Abstract
Background & Aims
The World Health Organization (WHO) HBV and HCV elimination targets, set in 2016 and based on projections to 2030, were unable to consider the impact of intervening factors. To evaluate the impact of the COVID-19 pandemic on viral hepatitis elimination programs, the European Association for the Study of the Liver (EASL) conducted a survey in liver centers worldwide in 2021.
Methods
A web-based questionnaire was distributed (May-July 2021) to all EASL members representing clinical units providing HBV and HCV hepatitis care. Results are expressed as absolute numbers and reduction rates for each care activity.
Results
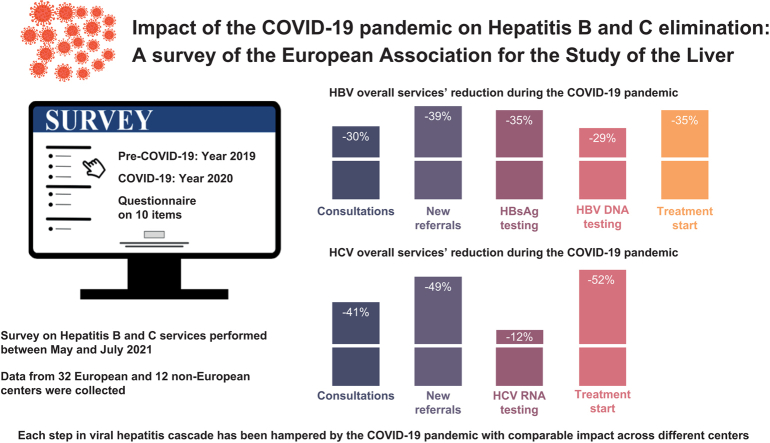
Data were collected from 32 European and 12 non-European clinical centers. Between January 2019 (pre-pandemic) and December 2020 (during the pandemic), chronic HBV consultations decreased by 32% and 26%, new referrals by 38% and 39%, HBV testing rates by 39% and 21% (for HBsAg detection) and 30% and 22% (for HBV DNA detection), and new HBV treatments by 20% and 44% (p = 0.328) in European and non-European centers, respectively. With regard to HCV during the same time frame, the overall reductions were 39% and 50% for consultations, 49% and 49% for new referrals, 11% and 38% for HCV RNA detection, and 51% and 54% for new HCV antiviral treatments for European and non-European Centers, respectively (p = 0.071).
Conclusions
All steps in the viral hepatitis care cascade have been hampered by the COVID-19 pandemic, with a comparable impact across different centers. These data reaffirm the pandemic’s major effect on global viral hepatitis elimination programs and suggest that actions to achieve the WHO 2030 targets should be reconsidered and revised to account for each country's progress relative to pre-pandemic values.
Lay summary
The EASL multinational survey conclusively shows that viral hepatitis elimination programs, expected to provide control of hepatitis B and hepatitis C worldwide by 2030, have been held back by the COVID-19 pandemic in clinical centers from several European and non-European countries, with a comparable impact across centers. Limitations in the cascade of care for both HBV and HCV were linked to limited access to screening, consultations, specific testing, and actual treatment. As restrictions for COVID-19 begin to lift, efforts to diagnose and provide treatment for viral hepatitis should remain high on the list of priorities for public health officials to maintain the WHO elimination efforts. Measures that have been put in place to control the COVID-19 pandemic could be transferred to increasing the diagnosis and linkage to care of people with hepatitis.
Keywords: Hepatitis B virus, Hepatitis C virus, COVID-19 pandemic, WHO elimination targets
Abbreviations: EASL, European Association for the Study of the Liver; EU Centers, European centers; Non-EU Centers, Non-European centers; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; WHO, World Health Organization
Graphical abstract
Highlights
-
•
Viral hepatitis elimination programs have been hampered by the COVID-19 pandemic.
-
•
A survey performed in several clinical centers shows the impact of COVID-19 on all steps of the viral hepatitis care cascade.
-
•
Measures used to control COVID-19 could be used to increase the diagnosis and linkage to care of people living with hepatitis B and C.
Introduction
Over the last decades, cirrhosis and hepatocellular carcinoma due to chronic infection with HBV or HCV have led to at least 1 million deaths every year worldwide.1,2 The WHO has set the goal of eliminating viral hepatitis as a global public health threat by 2030 through immunization, screening, and treatment programs.
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection and associated (COVID-19) pandemic disrupted the healthcare systems of most countries because of the overwhelming demand for COVID-19 care and the ensuing diversion of resources and public attention to this purpose. The WHO goals for HBV and HCV elimination are thus facing major hurdles regarding both screening and treatment, and are at risk of failure. Because of the pandemic, hospital liver care departments have reallocated health professionals and reduced or suspended outpatient care. Hepatitis elimination programs and interventions (screening, diagnosis, and treatment) have been reduced or halted altogether.[3], [4], [5], [6], [7] Moreover, social distancing, lockdown measures, quarantine rules, and the perceived risk of SARS-CoV-2 associated with hospital attendance have curtailed the number of outpatient visits for non-COVID conditions.[8], [9], [10]
To assess the impact of the COVID-19 pandemic on the viral hepatitis elimination goals in clinical centers of different countries, the European Association for the Study of the Liver (EASL) performed a survey to evaluate HBV and HCV diagnosis and treatment during 2019 (pre-COVID-19) and 2020 (during COVID-19). The data obtained address items that are directly connected to the criteria for measuring viral hepatitis elimination: separate evaluation of outpatient consultations, testing, new referrals, and the start of treatment for HBV and HCV infection. The survey’s ultimate goal was to provide insight into the health policies in place to tackle the challenges of COVID-19 at a critical moment of hepatitis elimination.
