Abstract
Sarcoidosis is a granulomatous disorder of multiple organs, with lungs and lymphatic systems being the most frequently affected sites of the body. It was first reported in 1877 and has continued to engross both clinicians and scientists since that time. Because sarcoidosis being a diagnosis of exclusion, it demands the physician to rule out all the possible diagnosis. Most of the patients remain asymptomatic and this makes the disease remain unnoticed for a prolonged period. Later after years, the disease could be diagnosed after witnessing the patient being symptomatic or suffering from organ failures. It could affect middle aged people of any sexes, often its clinical features correlate with tuberculosis. On immunological and histopathological examination, it reveals noncaseating granuloma in simple terms. Glucocorticoids remain the standard drug now and then. Further research has to be done to know the exact pathogenesis, early detection and betterment in treatment plan of sarcoidosis. The current review article gives a brief knowledge about etiopathogenesis, Clinical features, upgraded diagnostic methods such as biomarkers detection and the organized treatment plan to treat sarcoidosis.
Keywords: Asteroid bodies, glucocorticoids, granulomatous, kveim-slitzbach skin patch test, sarcoidosis, Schaumann bodies
INTRODUCTION
Sarcoidosis is a multisystem disorder of unrecognized etiology. It is also a chronic granulomatous disease primarily affecting lungs, lymphoid systems, and any organ system in the body. The histopathology of sarcoidosis reveals granulomas which are nonnecrotizing with a tightly packed macrophages in the center, epithelioid cells, multinucleated giant cells, and T-lymphocytes that are CD4 positive.[1,2] Since this is a diagnosis of exclusion, it is mandatory to exclude other granulomatous diseases. In 1877, Jonathan Hutchinson reported the first case of sarcoidosis at the King's College Hospital in London (United Kingdom). It still remains a challenge for clinicians to give sarcoidosis as a diagnosis even after many advancements have occurred. It has nonspecific symptoms and histopathology remains as a gold standard to confirm the diagnosis. This review article discusses about the etiology, pathogenesis, clinical manifestations, and the advancements in the management of sarcoidosis.[3]
EPIDEMIOLOGY
The prevalence of sarcoidosis is seen in people of all ages, regardless of race and ethnicity, with crest incidence seen in people aged between 20 and 39 years.[4,5] Highest incidence is seen among African Americans with an annual incidence of 17–35 per 100,000 population while the lowest annual incidence is observed among Asians and Hispanics (1–3/100,000).[6,7,8,9,10,11,12] There is a female predilection of 2:1 seen in Africans Americans.[12] It is more common among rural areas in particularly among nonsmokers.[13] Prevalence of sarcoidosis in India is 10–12 cases/1000 registrations yearly, as announced by a respiratory unit in western India.[14,15] Erythema nodosum (EN) in Europeans, chronic uveitis in U. S. blacks and lupus pernio in Puerto Ricans are the extra-thoracic manifestations encountered in specific populations. Unusual enitity in Blacks and Japanese is the sarcoid-related erythema nodosum.[16] Myocardial involvement is the frequent cause of death caused by sarcoidosis which is[17,18,19] followed by respiratory failure.[20,21] Mortality rate due to sarcoidosis is 1%–5%.
RISK FACTORS
The exact cause of sarcoidosis is unknown. The causes can be categorized under genetic factors, environmental factors, Infection and autoimmunity. Genetic factors that predispose to sarcoidosis includes the following risk loci like BTNL2, HLA-B, HLA-DPB1, ANXA11, IL23R, SH2B3/ATXN2, IL12B, NFKB1/MANBA and FAM177B. Environmental agents such as aluminium, zirconium, talc, pine tree pollen, clay, insecticide were the potent pathogens. Mycobacteria is the frequently and strongest pathogen associated with sarcoidosis, followed by Leptospira species, Mycoplasma, Chlamydia pneumoniae and Borrelia burgdorferi.
ETIOPATHOGENESIS OF SARCOIDOSIS
The etiology of sarcoidosis remains uncertain; however, there is improved understanding of its genetic factors, environmental associations, putative antigens and immunopathogenesis, and it probably results due to genetical susceptibility of individuals to specific environmental agents.[22]
Etiologic agents must be able to evoke the basic histologic hallmarks of sarcoidosis and account for the clinical heterogeneity and immunologic features of this disease.[23] The histological characteristic feature of sarcoidosis is well-organized, closely-packed, nonnecrotizing granulomas surrounded by lamellar hyaline collagen as described in Flow Chart 1.[24] Most researchers concur that environmental exposure, genetic factors, seemingly dysregulated immune system represented by an exaggerated T helper 1 (TH1) immune response are involved in pathogenesis of sarcoidosis.[25,26,27,28]
Flow Chart 1.
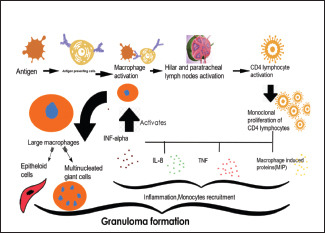
Explaining the mechanism involved in the granuloma formation
ROLE OF MYCOBACTERIA IN SARCOIDOSIS
There is similarity between sarcoidosis and tuberculosis (TB), in clinical, radiological and immunological features leading to the suggestion of mycobacteria as etiologic agent in sarcoidosis.[29,30,31,32,33] Favourable association between mycobacteria and sarcoidosis has been observed in various studies. Slow-growing mycobacteria species with low pathogenic potential, but with the ability of eliciting a type IV immune response, may be important in sarcoidosis.[34] Residues of Mycobacterial species are detected in the tissues of patients with sarcoidosis, in specific an intracellular protein, mycobacterial catalase– peroxidase (KatG), which could be a target of the adaptive immune response.[25] Other candidate mycobacterial antigens comprise superoxide dismutase and HSPs3,[25] early-secreted antigenic target of 6 kDa (ESAT6).
GENETIC FACTORS ASSOCIATED WITH SARCOIDOSIS
Sarcoidosis is a polygenic disease and various gene variants have been related with distinct phenotypes, prognosis and therapeutic response. The importance of interactions at the MHC binding site in the pathogenicity of sarcoidosis is supported by various studies.[24] Twin studies prove that monozygotic twins are more vulnerable for sarcoidosis than dizygotic twins.[35]
Human leucocyte antigen genotypes confer susceptibility, particularly a polymorphism in the butyrophilin-like 2 receptor gene (BTNL2-Costimulatory molecule within the MHC locus).[22] Hofmann and colleagues acknowledge an association of annexin A11 gene on chromosome 10q22.3. The annexin A11 gene is responsible for calcium signalling, vesicle trafficking, cell division, and apoptosis. Therefore, its deletion or dysfunction may influence apoptotic pathways in sarcoidosis.[35] The BTNL2 single-nucleotide polymorphism associated with sarcoidosis (rs2076530 G → A) may influence T-lymphocyte activation and regulation.[36] Sarcoidosis is linked with the DR subtypes of class II ANTIGENS. HLADRB1∗ 03, HLA-DRB1∗ 11, HLA-DRB1∗ 12, HLA-DRB1∗ 14 and HLA-DRB1∗ 15 promote the risk of sarcoidosis whereas HLA-DRB1∗ 01 AND HLA-DRB1∗ 04, are negatively linked with sarcoidosis. HLA-DRB1∗ 03 is associated with Lofgren's syndrome.[35]
IMMUNOLOGICAL HALLMARKS
Natural killer T cells
Reduced numbers of NKT cells have been associated with sarcoid blood and Broncho alveolar lavage (BAL) fluid. Blood NKT cells obtained from patients with sarcoidosis and stimulated with a potent glycolipid stimulator, a-galactosyl ceramide, exhibited impaired production of interferon gamma.[36]
Toll like receptors
BAL cells obtained from sarcoidosis patients showed increased cytokine responses to TLR2/1 ligand 19-kDa lipoprotein of Mycobacterium TB. eTLR-2 promotor polymorphism-16934AA have a higher risk of developing a course of chronic course due to increased production of tumor necrosis factor-alpha (TNF-α).[36]
CLINICAL MANIFESTATIONS
It has got nonspecific manifestations and it primarily affects lungs and lymphoid system of the body. It has got organ-specific manifestations. 50% had extra thoracic symptoms, 95% of patients had thoracic engagement, and 2% had unaccompanied extra thoracic sarcoidosis as reported by ACCESS.[37]
Sarcoidosis may be acute, subacute or chronic in presentation. Lofgren syndrome is a triad comprising erythema nodosum, bilateral lymphadenopathy and polyarthritis are present. Whereas individuals suffering from subacute sarcoidosis have nonspecific signs such as fever, weight loss, frailty along with arthralgia and peripheral lymphadenopathy. Chronic sarcoidosis is linked with persistent lung engagement.
