Abstract
Truncated variants of GTF-I from Streptococcus downei MFe28 were purified by means of a histidine tag. Sequential deletions showed that the C-terminal domain was not directly involved in the catalytic process but was required for primer activation. A fully active catalytic core of only 100 kDa was isolated.
Oral mutans streptococci play a key role in the induction of human dental caries, virulence being mediated by extracellular glucose polymers (glucans) synthesized from sucrose by secreted glucosyltransferases (GTFs) (EC 2.4.1.5) (8). GTFs catalyze cleavage of sucrose and the transfer of glucosyl residues to a growing glucan chain. It is also possible to observe sucrose hydrolysis (the transfer of glucose to water) or the synthesis of oligosaccharides resulting from the transfer of glucose to an alternative sugar acceptor (11).
GTFs are of high molecular mass, around 160 kDa. Except for DSR-A of Leuconostoc mesenteroides B1299 (10), they possess a signal peptide followed by a highly variable region of about 100 amino acids. They have a highly conserved catalytic core region of about 900 amino acids (19) followed by a C-terminal glucan-binding domain covering about 400 amino acids composed of series of tandem repeats designated A, B, C, and D repeats (14, 15). At least part of the C-terminal domain is required for glucan synthesis, though there are conflicting reports as to whether it is involved in sucrose splitting as well (1, 3, 4). The C terminus may influence the structure of the glucan (12, 20), though such studies have not been carried out with a GTF that makes an insoluble glucan. GTF-I expressed by Streptococcus downei produces a water-insoluble glucan containing α(1–3) glucosyl linkages (3, 16). Other almost identical gtfI genes have been isolated from Streptococcus sobrinus, which is taxonomically closely related to S. downei (1, 17). To extend earlier observations on the effect of C-terminal truncation on GTF-I activity (3) and to define what minimum size was required for glucan synthesis, we have introduced a rapid and efficient method for purifying recombinant GTF.
Construction of truncated GTF-I forms and expression in Escherichia coli.
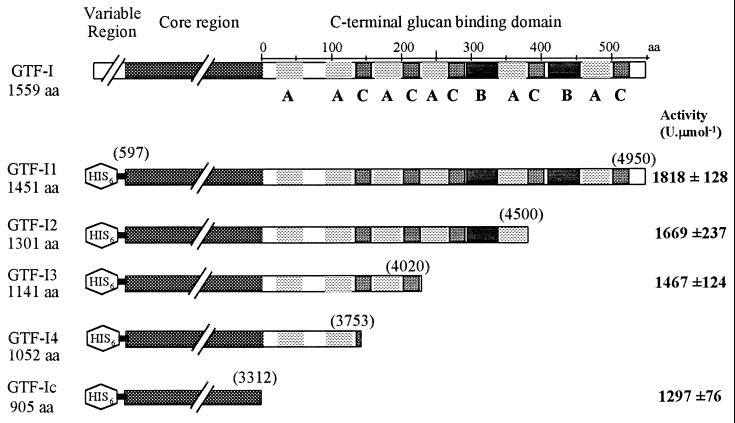
The signal peptide and N-terminal highly variable region are not required to have fully active GTF (1, 10). We therefore engineered modified gtfI genes encoding only the conserved core region (GTF-Ic) or the conserved core region with either full-length (GTF-I1) or truncated C-terminal domains (GTF-I2, GTF-I3, and GTF-I4) by subcloning PCR-amplified gtfI fragments using VENT polymerase (Biolabs Inc.) and primers designed upon the gtfI sequence (3) containing engineered restriction sites into pTrcHisA (Invitrogen). The resulting plasmids expressed fusion proteins containing an additional stretch of six histidine residues at the N terminus (Fig. 1). Overproduction of these GTF-I variants was achieved by culturing E. coli XL1-Blue in Luria broth. After induction with isopropyl-β-d-thiogalactopyranoside, protein extracts were obtained by sonication (Fig. 2A). Activity assays carried out using the dinitrosalicylic acid method specific for reducing sugars (9) showed very little difference between GTF-I4 and GTF-I3. Therefore, GTF-I4 was chosen for further study, as were GTF-I1, GTF-I2, and GTF-Ic.