Materials and methods
Between May 2021 and July 2021, SurveyMonkey-1 was used to distribute a web-based survey to all EASL members (European and non-European) with activities in hepatitis care. The survey consisted of a 10-item questionnaire including the identifying information and population volume of each center, and simple questions related to the impact of SARS-CoV-2 on the following items: total outpatient consultations for HBV and HCV; new HBV and HCV referrals; HBsAg, HBV DNA, and HCV RNA tests performed; the number of patients who started HCV and HBV treatment; and whether the center was also treating patients with COVID-19 (survey provided in the Supplementary information). Quantitative and qualitative changes occurring in these healthcare tasks were analyzed by comparing the activity during the pandemic period (January-December 2020) to that of the pre-pandemic period (January-December 2019).
Statistical analysis
Quantitative variables were analyzed with the Mann-Whitney U test or Student’s t test, as appropriate, and the Wilcoxon test in the case of paired samples. Results are expressed as the median and IQR. Categorical variables were compared using the chi-square test or Fisher’s exact test when frequencies were less than 5%, and are expressed as frequencies and percentages. Results were considered statistically significant at p values lower than 0.05. All statistical analyses were performed using IBM SPSS, version 26.0 (SPSS Inc., Armonk, NY, USA).
Results
Thirty-two clinical centers from the WHO European region (EU) and 12 from WHO non-European regions (Non-EU) are represented in the dataset (the list of centers is reported in the Acknowledgements). In 2 cases, 2 separate centers were affiliated with a single hospital complex, and the specific results were counted separately in the overall and EU and Non-EU center estimations. Potential differences between different respondents in the same hospital but from different clinical centers could not be evaluated.
Twenty-nine of the 32 EU centers (91%) and all 12 (100%) Non-EU centers also attended to patients with COVID-19 during the pandemic (p = 0.375). Overall, 32/44 (72.7%) participants answered all items on the survey. The activity performed in each center was recorded, evaluated separately, and included in the overall estimations for EU and Non-EU centers. There was a trend to a higher rate of complete survey answers in Non-EU than in EU centers (91.7% vs. 65.6%, p = 0.084). Regarding the HBV-related questions, all Non-EU centers and 65.6% of EU centers completed all the items (100% vs. 65.6%, p = 0.017), whereas there were no significant differences for the HCV-related items (Non-EU, 91.7% vs. EU, 78.1%; p = 0.287).
Impact of COVID -19 on hepatitis B programs
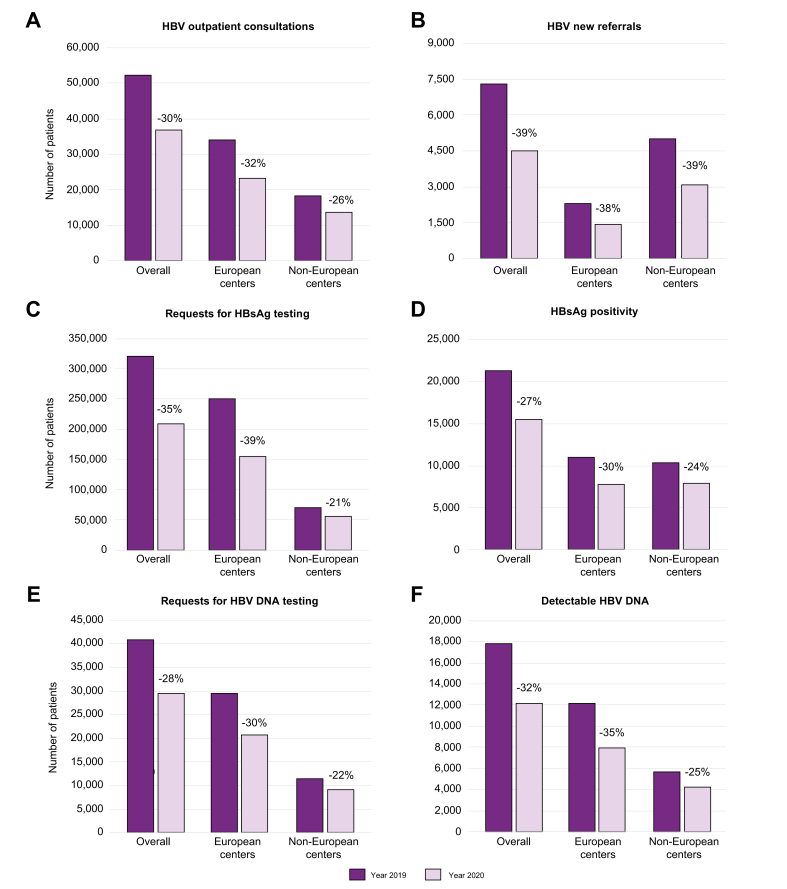
All 32 European centers reported data on both periods (pre-pandemic and during the pandemic) and on the overall number of consultations, new referrals, testing for HBV markers, and prescription of new treatments; however, not all questions were addressed by all centers. During the pandemic period, the number of consultations for chronic HBV infection decreased in 25 of 32 centers (78.1%) and new referrals of HBV patients decreased in 29 of 32 (90.6%) centers. The median reduction between 2019 and 2020 was 32% for consultations and 38% for new referrals (Fig. 1A,B); that is, a drop in numbers from 33,717 to 23,010 for overall consultations and from 2,295 to 1,425 for new referrals (median difference, p <0.001). As a consequence, the number of HBsAg-positive individuals observed in the European centers decreased from 10,847 to 7,642 (median difference, p <0.001) (Fig. 1D).