GENERALIZED SYMPTOMS
Majority of the sarcoidosis patients would be asymptomatic. Nonspecific symptoms like malaise, fatigue, fever and weight loss may occur in about one-third of sarcoidosis patients. Sarcoidosis seems to be an important and frequently neglected reason for fever of unknown origin.[38] Fever is generally low grade but temperature elevations of 39° to 40°C may be seen. Weight loss is usually bound to 2–6 kg during the 10–12 weeks before presentation. Occasionally, night sweats may occur.
PULMONARY SYMPTOMS
Lung with hilar and mediastinal lymph nodes is the most frequently affected organ (over 90% populations).[39] Fifty percent of the patients with pulmonary sarcoidosis are asymptomatic (stage) and rest of the patients will be presenting with dry cough, wheezing, dyspnea, chest tightness. Hemoptysis is rare. Certain atypical features like mucosal erythema, mucosal nodules, obstructive sleep apnea, hilar and mediastinal lymphadenopathy is seen. Conglomerate masses in the lungs will be well evident in radiographic image as linear opacities, ground-glass opacities[40,41] etc., [Table 1].
Table 1.
Scadding radiological staging of pulmonary sarcoidosis[40]
| Stages | Radiographic Features | Frequency at Presenation |
|---|---|---|
| I | Mediastinal and hilar adenopathy(usually bilaterla)without pulmonary infiltrates | 40-50% |
| II | Mediastinal and hilar adenopathy(usually bilateral) With pulmonary infiltrates | 30-40% |
| III | Pulmonary infiltrates wothout adenopathy | 15-20% |
| IV | Pulmonary fibrosis with volume loss,no adenopathy | 2-5% |
EXTRAPULMONARY MANIFESTATIONS
Cutaneous sarcoidosis
Most common extra thoracic manifestations of sarcoidosis. It has got an incidence of 20-40% individuals which can either be specific or non- specific.[42]
Sarcoidosis specific skin lesions
Papules/Plaques, subcutaneous nodules maybe present. Papule can be skin colored, violaceous, hypo/hyper pigmented, erythematous and are frequently found on extremities, head and neck region and least on the trunk. Subcutaneous nodules are due to Granulomatous inflammation of adipose tissue under the skin. These are multiple and painless nodules without overlying erythema, seen on extremities.[43] Other uncommon manifestations can be inflammation around scars, tattoos and lupus pernio.
Nonspecific skin lesions
Erythema nodosum. Painful erythematous nodules seen in anterior surface of lower extremities is the typical presentation. It usually represents acute form of sarcoidosis (i.e., Lofgren syndrome). Profuse sweating will also be present.
Scarring and non-scarring alopecia will be present. In nails, onycholysis, dystrophy, hyper keratosis and longitudinal ridging may be present.
Ocular sarcoidosis
Affects more than 40% of the individuals.[44,45,46] Affects any part of the eye, mostly causes uveitis and it is visualized on slit-lamp examination. Blindness results due to adhesions with the iris and lens.
According to involvement of eye, it can be further classified into anterior, posterior, intermediate and diffuse uveitis (PAN uveitis). Depending on the intraocular inflammation, it can be either anterior or posterior uveitis.
Anterior uveitis
Present with eye pain, erythematous around the limbus and visual loss. Usually seen in whites (over 80% cases).
POSTERIOR AND INTERMEDIATE UVEITIS
This is characterized by painless visual loss and floaters. It is more common in blacks.[47,48,49]
Nonuveitis ocular sarcoidosis
Conjunctivitis/:Scleritis, episcleritis, conjunctivitis/conjunctival nodules, lacrimal gland involvement, orbital mass, and optic neuritis. It won't affect visual acuity.[43] Other manifestations include pain, hyperemia and photophobia.
Renal sarcoidosis
It is rare and seen in <3% populations.[39,50] Patients with sarcoidosis should be observed for the existence of renal impairment to prevent chronic kidney disease. Hence investigations like serum creatinine, blood urea nitrogen, estimated glomerular filtration rate, protein and calcium in both serum and urine, and screening of the urinary sediment for casts of red or white blood cells. 25-hydroxvitamin D3,
1,25-dihydroxyvitamin D3, and parathyroid hormone should be measured in sarcoidosis patients.[51] Chronic kidney disease with or without abnormal urine, pyuria, proteinuria is the typical presentation. Granulomatous interstitial nephritis is seen in <20% sarcoidosis patients.[52,53] Nephrolithiasis and nephrocalcinosis arises owing to hypercalcemia and hypercalciuria. Renal biopsy remains the standard method for the diagnosis renal sarcoidosis.
Cardiac sarcoidosis
It occurs in 20%–27% of populations.[54,55] Initially, the patients may be asymptomatic initially after which symptoms like palpitations, syncope or even sudden cardiac death can occur. Cardiac failure occurs due to Granulomatous inflammation of myocardium manifested as arrhythmia (commonly AV block is seen in 50% of patients followed by ventricular tachycardia and supraventricular arrhythmia) and cardiomyopathy. Cardiac sarcoidosis constitutes for two-thirds of all cases.[56]
Neurosarcoidosis
Neurosarcoidosis is reported in <10% of patients.[57,58] Unilateral or bilateral cranial neuropathy of facial and optic nerve is the most common manifestations in neurosarcoidosis.[59,60] The mechanism involved in cranial neuropathy could be either granulomatous inflammation of the epineural/perineural nerve itself or compressing of nerve by leptomeninges.[61,62] The lesions are most commonly found in the hypothalamus and pituitary glands, and may result in endocrine manifestations, including diabetes insipidus, adrenal and pituitary failure, and amenorrhoea–galactorrhoea syndrome.[63,64,65] Psychiatric manifestations like psychosis may be present. Spinal cord involvement is a rare manifestation presenting with leg weakness, parenthesis most often thoracic segment is involved.[66] Symptoms ranging from mononeuritis multiplex to Guillain–Barré-like syndromes, as well as polyneuropathy or polyradiculopathy, can occur. Patients usually present with pain, burning sensation and paresthesia which may be migratory or intermittent.[67,68] Cerebrospinal fluid analysis reveals high protein level and increased monocyte cell count. 50% of renal biopsies reveal only one-fifth of cases.[69,70] Since taking biopsy is more invasive and difficult, brain MRI is considered as most sensitive noninvasive test for neurosarcoidosis.
Musculoskeletal involvement in sarcoidosis
It involves 1%-–13% of patients.[71,72] Acute arthritis with reference to sarcoidosis, most frequently arises in Lofgren syndrome (Bilateral hilar lymphadenopathy, Erythema nodosum and bilateral ankle swelling) which was explained in [Table 2]. Ankle swelling is predominantly due to soft tissue swelling and tenosynovitis. Chronic arthritis is extremely rare. Other manifestations like arthropathy, osteoporosis, osteopenia are usual. Nodular lesions, cystic lesions sffecting the joints, arthralgia may be present.[70,71] Axial sarcoidosis may involve the vertebral bodies or the joints of sacrum and ilium bones.
Table 2.
