Abstract
Objective:
Oral submucous fibrosis (OSMF) is a debilitating chronic disease of the oral cavity with a high potential for malignant transformation. The main etiological agent attributed to the development of OSMF is the use of smokeless tobacco products like areca nut. There is no known cure for the disease. Current modalities of treatment do not provide a complete cure and often prove invasive for the patient. Herbal preparations using natural compounds and medicinal plant extracts have long since been used in India, as an acceptable, noninvasive and cost-effective method in the treatment of various diseases. Hence, the present study aims to assess the anti-fibrotic effect of licorice in comparison with colchicine on areca nut-induced fibroblasts.
Materials and Methods:
Extracts of areca nut, licorice and colchicine were prepared in accordance with established protocols. Human fibroblast cell lines were procured from ATCC®(PSC-201-018). Fibroblast cultures were established, and upon reaching confluence the cells were subjected to the 25 μg/ml areca nut extract for 24 h to induce fibrosis, with CCl4 used as control fibrosing agent. The areca nut and CCl4 induced cells were then subjected to varying concentration of the test antifibrotic agent, licorice extract for the periods of 24 and 48 h, with colchicine used as positive control. Total collagen quantification was done using spectrophotometry.
Results:
Collagen accumulation decreased with increase in the concentration of licorice extract with maximum reduction seen at 200 μg/ml. Kruskal–Wallis test was done to analyze the difference in collagen accumulation. Analysis revealed that the P < 0.05 for both periods in both the areca and CCl4 induced cell lines following the addition of licorice extract. The data were found to be statistically significant.
Conclusion:
The current study proves the antifibrotic efficacy of licorice in areca nut induced cell lines and hence, this agent can be used for the therapeutic management of OSMF.
Keywords: Colchicine, licorice, malignant transformation, oral submucous fibrosis, phytochemical therapies
INTRODUCTION
Oral submucous fibrosis (OSMF) is a potentially malignant oral disorder that runs a chronic and indolent course within the oral cavity. The disease is characterized by inflammation and gradual hyalinization of the lamina propria and the deeper tissues of the oral cavity, pharynx and upper esophagus. Due to progressive fibrosis, stiffness ensues which eventually results in an inability to open the mouth, thus restricting food intake and impairing speech.[1] The main etiological agent attributed to the development of OSMF is the use of smokeless tobacco products like areca nut.
Areca nut is classified as a group I carcinogen for humans by International Agency for Research on Cancer because of its potential to cause carcinogenicity. Arecoline is the major alkaloid present in areca nut. Other alkaloids include arecadine, arecolidine, guyacoline and guacine. Metabolites derived from N-nitrosation of these alkaloids, results in the formation of cyanoethyl derivatives which binds to the DNA and have cytotoxic effects on cells. Prolonged exposure to these agents can thus result in malignant transformation.[2,3]
Although the disease has been prevalent for over half a century, little progress has been made toward the development of a permanent cure. Current modalities of treatment are the only palliative and often loaded with unnecessary side effects like rebound fibrosis and scarring.[4] Hence, the need of the hour is for noninvasive procedures to battle the disease. Herbal preparations using natural compounds and medicinal plant extracts have long since been used in India, as an acceptable, noninvasive and cost-effective method in the treatment of various diseases. Licorice is one such medicinal plant, used in traditional medicine. Evidence suggests that Glycyrrhizin, an active compound present in licorice directly interferes with transforming growth factor-β (TGF-β), a key mediator of fibrosis. Further, glycyrrhetinic acid, an aglycone of glycyrrhizin inhibits the accumulation of Smad 3, a key arbitrator in the TGF-β mediated fibrosis pathway.[5,6]
Hence, the present study was done to assess the anti-fibrotic effect of licorice on areca nut-induced fibroblast in comparison to colchicine to evaluate its therapeutic potential in the management of fibrosis.
MATERIALS AND METHODS
This study was approved by the ethical committee of SRM University. SRMDC/IRB/2018/MDS/No. 604.