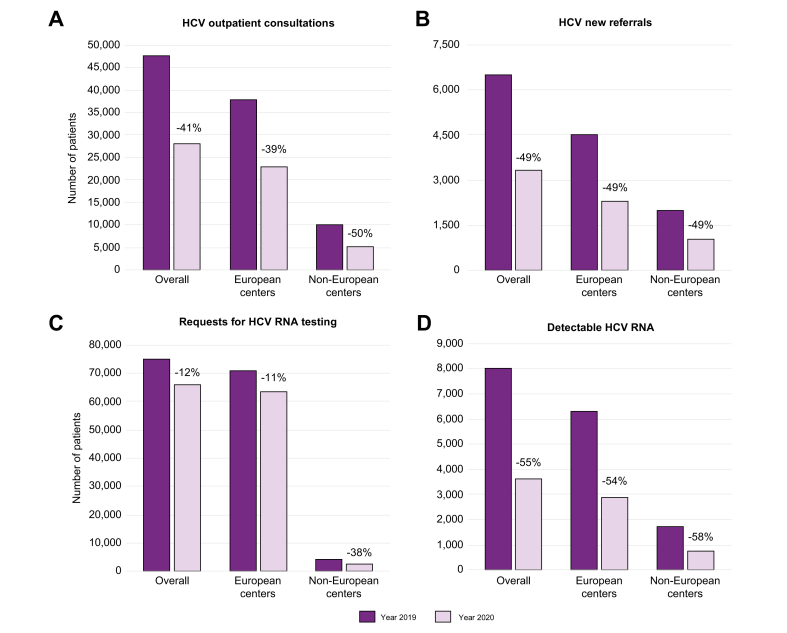
Fig. 1.
HBV consultations, new referrals, and markers testing before and during the COVID-19 pandemic.
The dark purple columns indicate numbers referring to the year 2019 (pre-pandemic) from the European and non-European centers. The light purple columns indicate numbers referring to the year 2020 (during the pandemic) from the European and non-European centers.
Requests for HBV testing were found to be highly affected. Between 2019 and 2020, HBsAg testing rates dropped in 18 of 27 (66.7%) centers and HBV DNA testing in 21 of 25 (84.0%) centers, with a 39% reduction in requests for HBsAg (a decrease from 249,509 to 153,140; median difference, p <0.006) (Fig. 1C) and 31% for HBV DNA (decrease from 29,384 to 20,523; median difference, p <0.001) (Fig. 1E). As a consequence, the number of patients with detectable HBV DNA dropped from 12,138 to 7,845 (median difference, p = 0.002) (Fig. 2F).
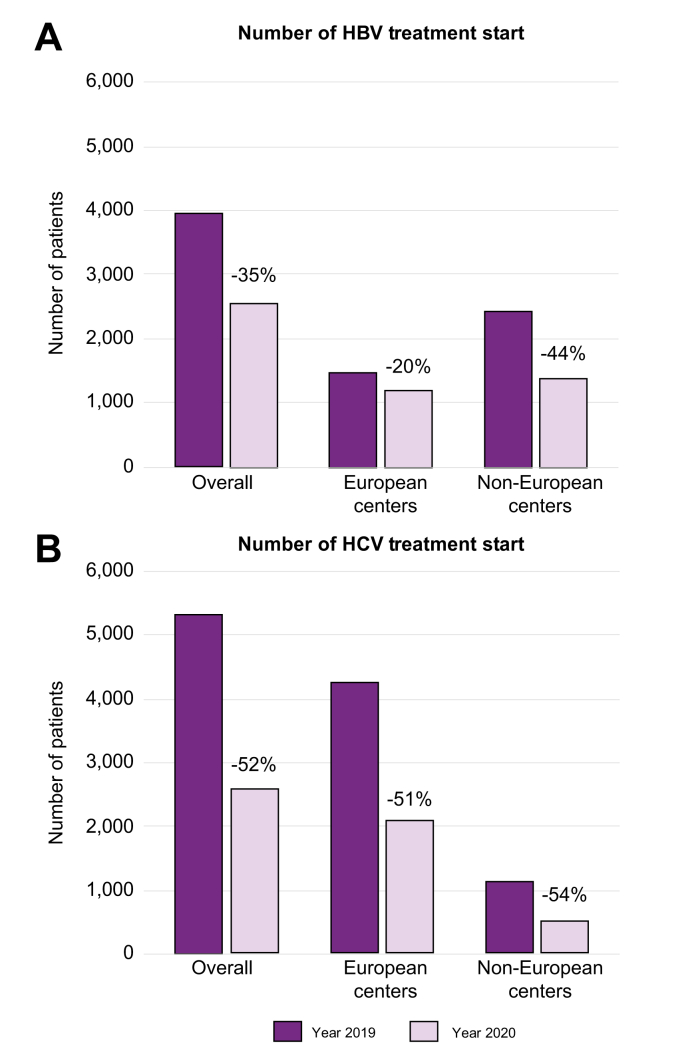
Fig. 2.
Number of patients starting HBV and HCV treatment before and during the COVID-19 pandemic.
The dark purple columns indicate numbers referring to the year 2019 (pre-pandemic) from the European and non-European centers. The light purple columns indicate numbers referring to the year 2020 (during the pandemic) from the European and non-European centers.