Criteria for diagnosing acute arthritis related to sarcoidosis
| Arthritis of ankle symmetrically |
| Symptomatic for <2 months |
| 40 years or below 40 years |
| EN reaching sensitivity and specificity of 93% and 99% |
EN: Erythema nodosum
Gastrointestinal and hepatic involvement: It accounts for 0%–3.4% of cases[72]
The most affected hollow organ is stomach. The pathological process involved in stomach is granulomatous infiltration of mucosa and muscular layer, which subsequently ends up in mucositis, ulcer, obstruction or strictures. About 20%of patients are asymptomatic and may present with sarcoid-related lesions. Patients with gastric sarcoidosis has tendency to present with epigastric pain. Other common symptoms include nausea, vomiting, diarrhea, weight loss etc. About 80%of patients were identified with granulomatous lesions in the liver biopsy on an autopsy study.[72] Common liver and spleen manifestations include hepatosplenomegaly, portal hypertension, intrahepatic cholestasis and impaired liver function.
Oral manifestation
Oral lesions are mostly asymptomatic and are not identified before the diagnosis is made. The most common extra-oral sites are salivary glands (parotid gland being affected 6%) and cervical lymph nodes. Buccal mucosa, lips, gingiva, tongue and palate are the most commonly affected intraoral sites. More than one site is involved only in few cases.[73] Oral lesions mostly evident as diffuse enlargements or nodular swellings, mostly localised at the sub mucosal level. Papule and superficial ulceration have also noted. Pain and dryness of tongue also evident in some rare cases.[74]
Endocrine and exocrine involvement
Its manifestations seen in 20%–50% of individuals.[75] Thyroid gland (5%) and parotid glands (5%–10%) are the frequently affected organs. Thyroid and parotid gland enlargement is most commonly seen.
Hypothermia, adrenal suppression hypothyroidism, hyperthyroidism, are rare.[22] It also influences hypothalamic-pituitary effects like diabetes insidious etc. Heerfordt's syndrome comprises the features of fever, parotid enlargement, facial palsy, and anterior uveitis.
Lymph node involvement
It is seen in 20% of patients. Peripheral lymphadenopathy is commonly seen. Cervical, axillary, epitrochlear, and inguinal are the most frequently involved lymph nodes. Affected lymph nodes are moderately swollen, and are usually nontender.[76,77,78] These are often round, granular in appearance, homogeneous echogenicity with distinct margin.[76]
HISTOPATHOLOGY
The typical feature of sarcoidosis would be well formed, noncaseating granuloma with mass of epithelioid cells and multinucleated giant cells. The granuloma is surrounded by lymphocytes and contains minimal or no central necrosis. Certain cytoplasmic inclusions like Asteroid bodies, Schaumann bodies, Hamazaki-Wesenberg bodies [illustrated in Figure 1 and Figure 2] calcium oxalate crystals will also be present.[79,80]
Figure 1.
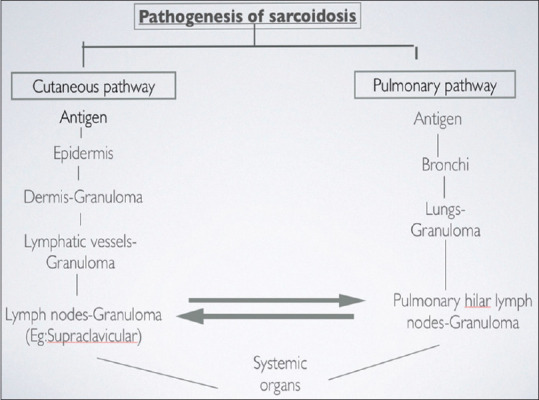
Pathogenesis of sarcoidosis
Figure 2.
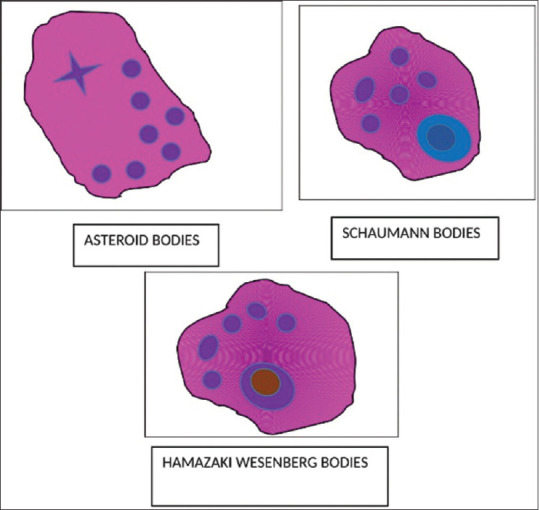
Inclusion bodies seen in sarcoidosis
Special stains can be used to differentiate sarcoidosis from other granulomatous diseases like fungal and mycobacterium diseases. Atypical mycobacterial infections and TB and can resemble sarcoidosis. These infections can be screened for by acid-fast staining. Fungal infections such as histoplasmosis should also be considered and staining has to be for the final diagnosis of sarcoidosis.
INVESTIGATIONS AND DIAGNOSIS
The various clinical manifestation exhibited by different organs and the investigations to be doneand their differential diagnosis are summarized in [Table 3].
Table 3.
Investigations and differential diagnosis of sarcoidosis
| Organ system | Clinical features | Investigations | Differential diagnosis |
|---|---|---|---|
| Lungs | Cough, dyspnoea | Chest radiograph, chest CT (may be necessary) | Noninfectious |
| Hypersensitivity pneumonitis | |||
| Hilar lymphadenopathy | Chest radiography and CT, endoscopic ultrasonographic with needle aspiration | Pneumoconiosis: Beryllium (chronic beryllium disease), titanium, aluminum | |
| Drug reactions | |||
| 18F-FDG PET (in selected patients), Gallium scan | Aspiration of foreign materials | ||
| Wegener’s granulomatosis | |||
| Chronic interstitial pneumonia like usual and lymphocytic interstitial pneumonia | |||
| Pulmonary hypertension | Brain natriuretic peptide, 6 min walk test, echocardiography, right heart catheterisation | NSG | |
| Infectious | |||
| Interstitial lung disease and pulmonary fibrosis | Chest radiograph, chest CT, bronchoscopy, surgical lung biopsy (if needed) | Tuberculosis | |
| Atypical mycobacteriosis | |||
| Cryptococcosis | |||
| To assess pulmonary involvement and disease severity | Pulmonary function test | Aspergillosis | |
| Histoplasmosis | |||
| Coccidioidomycosis | |||
| Blastomycosis | |||
| Pneumocystis carinii | |||
| Mycoplasma, etc. | |||
| Skin | Papules, nodules, plaques, erythe ma nodosum, lupus pernio | Skin biopsy if needed, except for EN and lupus pernio, which will usually be diagnosed clinically | Noninfectious |
| Reaction to foreign bodies: Beryllium zirconium, tattooing, paraffin, etc. | |||
| Rheumatoid nodules | |||
| Infectious | |||
| Tuberculosis | |||
| Atypical mycobacteriosis | |||
| Fungal infection | |||
| Heart | Conduction abnormalities, arrhythmia, ventricular tachycardia and ventricular fibrillation), sudden cardiac failure, death | Electrocardiograph, echocardiography, Holter monitoring, cardiac MRI, 18F-FDG PET, thallium scan (in selected patients) | Noninfectious |
| Giant cell myocarditis | |||
| Acute rheumatic heart disease | |||
| Granulomatosis with polyangiitis | |||
| Erdheim-Chester arrhythmogenic right ventricular dysplasia | |||
| Drugs/toxins | |||
| Granulomatous lesions of unknown significance | |||
| Infectious | |||
| Bacteria - Tuberculosis, syphilis, Tropheryma whippelii | |||
| Fungi - Aspergillosis | |||
| Nervous system | Cranial nerve | Brain MRI palsy | Noninfectious |
| Chronic variable immunodeficiency | |||
| Optic neuritis | Ophthalmologic evaluation | Rosai-Dorfman disease | |
| Lymphomatoid granulomatosis | |||
| Hypopituitarism | Hormonal studies | Granulomatosis with polyangiitis | |
| Rheumatoid nodules | |||
| Cognitive | Brain MRI, CSF dysfunction studies small finer | Amyloidosis | |
| Cholesterol granuloma | |||
| Foreign body | |||
| Drugs/toxins/heavy metals | |||
| Polyneuropathy | Electromyography, nerve conduction defects | Sarcoid-like reaction to tumor CNS malignancies | |
| Infectious | |||
| Bacteria - Tuberculosis, brucella | |||
| Fungi - Aspergillus, coccidioidomycosis, | |||
| cryptococcosis | |||
| Parasites - Amoeba, Toxoplasmosis, Schistosomiasis, | |||
| Taenia solium | |||
| Viruses: Varicella zoster, Herpes simplex | |||
| Kidney | Hypercalcemia | Biopsy, renal ultrasonography, CT nephrolithiasis, renal urography, renal stones, renal failure, function test | Noninfectious |
| Granulomatosis polyangiitis | |||
| Chronic lymphocytic leukemia | |||
| Infectious | |||
| Bacteria - Tuberculosis | |||
| Fungi - Histoplasmosis, Coccidioidomycosis | |||
| Virus - Adenovirus | |||
| Liver | Mostly asymptomatic | Liver biopsy, liver function test | Noninfectious |
| Crohn’s disease | |||
| Hodgkin’s disease | |||
| Non-Hodgkin’s lymphomas | |||
| GLUS syndrome | |||
| Infectious | |||
| Tuberculosis | |||
| Brucellosis | |||
| Schistosomiasis | |||