Source of samples
Fibroblast cell lines were procured from, ATCC® PSC-201-018
Preparation of aqueous extracts (licorice and colchicine)
Licorice barks were obtained from the Ayurveda Pharmacy, Chennai. Colchicine tablets were obtained from the local pharmacy. Both the raw materials were powdered until fine. To prepare the aqueous extract, the powder was dissolved in a suitable amount of distilled water and boiled at 50°C for 20 min.
Preparation of alcoholic extract (areca nut)
Areca extract was prepared using a soxhlet extractor. Areca nut flakes were obtained from the local market in Chennai. The flakes so obtained were loaded onto the soxhlet extractor. Alcohol was then added to about three times the quantity of the raw material and was heated to a temperature of 80°–85° for 3–4 h. The cycle was repeated for 48 h. The extract so formed was then filtered through a filter paper and transferred to a suitable container until further use.
Establishment of cell culture
Human fibroblast cell line was obtained from ATCC®(PSC-201-018). Morphological characteristics were used to confirm the fibroblast lineage of the procured cells. Trypsinization was carried out to passage the cell line. Trypsin-ethylenediaminetetraacetic acid solution was added to the T-25 flask containing the cell line and placed in the incubator for 1–2 min. The detached cells were then resuspended in Dulbecco's medium (Himedia® AT149) containing 10% fetal bovine serum (Himedia® RM9955). The medium was then aspirated using a micropipette and inoculated into culture plates. Cells from the fourth passage were used for the study. Five thousand cells were inoculated in each well along with Dulbeco's medium, 10% fetal bovine serum and antibiotics like streptomycin sulfate and benzylpenicillin. The culture plates were then incubated in CO2 incubator. The medium was replaced every 24 h until 80% confluence was achieved.
Induction of collagen secretion and addition of test herbal agent
Upon reaching 80% confluence, the cells were then subjected to 25 μg/ml of fibrosis-inducing agent, namely, areca nut extract for 24 h. The cells were then subjected to the test herbal specimen-licorice extract in succeeding concentrations of 20, 50, 100, 200 μg/ml at time intervals of 24 and 48 h. Colchicine was used as the positive control. The experimental protocol was repeated by using 25 μg/ml of carbon tetrachloride as the control fibrosis-inducing agent.
Quantification of collagen following fibrosis
At the time interval of 24 h and 48 h, the cell culture medium was removed using a micropipette. The cells were then fixed in methanol for 5 min and stained using 100 μL methylene blue for about 30 min. The cells were then washed in phosphate buffer solution thrice to remove excess stain. 100 μL 0.1 M HCL comprising 20% ethanol was used to elute methylene blue from the cell layers. Absorbance was then measured using a spectrophotometer at 650 nm.
Statistical analysis
Statistical analysis of the data obtained was performed using SPSS 22 software (IBM Corp, Armonk, NY). The descriptive statistics such as mean and standard deviations were calculated for the individual groups. The confidence interval was set at 95%. Since the data did not follow the normal distribution, Kruskal–Wallis test and Chi-square test were then done to analyze the difference in collagen accumulation between individual groups. Significance was kept at 0.05. Data <0.05 were considered statistically significant.
RESULTS
Assessment of collagen produced-24 h control
The cells were initially incubated for 24 h at a baseline concentration of 25 μg/ml of areca nut extract and CCl4 control respectively. At the baseline concentration of 25 μg/ml, the mean collagen produced was greater in the CCl4 control when compared to the areca nut group.
Quantification at different periods
Twenty-four hours following extract
Mean collagen accumulation decreased with an increase in the concentration of licorice extract with a maximum reduction seen at 200 μg/ml in both areca-induced cell lines and CCl4 induced cell lines. The control drug also showed a comparable decrease [Graphs 1 and 2].
Graph 1.
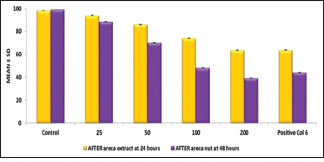
Comparison of effect of licorice extract on areca nut induced cell lines for time period of 24 h and 48 h
Graph 2.