HBV treatment rates also decreased: in European centers, 20% fewer patients with HBV were started on antivirals in the pandemic period than in the year before (Fig. 2A), and 20 of the 28 centers (71%) providing data on HBV treatment reported a reduction. The absolute drop in the number of HBsAg-positive individuals starting therapy at the European centers went from 1,495 to 1,196 (median difference, p <0.001) (Fig. 2A).
The trends seen in the 12 Non-EU centers were largely comparable (p >0.05) to those in the EU centers (Fig. 1, Fig. 2). Between 2019 and 2020 there was a 26% reduction in HBV consultations (from 18,250 to 13,543 patients; median difference, p = 0.007) and a 39% drop in new HBV referrals (from 4,994 to 3,037 patients; p = 0.004) (Fig. 1A,B). HBsAg testing requests dropped by 21% (from 69,220 to 54,492; p = 0.018) and HBV DNA requests by 22% (from 11,345 to 8,887; median difference, p = 0.018) (Fig. 1C,E). The number of HBsAg-positive patients seen at Non-EU centers decreased from 10,307 to 7,798 (median difference, p = 0.012) (Fig. 1D) and the number with detectable HBV DNA from 5,622 to 4,206 (median difference, p = 0.12) (Fig. 1F). There was a 44% reduction in HBV treatments, from 2,448 to 1,382 (median difference, p = 0.012) (Fig. 2B).
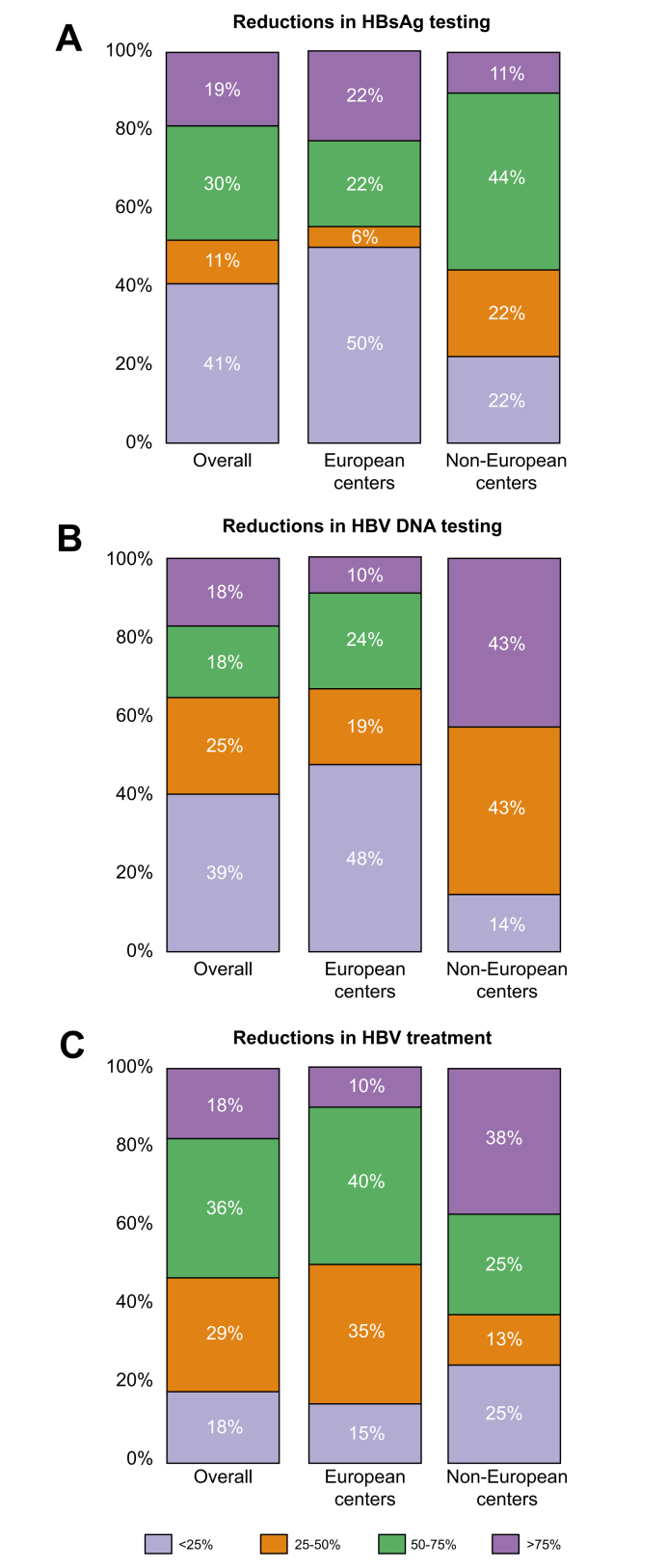
The order of magnitude of the reductions in HBV testing and treatment during the pandemic is shown in Fig. 3A-C. Overall, around 50% of centers reported a reduction of 50% or less in HBsAg (Fig. 3A) and HBV DNA testing (Fig. 3B) and more than 50% in the number of patients starting HBV treatment (Fig. 3C).
Fig. 3.
Reduction rates in HBV testing and treatment during the COVID-19 pandemic.
Different colors indicate the percent decrease for each marker analyzed. The percent decrease is expressed as the number inside each colored box on a scale of 100%.