| Spleen | Splenomegaly | Abdominal ultrasonography, abdominal CT | Noninfectious |
| Chronic variable immunodeficiency | |||
| Sarcoid-like reaction to tumor | |||
| Infectious | |||
| Bacteria - Tuberculosis | |||
| Fungi - Histoplasmosis | |||
| Parasites - Leishmaniasis | |||
| Eyes | Uveitis, retinal vascular changes, lacrima l gland enlargement, conjunctival nodules | Opthalmologic evaluation, lacrimal gland biopsy (if necessary), gallium scan (in selected patients) | Noninfectious |
| Inflammatory bowel disease | |||
| ANCA vasculitides | |||
| Vogt-Koyanagi-Harada diseases | |||
| Blau syndrome | |||
| Infectious | |||
| Perinaud oculoglandular syndrome | |||
| Bacteria - Tuberculosis, syphilis | |||
| Viruses - Cytomegalovirus, Varicella zoster | |||
| Fungi - Toxoplasmosis | |||
| Musculoske letal system | Proximal muscle weakness, myalgia, intramuscular nodules | Creatine kinase, MRI, 18F-FDG PET, possible muscle biopsy | Noninfectious |
| Non-Hodgkin lymphoma | |||
| Crohn’s disease | |||
| Thymoma-myasthenia gravis | |||
| Foreign body | |||
| Primary biliary cirrhosis (primary biliary cholangitis) | |||
| Infectious | |||
| Bacteria - Tuberculosis, syphilis, brucella | |||
| Fungi - Pneumocystis jirovecii, Cryptococcosis | |||
| Virus - Human T-lymphotrophic virus 1 | |||
| Hematologic | Anaemia, leukopenia | Complete blood count, bone marrow biopsy | Idiopathic thrombocytopenia purpura |
| Lymph nodes | Peripheral lymphadenopath y such as cervical lymph node enlargement Hilar and mediastinal lymph node enlargement |
Biopsy of most accessible and safest site Chest radiograph, chest CT, endoscopic ultrasonography with needle aspiration (endobronchial or esophageal), gallium scan, 18F-FDG PET (in selected patients) |
Noninfectious |
| Hodgkin’s disease | |||
| Non-Hodgkin’s | |||
| Lymphomas | |||
| Granulomatous | |||
| GLUS syndrome | |||
| Infectious | |||
| Tuberculosis | |||
| Atypical mycobacteriosis | |||
| Brucellosis | |||
| Toxoplasmosis | |||
| Granulomatous histiocytic necrotizing | |||
| lymphadenitis (Kikuchi’s disease) | |||
| Cat-scratch disease | |||
| Exocrine and endocrine glands | Thyroid gland enlargement Parotid enlargement, isolated or associated with Heerfordt syndrome (uveoparotid fever) |
FNAC, ultrasound is otope study Barium, gallium scan (in selected patients) |
Noninfectious |
| Granulomatosis polyangiitis | |||
| Ductal obstruction (calculus, tumor) | |||
| Crohn’s disease | |||
| Infectious | |||
| Bacteria | |||
| Tuberculosis | |||
| Atypical mycobacteria |
CT: Computed tomography, 18F-FDG PET: 18Fluorodeoxyglucose positron emission tomography, MRI: Magnetic resonance imaging, NSG: Necrotizing sarcoid granulomatosis, EN: Erythema nodosum, CSF: Cerebrospinal fluid, CNS: Central nervous system, GLUS: Granulomatous lesions of unknown significance, ANCA: Antineutrophilic cytoplasmic antibody, FNAC: Fine needle aspiration cytology
SERUM BIOMARKERS FOR SARCOIDOSIS
Numerous biomarkers are in investigational procedures for the accurate diagnosis and formulating a successful treatment plan. The summary of all the markers and their indications are tabulated in the [Table 4].
Table 4.
| Serial number | Biomarkers | Indications |
|---|---|---|
|
| ||
| Serum biomarkers for sarcoidosis | ||
| A) | Macrophages | |
| 1 | Serum angiotensin-converting enzyme | Well known serum biomarker correlates with granuloma burden and radiological Stages II and III |
| Sensitivity: 22%-86%; specificity: 54%-5% also increased in other inflammatory diseases like tuberculosis, histoplasmosis, Gaucher disease etc. | ||
| Not significant when ACE inhibitors is used by patients | ||
| 2 | Lysozyme | Prognostic tool |
| Mainly observed at the time of disease onset. Involved in granuloma formation | ||
| Low sensitivity for sarcoidosis | ||
| 3 | Serum CD163 | Prognostic tool |
| CD163 levels alter under the influence of inflammatory mediators | ||
| High sensitivity and low specificity | ||
| Also increased in diseases like rheumatoid arthritis, MS, Crohn’s disease | ||
| 4 | YKL40 | Marker for granuloma burden |
| Growth factor for fibroblast and vascular endothelial cells | ||
| Comparatively higher in active sarcoidosis | ||
| Patients | ||
| 5 | Neopterin | Nonspecific marker |
| Low specificity | ||
| 6 | Serum amyloid A | Produced by liver during acute phase of sarcoidosis |
| Clinical marker of inflammation | ||
| Also elevated in rheumatoid arthritis, Crohn’s disease etc. | ||
| 7 | CC chemokine Ligand 18 | Prognostic marker |
| Seen in patients with active disease Increased levels seen in most interstitial lung disease and gaucherie disease | ||
| 8 | Chitotriosidase | Good prognostic marker |
| Elevated in case of progressive disease high sensitivity and specificity | ||
| Also increased in Gaucher’s disease, malaria, multiple sclerosis, atherosclerosis, Alzheimer’s disease and tuberculosis | ||
| B) | Monocytes | Intermediate monocytes (CD14+/CD16+) and nonclassical monocytes (CD14−/CD16++) will be elevated |
| Low specificity | ||
| Also increased in cardiovascular diseases and interstitial lung disease | ||
| C) | T-cell | |
| 1 | Serum soluble interleukin 2 receptor | Diagnostic marker |
| Also elevated in some hematological disorders, autoimmune diseases, also in patients with impaired renal function | ||
| D) | B cell | |
| 1 | B-cell activating factor | Low specificity |
| Elevated levels seen in the multiple organ involvement (i.e., skin and eye involvement), decline in pulmonary function and more advanced chest radiographic stages (II/III) | ||
| 2 | Naive and memory B-cells | Naive B-cells increase |
| Memory B-cells downregulated | ||
| 3 | Regulatory B-cells | Elevated in active sarcoidosis |
|
| ||
| Bronchoalveolar lavage fluid biomarkers | ||
|
| ||
| 1 | CD4/CD8 ratio | Not a specific biomarker |
| Sensitivity: 54%-80% and specificity: 59%-80% | ||
| 2 | CD 103+CD 4+/CD4+ratio | Diagnostic tool |
| 3 | T-helper 17.1 cells | Immunological marker |
| 4 | Regulatory T-cells | Treg/Th17 ratio inversely related to disease activity |
| 5 | Neutrophils | Elevated in radiological stage (II/III) |
| 6 | Natural killer cells | Elevated in patients with impaired lung function |
| 7 | Natural-killer T cells | Reduced number of NKT cells seen |
| 8 | CXCL9, CXCL10, and CXCL11 | Prognostic marker |
| CXCL9 and CXCL11 associated with number of organs involved | ||
| CXCL10 associated with higher dyspnea scores | ||
| 9 | krebs Von den lungen-6 | Reflects damaged or regenerating Type II pneumocytes |
| Elevated in radiological Stage IV pulmonary sarcoidosis (marker of severity) | ||
|
| ||
| Future biomarkers of sarcoidosis | ||
|
| ||
| 1 | JAK/STAT signaling | |
| 2 | mTOR signaling | |
| 3 | Hair cortisol | |
| 4 | Labeled PET-Tracers | |
ACE: Angiotensin-converting enzyme, MS: Multiple sclerosis, NKT: Natural-killer T, JAK: Janus kinase, STAT: Signal transducer and activator of transcription, mTOR: Mammalian target of rapamycin, PET: Positron emission tomography
Treatment
Sarcoidosis is a life-threatening disease. Hence, timely diagnosis influences the prognosis of the sarcoidosis patients. In sarcoidosis patients, the medical intervention has to becarried out when the patient develops specific symptoms (worsening functional status) which fails to regress on its own, along with the imaging abnormalities. Management modality for sarcoidosis are tabulated in the Table 5. Glucocorticoids acts as a first line drug treatment and also has several side effects.[83,84]
Table 5.
