortality: A systematic review and meta-analysis? Mediators Inflamm. 2015;2015:7. doi: 10.1155/2015/764250. doi: 10.1155/2015/764250. [DOI] [PMC free article] [PubMed] [Google Scholar]