Impact of COVID -19 on hepatitis C programs
In European countries, the number of consultations for chronic HCV infection decreased during the pandemic period in 27 (87%) of the 31 centers that replied to this question. The reduction during 2020 vs. 2019 was 39% for consultations (from 37,590 to 22,908) and 49% for new referrals (from 4,490 to 2,271), with a highly significant median difference for both (p <0.001) (Fig. 4A,B).
Fig. 4.
HCV consultations, new referrals, and HCV RNA testing before and during the COVID-19 pandemic.
The dark purple columns indicate numbers referring to the year 2019 (pre-pandemic) from the European and non-European centers. The light purple columns indicate numbers referring to the year 2020 (during the pandemic) from the European and non-European Centers.
Requests for HCV RNA testing were greatly affected, with rates dropping in 21 of the 26 (81%) centers that replied to this question. There was an 11% reduction in HCV RNA test requests between 2019 and 2020, with a decrease in numbers from 70,761 to 63,161 (median difference, p = 0.001) (Fig. 4C).
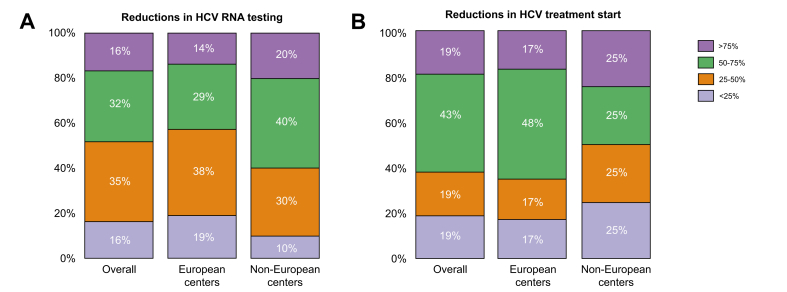
In total, 43% of centers reported a greater than 50% reduction in HCV RNA testing (Fig. 5A). The number of HCV RNA-positive patients detected dropped from 1,709 to 722 (median difference, p = 0.005) (Fig. 4D).
Fig. 5.
Reduction rates in HCV testing and treatment during COVID-19 pandemic.
Different colors indicate the percent decrease for each marker analyzed. The percent decrease is expressed as the number inside each colored box on a scale of 100%.
HCV treatment rates in 31 of the 32 centers providing this data were much reduced; 29 of 31 centers (93.5%) reported that 51% fewer patients with HCV (from 4,209 to 2,047) were started on antiviral treatment during the pandemic period compared to the year before (Fig. 2B, median difference, p <0.001).
As was seen for HBV, trends in the HCV data reported by the 12 non-European centers were largely comparable to those of European centers that replied in this survey (p >0.05) (Fig. 2, Fig. 4). Compared to the situation in 2019, there was a significant 50% reduction in HCV consultations (from 9,895 to 4,972 patients) and a 49% drop in new referrals (from 1,987 to 1,023 patients) in 2020 (median differences, p = 0.002 and p = 0.003, respectively) (Fig. 4A,B). Requests for HCV RNA testing dropped by 38% (from 3,820 to 2,360; median difference, p = 0.005) (Fig. 4C). The number of HCV RNA-positive patients seen at Non-EU centers decreased by 58%, from 1,709 to 722 (Fig. 4D), and there was a 54% reduction in HCV treatments, from 1,089 to 499 (median differences, p = 0.005 and p = 0.011, respectively) with more than 60% of centers reporting a more than 50% reduction in HCV treatment start (Fig. 2, Fig. 4B).
Discussion
An essential step in eliminating viral hepatitis is screening and diagnosis, to enable the prompt initiation of treatment to control hepatitis B infection, cure hepatitis C, and prevent onward transmission. Underdiagnosis is a key hurdle in chronic HBV and HCV infection, as it has a long-term impact on transmission probability, progression of liver disease, and morbidity and mortality rates. COVID-19 has placed a major strain on national healthcare systems at a critical time in the global effort to eliminate viral hepatitis. Previous modeling studies reported that the COVID-19 pandemic would potentially have a great impact on the HCV liver disease burden worldwide. This notion has been supported by several surveys and studies describing the effect of the pandemic on hepatitis C-related health care.[5], [6], [7],[11], [12], [13], [14] This EASL survey adds real-life data regarding each step of the care cascade, not only for HCV but also with specific regard for HBV infection, derived from units focusing on HBV and HCV clinical care in various centers worldwide. Based on the survey data, the pandemic has had a considerable impact on hepatitis consultations and referral of new patients with HBV or HCV infection, with reductions of 31% and 38%, respectively. This is likely due to the reduction in screening activity at the time of the pandemic, the first step toward diagnosis and treatment. These findings are in line with data from the World Health Alliance survey, performed across 32 low- and middle-income countries, in which only 36% of respondents reported that people were accessing testing services, either because of closures or avoidance of these facilities due to COVID-19.7 A single-center experience11 found that hospital-based anti-HCV testing decreased by 49.6% and identification of new HCV-infected patients by 42.1%. Thus, public health initiatives for testing and screening have fallen behind both in developed and developing nations, indicating a need for re-launch strategies.