FIG. 1.
Schematic representation of protein sequence of GTF-I and the truncated variants constructed. Molecular sizes of GTF-I, GTF-I1, GTF-I2, GTF-I3, GTF-I4, and GTF-Ic are indicated. aa, amino acids. Repeat units (A, B, and C) are also localized in the C-terminal glucan-binding domain. Numbers in brackets represent the end points of the different variants in the gtfI sequence (3). Activity obtained with purified GTF-I1, GTF-I2, GTF-I3, and GTF-Ic is indicated at right in boldface.
FIG. 2.
Expression of the GTF-I-derived forms truncated at the C terminus. Shown is sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of GTF-I1, GTF-I2, GTF-I3, and GTF-Ic in E. coli XL1-Blue (A) and after purification (B). Experimentally determined sizes were 160, 150, 135, and 110 kDa for GTF-I1, GTF-I2, GTF-I3, and GTF-Ic, respectively, and were in good agreement with the predicted values.
Purification of GTF-I variants.
Previous processes for purification of GTFs were based upon their glucan binding ability and necessitated the use of denaturing agents or the addition of dextran for elution (2, 6, 15). His-tagged GTF-I variants were purified under native conditions using Ni-nitrilotriacetic acid agarose resin (Qiagen). Binding was realized with sodium phosphate buffer (pH 7.8) (500 mM NaCl in the presence of 5 mM imidazole) followed by washing with sodium phosphate buffer (pH 6.0) (500 mM NaCl in the presence of 20 mM imidazole). Elution was performed with 200 mM imidazole. Proteins present in the elution peak were dialyzed overnight at 4°C against 50 mM Tris-HCl buffer (pH 7.5). Overall, 200-ml cultures yielded about 4 mg of protein purified to a high degree of purity (Fig. 2B).
Characterization of GTF-I variants.
Reactions were performed at 37°C in 50 mM Tris-HCl buffer (pH 7.5) containing 100 g of sucrose liter−1. One unit was defined as the amount of enzyme that catalyzed the formation of 1 μmol of fructose per min. An activity assay (Fig. 1) showed there is a slight decrease of activity in variants with truncated C-terminal domains but that it is less extensive than those reported in earlier studies (1, 3, 4). GTF-Ic retained almost 70% of the activity obtained with GTF-I1. This difference may be attributable to the fact that previously activity has only been estimated from crude E. coli extracts with no allowance for possible variations in expression levels, whereas we used purified enzyme. For GTFs producing α(1–6)-rich glucan, a large part of the C-terminal domain is required (7, 9). In contrast, the present results show that for a GTF producing α(1–3)-rich glucan, the core region alone is active.
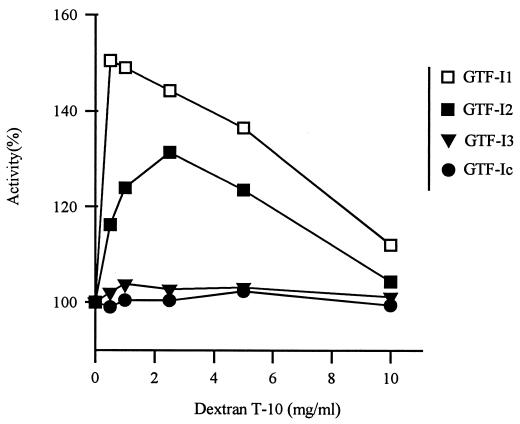
Kinetic analysis showed that the Km value for sucrose remained unchanged (53.5 ± 3.7 mM) and only kcat values were affected by deletions in a significant manner (from 43.4 ± 1.5 s−1 for GTF-I1 to 36.0 ± 3.0 s−1 for GTF-Ic). This suggested that the turnover rate of the reaction but not the ability for substrate binding was modified by the truncations in the C-terminal domain. This domain is, however, required for activation by dextran T-10 (Fig. 3). Addition of 0.5 mg of dextran T-10 ml−1 to full-size GTF-I1 resulted in an activity increase of 1.5. This induction level was similar to those obtained with entire GTF-I from S. sobrinus OMZ176 (5) or S. downei MFe28 (13). GTF-I2 was less sensitive, and the activator effect was abolished with GTF-I3 and GTF-Ic. The results clearly show that dextran T-10 binds at a site separate from the sucrose binding site present in the core region.