Explaining the management of sarcoidosis

|
Pulmonary sarcoidosis
In pulmonary sarcoidosis, the granulomatous inflammation in lungs leads to reduced forced vital capacity and diffusing capacity of lung for carbon monoxide from its baseline (10%–20% or more) denoting significant impairment of lung functions.[85]
First line treatment: Glucocorticoids like prednisolone 20–40 mg/day for 1–3 months has to be given. Tapered dose of 5–10 mg daily for every 1–3 months, until a maintenance dose of 5–10 mg/d for approximately 1 year. Relapse may occur in 30% of patients after discontinuing or tapering steroids.[85] Most importantly, patients start depending on corticosteroid drugs
Second line drugs: To overcome glucocorticoid toxicity, disease modified anti-rheumatic drugs (DMARD's) are recommended.[86,87] Methotrexate (10-25 mg weekly, oral or intramuscular) is most commonly used drug in pulmonary sarcoidosis.[88,89,90,91] Folic acid supplements have to be given along with methotrexate. Patient can develop complications like hepatotoxicity, bone marrow suppression. Onset of action is slow (i.e. 2–3 months). Other DMARD's with less efficacy are leflunomide (10–20 mg/day), azathioprine (50–200 mg/day), mycophenolate (500–3000 mg/day)
Third line drugs: Another class of drugs would be TNF-α inhibitor like Infliximab, adalimumab etc. Infliximab is given intravenously at a dosage of 5 mg/kg body weight at 0, 2 and every 4–8 weeks thereafter. Adalimumab 40 mg subcutaneously for every 1–2 weeks can be given. Adverse reactions of these drugs have to be consider while administering drugs.
Extra pulmonary sarcoidosis
Skin: Most of the skin lesions like erythema nodosum are self-regressing lesions. Hence no treatment is needed, in most of the patients. For some patients presenting with pain, either short course nonsteroidal anti-inflammatory drugs (NSAIDs) or glucocorticoids can be prescribed.[61,92] Topical or intralesional administration of corticosteroids is the most preferred route for better efficacy and to reduce systemic toxicity. In severe cases, oral administrations also preferred. The second line drugs such as hydroxychloroquine and chloroquine also be prescribed.[61,93,94] Infliximab is prescribed when both the above-mentioned drugs fail to act upon the lesion. Other topical formulations like clobetasol, halobetasol, propionate can also be used
Eyes: Uveitis is the most common eye lesion in sarcoidosis. Glucocorticoids in the form of eye drops can be used for anterior uveitis and periocular/intravitreal injection or implant for posterior uveitis. Other second and third line drugs like Azathioprine, Infliximab can also be used. Orbital debulking/decompression surgery is also needed[44]
Joints: NSAIDs are the first line drug used in sarcoid arthropathy.[61] 1n unresponsive cases, hydroxychloroquine and methotrexate can be used
Heart: Granulomatous inflammation of the myocardium results in arrhythmia, conduction defect, left ventricular dysfunction or right ventricular dysfunction.[95] Immunosuppressants like Glucocorticoids are the drug of choice in cardiac sarcoidosis. Prednisolone initial dose 40–60 mg daily with taper regimen has to be given. Some experts suggest taking cardiac fluorodeoxyglucose positron emission tomography (FDG PET) scan before initiating immunosuppressants.[96] In case of corticosteroids intolerance, methotrexate, azathioprine, mycophenolate has to be prescribed.
Other TNF-alpha inhibitors like Rituximab can be used. Infliximab is not used since it tends to exacerbate heart failure. In case of cardiac failure, other drugs like diuretics, beta blockers, angiotensin converting enzyme inhibitors also used.[97] For advanced cardiac failure, implantable cardioverter-defibrillator is used.
Nervous system: Curative treatment is done only for transient lesions whereas palliative treatment is carried out for permanent neurological deficit like facial nerve palsy etc.[98] A short course of intravenous methyl prednisolone 1000 mg daily should be given in patients with severe manifestations like visual loss, altered mental status etc. Moderate dose of prednisone 0.5 mg/kg/day is prescribed for patients with peripheral nerve involvement. Higher dose of corticosteroids (Prednisone 1.0 mg/kg/day) should be given for patients with central nervous system involvement. Prednisone 20–25 mg/daily should be given along with tapering dose. Drugs can be given in combinations like “Prednisolone+ DMARD's (Methotrexate).”