The present survey data show that requests for HBsAg and HBV DNA testing decreased by 31% and 39%, respectively, in the pandemic period, whereas requests for HCV RNA dropped by only 11%. There may be several explanations for the difference between HBV and HCV testing rates: low population or physician awareness of the likelihood of infection, less strict follow-up of HBsAg carriers during the pandemic, greater use of reflex HCV RNA testing, or low levels of HCV testing during the pre-pandemic period. Modest pre-pandemic HCV testing rates in 2019 have been cited as an existing challenge to the achievement of the HCV elimination target.[15], [16], [17] Data from a large national clinical reference laboratory show that despite an increase in anti-HCV-positive test results following the first pandemic wave, the rates of HCV RNA-positive individuals and prescriptions for direct-acting antivirals (DAAs) have not returned to pre-pandemic levels, remaining low in comparison to 2018 and 2019.12 This suggests that many patients who returned for testing in June and July 2020 might have been at lower risk, implying a potentially significant gap regarding higher-risk patients.13
In addition, the survey shows that the number of patients who started HBV or HCV treatment in 2020 decreased by 20% and 52%, respectively, compared to 2019. These figures agree with those of the World Hepatitis Alliance survey, which reported that treatment access significantly deteriorated in low- and middle-income countries due to COVID-19 in 15 (52%) of 29 respondents, and the Coalition for Global Hepatitis Elimination (CGHE) survey, in which 39% and 21% of respondents reported a more than 50% decline in treatment volumes for HCV and HBV, respectively.7,13
Even before the COVID-19 pandemic, the progress toward hepatitis elimination was slowing. Very few countries were on track to reach the 2030 hepatitis C elimination goals set by the WHO,17 with the main factor for “on-track” status being maintenance of a high HCV treatment rate through screening. A web-based survey performed in Italy during the first COVID-19 wave showed that initiation of antiviral therapy had been deferred in 23% of the centers included and that treatment had been started in only 20% to 28%, even in patients considered at high risk for progression.5 In a follow-up survey performed in the same 55 centers 1 year later,14 routine prescription of DAAs had been restarted in 60.0% of sites, compared to 12.6% in the first wave. Nonetheless, the number of patients treated in 2020 reported by the Italian Medicines Agency was still less than half (57%) of those treated in 2019 (36,348 treated in 2019 vs. 15,664 treated in 2020). As Italy still has an estimated pool of 400,000 potentially treatable viremic persons with HCV infection,18 it is reasonable to infer that the bulk of missing treatments is mainly due to deficient implementation of screening campaigns. The situation in Italy may be comparable to that of Spain, where the number of cases treated decreased by 47% (as reported by the Spanish National Health Registry: 15,859 HCV-infected patients were DAA-treated in 2019 vs. 8,440 in 2020) and that of Slovenia, (as reported by the National Viral Hepatitis Expert Board), where a 52% decrease in the number of treated patients with HCV was reported during the pandemic (216 patients treated in 2019 vs. 104 in 2020).
Interestingly, the survey showed that the COVID-19 pandemic has had a comparable impact across different centers, even though there is regional variation in the approach to screening, treatment, and follow-up. Razavi et al.19 have suggested a change to absolute targets for HCV hepatitis elimination to allow countries to achieve the targets with their own optimized service coverage initiatives. The results of this survey could be helpful to evaluate these absolute targets, considering the great impact of COVID-19 on almost all steps of viral hepatitis care in several clinical settings and various countries.
Our study has some of the obvious limitations of an online survey requested by invitation, with almost all responses coming from hospitals and only one participating community center. The centers included are heterogeneous, they belong to different health care systems, and in some cases, the full requested dataset was not completed. Thus, they may not be representative of the overall national situation in each case. Furthermore, the results are based only on the data from centers responding to the survey and the conclusions may not apply to all countries. Another limitation is possible time-related bias. The spread of the COVID-19 pandemic in subsequent waves had different timings in the various countries, and this may have caused regional differences. Notwithstanding these potential biases, this EASL survey outlines many missed opportunities for hepatitis testing and treatment during the COVID-19 pandemic, which could have serious consequences for patients with chronic hepatitis B and C and those at risk of infection through contact with undiagnosed or untreated people. The figures reported here, showing the trends in the decrease of clinical care activities for HBV and HCV during the pandemic, can be useful to evaluate whether their potential recovery in coming years will suffice to achieve the viral hepatitis elimination targets.
The burden of the COVID-19 pandemic will extend beyond the morbidity and mortality related to SARS-CoV-2 infection. Each step in the viral hepatitis care cascade has been affected. The total number of deaths due to hepatitis B and C is estimated to increase from 434,724 in 2017 to 527,829 in 2030 if there is no implementation of tailored interventions. The mathematical model projection reported by Blach et al.20 has estimated that a 1-year delay in viral hepatitis elimination programs will result in 44,800 (95% uncertainty interval [UI]: 43,800-49,300) excess hepatocellular carcinoma cases and 72,300 (95% UI: 70,600-79,400) excess liver-related deaths, relative to the no-delay scenario globally, from 2020 to 2030. The impact of COVID-19 will be different for each country, but the increased disease and mortality burden will also occur to varying degrees in high-income countries.21 Data from this survey could be useful for updates of the global models on viral hepatitis elimination, providing potentially stronger input derived from estimation of specific real-life activities related to the care of patients with viral hepatitis.