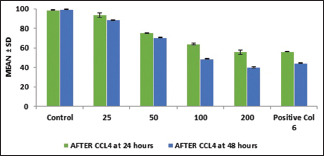
Comparison of effect of licorice extract on CCl4 induced cell lines for time period of 24 h and 48 h
Forty-eight hours following extract
A similar trend was also seen when the cells were incubated for 48 h with licorice extract. The mean collagen accumulation decreased with an increase in the concentration of licorice extract with a maximum reduction achieved at 200 μg/ml [Graphs 1 and 2].
Comparison between the time periods
At baseline, collagen production by fibroblasts increased with increasing time period and showed maximum collagen production at 48 h on induction with fibrosing agents. The CCl4 group showed greater collagen production when compared to the arecoline group.
Following, the addition of the test herbal agent, the collagen production successively decreased in both periods, with maximum reduction obtained after 48 h following treatment with 200 μg/ml of licorice extract. The data set was also found to be statistically significant with P values were <0.05 for both the periods of 24 h [Tables 1 and 3] and 48 h [Tables 2 and 4] in both areca nut and CCl4 induced cells.
Table 1.
Comparison of total collagen accumulation following addition of licorice extract in areca nut induced cell lines at 24 h
| After areca extract at 24 h | Mean | SD | SE | Mean rank | χ 2 | df | P |
|---|---|---|---|---|---|---|---|
| Control | 98.4167 | 0.10786 | 0.06227 | 17.00 | 16.191 | 5 | 0.006 |
| 25 | 93.9833 | 0.30534 | 0.17629 | 14.00 | |||
| 50 | 86.1067 | 0.38214 | 0.22063 | 11.00 | |||
| 100 | 74.1267 | 0.23459 | 0.13544 | 8.00 | |||
| 200 | 63.5633 | 0.38280 | 0.22101 | 3.00 | |||
| Positive control 6 | 63.7667 | 0.23459 | 0.13544 | 4.00 | |||
| Total | 79.9939 | 14.18689 | 3.34388 |
SD: Standard deviation, SE: Standard error
Table 3.
Comparison of total collagen accumulation following addition of licorice extract in areca nut induced cell lines at 48 h
| After areca nut at 48 h | Mean | SD | SE | Mean rank | χ 2 | df | P |
|---|---|---|---|---|---|---|---|
| Control | 99.0767 | 0.08737 | 0.05044 | 17.00 | 16.613 | 5 | 0.005 |
| 25 | 88.4100 | 0.29445 | 0.17000 | 14.00 | |||
| 50 | 70.1067 | 0.19858 | 0.11465 | 11.00 | |||
| 100 | 48.1433 | 0.26502 | 0.15301 | 8.00 | |||
| 200 | 39.3567 | 0.39716 | 0.22930 | 8.00 | |||
| Positive control 6 | 44.1367 | 0.15011 | 0.08667 | 5.00 | |||
| Total | 64.8717 | 23.43636 | 5.52400 |
SD: Standard deviation, SE: Standard error
Table 2.
Comparison of total collagen accumulation following addition of licorice extract in Carbon tetrachloride induced cell lines at 24 h
| After CCl4 at 24 h | Mean | SD | SE | Mean rank | χ 2 | Df | P |
|---|---|---|---|---|---|---|---|
| Control | 98.7433 | 0.11590 | 0.06692 | 17.00 | 16.145 | 5 | 0.006 |
| 25 | 93.6067 | 2.14197 | 1.23667 | 14.00 | |||
| 50 | 75.2100 | 0.55245 | 0.31896 | 11.00 | |||
| 100 | 63.9600 | 0.85082 | 0.49122 | 8.00 | |||
| 200 | 55.5800 | 20.38937 | 10.37950 | 3.83 | |||
| Positive control 6 | 55.9300 | 0.27622 | 0.15948 | 3.17 | |||
| Total | 73.8383 | 17.68446 | 4.16827 |
SD: Standard deviation, SE: Standard error, CCl4: Carbon tetrachloride
Table 4.