Neurosarcoidosis has got a high recurrence rate. In a retrospective study, they found that infliximab has got high efficacy over patients with refractory sarcoidosis. Intravenous immunoglobulin and TNF-alpha inhibitors also appears to be more effective options because about 70% of patients who received one of them or a combination of them did experience improvement within the 1st month of therapy.[99] For seizure experiencing patients, anti-epileptics has to be prescribed.[103]
Kidneys: The ultimate risk of renal sarcoidosis is chronic kidney disease. Glucocorticoids along with DMARDs can also be taken.[100] In prolonged Glucocorticoids intake, hypercalcemia has to be checked periodically. Calcium levels become normal after inhaling corticosteroids 20-40 MD.=
GIT and liver involvement: It is rarely affected and hence the treatment remains unclear. Glucocorticoids can be used as a first line of choice[43]
Oral cavity: Asymptomatic lesions would heal slowly and require no treatment. Surgery is the first choice for nodular lesions. Corticosteroids be given for painful or progressive lesions.[101,102]
CONCLUSION
Sarcoidosis is a diagnosis of exclusion. After many research studies done in sarcoidosis patients, the exact etiology of sarcoidosis still remains inconclusive. Since it has got nonspecific symptoms and multi-organ involvement, diagnosis cannot be given purely based on clinical history. A lot of investigations like Kveim-slitzbach skin patch test, imaging tests like chest X-ray, computed tomography, magnetic resonance imaging and 18f FDG-PET scan plays a major role in arriving at a diagnosis of sarcoidosis. However, presence of noncaseating granuloma in histopathology gives a clue for the diagnosis. Glucocorticoids remains the first line drugs in treating sarcoidosis. Methotrexate, Infliximab also has good efficacy and used as second and third line of drug treatment. This review article gives a clear idea about the clinical manifestations, differential diagnosis and treatment plan for sarcoidosis. It will help clinicians for early and easy diagnosis and prompt treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Thomas KW, Hunninghake GW. Sarcoidosis. JAMA. 2003;289:3300–3. doi: 10.1001/jama.289.24.3300. [DOI] [PubMed] [Google Scholar]
- 2.Chen ES, Moller DR. Sarcoidosis – Scientific progress and clinical challenges. Nat Rev Rheumatol. 2011;7:457–67. doi: 10.1038/nrrheum.2011.93. [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson J. Anomalous disease of the skin of the fingers: Case of livid papillary psoriasis. Illus. Clin Surg. 1877;1:42–3. [Google Scholar]
- 4.Statement on Sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 5.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–65. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 6.Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: A 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–41. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 7.Baughman RP, Field S, Costabel U, Crystal RG, Culver DA, Drent M, et al. Sarcoidosis in America: Analysis based on health care use. Ann Am Thorac Soc. 2016;13:1244–52. doi: 10.1513/AnnalsATS.201511-760OC. [DOI] [PubMed] [Google Scholar]
- 8.Ungprasert P, Carmona EM, Utz JP, Ryu JH, Crowson CS, Matteson EL. Epidemiology of sarcoidosis 1946-2013: A population-based study. Mayo Clin Proc. 2016;91:183–8. doi: 10.1016/j.mayocp.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gribbin J, Hubbard RB, Le Jeune I, Smith CJ, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006;61:980–5. doi: 10.1136/thx.2006.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arkema EV, Grunewald J, Kullberg S, Eklund A, Askling J. Sarcoidosis incidence and prevalence: A nationwide register-based assessment in Sweden. Eur Respir J. 2016;48:1690–9. doi: 10.1183/13993003.00477-2016. [DOI] [PubMed] [Google Scholar]
- 11.Morimoto T, Azuma A, Abe S, Usuki J, Kudoh S, Sugisaki K, et al. Epidemiology of sarcoidosis in Japan. Eur Respir J. 2008;31:372–9. doi: 10.1183/09031936.00075307. [DOI] [PubMed] [Google Scholar]
- 12.Park JE, Kim YS, Kang MJ, Kim CJ, Han CH, Lee SM, et al. Prevalence, incidence, and mortality of sarcoidosis in Korea, 2003-2015: A nationwide population-based study. Respir Med. 2018;144S:S28–34. doi: 10.1016/j.rmed.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 13.Peros-Golubicić T, Ljubić S. Cigarette smoking and sarcoidosis. Acta Med Croatica. 1995;49:187–93. [PubMed] [Google Scholar]
- 14.Sharma S, Mohan A. Sarcoidosis in India: Not so rare. J Indian Acad Clin Med. 2004;5:12–21. [Google Scholar]
- 15.Gupta SK, Gupta S. Sarcoidosis in India: A review of 125 biopsy-proven cases from eastern India. Sarcoidosis. 1990;7:43–9. [PubMed] [Google Scholar]
- 16.Pietinalho A, Ohmichi M, Hiraga Y, Löfroos AB, Selroos O. The mode of presentation of sarcoidosis in Finland and Hokkaido, Japan. A comparative analysis of 571 Finnish and 686 Japanese patients. Sarcoidosis Vasc Diffuse Lung Dis. 1996;13:159–66. doi: 10.1007/BF00389839. [DOI] [PubMed] [Google Scholar]
- 17.Iwai K, Sekiguti M, Hosoda Y, DeRemee RA, Tazelaar HD, Sharma OP, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis. 1994;11:26–31. [PubMed] [Google Scholar]
- 18.Iwai K, Tachibana T, Takemura T, Matsui Y, Kitaichi M, Kawabata Y. Pathological studies on sarcoidosis autopsy. I. Epidemiological features of 320 cases in Japan. Acta Pathol Jpn. 1993;43:372–6. doi: 10.1111/j.1440-1827.1993.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 19.Iwai K, Takemura T, Kitaichi M, Kawabata Y, Matsui Y. Pathological studies on sarcoidosis autopsy. II. Early change, mode of progression and death pattern. Acta Pathol Jpn. 1993;43:377–85. doi: 10.1111/j.1440-1827.1993.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Lower EE, Li H, Farhey Y, Baughman RP. Clinical characteristics of patients with bone sarcoidosis. Semin Arthritis Rheum. 2017;47:143–8. doi: 10.1016/j.semarthrit.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Bell NH. Endocrine complications of sarcoidosis. Endocrinol Metab Clin North Am. 1991;20:645–54. [PubMed] [Google Scholar]
- 22.Ahmadzai H, Huang S, Steinfort C, Markos J, Allen RK, Wakefield D, et al. Sarcoidosis: A state of the art review from the Thoracic Society of Australia and New Zealand. Med J Aust. 2018;208:499–504. doi: 10.5694/mja17.00610. [DOI] [PubMed] [Google Scholar]
- 23.Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365–77. doi: 10.1016/j.ccm.2008.03.011. vii. [DOI] [PubMed] [Google Scholar]
- 24.Bennett D, Bargagli E, Refini RM, Rottoli P. New concepts in the pathogenesis of sarcoidosis. Expert Rev Respir Med. 2019;13:981–91. doi: 10.1080/17476348.2019.1655401. [DOI] [PubMed] [Google Scholar]
- 25.Grunewald J, Grutters JC, Arkema EV, Saketkoo LA, Moller DR, Müller-Quernheim J. Sarcoidosis. Nat Rev Dis Primers. 2019;5:45. doi: 10.1038/s41572-019-0096-x. [DOI] [PubMed] [Google Scholar]
- 26.Fischer A, Ellinghaus D, Nutsua M, Hofmann S, Montgomery CG, Iannuzzi MC, et al. Identification of immune-relevant factors conferring sarcoidosis genetic risk. Am J Respir Crit Care Med. 2015;192:727–36. doi: 10.1164/rccm.201503-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kucera GP, Rybicki BA, Kirkey KL, Coon SW, Major ML, Maliarik MJ, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527–35. doi: 10.1378/chest.123.5.1527. [DOI] [PubMed] [Google Scholar]
- 28.Newman LS, Rose CS, Bresnitz EA, Rossman MD, Barnard J, Frederick M, et al. A case control etiologic study of sarcoidosis: Environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324–30. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 29.Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145–50. doi: 10.1097/ACI.0b013e3283515173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grunewald J, Kaiser Y, Ostadkarampour M, Rivera NV, Vezzi F, Lötstedt B, et al. T-cell receptor-HLA-DRB1 associations suggest specific antigens in pulmonary sarcoidosis. Eur Respir J. 2016;47:898–909. doi: 10.1183/13993003.01209-2015. [DOI] [PubMed] [Google Scholar]