FIG. 3.
Effect of dextran T-10 on GTF-I1, GTF-I2, GTF-I3, and GTF-Ic activities, which were determined at different dextran T-10 concentrations. Activity obtained without dextran T-10 was defined as 100% activity for each enzyme.
Previous studies indicated that the ability to transfer the glucose moiety to glucan or another acceptor may be affected by C-terminal truncation (1, 4). We therefore assayed glucan synthesis after the complete depletion of sucrose by measuring the mass of the total polymer produced and assayed the rate of hydrolysis by measuring the concentration of free glucose with the TC d-glucose/d-fructose kit (Boehringer Mannheim). In addition, the acceptor reaction was determined by using high-performance liquid chromatography to measure the yield of leucrose (5-O-α-d-glucopyranosyl-d-fructopyranose), which results from the transfer of glucosyl residues onto fructose (18). All the GTF-I variants, even the core molecule GTF-Ic, exhibited the same yields of glucan synthesis, sucrose hydrolysis, and leucrose (Table 1). Glucan synthesized by GTF-I variants was analyzed by 13C nuclear magnetic resonance spectra and recorded with a Bruker AC 300 spectrometer. This analysis showed that they contained only α(1–3) glucosyl linkages, as reported for intact GTF-I (14), so the presence of the C-terminal glucan-binding domain of GTF-I does not influence the structure of the glucan produced.
TABLE 1.
Relative distribution of glucosyl residues during mutan synthesis reaction with GTF-I1 and variants with truncated C termini
| Glucose | Yield (%) (mean ± SD) in:
|
|||
|---|---|---|---|---|
| GTF-I1 | GTF-I2 | GTF-I3 | GTF-Ic | |
| Free | 11.1 ± 1.7 | 11.5 ± 1.9 | 11.6 ± 1.7 | 10.9 ± 1.6 |
| Incorporated into mutan | 58.1 ± 2.1 | 57.0 ± 4.0 | 58.6 ± 3.3 | 58.4 ± 1.8 |
| Incorporated into leucrose | 27.5 ± 3.9 | 29.6 ± 2.4 | 28.8 ± 4.1 | 27.2 ± 2.7 |
In conclusion, the core region of GTF-I contains all the determinants necessary for the catalytic mechanism, and the glucan-binding domain played no role in the mechanism controlling the specificity for substrate or acceptor, though its presence was required for activation by dextran T-10. This has allowed us to isolate a fully active and stable catalytic domain of only 100 kDa from GTF-I that will be a suitable model for structure studies by X-ray crystallography. However, it is now clear that deletions of the C-terminal domains of different GTFs have different effects. The reason for this variation remains unknown, and a full explanation may require knowledge of the three-dimensional structure of a number of GTFs.
Acknowledgments
This work was supported by Wellcome Trust grant 04539 and the European Project BIOTECH CT98-0022.
We are indebted to M. Vignon (CERMAV, Grenoble, France) for performing the nuclear magnetic resonance analyses.