Comparison of total collagen accumulation following addition of licorice extract in carbon tetrachloride cell lines at 48 h
| After CCl4 at 48 h | Mean | SD | SD | Mean rank | χ 2 | df | P |
|---|---|---|---|---|---|---|---|
| Control | 99.4967 | 0.04509 | 0.02603 | 17.00 | 16.596 | 5 | 0.005 |
| 25 | 88.5600 | 0.29445 | 0.17000 | 14.00 | |||
| 50 | 70.4900 | 0.19313 | 0.11150 | 11.00 | |||
| 100 | 48.7600 | 0.19313 | 0.11150 | 8.00 | |||
| 200 | 40.0500 | 0.51000 | 0.29445 | 2.00 | |||
| Positive control 6 | 44.1600 | 0.25515 | 0.14731 | 5.00 | |||
| Total | 65.2528 | 23.37314 | 5.50910 |
SD: Standard deviation, SE: Standard error, CCl4: Carbon tetrachloride
Comparison of antifibrotic potential between licorice and colchicine
The antifibrotic potential of licorice was comparable to colchicine at the concentration of 200 μg/ml in both areca nut-induced cell lines and CCl4 induced cell lines.
DISCUSSION
“OSMF” is a potentially malignant disorder whose potential for malignant transformation ranges between 2% and 8%.[7] Even in the current era of modern diagnostics and treatment modalities, the antifibrotic treatment of fibrosis represents an unconquered zone for drug development. Various preclinical studies have revealed numerous profibrotic genes that could be targeted by antifibrotic therapy. Chief signaling cascades, primarily mediated by TGF-β, and its downstream signaling molecules bring about excess extracellular matrix deposition by altering fibroblasts to myofibroblasts. TGF-β thus offers a potential gene target for the binding of anti-fibrotic drugs.[8,9] Accordingly, the current study was put forth with an agenda to find a novel agent that could prevent or treat OSMF by blocking this fibrotic cascade.
In the current study, licorice was used as the phytochemical of choice, to determine its anti-fibrotic potential within the oral cavity. Licorice is a medicinal herb used for centuries in traditional medicine commonly used for the treatment of a wide variety of disorders such as sore throat, ulcers, etc., due to its potent anti-inflammatory and anti-microbial properties.[10,11] Licorice contains numerous active ingredients comprising of agents like Glycyrrhizin and isoflavonoids like Glabrene, Isoliquiritigenin and glabridin. Numerous in vitro studies have shown the potential antifibrotic action of licorice in hepatic and renal fibrosis models.[12,13]
The literature is however scarce on the efficacy of licorice as an antifibrotic agent in the treatment of OSMF induced by arecoline present in areca nut. Hence our study exploited this phytochemical agent to assess its antifibrotic potential.
In the current study, we had decided to use the aqueous extract of licorice. Once the desired amount of fibrosis was achieved using both areca nut extract and CCL4, the culture plates were incubated with various concentrations of freshly prepared aqueous extract of licorice. Successive concentrations of 20, 50, 100, 200 μg/ml of licorice extract were utilized for two different periods of 24 h and 48 h.
Colchicine was used as the control drug in the present study. Colchicine is an ancient drug that was used in the treatment of fibrotic conditions such as hepatic fibrosis, pulmonary fibrosis and scleroderma. A recent study by Daga et al., also found oral colchicine when administered along with intralesional hyaluronidase was effective in the treatment of OSMF and aided to ameliorate burning sensation and improved the mouth opening in such patients.[14] A comparative study by Neupane et al., to evaluate the role of colchicine in OSMF in comparison to intralesional dexamethasone and hyaluronidase found that mouth opening significantly increased in both groups in a similar manner thus prompting the attention to the use of oral colchicine for the treatment of OSMF.[15] Since no exclusive drug has been systemically approved for the treatment of OSMF, we considered the use of colchicine as our control drug, which was well documented as an antifibrotic drug in a variety of other fibrotic diseases, with a potential role even in OSMF. 6 μg/ml of colchicine used as the positive control in the study.