- 31.Wahlström J, Dengjel J, Winqvist O, Targoff I, Persson B, Duyar H, et al. Autoimmune T cell responses to antigenic peptides presented by bronchoalveolar lavage cell HLA-DR molecules in sarcoidosis. Clin Immunol. 2009;133:353–63. doi: 10.1016/j.clim.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Zissel G, Müller-Quernheim J. Specific antigen(s) in sarcoidosis: A link to autoimmunity? Eur Respir J. 2016;47:707–9. doi: 10.1183/13993003.01791-2015. [DOI] [PubMed] [Google Scholar]
- 33.Beijer E, Veltkamp M, Meek B, Moller DR. Etiology and immunopathogenesis of sarcoidosis: novel insights. InSeminars in Respiratory and Critical Care Medicine 2017 Aug (Vol. 38, No. 04, pp. 404-416) Thieme Medical Publishers. doi: 10.1055/s-0037-1603087. [DOI] [PubMed] [Google Scholar]
- 34.Mortaz E, Adcock IM, Barnes PJ. Sarcoidosis: Role of non-tuberculosis mycobacteria and Mycobacterium tuberculosis. Int J Mycobacteriol. 2014;3:225–9. doi: 10.1016/j.ijmyco.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Loke WS, Herbert C, Thomas PS. Sarcoidosis: Immunopathogenesis and Immunological Markers. Int J Chronic Dis. 2013;2013:928601. doi: 10.1155/2013/928601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgenthau AS, Iannuzzi MC. Recent advances in sarcoidosis. Chest. 2011;139:174–82. doi: 10.1378/chest.10-0188. [DOI] [PubMed] [Google Scholar]
- 37.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Jr, Bresnitz EA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–9. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 38.Telenti A, Hermans PE. Idiopathic granulomatosis manifesting as fever of unknown origin. Mayo Clin Proc. 1989;64:44–50. doi: 10.1016/s0025-6196(12)65302-6. [DOI] [PubMed] [Google Scholar]
- 39.Judson MA, Boan AD, Lackland DT. The clinical course of sarcoidosis: Presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:119–27. [PubMed] [Google Scholar]
- 40.Jain R, Yadav D, Puranik N, Guleria R, Jin JO. Sarcoidosis: Causes, diagnosis, clinical features, and treatments. J Clin Med. 2020;9:1081. doi: 10.3390/jcm9041081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Criado E, Sánchez M, Ramírez J, Arguis P, de Caralt TM, Perea RJ, et al. Pulmonary sarcoidosis: Typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics. 2010;30:1567–86. doi: 10.1148/rg.306105512. [DOI] [PubMed] [Google Scholar]
- 42.Mangas C, Fernández-Figueras MT, Fité E, Fernández-Chico N, Sàbat M, Ferrándiz C. Clinical spectrum and histological analysis of 32 cases of specific cutaneous sarcoidosis. J Cutan Pathol. 2006;33:772–7. doi: 10.1111/j.1600-0560.2006.00563.x. [DOI] [PubMed] [Google Scholar]
- 43.Ungprasert P, Ryu JH, Matteson EL. Clinical manifestations, diagnosis, and treatment of sarcoidosis. Mayo Clin Proc Innov Qual Outcomes. 2019;3:358–75. doi: 10.1016/j.mayocpiqo.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36:669–83. doi: 10.1016/j.ccm.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raevis JJ, Antonova N, Agemy S. Ocular involvement in sarcoidosis. J Rheumatol. 2018;45:580. doi: 10.3899/jrheum.171058. [DOI] [PubMed] [Google Scholar]
- 46.Kansal V, Dollin M. Ocular involvement in sarcoidosis. CMAJ. 2017;189:E609. doi: 10.1503/cmaj.160569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Birnbaum AD, French DD, Mirsaeidi M, Wehrli S. Sarcoidosis in the national veteran population: Association of ocular inflammation and mortality. Ophthalmology. 2015;122:934–8. doi: 10.1016/j.ophtha.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groen F, Rothova A. Ocular involvement in sarcoidosis. Semin Respir Crit Care Med. 2017;38:514–22. doi: 10.1055/s-0037-1602382. [DOI] [PubMed] [Google Scholar]
- 49.Evans M, Sharma O, LaBree L, Smith RE, Rao NA. Differences in clinical findings between Caucasians and African Americans with biopsy-proven sarcoidosis. Ophthalmology. 2007;114:325–33. doi: 10.1016/j.ophtha.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 50.Cozier YC, Berman JS, Palmer JR, Boggs DA, Serlin DM, Rosenberg L. Sarcoidosis in black women in the United States: Data from the Black Women's Health Study. Chest. 2011;139:144–50. doi: 10.1378/chest.10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Kofahi K, Korsten P, Ascoli C, Virupannavar S, Mirsaeidi M, Chang I, et al. Management of extrapulmonary sarcoidosis: Challenges and solutions. Ther Clin Risk Manag. 2016;12:1623–34. doi: 10.2147/TCRM.S74476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berliner AR, Haas M, Choi MJ. Sarcoidosis: The nephrologist's perspective. Am J Kidney Dis. 2006;48:856–70. doi: 10.1053/j.ajkd.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 53.Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine (Baltimore) 1952;31:1–132. [PubMed] [Google Scholar]
- 54.Birnie DH, Kandolin R, Nery PB, Kupari M. Cardiac manifestations of sarcoidosis: Diagnosis and management. Eur Heart J. 2017;38:2663–70. doi: 10.1093/eurheartj/ehw328. [DOI] [PubMed] [Google Scholar]
- 55.Lynch JP, 3rd, Hwang J, Bradfield J, Fishbein M, Shivkumar K, Tung R. Cardiac involvement in sarcoidosis: Evolving concepts in diagnosis and treatment. Semin Respir Crit Care Med. 2014;35:372–90. doi: 10.1055/s-0034-1376889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, et al. Cardiac sarcoidosis: Epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–32. doi: 10.1161/CIRCULATIONAHA.114.011522. [DOI] [PubMed] [Google Scholar]
- 57.Ibitoye RT, Wilkins A, Scolding NJ. Neurosarcoidosis: A clinical approach to diagnosis and management. J Neurol. 2017;264:1023–8. doi: 10.1007/s00415-016-8336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lacomis D. Neurosarcoidosis. Curr Neuropharmacol. 2011;9:429–36. doi: 10.2174/157015911796557975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stern BJ, Krumholz A, Johns C, Scott P, Nissim J. Sarcoidosis and its neurological manifestations. Arch Neurol. 1985;42:909–17. doi: 10.1001/archneur.1985.04060080095022. [DOI] [PubMed] [Google Scholar]
- 60.Pawate S, Moses H, Sriram S. Presentations and outcomes of neurosarcoidosis: A study of 54 cases. QJM. 2009;102:449–60. doi: 10.1093/qjmed/hcp042. [DOI] [PubMed] [Google Scholar]
- 61.Ungprasert P, Matteson EL. Neurosarcoidosis. Rheum Dis Clin North Am. 2017;43:593–606. doi: 10.1016/j.rdc.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 62.Carlson ML, White JR, Jr, Espahbodi M, Haynes DS, Driscoll CL, Aksamit AJ, et al. Cranial base manifestations of neurosarcoidosis: A review of 305 patients. Otol Neurotol. 2015;36:156–66. doi: 10.1097/MAO.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 63.Murialdo G, Tamagno G. Endocrine aspects of neurosarcoidosis. J Endocrinol Invest. 2002;25:650–62. doi: 10.1007/BF03345093. [DOI] [PubMed] [Google Scholar]
- 64.Tamagno G, Murialdo G. Amenorrhea-galactorrhea syndrome as an uncommon manifestation of isolated neurosarcoidosis. Ann Ital Med Int. 2001;16:260–6. [PubMed] [Google Scholar]
- 65.Bullmann C, Faust M, Hoffmann A, Heppner C, Jockenhövel F, Müller-Wieland D, et al. Five cases with central diabetes insipidus and hypogonadism as first presentation of neurosarcoidosis. Eur J Endocrinol. 2000;142:365–72. doi: 10.1530/eje.0.1420365. [DOI] [PubMed] [Google Scholar]
- 66.Sohn M, Culver DA, Judson MA, Scott TF, Tavee J, Nozaki K. Spinal cord neurosarcoidosis. Am J Med Sci. 2014;347:195–8. doi: 10.1097/MAJ.0b013e3182808781. [DOI] [PubMed] [Google Scholar]