In the long-term, the negative economic effects of the pandemic will exert extra pressure on viral hepatitis-related public health initiatives, further dampening hopes of a hepatitis-free world. It is crucial to ensure that policymakers do not lose sight of the immense benefits of viral hepatitis elimination and that the opportunities presented by the current crisis are identified and seized upon when possible. The hindrance to HCV testing prior to the pandemic is related, in part, to the paucity of hepatitis awareness programs, and the measures taken during the pandemic have further led to closure of community-based education and screening programs and in-person events. The problems related to HBV and HCV diagnosis and screening can only be overcome by raising awareness, favoring partnerships, and allocating resources. There may be a window of opportunity in combining HBV and HCV detection with COVID-19 vaccination efforts. Further studies are needed to evaluate the duration of the decline in the hepatitis cascade of care, particularly in light of the health disparities in historically underserved communities with more limited access to health care.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contributions
All authors made substantial contributions to the conceptualization and design, the analysis, and data interpretation. AC and MB: conceptualization, data interpretation, and review and editing of the manuscript. LAK and AC: analysis and interpretation of the data, writing the original draft of the manuscript. MRB: formal statistical analysis and data interpretation. MM, FN and TB: conceptualization, design, and critical revision of the manuscript. All authors have approved the final version of the manuscript.
Data availability statement
The EASL Survey questions are reported in the Supplementary material. Data provided by each center can be accessed by request to the EASL secretary.
Disclaimer
Preliminary data from this survey were presented at the annual AASLD meeting (November 2020), abstract # 41.
Conflicts of interest
LK: Received speaker fees from Gilead and Abbvie and consultancy fees from Abbvie. MB: Received grants/research support from Abbvie, BMS, and Gilead. Received honoraria/consultation fees or participated in an advisory board for Abbvie, Gilead, GSK, Janssen, MSD, and Roche. Participated in a company-sponsored speaker’s bureau for Abbvie, Gilead, and Janssen. MRB: Received research funding from Gilead and served as a speaker for Gilead and AbbVie. MM: Nothing to declare. TB: Received grants/research support from Abbvie, BMS, Gilead, MSD/Merck, Humedics, Intercept, Merz, Norgine, Novartis, Orphalan, and Sequana Medical. Received honoraria/consultation fees or served on the advisory board for Abbvie, Alexion, Bayer, Gilead, GSK, Eisai, Enyo Pharma, Falk Foundation, HepaRegeniX GmbH, Humedics, Intercept, Ipsen, Janssen, MSD/Merck, Novartis, Orphalan, Roche, Sequana Medical, SIRTEX, SOBI, and Shionogi. Participated in a company-sponsored speaker’s bureau for Abbvie, Alexion, Bayer, Gilead, Eisai, Intercept, Ipsen, Janssen, MedUpdate GmbH, MSD/Merck, Novartis, Orphalan, Sequana Medica, SIRTEX, and SOBI. FN: Advisor for Gilead and AbbVie, and received speaker fees from Roche Diagnostics. AC: Received grants and research support from AbbVie, Alfasigma, Bayer, BMS, Gilead Sciences, Intercept, MSD, and Roche. Served on advisory committees for AbbVie, Alfasigma, Bayer, BMS, Gilead Sciences, Intercept, MSD, and Roche. Participated in speaking and teaching activities for AbbVie, Alfasigma, Bayer, BMS, and Gilead Sc.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The authors wish to thank Yoanna Nedelcheva and Morgane Guex of EASL Policy, Advocacy, and Public Health for their assistance in the development of the survey.
Gratitude is also expressed to all the clinical centers participating in the survey, as follows: Klinik Ottakring,Vienna, Austria; Gaffrée e Guinle University Hospital, Rio de Janeiro, Brasil; University of Toronto, Toronto, Canada; Siham Abdel Rehim, Alexandria, Egypt; West Tallinn Central Hospital, Tallinn, Estonia; Hopital de la Croix Rousse, Lyon, France; Santosa Hospital Bandung Central, Bandung, Indonesia; Policlinico di Bari, Bari, Italy; Humanitas Research Hospital, Milan Italy; Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy, University of Naples Federico II; Naples, Italy; Azienda Ospedaliera Università di Padova, Padova, Italy; University Hospital of Messina, Sicilia, Italy; Azienda Ospedaliera Universitaria Policlinico Paolo, Palermo, Italy; Azienda Ospedaliera Ospedali Riuniti Villa Sofia Cervello, Palermo, Italy; Novara Hospital, Italy; University Hospital of Pisa, Pisa, Italy; Fondazione Policlinico Universitario Agostino Gemelli IRCCS - Università Cattolica del Sacro Cuore; Rome, Italy; University of Salerno, Baronissi, Italy; Hospital of Siena, Siena, Italy; Babylon GIT center, Hillah Babylon, Iraq; Ogaki Municipal Hospital, Japan; Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico; Sabai Phyu, Bahan Township (Myanmar); NAMS, Bir Hospital, Kathmandu, Nepal; Department of Infectious Diseases and Hepatology, Medical University of Bialystok, Bialystok, Poland; Centro Hospitalar Universitário Porto, Portugal; University of Novo Sad, Novo Sad,Serbia; Clinic for Infectious Diseases and Febrile Illnesses, University Medical Center Ljubljana, Ljubljana Slovenia; Hospital Universitario Valle Hebrón, Barcelona, Spain; Hospital Clinic Barcelona, Barcelona, Spain; Hospital Ramon y Cajal, Madrid Spain; Marques de Valdecilla University Hospital, Santander, Spain; Mahidol University, Siriraj hospital, Bangkok, Thailand; Faculty of Medicine, Mahidol University, Bangkok, Thailand; Ankara University School of Medicine, Ankara, Turkey; Ankara Şehir Hastanesi, Ankara Turkey; Kocaeli University Gastroenterology Department, Izmir, Turkey;Central Hospital №1 Zhytomyr, Ukraine; Queen Elizabeth Hospital Birmingham, Birmingham United Kingdom; Nottingham University Hospital Nottingham United Kingdom; Hep C Positive, Swindon, United Kingdom.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100531.