In our study, total collagen was measured calorimetrically using spectrophotometry following degradation of collagen and subsequent staining as done by Krishnakumar et al.[16] Our study showed that Licorice significantly reduced collagen accumulation in a dose-dependent manner in both areca nut induced cell lines and CCL4 induced cells lines.
In the areca nut-induced cell line collagen production decreased with an increase in the concentration of licorice extract. At 24 h maximum reduction was seen at 200 μg/ml with 63.56% of collagen. The control drug also showed a comparable decrease of 63.76%. At 48 h, again maximum reduction was seen 200 μg/ml with 44.13% of collagen. The control drug also showed a similar decrease with 44.13%.
In the CCL4-induced cell line, a similar trend was noticed. Collagen production decreased with an increase in the concentration of licorice extract with the maximum reduction seen at 200 μg/ml with 54.96% collagen at 24 h and 40.05% of collagen at 48 h. The control drug also showed a comparable decrease with 55.93% at 24 h and 44.16% at 48 h.
Statistical analysis done to check for significance, revealed that the P < 0.05 for both the period of 24 h and 48 h indicating that the obtained data were statistically significant. The data demonstrated that the collagen accumulation significantly reduced following incubation with licorice for the period of 24 and 48 h.
On comparing the efficacy of licorice and colchicine the data was found to be statistically significant at both 24 and 48 h, signifying that the antifibrotic efficacy of licorice is similar, if not better than that of colchicine.
In a similar, but the extensive study by Hu et al., the antifibrotic potential of flavonoids and nonflavonoids present in 21 Chinese herbs were assessed in vitro. Rat kidney fibroblasts were induced to undergo fibrosis by use of TGF-β1. The total collagen accumulation was assessed using calorimetric methods. In addition to this, they had also performed immunofluorescence staining for collagen 1 and alpha-smooth muscle actin and reverse transcription-polymerase chain reaction (RT-PCR) analysis for COL1A2 mRNA expression. Their study found that 5 compounds namely quercetin, baicalein, baicalin, salvianolic acid B and emodin showed antifibrotic potential by decreasing total collagen accumulation as assessed by spectrophotometry and suppressed collagen 1 and alpha-smooth muscle actin both at protein and mRNA levels.[17] Our study also analyzed total collagen using calorimetric methods similar to this study and found licorice to be an effective antifibrotic agent which reduced collagen accumulation in a dose-dependent manner.
In a study by Liu et al. to assess the antifibrotic potential of curcumin (Turmeric) in TGF-β2 induced mouse lung fibroblasts, they established that curcumin considerably mitigated the production of collagen 1 as evaluated by ELISA. The expression of alpha-smooth muscle actin was also analyzed by immunofluorescence to check for myofibroblast activity. They concluded that the usage of curcumin could prevent the conversion of fibroblasts to myofibroblasts thereby inhibiting fibrosis.[18] In contrast to this study, where collagen 1 was specifically assessed, our study assessed total collagen without any specification for its type.
In a study by Adtani et al., extracts of Centella asiatica Linn and its bioactive compound-Asiatic acid were used to assess the antifibrotic activity in buccal fibroblasts induced with arecoline in vitro. They concluded that both the extracts were effective in down regulating fibroblastic markers like TGF-β and collagen genes, COL1A2 and COL3A1 thus ameliorating fibrosis.[19]
In another recent study by Adtani et al., extracts of Ocimum basilicum (Tulasi) and linalool were used to study antifibrotic activity in buccal fibroblasts in vitro. They determined the antifibrotic potential of these extracts by use of RT-PCR expression of TGF-β and collagen genes, COL1A2 and COL3A1. The study found that the expression of TGF-β and collagen genes was significantly downregulated by the use of these herbal extracts thus exhibiting antifibrotic activity. Masson's trichrome staining was also carried out to study the morphology of the altered fibroblasts.[20] In our study, the estimation of total collagen was done only by calorimetric methods which showed the antifibrotic potential of licorice in decreasing total collagen accumulation in a concentration-dependent manner.