- 67.Nozaki K, Judson MA. Neurosarcoidosis: Clinical manifestations, diagnosis and treatment. Presse Med. 2012;41:e331–48. doi: 10.1016/j.lpm.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 68.Tavee JO, Karwa K, Ahmed Z, Thompson N, Parambil J, Culver DA. Sarcoidosis-associated small fiber neuropathy in a large cohort: Clinical aspects and response to IVIG and anti-TNF alpha treatment. Respir Med. 2017;126:135–8. doi: 10.1016/j.rmed.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 69.Joseph FG, Scolding NJ. Neurosarcoidosis: A study of 30 new cases. J Neurol Neurosurg Psychiatry. 2009;80:297–304. doi: 10.1136/jnnp.2008.151977. [DOI] [PubMed] [Google Scholar]
- 70.Nessrine A, Zahra AF, Taoufik H. Musculoskeletal involvement in sarcoidosis. J Bras Pneumol. 2014;40:175–82. doi: 10.1590/S1806-37132014000200012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conte G, Zugni F, Colleoni M, Renne G, Bellomi M, Petralia G. Sarcoidosis with bone involvement mimicking metastatic disease at (18) F-FDG PET/CT: Problem solving by diffusion whole-body MRI. Ecancermedicalscience. 2015;9:537. doi: 10.3332/ecancer.2015.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hercules HD, Bethlem NM. Value of liver biopsy in sarcoidosis. Arch Pathol Lab Med. 1984;108:831–4. [PubMed] [Google Scholar]
- 73.Radochová V, Radocha J, Laco J, Slezák R. Oral manifestation of sarcoidosis: A case report and review of the literature. J Indian Soc Periodontol. 2016;20:627–9. doi: 10.4103/jisp.jisp_378_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gupta S, Tripathi AK, Kumar V, Saimbi CS. Sarcoidosis: Oral and extra-oral manifestation. J Indian Soc Periodontol. 2015;19:582–5. doi: 10.4103/0972-124X.167167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Porter N, Beynon HL, Randeva HS. Endocrine and reproductive manifestations of sarcoidosis. QJM. 2003;96:553–61. doi: 10.1093/qjmed/hcg103. [DOI] [PubMed] [Google Scholar]
- 76.Ozgul M, Cetinkaya E, Kirkil G, Ozgul G, Abul Y, Acat M, et al. Lymph node characteristics of sarcoidosis with endobronchial ultrasound. Endosc Ultrasound. 2014;3:232–7. doi: 10.4103/2303-9027.144541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koo HJ, Kim MY, Shin SY, Shin S, Kim SS, Lee SW, et al. Evaluation of mediastinal lymph nodes in sarcoidosis, sarcoid reaction, and malignant lymph nodes using CT and FDG-PET/CT. Medicine (Baltimore) 2015;94:e1095. doi: 10.1097/MD.0000000000001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robinson LA, Smith P, Sengupta DJ, Prentice JL, Sandin RL. Molecular analysis of sarcoidosis lymph nodes for microorganisms: A case-control study with clinical correlates. BMJ Open. 2013;3:e004065. doi: 10.1136/bmjopen-2013-004065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Judson MA. The diagnosis of sarcoidosis. Clin Chest Med. 2008;29:415–27. doi: 10.1016/j.ccm.2008.03.009. viii. [DOI] [PubMed] [Google Scholar]
- 80.Govender P, Berman JS. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36:585–602. doi: 10.1016/j.ccm.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 81.Crouzer ED, Maier LA, Wilson KC, Bonham CA, Morgenthau AS, Patterson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26–51. doi: 10.1164/rccm.202002-0251ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kraaijvanger R, Janssen Bonás M, Vorselaars AD, Veltkamp M. Biomarkers in the diagnosis and prognosis of sarcoidosis: Current use and future prospects. Front Immunol. 2020;11:1443. doi: 10.3389/fimmu.2020.01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Migita K, Sasaki Y, Ishizuka N, Arai T, Kiyokawa T, Suematsu E, et al. Glucocorticoid therapy and the risk of infection in patients with newly diagnosed autoimmune disease. Medicine (Baltimore) 2013;92:285–93. doi: 10.1097/MD.0b013e3182a72299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maradit Kremers H, Reinalda MS, Crowson CS, Davis JM, 3rd, Hunder GG, Gabriel SE. Glucocorticoids and cardiovascular and cerebrovascular events in polymyalgia rheumatica. Arthritis Rheum. 2007;57:279–86. doi: 10.1002/art.22548. [DOI] [PubMed] [Google Scholar]
- 85.Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149–73. [PubMed] [Google Scholar]
- 86.Carmona EM, Kalra S, Ryu JH. Pulmonary sarcoidosis: Diagnosis and treatment. Mayo Clin Proc. 2016;91:946–54. doi: 10.1016/j.mayocp.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 87.Schutt AC, Bullington WM, Judson MA. Pharmacotherapy for pulmonary sarcoidosis: A Delphi consensus study. Respir Med. 2010;104:717–23. doi: 10.1016/j.rmed.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 88.Paramothayan S, Lasserson T. Treatments for pulmonary sarcoidosis. Respir Med. 2008;102:1–9. doi: 10.1016/j.rmed.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 89.Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: Results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60–6. [PubMed] [Google Scholar]
- 90.Lower EE, Baughman RP. The use of low dose methotrexate in refractory sarcoidosis. Am J Med Sci. 1990;299:153–7. doi: 10.1097/00000441-199003000-00002. [DOI] [PubMed] [Google Scholar]
- 91.Vucinic VM. What is the future of methotrexate in sarcoidosis. A study and review? Curr Opin Pulm Med. 2002;8:470–6. doi: 10.1097/00063198-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 92.Marchell RM, Judson MA. Cutaneous sarcoidosis. Semin Respir Crit Care Med. 2010;31:442–51. doi: 10.1055/s-0030-1262212. [DOI] [PubMed] [Google Scholar]
- 93.Zic JA, Horowitz DH, Arzubiaga C, King LE., Jr Treatment of cutaneous sarcoidosis with chloroquine. Review of the literature. Arch Dermatol. 1991;127:1034–40. [PubMed] [Google Scholar]
- 94.Webster GF, Razsi LK, Sanchez M, Shupack JL. Weekly low-dose methotrexate therapy for cutaneous sarcoidosis. J Am Acad Dermatol. 1991;24:451–4. doi: 10.1016/0190-9622(91)70071-9. [DOI] [PubMed] [Google Scholar]
- 95.Hamzeh NY, Wamboldt FS, Weinberger HD. Management of cardiac sarcoidosis in the United States: A Delphi study. Chest. 2012;141:154–62. doi: 10.1378/chest.11-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chareonthaitawee P, Beanlands RS, Chen W, Dorbala S, Miller EJ, Murthy VL, et al. Joint SNMMI-ASNC expert consensus document on the role of 18F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Cardiol. 2017;24:1741–58. doi: 10.1007/s12350-017-0978-9. [DOI] [PubMed] [Google Scholar]
- 97.Hamzeh N, Steckman DA, Sauer WH, Judson MA. Pathophysiology and clinical management of cardiac sarcoidosis. Nat Rev Cardiol. 2015;12:278–88. doi: 10.1038/nrcardio.2015.22. [DOI] [PubMed] [Google Scholar]
- 98.Ungprasert P, Crowson CS, Matteson EL. Characteristics and long-term outcome of neurosarcoidosis: A population-based study from 1976-2013. Neuroepidemiology. 2017;48:87–94. doi: 10.1159/000477300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ehrhart IC, Parker PE, Weidner WJ, Dabney JM, Scott JB, Haddy FJ. Coronary vascular and myocardial responses to carotid body stimulation in the dog. Am J Physiol. 1975;229:754–60. doi: 10.1152/ajplegacy.1975.229.3.754. [DOI] [PubMed] [Google Scholar]
- 100.Krumholz A, Stern BJ, Stern EG. Clinical implications of seizures in neurosarcoidosis. Arch Neurol. 1991;48:842–4. doi: 10.1001/archneur.1991.00530200084023. [DOI] [PubMed] [Google Scholar]
- 101.Afshar K, BoydKing A, Sharma OP, Shigemitsu H. Gastric sarcoidosis and review of the literature. J Natl Med Assoc. 2010;102:419–22. doi: 10.1016/s0027-9684(15)30577-0. [DOI] [PubMed] [Google Scholar]
- 102.Kasamatsu A, Kanazawa H, Watanabe T, Matsuzaki O. Oral sarcoidosis: Report of a case and review of literature. J Oral Maxillofac Surg. 2007;65:1256–9. doi: 10.1016/j.joms.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 103.Suresh L, Radfar L. Oral sarcoidosis: A review of literature. Oral Dis. 2005;11:138–45. doi: 10.1111/j.1601-0825.2005.01014.x. [DOI] [PubMed] [Google Scholar]