Supplementary data
The following are the supplementary data to this article:
References
- 1.The Lancet null Towards elimination of viral hepatitis by 2030. Lancet. 2016;388:308. doi: 10.1016/S0140-6736(16)31144-8. [DOI] [PubMed] [Google Scholar]
- 2.Thomas D.L. Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041–2050. doi: 10.1056/NEJMra1810477. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum L. The untold toll - the pandemic’s effects on patients without Covid-19. N Engl J Med. 2020;382:2368–2371. doi: 10.1056/NEJMms2009984. [DOI] [PubMed] [Google Scholar]
- 4.Fagiuoli S., Lorini F.L., Remuzzi G. Covid-19 Bergamo hospital crisis unit. Adaptations and lessons in the Province of Bergamo. N Engl J Med. 2020;382:e71. doi: 10.1056/NEJMc2011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aghemo A., Masarone M., Montagnese S., Petta S., Ponziani F.R., Russo F.P., et al. Assessing the impact of COVID-19 on the management of patients with liver diseases: a national survey by the Italian association for the study of the liver. Dig Liver Dis. 2020;52:937–941. doi: 10.1016/j.dld.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buti M., Domínguez-Hernández R., Casado M.A. Impact of the COVID-19 pandemic on HCV elimination in Spain. J Hepatol. 2021;74:1246–1248. doi: 10.1016/j.jhep.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wingrove C., Ferrier L., James C., Wang S. The impact of COVID-19 on hepatitis elimination. Lancet Gastroenterol Hepatol. 2020;5:792–794. doi: 10.1016/S2468-1253(20)30238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viganò M., Voza A., Harari S., Eusebio A., Ripoll Pons M., Bordonali M., et al. Letter to the Editor: Clinical management of nonrespiratory diseases in the COVID-19 pandemic: what have we done and what needs to be done? Telemed J E Health. 2020;26:1206–1208. doi: 10.1089/tmj.2020.0148. [DOI] [PubMed] [Google Scholar]
- 9.De Filippo O., D’Ascenzo F., Angelini F., Bocchino P.P., Conrotto F., Saglietto A., et al. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in Northern Italy. N Engl J Med. 2020;383:88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Lancet Oncology null COVID-19 and cancer: 1 year on. Lancet Oncol. 2021;22:411. doi: 10.1016/S1470-2045(21)00148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperring H., Ruiz-Mercado G., Schechter-Perkins E.M. Impact of the 2020 COVID-19 pandemic on ambulatory hepatitis C testing. J Prim Care Community Health. 2020;11 doi: 10.1177/2150132720969554. 2150132720969554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufman H.W., Bull-Otterson L., Meyer W.A., Huang X., Doshani M., Thompson W.W., et al. Decreases in hepatitis C testing and treatment during the COVID-19 pandemic. Am J Prev Med. 2021;61:369–376. doi: 10.1016/j.amepre.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laury J., Hiebert L., Ward J.W. Impact of COVID-19 response on hepatitis prevention care and treatment: results from global survey of providers and program managers. Clin Liver Dis (Hoboken) 2021;17:41–46. doi: 10.1002/cld.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponziani F.R., Aghemo A., Cabibbo G., Masarone M., Montagnese S., Petta S., et al. Management of liver disease in Italy after one year of the SARS-CoV-2 pandemic: a web-based survey. Liver Int. 2021;41:2228–2232. doi: 10.1111/liv.14998. [DOI] [PubMed] [Google Scholar]
- 15.Hatzakis A., Lazarus J.V., Cholongitas E., Baptista-Leite R., Boucher C., Busoi C.-S., et al. Securing sustainable funding for viral hepatitis elimination plans. Liver Int. 2020;40:260–270. doi: 10.1111/liv.14282. [DOI] [PubMed] [Google Scholar]
- 16.Accelerating access to hepatitis C diagnostics and treatment n.d. https://www.who.int/publications-detail-redirect/9789240019003 (accessed April 18, 2022).
- 17.Gamkrelidze I., Pawlotsky J.-M., Lazarus J.V., Feld J.J., Zeuzem S., Bao Y., et al. Progress towards hepatitis C virus elimination in high-income countries: an updated analysis. Liver Int. 2021;41:456–463. doi: 10.1111/liv.14779. [DOI] [PubMed] [Google Scholar]
- 18.Kondili L.A., Andreoni M., Alberti A., Lobello S., Babudieri S., Roscini A.S., et al. Estimated prevalence of undiagnosed HCV infected individuals in Italy: a mathematical model by route of transmission and fibrosis progression. Epidemics. 2021;34 doi: 10.1016/j.epidem.2021.100442. [DOI] [PubMed] [Google Scholar]
- 19.Polaris Observatory Collaborators The case for simplifying and using absolute targets for viral hepatitis elimination goals. J Viral Hepat. 2021;28:12–19. doi: 10.1111/jvh.13412. [DOI] [PubMed] [Google Scholar]
- 20.Polaris Observatory Collaborators Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022 doi: 10.1016/S2468-1253(21)00472-6. 0. [DOI] [PubMed] [Google Scholar]
- 21.Mennini F.S., Marcellusi A., Robbins Scott S., Montilla S., Craxi A., Buti M., et al. The impact of direct-acting antivirals on hepatitis C virus disease burden and associated costs in four european countries. Liver Int. 2021;41:934–948. doi: 10.1111/liv.14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The EASL Survey questions are reported in the Supplementary material. Data provided by each center can be accessed by request to the EASL secretary.