In a study by Kim et al., to study the antifibrotic potential of Cuscuta chinensis extract on hepatic stellate cells and mouse models induced by thioacetamide, they found that the herbal extract reduced the expression of α-SMA, Col1α1 and TGF-β1 expression analyzed by RT-PCR.[21] Our study, in contrast, did not focus on the molecular aspects of fibrinogenesis.
Limitations
The current study is not short of limitations. First, since the fibroblasts were procured from an outside source and induced to undergo fibrosis, it may not completely mimic the oral environment of patients as when primary cultures are performed from fibroblasts of OSMF patients. Second, since the study was conducted in vitro, confounding factors that could occur in the oral environment could not be assessed. Third, due to monetary constraints, assessment of fibrosis was limited to total collagen assessment done by spectrophotometry which is often less sensitive and specific when compared to other molecular methods like PCR and ELISA. Although limitations exist, the current study unlocks fresh avenues for exploration and provides scope for further research in this area.
CONCLUSION AND FUTURE DIRECTION
This study unravels wide opportunities for the therapy of OSMF using phytochemical derivatives. Unlike the commercially available antifibrotic drugs such as colchicine and pirfenidone, phytochemical agents do not possess any adverse drug reactions and hence can be utilized as topical formulations for managing OSMF. However, further animal studies are required to validate the efficacy of this phytochemical drug for oral consumption to identify the appropriate dosage of administration. Further studies are also required to identify the exact mechanism of action of licorice on fibroblast cells to aid in more targeted therapy.
Ethics
This study was approved by the ethical committee of SRM University. SRMDC/IRB/2018/MDS/No. 604.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tilakaratne WM, Klinikowski MF, Saku T, Peters TJ, Warnakulasuriya S. Oral submucous fibrosis: Review on aetiology and pathogenesis. Oral Oncol. 2006;42:561–8. doi: 10.1016/j.oraloncology.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Chiang SL, Jiang SS, Wang YJ, Chiang HC, Chen PH, Tu HP, et al. Characterization of arecoline-induced effects on cytotoxicity in normal human gingival fibroblasts by global gene expression profiling. Toxicol Sci. 2007;100:66–74. doi: 10.1093/toxsci/kfm201. [DOI] [PubMed] [Google Scholar]
- 3.Khan S, Chatra L, Prashanth SK, Veena KM, Rao PK. Pathogenesis of oral submucous fibrosis. J Cancer Res Ther. 2012;8:199–203. doi: 10.4103/0973-1482.98970. [DOI] [PubMed] [Google Scholar]
- 4.Hebbar PB, Sheshaprasad R, Gurudath S, Pai A, Sujatha D. Oral submucous fibrosis in India: Are we progressing? Indian J Cancer. 2014;51:222–6. doi: 10.4103/0019-509X.146724. [DOI] [PubMed] [Google Scholar]
- 5.Kao TC, Wu CH, Yen GC. Bioactivity and potential health benefits of licorice. J Agric Food Chem. 2014;62:542–53. doi: 10.1021/jf404939f. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Lim SS, Lee ES, Gong JH, Shin D, Kang IJ, et al. Isoangustone A suppresses mesangial fibrosis and inflammation in human renal mesangial cells. Exp Biol Med (Maywood) 2011;236:435–44. doi: 10.1258/ebm.2010.010325. [DOI] [PubMed] [Google Scholar]