REFERENCES
- 1.Abo H, Matsumura T, Kodama T, Ohta H, Fukui K, Kato K, Kagawa H. Peptide sequences for sucrose splitting and glucan binding within Streptococcus sobrinus glucosyltransferase (water-insoluble glucan synthetase) J Bacteriol. 1991;173:989–996. doi: 10.1128/jb.173.3.989-996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devulapalle K S, Mooser G. Subsite specificity of the active site of glucosyltransferases from Streptococcus sobrinus. J Biol Chem. 1994;269:11967–11971. [PubMed] [Google Scholar]
- 3.Ferretti J J, Gilpin M L, Russell R R B. Nucleotide sequence of a glucosyltransferase gene from Streptococcus sobrinus MFe28. J Bacteriol. 1987;169:4271–4278. doi: 10.1128/jb.169.9.4271-4278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato C, Kuramitsu H K. Carboxyl-terminal deletion analysis of the Streptococcus mutans glucosyltransferase-I enzyme. FEMS Microbiol Lett. 1990;60:299–302. doi: 10.1016/0378-1097(90)90321-g. [DOI] [PubMed] [Google Scholar]
- 5.Koga S, Sato S, Inoue M, Takeuchi K, Furuta T, Hamada S. Role of primers in glucan synthesis by glucosyltransferases from Streptococcus mutans strain OMZ176. J Gen Microbiol. 1983;129:751–754. doi: 10.1099/00221287-129-3-751. [DOI] [PubMed] [Google Scholar]
- 6.Kuramitsu H K. Characterization of extracellular glucosyltransferase activity of Streptococcus mutans. Infect Immun. 1975;12:738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lis M, Shiroza T, Kuramitsu H K. Role of C-terminal direct repeating units of the Streptococcus mutans glucosyltransferase-S in glucan binding. Appl Environ Microbiol. 1995;61:2040–2042. doi: 10.1128/aem.61.5.2040-2042.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loesche W J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monchois V, Reverte A, Remaud Simeon M, Monsan P, Willemot R M. Effect of Leuconostoc mesenteroides NRRL B-512F dextransucrase carboxy-terminal deletions on dextran and oligosaccharide synthesis. Appl Environ Microbiol. 1998;64:1644–1649. doi: 10.1128/aem.64.5.1644-1649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monchois V, Willemot R M, Remaud Simeon M, Croux C, Monsan P. Cloning and sequencing of a gene coding for a novel dextransucrase from Leuconostoc mesenteroides NRRL B-1299 synthesizing only α(1–6) and α(1–3) linkages. Gene. 1996;182:23–32. doi: 10.1016/s0378-1119(96)00443-x. [DOI] [PubMed] [Google Scholar]
- 11.Mooser G. Glycosidases and glycosyltransferases. In: Sigman D, editor. The enzymes. XX. London, United Kingdom: Academic Press Inc.; 1992. pp. 187–221. [Google Scholar]
- 12.Nakano Y J, Kuramitsu H K. Mechanism of Streptococcus mutans glucosyltransferases: hybrid-enzyme analysis. J Bacteriol. 1992;174:5639–5646. doi: 10.1128/jb.174.17.5639-5646.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remaud-Simeon, M. Personal communication.
- 14.Russell R R B. Molecular genetics of glucan metabolism in oral streptococci. Arch Oral Biol. 1990;35(Suppl.):53S–58S. doi: 10.1016/0003-9969(90)90131-s. [DOI] [PubMed] [Google Scholar]
- 15.Russell R R B, Coleman D, Dougan G. Expression of a gene for glucan-binding protein from Streptococcus mutans in Escherichia coli. J Gen Microbiol. 1985;131:295–299. doi: 10.1099/00221287-131-2-295. [DOI] [PubMed] [Google Scholar]
- 16.Russell R R B, Gilpin M L, Mukasa H, Dougan G. Characterization of glucosyltransferase expressed from a Streptococcus sobrinus gene cloned in Escherichia coli. J Gen Microbiol. 1987;133:935–944. doi: 10.1099/00221287-133-4-935. [DOI] [PubMed] [Google Scholar]
- 17.Sato S, Inoue M, Hanada N, Aizawa Y, Isobe Y, Katayama T. DNA sequence of the glucosyltransferase gene of serotype d Streptococcus sobrinus. DNA Seq. 1993;4:19–27. doi: 10.3109/10425179309015618. [DOI] [PubMed] [Google Scholar]
- 18.Stolada F H, Sharpe E H, Koepsell H J. The preparation properties and structure of the disaccharide leucrose. J Am Chem Soc. 1956;78:2514–2518. [Google Scholar]
- 19.Tsumori H, Minami T, Kuramitsu H K. Identification of essential amino acids in the Streptococcus mutans glucosyltransferases. J Bacteriol. 1997;179:3391–3396. doi: 10.1128/jb.179.11.3391-3396.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vickerman M M, Sulavik M C, Minick P E, Clewell D B. Changes in the carboxyl-terminal repeat region affect extracellular activity and glucan products of Streptococcus gordonii glucosyltransferase. Infect Immun. 1996;64:5117–5128. doi: 10.1128/iai.64.12.5117-5128.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]