- 7.Ray JG, Ranganathan K, Chattopadhyay A. Malignant transformation of oral submucous fibrosis: Overview of histopathological aspects. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:200–9. doi: 10.1016/j.oooo.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Rajalalitha P, Vali S. Molecular pathogenesis of oral submucous fibrosis–A collagen metabolic disorder. J Oral Pathol Med. 2005;34:321–8. doi: 10.1111/j.1600-0714.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 9.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li P, Li Y, Jiang H, Xu Y, Liu X, Che B, et al. Glabridin, an isoflavan from licorice root, ameliorates imiquimod-induced psoriasis-like inflammation of BALB/c mice. Int Immunopharmacol. 2018;59:243–51. doi: 10.1016/j.intimp.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Sidhu P, Shankargouda S, Rath A, Hesarghatta Ramamurthy P, Fernandes B, Kumar Singh A. Therapeutic benefits of liquorice in dentistry. J Ayurveda Integr Med. 2020;11:82–8. doi: 10.1016/j.jaim.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bean P. The use of alternative medicine in the treatment of hepatitis C. Am Clin Lab. 2002;21:19–21. [PubMed] [Google Scholar]
- 13.Li J, Kang SW, Kim JL, Sung HY, Kwun IS, Kang YH. Isoliquiritigenin entails blockade of TGF-beta1-SMAD signaling for retarding high glucose-induced mesangial matrix accumulation. J Agric Food Chem. 2010;58:3205–12. doi: 10.1021/jf9040723. [DOI] [PubMed] [Google Scholar]
- 14.Daga D, Singh RK, Pal US, Gurung T, Gangwar S. Efficacy of oral colchicine with intralesional hyaluronidase or triamcinolone acetonide in the Grade II oral submucous fibrosis. Natl J Maxillofac Surg. 2017;8:50–4. doi: 10.4103/njms.NJMS_5_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neupane GP, Rai M, Rathore RS, Bhargava VK, Mahat AK, Dhami DB. Comparative study of intralesional dexamethasone plus hyaluronidase and oral colchicine in patients with oral submucous fibrosis. J Nepalgunj Med Coll. 2018;14:60–5. [Google Scholar]
- 16.Krishnakumar K, Ramadoss R, Krishnan R, Sukhija H. In vitro quantification of collagen and snail1 gene expression in experimentally induced fibrosis by arecoline and commercial smokeless tobacco products. Asian Pac J Cancer Prev. 2020;21:1143–8. doi: 10.31557/APJCP.2020.21.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Q, Noor M, Wong YF, Hylands PJ, Simmonds MS, Xu Q, et al. In vitro anti-fibrotic activities of herbal compounds and herbs. Nephrol Dial Transplant. 2009;24:3033–41. doi: 10.1093/ndt/gfp245. [DOI] [PubMed] [Google Scholar]
- 18.Liu D, Gong L, Zhu H, Pu S, Wu Y, Zhang W, et al. Curcumin inhibits transforming growth factor β induced differentiation of mouse lung fibroblasts to myofibroblasts. Front Pharmacol. 2016;7:419. doi: 10.3389/fphar.2016.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adtani PN, Narasimhan M, Punnoose AM, Kambalachenu HR. Antifibrotic effect of Centella asiatica Linn and asiatic acid on arecoline-induced fibrosis in human buccal fibroblasts. Journal of investigative and clinical dentistry. 2017;8:e12208. doi: 10.1111/jicd.12208. [DOI] [PubMed] [Google Scholar]
- 20.Adtani P, Malathi N, Ranganathan K, Lokeswari S, Punnoose AM. Antifibrotic effect of Ocimum basilicum L. and linalool on arecolineinduced fibrosis in human buccal fibroblasts: An in vitro study. Translational Research in Oral Oncology. 2018;3:1–9. [Google Scholar]
- 21.Kim JS, Koppula S, Yum MJ, Shin GM, Chae YJ, Hong SM, et al. Anti-fibrotic effects of Cuscuta chinensis with in vitro hepatic stellate cells and a thioacetamide-induced experimental rat model. Pharm Biol. 2017;55:1909–19. doi: 10.1080/13880209.2017.1340965. [DOI] [PMC free article] [PubMed] [Google Scholar]


